Abstract
In chronic kidney disease (CKD), a higher body mass index (BMI) is associated with a lower risk for death, but cause-specific death details are unknown across the BMI range. To define this, we studied 54,506 patients with CKD (stage 3 CKD- 91.5%) from our institutional electronic medical record based-registry. We examined the associations among various causes of death (cardiovascular, malignancy and non-cardiovascular/non-malignancy related deaths) across the BMI range using Cox proportional hazards and competing risks regression models. During a median follow-up of 3.7 years, 14,518 patients died. In the multivariable model, an inverted J-shaped association was noted between BMI and cardiovascular, malignancy-related, and non-cardiovascular/non-malignancy related deaths. Similar associations were noted for BMI 25–29.9, 30–34.9, and 35–39.9 kg/m2 categories. A BMI over 40 kg/m2 was not associated with cardiovascular and non-cardiovascular/non-malignancy related deaths in CKD. Sensitivity analyses yielded similar results even after adjusting for proteinuria, and excluding diabetes and hypertension from the models. In CKD, compared to BMI of 18.5–24.9 kg/m2, those who are overweight, class 1 and 2 obesity are associated with lower risk for cardiovascular, malignancy-related and non-cardiovascular/non-malignancy related deaths. Future studies should examine the associations of other measures of adiposity with outcomes in CKD.
Keywords: obesity, kidney disease, cardiovascular deaths and mortality
Background
Chronic kidney disease (CKD) is associated with increased risk of cardiovascular disease and mortality (1, 2). Over two-thirds of CKD patients are either overweight or obese, and both obesity and CKD contribute to substantial public health burden (3). Obesity is an independent risk factor for the development of kidney disease in the general population (4). While earlier studies report obesity as an independent risk factor for the development of end stage renal disease, a recent large study that included the US Veteran population reported slower progression of kidney disease among those in the higher BMI categories (5, 6). Similar to recent reports for type 2 diabetes, some groups have observed that obesity, assessed using body mass index (BMI), in non-dialysis dependent CKD is associated with lower risk for all-cause mortality (7–11). While there is some disagreement regarding the best predictor of obesity, BMI continues to be the mostly widely used metric (12–14).
Cardiovascular disease and malignancy are leading causes of death in the general population (15). Recent reports from Canada and our group suggest that cardiovascular disease and malignancy accounts for the majority of deaths in the CKD population (16, 17). While obesity is associated with higher cardiovascular mortality in the general population, it is not associated with cancer and non-cardiovascular/non-malignancy related deaths (18). The specific etiology and biological mechanisms that explain the association between BMI and lower all-cause mortality are unclear, and whether overweight/obese CKD patients sustain differential (higher or lower) rates of deaths due to cardiovascular disease, malignancy and other diseases is unknown. Such information would help us understand the relative contribution of various diseases to mortality rates in CKD, and provide a platform for developing preventive and therapeutic strategies in this high risk cohort.
Therefore, we examined associations between various causes of death (cardiovascular, malignancy and non-cardiovascular/non-malignancy related deaths) in CKD patients with a full range of obesity, as reflected in BMI values, who were followed in a large health care system and had cause-specific death data.
Results
Patient characteristics
The study population comprised of 54,506 CKD patients who were not on dialysis, or had renal transplant. Mean age was 72.0 ± 11.7 years, with 44.2% being males and 12.7% blacks. Mean BMI of the study cohort was 29.4 ± 6.6 kg/m2. Prevalence of diabetes, hypertension, malignancy and coronary artery disease were 23.3%, 86.1%, 25% and 20.7% respectively. Mean eGFR was 47.8 ml/min/1.73 m2 with 67.9% of them in stage 3a, 24.5% in stage 3b and 7.5% with stage 4 CKD. Other details of the study population based on BMI categories are outlined in Table 1.
Table 1.
Characteristics of CKD patients based on BMI levels
| Variable | Summary N=54506 |
<18.5 kg/m2 (N=710) |
18.5–24.9 kg/m2 (N=13019) |
25–29.9 kg/m2 (N=19388) |
30–34.9 kg/m2 (N=12097) |
35–39.9 kg/m2 (N=5477) |
>40 kg/m2 (N=3815) |
|---|---|---|---|---|---|---|---|
| Age | 72.0±11.7 | 74.4±15.3 | 75.1±12.5 | 73.4±11.0 | 70.5±10.8 | 68.0±10.5 | 64.2±10.6 |
| Male gender (%) | 44.2 | 24.9 | 39.6 | 51.3 | 46.8 | 37.9 | 27.8 |
| African American (%) | 12.7 | 19.2 | 10.6 | 11.1 | 13.5 | 16.6 | 18.7 |
| BMI (kg/m2) | 29.4±6.6 | 17.4±0.99 | 22.7±1.7 | 27.5±1.4 | 32.2±1.4 | 37.2±1.4 | 45.2±5.0 |
| Diabetes (%) | 23.3 | 7.2 | 13.2 | 19.8 | 28.0 | 37.1 | 44.4 |
| Malignancy (%) | 25.0 | 28.0 | 27.2 | 26.9 | 23.8 | 20.9 | 17.0 |
| Hypertension (%) | 86.1 | 82.1 | 81.9 | 86.5 | 88.5 | 88.7 | 87.9 |
| Hyperlipidemia (%) | 7.9 | 55.9 | 71.5 | 79.2 | 81.7 | 81.6 | 79.9 |
| Coronary artery disease (%) | 20.7 | 12.7 | 18.8 | 22.5 | 21.8 | 20.4 | 16.7 |
| Congestive heart failure (%) | 7.9 | 8.9 | 8.2 | 7.2 | 7.5 | 8.7 | 10.3 |
| Cerebrovascular disease (%) | 9.1 | 10.1 | 10.1 | 10.0 | 9.0 | 6.6 | 4.8 |
| Peripheral vascular disease (%) | 3.1 | 6.1 | 3.4 | 3.3 | 2.8 | 2.5 | 1.8 |
| ACEI/ARB use (%) | 63.9 | 48.2 | 54.9 | 62.5 | 68.5 | 74.1 | 75.7 |
| Statins use (%) | 58.1 | 34.6 | 49.4 | 59.5 | 63.0 | 63.7 | 60.7 |
| Beta Blocker use (%) | 58.5 | 52.1 | 55.1 | 58.0 | 60.6 | 63.1 | 60.3 |
| eGFR (ml/min/1.73 m2) | 47.8±10.2 | 45.6±11.3 | 47.3±10.5 | 48.1±10.0 | 48.3±10.0 | 47.7±10.2 | 47.1±10.4 |
| eGFR categories | |||||||
| 45–59 ml/min/1.73 m2 | 67.9 | 60.4 | 65.8 | 68.8 | 69.9 | 68.1 | 65.4 |
| 30–44 ml/min/1.73 m2 | 24.5 | 28.3 | 25.5 | 24.2 | 23.5 | 24.2 | 26.3 |
| 15–29 ml/min/1.73 m2 | 7.5 | 11.3 | 8.7 | 7.0 | 6.6 | 7.7 | 8.3 |
| Albumin (g/dL | 4.1±0.79 | 4.0±0.55 | 4.1±1.4 | 4.1±0.45 | 4.1±0.45 | 4.1±0.44 | 4.0±0.46 |
| Hemoglobin (mg/dl) | 12.8±1.8 | 12.2±1.9 | 12.6±1.8 | 13.0±1.8 | 13.0±1.8 | 12.8±1.8 | 12.5±1.7 |
| Insurance (%) | |||||||
| Medicaid | 0.7 | 1.7 | 0.67 | 0.47 | 0.73 | 0.79 | 1.5 |
| Medicare | 78.5 | 78.7 | 82.1 | 81.0 | 76.9 | 73.1 | 66.7 |
| Missing | 2.8 | 3.4 | 2.6 | 2.4 | 2.9 | 3.4 | 4.5 |
| Other | 17.9 | 16.2 | 14.7 | 16.1 | 19.4 | 22.6 | 27.3 |
| Smoking (%) | |||||||
| No | 79.6 | 68.9 | 76.6 | 80.5 | 80.9 | 80.8 | 80.3 |
| Yes | 7.1 | 16.9 | 8.4 | 6.6 | 6.6 | 6.4 | 6.1 |
| Missing | 13.3 | 14.2 | 14.9 | 12.9 | 12.5 | 12.7 | 13.7 |
Age and gender adjusted mortality rates and causes of death
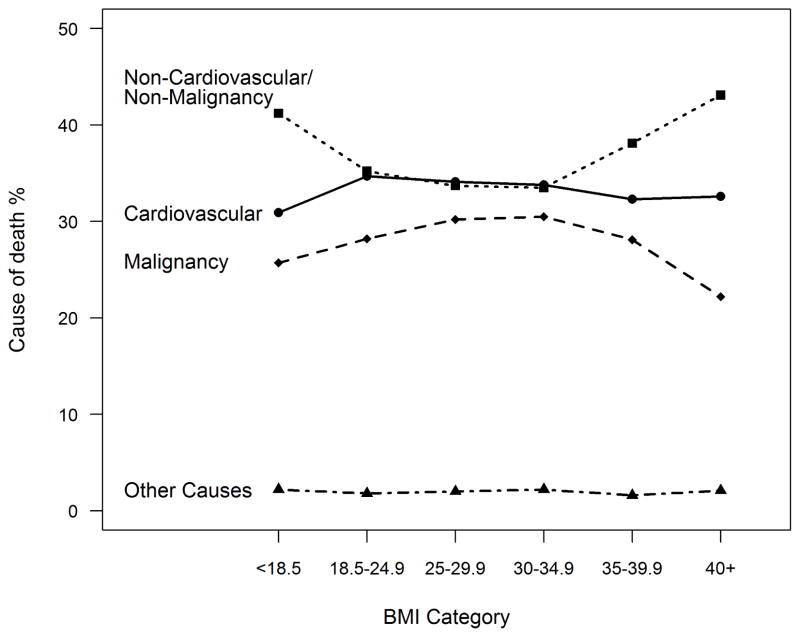
During a median follow-up of 3.7 years (25th percentile 1.8, and 75th percentile 5.8), 14,518 died. Figure 1 shows the proportion of various causes of death among those in different BMI categories. Table 2 and Figure 1 outline various causes of death within the cardiovascular and non-cardiovascular/non-malignancy related deaths. Overall, cardiovascular diseases (34.0%) and malignant neoplasms (28.9%) were the leading causes of death among the study population.
Figure 1.
Various causes of death among those in different BMI categories.
Table 2.
Leading causes of death based on BMI levels in CKD
| Cause of death | Summary N=14321 |
<18.5 kg/m2 (N=362) |
18.5–24.9 kg/m2 (N=4506) |
25–29.9 kg/m2 (N=4996) |
30–34.9 kg/m2 (N=2592) |
35–39.9 kg/m2 (N=1090) |
>40 kg/m2 (N=775) |
|---|---|---|---|---|---|---|---|
| All Cardiovascular Diseases | 34.0 | 30.9 | 34.7 | 34.1 | 33.8 | 32.3 | 32.6 |
| Ischemic Heart Disease | 16.8 | 15.2 | 16.8 | 17.1 | 17.2 | 16.4 | 15.5 |
| Heart Failure | 2.6 | 2.2 | 2.9 | 2.4 | 2.9 | 2.2 | 1.8 |
| Cerebrovascular disease | 4.0 | 3.3 | 4.5 | 4.1 | 3.5 | 3.2 | 3.0 |
| All Other Cardiovascular | 10.5 | 10.2 | 10.5 | 10.5 | 10.1 | 10.5 | 12.4 |
| Malignant Neoplasms | 28.9 | 25.7 | 28.2 | 30.2 | 30.5 | 28.1 | 22.2 |
| Non-cardiovascular/Non-malignancy diseases | 35.2 | 41.2 | 35.2 | 33.7 | 33.5 | 38.1 | 43.1 |
| Chronic lower respiratory diseases | 4.6 | 9.7 | 4.8 | 3.9 | 4.4 | 5.5 | 5.8 |
| Diabetes mellitus | 3.7 | 1.7 | 2.4 | 3.0 | 5.1 | 6.9 | 8.3 |
| Nephritis, nephrotic syndrome and | 2.6 | 1.7 | 2.2 | 2.5 | 2.9 | 3.9 | 3.0 |
| Alzheimer’s disease | 2.0 | 4.1 | 2.8 | 2.1 | 1.0 | 1.0 | 0.26 |
| Influenza and pneumonia | 1.8 | 1.1 | 2.0 | 1.6 | 1.7 | 1.9 | 1.9 |
| Septicemia | 1.9 | 1.1 | 1.6 | 1.7 | 2.2 | 2.2 | 4.3 |
| Chronic liver disease and cirrhosis | 1.1 | 1.7 | 0.93 | 1.1 | 1.2 | 1.3 | 1.7 |
| Pneumonitis | 0.89 | 0.55 | 1.1 | 0.96 | 0.69 | 0.64 | 0.65 |
| Parkinson’s disease | 0.52 | 0.55 | 0.75 | 0.48 | 0.42 | 0.18 | 0.13 |
| All other diseases | 16.0 | 19.1 | 16.7 | 16.3 | 13.8 | 14.6 | 17.2 |
| Other causes | 2.0 | 2.2 | 1.8 | 2.0 | 2.2 | 1.6 | 2.1 |
e.g homicide, suicide, accidents
BMI and cause-specific death
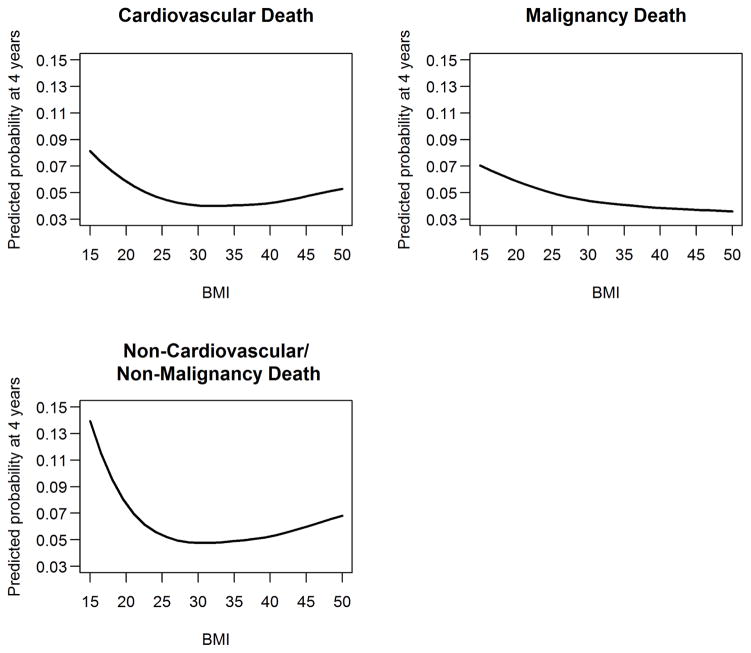
In the models adjusting for relevant confounding variables, BMI 25–29.9 kg/m2 was associated with lower hazards for overall mortality (HR 0.78, 95% CI 0.75, 0.81, p <0.001), and lower subhazards for cardiovascular (HR 0.83, 95% CI 0.77, 0.89, p <0.001), malignancy-related (HR 0.84, 95% CI 0.78, 0.91, p <0.001) and non-cardiovascular/non-malignancy related deaths (HR 0.81, 95% CI 0.75, 0.87, p <0.001). BMI 30–34.9 kg/m2 was associated with lower hazards for overall mortality (HR 0.73, 95% CI 0.70, 0.77, p <0.001), and lower subhazards for cardiovascular (HR 0.81, 95% CI 0.74, 0.89, p <0.001), malignancy-related (HR 0.80, 95% CI 0.73, 0.88, p <0.001) and non-cardiovascular/non-malignancy related deaths (HR 0.75, 95% CI 0.69, 0.82, p <0.001). Similarly, BMI 35–39.9 kg/m2 was associated with lower hazards for overall mortality (HR 0.73, 95% CI 0.68, 0.78, p <0.001), and lower subhazards for cardiovascular (HR 0.80, 95% CI 0.71, 0.91, p <0.001), malignancy-related (HR 0.73, 95% CI 0.64, 0.83, p <0.001) and non-cardiovascular/non-malignancy related deaths (HR 0.83, 95% CI 0.74, 0.93, p=0.001). However, BMI >40 kg/m2 was not associated with cardiovascular and non-cardiovascular/non-malignancy related deaths (Table 3 and 4). Lower BMI category (<18.5 kg/m2) was associated with higher risk for all-cause (HR 1.80, 95% CI 1.6, 2.0, p <0.001), cardiovascular (HR 1.40, 95% CI 1.14, 1.71, p =0.001), malignancy-related (HR 1.31, 95% CI 1.04, 1.66, p=0.02) and non-cardiovascular and non-malignancy related deaths (HR 1.75, 95% CI 1.46, 2.09, p <0.001) (Table 3 and 4). Figure 2 shows the associations between BMI (as a continuous measure) and various causes of death.
Table 3.
Associations of Overall mortality according to various levels of BMI in CKD (using Cox proportional hazards model)
| HR (95%CI) adjusted* N=54,506 |
p-value | |
|---|---|---|
| BMI <18.5 kg/m2vs. 18.5–24.9 kg/m2 | 1.8 (1.6, 2.0) | <0.001 |
| BMI 25–29.9 kg/m2 vs. 18.5–24.9 kg/m2 | 0.78 (0.75, 0.81) | <0.001 |
| BMI 30–34.9 kg/m2 vs. 18.5–24.9 kg/m2 | 0.73 (0.70, 0.77) | <0.001 |
| BMI 35–39.9 kg/m2 vs. 18.5–24.9 kg/m2 | 0.73 (0.68, 0.78) | <0.001 |
| BMI >40 kg/m2 vs. 18.5–24.9 kg/m2 | 0.79 (0.73, 0.86) | <0.001 |
Model includes race, age, gender, diabetes, HTN, hyperlipidemia, BMI group, albumin and hemoglobin, malignancy, CAD, CHF, cerebrovascular disease, peripheral vascular disease, insurance, ACE/ARB, statin, beta blocker, smoking, and CKD stage. For albumin and hemoglobin we used mean value imputation with dummy indicators for missing values.
Table 4.
Associations of cause-specific mortality according to various levels of BMI in CKD (using competing risk analyses)
| SHR (95%CI) Adjusted* N=54,309 |
p-value | |
|---|---|---|
| BMI <18.5 kg/m2 vs. 18.5–24.9 kg/m2 | ||
| Cardiovascular death | 1.40 (1.14, 1.71) | 0.001 |
| Malignancy death | 1.31 (1.04, 1.66) | 0.021 |
| Non-cardiovascular and non-malignancy related death | 1.75 (1.46, 2.09) | <0.001 |
| BMI 25–29.9 kg/m2 vs. 18.5–24.9 kg/m2 | ||
| Cardiovascular death | 0.83 (0.77, 0.89) | <0.001 |
| Malignancy death | 0.84 (0.78, 0.91) | <0.001 |
| Non-cardiovascular and non-malignancy related death | 0.81 (0.75, 0.87) | <0.001 |
| BMI 30–34.9 kg/m2 vs. 18.5–24.9 kg/m2 | ||
| Cardiovascular death | 0.81 (0.74, 0.89) | <0.001 |
| Malignancy death | 0.80 (0.73, 0.88) | <0.001 |
| Non-cardiovascular and non-malignancy related death | 0.75 (0.69, 0.82) | <0.001 |
| BMI 35–39.9 kg/m2 vs. 18.5–24.9 kg/m2 | ||
| Cardiovascular death | 0.80 (0.71, 0.91) | <0.001 |
| Malignancy death | 0.73 (0.64, 0.83) | <0.001 |
| Non-cardiovascular and non-malignancy related death | 0.83 (0.74, 0.93) | 0.001 |
| BMI >40 kg/m2 vs. 18.5–24.9 kg/m2 | ||
| Cardiovascular death | 0.93 (0.81, 1.08) | 0.33 |
| Malignancy death | 0.66 (0.56, 0.78) | <0.001 |
| Non-cardiovascular and non-malignancy related death | 0.98 (0.87, 1.12) | 0.81 |
Model includes race, age, gender, diabetes, HTN, hyperlipidemia, BMI group, albumin and hemoglobin, malignancy, CAD, CHF, cerebrovascular disease, peripheral vascular disease, insurance, ACE/ARB, statin, beta blocker, smoking, and CKD stage. For albumin and hemoglobin we used mean value imputation with dummy indicators for missing values.
Figure 2.
Predicted mortality from competing risks model at 4 years due to cardiovascular, malignancy and non-cardiovascular/non-malignancy diseases based on BMI in CKD
40,354 patients had a weight measurement at least 1 year before the baseline weight and were included in analyses adjusting for weight change over time. The median weight change per year was a decrease of −0.3 kg/yr. The associations between BMI categories and all cause- mortality remained similar while adjusting for weight change per year (Supplemental table 1).
Sensitivity analyses
BMI 21.5–24.9 kg/m2 as reference range
In a model with BMI 21.5–24.9 kg/m2 as the reference range, similar associations between BMI categories and various causes of death were noted (Supplemental Table 2).
BMI 25–29.9 kg/m2 as reference range
In a model with BMI 25–29.9 kg/m2 as the reference range, BMI <18.5, 18.5–21.4 and 21.5–24.9 kg/m2 were associated with higher sub-hazards of each cause-specific death. However, other BMI categories were not associated with higher sub-hazards of death, except for BMI>40 kg/m2 for non-cardiovascular/non-malignancy related causes of death (Supplemental Table 2).
Adjustment for proteinuria
In a subgroup of patients who had proteinuria measurements, similar associations between BMI categories and various causes of death were noted (Supplemental Table 3).
Exclusion of Diabetes and Hypertension in the Cox model
In the competing-risk model in which diabetes and hypertension were not adjusted for, results similar to the primary analysis were noted (Supplemental Table 3).
Censoring patients who reached dialysis
In the analysis in which we censored patients as of 09/2009 (to exclude any patients who would have gone on to dialysis), results similar to the primary analysis were noted (Supplemental Table 3).
Interactions
The two-way interactions between BMI group and diabetes and smoking on overall mortality were not significant. The two-way interactions between BMI group and sex, hypertension, and age were significant on overall mortality. These interactions showed that the effects were somewhat stronger among males compared to females, among patients with hypertension compared to those without, and among younger patients compared to older. We found no significant interactions between BMI group and either sex, diabetes, hypertension or smoking on each cause-specific mortality model. We found a significant interaction between BMI group and age only on non-cardiovascular/non-malignancy related deaths which showed stronger effects among younger patients (data not shown).
Discussion
Chronic kidney disease is associated with higher risk for all-cause and cardiovascular related death. Among those with CKD, having a higher BMI is associated with lower risk of all-cause mortality. In this report, we provided details about the specific causes of death among CKD patients with different BMI cut-offs. Higher BMI (except for those >40 kg/m2) is associated with lower risk for cardiovascular, malignancy and non-cardiovascular/non-malignancy related death, while lower BMI (<18.5 kg/m2) is associated with higher risk for these deaths.
In the general population, using data from NHANES I–III, Flegal et al. reported that being overweight was significantly correlated with a lower risk for non-cardiovascular and non-malignancy related death, but not with cancer and cardiovascular related death. Obesity on the other hand was associated with increased risk for cardiovascular mortality (18). Data from the Canadian Heart Health survey shows that a BMI >30 kg/m2 is associated with all-cause, cardiovascular and cancer mortality (19). To our knowledge, associations between BMI and various causes of death have not been examined in CKD. The “obesity paradox” was reported in dialysis patients several years ago and more recent data also show that higher BMI is associated with lower hazards of death among those with CKD (20). It has been argued that BMI is not an ideal measure for assessing risk in CKD as these patients are more likely to develop fluid retention and BMI fails to differentiate this factor. Few studies have used BMI 25–29.9 kg/m2 as the reference range in CKD. It might be that in CKD, the protective nutritional effects (higher BMI reflecting the better nutritional status) might dominate the harmful metabolic effects that are usually noted in obesity (21, 22). This could explain the apparent protective associations noted in Class 1 and 2 obesity. In any case, morbid obesity was not associated with various causes of death in CKD. It is also important to point out that BMI <18.5 kg/m2 was associated with higher all-cause and various cause-specific mortality rates (Table 3).
Despite the known association between CKD and cardiovascular disease, it is surprising that the cardiovascular death risk is lower in those with higher BMI categories (except for those with morbid obesity). Recent data from Atherosclerosis Risk In Communities (ARIC) study showed that patients who were overweight or obese before developing heart failure had lower mortality after they were diagnosed with heart failure (23). Whether such a phenomenon exists in CKD is unclear. As discussed above, other than the protective nutritional effects in CKD, better and more frequent care for those who are overweight and obese, and better adoption of healthy lifestyle including higher activity levels (since they are already overweight and obese) could explain the paradoxical associations that we observed, however further study is warranted to test this hypothesis. Emerging data suggest that obesity is a risk factor for development of cancer. It is possible that patients who are overweight and obese may be able to tolerate the side-effects of chemotherapy and other interventions (24). This could be one of the factors that contribute to the protective effects of overweight and obesity for cancer-related mortality in CKD.
Several strategies have been proposed to reduce the reverse causation in epidemiological investigations of obesity and mortality (9, 25, 26). They include excluding deaths that occur earlier on during follow-up in a sick population. This is important as this analysis included patients followed in our health care system who could have presented for care of chronic conditions including cancer. The metabolic status of the individual (irrespective of their BMI) could influence outcomes and in CKD, metabolic abnormalities could attenuate the observed associations (21, 27). In order to address this potential confounder, we performed a subgroup analysis comparing those with and without metabolic syndrome and found similar results (data not shown).
Obesity is associated with incident CKD and ESRD and previous analyses have excluded diabetes and hypertension from the models as they could be in the causal pathway of developing kidney disease (5). In those with established CKD, whether diabetes and hypertension continue to act as a mediator for adverse outcome is unclear. In a model that excluded diabetes and hypertension, we observed similar lower hazards for cardiovascular, malignancy-related, and non-cardiovascular and non-malignancy related deaths (Supplemental table 3). Weight changes, particularly weight loss with progression of kidney disease, are common in those with kidney disease and hence could influence the observed associations between BMI and mortality. While we adjusted for changes in weight prior to the onset of kidney disease, we did not adjust for changes in BMI after the development of CKD. We did not have waist circumference data (which reflects visceral adiposity) and future studies should compare the causes of death among those with different BMI and WC (13, 21). Importantly, sarcopenic obesity is more common among those with CKD and availability of body composition data would have helped us to assess the differential associations between muscle mass, adipose tissue and various causes of death in CKD (28–30). Cumulatively, our study results argue for future studies that compare various components of body composition including muscle mass and various adipose tissue measures and various causes of death in those with CKD. These studies should also include measures of physical activity and physical fitness, determinants of adverse outcomes in CKD (14, 31).
A large cohort of CKD population who had two eGFR <60 ml/min/1.73 m2 from a validated CKD registry and the availability of cause specific death data from the Ohio State department of health are major strengths of this study. These patients are followed in a health care system and had underlying comorbid conditions. Given the clinical nature of the cohort, we lacked dietary data, but we did adjust for related confounding variables including serum albumin in order to address nutritional influences. The data include patients from a single health care system but it is unlikely that CKD patients who live in the State of Ohio are substantially different from CKD patients cared for in other health care systems in other parts of the country. Future studies should examine whether such associations are noted in other CKD cohorts. Proteinuria data was missing for >50% of the study population but a sensitivity analysis restricting it to those with available urinary data showed similar findings. It important to point out that we did not have details related to screening procedures for cancer, and treatment details for underlying cancer. Further, we also lacked details on measures of inflammation (such as CRP) to adjust for inflammation which is a known confounder (32, 33). Cause-specific death data were obtained from the State of Ohio Department of Health mortality files. The National Death Index obtains data from individual States and these death indexes have been used by several studies in the past and are considered reliable (34–36). For our earlier analysis, we performed a validation for those who died within our hospital system and this analysis confirmed the reliability of death certificate derived data.
In summary, compared to a BMI of 18.5–24.9 kg/m2, those who are overweight, or have Class 1 or 2 obesity, are associated with lower risk for cardiovascular, malignancy-related and non-cardiovascular and non-malignancy related deaths. Further studies are needed to confirm these findings and examine the associations of other measures of adiposity with various causes of deaths in CKD.
Methods
Patient population
This analysis was conducted using the Cleveland Clinic’s pre-existing electronic health record (EHR)-based CKD registry. The development and validation of the registry was described in detail elsewhere (37). Patients who: a) had at least one face-to-face outpatient encounter with a Cleveland Clinic health care provider and had at least two estimated glomerular fitration rate (eGFR) measures 15–59.9 ml/min/1.73 m2, more than 90 days apart between January 1, 2005 and December 31, 2012, and not on dialysis or had renal transplant, b) were residents of the State of Ohio, and c) had BMI data, were included in this analysis.
Covariates
Demographic details (age, gender, race, insurance details) were extracted from the EHR. Comorbid conditions such as diabetes mellitus, hypertension, coronary artery disease, malignancy, congestive heart failure, and hyperlipidemia were defined using prespecified criteria and were validated in an earlier publication (37). These conditions existed prior to the second eGFR <60 ml/min/1.73 m2. We also extracted relevant laboratory data (serum albumin and hemoglobin) from the EHR.
Kidney function
All serum creatinine measurements were performed in the same clinical laboratory using a Hitachi D 2400 Modular Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN). CKD was classified into the following stages: stage 3 CKD (eGFR 30–59 ml/min/1.73 m2) and stage 4 CKD (eGFR 15–29 ml/min/1.73 m22). We calculated eGFR using the CKD-EPI equation (38). We further categorized stage 3 into CKD stage 3a (eGFR 45–59 ml/min/1.73 m2) and stage 3b (eGFR 30–44 ml/min/1.73 m2). To reflect clinical practice, patients who had a urine dipstick measurement, urine albumin-to-creatinine ratio (UACR), urine protein-to-creatinine ratio (UPR), and 24 hour urine studies were included to assess whether they had proteinuria or not. The following cut-offs were considered in determining whether someone had proteinuria: presence of ≥1+ proteinuria in dipstick studies, >30 mg/g in those who had UACR and UPR studies, and >30 mg proteinuria in 24 hour studies.
BMI and weight change
The body weight measure that was closest to the second eGFR <60 ml/min/1.73 m2 (baseline weight) was used to calculate BMI for the primary analysis. For patients with available data, we selected a weight measurement at least 1 year prior to the baseline weight value, and we estimated the weight change per year from the prior to the baseline weight while dividing by the amount of time elapsed between the two.. This weight change per year was included in the analyses where weight change (increase or decrease) was taken into account.
Ascertainment of death and its causes
We ascertained mortality from our EMR, and linked our CKD registry with the Ohio Department of Health mortality data to obtain details about mortality rates and cause specific mortality details. The underlying cause of death was coded according to the International Classification of Diseases, Tenth Revision (ICD-10). We grouped the underlying causes of death according to the National Center for Health Statistics for each coding system, except for some changes as outlined below. We classified deaths into four major categories: a) cardiovascular deaths, b) malignancy, c) non-cardiovascular/ non-malignancy related, and d) other causes (such as homicide/suicide/accidents). We defined cardiovascular deaths as deaths due to diseases of the heart, essential hypertension, cerebrovascular disease, atherosclerosis or other diseases of the circulatory system (ICD-10 codes I00–I78). We also categorized the cardiovascular deaths into the following clinically meaningful sub-categories: ischemic heart disease (I20– I25), heart failure (I50), cerebrovascular diseases (I60s) and all other cardiovascular disease (include all others from I00 to I-78 except I20–I25, I50 and I60). Patients with death noted in the EMR but not found in Ohio mortality files were included in the analysis of all-cause mortality and excluded from the cause-specific analysis (n=197).
Statistical analysis
We compared baseline characteristics among patients in various categories of BMI using Chi-square and ANOVA tests for categorical and continuous variables, respectively. We summarized the leading causes of death for various BMI categories as percent of total deaths observed. We evaluated the relationship between various BMI categories and overall mortality using a Cox proportional hazards model and the relationship between BMI and cause specific deaths using competing risks regression models. We inspected log-log plots for violations of the proportional hazards assumption. We fitted Cox proportional hazards models for all-cause deaths while adjusting for the following covariates: eGFR group, age, race, gender, diabetes, hypertension, hyperlipidemia, malignancy, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, insurance, use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs), statins and beta blockers, albumin, hemoglobin, and smoking. We also fitted separate competing risk regression models for each cause of death outcome (deaths due to cardiovascular disease, malignancy, and non-cardiovascular/ non-malignant deaths) to estimate the subdistribution hazards while adjusting for the variables mentioned earlier. We also evaluated the association between continuous BMI and each cause-specific mortality using splines at the 10th, 50th and 90th percentile of BMI. We then plotted continuous BMI vs. the estimated subdistribution values at 4 years of follow up while fixing all covariates in the model at their mean values.. We evaluated two-way interactions between BMI group and each of the following: sex, diabetes, hypertension, smoking and age. Approximately 19% of patients were missing serum albumin, 17% were missing hemoglobin data, 13% were missing smoking status, and 3% did not have insurance. For hemoglobin and albumin we used mean value imputation along with dummy indicators of missing values. To evaluate the effect of mean value imputation we also fitted models using only patients with complete data.
To evaluate the effect of BMI while adjusting for weight change over time, we selected patients who had a weight measurement at least 1 year prior to the baseline weight value, and we estimated the weight change per year from the prior to the baseline weight. We then fitted models for all-cause and cause-specific mortality while adjusting for patients’ weight change per year plus all previously mentioned variables. To relax linearity assumptions weight change was modeled with splines at the 10th, 50th and 90th percentile.
We conducted the following sensitivity analyses to confirm the primary findings: a) by considering the reference group as those with BMI 25–29.9 kg/m2 as reported by others (39), b) by considering the reference group as those with BMI 21.5–24.9 kg/m2 (40, c) by excluding diabetes and hypertension in the models as they could be in the causal pathway of obesity and adverse outcomes in CKD (5), d) by including only those with proteinuria data, e) by censoring all follow-up as of Sep 2009 (until when we have the USRDS data to confirm that patients were not on dialysis.
All analyses were conducted using Unix SAS version 9.4 (SAS Institute, Cary, NC), and graphs were created using R 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria) and cmprsk package. This study and the CKD registry were both approved by the Cleveland Clinic Institutional Review Board.
Supplementary Material
Acknowledgments
The authors wish to thank Welf Saupe, Vicky Konig, Dr. Stacey Jolly and John Sharp of Cleveland Clinic who helped in data extraction during the development of the registry.
Grant support:
SDN: R01DK101500; JVN: DK094112. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have no relevant financial interest related to the contents of data presented in this study. The creation of the CCF CKD registry was funded by an unrestricted grant from Amgen, Inc to the Cleveland Clinic - Department of Nephrology and Hypertension Research and Education Fund. Results of this manuscript will be presented as an abstract at the annual American Society of Nephrology meeting to be held in San Diego, CA in November 2015.
Footnotes
Disclosures:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Chen X, Song Y, et al. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Lu JL, Kalantar-Zadeh K, Ma JZ, et al. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25:2088–2096. doi: 10.1681/ASN.2013070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol. 2013;8:945–952. doi: 10.2215/CJN.09870912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49:581–591. doi: 10.1053/j.ajkd.2007.02.277. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W, Katzmarzyk PT, Horswell R, et al. Body mass index and the risk of all-cause mortality among patients with type 2 diabetes mellitus. Circulation. 2014;130:2143–2151. doi: 10.1161/CIRCULATIONAHA.114.009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Katzmarzyk PT, Horswell R, et al. Body mass index and stroke risk among patients with type 2 diabetes mellitus. Stroke. 2015;46:164–169. doi: 10.1161/STROKEAHA.114.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer H, Shoham D, McClure LA, et al. Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2011;58:177–185. doi: 10.1053/j.ajkd.2011.02.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsayed EF, Tighiouart H, Weiner DE, et al. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am J Kidney Dis. 2008;52:49–57. doi: 10.1053/j.ajkd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navaneethan SD, Kirwan JP, Arrigain S, Schold JD. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999–2004. BMC Nephrol. 2014;15:108-2369-15-108. doi: 10.1186/1471-2369-15-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Disease Control and Prevention. Deaths: Preliminary data for 2011. 2014;2014 [Google Scholar]
- 16.Thompson S, James M, Wiebe N, et al. Cause of Death in Patients with Reduced Kidney Function. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaneethan SD, Schold JD, Arrigain S, Jolly S, Nally JV. Cause-specific deaths in non-dialysis dependent chronic kidney disease. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2014101034. Accepted- In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 19.Katzmarzyk PT, Reeder BA, Elliott S, et al. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can J Public Health. 2012;103:147–151. doi: 10.1007/BF03404221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 21.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 22.Beddhu S, Abraham J. Risk factor paradox in CKD and ESRD: does a healthy lifestyle matter? Clin J Am Soc Nephrol. 2013;8:515–517. doi: 10.2215/CJN.02030213. [DOI] [PubMed] [Google Scholar]
- 23.Khalid U, Ather S, Bavishi C, et al. Pre-morbid body mass index and mortality after incident heart failure: the ARIC Study. J Am Coll Cardiol. 2014;64:2743–2749. doi: 10.1016/j.jacc.2014.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 25.Tobias DK, Hu FB. Does being overweight really reduce mortality? Obesity (Silver Spring) 2013;21:1746–1749. doi: 10.1002/oby.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson WR, Furberg H, Banack HR. Selection bias: a missing factor in the obesity paradox debate. Obesity (Silver Spring) 2014;22:625. doi: 10.1002/oby.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanks LJ, Tanner RM, Muntner P, et al. Metabolic subtypes and risk of mortality in normal weight, overweight, and obese individuals with CKD. Clin J Am Soc Nephrol. 2013;8:2064–2071. doi: 10.2215/CJN.00140113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrero JJ. Misclassification of obesity in CKD: appearances are deceptive. Clin J Am Soc Nephrol. 2014;9:2025–2027. doi: 10.2215/CJN.10361014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma D, Hawkins M, Abramowitz MK. Association of sarcopenia with eGFR and misclassification of obesity in adults with CKD in the United States. Clin J Am Soc Nephrol. 2014;9:2079–2088. doi: 10.2215/CJN.02140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal R, Bills JE, Light RP. Diagnosing obesity by body mass index in chronic kidney disease: an explanation for the “obesity paradox? ” Hypertension. 2010;56:893–900. doi: 10.1161/HYPERTENSIONAHA.110.160747. [DOI] [PubMed] [Google Scholar]
- 31.Kokkinos P, Faselis C, Myers J, et al. Cardiorespiratory fitness and the paradoxical BMI-mortality risk association in male veterans. Mayo Clin Proc. 2014;89:754–762. doi: 10.1016/j.mayocp.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Beddhu S. The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial. 2004;17:229–232. doi: 10.1111/j.0894-0959.2004.17311.x. [DOI] [PubMed] [Google Scholar]
- 33.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J Am Soc Nephrol. 2007;18:304–311. doi: 10.1681/ASN.2006060674. [DOI] [PubMed] [Google Scholar]
- 34.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 35.Lee TA, Pickard AS, Au DH, et al. Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med. 2008;149:380–390. doi: 10.7326/0003-4819-149-6-200809160-00004. [DOI] [PubMed] [Google Scholar]
- 36.Iso H, Jacobs DR, Jr, Goldman L. Accuracy of death certificate diagnosis of intracranial hemorrhage and nonhemorrhagic stroke. The Minnesota Heart Survey. Am J Epidemiol. 1990;132:993–998. doi: 10.1093/oxfordjournals.aje.a115742. [DOI] [PubMed] [Google Scholar]
- 37.Navaneethan SD, Jolly SE, Schold JD, et al. Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6:40–49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer H, Shoham D, McClure LA, et al. Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2011;58:177–185. doi: 10.1053/j.ajkd.2011.02.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–244. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.