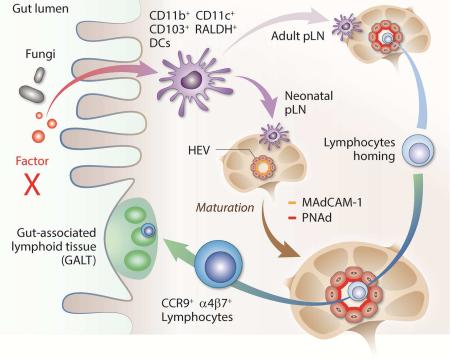
Summary
Lymphocyte homing to draining lymph nodes is critical for the initiation of immune responses. Secondary lymphoid organs of germ-free mice are underdeveloped. How gut commensal microbes remotely regulate cellularity and volume of secondary lymphoid organs remains unknown. We report here that driven by commensal fungi, a wave of CD45+CD103+RALDH+ cells migrated to the peripheral lymph nodes after birth. The arrival of these cells introduced a high amounts of retinoic acid, mediated the neonatal to adult addressin switch on endothelial cells, and directed the homing of lymphocytes to both gut-associated lymphoid tissues and peripheral lymph nodes. In adult mice, a small number of these RALDH+ cells may serve to maintain the volume of secondary lymphoid organs. Homing deficiency of these cells was associated with lymph node attrition in Vitamin A-deficient mice, suggesting a perpetual dependence on retinoic acid signaling for structural and functional maintenance of peripheral immune organs.
Graphical Abstract
Introduction
In prenatal lymphogenesis, lymph node (LN) anlagen develop from primitive lymph sacs that appear during lymphatic vascularization, upon the arrival of lymphoid tissue-inducer cells (LTi, RORγt and ID2-dependent (Eberl et al., 2004; Sun et al., 2000; Yokota et al., 1999), CD4+CCR6+NKp46− subset of group 3 innate lymphoid cells (Spits et al., 2013)). LTi mediate the initial development of these structures after receiving cues from retinoid acid (RA) released from neuronal termini in the vicinity (van de Pavert et al., 2009), and LTi cell-autonomous RA is critical for LN development in utero (van de Pavert et al., 2014). After birth, LTi are no longer found in LN (Kim et al., 2003), yet gut-associated lymphoid tissues (GALT) and peripheral LN (pLN) continue to expand in size and cellularity. By the second week after birth, pLN form adult-like clear demarcation of T cell zones and B cell follicles (Mebius et al., 1996). However, neonatal pLN developments are arrested in germ-free (GF) animals (Bauer et al., 1963; Inagaki et al., 1996). In GALT of GF mice, Peyer's patches (PP), isolated lymphoid follicles, and mesenteric LN (mLN) are fewer in number and less cellular; pLN are relatively structureless. These defects suggest a critical role of commensal microbes in the development of SLO after birth (Macpherson and Harris, 2004). How the microbiota promotes these changes, particularly at distal LN, remains unknown.
Blood lymphocytes travel into LN through high endothelial venules (HEV) (Masopust and Schenkel, 2013), attracted by addressins such as MAdCAM-1 (receptor for α4β7) and peripheral lymph node addressin (PNAd, receptor for CD62L (Rosen, 2004)). In neonatal specific pathogen free (SPF) mice shortly after birth, MAdCAM-1 expression is high (Mebius et al., 1996), followed by a switch to PNAd-dominated profile over a course of 2-3 weeks (Hemmerich et al., 2001; van Zante et al., 2003), gradually initiating L-selectin-based adult lymphocyte homing. A “non-T cell” population has been found immediately before B cell follicle formation (Cupedo et al., 2004); the identity of those mysterious cells and how they might contribute to LN structural buildup remain elusive. In steady state pLN, two reports suggest that semi-mature chemokine receptor CCR7+ (Wendland et al., 2011) and CD11c+ (Moussion and Girard, 2011) DCs regularly enter SLO to promote the development of HEV and attract lymphocyte homing. Questions remain regarding how much these three types of cells functionally and phenotypically overlap, and more importantly, whether they are associated with gut microbiota in inducing LN development.
In specific pathogen-free (SPF) mice, GALT are modulated by a stream of incoming ID2, IRF8, Batf3, IRF4 and Notch2 transcription factor-dependent RALDH+ (Retinaldehyde Dehydrogenase) CD11b+CD103+ DCs from the gut, mainly lamina properia (LP) (Helft et al., 2010), with a mirroring population in humans (Watchmaker et al., 2014). These DCs are phenotypically unique as in the periphery of mice, such as skin, CD103 and CD11b expressions are mutually exclusive (Ginhoux et al., 2009). Arriving via CCR7 (Jang et al., 2006; Worbs et al., 2006), these cells have been reported to convert CD4+ T cells into inducible regulatory T cells in concert with TGF-β (Coombes et al., 2007), mediate T helper-17 (Th17) cell homeostasis (both positively and negatively) (Cha et al., 2010; Elias et al., 2008; Mucida et al., 2007; Wang et al., 2010) and imprint lymphocytes for gut homing (Iwata et al., 2004; Mora et al., 2003). Therefore, a coherent scheme linking gut microbiota, those seemingly disparate “visitors”, and neonatal LN structural development is highly desired.
We report here that in comparison to GF, RA-related genes were highly expressed in SPF pLN. Notably, a group of RALDH+ cells appeared in pLN following birth or in GF LN after cohousing with SPF mice. Transfer of these cells completely restored the structural and cellularity defects and triggered HEV MAdCAM-1 to PNAd addressin profile change in GF mice. In early neonatal weeks, these cells imprinted peripheral lymphocytes for gut homing, in sync with the rapid establishment of GALT. In adult mice, this signal was disrupted in Vitamin A (VitA)-restricted mice, linking this regulation to immune dysfunctions associated with VitA deficiency. Our work therefore unveils the path linking gut microbiota to peripheral immune functions and establishes the instrumental role of RA in the birth, development, and health of SLO.
Results
Germ-free mice have reduced lymphocyte homing in peripheral lymph nodes
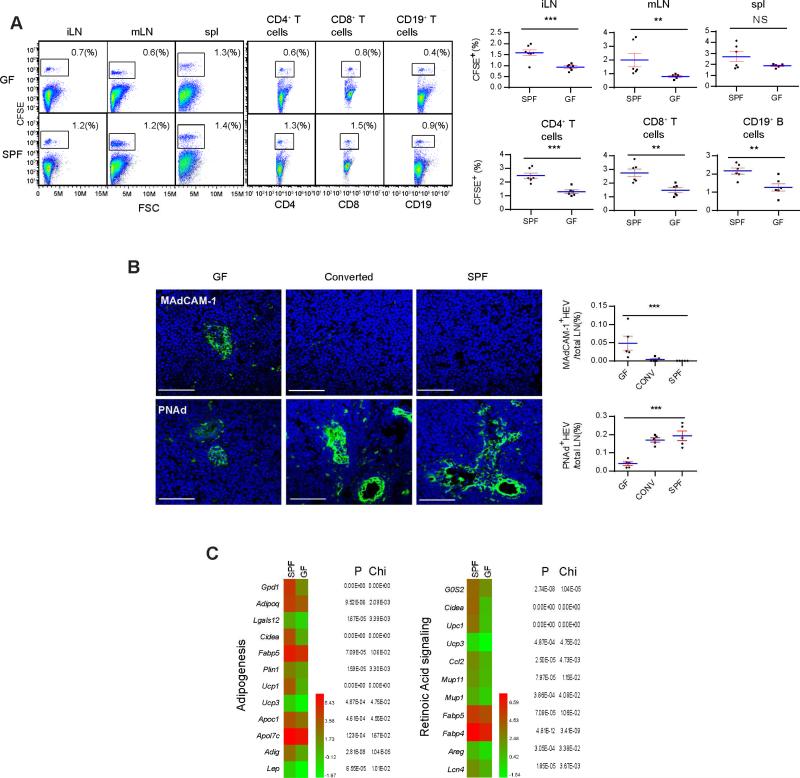
To confirm that reduced lymphocyte homing was the cause of pLN defects in GF mice, 107 SPF total LN lymphocytes were labeled with CFSE and injected into tail veins of GF and control SPF mice (see Experimental Procedures for operational detail of i.v. infusion and surgery in GF isolators). In SPF mice, CFSE+ cells were detected in iLN (inguinal), mLN and the spleen 24 hrs after the transfer. This afflux was greatly reduced in iLN (Figure 1A). In iLN, CD4+, CD8+ T and CD19+ B cells also showed a similar homing reduction (Figure 1A). HEV in iLN of 5 week-old GF mice were smaller and maintained expression of MAdCAM-1 mixed with a modest presence of PNAd (Rosen, 2004) (Figure 1B). Total size of lumen area and number of lumen per node were also smaller in GF mice (Figure S1A). Cohousing with SPF mice for 1 week, the MAdCAM-1 disappeared, with a robust expression of PNAd surrounding enlarged HEV portals (Figure 1B), suggesting that this change was driven by the microbiota and was accelerated in adult mice. To globally analyze the impact of gut flora on the development of HEV, iLN, axillary (aLN) and submandibular LN mRNA was isolated from 5 week-old GF and SPF mice and was subjected to Illumina transcriptome sequencing (details in Figure S1B, overall heat map). Transcripts with the largest differences in abundance and X2 values less than 0.05 between GF and SPF LN were graphed by functions (Figure 1C, and Figure S1C and S1D, the database is available online). RNAseq did not detect statistically significant difference in MAdCAM-1 mRNA expression. The abundance of mRNA transcripts of enzymes involved in PNAd expression also did not change (data not shown) (Sperandio, 2006), suggesting post-translational modification. Two groups with the greatest increases in SPF mice were associated with adipogenesis and RA signaling (Figure 1C). For example, Fabp5, Plin1, Apoc1, and Apol7c are involved in lipid transportation and G0S2, Cidea, CCL2, Areg, UCP1 are RA transcriptional targets (Balmer and Blomhoff, 2002). GF mice are lipoatrophic (Backhed et al., 2004; Backhed et al., 2007). Transplantation of visceral epididymal fat tissue from SPF mice to the lower region of abdomen of GF mice led to an increase in the size of ipsilateral but not contralateral or sham-operated iLN within 2 weeks (Figure S1E and data not shown). However we failed to isolate a population of cells from the transplant that supported LN size expansion, implicating the involvement of a soluble effector stored in the graft.
Figure 1. Homing of lymphocytes to peripheral lymph nodes is defective in germ-free mice.
(A) 107 CFSE-labeled total SPF pLN lymphocytes were isolated from C57BL/6 SPF mice and i.v. injected into GF or SPF recipients. 24 hrs later, the arrival of these cells in iLN, mLN and the spleen, and CD4+, CD8+ and CD19+ subpopulations in iLN were analyzed by flow cytometry. The right panels show results from multiple mice. Student's t analysis results for this and all subsequent figures are indicated as follows: p<0.001: ***; p<0.01: **; p<0.05: *; p at 0.05 or larger was considered non-significant: N.S.. The data are representative of four independent experiments. (B) iLN of GF, SPF-cohoused GF (1 week) and SPF iLN were stained with DAPI (blue) and either MECA-367 (anti-MAdCAM-1) or MECA-79 (anti-PNAd) (green) antibody, and analyzed by confocal imaging. The scale bars in this and all subsequent figures are 50 μm. See Figure S1A for lumen area size and number analyses. Shown is a representative slide from a total of at least 30 sections, and sections were collected throughout entire lymph nodes. Images were processed with Image J and surface areas were calculated by the ruler-transformed pixels. (C) Total mRNA from pooled pLN (excluding mLN) was extracted from 5 week-old C57BL/6 GF and SPF mice and analyzed as detailed in the methods. mRNA ratios between GF and SPF reaching statistical significance were displayed by function groups. P and X2 values from RNAseq assay are shown to the right. Shown are the heat maps for gene functions associated with adipogenesis and RA signaling, corresponding data shown as bar graphs are in Figure S1D. See Figure S1B and S1C for the total heat map and other gene function groups. The data are representative of two independent experiments. Also see Figure S1.
Germ-free mice have reduced RALDH activities in secondary lymphoid tissues
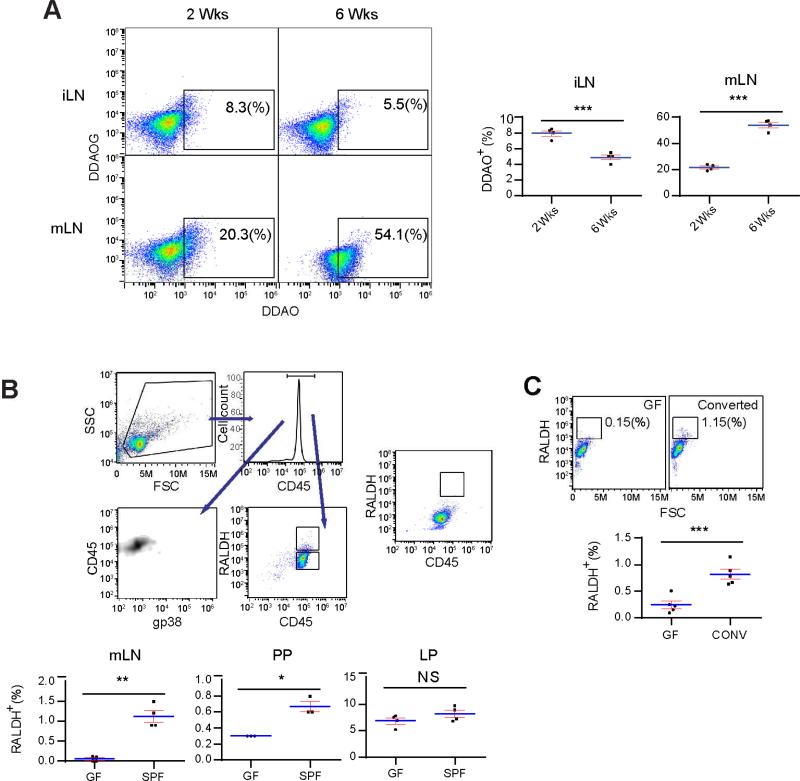
Vitamin A, enriched in adipocytes, can initiate RA signaling in tissue development (Hall et al., 2011b; Molenaar et al., 2011). RALDH produces RA from retinaldehyde, a derivative of VitA (Hall et al., 2011b). To assess the presence of this enzyme in SPF LN, total iLN cells from a CD1 strain with a β-galactosidase reporter under the control of retinoic acid responsive element (RARE) were labeled with DDAOG and incubated in vitro. Measured by DDAOG to DDAO conversion (Tung et al., 2004), Figure 2A shows increased RA activities in iLN of 2 week-old mice, in comparison with a modest presence in 6 week-old counterparts. In line with RA-dependent regulations in adult gut lymphoid tissues, DDAO conversion continued to rise from 2 to 6 weeks in mLN (Figure 2A). RALDH2 mRNA was highly expressed in RALDH+ cells from iLN and mLN (Figure S2A) (Iwata et al., 2004). We failed to notice any difference in the frequency of RALDH+ cells in Raldha1−/− and Raldha3−/− LN (data not shown).
Figure 2. CD45+RALDH+ cells in pLN are associated with the increased number of lymphocytes following establishment of gut microbiota.
(A) Left panel: lymphocytes from 2 and 6 week-old SPF iLN and mLN were labeled with DDAOG, cultured in vitro for 24 hrs, the percentages of DDAO-positive populations were identified by flow cytometry. Right panel: the percentage values in the boxes in the left panel were graphed with results from multiple mice. (B) GF and SPF mouse mLN, PP and LP cells were gated on CD45 expression and then on RALDH (Aldefluor). Top panel shows the gating steps. The CD45+ population indicated in the window of top right flow cytometry plot was also stained for their gp38 expression to rule out the presence of stromal cells. DEAB-treated control is shown to the right. The percentages of high RALDH expression cells as indicated by the RALDH high box are shown in the lower panels. (C) Adult GF mice were transferred to and cohoused with SPF mice for 1 week. The percentages of high RALDH expressers were compared with GF mice without the cohousing. All panels are representative of at least three independent experiments. Also see Figure S2.
To identify the bearer of RALDH2, mLN cells from 5 week-old SPF mice were treated with Aldefluor (a RALDH fluorometric substrate) (Moreb et al., 2012). Figure 2B shows that among gp38−CD45+ (lymphoid) cells, on average 1.13 +/− 0.14 % of SPF mLN cells were RALDH+. This population was barely detectable in GF mice. The number was also reduced in PP (Figure 2B), while remaining roughly the same in LP. Diethylaminobenzaldehyde (DEAB), a RALDH inhibitor (Moreb et al., 2012), blocked the Aldefluor staining (Figure 2B). It was important to confirm that gut microbiota was not required for the appearance of RALDH+ cells in LP, as microbial fermentation product butyrate has been reported to upregulate RALDH1 (skin-specific RALDH) mRNA in cultured DCs in vitro (Singh et al., 2014), an event clearly unrelated to RALDH2 expression in CD45+RALDH+ DCs in LP. 1 week after GF mice were transferred to SPF housing, a spike of these cells was detected in iLN (Figure 2C, 0.82 +/− 0.09 % vs 0.24 +/− 0.07 % in the untreated, p<0.001).
We performed the RNAseq on the Swiss ICR strain of GF and SPF mice to confirm that RA signaling is a common theme in LN development (Rice and O'Brien, 1980). The upregulation of RA-associated transcripts was also seen 1 week after GF to SPF conversion (Figure S2B and S2C, overall and RA signaling-related heatmaps and numerical display, respectively), suggesting that RA signaling was involved in regulating LN structure in outbred mice. The appearance of CD45+RALDH+ cells was normal in mLN and PP from Nfkb1−/−, Myd88−/−, Tlr4−/−, and Il1r1−/−-deficient SPF mice (Figure S2D), indicating that these pathways were not essential for the appearance of CD45+RALDH+ cells in neonatal SLO.
RALDH+ migratory cells restore the cellularity of peripheral lymph nodes in GF mice
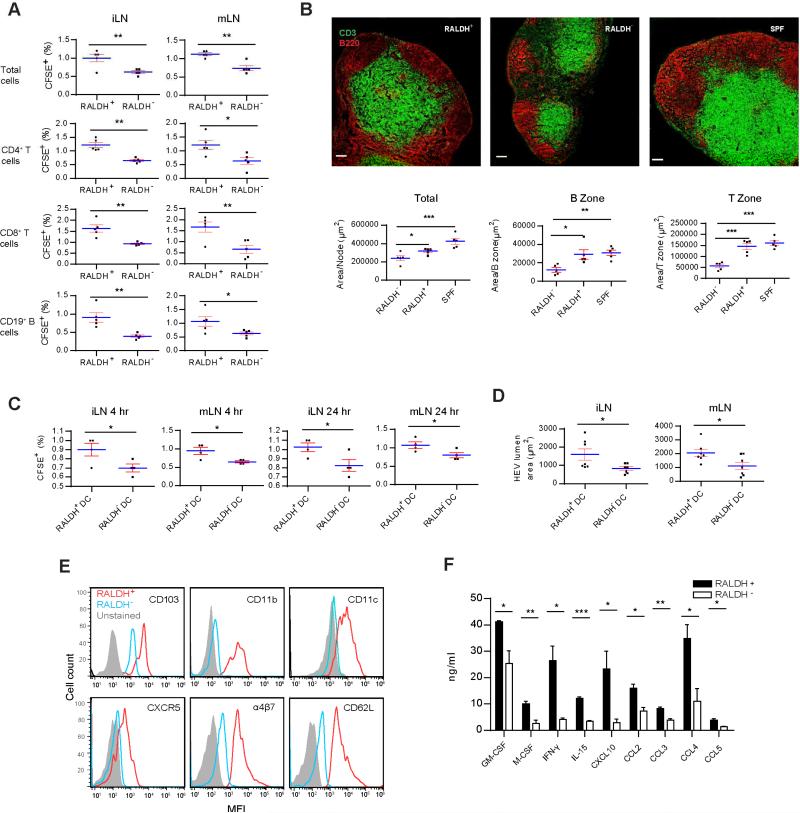
Because RALDH+ cells are regarded mostly as being unique to the gut (Hall et al., 2011b), their appearance in pLN suggested a previously unexplored role by these cells in postnatal SLO expansion. CD45+RALDH+ cells were purified from 2 week-old SPF pLN, and 2×105 cells were i.v. transferred into 5 week-old GF mice. 7 days later, 107 CFSE-labeled total SPF LN cells were similarly injected and their homing pattern was monitored after 24 hrs. Figure 3A shows that more CFSE+ cells migrated to iLN and mLN in RALDH+ recipients than in RALDH− controls. The same trend was seen with CD4+, CD8+ and CD19+ lymphocytes (Figure 3A). In addition, iLN in RALDH+ recipients showed mature structures with enlarged B cell follicles, denser T cells, and a clear demarcation between T and B zones (Figure 3B). Quantitatively, the overall size of LN, and areas of definable B and T cell zones were also increased after RALDH+ cell infusion (Figure 3B). When bone marrow DCs (BMDCs, RALDH−) were used for comparison, CD45+RALDH+ cells were still more robust in triggering lymphocyte homing in both 6 and 24 hrs (Figure 3C), suggesting that not all DCs were able to efficiently support pLN development. In addition, total HEV area was larger in RALDH+ cell-infused group (Figure 3D).
Figure 3. CD45+RALDH+ cells are phenotypically similar to mLN migratory DCs.
(A) 2 × 105 CD45+RALDH+ and CD45+RALDH− (lower RALDH box in Figure 2B) cells were isolated by cell sorting from SPF mice and i.v. injected in containment into tail veins of 5 week-old GF mice. 7 days later, CFSE-labeled 107 SPF pLN total lymphocytes were similarly infused into the recipients. 24 hrs later, the percentages of CFSE+ cells as well as their CD4+, CD8+ and CD19+ subsets in iLN and mLN were analyzed. The data are presentative of four independent experiments. (B) CD45+RALDH+ or CD45+RALDH− cell-infused GF mice were rested for 7 days and their iLN were stained for anti-B220 and anti-CD3 and analyzed by confocal imaging. SPF iLN was used as the control. Overall surface areas of LN and accumulative T zone defined by dense anti-CD3 stain in one LN are shown in lower panels. For B cell area, single B cell zone defined by dense anti-B220 stain was counted. (C) Identical to A except that 5 day BMDCs sorted for RALDH− stain were used in place of RALDH− cells. Overall lymphocyte homing to iLN and mLN in 6 and 24 hrs is shown. (D) Individual surface areas of HEV staining per lumen are shown. All images are representative of at least 30 independent photos, and the statistics are shown as the pooled data. (E) CD45+RALDH+ cells and CD45+RALDH− control from 2 week-old SPF iLN were gated and further analyzed for CD103, CD11b, CD11c, CXCR5, α4β7 and CD62L. Secondary antibody-only staining of CD45+RALDH+ cells was used as the background control. See Figure S3B for additional markers. (F) 2 × 105 CD45+RALDH+ cells from 2 week-old SPF iLN were isolated and rested in tissue culture media overnight. Their cytokine secretion was analyzed. Qualitative differences in secretion reaching t test p value < 0.05 are shown. E and F are representative of three independent experiments. See Figure S3C for the secretion of additional 22 cytokines that did not reach statistically significant difference (p > 0.05). Also see Figure S3.
All RALDH+ cells identified in Figure 2B also expressed CD103, CD11c and MHC class II (Figure S3A). In comparison with CD45+RALDH− cells, CD45+RALDH+ cells from 2 week-old SPF mice expressed higher CD103, CD11b, CD11c, CXCR5, CD62L, and α4β7 (Figure 3E), resembling the CD103+ DC population in the gut. MHC class II, F4/80, complement receptor 1, mannose receptor, CCR7, ICAM-1, VCAM-1, CD11a, CD80, CD86, and CD83 were higher as well, although the difference in expression was minimal for CD127, CD45RB, CD19, and Ly6G (Figure S3B). CD8 expression however was much lower in comparison (Figure S3B). No bimodal expression was detected for the tested markers (Figure 3E and Figure S3B). Therefore CD45+RALDH+ cells isolated during the initial expansion of LN appeared to be homogeneous. While IL-1α, IL-1β, IL-6, IL-10, IL-17 and additional 17 cytokines from the untreated in vitro culture were similar (Figure S3C, p>0.05), CD45+RALDH+ cells showed significantly higher production of IFN-γ, GM-CSF, M-CSF, IL-5, CCL2, CCL3, CCL4, CCL5 and CXCL10, factors involved in either chemotaxis or stromal cell addressin regulation, when compared to CD45+RALDH− cells (Figure 3F). In comparison with RALDH+ cells, RALDH− cells should represent all other cells identified in our initial size and granularity gating. Although we did not pursue those cells further, overall results suggested that RALDH+ cells contributed to pLN structural development.
Discussed in the context of the gut (Hall et al., 2011b); RALDH+ cells have never been reported to travel long distance to initiate pLN development following gut colonization or to regulate mLN development after birth. A comparison of 14 surface markers showed that CD45+RALDH+ cells in iLN and mLN of 2 week-old SPF mice were similar (Figure S3D). In addition, RALDH+ cells in 2 week-old iLN were found to be also positive for CD45, CD103, CD11c and MHC class II, suggesting the unique role of these cells at the early development of SLO (Figure S3E).
Gut RALDH+ migratory cells are driven by commensal fungi
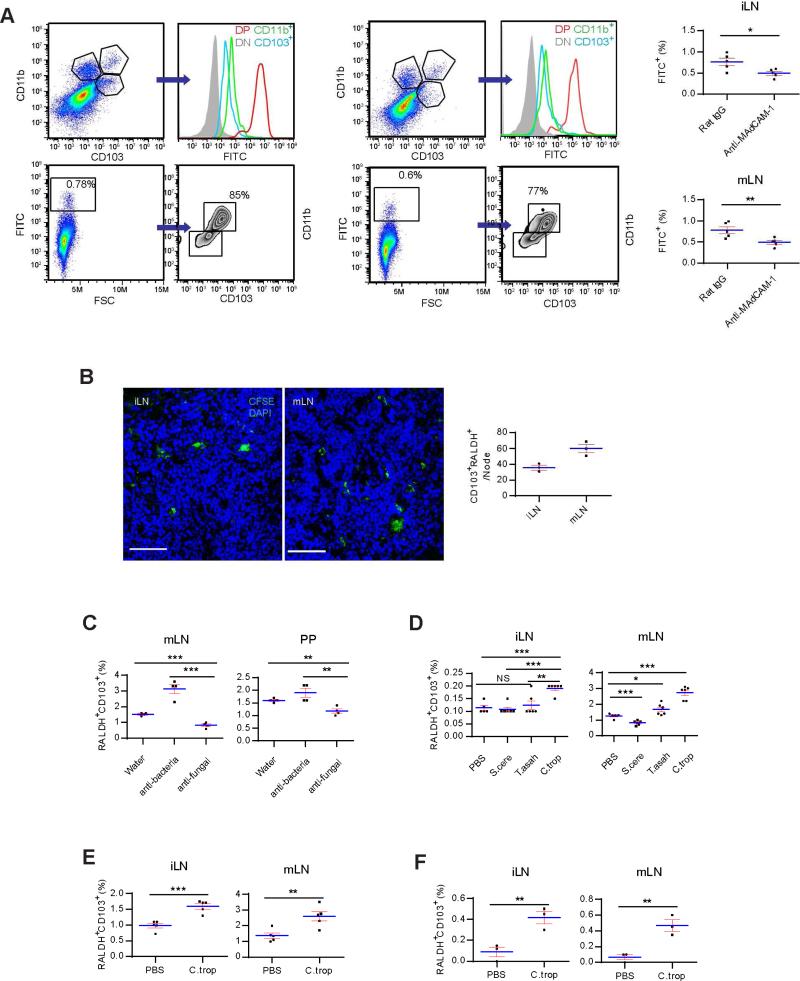
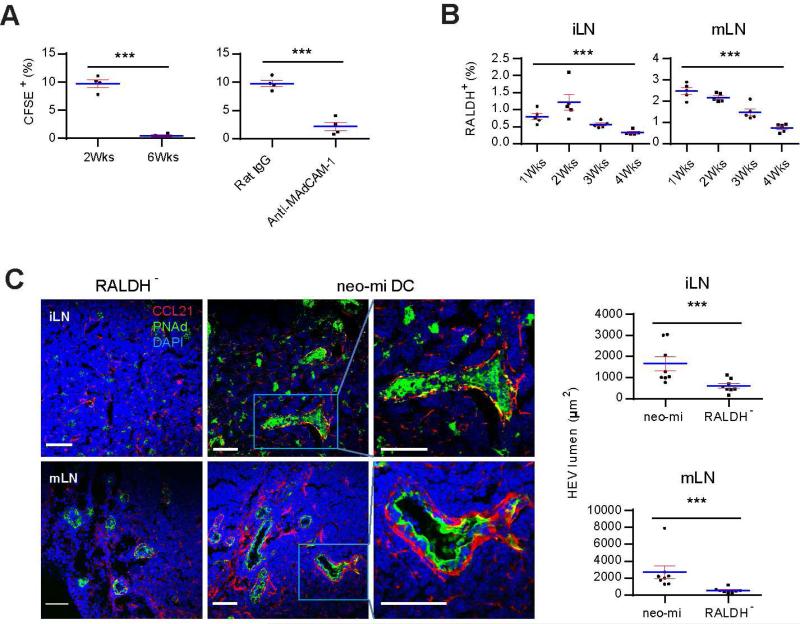
To verify that neonatal pLN CD45+RALDH+ cells were indeed CD103+CD11b+ DCs from the gut, 2 week-old SPF mice were fasted, and orally gavaged with FITC-dextran 2000KD. 6 hrs later, iLN cells were analyzed by flow cytometry. In contrast with CD103 and CD11b double-negative and single-positive cells, the double-positive population was FITChigh, suggesting their prior exposure to the label in the gut (Figure 4A). A nearly identical profile was seen in mLN at this age (Figure 4A). Injection of anti-MAdCAM-1 antibody reduced the number of FITC-labelled double positive cells in LN (Figure 4A). These data indicated that the initial exodus of gut DCs targeted all SLO, and their preference for GALT was an “acquired” feature established later. FITC-labelled DCs reached LN in 6 hrs in neonatal mice. This was likely due to the higher gastrointestinal permeability, a different gastric emptying time and greater basal metabolic rates in these young animals (Beach et al., 1982; Catassi et al., 1995; Shearer et al., 1995). Further supporting their similar identity, CD103+RALDH+ cells purified from 5 week-old LP readily homed to 9 day-old iLN (Figure 4B). These long-distance migratory cells were henceforth called neonatal migratory DCs (neo-mi DCs) to functionally distinguish them from the sibling steady-state migration to GALT in SPF mice.
Figure 4. RALDH+ DC migration is driven by commensal fungi.
(A) 2 week-old SPF mice were fasted, orally forced fed with FITC-dextran 2000KD, and iLN and mLN were harvested after 6 hrs. Left: The FITC intensity in iLN was compared in CD103 and CD11b double-negative, double-positive and single-positive populations (upper panels). All FITC+ cells were also analyzed for their CD103 and CD11b expression (lower panels). Right flow cytometry plots: mLN data. The charts show the percentages of FITC+-DP cells in anti-MAdCAM-1 antibody-injected mice in comparison with rat IgG-injected control. (B) 2 × 105 CFSE-labeled CD103+CD11b+RALDH+ cells isolated from adult SPF LP were i.v. injected into 9 day-old SPF mice. 24 hrs later, the presence of these cells in iLN and mLN was analyzed by confocal imaging. Right panel is the cell counts per LN. (C) Adult SPF mice were treated with AVNM or fluconazole mixed in drinking water for 3 weeks, and the percentages of RALDH+CD103+ cells in mLN and PP were determined by flow cytometry. (D) Untreated adult SPF mice were given 108 indicated live fungal strains by gavage and LN were analyzed as in C after 24 hrs. (E) As in D, 2 week-old SPF mice were used in place of the adult mice. (F) 8 week-old GF mice were given 108 C. tropicalis gavage and neo-mi DCs in LN were analyzed after 48 hrs. All data are representative of at least three independent experiments. Also see Figure S4.
Neo-mi DC migrated out of LP in response to some components in gut microbiota. We first treated adult SPF mice with an anti-bacterial cocktail (ampicillin, vancomycin, neomycin and metronidazole, AVNM) or fluconazole (anti-fungal) added to drinking water. Percentages of RALDH+ cells in mLN and PP were analyzed after 3 weeks. Fluconazole significantly inhibited their numbers in mLN and PP (Figure 4C). Anti-bacterial treatment had an opposite effect (Figure 4C). Candida tropicalis, Saccharomyces cerevisiae and several strains of Trichosporon are most abundantly found in the gut (Iliev et al., 2012). 4 week-old SPF mice were force-fed with those live fungi produced from purified cultures. Candida tropicalis not only enhanced the steady migration of RALDH+ cells to mLN (Figure 4D), but also revived the “neo-mi” DC-like migration in adult mice (Figure 4D). To confirm that C. tropicalis induced neo-mi DC migration, 2 week-old mice were similarly treated. In comparison with PBS control, C. tropicalis significantly increased percentages of neo-mi DCs in iLN and mLN (Figure 4E). In GF mice receiving the same gavage in confinement, numbers of neo-mi DCs in iLN were also significantly higher, mimicking the natural neonatal migration in SPF mice or during GF to SPF conversion (Figure 4F). Therefore, commensal contents, particularly those from Candida tropicalis, were capable of triggering neo-mi DC migration to both mLN and pLN in C57BL/6 mice. Functionally, C. tropicalis may regulate neo-mi DCs either by functionally upregulating RALDH expression in a population of DCs, or driving a preexisting CD103+RALDH+ cells to leave LP and enter SLO. Figure S4A and B show that neither BMDCs nor RALDH−CD11c+ cells from LP expressed RALDH after co-culture with killed C. tropicalis. Conversely, anti-fungi treatment slightly retained neo-mi DCs in LP with a corresponding decreased of these cells in iLN (Figure S4C). Following oral gavage of this strain, CD103+RALDH+ cells were reduced in cell number in LP with an ensuing increase of these cells in iLN (Figure S4D). Collectively, these data suggest that C. tropicalis likely triggers migration of neo-mi DCs rather than induced RALDH expression in DCs in LP.
RALDH+ cells induce pLN expansion and neonatal to adult addressin switch
As α4β7 expression on peripheral lymphocytes was reduced in GF mice (Figure S5A), MAdCAM-1 expression alone was clearly not sufficient to permit their homing to pLN. CD103+ cells appeared in iLN of Rag1−/− mice (Figure S5B), suggesting they were functionally upstream to the lymphocyte homing and the establishment of pLN volume. This raised the possibility that MAdCAM-1 mediated the initial homing of neo-mi DCs and the latter subsequently attracted bona fide lymphocyte accumulation in pLN. Indeed, neo-mi DCs readily homed to pLN of 2 week-old SPF mice but not as efficiently to those of 6 week-old (Figure 5A). Infusion of MAdCAM-1 antibody blocked this migration (Figure 5A right panel and Figure S5C). In 1 week-old SPF mice, relative percentage of these cells in iLN was low, followed by a rise by week 2 (Figure 5B). Numbers of these cells became lower after 2 weeks, retaining only 0.33 +/− 0.07 % at week 4 (Figure 5B, One way ANOVA p<0.001). In mLN, the downward trend was similar, although the percentages were higher at both 2 and 4 weeks (Figure 5B).
Figure 5. Neo-mi DCs induce neonatal to adult pLN addressin switch and HEV portal enlargement.
(A) Homing of neo-mi DCs into 2 and 6 week-old iLN. Left panel, 2 × 105 CD45+RALDH+ neo-mi DCs were isolated from pLN of 2 week-old SPF mice and labeled with CFSE, and their migration to 2 and 6 week-old iLN was analyzed after 24 hrs as a percentage of total CD103+ cells in LN. Right panel, similar to the left, 2 week-old mice were injected with MAdCAM-1-blocking antibody as detailed in the methods, the migration of neo-mi DCs to iLN of 2 week-old mice after 24 hrs was compared to a rat IgG-injected control. The data are representative of three independent experiments. (B) The percentages of neo-mi DCs in iLN and mLN of 1, 2, 3 and 4 week-old SPF mice were analyzed by flow cytometry. The data are representative of at least five independent experiments. (C) 2 × 105 neo-mi DCs were isolated from 2 week-old iLN of SPF mice, and i.v. injected into tail veins of 5 week-old GF mice in containment. The expression of PNAd and CCL21 in iLN and mLN was analyzed by confocal imaging after 7 days. Right panels: HEV portal areas (pixels surround by PNAd) were counted from at least 8 random images (3 mice per group). The images are representative of at least 30 photos. The statistics are shown from the pooled data. Also see Figure S5.
The arrival of these cells was associated with a dramatically altered appearance of HEV in pLN. 6 days after an infusion of neo-mi DCs, both iLN and mLN of GF mice displayed robust HEV portals, coated by thick layers of PNAd, while being surrounded by CCL21-expressing cells (Figure 5C) (Wendland et al., 2011). None of these features was present in pLN of CD45+RALDH− recipients (Figure 5C). Injection of anti-MAdCAM-1 antibody greatly reduced the number of HEV in iLN in comparison with Rat IgG control (Figure S5D), suggesting that CD103+RALDH+ cells were important in pLN expansion.
Neonatal RALDH+ cells imprint peripheral lymphocytes for gut homing
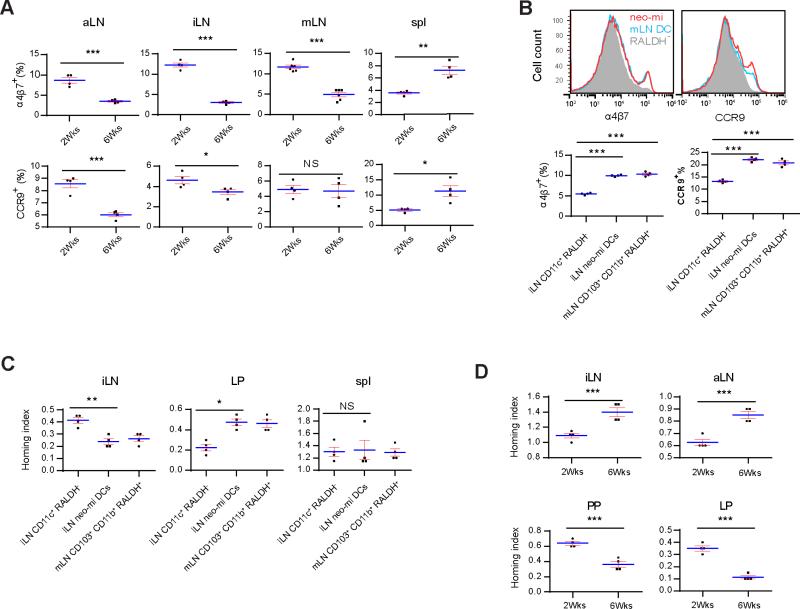
Although RALDH+ cells are mostly found in the gut, in pLN, a small percentage (about 10%) of CD103−CD8α+ DCs express RALDH under steady state (Guilliams et al., 2010; Molenaar et al., 2011). This raised the question why LP-originated neo-mi DCs were necessary in the neonatal expansion of pLN. In the digestive tract, abundance of food-derived VitA induces RALDH2 expression in DCs, creating a self-amplification loop and promoting a unique homing pattern towards the gut via upregulation of α4β7 and CCR9 in transiting lymphocytes (Bauer et al., 1963). In 2 week-old SPF mice, overall percentage of α4β7+ cells in pLN was higher than 6 week-old mice (Figure 6A). CCR9+ cells showed a similar trend in aLN and iLN, but not in mLN (Figure 6A). Overall, the data suggested that neo-mi DCs might redirect lymphocytes passing through pLN to the gut in early weeks after birth. Indeed, co-culture of neo-mi DCs from 2 week-old iLN and aLN with OT-II CD4+ T cells induced higher α4β7 and CCR9 expression on the latter (both p<0.001), similar to that of mLN counterpart (Figure 6B). For verification, a homing index assay was performed (Mora et al., 2003): OT-II T cells were co-cultured with neo-mi DCs from 2 week-old SPF pLN in the presence of OVA, labeled with CFSE, and i.v. co-delivered with an equal number of untreated, DDAO-labeled adult CD4+ T cells into 5 week-old SPF recipients. The ratio (CFSE over DDAO) of cells recovered from pLN showed that neo-mi DCs promoted better OT-II cell migration to LP than to iLN in adult SPF mice, compared with CD11c+RALDH− co-cultured OT-II cells, and were indistinguishable from mLN CD103+CD11b+RALDH+ cells (Figure 6C). In a similar homing index assay, pLN lymphocytes from 2 week-old mice migrated efficiently to PP and LP, and less so to iLN and aLN of 5 week-old SPF mice (Figure 6D). However, pLN lymphocytes from 6 week-old mice had lost this ability, instead showed a better migration to pLN than to GALT (Figure 6D). Therefore, our data imply that the presence of neo-mi DCs in pLN of neonatal mice contribute to the imperative buildup of GALT.
Figure 6. Neo-mi DCs imprint lymphocytes for gut homing.
(A) α4β7+ and CCR9+ cells among the total cells from aLN, iLN, mLN and the spleen of 2 and 6 week-old SPF mice were analyzed by flow cytometry. (B) Top panel: 5 × 105 neo-mi DCs were cocultured with purified CD4+ iLN cells (1:4) of OT-II mice. 7 days later the presence of α4β7 and CCR9 was determined by flow cytometry. Identical numbers of CD45+RALDH− cells from the same isolation and mLN CD45+RALDH+ cells were used as controls. Bottom panels: percentages of α4β7high and CCR9high cells from multiple mice are shown. (C) CD4+ cells isolated from OT-II iLN were cultured with neo-mi DCs in the presence of 200 μg/ml OVA. These T cells were labeled with CFSE. 2 × 106 cells from this mixture were then injected in combination with an equal number of DDAO-labeled CD4+ T cells isolated from SPF iLN into 2 week-old SPF mice. The homing index was determined by the CSFE+/DDAO+ ratios in iLN, mLN and the spleen. (D) Similar to C, untreated total pLN cells from 2 and 6 week-old SPF mice were used in place of OT-II T cells. Homing index was determined as in C. All panels are representative of at least three independent experiments.
RALDH+ cell migration is reduced in Vitamin A deficiency-associated immune malfunction
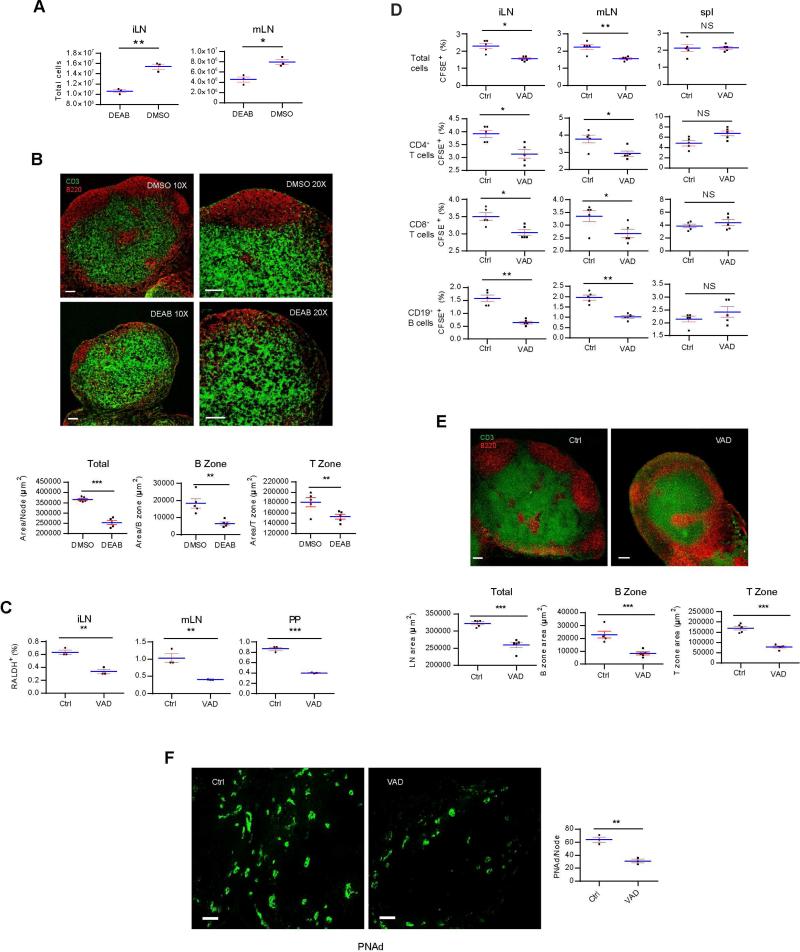
VitA deficiency is associated with a number of infections, reduced mucosal immune activation, limited T helper-1 (Th1) cell response, and one to two million yearly mortalities worldwide (Humphrey et al., 1992). Since a fraction of neo-mi DCs remained in pLN (Figure 5B), it was perceivable that a long-term maintenance function was fulfilled by those residual cells which could be impacted by VitA restriction. As, Aldh1a2−/− mice do not survive, we instead injected 5 week-old SPF mice with DEAB. While the total body weight of the treated mice was no different from the untreated (Figure S6A), LN in the treated group were visibly smaller (Figure S6B). DEAB significantly reduced the number of cells in iLN and mLN (Figure 7A). Anti CD3 and 220 staining of LN from DEAB-treated mice showed no distinguishable B cell zones and reduced cellularity (Figure 7B). These results suggested that RA was a tonic signal in adult LN maintenance. We fed SPF breeder mice with VitA-deficient (VAD) diet from the 9th day into gestation, and detected a reduction in the percentage of neo-mi DC population in 2 week-old LN (Figure 7C). This was accompanied by a reduction of lymphocyte numbers in iLN and mLN in 5 week-old mice weaned onto VAD diet (Figure 7D). The same pattern was seen for CD4+, CD8+ and CD19+ cells (Figure 7D). VitA restriction severely disrupted the pLN architecture (Figure 7E) and inhibited the expression of PNAd in iLN (Figure 7F) in 5 week-old mice, providing the mechanistic base for the reduced lymphocyte afflux. The total lymphocytes, and their CD4+, CD8+ and CD19+ subsets isolated from VitA-restricted mice travelled to SPF pLN, mLN and the spleen with either similar or in most cases increased efficiency compared with the cells from untreated mice (Figure S6C), pointing out that the VitA deficiency-induced homing defect was in the pLN rather than an altered homing capacity of lymphocytes. In addition, anti-CD3-treated LN cells from mice under a continuous treatment of DEAB showed a reduced percentage of intracellular IFN-γ+ cells, without altering IL-4+ cell numbers (Figure S6D), suggesting that a lack of proper RA signaling was associated the Th2 cell bias long observed in VitA-deficient human populations (Hall et al., 2011a; Stephensen et al., 2004).
Figure 7. Vitamin A deficiency causes a reduced homing of neo-mi DCs into pLN and the lack of maintenance of pLN structures.
(A) Single-cell suspensions of iLN and mLN from DEAB-treated 5 week-old SPF mice were counted for total cell numbers. (B) The same LN in A was stained for anti-CD3 and anti-B220. Overall surface area of LN and accumulative T zone defined by dense anti-CD3 stain in one LN are shown in lower panels. For B cell area, single B cell zone defined by dense anti-B220 stain was counted. (C) iLN, mLN, and PP from 2 week-old VAD and control-fed mice were analyzed by flow cytometry for the presence of neo-mi DCs. (D) CFSE-labeled 107 total lymphocytes were i.v. injected into VAD and control-fed mice, and the presence of these cells and their CD4+, CD8+ and CD19+ subsets in iLN, mLN and the spleen was analyzed by flow cytometry after 24 hrs. (E) Similar to B, iLN of VAD and control-mice were stained with anti-CD3 and anti-B220 and analyzed by confocal imaging. Area analysis as in B is shown in the lower panels. (F) iLN of VAD and control-fed mice were stained for PNAd expression. Scale bar: 100 μm. Right panel is the cell counts per LN. All data are representative of at least three independent experiments. Also see Figure S6.
Discussion
Our data reveal that driven by inoculation of gut microbiota, CD103+RALDH+ (neo-mi) DCs become the neonatal baton carrier after embryonic LTi to continue the buildup of SLO's volume and cellularity in a RA-dependent manner. Neo-mi DCs migrate out the pool of CD103+CD11b+RALDH+ DCs present in LP. The migration process takes advantage of default neonatal addressin expression in pLN to guide neo-mi DCs for their exodus from LP to the periphery. The gut microbiota-driven migration likely remains in adult mice at reduced intensity; however, the importance of this lingering patronage to pLN becomes evident under VitA restriction: failure to deliver a tonic RA signal causes severe structural defects in distal SLO.
Upon arrival at pLN, RALDH+ DCs imprint peripheral lymphocytes for their homing to the gut, which seems logical as the microbial burden increases exponentially in the gastrointestinal tract at this moment in contrast to the rest of the body (Adlerberth and Wold, 2009). In addition, they alter pLN-targeting characteristics from MAdCAM-1-mediated to PNAd-mediated homing profile, and drastically expand HEV portals. These two changes initiate L-selectin-based homing of lymphocytes seen in adult mice, and paradoxically reduce the attraction of neo-mi DCs to pLN. Without the arrival of neo-mi DCs, as in GF mice, the MAdCAM-1 to PNAd switch is halted, arresting neonatal pLN development.
Gut microbiota changes the overall cytokine production in the host and provides additional stimulatory factors such as peptidoglycan (Ganal et al., 2012). The role of RA in pLN development however may be the most prominent. In addition to altering lymphocyte homing markers in co-culture assay, infusion of neo-mi DCs in GF mice can completely restore the pLN structures; inhibition of RALDH or VitA deficiency result in pLN atrophy. These findings are consistent with the regulatory functions of RALDH+ cells in SLO development at least in neonatal mice, although gut microbiota may modulate phagocyte activities in the periphery in adult mice (Ganal et al., 2012). On the other hand, it is important to note that although a small percentage of skin- originated CD103− DCs are also RALDH+, they are relatively fewer in number (Guilliams et al., 2010). Nonetheless, our results do not rule out other factors potentially participating in SLO development.
The decisive role of gut fungi, particularly Candida tropicalis, in driving neo-mi DC migration was notable. In a dextran sodium sulfate (DSS)-induced colitis model, Clec7a−/− mice develop a more severe pathology, suggesting fungal components mediate a suppressive effect on inflammation (Iliev et al., 2012). MGL1, mannose binding lectin (MBL), and SIGNR1, all involved in fungal cell wall component recognition, also demonstrate a similar suppressive function in the mouse model or human ulcerative colitis (Eriksson et al., 2013; Rector et al., 2001; Saba et al., 2009). Therefore, emerging evidence points to a definitive connection between commensal fungi and immune regulation. As neo-mi DC migration is possibly a tonic signal in pLN maintenance, it is likely that we will identify additional regulatory functions by gut fungi in modulating peripheral immune responses in the future. While the role of gut commensal fungi in neonatal LN development is observed in our setting, Candida tropicalis may be merely a representative strain in B6 mouse model. In human, this strain is uncommon in the gut microbiota (Hoffmann et al., 2013). The key to establish causality in the future is to identify the biochemical products that drive the neo-mi DCs’ migration.
It has been reported that retinoic acid signaling is critical for the development of gut CD103+CD11b+ cells, in line with our findings (Klebanoff et al., 2013), and suggests in VitA-deficient animals, the lack of CD103+CD11b+ cells rather than their defect in migration underlies the pathology. It should be noted that limited VitA availability may affect other cellular functions that are essential to pLN maintenance, a possibility that we did not explore. On the other hand, in several reports, VitA supplement did not improve overall human survival in developing countries (Benn et al., 2005; Lund et al., 2014), and sometimes is associated with atopy in some populations (Aage et al., 2015). How much neo-mi DCs are associated VitA-related human health issues awaits further analyses.
A revelation of our work is that all LN (mLN and pLN), at the moment of birth, are similar in attracting RALDH+ DCs and these cells mediate the MAdCAM-1 to PNAd addressin switch. Draining the gut, mLN in adult mice continue by default to receive large numbers of CD103+RALDH+ DCs. The latter serves as the basis for the reported, RA-mediated functions such as regulatory T (Treg) cell induction, imprinting lymphocytes for gut homing and IgA production (Cassani et al., 2012). In contrast to pLN, it is possible that additional factors present in GALT such as TNFα, IL-1, lymphotoxin and LPS help maintain the MAdCAM-1 expression to continue to attract RALDH+ DCs (Cuff et al., 1998; Sikorski et al., 1993). In contrast in adult mice, additional factors in pLN, such as T zone fibroblastic reticular cells, provide essential assistance (CCR7 and CCL19) to maintain the peripheral homeostasis under much reduced RA signaling (Link et al., 2007), creating some times diametrically different immune regulations in pLN and GALT towards activation and tolerance.
Although several cell types have been proposed to be essential in maintenance of peripheral SLO, none of previous reports attempted to address the dependence of postnatal pLN development on gut microbiota. Those preceding observations can be appreciated in great accordance in hindsight. It is very likely these analyses approached a similar question from different viewing angles. Neo-mi DCs are also CD11c and CCR7 positive (Moussion and Girard, 2011; Wendland et al., 2011), and indeed CD3-negative cells (Cupedo et al., 2004). Neo-mi DCs are most certainly a part of these documented populations. This work however represents a conceptual leap in identifying the exact subset of cells responsible for pLN development, their homing kinetics, their dependence on RA signaling, and their continuing role in adult pLN maintenance, which may lie underneath immune dysfunctions associated with Vitamin A deficiency.
Experimental Procedures
Mice
Tg (RARE-Hspa1b/lacZ)12Jrt/J strain was obtained from Jackson Laboratories and maintained as homozygotes to retain the reporter activity. These mice, along with C57BL/6, OT-II, Rag1−/−, Il1r1−/−, Nfkb1−/−, Tlr4−/−, Myd88−/−, Raldha1−/− and Raldha3−/− in C57BL/6 background and ICR (Swiss CD1) mice were bred and housed at Tsinghua University Animal Facilities. C57BL/6 and ICR GF mice were obtained from Shanghai Slac Laboratory Animal Co. Ltd, the experimental animal center of the Third Military Medical University and the Institute of Laboratory Animal Sciences, Peking Union Medical College and maintained in GF isolators, which were ventilated with sterile filtered air under positive pressure. GF mice were fed with autoclaved standard chow and water. For gut flora colonization, GF mice were transferred to regular isolators and cohoused with SPF mice for 1 week. For VitA-deficient (VAD) mice, C57BL/6 pregnant females were given TD.86143 diet (Harlan Laboratories), starting at 8–9 d of gestation vs a control VitA-sufficient diet provided by the same manufacturer. The pups were weaned at 3 weeks of age and maintained on the same diet before analysis. The Animal Experiments Committee of the Tsinghua University approved all of the experiments reported in this study.
Reagents
CD45 (30-F11), gp38 (8.1.1), CD19 (6D5), CR1 (7E9), CD11b (M1/70), CD127 (A7R34), CD206 (mannose receptor or MR, C068C2), F4/80 (BM8), VACM1 (429) antibodies and CFSE (Carboxy fluorescein succinimidyl ester) were obtained from BioLegend; CD103 (2E7), MAdCAM-1 (MECA-367), and purified MAdCAM-1 blocking (MECA-367), α4β7 (DATK32), CCR7 (4B12), CXCR5 (SPRCL5), CD62L (MEL-14), ICAM1 (KAT1), CCR9 (CW1.2), CD11c (N418), CD3 (17A2), B220 (RA3-6B2), CD45RB (C363.16A), CD8 (53-6.7), CD40 (1C10), CD80 (16-10A1), CD86 (GL1), MHC-II (M5/114.15.2), and PNAd (MECA-79) antibodies were from eBioscience; CD11a (M17/4), LY6G (RB6-8C5), and CD83 (Michel-19) antibodies were from Sungene Biotech; CCL21 goat polyclonal antibody was from R&D Systems; DAPI, DEAB, OVA, ampicillin, vancomycin, neomycin sulfate, metronidazole, fluconazole and RA were from Sigma; DDAO and DDAO-galactoside were from Life Technologies, and Aldefluor kit was from StemCell. All secondary antibodies (AlexaFluor 488, 555, and 647) were from Jackson ImmunoResearch. All in vitro cell cultures were carried out with standard culture media (RPM 1640 + 10% FBS, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-ME).
Microbial component-driven RALDH+CD103+ cell migration
For depletion of intestinal bacteria or fungi, mice were given an antibiotic cocktail, containing ampicillin (1 g/l), vancomycin (500 mg/l), neomycin sulfate (1 g/l), and metronidazole (1 g/l), or fluconazole (0.5mg/ml) in drinking water for 3 weeks. Fungal strains, Trichosporon asahii, Candida tropicalis, and Saccharomyces cerevisiae were purchased from China General Microbiological Culture Collection Center (CGMCC), and were cultured in YM Broth Medium for 48 hrs. Live fungi were purified by multiple washes with cold PBS, and were delivered as PBS suspension into recipient mice by gavage. 48 hrs later, mice were sacrificed and LN and LP were removed and single cells suspension was prepared for flow cytometry staining.
Statistics
For all assays, a minimum of 4 mice were used in each conditions and all experiments were repeated at least three times. All plot graphs show means with SEM. Statistical analysis for each independent experiment was performed with an unpaired, Student's t test. A P value of less than 0.05 was considered significant. *: <0.05; **:<0.01; ***: <0.001; N.S.: not significant. One-Way ANOVA was used for percentage change of neo-mi DCs in LN over time and LN histology analyses. Same labels were used to indicate different degrees of statistical significance.
Supplementary Material
Highlights.
Gut microbiota is required for secondary lymphoid organ development
Gut RALDH+ DCs move to peripheral lymph nodes following gut fungal colonization
These DCs initiates LN structural development via retinoic acid signaling
Neonatal lymphocytes are imprinted for gut homing by these DCs
Acknowledgements
We thank Drs. Hai Qi, Li Wu, Chen Dong, and Wei Jin of Tsinghua University for insightful comments and critiques, Shanghai and Chongqing animal facility and Drs. Chen Dong and Wei Jin for technical assistance, Libing Mu for graphic abstract, and Tsinghua University Animal Facilities and Dr. Zai Chang for animal services. This work was supported by Joint Peking-Tsinghua Center of Life Sciences and by grants from US National Institutes of Health (R01AI098995), Natural Sciences and Engineering Research Council of Canada (RGPIN-355350/396037) and Canadian Institutes for Health Research (MOP-119295).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Z.D.Z. performed all the experiments unless noted otherwise with assistance from J.L., and analyzed the data. Z.H., G.Z. and W.Z. provided mouse husbandry and some cell isolation assistance. X.F.W. produced graphic presentation of RNAseq data. Y.G. and C.Q. provided C57BL/6 mice and technical assistance in GF mouse handling. Y.S. conceptualized the project, designed experiments with Z.D.Z. and wrote the manuscript.
See Supplemental Experimental Procedures for adipose tissue isolation and implanting, FITC-dextran gavage, and other methods and protocols.
References
- Aage S, Kiraly N, Da Costa K, Byberg S, Bjerregaard-Andersen M, Fisker AB, Aaby P, Benn CS. Neonatal vitamin A supplementation associated with increased atopy in girls. Allergy. 2015;70:985–994. doi: 10.1111/all.12641. [DOI] [PubMed] [Google Scholar]
- Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta paediatrica. 2009;98:229–238. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. Journal of lipid research. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- Bauer H, Horowitz RE, Levenson SM, Popper H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. The American journal of pathology. 1963;42:471–483. [PMC free article] [PubMed] [Google Scholar]
- Beach RC, Menzies IS, Clayden GS, Scopes JW. Gastrointestinal permeability changes in the preterm neonate. Archives of disease in childhood. 1982;57:141–145. doi: 10.1136/adc.57.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn CS, Martins C, Rodrigues A, Jensen H, Lisse IM, Aaby P. Randomised study of effect of different doses of vitamin A on childhood morbidity and mortality. Bmj. 2005;331:1428–1432. doi: 10.1136/bmj.38670.639340.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani B, Villablanca EJ, De Calisto J, Wang S, Mora JR. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Molecular aspects of medicine. 2012;33:63–76. doi: 10.1016/j.mam.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. Journal of pediatric gastroenterology and nutrition. 1995;21:383–386. doi: 10.1097/00005176-199511000-00003. [DOI] [PubMed] [Google Scholar]
- Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. Journal of immunology. 2010;184:6799–6806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff CA, Schwartz J, Bergman CM, Russell KS, Bender JR, Ruddle NH. Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. Journal of immunology. 1998;161:6853–6860. [PubMed] [Google Scholar]
- Cupedo T, Lund FE, Ngo VN, Randall TD, Jansen W, Greuter MJ, de Waal-Malefyt R, Kraal G, Cyster JG, Mebius RE. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. Journal of immunology. 2004;173:4889–4896. doi: 10.4049/jimmunol.173.8.4889. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nature immunology. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O'Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Johannssen T, von Smolinski D, Gruber AD, Seeberger PH, Lepenies B. The C-Type Lectin Receptor SIGNR3 Binds to Fungi Present in Commensal Microbiota and Influences Immune Regulation in Experimental Colitis. Frontiers in immunology. 2013;4:196. doi: 10.3389/fimmu.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, Diefenbach A. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. The Journal of experimental medicine. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011a;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011b;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunological reviews. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Bistrup A, Singer MS, van Zante A, Lee JK, Tsay D, Peters M, Carminati JL, Brennan TJ, Carver-Moore K, et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15:237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PloS one. 2013;8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JH, West KP, Jr., Sommer A. Vitamin A deficiency and attributable mortality among under-5-year-olds. Bulletin of the World Health Organization. 1992;70:225–232. [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H, Suzuki T, Nomoto K, Yoshikai Y. Increased susceptibility to primary infection with Listeria monocytogenes in germfree mice may be due to lack of accumulation of L-selectin+ CD44+ T cells in sites of inflammation. Infection and immunity. 1996;64:3280–3287. doi: 10.1128/iai.64.8.3280-3287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, Guo Z, Umemoto E, Ebisuno Y, Yang BG, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. Journal of immunology. 2006;176:803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JA, et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. The Journal of experimental medicine. 2013;210:1961–1976. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nature immunology. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- Lund N, Biering-Sorensen S, Andersen A, Monteiro I, Camala L, Jorgensen MJ, Aaby P, Benn CS. Neonatal vitamin A supplementation associated with a cluster of deaths and poor early growth in a randomised trial among low-birth-weight boys of vitamin A versus oral polio vaccine at birth. BMC pediatrics. 2014;14:214. doi: 10.1186/1471-2431-14-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nature reviews. Immunology. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nature reviews. Immunology. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3-cells to colonize lymph nodes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11019–11024. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O'Toole T, Mebius RE. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. Journal of immunology. 2011;186:1934–1942. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Moreb JS, Ucar D, Han S, Amory JK, Goldstein AS, Ostmark B, Chang LJ. The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chemico-biological interactions. 2012;195:52–60. doi: 10.1016/j.cbi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–546. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Rector A, Lemey P, Laffut W, Keyaerts E, Struyf F, Wollants E, Vermeire S, Rutgeerts P, Van Ranst M. Mannan-binding lectin (MBL) gene polymorphisms in ulcerative colitis and Crohn's disease. Genes and immunity. 2001;2:323–328. doi: 10.1038/sj.gene.6363784. [DOI] [PubMed] [Google Scholar]
- Rice MC, O'Brien SJ. Genetic variance of laboratory outbred Swiss mice. Nature. 1980;283:157–161. doi: 10.1038/283157a0. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annual review of immunology. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Saba K, Denda-Nagai K, Irimura T. A C-type lectin MGL1/CD301a plays an anti-inflammatory role in murine experimental colitis. The American journal of pathology. 2009;174:144–152. doi: 10.2353/ajpath.2009.080235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer WT, Duliege AM, Kline MW, Hammill H, Minkoff H, Ammann AJ, Chen S, Izu A, Mordenti J. Transport of recombinant human CD4-immunoglobulin G across the human placenta: pharmacokinetics and safety in six mother-infant pairs in AIDS clinical trial group protocol 146. Clinical and diagnostic laboratory immunology. 1995;2:281–285. doi: 10.1128/cdli.2.3.281-285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer's patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. Journal of immunology. 1993;151:5239–5250. [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. The FEBS journal. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews. Immunology. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Stephensen CB, Jiang X, Freytag T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. The Journal of nutrition. 2004;134:2660–2666. doi: 10.1093/jn/134.10.2660. [DOI] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Tung CH, Zeng Q, Shah K, Kim DE, Schellingerhout D, Weissleder R. In vivo imaging of beta-galactosidase activity using far red fluorescent switch. Cancer research. 2004;64:1579–1583. doi: 10.1158/0008-5472.can-03-3226. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Hopken UE, Lipp M, Niederreither K, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nature immunology. 2009;10:1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zante A, Gauguet JM, Bistrup A, Tsay D, von Andrian UH, Rosen SD. Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse. The Journal of experimental medicine. 2003;198:1289–1300. doi: 10.1084/jem.20030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Kang SG, HogenEsch H, Love PE, Kim CH. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. Journal of immunology. 2010;184:5519–5526. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ, Zeng R, Dent A, Ansel KM, Diamond B, et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nature immunology. 2014;15:98–108. doi: 10.1038/ni.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M, Willenzon S, Kocks J, Davalos-Misslitz AC, Hammerschmidt SI, Schumann K, Kremmer E, Sixt M, Hoffmeyer A, Pabst O, Forster R. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity. 2011;35:945–957. doi: 10.1016/j.immuni.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. The Journal of experimental medicine. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.