Abstract
Background
Carbamylated hemoglobin (carbHb) is reported to interfere with measurement and interpretation of HbA1c in diabetic patients with chronic renal failure (CRF). There is also concern that HbA1c may give low results in these patients due to shortened erythrocyte survival.
Methods
We evaluated the effect of carbHb on HbA1c measurements and compared HbA1c with glycated albumin (GA) in patients with and without renal disease to test if CRF causes clinically significant bias in HbA1c results using 11 assay methods. Subjects included those with and without renal failure and diabetes. Each subject’s estimated glomerular filtration rate (eGFR) was used to determine the presence and degree of renal disease. A multiple regression model was used to determine if the relationship between HbA1c results obtained from each test method and the comparative method were significantly (p<0.05) affected by eGFR. These methods were further evaluated for clinical significance using difference between the eGRF quartiles of >7% at 6 or 9% HbA1c. The relationship between HbA1c and glycated albumin (GA) in patients with and without renal failure was also compared.
Results
Some methods showed small but statistically significant effects of eGFR; none of these differences were clinically significant. If GA is assumed to better reflect glycemic control, then HbA1c was approximately 1.5% HbA1c lower in patients with renal failure.
Conclusions
Although most methods can measure HbA1c accurately in patients with renal failure, healthcare providers must interpret these test results cautiously in these patients due the propensity for shortened erythrocyte survival in renal failure.
Keywords: carbamylated hemoglobin, HbA1c, chronic renal failure, interference, glycated albumin
1. Introduction
Renal failure is common in patients with diabetes, and HbA1c is widely used as an index of mean blood glucose in these patients. Many factors can affect interpretation of HbA1c measurements in patients with chronic renal failure (CRF). Several reports have suggested that erythrocyte survival is substantially lowered in most patients with CRF; this would be expected to lower HbA1c results [1–3]. Although shortened erythrocyte lifespan would presumably not interfere with the measurement of HbA1c, it could adversely affect the interpretation of HbA1c results.
Carbamylated Hb (carbHb) is formed by non-enzymatic condensation of cyanate with the N-terminal valine of hemoglobin. In chronic renal failure carbHb is increased due to elevated urea, which is dissociated in vivo to yield cyanate ions [4]. A number of old reports have suggested that HbA1c methods, especially those based on charge separation (e.g. ion-exchange HPLC) may have interference from carbHb that would be expected to falsely increase HbA1c results [5–7], but many of these methods are no longer in use. Subsequent reports evaluated newer ion-exchange HPLC assay methods which showed improved separation of the HbA1c fraction from other hemoglobin adducts [8,9] and therefore did not show interference from carbHb.
The present study is twofold; we first evaluated several current HbA1c methods for interference from carbHb in patients with and without renal failure. Although carbHb was not measured directly in the present study, there is a large amount of data showing that this hemoglobin modification is significantly increased in patients with renal failure and the carbamylated fraction (HbA1d3) as well as other measures of carbHb (measurement of valine hydantoin by HPLC) are correlated with plasma creatinine, serum urea and time-averaged urea concentrations [10–12]. Studies have also shown that the amount of carbHb depends upon both the duration and severity of real failure [13–15]. We therefore used eGFR as an indicator of overall renal function in place of direct measurement of carb Hb. We used a boronate affinity chromatography HPLC method as our reference method since this method has been shown to have no interference from carbHb [6,7,16,17].
In addition to the possible method-specific interference of carbHb, CRF, especially end stage renal disease, may also cause changes in erythrocyte lifespan which might alter the interpretation of HbA1c results. Several studies propose the use of glycated albumin (GA) measurement in place of HbA1c as a more accurate assessment of glycemic control in patients with renal disease. One study showed that GA was a better predictor of risk of death and hospitalization in these patients, compared to HbA1c [18]. Serum GA levels were also shown to be better correlated with average glucose (based on 4-point profiles, 3 days per week for 4 weeks) than HbA1c [19]. In this present study, we investigated the relationship between HbA1c and glycated albumin (GA) in patients with and without renal failure using the same patient samples as for the method-specific carbHb interference study.
2. Methods
We evaluated eight ion-exchange HPLC methods: G7 and G8 (Tosoh Bioscience), Variant II NU, Variant II Turbo, Variant II Turbo 2.0, D-10 and D-10 Dual (Bio-Rad Laboratories), HA-8160 (A. Menarini Diagnostics), two immunoassay methods: Tina-quant HbA1c gen.2 on Integra 800 (Roche Diagnostics) and DCA 2000 (Siemens Healthcare Diagnostics), and one enzymatic method: Direct Enzymatic HbA1c (Diazyme Laboratories) on the Hitachi 917 (Roche Diagnostics). Presumably, hemoglobin species modified by reactants other than glucose and not displaying a cis-1,1-diol group should not interfere with measurement of HbA1c by boronate affinity methods. Published data support this lack of interference of carbHb with boronate affinity methods [6,16,17]. Therefore, we used the boronate affinity ultra2 HPLC (Trinity Biotech) as our comparative method.
This study was approved by the ethics review committee at DynaLIFEDX in Edmonton, Canada where the samples originated. Whole blood samples (n=120) from subjects normal renal function and subjects in various stages of renal failure were residual samples from routine testing that had been collected in EDTA-containing tubes. The samples were shipped on cold packs to the Diabetes Diagnostic Laboratory at the University of Missouri (Columbia, MO). Several small whole blood aliquots were made from each sample and stored at −70°C until they were shipped on dry ice to various sites for analysis. One aliquot was centrifuged and the plasma was separated and stored at −70°C until analysis of GA. Each patient’s eGFR, calculated using the MDRD equation, was used to estimate the degree of renal disease and the level of carbHb. A multiple regression model was used to determine if the relationship between HbA1c results obtained from each test method and the ultra2 method were significantly (p<0.05) affected by eGFR. For those methods’ results that were significantly affected by eGFR, results were evaluated for clinical significance by dividing the samples into quartiles based on eGFR results (eGFR ≤11, 11< eGFR ≤ 45, 45< eGFR ≤ 84, eGFR >84). Deming regression was then used to compare the relationships between each method and the ultra2 for the highest and lowest quartiles; a difference between the quartiles of >7% at 6 or 9% HbA1c was defined as being clinically significant [20]. The relationship between HbA1c (ultra2 HPLC) and GA was evaluated comparing patients with normal eGFR (eGFR>90 ml/min, no renal disease, n=18) and those with renal failure (eGFR<60 ml/min, n=73). Data analyses were performed using SAS and Excel.
3. Results and Discussion
The D-10, D-10 Dual, DCA 2000, G7 and Direct Enzymatic methods showed very small but statistically significant effects of eGFR; clinical significance was therefore evaluated. The differences in HbA1c from the reference method between the lowest and highest eGFR quartiles is shown in figure 1 as a box plot of the HbA1c simple linear regression residuals for each method. In this way, any inherent calibration bias is removed and only the difference between the highest and lowest quartiles can be seen. Importantly, none of the methods evaluated showed any clinically significant effects of eGFR.
Figure 1.
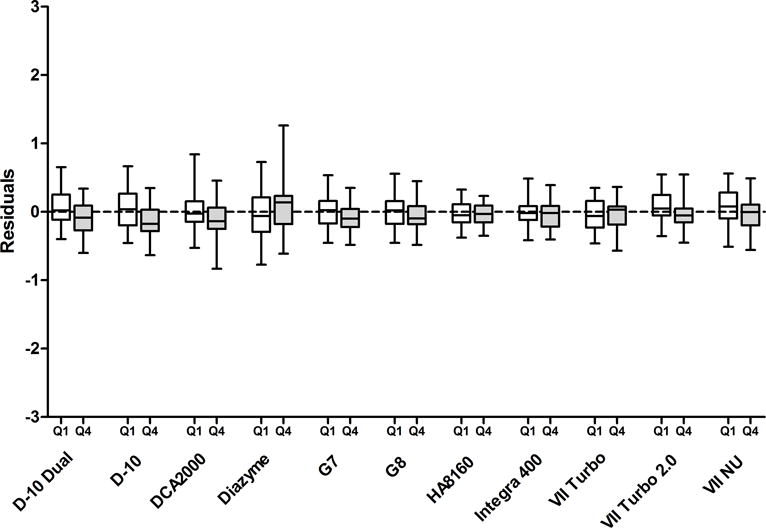
Box plots of the residuals of the regression of HbA1c compared to the comparative method for the lowest and highest (shaded) eGFR quartiles. The horizontal line within each box is the median of the residuals. The upper and lower limits of each box correspond to the 25th and 75th percentile of the residuals. The highest and lowest whiskers represent the minimal and maximal residuals. Q1, lowest eGFR quartile; Q4, highest eGFR quartile.
The relationship between HbA1c and GA is shown in figure 2. The difference in the relationship between the normal and renal failure groups was both statistically (Linear regression, p<0.0001) and clinically significant. If we assume that GA is providing an accurate measure of glycemic control in patients with renal failure, HbA1c results are lowered by approximately 1.5% HbA1c in patients with renal failure at critical treatment levels. These results are consistent with the findings of others that have found lower HbA1c results in renal failure when compared to measures of glycated plasma protein or plasma albumin [2,3]. The studies showing that GA is superior to HbA1c use in CRF are somewhat convincing but far from definitive [21]. There are studies showing a linear increase in all-cause mortality with increasing HbA1c levels [22,23] and there is no evidence as yet that physicians can achieve better glycemic control using GA instead of HbA1c in these patients. In addition, as with HbA1c, there may be factors (e.g. proteinuria, altered albumin homeostasis) that interfere with measurement or interpretation of GA. There is ongoing debate about which assay is most useful for monitoring glycemic control in this vulnerable population [24,25].
Figure 2.
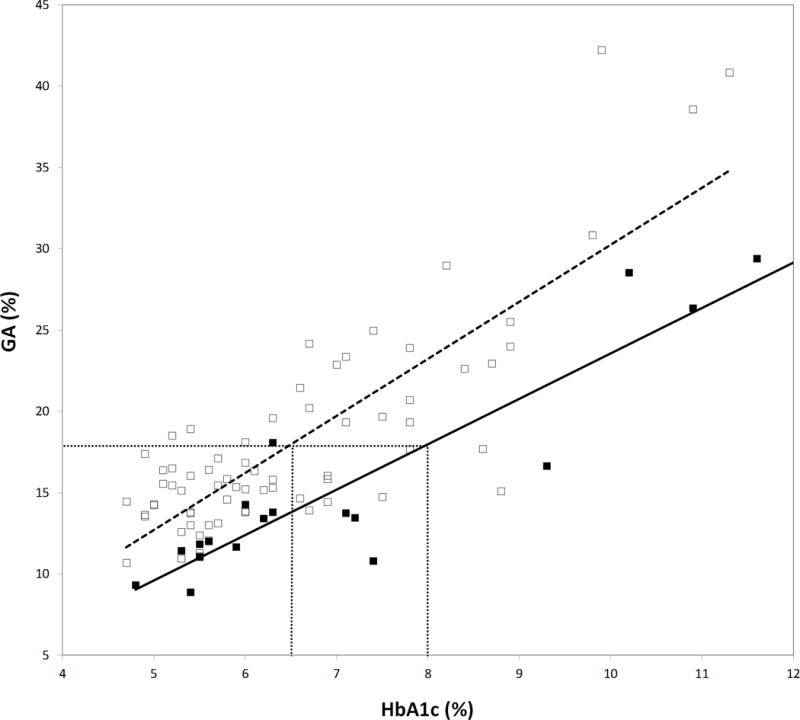
Relationship between GA and HbA1c in patients with chronic renal failure (□, eGFR <60; —, y=3.51x − 4.88; r2=0.68) and without renal disease (■, eGFR>90; y=2.79x − 4.34; r2=0.83). Dotted horizontal and vertical lines indicate the difference in HbA1c between the two groups at a fixed GA.
4. Conclusions
We conclude that most current HbA1c methods can provide valid analytical results for patients with CRF. However, healthcare providers need to be aware of potential interferences when interpreting HbA1c results in clinical settings due to alteration in erythrocyte lifespan in many patients with chronic renal failure which can cause falsely lowered HbA1c results.
Acknowledgments
We thank Bio-Rad Laboratories and Diazyme Laboratories for analyzing HbA1c in these samples using their methods. We thank Asahi Kasei Pharma Corporation for donation of their Lucica GA-L assay reagents. We also thank the chemistry staff at DynaLIFEDX who diligently collected these samples for our study.
Abbreviations
- eGFR
estimated glomerular filtration rate
- carbHb
carbamylated hemoglobin
Contributor Information
Randie R. Little, Email: littler@health.missouri.edu.
Curt L. Rohlfing, Email: rohlfingc@health.missouri.edu.
Alethea L. Tennill, Email: tennilla@health.missouri.edu.
Steven E. Hanson, Email: hansons@health.missouri.edu.
Shawn Connolly, Email: connollyc@health.missouri.edu.
Trefor Higgins, Email: trefor.higgins@dynalifedx.com.
Charles E. Wiedmeyer, Email: wiedmeyerc@missouri.edu.
Cas W. Weykamp, Email: cweykamp@skbwinterswijk.nl.
Richard Krause, Email: richard.krause@cls.ab.ca.
References
- 1.Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73:1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 2.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 3.Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Peritoneal Dialysis International. 2010;30:72–79. doi: 10.3747/pdi.2008.00243. [DOI] [PubMed] [Google Scholar]
- 4.Nigen AM, Bass BD, Manning JM. Reactivity of cyanate with valine-1 (alpha) of hemoglobin. A probe of conformational change and anion binding. J Biol Chem. 1976;251:7638–7643. [PubMed] [Google Scholar]
- 5.Herruer MH, van Kooten EA, Sluiter HE, Zuijderhoudt FM. Influence of uraemia on the determination of blood glycohaemoglobin by HPLC, electrophoresis and affinity chromatography in diabetic and non-diabetic patients. Eur J Clin Chem Clin Biochem. 1994;32:361–364. doi: 10.1515/cclm.1994.32.5.361. [DOI] [PubMed] [Google Scholar]
- 6.Bannon P, L F, Lepage R, Joly J, Dufresne L. Glycated Hemoglobin in Uremic Patients as Measured by Affinity and Ion-Exchange Chromatography. Clin Chem. 1984;30:485–486. [PubMed] [Google Scholar]
- 7.Little R, A A, Tennill A, England J, Khanna R, Goel S, Ou C, Goldstein D. Can Glycohemoglobin (GHB) be Used to Accurately Assess Glycemic Control in Patients with Chronic Renal Failure (CRF)? Clin Chem. 1999;45:A4. [PubMed] [Google Scholar]
- 8.Little RR, Tennill AL, Rohlfing C, et al. Can glycohemoglobin be used to assess glycemic control in patients with chronic renal failure? Clin Chem. 2002;48:784–786. [PubMed] [Google Scholar]
- 9.Chachou A, Randoux C, Millart H, Chanard J, Gillery P. Influence of in vivo hemoglobin carbamylation on HbA1c measurements by various methods. Clin Chem Lab Med. 2000;38:321–326. doi: 10.1515/CCLM.2000.046. [DOI] [PubMed] [Google Scholar]
- 10.Kwan JTC, C E, Neal AD, Burdon J, Raftery MJ, Marsh FP, Barron JL, Bending MR. Carbamylated Haemoglobin, Urea Kinetic Modelling and Adequacy of Dialysis in Haemodialysis Patients. Nephrol Dial Transplant. 1991;6:38–43. doi: 10.1093/ndt/6.1.38. [DOI] [PubMed] [Google Scholar]
- 11.Bisse E, Huaman-Guillen P, Wieland H. Chromatographic evaluation of minor hemoglobins: clinical significance of hemoglobin A1d, comparison with hemoglobin A1c, and possible interferences. Clin Chem. 1995;41:658–663. [PubMed] [Google Scholar]
- 12.Fluckiger R, Harmon W, Meier W, Loo S, Gabbay KH. Hemoglobin carbamylation in uremia. N Engl J Med. 1981;304:823–827. doi: 10.1056/NEJM198104023041406. [DOI] [PubMed] [Google Scholar]
- 13.Davenport A, Jones S, Goel S, Astley JP, Feest TG. Carbamylated hemoglobin: a potential marker for the adequacy of hemodialysis therapy in end-stage renal failure. Kidney Int. 1996;50:1344–1351. doi: 10.1038/ki.1996.447. [DOI] [PubMed] [Google Scholar]
- 14.Davenport A, Jones SR, Goel S, Astley JP, Hartog M. Differentiation of acute from chronic renal impairment by detection of carbamylated haemoglobin. Lancet. 1993;341:1614–1617. doi: 10.1016/0140-6736(93)90757-8. [DOI] [PubMed] [Google Scholar]
- 15.Smith WGJ, H M, Benton M, Brown CB. Glycosylated and Carbamylated Haemoglobin in Uraemia. Nephrol Dial Transplant. 1989;4:96–100. [PubMed] [Google Scholar]
- 16.Bruns DE, W M. Glycosylation and Uraemia. The Lancet. 1984;324:344. doi: 10.1016/s0140-6736(84)92705-3. [DOI] [PubMed] [Google Scholar]
- 17.Morgan LJ, Marenah CB, Morgan AG, Burden RP, John WG. Glycated haemoglobin and fructosamine in non-diabetic subjects with chronic renal failure. Nephrol Dial Transplant. 1990;5:868–873. doi: 10.1093/ndt/5.10.868. [DOI] [PubMed] [Google Scholar]
- 18.Freedman BI, Andries L, Shihabi ZK, et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clinical Journal of The American Society of Nephrology: CJASN. 2011;6:1635–1643. doi: 10.2215/CJN.11491210. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Park JT, Oh HJ, et al. Estimating average glucose levels from glycated albumin in patients with end-stage renal disease. Yonsei Med J. 2012;53:578–586. doi: 10.3349/ymj.2012.53.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CN, Emery TJ, Little RR, et al. Effects of hemoglobin C, D, E, and S traits on measurements of HbA1c by six methods. Clin Chim Acta. 2012;413:819–821. doi: 10.1016/j.cca.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrotra R, Kalantar-Zadeh K, Adler S, Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin? Clinical Journal of The American Society of Nephrology: CJASN. 2011;6:1520–1522. doi: 10.2215/CJN.04210511. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30:1049–1055. doi: 10.2337/dc06-2127. [DOI] [PubMed] [Google Scholar]
- 23.Duong U, Mehrotra R, Molnar MZ, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:1041–1048. doi: 10.2215/CJN.08921010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman BI, Freedman BI. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: time to dispense with the hemoglobin A1C in end-stage kidney disease. Diabetes Care. 2012;35:1621–1624. doi: 10.2337/dc12-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: A1C remains the gold standard outcome predictor in diabetic dialysis patients. Counterpoint Diabetes Care. 2012;35:1625–1628. doi: 10.2337/dc12-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]


