Abstract
Secreted Wnts play diverse roles in a non-cell-autonomous fashion. However, the cell-autonomous effect of unsecreted Wnts remains unknown. Endoplasmic reticulum (ER) stress is observed in specialized secretory cells and participates in pathophysiological processes. The correlation between Wnt secretion and ER stress remains poorly understood. Here, we demonstrated that Drosophila miR-307a initiates ER stress specifically in wingless (wg)-expressing cells through targeting wntless (wls/evi). This phenotype could be mimicked by retromer loss-of-function or porcupine (porc) depletion, and rescued by wg knockdown, arguing that unsecreted Wg triggers ER stress. Consistently, we found that disrupting the secretion of human Wnt5a also induced ER stress in mammalian cells. Furthermore, we showed that a C-terminal KKVY-motif of Wg is required for its retrograde Golgi-to-ER transport, thus inducing ER stress. Next, we investigated if COPI, the regulator of retrograde transport, is responsible for unsecreted Wg to induce ER stress. To our surprise, we found that COPI acts as a novel regulator of Wg secretion. Taken together, this study reveals a previously unknown Golgi-to-ER retrograde route of Wg, and elucidates a correlation between Wnt secretion and ER stress during development.
Wnt proteins are secreted glycoproteins that regulate multiple processes during development and adult tissue homeostasis1. Over the last three decades, the signaling events that occur downstream of Wnt receptors have been well elucidated. However, the mechanisms underlying Wnt secretion remain largely unknown. Recent attention has been drawn to this process due to the association of aberrant Wnt levels with various diseases2,3.
Endoplasmic reticulum (ER) protein Porcupine (Porc) was the first identified regulator of Wnt secretion4,5. In Drosophila, Porc mediates the lipidation of Wnt proteins and facilitates their recognition by Wls6,7. Wls is a conserved transmembrane protein that regulates Wnt exocytosis from the trans-Golgi network (TGN) to the cell membrane8,9,10,11,12. In the absence of Wls, Wnt proteins are retained in their expressing cells. Once Wnts are exocytosed, Wls is internalized by Clathrin-mediated endocytosis13 and transported to TGN by the retromer complex. Retromer is a conserved multi-protein complex that sorts cargos from early endosomes (EE) back to TGN14. In 2008, five groups demonstrated that Wls is the target of retromer15,16,17,18,19. In the absence of retromer, Wls is eventually trapped into lysosomes for degradation. Later studies described an unconventional SNX3-retromer that is specifically required for Wls recycling20,21. Recently, the P24 family of proteins was identified as regulators of Wg secretion by controlling its ER export22,23. Drosophila miR-315 was demonstrated to activate Wg signaling by targeting axin and notum24. MiR-8 was reported to negatively regulate Wnt signaling at multiple levels25. Together, these studies indicate that the above regulators hold great potential for therapeutic targeting. However, the cell autonomous role of unsecreted Wnts is still unknown. Abnormal protein accumulation in the secretory cells leads to ER stress26. Upon ER stress, cells activate an integrated response, termed unfolded protein response (UPR)27. The ER chaperone Bip/Grp78 was upregulated upon UPR. In Drosophila, BiP is encoded by hsc70-328. It is broadly expressed in embryos, with higher expression levels found in developing secretory organs29. Bip is a downstream target of xbp1, which is spliced and activated in response to ER stress. So, Bip is usually used as an ER marker, and its upregulation can be used an indicator of ER stress29. Given this information, in our genetic screen we focused on Wg secretion regulators, we also paid attention to their roles in regulating ER stress.
In this study, we identified that miR-307a inhibits Wg secretion through targeting wls. Interestingly, we found that ectopic expression of miR-307a initiates ER stress specifically in wg-expressing cells. Furthermore, we demonstrated that the C-terminal KKVY-motif of Wg mediates its Golgi-to-ER retrieval. Given that KKVY-motif is the sorting signal of the COPI complex, we investigated if COPI is responsible for unsecreted Wg to induce ER stress. Surprisingly, we found that COPI loss-of-function resulted in a Wg secretion defect and induces ER stress as well. Altogether, we discovered a novel retrograde route of Wg from the Golgi to the ER and yielded a new concept that unsecreted Wg cell-autonomously triggers ER stress.
Results
MiR-307a regulates Wg secretion and initiates ER stress in wg-expressing cells
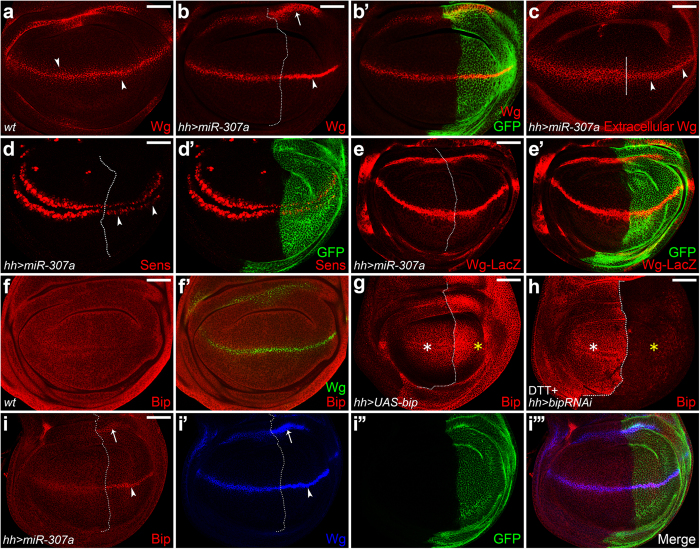
To identify new regulators of Wg secretion, we performed a genetic screen which mainly focuses on vesicle trafficking related proteins and a microRNA library described previously30. The schematic representation of the miRNA construct was shown in (Supplementary Fig. S1a). In the screen, we identified that ectopic expression of miR-307a resulted in wing notches (Supplementary Fig. S1b-b’) and loss of dorsal thoracic bristles (Supplementary Fig. S1c-c’, red box in S1c’), raising the possibility that Wg signaling is disrupted. Next, we examined the role of miR-307a in Wg signaling in the wing imaginal discs. In the wing disc, the Wg protein is produced at the dorsal/ventral (D/V) boundary and forms a gradient along the D/V axis (Fig. 1a). Overexpression of miR-307a resulted in accumulation of Wg in its expressing cells (Fig. 1b-b’). The accumulation was not due to the increased transcription of the wg gene as the expression of wg-lacZ was unaffected (Fig. 1e-e’). In contrast to accumulated Wg within its expressing cells, extracellular Wg levels were reduced with miR-307a overexpression (Fig. 1c). Consistent with this, expression of senseless (Sens), a short-range target gene of Wg signaling31, is reduced by miR-307a overexpression (Fig. 1d-d’). Together, our data demonstrate that miR-307a is a negative regulator of Wg secretion.
Figure 1. MiR-307a regulates Wg secretion and initiates ER stress in wg-expressing cells.
All the wing discs hereafter are oriented anterior left, dorsal up. The dotted lines were used to indicate the Anterior/Posterior (A/P) compartment boundary. (a) Immunostaining of Wg in wild-type wing disc. (b-e’, i-i”’) Expression of UAS-miR-307a (marked by GFP) was induced in the P compartment using hhGal4 driver. (b-b’) The arrow and arrowhead indicate Wg is accumulated in its expressing cells. (c) Immunostaining of extracellular Wg. The arrowheads indicate reduction of extracellular Wg staining. (d-d’) Immunostaining of Sens (red). The arrowheads indicate the loss of Sens expression in the miR-307a-expressing P compartment. (e-e’) Transcription of wg (wg-lacZ) is not regulated by miR-307a overexpression. (f-f’) Immunostaining of Bip and Wg in wild-type wing disc. (g, h) Expression of UAS-bip or UAS-bipRNAi transgene was induced using hhGal4. (g) The yellow asterisk indicates the increasing of Bip levels. (h) The wing discs were treated with 5 mM DTT (in M3 medium) for 16 h before immunostaining for inducing ER stress. Upregulation of Bip occurs in the wild-type tissue (white asterisk) but not in bip depletion tissue (yellow asterisk). (i-i”’) Overexpression of miR-307a causes an ectopic expression of Bip specifically in the wg-expressing cells (arrow and arrowhead in i). Scale bar = 50 μm.
Interestingly, we found that miR-307a regulates the initiation of ER stress as indicated by Bip staining. In the wild-type wing disc, Bip is ubiquitously expressed (Fig. 1f-f’). To test the specificity of the Bip antibody we used, we overexpressed bip by using hhGal4 and found the staining signal of Bip antibody was obviously increased in the posterior (P) compartment (Fig. 1g). Next, we used Dithiothreitol (DTT), an ER-stress-causing agent, to treat the wing disc to elevate the basal levels of Bip (as described in29) since the endogenous level of Bip is relatively low (as shown in Fig.1f-f’). We found that the upregulation of Bip occurs in the wild-type anterior tissue but not in the bip-depleted posterior compartment (Fig. 1h). These experiments confirmed that the Bip antibody is sensitive enough for detecting the ectopic Bip levels. Through Bip staining, we found that overexpression of miR-307a by hhGal4 caused ectopic expression of Bip specifically in the wg-expressing cells of the posterior wing disc (Fig. 1i-i”’).
MiR-307a initiates ER stress through targeting of wls
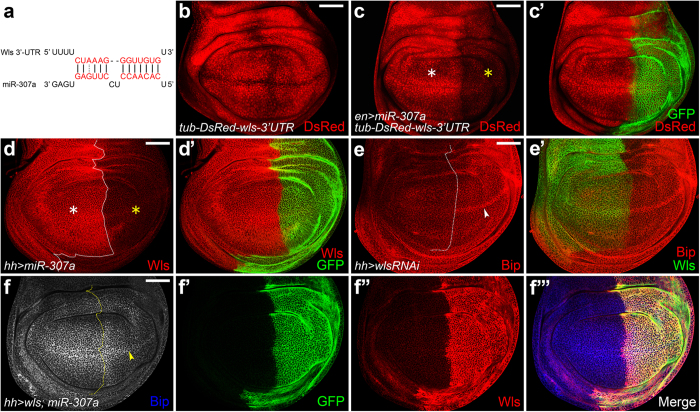
To investigate the possible correlation between miR-307a and ER stress, we searched for targets of miR-307a using miRanda and TargetScan32,33. We found wls is one of the predicted targets of miR-307a (Fig. 2a). To confirm this prediction, we made a wls-sensor transgenic fly. The expression pattern of wls-sensor was shown in (Fig. 2b). In the wing disc, overexpression of miR-307a using enGal4 blocked expression of wls-sensor in the P compartment (Fig. 2c-c’). Consistently, overexpression of miR-307a causes a remarkable reduction in Wls protein levels (Fig. 2d-d’). These data demonstrated that wls is a target of miR-307a.
Figure 2. MiR-307a initiates ER stress through targeting Wls.
(a) The 3’UTR of wls mRNA is a predicted miR-307a binding site. (b) tub-DsRed-wls-3’UTR (wls-sensor) was generated by cloning a 609 bp fragment of wls 3’UTR downstream of pCaSpeR-tub-DsRed. (c-c’) Overexpression of miR-307a using enGal4 strongly inhibits the expression of wls-sensor (yellow asterisk in c). (d-d’) The yellow asterisk indicates the loss of wls expression in the miR-307a-expressing P compartment. (e-e’) UAS-wlsRNAi was expressed using hhGal4. An ectopic Bip staining was observed in the wg-expressing cells (arrowhead in e). (f-f”’) UAS-wls and UAS-miR-307a were co-expressed using hhGal4. The ectopic expression of Bip was suppressed by wls overexpression (arrowhead in f). Scale bar = 50 μm.
Subsequently, we generated the miR-307a-sensor (Supplementary Fig. S2b-b”) and miR-307a-sponge (Supplementary Fig. S2c) transgenes for monitoring the endogenous expression levels of miR-307a. Comparing with the wild-type control (Supplementary Fig. S2a), the miR-307a-sensor shows decreased expression in the wing pouch with striking reduction at the A/P and D/V boundaries (Supplementary Fig. S2b-b”). This pattern is similar with that of wls-sensor, indicating that miR-307a is endogenously expressed in these regions. Further, overexpression of miR-307a-sponge enhanced the signal of miR-307a-sensor (Supplementary Fig. S2d-d’). Taken together, these data suggested that miR-307a acts as an endogenous regulator in the wing pouch.
Next, we asked whether miR-307a triggered ER stress through targeting of wls. We found that knockdown of wls also induced ectopic Bip staining in the wg-expressing cells (Fig. 2e-e’). Moreover, overexpression of wls suppressed the ectopic Bip pattern induced by miR-307a overexpression (Fig. 2f-f”’). These data indicated that Wls acts as a negative regulator of ER stress in the wg-expressing cells.
Unsecreted Wg induces ER stress in its expressing cells
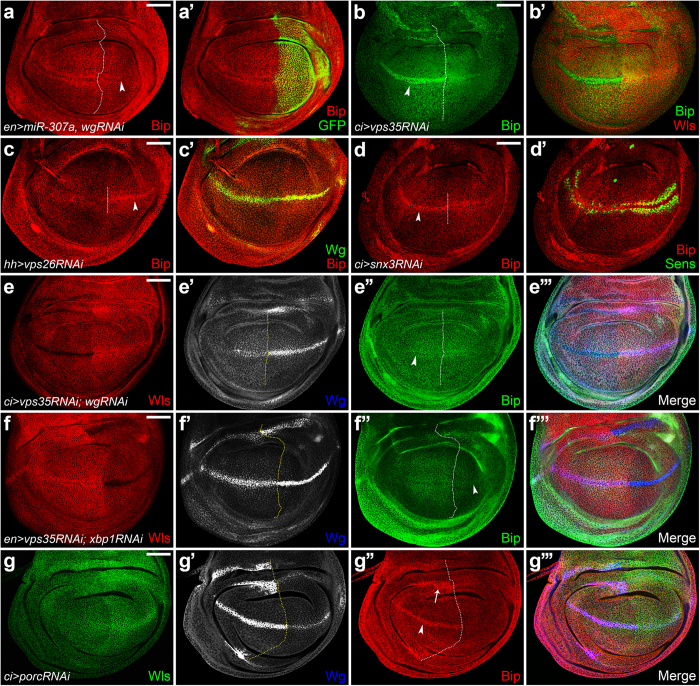
Given that Wls dysfunction-induced ER stress is only observed in wg-expressing cells, we asked whether Wg was the initiator. We found that knockdown of wg suppressed the ectopic expression of Bip induced by miR-307a (Fig. 3a-a’). Vps35 knockdown mimicked the Bip induced phenotype caused by miR-307a (Fig. 3b-b’). Consistently, depleting the other components of the retromer complex, vps26 or snx3, resulted in the same phenotype with vps35 knockdown (Fig. 3c-d’). Further, knockdown of wg in a vps35 depletion background efficiently inhibited the ectopic Bip expression (Fig. 3e-e”’). Moreover, increasing the ER retention of Wg by porc knockdown also induced ectopic Bip expression in the Wg-accumulating cells (Fig. 3g-g”’). These data demonstrated that unsecreted Wg triggers ER stress. Furthermore, depletion of xbp1 blocked the ectopic Bip expression caused by vps35 knockdown (Fig. 3f-f”’), suggesting that unsecreted Wg induces ER stress through the activation of Xbp1.
Figure 3. Unsecreted Wg induces ER stress in its expressing cells.
(a-a’) UAS-wgRNAi and UAS-miR-307a were co-expressed using enGal4. miR-307a-induced ectopic Bip staining was suppressed by wg knockdown (arrowhead in a). (b-b’) UAS-vps35RNAi was expressed in the A compartment using ciGal4. Ectopic Bip staining was observed in wg-expressing cells (b, arrowhead). (c-c’) vps26 RNAi was induced using hhGal4. Arrowhead in (c) indicates ectopic expression of Bip in the wg-expressing cells of P compartment. (d-d’) snx3 RNAi was induced using ciGal4. Arrowhead in (d) indicates ectopic expression of Bip in the wg-expressing cells of A compartment. (e-e”’) Knockdown of wg in a vps35 depletion background driven by ciGal4, the ectopic Bip staining was dramatically reduced (e”, arrowhead). (f-f”’) UAS-vps35RNAi and UAS-xbp1RNAi were co-expressed using enGal4. The ectopic Bip expression induced by vps35 knockdown was dramatically suppressed by xbp1 depletion (indicated by arrowhead in f”). (g-g”’) UAS-porcRNAi was expressed using ciGal4. Wg was accumulated in its expressing cells (g’). Bip was ectopically expressed in the wg-expressing cells (g”, arrow and arrowhead). Scale bar = 50 μm.
Human Wnt5a acts as an ER stress initiator in mammalian cells
Next, we investigated whether the Wg-induced ER stress is conserved in mammalian cells. We generated a xbp1-GFP reporter for ER stress as described in34. In metazoans, a 26-nucleotide intron of xbp1 mRNA is spliced out during ER stress, causing a shift in the codon reading frame34. The spliced xbp1 mRNA is translated into the mature XBP1 protein in response to ER stress34. In this case, we fused the gene encoding GFP downstream of a partial sequence of human xbp1, including the 26-nt ER stress-specific intron. Under normal conditions, the mRNA of the fusion gene would not be spliced, and that its translation would be terminated at the stop codon near the joint between the XBP1 and GFP. During ER stress, the 26-nt intron should be spliced out. Thus, a fusion protein of XBP1-GFP should be produced in cells. To test whether xbp1-GFP works as an indicator for ER stress, we transfected HEK293T cells with xbp1-GFP, and then treated them with Thapsigargin (an agent that promotes ER stress by depletion of lumenal calcium storage). The splicing of xbp1 was detected by anti-GFP antibody (Fig. 4a) or GFP fluorescence (the upper panels of Fig. 4c), indicating that the xbp1-GFP works well. Next, We co-transfected HeLa cells with xbp1-GFP and hwnt5a-flag-KDEL (an ER retention form of Wnt5a). A remarkable splicing of xbp1 was observed (Fig. 4b and the lower panels of Fig. 4c). Through quantification, we found the percentage of cells with visible GFP expression was significantly increased by hwnt5a-flag-KDEL overexpression (Fig. 4d). These data indicated that ER stress is triggered. Furthermore, Knockdown of wls in HEK293T cells which stably expressed hWnt5a. The knockdown efficiency of two Wls RNAi could reach 50% (Fig. 4f). The lysates were immunobloted with Flag antibody. The amount of Wnt5a in cell lysates was increased upon Wls knockdown (Fig. 4e, middle panel), indicating that Wnt5a was accumulated in the cells. Also, an upregulation of Grp78 was observed (up panel of Fig. 4e,g). Taken together, these data suggested that hWnt5a act as an initiator of ER stress when its secretion route is disrupted.
Figure 4. Human Wnt5a acts as an ER stress initiator in mammalian cells.
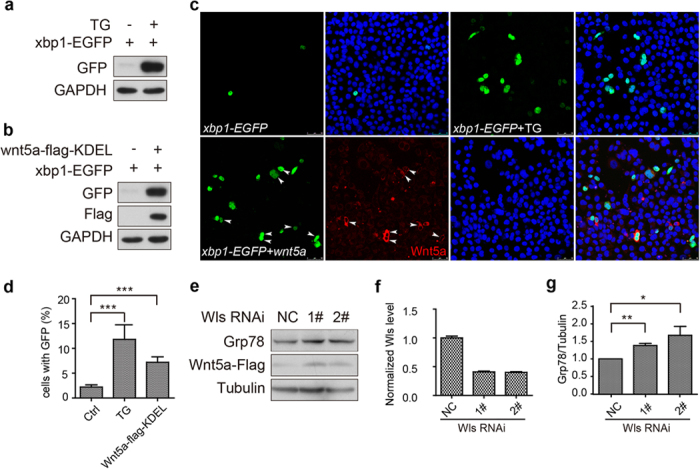
(a) HEK293T cells were transfected with xbp1-GFP, and then were treated with Thapsigargin (TG, 2 μM) for 12 h at 24h post-transfection for inducing ER stress. The splicing of xbp1 was detected by anti-GFP antibody. (b) HEK293T cells were co-transfected with xbp1-GFP and wnt5a-flag-KDEL. GFP and Flag antibodies were used for detecting the spliced Xbp1 and hWnt5a, respectively. (c) HeLa cells were transfected with xbp1-GFP, or together with hWnt5a-flag-KDEL at a ratio of 1:3. Portions of xbp1-GFP-transfected wells were treated with TG as positive control. The spliced Xbp1 was indicated by GFP signal. The expression of hWnt5a was indicated by Flag staining (red). (d) The cells with visible GFP expression were calculated and quantified. Data represent the Mean ± SEM (t-test, ***P < 0.001, n = 10 random selected fields). (e) HEK293T cells that stably expressed hWnt5a-Flag were transfected with hWls siRNA. The transfected cells were washed twice at 72hr post-transfection for lysis. Lysate was immunobloted with Grp78, Flag and Tubulin antibodies. (f) The knockdown efficiency of hWls siRNAs was detected by real-time PCR. (g) The relative levels of GRP78 as shown in (e) were quantified after normalization against Tubulin. Values represent the Mean ± S.E.M. (student’s t-test, n = 3, *P < 0.05, **P < 0.01).
A C-terminal KKVY-motif mediates the Golgi-to-ER retrieval of Wg
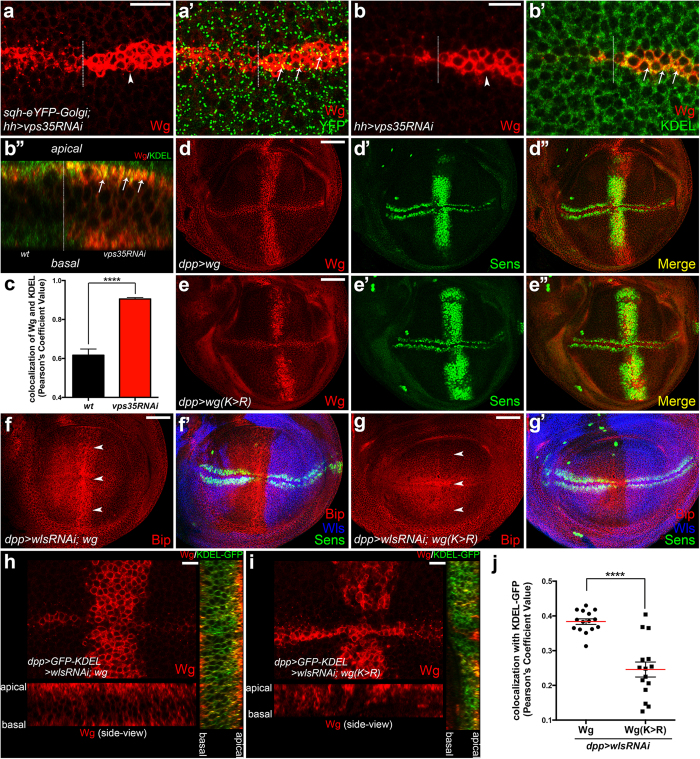
As Wls exports Wg from Golgi to the cell membrane, we asked why Wls dysfunction could induce ER stress. In the vps35-depleted wg-expressing cells, we observed the subcellular co-localization of Wg with a Golgi marker was enhanced (Fig. 5a-a’). Interestingly, an increased co-localization of Wg with an ER marker was also observed (Fig. 5b,c and Supplementary Fig S3a,b). We hypothesized that the transient accumulation of Wg in Golgi triggers its retrieval to the ER. Previous studies demonstrated that COPI complex retrieves the cargos containing the canonical C-terminal KKxx- or KxKxx-motif as well as the non-canonical KxHxx- or RKxx-motif35,36. Coincidently, there is a KKVY-motif in the C-terminal of Wg. We generated the UAS-wg(K334K > R334R) and UAS-wg transgenic flies using the PhiC31 integrase-mediated site-specific transgenesis system. Both of these are integrated into the 3R 86F locus to ensure that at least the transcripts have the same expression levels. We found that the K-to-R mutant form of Wg maintained its signaling transduction ability (Fig. 5d,e), indicating its secretion and transporting functions were unaffected under normal conditions. Next, we co-expressed UAS-wg and UAS-wg(K334K > R334R) with UAS-wlsRNAi using dppGal4. In this context, we found the unsecreted wild-type Wg caused obvious ER stress (Fig. 5f-f’). However, the phenotype was not observed in the mutant form of Wg (Fig. 5g-g’). Furthermore, the K-to-R mutant form of Wg showed weaker colocalization with the ER marker (Fig. 5h–j) but stronger colocalization with the Golgi marker (Supplementary Fig. S3c–e) compared with the wild-type of Wg. Taken together, these data suggested that the KKVY-motif mediates the retrieval of unsecreted Wg from Golgi to ER, thus inducing ER stress.
Figure 5. A C-terminal KKVY-motif of Wg mediates its Golgi-to-ER retrieval.
(a-a’) UAS-vps35RNAi was driven by hhGal4 carrying sqh-eYFP-Golgi transgene. Wg was accumulated in the posterior wg-expressing cells (arrowhead in a). The colocalization of unsecreted Wg with Golgi marker was mildly enhanced (arrows in a’) compared with the wild-type control. (b-b”’) UAS-vps35RNAi was expressed using hhGal4. The unsecreted Wg shows increasing of colocalization with the ER marker, KDEL (arrows in b’,b”). (b”) shows the side-view of Wg-expressing cells. Z-section images were taken from apical to basal of (b). (c) Cross sections were cut along the Wg-expressing region for colocalization analysis. The Pearson’s Correlation (PC) value represents the colocalization of Wg with KDEL. The colcalization of unsecreted Wg with KDEL was significantly increased compared with the wild-type control. Data represent the Mean ± SEM (t-test, n = 10, ****P < 0.0001). (d-e”) Ectopic expression of Sens can be induced by either UAS-wg or UAS-wg(K334K > R334R) driven by dppGal4. (f-g’) UAS-wg or UAS-wg(K334K > R334R) was co-expressed with UAS-wlsRNAi using dppGal4, respectively. The genetic crosses and immunostaining were performed under the same condition. Images were taken with same laser setting on the confocal microscope. The accumulation of wild-type Wg in its expressing cells induces remarkable ectopic Bip expression (arrowheads in f). Whereas, the K-to-R mutant form of Wg does not show obvious induction of ectopic Bip (arrowheads in g). (h-i) UAS-wg or UAS-wg(K334K > R334R) was co-expressed with UAS-wlsRNAi and UAS-GFP-KDEL using dppGal4, respectively. Z-stack sections were taken with same laser setting on the confocal microscope. The subcellular distribution of Wg or Wg(K > R) was shown in the lower panel. The colocalization of Wg or Wg(K > R) with GFP-KDEL was shown in the right panel. (j) Cross sections were cut along the dppGal4-expressing region for colocalization analysis. Comparing with the wild-type Wg, the K-to-R mutant form of Wg shows weaker colocalization with GFP-KDEL. Data represent the Mean ± SEM (t-test, n = 15, ****P < 0.0001). The Wg dilution used in this Figure was 1:20. Scale bars in (a,b,h,i) 10 μm; (d–g) 50 μm.
COPI regulates Wg secretion and ER stress initiation
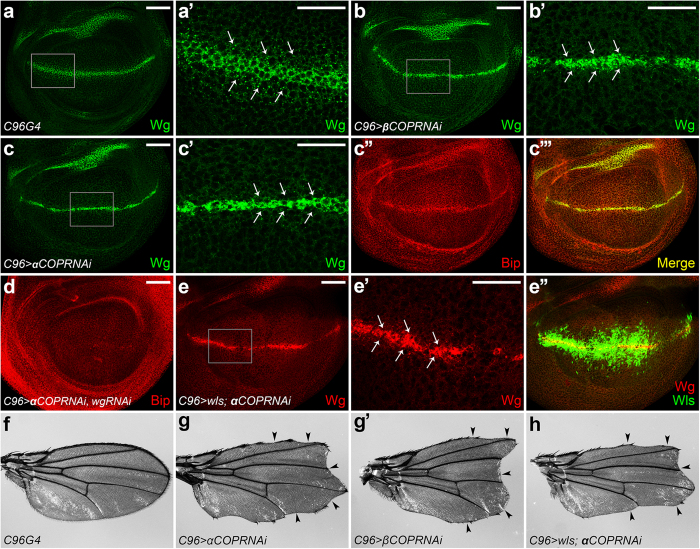
Since COPI is the key regulator of the retrograde Golgi-to-ER transport, we asked if COPI is responsible for unsecreted Wg to induce ER stress. To investigate this, we performed the epistasis tests between Wls and the COPI subunits, αCOP and βCOP37, respectively. Surprisingly, knockdown of either αCOP or βCOP alone could induce Wg secretion defect (Fig. 6a-c”’). Consistently, the adult flies displayed notched wings (Fig. 6f-g’), suggesting the deficient of Wg signaling. In addition, an ectopic Bip expression was observed in the αCOP-depleted wg-expressing cells (Fig. 6c”). This phenotype could be suppressed by wg knockdown (Fig. 6d), suggesting that COPI regulates ER stress initiation through controlling Wg secretion. Taken together, these data revealed a novel role for COPI that regulated Wg secretion and ER stress initiation as well.
Figure 6. COPI regulates Wg secretion and ER stress initiation.
(a) Wg staining in the wild-type wing disc. (a’) Enlarged view of the gray box region in (a). (b-b’) UAS-βCOPRNAi was expressed using C96Gal4. Wg secretion was disrupted by βCOP knockdown. The secreted punctate structures of Wg were dramatically reduced (indicated by arrows in b’). (c-c”’) Depletion of αCOP using C96Gal4 generated same phenotype (shown in c’). Ectopic Bip induction was also observed along the D/V boundary (shown in c”). (d) The ectopic Bip expression was suppressed by wg knockdown. (e-e”) UAS-wls and UAS-αCOPRNAi were co-expressed by C96Gal4 driver. αCOP depletion-induced Wg secretion defect cannot be rescued by wls overexpression (arrows in d’). (f) Adult wing of C96Gal4. (g-g’) UAS-αCOPRNAi or UAS-βCOPRNAi was expressed using C96Gal4. Knockdown either of them induced obvious wing notches (indicated by arrowheads in d’,d”). (h) Overexpression of wls failed to rescue the wing notching phenotype induced by αCOP knockdown. Scale bars in (a–e) 50 μm; (a’,b’,c’,e’) 20 μm.
Discussion
Wnt secretion and ER stress are two fundamental biological processes that participate in diverse biological and pathophysiological processes. The correlation between these processes remains poorly understood. Wnt proteins play roles in a cell non-autonomous fashion. However, the cell-autonomous effect of unsecreted Wnts is still unknown. It has been reported that deletion of p24 genes activates an ER stress response which alleviates the deleterious effects of the p24 deletion38,39. As the P24 family of proteins controls Wg secretion by regulating the ER export of Wg22,23, this data led us to pursue the possibility that unsecreted Wg triggers ER stress. Our new findings yielded a new concept that unsecreted Wg can cell-autonomously trigger ER stress.
In this study, we identified that miR-307a inhibits Wg secretion through targeting wls. Intriguingly, we found that overexpression of miR-307a induces ER stress specifically in the wg-expressing cells. This phenotype could be mimicked by retromer loss-of-function or porc depletion and could be rescued by wg knockdown, suggesting that unsecreted Wg triggers ER stress. We hypothesized that the transiently Golgi-accumulated Wg was retrieved to ER thereby inducing ER stress. Previous studies have demonstrated that COPI governs the retrograde Golgi-to-ER transport by recognition of the C-terminal KKxx-motif of type I transmembrane proteins35,36. Coincidently, there is a KKVY sequence in the C-terminal of Wg. By further in vivo assay, we found that the K-to-R mutant form of Wg lost the ability to trigger ER stress, suggesting that the KKVY-motif of Wg is required for its retrieval and ER stress induction. Since Wg is a protein on the luminal side, it is most unlikely that Wg was directly recognized by COPI. Given this, we suppose that there could be another transmembrane bridge protein(s) mediating the interaction of Wg with COPI. Through the alignment of Drosophila P24 family of proteins, we found a typical COPI-recognized KKxx-motif (KKLV) in the C-terminal of Éclair (Eca). Interestingly, it has been demonstrated that Eca is required for the ER-to-Golgi anterograde of Wg23. Together, it raised a possibility that Eca is retrieved back to the ER by COPI for reuse. The interaction between Eca and Wg may facilitate the retrieval of Wg. However, whether the KKVY-motif of Wg mediates its interaction with Eca in the retrieval route needs to be further investigated.
Next, we tested whether knockdown of COPI can suppress the ER stress caused by wls depletion. But, we surprisingly found that downregulation of COPI unexpectedly disrupted Wg secretion and triggered ER stress. This phenotype raised two possibilities: First, the regulators that control the ER-to-Golgi transport of Wg might be retrieved to the ER for reuse by COPI (as discussed above). In this case, COPI loss-of-function could block the retrieval of these regulators, thereby indirectly inhibiting Wg secretion and inducing ER stress. Second, Wls is retrieved from Golgi to ER by COPI to facilitate Wg secretion. In this case, COPI loss-of-function will block the retrieval of Wls, thus increasing the ER retention of newly synthesized Wg and initiating ER stress. The second possibility was supported by a recent study from Virshup lab. In that study, they identified a C-terminal RKEAQE-motif of human Wls that can be recognized by COPI for retrieval40. However, this motif is not very conserved in Drosophila Wls. Interestingly, they showed that Wls overexpression rescued the effects of ERGIC2 (a subunit of vertebrate COPI) depletion in X. laevis embryos40. However, in Drosophila, we found that Wls overexpression failed to rescue the Wg secretion defect induced by αCOP RNAi (Fig. 6e-e”), which might be due to general effect upon COPI depletion. Alternatively, Wls might require COPI for the function. In addition, previous study has shown that Wg colocalized with Golgi-localized Wls18, and the Golgi-localization of the EE-to-TGN recycled Wls depends on the levels of Wg18. These data suggested that Wls encounters with Wg predominantly in the Golgi apparatus and the stability of Wls might be depended on its interaction with Wg. Moreover, if Wls is indeed retrieved to the ER to bind Wg, blocking the ER export of Wg should not influence the interaction of Wls with Wg. However, blocking the ER export of Wg by knockdown of P24 proteins reduced the punctuate pattern of Wls in wg-expressing cells23, suggesting that the interaction of Wls with Wg was decreased. Consistently, we found that increasing the ER retention of Wg by porc knockdown also reduced the Wls levels (Fig. 3g). These data suggested consistent with the idea that Drosophila Wls may not be retrieved to the ER by COPI. The molecular mechanism regarding the Drosophila Wls retrieval in vivo needs to be further investigated.
Several components of the UPR have been shown to play a protective role against the progression of disease41. In this study, we found that Wls dysfunction-induced ER stress buffers the toxicity of unsecreted Wg during development, while the detailed mechanisms need to be further addressed. Additionally, previous study reported that tumour hypoxia blocks Wnt secretion through inducing ER stress42. Together with our findings, whether a feedback loop exists between Wnt secretion and ER stress remains to be elucidated. Mutations in human porcupine (porcn) cause the X-linked dominant disorder focal dermal hypoplasia (FDH, also known as Goltz syndrome)43,44. Vps35, a component of the retromer complex, has been found to be often mutated in PD patients45,46. Whether unsecreted Wg-induced ER stress plays a role in these diseases is a promising topic. In summary, we discovered a retrograde route of Wg from the Golgi to the ER and elucidated a correlation between Wnt secretion and ER stress. Given the critical roles of aberrant Wnt signaling and ER stress in the pathogenesis of human diseases, identifying regulators that connect these two processes will facilitate approaches for therapeutic intervention.
Methods
Drosophila strains
The enGal4, hhGal4, apGal4, ciGal4, dppGal4, C96Gal4 and wg-lacZ were as described in FlyBase. The UAS-wlsRNAi (103812), UAS-wlsRNAi (5214), UAS-porcRNAi (47864), UAS-snx3RNAi (104494), UAS-vps26RNAi (18396), UAS-αCOPRNAi (35305) and UAS-βCOPRNAi (15418) were obtained from the Vienna Drosophila RNAi Center. The UAS-bipRNAi (HMS00397), UAS-xbp1RNAi (HMS03015) and UAS-wgRNAi (HMS00844) were obtained from the Drosophila RNAi Screen Center at Harvard Medical School. UAS-bip (5843), sqh-eYFP-golgi (7193), sqh-eYFP-ER (7195), UAS-GFP-KDEL (9898) and UAS-GRASP65-GFP (8507) was obtained from Bloomington Drosophila Stock Center. UAS-vps35RNAi and UAS-wls were described previously15. UAS-GFP-miR-307a, UAS-DsRed-miR-307a-sponge, UAS-wg, UAS-wg(K334K > R334R), tub-EGFP, tub-EGFP-miR-307a-sensor and tub-DsRed-wls-3′UTR transgenes were generated in this study.
Plasmid construction
To generate the pWALIUM10-moe-GFP-GPI-miR-307a, 714 bp of genomic DNA surrounding miR-307a was amplified by PCR and cloned downstream of GFP-GPI in the NheI site of pWALIUM10-moe-GFP-GPI vector30.
Forward: 5′-CGGCTAGCCGGAACGAGGATTCTG-3′
Reverse: 5′-CGGCTAGCCCTGATGGTTTAAGTCCTG-3′
To generate the pWALIUM10-moe-DsRed-miR-307a-sponge, the following sequence was designed as described in47, and then directly cloned into pWALIUM10-moe-DsRed through BglII and NdeI sites. The sequence is:
5′-AGATCTCTCACTCAGATGGTTGTGAAATCCTCACTCAGATGGTTGTGAAATCCTCACTCAGATGGTTGTGAAATCCTCACTCAGATGGTTGTGAAATCCTCACTCAGATGGTTGTGAAATCCTCACTCAGATGGTTGTGAAATCCTCACTCAGATGGTTGTGAAATCCTCACTCAGATGGTTGTGACATATG-3′
To generate the pCaSpeR-tub-EGFP-miR307a-sensor, the following primers were annealed and then cloned into pCaSpeR-tub-EGFP vector (a gift from T. Kai) through NotI and XhoI sites. The primers are:
Forward: 5′-GGCCGCCTCACTCAAGGAGGTTGTGAAATCACACCTCACTCAAGGAGGTTGTGAC-3′
Reverse: 5′-TCGAGTCACAACCTCCTTGAGTGAGGTGTGATTTCACAACCTCCTTGAGTGAGGC-3′
To generate the pCaSpeR-tub-DsRed-wls-3′UTR, a 609 bp fragment of the Wls 3′UTR was amplified by PCR from wild-type genomic DNA and cloned downstream of pCaSpeR-tub-DsRed through NotI and XhoI sites. The primers are:
Forward: 5′-AAAGCGGCCGCGCGGAAGGACTCGAATTATTG-3′
Reverse: 5′-GGGCTCGAGCAATATTGCTTTTTATTCGATGCA-3′
To generate the UAS-wg construct, the full-length cDNA of wg was cloned into the pUAST-attB vector through NotI and XhaI sites. The primers are:
Forward: 5′-AAAGCGGCCGC ATGGATATCAGCTATATCTTCGTC-3′
Reverse: 5′-GGGTCTAGATTACAGACACGTGTAGATGACC-3′
A similar strategy was used to make the UAS-wg(K334K > R334R). The point mutation was generated by PCR using the following primers:
Forward: 5′-GCTGTGTCGGACCCGACGGGTCATCTACACGTGTCTGTAATCTAG-3′
Reverse: 5′-CACGTGTAGATGACCCGTCGGGTCCGACACAGCTTGCACTTCACCTCG-3′
To generate the pEGFP-N1-xbp1, a 596 bp fragment of xbp1 (from 49 to 644) was cloned into pEGFP-N1 through EcoRI and BamHI sites. The primers are:
Forward: 5′-GGGGAATTCATGGTGGTGGTGGCAGCC-3′
Reverse: 5′-AAAGGATCCCGTGAATCTGAAGAGTCAATACCGCC-3′
To generate pSIN-wnt5a-flag, primers containing flag sequence were designed to amplify wnt5a from the plasmid pcDNA3.1-wnt5a (a gift from Q. Tao). The primers are:
Forward: 5′-AAAGAATTCTTACAGTTCATCCTTCTTGCACACAAACTGGTCCAC-3′
Reverse: 5′-AAAGAATTCTTACAGTTCATCCTTCTTGCAGGTGTGCACGTCG-3′
Similar strategy was used to generate the pcDNA3.1-wnt5a-flag-KDEL. The primers used are:
Forward: 5′-GGGACTAGTATGGCTGGAAGTGCAATGTC-3′
Reverse: 5′-AAAGGATCCTCACTTATCGTCGTCATCCTTGTAATCCTTGCACACAAACTGGTCCAC-3′
Cell culture, transfection and western blot
HEK293T and HeLa cell lines were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco), 50U/ml penicillin, 50 μg/ml streptomycin, in 5% CO2 atmosphere at 37 °C. Plasmid transfection was carried out using LipofectAMINE (Invitrogen) according to manufacturer’s instructions. 2 μM Thapsigargin (Enzo Life Sciences) was used to induce ER stress. The primary antibodies used for western blot were rabbit anti-Grp78 (Cell Signaling Technology), mouse anti-Flag (Sigma), rabbit anti-GFP (Invitrogen) and mouse anti-β-Tubulin (CWBiotech).
Immunostaining and microsopy
Antibody staining of wing imaginal discs or cells was performed using standard protocols. The following primary antibodies were used: rat anti-Bip (MAC143, Abcam), mouse anti-Wg (4D4; DSHB), guinea pig anti-Sens31, rabbit anti-Wls21, rabbit anti-GFP Alexa Fluor 488 (Molecular Probe), mouse anti-lacZ (Abmart), Rat anti-KDEL (Abcam), and Rabbit anti-GM130 (Abcam). The nuclei were stained by Hoechst 33258 (Sigma). The primary antibodies were detected by fluorescent-conjugated secondary antibodies from Jackson ImmunoResearch Laboratories. Confocal images were collected using a Lecia TCS SP5 confocal microscope with 40X/1.25 oil objectives. Adult thoracic bristle and wing images were obtained using a Nikon SMZ1500 microscope. Z-section images were taken with a Zeiss LSM 780 confocal microscope with 40X/1.3 oil objectives for the colocalization analysis. For determination of the Pearson’s correlation coefficient, the JACoP plugin48 for ImageJ was applied. Images were processed with ImageJ and Adobe Photoshop.
Statistical analysis
Statistical analyses were performed using the Graphpad Prism 6 software package. Statistical significance (P values) of the results was calculated by unpaired two-tailed Student’s t test. Data are presented as the Mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 denote statistical significance.
Additional Information
How to cite this article: Zhang, P. et al. Dysfunction of Wntless triggers the retrograde Golgi-to-ER transport of Wingless and induces ER stress. Sci. Rep. 6, 19418; doi: 10.1038/srep19418 (2016).
Supplementary Material
Acknowledgments
We thank H. Bellen and the Iowa Developmental Studies Hybridoma Bank for antibodies; the Bloomington Stock Center and Vienna Drosophila RNAi Centre for fly stocks; T. Kai and Q. Tao for reagent; and L. Ray for comments on the manuscript. I would like to thank H. Lorenz from the ZMBH Imaging Facility for assistance. This work was supported by grants from the National Natural Science Foundation of China (81200993 to P.Z., 81125010 and 81030025 to Z.Y.), and the Ministry of Science and Technology of China (973-2012CB910701 and 2013DFA31990 to Z.Y.). X.L. is supported by grants from the National Basic Research Program of China (2011CB943901), and from NIH (2R01 GM063891).
Footnotes
Author Contributions P.Z. and Z.Y. conceived the project. P.Z. designed the experiments. The experiments were performed by P.Z., L.Z. and C.P. The data were analysed by P.Z., L.Z. and X.L provided reagents and comments on the manuscript. P.Z. wrote the manuscript.
References
- MacDonald B. T., Tamai K. & He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell 17, 9–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. & Nusse R. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 (2012). [DOI] [PubMed] [Google Scholar]
- Herr P., Hausmann G. & Basler K. WNT secretion and signalling in human disease. Trends in molecular medicine 18, 483–493 (2012). [DOI] [PubMed] [Google Scholar]
- Takada R. et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Developmental cell 11, 791–801 (2006). [DOI] [PubMed] [Google Scholar]
- van den Heuvel M., Harryman-Samos C., Klingensmith J., Perrimon N. & Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. The EMBO journal 12, 5293–5302 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr P. & Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Developmental biology 361, 392–402 (2012). [DOI] [PubMed] [Google Scholar]
- Tang X. et al. Roles of N-glycosylation and lipidation in Wg secretion and signaling. Developmental biology 364, 32–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C. et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509–522 (2006). [DOI] [PubMed] [Google Scholar]
- Bartscherer K., Pelte N., Ingelfinger D. & Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125, 523–533 (2006). [DOI] [PubMed] [Google Scholar]
- Goodman R. M. et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development 133, 4901–4911 (2006). [DOI] [PubMed] [Google Scholar]
- Fu J., Jiang M., Mirando A. J., Yu H. M. & Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proceedings of the National Academy of Sciences of the United States of America 106, 18598–18603 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. et al. Xenopus Wntless and the retromer complex cooperate to regulate XWnt4 secretion. Molecular and cellular biology 29, 2118–2128 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasnereau I., Herr P., Chia P. Z., Basler K. & Gleeson P. A. Identification of an endocytosis motif in an intracellular loop of Wntless protein, essential for its recycling and the control of Wnt protein signaling. The Journal of biological chemistry 286, 43324–43333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S. & Hurley J. H. Retromer. Current opinion in cell biology 20, 427–436 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya T. Y. et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Developmental cell 14, 120–131 (2008). [DOI] [PubMed] [Google Scholar]
- Franch-Marro X. et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nature cell biology 10, 170–177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C. L. et al. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Developmental cell 14, 132–139 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F. et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nature cell biology 10, 178–185 (2008). [DOI] [PubMed] [Google Scholar]
- Yang P. T. et al. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Developmental cell 14, 140–147 (2008). [DOI] [PubMed] [Google Scholar]
- Harterink M. et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nature cell biology 13, 914–923 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Wu Y., Belenkaya T. Y. & Lin X. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell research 21, 1677–1690 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechling T., Chaudhary V., Spirohn K., Weiss M. & Boutros M. p24 proteins are required for secretion of Wnt ligands. EMBO reports 12, 1265–1272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F., Hausmann G. & Basler K. A genome-wide RNA interference screen uncovers two p24 proteins as regulators of Wingless secretion. EMBO reports 12, 1144–1152 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. J., Hagen J. W., Okamura K., Perrimon N. & Lai E. C. Functional screening identifies miR-315 as a potent activator of Wingless signaling. Proceedings of the National Academy of Sciences of the United States of America 104, 18151–18156 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell J. A., Gerin I., MacDougald O. A. & Cadigan K. M. The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America 105, 15417–15422 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature reviews. Molecular cell biology 13, 89–102 (2012). [DOI] [PubMed] [Google Scholar]
- Matus S., Glimcher L. H. & Hetz C. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Current opinion in cell biology 23, 239–252 (2011). [DOI] [PubMed] [Google Scholar]
- Rubin D. M. et al. Genomic structure and sequence analysis of Drosophila melanogaster HSC70 genes. Gene 128, 155–163 (1993). [DOI] [PubMed] [Google Scholar]
- Ryoo H. D., Domingos P. M., Kang M. J. & Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. The EMBO journal 26, 242–252 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. F. et al. Drosophila miR-5 suppresses Hedgehog signaling by directly targeting Smoothened. FEBS letters 586, 4052–4060 (2012). [DOI] [PubMed] [Google Scholar]
- Nolo R., Abbott L. A. & Bellen H. J. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349–362 (2000). [DOI] [PubMed] [Google Scholar]
- Enright A. J. et al. MicroRNA targets in Drosophila. Genome biology 5, R1 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby J. G. et al. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome research 17, 1850–1864 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwawaki T., Akai R., Kohno K. & Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nature medicine 10, 98–102 (2004). [DOI] [PubMed] [Google Scholar]
- Duden R. ER-to-Golgi transport: COP I and COP II function (Review). Molecular membrane biology 20, 197–207 (2003). [DOI] [PubMed] [Google Scholar]
- Ma W. & Goldberg J. Rules for the recognition of dilysine retrieval motifs by coatomer. The EMBO journal 32, 926–937 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor E. C., Graham T. R. & Emr S. D. COPI in ER/Golgi and intra-Golgi transport: do yeast COPI mutants point the way ? Biochimica et biophysica acta 1404, 33–51 (1998). [DOI] [PubMed] [Google Scholar]
- Belden W. J. & Barlowe C. Deletion of yeast p24 genes activates the unfolded protein response. Molecular biology of the cell 12, 957–969 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz K. A. & Carney G. E. Loss of p24 function in Drosophila melanogaster causes a stress response and increased levels of NF-kappaB-regulated gene products. BMC genomics 9, 212 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. et al. WLS Retrograde Transport to the Endoplasmic Reticulum during Wnt Secretion. Developmental cell 29, 277–291 (2014). [DOI] [PubMed] [Google Scholar]
- Wang S. & Kaufman R. J. The impact of the unfolded protein response on human disease. The Journal of cell biology 197, 857–867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verras M., Papandreou I., Lim A. L. & Denko N. C. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Molecular and cellular biology 28, 7212–7224 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik K. H. et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nature genetics 39, 833–835 (2007). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nature genetics 39, 836–838 (2007). [DOI] [PubMed] [Google Scholar]
- Vilarino-Guell C. et al. VPS35 mutations in Parkinson disease. American journal of human genetics 89, 162–167 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A. et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. American journal of human genetics 89, 168–175 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert M. S., Neilson J. R. & Sharp P. A. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature methods 4, 721–726 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S. & Cordelieres F. P. A guided tour into subcellular colocalization analysis in light microscopy. Journal of microscopy 224, 213–232 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.