Abstract
Anti-inflammatory polyphenols, such as epigallocatechin-3-gallate (EGCG), have been shown to protect against the toxicity of environmental pollutants. It is well known that bioactive food compounds such as polyphenols may exert their protection by modulating inflammatory pathways regulated through nuclear factor-kappa B (NF-κB) signaling. EGCG has been reported to inhibit NF-κB activation. We hypothesize that EGCG can protect against PCB-induced endothelial inflammation in part through epigenetic regulation of NF-kB-regulated inflammatory genes. In order to test this hypothesis, human endothelial cells (EA.hy926) were exposed to physiologically relevant levels of coplanar PCB 126 and/or 15 or 30 μM of EGCG, followed by quantification of NF-kB subunit p65, histone acetyltransferase (HAT) p300 and histone deacetylases (HDACs) accumulation through ChIP assay in the promotor region of inflammatory genes. In addition, the enrichment of the acetylated H3 (ac-H3) was also quantified. PCB 126 exposure increased the expression of vascular inflammatory mediators, including interleukin (IL)-6, C-reactive protein (CRP), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and IL-1α/β, which were prevented by pre-treatment with EGCG. This inhibitory effect by EGCG correlated with abolished nuclear import of p65, decreased chromatin binding of p65 and p300, as well as increased chromatin binding of HDAC1/2. Furthermore, EGCG induced hypo-acetylation of H3, which accounts for deactivation of downstream genes. These data suggest that EGCG-induced epigenetic modifications can decrease PCB-induced vascular toxicity.
Keywords: EGCG, vascular inflammation, PCB 126, p65, p300, HDACs
Introduction
Cardiovascular diseases (CVD), such as atherosclerosis, is a multifactorial, multistep disease that involves chronic inflammation from initiation to progression and, eventually, plaque rupture [1, 2]. A large variety of cytokines and adhesion molecules are well-established contributors to CVD, including interleukin (IL)-6, IL-1, C-reactive protein (CRP), vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) [3–6]. Besides the involvement of immune cells in the development of atherosclerosis [6], the endothelial cell also participates and its dysfunction is a critical underlying cause of CVD [7]. Epidemiological studies provide substantial evidence that the pathology of CVD is associated with exposure to environmental pollutants, such as polychlorinated biphenyls (PCBs) [8–10]. Importantly, PCB exposure is believed to cause vascular inflammation through expression of several inflammatory markers, cytokines and adhesion molecules [11–13]. Thus, these inflammatory markers may become potential therapeutic targets for nutritional intervention against CVD, which is triggered or enhanced by exposure to environmental pollutants.
Epigallocatechin gallate (EGCG) is a polyphenolic compound abundant in green tea and it has gained significant attention for its vascular protective effects [14, 15]. A human study demonstrated that EGCG can reverse endothelial dysfunction and improve flow-mediated dilation in patients with coronary artery disease [16]. EGCG has been reported to have an anti-NF-κB transactivation activity in a broad range of human malignancies, such as colon cancer, breast cancer, lung cancer, and in chronic inflammation [17–20]. Recently, an in vivo study utilizing db/db mice showed that EGCG reduced vascular inflammation through a NF-κB-mediated mechanism [21]. Although EGCG is known to protect endothelial cells against PCB 126-induced vascular inflammation [22, 23], it is not clear if epigenetic regulation of the NF-κB pathway is involved in the protective properties of EGCG.
NF-κB plays a major role in governing the vascular inflammatory process by directly up-regulating cytokines and adhesion molecules [24]. The most abundant form of NF-κB is a p50/p65 heterodimer, in which p65 contains the transcriptional activation domain [24–26]. NF-κB activation may arise from the increased nuclear importation of the p65 subunit [25], and PCB 126 has been shown to increase nuclear localization of p65 (Liu et al., unpublished observations). The NF-κB subunit p65 has been shown to interact with p300 and histone deacetylases (HDACs) 1–3, which seems to be crucial for the epigenetic regulation of NF-κB-regulated genes through reversible histone acetylation [27–29]. This reversible acetylation may be caused by exchange between the HDACs and HATs in the promoter region of target genes. Therefore, we hypothesize that the balance between HDAC and HAT activity determines subsequent expression levels of NF-κB-regulated genes. In the present study, we investigated the role of EGCG in the prevention of PCB 126-induced vascular inflammation and further examined the epigenetic mechanism by which EGCG decreases vascular toxicity of PCB 126. Our data show that EGCG can reduce PCB 126-induced expression of p65, prevent PCB 126-induced p65 nuclear translocation, down-regulate the expression of NF-κB-regulated genes, and modulate HAT and HDAC chromatin recruitment and histone hypo-acetylation, which may further suppress vascular inflammation.
Material and Methods
Cell cultures and treatments
EA.hy926 human endothelial cells (ATCC, Manassas, VA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT) as described [30]. Once confluent, cells were synchronized by culturing in DMEM supplemented with 1% FBS overnight before further treatments were initiated.
Stock solution of PCB 126 (AccuStandard, New Haven, CT) was prepared in DMSO. All cultures contained the same amount of DMSO and concentrations in the media were kept below 0.05%. Cells were pre-treated with 15 or 30 μM of EGCG (Sigma, St. Louis, MO) for 8 h, followed by 16 h treatment with 0.03 nM of PCB 126, which represents the plasma PCB 126 level of individuals with long-time PCB exposure [12, 31]. Plasma concentrations in humans of the parent compound EGCG are usually in the low μM range (less than 1 μM) [32, 33]. Concentrations of EGCG in our study were chosen based on our past data showing maximum endothelial cell protection against PCB exposure [23]. Such high concentrations can be considered supraphysiological.
Immunocytochemistry
To investigate the effect of EGCG on NF-κB activation as evidenced by p65 translocation, nuclear localization of p65 was assessed by immunocytochemical staining. Endothelial cells were seeded in chamber slides and pre-treated with EGCG for 8 h, followed by treatment with PCB 126 in fresh medium for 8 h. Tumor necrosis factor alpha (TNF-α) (10 ng/ml) was used as a positive control. After washing with PCB, cells were fixed in 4% paraformaldehyde for 10 min and permeablized with 0.25% Triton X-100 at room temperature. After blocking with 1% bovine serum albumin, cells were first incubated with mouse monoclonal anti-p65 (1:500) overnight at 4°C and then with Alexa Fluor-labeled secondary antibody (1:1000) for 1 h at room temperature in the dark. The nucleus were stained with 4, 6-diamidino-2-phenylindole (DAPI), and then images were viewed under the fluorescence microscope.
Quantitative real-time PCR (qRT-PCR)
Quantitative RT-PCR was carried out using SYBR Green master mix (Applied Biosystems, Carlsbad, CA) to evaluate the abundance of mRNA transcripts and amplicons in immunoprecipitated chromatin. The relative amount of each gene was normalized using housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The relative occupancy of epigenetic marks was calculated as described previously [34] and values are reported as fold enrichment. Primer pairs are listed in Table 1.
Table 1.
Oligonucleotide primers used for quantitative real-time PCR
| Gene1 | Sequence (5′-3′) | F/R2 | Template |
|---|---|---|---|
| CRP | GCCCTTCAGTCCTAATGTCCTG | F | cDNA |
| AGCATAGTTAACGAGCTCCCAGA | R | ||
| CRP | CGCTCCTATGATCCACCCA | F | gDNA |
| CCAAAGTGCTGGGATTACAGGCA | R | ||
| GAPDH | TCCACTGGCGTCTTCACC | F | cDNA |
| GGCAGAGATGATGACCCTTT | R | ||
| GAPDH | GCAAGGTCATCCCTGAGCTGAA | F | gDNA |
| GGGTGTCGCTGTTGAAGTCAGA | R | ||
| ICAM-1 | GCCACCCCAGAGGACAA | F | cDNA |
| CCATTATGACTGCGGCTGCTA | R | ||
| ICAM-1 | CAGGATTTTCCCAGGCCTTCTGA | F | gDNA |
| GGAGGTCGCGCAGCAGAA | R | ||
| IL-1α | ACAAAAGGCGAAGAAGACTGA | F | cDNA |
| GGAACTTTGGCCATCTTGAC | R | ||
| IL-1α | CGATTTCTCCCTTCCTCCTAGAAACTTGA | F | gDNA |
| CGGGGAATTTACAGGGAAGAATTCAGT | R | ||
| IL-1β | CTGTCCTGCGTGTTGAAAGA | F | cDNA |
| TTGGGTAATTTTTGGGATCTACA | R | ||
| IL-1β | GATTTGGAAAGTCCCAGTACTACCCTGA | F | gDNA |
| CCTCTGTCCCCTAAATGTTTCCACA | R | ||
| IL-6 | CAATGAGGAGACTTGCCTGGTGA | F | cDNA |
| TGGCATTTGTGGTTGGGTCAG | R | ||
| IL-6 | GACATGCCAAAGTGCTGAGTCACTA | F | gDNA |
| ATTGAGACTCATGGGAAAATCCCACA | R | ||
| p65 | GGCCATGGACGAACTGTTCC | F | cDNA |
| GAGGGTCCTTGGTGACCAG | R | ||
| VCAM-1 | GTCTTGGTCAGCCCTTCCT | F | cDNA |
| ACATTCATATACTCCCGCATCCTTC | R | ||
| VCAM-1 | GGACAGAGAGAGGAGCTTCAGCA | F | gDNA |
| GGCGGAGGGAAATCCCTTCAA | R |
Genbank entries: CRP = Homo sapiens c-reactive protein (NM_000567.2 for cDNA, NC_000001.10 for gDNA); GAPDH = Homo sapiens glyceraldehyde-3-phosphate dehydrogenase (NM_002046.5 for cDNA, NC_000012.11 for gDNA); ICAM-1 = Homo sapiens intercellular adhesion molecule 1 (NM_000201.2 for cDNA, NC_000019.9 for gDNA); IL-1α = Homo sapiens interleukin-1 alpha (NM_000575.3 for cDNA, NC_000002.11 for gDNA); IL-1β =Homo sapiens interleukin-1 beta (NM_000576.2 for cDNA, NC_000002.11 for gDNA); IL-6 = Homo sapiens interleukin-6 (NM_000600.3 for cDNA, NC_000007.13 for gDNA); p65 (also known as RelA) = Homo sapiens v-rel avian reticuloendotheliosis viral oncogene homolog A (NM_021975.3 for cDNA); VCAM-1 = Homo sapiens vascular cell adhesion molecule 1 (NM_001078.3 for cDNA, NC_000001.10 for gDNA).
Abbreviations: cDNA, complementary DNA (for mRNA quantification); F, forward; gDNA, genomic DNA (for ChIP assays); R, reverse.
Chromatin immunoprecipitation (ChIP)
ChIP assay was conducted to quantify the abundance of p65, p300, HDAC1-3, and ac-H3 in the promotor regions of target genes [35]. Sheared chromatin was immuneprecipitated with antibodies to p65 (Santa Cruz Biotechnology, Santa Cruz, CA), p300 (Santa Cruz Biotechnology), HDAC1 (Santa Cruz Biotechnology), HDAC2 (Santa Cruz Biotechnology), HDAC3 (Santa Cruz Biotechnology), rabbit IgG (Santa Cruz Biotechnology), ac-H3 (Abcam), and H3 (Santa Cruz Biotechnology). Non-specific IgG was used as a negative control and produced signals much less than those produced by target-specific antibodies. H3 was used to normalize for ac-H3 occupancy. The promoter regulating the expression of GAPDH localizes in euchromatin and was used as a control locus. Data are expressed as fold enrichment, representing the ChIP signal as the fold increase in signal relative to the background signal.
Immunoblotting
Protein levels of p65, IκB, and I kappa B kinase (IKK) in whole cell extracts were determined by immunoblotting as described previously [12], using antibodies to p65, IKK-α/β, phosphorylated IκB-α and phosphorylated IκB-β, respectively. All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and the dilution factor of all antibodies was 1:1500). Actin (Santa Cruz Biotechnology) was used as a loading control.
Statistical analysis
Data were tested for normal distributions and homogenous variances. Statistical significance was assessed by one-way ANOVA and Fisher’s protected least significant difference posthoc test [36]. JMP 10.0.0 (SAS Institute, Cary, NC) was used to perform all analysis. The results are expressed as means ± SD, and a probability value of p ≤ 0.05 was considered statistically significant.
Results
EGCG decreases PCB 126-induced p65 expression and its nuclear translocation
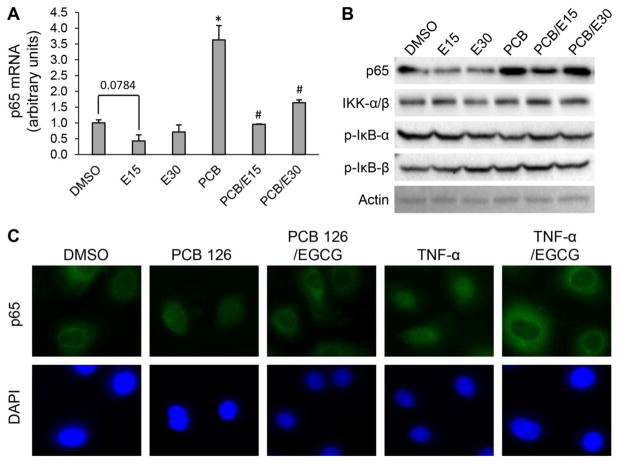
The NF-κB transcription factor is known as a signal integrator that controls two initial steps in the process of vascular inflammation [37]. To determine whether EGCG can modulate PCB-mediated induction of NF-κB subunit p65, endothelial cells were pre-treated with EGCG and followed by treatment with PCB 126. Concentrations of EGCG were chosen based on preliminary data showing maximum endothelial cell protection against PCB 126 exposure without cell death (data not shown). Exposure to PCB 126 significantly increased expression of p65 at the transcription level, and the PCB 126-induced p65 mRNA was markedly decreased when cells were pre-treated with EGCG at either 15 or 30 μM (Fig 1A). Protein level of p65 detected by immunoblotting was consistent with its mRNA transcripts (Fig 1B). In addition, protein levels of IKK and phosphorylated IκB were not affected by PCB 126 or EGCG treatment (Fig 1B).
Figure 1. EGCG decreases PCB-induced NF-κB activation.
(A) EGCG decreased PCB 126-induced p65 expression. Cellular p65 mRNA level was measured in endothelial cells which were pre-treated with 15 or 30 μM of EGCG (E15, E30) for 8 h, and then treated in fresh medium with 0.03 nM of PCB 126 for 16 h. Values are means ± SD with n=3. *Significantly increased compared to the DMSO control. #Significantly decreased compare to the PCB treatment group. (B) Protein levels of p65, IKK-α/β, IκB-α and -β were measured by immunoblotting. Actin was used as the loading control. (C) EGCG decreased PCB 126-mediated p65 nuclear translocation. The intracellular localization of endogenous p65 was analyzed by immunocytochemical staining using anti-p65 (green) and the nucleus was visualized by DAPI staining (blue).
Since the activation of the NF-κB pathway is associated with the intracellular localization of its subunits, p65 nuclear translocation is commonly used as a marker of activation [38]. The intracellular localization of endogenous p65 was analyzed by immunocytochemical staining with anti-p65 antibody, and nuclear DNA was revealed by DAPI staining (Fig 1C). The p65 subunit localized to the nucleus after exposure of PCB 126 for 8 h, leading to a substantial increase in the amount of p65 found in the nucleus. However, EGCG reversed PCB 126-induced nuclear translocation of p65 or enhanced its nuclear export (Fig 1C). Cells stimulated with TNF-α were used as a positive control. Taken together, these results suggest that EGCG reduced PCB 126-mediated induction of p65 and suppressed NF-κB signaling via preventing PCB 126-induced p65 nuclear translocation in endothelial cells.
EGCG attenuates PCB 126-mediated induction of NF-κB-regulated inflammatory genes
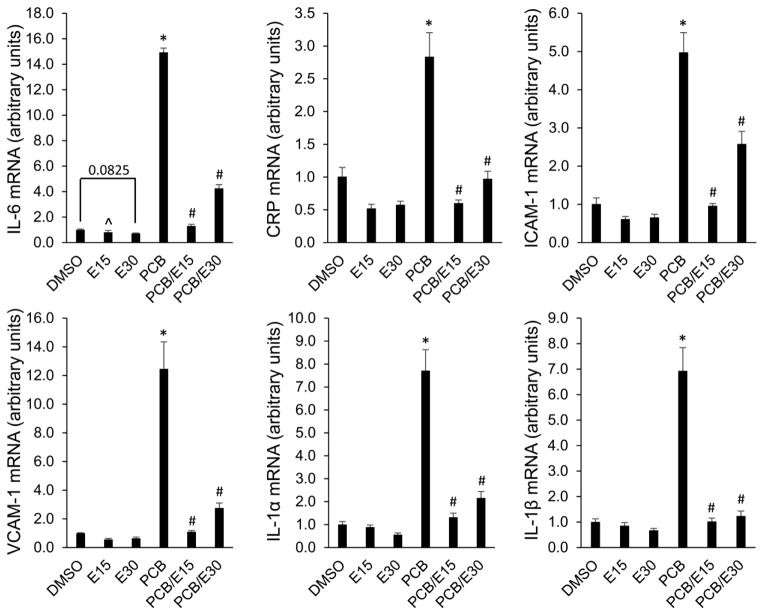
To test whether EGCG can protect endothelial cells against PCB 126-induced vascular inflammation, expression of NF-κB-regulated inflammatory genes IL-6, CRP, ICAM-1, VCAM-1, IL-1α, and IL-1β were quantified using qRT-PCR. IL-6 is a potent proatherogenic cytokine involved in many pathologic processes characterized by vascular inflammation and has been shown to enhance fatty lesion development [39]. As shown, a significant increase of IL-6 mRNA was observed following exposure to PCB 126 and pre-treatment with EGCG significantly reduced PCB 126-induced IL-6 mRNA expression (Fig 2). In addition, PCB 126-induced expression of CRP, ICAM-1, VCAM-1, and IL-1α/β were also significantly attenuated by EGCG. It is also worth noting that EGCG treatment markedly decreased the expression of these inflammatory genes at base-line levels. Overall, these data imply that EGCG can protect endothelial cells from PCB 126-mediated vascular inflammation through down-regulation of proatherogenic cytokines and adhesion molecules.
Figure 2. EGCG attenuates PCB 126-induced inflammatory gene expression.
The mRNA level of inflammatory genes was analyzed using qRT-PCR. Endothelial cells were pretreated with 15 or 30 μM of EGCG for 8 h, and followed by treatment in fresh medium with 0.03 nM of PCB 126 for 16 h. Values are the means ± SD with n=3. *Significantly increased compared to the DMSO control. ^Significantly decreased compared to the DMSO control. #Significantly decreased compare to the PCB treatment group.
EGCG reverses PCB 126-induced chromatin binding of acetylation enzymes and induces H3 hypo-acetylation
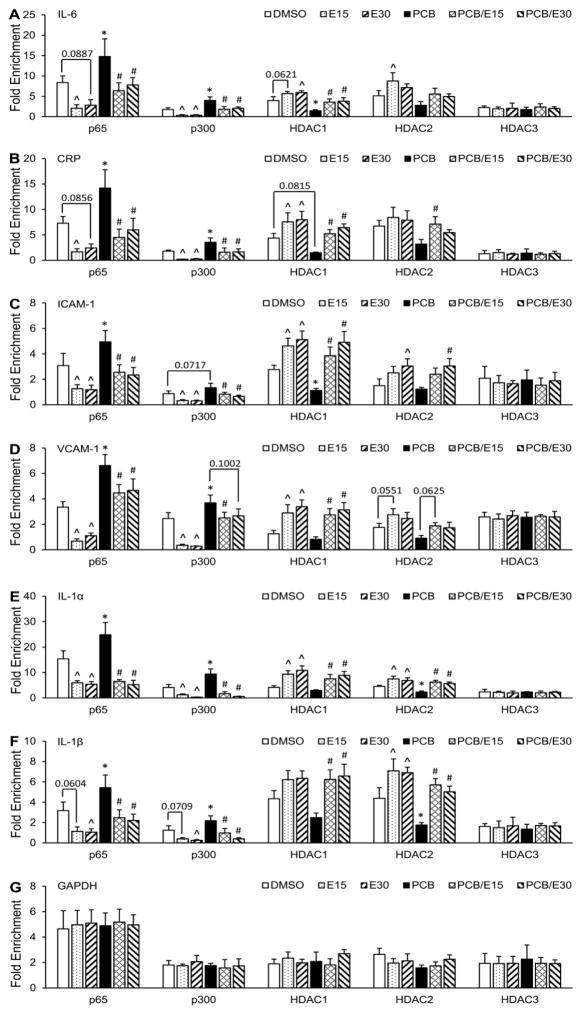
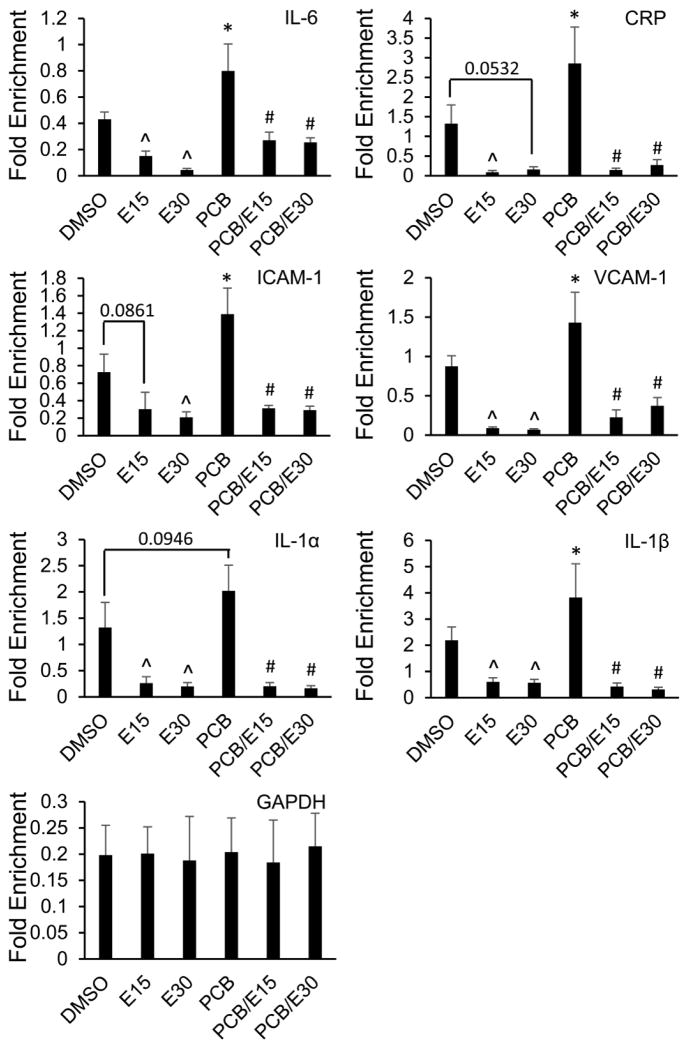
NF-κB subunit p65 has also been shown to interact with HDACs 1–3 and p300, which seem to be crucial for the acetylation/deacetylation of particular histones of target genes [27–29]. The reversible acetylation caused by the exchange between the HAT and HDAC binding in the promoter region of target genes determines the subsequent expression level of NF-κB-regulated genes. In order to test this hypothesis, the recruitment of p300 and HDAC 1–3 to the promoter region of NF-κB-regulated genes were assessed. The promoters of NF-κB-regulated genes, such as IL-6, CRP, ICAM-1, VCAM-1, and IL-1α/β, contain well-characterized NF-κB-binding sites. ChIP assay was used to determine whether p65, p300 or HDAC 1–3 were associated with those promoters in PCB 126-treated endothelial cells with or without EGCG. PCB 126 exposure led to a significant increase in binding of p65 and p300 to the NF-κB-binding site of the IL-6 promoter. However, addition of EGCG resulted in a markedly decreased recruitment of both p65 and p300 to the NF-κB-binding site (Fig 3A left). The recruitment of HDAC1/2 was modulated by treatment with PCB 126/EGCG oppositely compared to p65 and p300. Specifically, PCB 126 exposure decreased the chromatin binding of HDAC1/2 and pre-treatment with EGCG significantly enhanced HDAC1/2 recruitment in the promoter region of IL-6 (Fig 3A right). Changes in the chromatin recruitment of HDAC3 was not affected by either PCB 126 or EGCG treatment. Similar epigenetic modification pattern were observed in the promoter region of other NF-κB-regulated genes CRP, ICAM-1, VCAM-1, and IL-1α/β (Fig 3B–G). Furthermore, EGCG reduced acetylation of H3, which is an activation mark, and substantially resulted in the deactivation of the inflammatory genes described above (Fig 4). These data suggest that EGCG can prevent vascular inflammation through exchange of HAT and HDAC chromatin recruitment and histone hypo-acetylation events, further confirming the importance of the balance between HAT and HDAC to the NF-κB-mediated inflammatory signaling.
Figure 3. EGCG reverses PCB 126-induced chromatin binding of acetylation enzymes.
The recruitment of p65, p300, and HDAC1-3 was modulated by EGCG in the promoters of IL-6 (A), CRP (B), ICAM-1 (C), VCAM-1 (D), IL-1α (E), IL-1β (F) and GAPDH (G). Sheared chromatin from whole-cell lysates was immunoprecipitated with antibodies against the indicated proteins. IgG was used as a negative control. The GAPDH promoter was used as control locus. Values are means ± SD with n=3. *Significantly increased compared to the DMSO control. ^Significantly decreased compared to the DMSO control. #Significantly decreased compared to the PCB treatment group.
Figure 4. EGCG induces H3 hypo-acetylation as a result of HAT/HDAC binding exchange.
The H3 acetylation was enhanced by PCB 126 and decreased by the pre-treatment with EGCG in the NF-κB-regulated genes. H3 is the control for nucleosomal occupancy. Values are means ± SD with n=3. *Significantly increased compared to the DMSO control. ^Significantly decreased compared to the DMSO control. #Significantly decreased compared to the PCB treatment group.
Discussion
Atherosclerosis is a chronic inflammatory disease and the underlying cause of most CVD [40]. Dysfunction of the endothelium is regarded as an important factor in the pathology of atherosclerosis [7]. There is accumulating evidence that persistent organic pollutants such as PCBs can accelerate an inflammatory response in vascular tissues [11–13, 22, 23, 41]. In the current studies, we demonstrated that EGCG significantly attenuated PCB 126-mediated induction of proatherogenic cytokines and adhesion molecules such as IL-6, CRP, ICAM-1, VCAM-1, and IL-1α/β in human endothelial cells. Although the EGCG concentrations used in our study are lower than in previous studies [23], our preliminary data showed that these concentrations exerted maximum endothelial cell protection against PCB exposure.
Inflammatory cytokines and adhesion molecules are directly regulated by the activation of NF-κB [24]. In the nucleus, NF-κB p50/p65 dimers bind to the promoters of NF-κB-dependent inflammatory genes and regulate a large number of genes that are involved in the pathology of atherosclerosis, such as ICAM-1, VCAM-1, and IL-1 [42–45]. NF-κB activation and nuclear localization of p65 were detected in human atherosclerotic lesions in endothelial cells, macrophages and vascular smooth muscle cells [46]. Coplanar PCBs can enhance the activation of NF-κB, which then induces the expression of inflammatory genes encoding a number of mediators of atherogenesis such as inflammatory cytokines and adhesion molecules [47]. Moreover, EGCG has been shown to prevent NF-κB transactivation activity and thus can reduce vascular inflammation [20, 21, 48–50]. In our present study, EGCG prevented PCB 126-induced NF-κB activation by greatly suppressing the increased p65 nuclear translocation. In fact, PCB 126 increased p65 nuclear translocation and its activation similar to TNF-α, an inflammatory cytokine used as a positive control in our experimental settings.
Recent evidence suggests that EGCG can contribute to epigenetic metabolic regulation due to its inhibitory activities to polycomb group proteins, DNA methyltransferases, HDACs, and HATs [51–55], although the possible relevance of the inhibitory activity to its anti-cancer or atheroprotective properties is not well established. The HDAC or HAT inhibitory activity of EGCG has been shown to lead to hyper- or hypo-acetylation of non-histone proteins such as p53, p65, androgen and estrogen receptors [52, 54–56]. Even though in our study p65 acetylation was not diminished by EGCG (data not shown) as demonstrated previously [52], we found that EGCG can reduce the binding of p65 and p300 to the promoter region of NF-κB-regulated genes with an increased recruitment of HDAC1/2. This highlights the importance of the balance between HATs and HDACs in the NF-κB-mediated inflammatory signaling pathway. In general, histone acetylation is a dynamic and reversible post-translational modification regulated by the opposing activities of HATs and HDACs [57]. Acetylation catalyzed by HATs usually marks transcriptionally active genes, as it contributes to the decondensed chromatin state and maintains the unfolded structure of the transcribed nucleosome [57]. In contrast, HDACs are found in corepressor complexes catalyzing deacetylation of histones and resulting in the formation of a compacted, transcriptionally repressed chromatin structure [58]. In our study EGCG modulated HAT and HDAC chromatin recruitment leading to histone hypo-acetylation in downstream target genes, which is consistent with the endpoints of an inflammatory response. Histone hypo-acetylation creates transcriptionally repressed chromatin structure and causes deactivation of downstream genes. HDACs are typically present in multiple distinct complexes while they have relatively low substrate specificity if operating independently of other proteins, and thus may deacetylate multiple lysine residues in histones [59–61]. Therefore, we examined the overall acetylation level of histone H3 instead of specific lysine residues in our study.
Some future studies may be needed to determine the effect of EGCG on histone acetylation in loci not related to inflammation. Our study focused on endothelial inflammation, thus we did not expand to non-inflammatory targets. However, previous studies have shown that EGCG can prevent the expression of the proapoptotic genes Bax and Bad while inducing antiapoptotic genes in a model of 6-hydroxydopamine-induced apoptosis in human neuroblastoma cells [62, 63], which indicates that EGCG could also function in non-inflammatory pathways. Additionally, it is not known if other polyphenols can lead to similar epigenetic changes. Thus, we do not know for certain whether the anti-inflammatory effects of EGCG can be generalized to all polyphenols.
In conclusion, our findings in this study demonstrate that EGCG, the major bioactive polyphenol present in green tea, attenuates PCB-induced vascular inflammation via a repressive epigenetic effect on the NF-κB signaling pathway. These findings indicate that EGCG has anti-inflammatory properties in vascular diseases, which may be in part via epigenetic modifications of NF-κB target genes. Thus, epigenetic regulation of vascular inflammation by EGCG may explain in part its protective mechanisms and thus support the consumption of green tea as a potential candidate for the treatment and prevention of vascular inflammation and atherosclerosis against environmental pollutants.
Highlights.
EGCG reduced PCB 126-induced expression of p65 and repressed NF-κB signaling by preventing PCB 126-induced p65 nuclear translocation.
EGCG down-regulated the expression of proatherogenic cytokines and adhesion molecules to protect endothelial cells from PCB 126-mediated vascular inflammation.
EGCG prevented vascular inflammation through modified HAT and HDAC chromatin recruitment and histone hypo-acetylation, which represses expression of NF-κB-regulated genes.
Acknowledgments
Research reported in this publication was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [P42ES007380], National Institute of General Medical Sciences NIH grant 8 P20 GM103527, and the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project KY007069 under 0220363.
Abbreviations
- ac-H3
acetylated H3
- ChIP
chromatin immunoprecipitation
- CRP
C-reactive protein
- CVD
cardiovascular diseases
- DAPI
4, 6-diamidino-2-phenylindole
- EGCG
epigallocatechin-3-gallate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- ICAM-1
intercellular adhesion molecule-1
- IKK
I kappa B kinase
- IL
interleukin
- NF-κB
nuclear factor-kappa B
- PCBs
polychlorinated biphenyls
- qRT-PCR
quantitative real-time PCR
- TNF-α
tumor necrosis factor alpha
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hansson GK, et al. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91(4):281–91. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Riegsecker S, et al. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013;93(8):307–12. doi: 10.1016/j.lfs.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi C, et al. Pathogen-mediated inflammatory atherosclerosis is mediated in part via Toll-like receptor 2-induced inflammatory responses. J Innate Immun. 2010;2(4):334–43. doi: 10.1159/000314686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakashima Y, et al. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18(5):842–51. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Crother TR, Arditi M. Emerging role of IL-17 in atherosclerosis. J Innate Immun. 2010;2(4):325–33. doi: 10.1159/000314626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Goncharov A, et al. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res. 2008;106(2):226–39. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sergeev AV, Carpenter DO. Residential proximity to environmental sources of persistent organic pollutants and first-time hospitalizations for myocardial infarction with comorbid diabetes mellitus: a 12-year population-based study. Int J Occup Med Environ Health. 2010;23(1):5–13. doi: 10.2478/v10001-010-0010-y. [DOI] [PubMed] [Google Scholar]
- 10.Perkins JT, et al. Polychlorinated biphenyls and links to cardiovascular disease. Environ Sci Pollut Res Int. 2015 doi: 10.1007/s11356-015-4479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eske K, et al. PCB 77 dechlorination products modulate pro-inflammatory events in vascular endothelial cells. Environ Sci Pollut Res Int. 2014;21(10):6354–64. doi: 10.1007/s11356-013-1591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han SG, et al. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol Appl Pharmacol. 2010;246(1–2):74–82. doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majkova Z, et al. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237(1):1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82(12):1807–21. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr. 2007;26(4):373S–388S. doi: 10.1080/07315724.2007.10719626. [DOI] [PubMed] [Google Scholar]
- 16.Widlansky ME, et al. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr. 2007;26(2):95–102. doi: 10.1080/07315724.2007.10719590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CS, et al. Inhibition of lung tumorigenesis by tea. Exp Lung Res. 2005;31(1):135–44. doi: 10.1080/01902140490495525. [DOI] [PubMed] [Google Scholar]
- 18.Doss MX, et al. Trapping of growth factors by catechins: a possible therapeutical target for prevention of proliferative diseases. J Nutr Biochem. 2005;16(5):259–66. doi: 10.1016/j.jnutbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Navarro-Peran E, et al. The anti-inflammatory and anti-cancer properties of epigallocatechin-3-gallate are mediated by folate cycle disruption, adenosine release and NF-kappaB suppression. Inflamm Res. 2008;57(10):472–8. doi: 10.1007/s00011-008-8013-x. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, et al. EGCG attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-kappaB signaling in human umbilical vein endothelial cells. Life Sci. 2013;92(10):589–97. doi: 10.1016/j.lfs.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Babu PV, Si H, Liu D. Epigallocatechin gallate reduces vascular inflammation in db/db mice possibly through an NF-kappaB-mediated mechanism. Mol Nutr Food Res. 2012;56(9):1424–32. doi: 10.1002/mnfr.201200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newsome BJ, et al. Green tea diet decreases PCB 126-induced oxidative stress in mice by up-regulating antioxidant enzymes. J Nutr Biochem. 2014;25(2):126–35. doi: 10.1016/j.jnutbio.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han SG, et al. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol Appl Pharmacol. 2012;261(2):181–8. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 26.Perkins ND. The Rel/NF-kappa B family: friend and foe. Trends Biochem Sci. 2000;25(9):434–40. doi: 10.1016/s0968-0004(00)01617-0. [DOI] [PubMed] [Google Scholar]
- 27.Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21(20):7065–77. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong H, et al. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9(3):625–36. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 29.Sankar N, et al. p300 provides a corepressor function by cooperating with YY1 and HDAC3 to repress c-Myc. Oncogene. 2008;27(43):5717–28. doi: 10.1038/onc.2008.181. [DOI] [PubMed] [Google Scholar]
- 30.Lim EJ, et al. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293(6):H3340–7. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- 31.Schettgen T, et al. Plasma polychlorinated biphenyls (PCB) levels of workers in a transformer recycling company, their family members, and employees of surrounding companies. J Toxicol Environ Health A. 2012;75(8–10):414–22. doi: 10.1080/15287394.2012.674905. [DOI] [PubMed] [Google Scholar]
- 32.Umegaki K, et al. Analytical method of measuring tea catechins in human plasma by solid-phase extraction and HPLC with electrochemical detection. J Nutr Sci Vitaminol (Tokyo) 2001;47(6):402–8. doi: 10.3177/jnsv.47.402. [DOI] [PubMed] [Google Scholar]
- 33.Yang CS, et al. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7(4):351–4. [PubMed] [Google Scholar]
- 34.Wei CL, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124(1):207–19. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Carey MF, Peterson CL, Smale ST. Chromatin immunoprecipitation (ChIP) Cold Spring Harb Protoc. 2009;2009(9):pdb prot5279. doi: 10.1101/pdb.prot5279. [DOI] [PubMed] [Google Scholar]
- 36.Institute, S. StatView Reference. 3. Cary, NC: SAS Publishing; 1998. [Google Scholar]
- 37.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86(2):211–8. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maguire O, et al. Quantifying nuclear p65 as a parameter for NF-kappaB activation: Correlation between ImageStream cytometry, microscopy, and Western blot. Cytometry A. 2011;79(6):461–9. doi: 10.1002/cyto.a.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber SA, et al. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19(10):2364–7. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 40.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 41.Petriello MC, et al. PCB 126 toxicity is modulated by cross-talk between caveolae and Nrf2 signaling. Toxicol Appl Pharmacol. 2014;277(2):192–9. doi: 10.1016/j.taap.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiscott J, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13(10):6231–40. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–91. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 44.Iademarco MF, et al. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) J Biol Chem. 1992;267(23):16323–9. [PubMed] [Google Scholar]
- 45.DeGraba TJ, et al. Increased endothelial expression of intercellular adhesion molecule-1 in symptomatic versus asymptomatic human carotid atherosclerotic plaque. Stroke. 1998;29(7):1405–10. doi: 10.1161/01.str.29.7.1405. [DOI] [PubMed] [Google Scholar]
- 46.Brand K, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97(7):1715–22. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hennig B, et al. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181(3):174–83. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- 48.Aneja R, et al. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004;10(1–6):55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramesh E, Geraldine P, Thomas PA. Regulatory effect of epigallocatechin gallate on the expression of C-reactive protein and other inflammatory markers in an experimental model of atherosclerosis. Chem Biol Interact. 2010;183(1):125–32. doi: 10.1016/j.cbi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Ludwig A, et al. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2004;316(3):659–65. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- 51.Fang MZ, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation–silenced genes in cancer cell lines. Cancer Res. 2003;63(22):7563–70. [PubMed] [Google Scholar]
- 52.Choi KC, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69(2):583–92. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 53.Balasubramanian S, Adhikary G, Eckert RL. The Bmi-1 polycomb protein antagonizes the (–)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival. Carcinogenesis. 2010;31(3):496–503. doi: 10.1093/carcin/bgp314. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 54.Thakur VS, Gupta K, Gupta S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int J Oncol. 2012;41(1):353–61. doi: 10.3892/ijo.2012.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, et al. Synergistic epigenetic reactivation of estrogen receptor-alpha (ERalpha) by combined green tea polyphenol and histone deacetylase inhibitor in ERalpha-negative breast cancer cells. Mol Cancer. 2010;9:274. doi: 10.1186/1476-4598-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YH, et al. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int J Mol Med. 2012;30(1):69–74. doi: 10.3892/ijmm.2012.966. [DOI] [PubMed] [Google Scholar]
- 57.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 58.Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26(37):5310–8. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 59.Patel J, Pathak RR, Mujtaba S. The biology of lysine acetylation integrates transcriptional programming and metabolism. Nutr Metab (Lond) 2011;8:12. doi: 10.1186/1743-7075-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Ruijter AJ, et al. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370(Pt 3):737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9(3):206–18. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levites Y, et al. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappaB) activation and cell death by tea extracts in neuronal cultures. Biochem Pharmacol. 2002;63(1):21–9. doi: 10.1016/s0006-2952(01)00813-9. [DOI] [PubMed] [Google Scholar]
- 63.Weinreb O, et al. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J Nutr Biochem. 2004;15(9):506–16. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]