Abstract
Prostate cancer is the second most common form of cancer in males, affecting one in eight men by the time they reach the age of 70. Current diagnostic tests for prostate cancer have significant problems with both false negatives and false positives, necessitating the search for new molecular markers. A recent investigation of endosomal and lysosomal proteins revealed that the critical process of endosomal biogenesis might be altered in prostate cancer. Here, a panel of endosomal markers was evaluated in prostate cancer and non-malignant cells and a significant increase in gene and protein expression was found for early, but not late endosomal proteins. There was also a differential distribution of early endosomes, and altered endosomal traffic and signalling of the transferrin receptors (TFRC and TFR2) in prostate cancer cells. These findings support the concept that endosome biogenesis and function is altered in prostate cancer. Microarray analysis of a clinical cohort confirmed the altered endosomal gene expression observed in cultured prostate cancer cells. Furthermore, in prostate cancer patient tissue specimens, the early endosomal marker and adaptor protein APPL1 showed consistently altered basement membrane histology in the vicinity of tumours and concentrated staining within tumour masses. These novel observations on altered early endosome biogenesis provide a new avenue for prostate cancer biomarker investigation and suggest new methods for the early diagnosis and accurate prognosis of prostate cancer.
Keywords: Prostate cancer, endosomes, biomarkers, diagnosis, prognosis
Introduction
Prostate cancer is the most common form of cancer in males from developed countries, and the incidence of this disease is predicted to double globally by 2030 (WCRF prostate cancer statistics; http://globocan.iarc.fr). Prostate cancer affects approximately one in eight men globally by the time they reach the age of 70 (1). The prostate-specific antigen test is currently used for prostate cancer screening, however, this assay suffers from a high percentage of false-positive results (see for example: 2) and recently there have been recommendations to abandon this procedure, particularly in older men (3). In addition, the digital rectal examination, which manually checks the prostate for abnormalities, is limited by the inability to assess the entire gland and to some degree the size of the tumour. Understanding the cell biology of prostate cancer is important in order to develop new biomarkers for the early diagnosis and accurate prognosis of prostate cancer.
There is mounting evidence for a central role of endosome-lysosome compartments in cancer cell biology (see: 4–6). Endosomes and lysosomes are directly involved in the critical processes of energy metabolism (7), cell division (8) and intracellular signalling (see for example: 9) and would therefore have a direct role in cancer pathogenesis. The endosome-lysosome system has a specific capacity to respond to environmental change, acting as an indicator of cellular function and will consequently be altered in cancer (10). Moreover, the endosome-lysosome system has a critical role in controlling the secretion of proteins into extracellular fluids (see for example: 11), making it an ideal system to identify new biomarkers that are released from cancer cells. Cumulative evidence from patient data and cell lines suggested that the process of lysosomal biogenesis might be altered in prostate cancer (see for example: 12, 13). However, we recently demonstrated that a panel of lysosomal proteins were unable to effectively discriminate between a set of non-malignant and prostate cancer cells (14). In contrast, the endosomal related proteins cathepsin B and acid ceramidase displayed increased gene and protein expression in prostate cancer cells and demonstrated some discriminatory capacity when compared to non-malignant cells. Acid ceramidase was previously shown to be upregulated in prostate cancer tissues and the over-expression of this enzyme has been implicated in advanced and chemo-resistant prostate cancer (15). Importantly, we also showed that LIMP-2, a critical regulator of endosome biogenesis (16), had increased gene and protein expression in prostate cancer cells (14), leading us to postulate that endosome biogenesis is altered in prostate cancer.
Endosome biogenesis involves the synthesis and organisation of structural elements of the endosome system to form an integrated set of functional organelles that eventually interact with lysosomes (see for example: 17). There are two main endosomal pathways: first, from the biosynthetic compartments (endoplasmic reticulum and Golgi apparatus) via specific vesicular traffic towards distal elements of the endosomal network, including early endosomes, late endosomes and multivesicular bodies; and from the cell surface through early endosomes to either recycling endosomes or towards late endosomes. In each case, the formation and movement of these dynamic vesicular compartments is controlled by specific targeting signals and trafficking machinery (see for example: 17, 18). This vesicular machinery can be used to define individual compartments; including for example, the small GTPase Rab5 on early endosomes and the small GTPase Rab7 on late endosomes (18, 19). Here we have investigated the gene expression, amount of protein and intracellular distribution of a panel of endosomal proteins in prostate cancer and non-malignant cell lines, to determine if endosome biogenesis is altered in prostate cancer cells and to identify potential new biomarkers.
Materials and Methods
Antibody reagents
A LIMP-2 sheep polyclonal antibody was generated against the peptide sequence CKKLDDFVETGDIRTMVFP (Mimotopes Pty. Ltd., VIC, Australia). Rabbit polyclonal antibodies (Abcam PLC, Cambridge United Kingdom) were against Appl1 (0.4 μg/mL), Appl2 (0.4 μg/mL), Rab4 (1 μg/mL), TGN46 (10 μg/mL), TfR1 (1 μg/mL), TfR2 (1 μg/mL). Akt (1/1000) and phospho-Akt (Thr308 1/1000) from Cell Signalling Technology Inc., MA, USA, and HRP-conjugated anti-GAPDH (1/20000 Sigma Aldrich Pty. Ltd., NSW, Australia). Goat polyclonal antibodies (Santa Cruz Biotechnology, CA, USA) were against Rab5 (1 μg/mL), Rab7 (1 μg/mL) and EEA1 (1 μg/mL). A LAMP-1 (1 μg/mL) mouse monoclonal BB6 was provided by Professor Sven Carlsson (Umea University, Sweden). HRP-conjugated secondary antibodies for Western blot analysis included anti-goat/sheep (1/2000, Merck Millipore Pty. Ltd., VIC, Australia), anti-rabbit (1/2000) and anti-mouse (1/2000) (Sigma Aldrich). The secondary and other antibody conjugated fluorophores that were used included Alexa Fluor® 488 (1/250), Alexa Fluor® 633 (1/250), Transferrin-633 (1/1000), Phalloidin-488 (1/100), and LysoTracker® (5 μM); all from Life Technologies Pty. Ltd., VIC, Australia.
Cell lines and culture conditions
The non-malignant cell lines PNT1a and PNT2 and prostate cancer cell lines 22RV1 and LNCaP (clone FCG) were obtained from the European Collection of Cell Cultures via CellBank Australia (Children’s Medical Research Institute, NSW, Australia). These cell lines were absent from the list of cross-contaminated or misidentified cell lines, version 6.8 (9th March 2012) (20).
Cell lines were cultured in 75 cm2 tissue culture flasks and maintained in Roswell Park Memorial Institute (RPMI) 1640 culture medium (Gibco®, Life Technologies), supplemented with 10% foetal calf serum (In Vitro Technologies Pty. Ltd., VIC, Australia) and 2 mM L-glutamine (Sigma Aldrich Pty. Ltd., NSW, Australia). Cells were incubated at 37°C with 5% CO2 in a Sanyo MCO-17AI humidified incubator (Sanyo Electric Biomedical Co., Ltd., Osaka, Japan). Cells were cultured to approximately 90% confluence before passage, by washing with sterile PBS (Sigma Aldrich), trypsin treatment (Trypsin-EDTA solution containing 0.12% trypsin, 0.02% EDTA; SAFC®, Sigma Aldrich) to dissociate the cells from the culture surface and then resuspension in supplemented culture medium.
Preparation of cell extracts and conditioned culture media for protein detection
The culture medium was aspirated from cultures at 80–90% confluence, the cells washed once with PBS and then incubated with 800 μL of a 20 mM Tris (pH 7.0) containing 500 mM sodium chloride and 2% (w/v) SDS. Cells were harvested and an extract prepared by heating to 95 °C and sonication for one minute. The lysate was then passaged six times through a 25-guage needle. Total protein in the cell extracts was quantified using a bicinchoninic acid assay according to the manufacturer’s instructions (Micro BCA kit, Pierce, Rockford, IL, USA). Samples were quantified using a Wallac Victor™ optical plate-reader and Workout software v2.0 (Perkin-Elmer Pty, Ltd., VIC, Australia), using a 5-point parameter standard curve. Cell extracts were stored at −20°C.
Protein was recovered from conditioned culture media, collected at the time of cell harvesting, using trichloroacetic acid precipitation. Briefly, cell debris was removed from the culture media by centrifugation (1000 g for 10 minutes), sodium deoxycholate (Sigma Aldrich) added to a final concentration of 0.02 % (v/v) and incubated on ice for 30 minutes. Trichloroacetic acid (Sigma Aldrich) was then added to a final concentration of 15 % (v/v) and incubated on ice for two hours. Protein was collected by centrifugation at 4 °C (5,500 g for 30 minutes), washed twice with ice-cold acetone and resuspended in SDS-sample buffer/PBS solution, and stored at −20 °C.
Gene expression
Relative amounts of mRNA from non-malignant and prostate cancer cell lines were defined by quantitative PCR (qPCR). Briefly, cells were lysed with TRI reagent® (Applied Biosystems®, Life Technologies) and RNA extraction performed using RNeasy® (Qiagen Pty. Ltd., VIC, Australia) according to the manufacturer’s instructions. Two micrograms of total RNA was reverse-transcribed using a High Capacity RNA-to-cDNA Kit (Life Technologies) following the manufacturer’s instructions. qPCR was performed with 2 μL of a 1:25 dilution of cDNA in 10 μL of reaction mixture; containing 5 μL Power SYBR® Green PCR Master Mix (Life Technologies) and 0.5 μL of both 10 nM forward and reverse primer. qPCR was performed using a 7500 Fast Real-Time PCR System (Life Technologies). Each target was assessed in triplicate on a single plate and quantified relative to GAPDH endogenous control for each plate, with triplicate biological replicates run independently. Oligonucleotides (GeneWorks Pty. Ltd., Adelaide, SA, Australia) were as follows: GAPDH TGCACCACCAACTGCTTAGC (Fwd) GGCATGGACTGTGGTCATGAG (Rev) (21); LAMP1 ACGTTACAGCGTCCAGCTCAT (Fwd) TCTTTGGAGCTCGCATTGG (Rev) (21); LIMP2 AAAGCAGCCAAGAGGTTCC (Fwd) GTCTCCCGTTTCAACAAAGTC (Rev); APPL1 ACTTGGGTACATGCAAGCTCA (Fwd) TCCCTGCGAACATTCTGAACG (Rev); APPL2 AGC TGATCGCGCCTGGAACG (Fwd) GGGTTGGTACGCCTGCTCCCT (Rev); EEA1 CCCAACTTGCTACTGAAATTGC (Fwd) TGTCAGACGTGTCACTTTTTGT (Rev); RAB5 AGACCCAACGGGCCAAATAC (Fwd) GCCCCAATGGTACTCTCTTGAA (Rev); RAB4 GGGGCTCTCCTCGTCTATGAT (Fwd) AGCGCATTGTAGGTTTCTCGG (Rev); RAB7 GTGTTGCTGAAGGTTATCATCCT (Fwd) GCTCCTATTGTGGCTTTGTACTG (Rev).
Western blotting
Ten micrograms of total protein for whole cell lysates or the secreted protein from approximately 3×106 cells, was heat-denatured (5 minutes at 100 °C in NuPAGE® LDS Sample Buffer and reducing agent), then electrophoresed at 120 V for 1.5 hours using pre-cast gels in an XCell SureLock Mini-Cell system (Life Technologies). The protein was then transferred to polyvinylidene difluoride membranes (Polyscreen®, PerkinElmer, VIC, Australia). The transfer membranes were blocked for 1 hour at room temperature using a 5% (w/v) skim milk solution in 0.1% (v/v) TBS-tween (blocking solution) and incubated with primary antibody overnight at 4 °C. The membranes were washed in 0.1% (v/v) TBS-tween and then incubated with the appropriate HRP-conjugated secondary antibody diluted 1/2000 in blocking solution. The membranes were developed using a Novex® ECL chemiluminescent substrate reagent kit (Life Technologies), and proteins visualised using an ImageQuant™ LAS 4000 imager, software version 1.2.0.101 (GE Healthcare Pty. Ltd., NSW, Australia). Triplicate samples were analysed and images quantified relative to a reference GAPDH loading control using AlphaViewSA™ software v3.0 (ProteinSimple Pty. Ltd., Santa Clara, CA, USA).
Immune fluorescence
Cells were cultured on 22 mm glass coverslips, fixed with 4% (v/v) formaldehyde in PBS for 20 minutes at room temperature, then permeabilised with 0.1% Triton-X (v/v) in PBS for 10 minutes. Non-specific antibody reactivity was blocked by incubation with 5% (w/v) bovine serum albumin in PBS for two hours at room temperature. Cells were incubated with primary antibody in 5% BSA for two hours at room temperature, followed by fluorophore-conjugated secondary antibody in the dark for one hour at room temperature. Unbound antibody was removed by three PBS washes and coverslips mounted with ProLong® Gold Antifade Reagent containing DAPI nuclear stain (Life Technologies). Confocal microscopy was performed using a Zeiss LSM 710 META NLO laser scanning microscope and associated Carl Zeiss Zen 2009 software. Laser lines of 370, 488, 543 and 633 nm were utilised for DAPI, Alexa Fluor® 488, Cy3 and Alexa Fluor® 633 fluorescence, respectively. Images were exported as greyscale 16-bit TIFF files and processed using Adobe® Photoshop® CS5 (Adobe Systems Inc., San Jose, CA, USA).
Immunohistochemistry
Matched human non-malignant and tumour prostate tissue sections (3 μm) were mounted on Superfrost Ultra Plus® slides (Menzel-Gläser GmbH, Braunschweig, Germany) and heated overnight at 50 °C. Sections were then dewaxed in xylene, rehydrated in ethanol and incubated in 0.3% H2O2 in PBS for 15 min at room temperature. HIER was carried out using 10 mM citrate buffer (pH 6.5) in a Decloaking Chamber (Biocare Medical LLC, Concord, CA, USA) for 5 min at 125 °C. Slides were blocked first using an Invitrogen Avidin/Biotin Kit (as per manufacturer’s instructions) and then in 5% blocking serum (Sigma Aldrich) for 30 min at room temperature in a humid chamber. Sections were then incubated with primary antibody overnight at 4 °C in a humid chamber, followed by incubation with the appropriate biotinylated secondary antibody (1/400; Dako Australia Pty. Ltd., NSW, Australia) for one hour at room temperature in a humid chamber, then streptavidin-horseradish peroxidise (1/500; Dako Australia Pty. Ltd.) for one hour at room temperature in a humid chamber and finally with DAB/H2O2. The tissue sections were then counterstained with Lillie-Mayer’s haemotoxylin, rinsed in water, rehydrated and mounted on slides with DPX mounting media (Merck Millipore Pty. Ltd., VIC, Australia). Images were obtained by scanning slides using a NanoZoomer (Hamamatsu Photonics K.K., Hamamatsu City, Shizuoka Pref., Japan).
Data analysis
Quantities of target-gene and endogenous-control (GAPDH) were calculated from a standard curve from serial dilutions of control material (LNCaP cDNA). Kruskal-Wallis non-parametric analysis of variance statistical analyses were performed using Stata/SE v11.2 (StataCorp LP, Texas, USA) to determine the significance between non-malignant control (PNT1a and PNT2) and prostate cancer (22RV1 and LNCaP) cell lines (95% confidence limit; P ≤ 0.05) for intracellular or secreted protein amount, and gene expression. The Taylor microarray cohort (GSE21034) consisted of patients treated by radical prostatectomy at the Memorial Sloan-Kettering Cancer Center (MSKCC) (22), profiling 150 prostate cancer and 29 non-malignant tissue samples which was performed using Affymetrix Human Exon 1.0 ST arrays. Statistical analysis of microarray gene expression was performed using a two-tailed unpaired t-test with Welch’s correction using GraphPad Prism 5.03 (GraphPad Software Inc., CA, USA).
Results
Increased endosome related gene and protein expression in prostate cancer cells
The expression of endosome and lysosome related genes was quantified by qPCR in control and prostate cancer cells and normalised to the expression of GAPDH mRNA. The amounts of LIMP2, APPL1, APPL2, RAB5A, EEA1 and RAB4 mRNA were significantly increased in prostate cancer when compared to non-malignant control cell lines (P ≤ 0.05; Figure 1). In each case there was an approximately 2–3 fold increase in mRNA expression. There was no significant difference in the amount of either RAB7 or LAMP1 mRNA detected in prostate cancer cells compared to non-malignant controls. Western analysis (Figure 2A) demonstrated significant increases in the amount of LIMP-2, Appl1, Appl2, EEA1, and Rab4 protein in extracts from prostate cancer cells when compared to non-malignant control cells (P ≤ 0.05; Figure 2B). Moreover, for both LIMP-2 and Rab4 the increase was approximately 2–4 fold for prostate cancer when compared to non-malignant cells (Figure 2B). There was no significant difference in the amount of Rab5, Rab7 and LAMP-1 protein detected in non-malignant compared to prostate cancer cells (Figure 2B).
Figure 1. Quantification of endosomal and lysosomal gene expression in control and prostate cancer cell lines.
Levels of mRNA transcripts in non-malignant control cell lines (white bars) and prostate cancer cell lines (black bars) were evaluated by qPCR in triplicate experiments. Data was expressed relative to GAPDH endogenous control and analysed by Kruskal-Wallis rank sum method. Statistical significance (P ≤ 0.05) is represented by an asterisk.
Figure 2. Detection and quantification of intracellular lysosomal proteins in non-malignant control and prostate cancer cell lines.
(A) Representative images from western blot analysis of 10 μg whole cell lysate from non-malignant control cell lines PNT1a and PNT2, and cancer cell lines 22RV1 and LNCaP, examined in triplicate. (B) Protein amount was quantified by densitometry relative to a GAPDH endogenous control. Data was analysed by Kruskal-Wallis rank sum method with statistical significance (P ≤ 0.05) represented by an asterisk.
Altered distribution of endosomes and lysosomes in prostate cancer cells
Representative confocal images for the distribution of endosomes and lysosome proteins (Figure 3), show evidence of increased staining and altered distribution in prostate cancer compared to the non-malignant controls. In non-malignant control cells LIMP-2 was concentrated in the perinuclear region, with some punctuate vesicular staining in the remainder of the cytoplasm. In contrast, prostate cancer cells displayed relatively smaller LIMP-2 compartments, which had an even distribution throughout the cytoplasm. Appl1 positive endosomes were detected throughout the cell cytoplasm of non-malignant control cells, whereas in prostate cancer cells these compartments were more concentrated at the cell periphery, particularly near the plasma membrane in cellular extensions/pseudopodia. In non-malignant control cells both Rab5 and its effector EEA1 were concentrated in the perinuclear region, while in prostate cancer cells these endosomal compartments were found throughout the cytoplasm, with some compartments located towards the cell periphery in cellular extensions. Rab7 positive endosomes were located mainly in the perinuclear region of both non-malignant control and prostate cancer cells. In non-malignant control cells, LAMP-1 compartments were concentrated in the perinuclear region, whereas in prostate cancer cells the LAMP-1 compartments were distributed away from the perinuclear region and concentrated in cellular extensions. Consistent with the LAMP-1 staining, LysoTracker™ positive acidic compartments were concentrated mainly in the perinuclear region of non-malignant control cells, whereas in prostate cancer cells these compartments were detected in both the perinuclear region and in cytoplasmic extensions (Figure 3).
Figure 3. Confocal micrographs of endosomal markers in prostate cancer cell lines compared to non-malignant control cell lines.
Fixed cells were probed for endosome markers (green) and counterstained with DAPI nuclear stain (blue) and visualised by laser-scanning confocal microscopy. Cell outlines were visualised by transmitted light illumination and cell periphery depicted by white dotted line. Antibody labelling was performed in triplicate experiments and a minimum of five cells were visualised in each cell line for each assay. Similar fluorescence staining was observed in all cells for each cell line/antibody label. An additional confocal micrograph from an independent labelling assay is presented in Supplementary Figure 1.
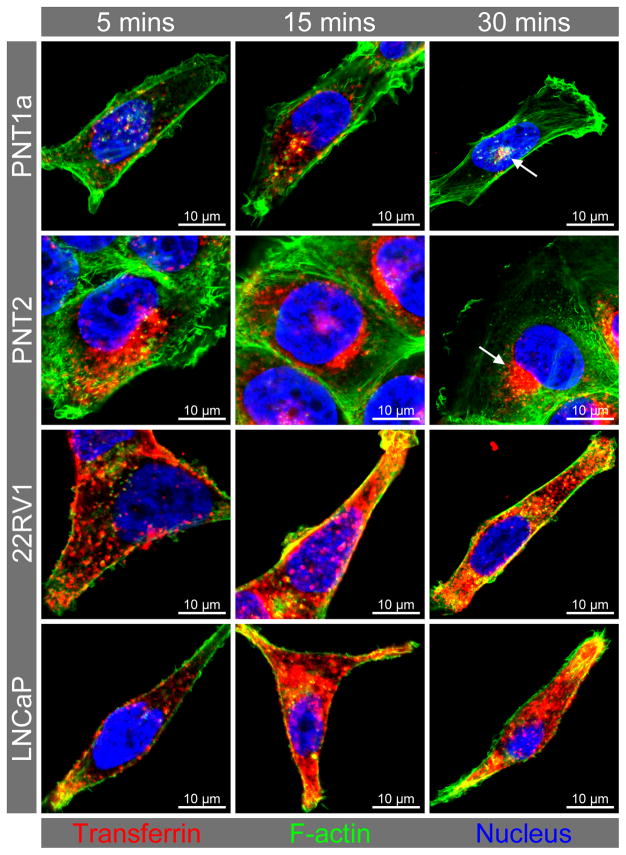
Altered distribution of endocytosed transferrin in prostate cancer cells
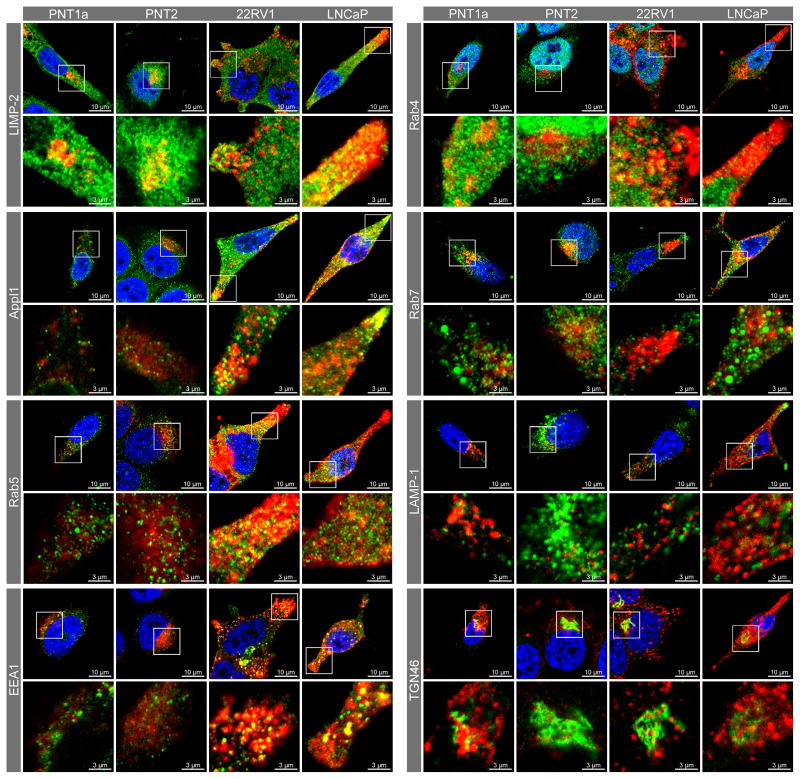
Previous studies have reported increased uptake of transferrin in prostate cancer cells, prompting the investigation of receptor expression and transferrin endocytosis in relation to the observed increase in endosome protein expression and altered endosome distribution. In non-malignant control cells, endocytosed transferrin was observed in punctate intracellular structures after five minutes and in the perinuclear region at 15 and 30 minutes (Figure 4). The prostate cancer cells endocytosed relatively more transferrin than the non-malignant control cell lines within the first five minutes of incubation and at the thirty minute incubation point. In non-malignant cells at 30 minutes (Figure 4) and 20 minutes (Figure 5) this transferrin was tightly concentrated in close proximity to the nucleus. After 15 minutes of incubation, the internalised transferrin was not as concentrated in the perinuclear region of prostate cancer cells, with more transferrin-labelled compartments in the cell periphery and distributed throughout the cytoplasm when compared to the non-malignant cells (Figure 4). There was also a marked reduction in actin staining for the prostate cancer compared to the non-malignant control cell lines (Figure 4). In the non-malignant control cells, transferrin was clustered mainly in LIMP-2 and Rab7-positive endosomes localised in the perinuclear region (Figure 5). While the prostate cancer cells had some LIMP-2 and transferrin positive staining in the perinuclear region and some co-localisation of transferrin with the Golgi marker TGN46 (yellow colocalisation), the majority of transferrin was localised in different endosomal compartments (i.e. Appl1, Rab5, EEA1) distributed throughout the cytoplasm and in cellular extensions (Figure 5). The Rab4 recycling endosomes and LAMP-1 positive lysosomes had similar patterns of transferrin staining for the prostate cancer and non-malignant control cell lines (Figure 5). Further analysis of the transferrin receptors revealed variable gene and protein expression for TfR1 (TFRC) and TfR2 (TFR2) (Figure 6A & B). There was a significant increase in TFRC gene expression (P ≤ 0.05) in prostate cancer cells when compared to non-malignant controls (Figure 6A), but only a qualitative increase in TfR1 protein in the prostate cancer cell line 22RV1 and not for LNCaP (Figure 6B). While there was significantly more TfR2 protein detected in prostate cancer cells when compared to the non-malignant cells (P ≤ 0.05), there was only an increase in TFR2 gene expression in the LNCaP cancer cell line (Figure 6A). Co-localisation of TfR1 and transferrin was observed in all cell lines, and was in a perinuclear location in non-malignant cell lines PNT1a and PNT2 compared to a broader cytoplasmic distribution in the cancer cell lines 22RV1 and LNCaP. Conversely, there appeared to be no co-localisation of transferrin with TfR2 in non-malignant cells and limited co-localisation of transferrin with TfR2-compartments in the prostate cancer cells.
Figure 4. Time-course of transferrin uptake in prostate cell lines.
Representative confocal micrographs from triplicate staining experiments showing increased uptake and altered distribution of transferrin in prostate cancer cell lines compared to non-malignant control cell lines. Cell cultures were incubated with transferrin Alexa Fluor® 633 conjugate (red) for a period of 5, 15 and 30 minutes prior to cell fixation and F-actin labelled with phalloidin Alexa Fluor® 488 (green). Arrows in the control cells depict transferrin that was concentrated in close proximity to the nucleus at 30 minutes. Additional confocal micrographs of transferrin uptake are presented in Supplementary Figure 2.
Figure 5. Transferrin and endosome/lysosome marker co-fluorescence.
Confocal micrographs and enlargements showing transferrin (red; endocytosed for 20 minutes) and endosome/lysosome marker (green) in non-malignant control cell lines PNT1a and PNT2, and prostate cancer cell lines 22RV1 and LNCaP. Co-localisation of markers is depicted by yellow fluorescence.
Figure 6. Analysis of transferrin receptor expression and localisation with transferrin.
(A) Quantification of transferrin receptor 1 (TFRC) and transferrin receptor 2 (TFR2) gene expression in non-malignant and prostate cancer cell lines. (B) Western blot analysis and protein quantification of transferrin receptor 1 (TfR1) and transferrin receptor 2 (TfR2). Quantification of gene and protein expression was relative to GAPDH gene and protein, respectively. (C) Confocal micrographs and enlargements showing transferrin (red) and transferrin receptor (green) in non-malignant cell lines PNT1a and PNT2, and prostate cancer cell lines 22RV1 and LNCaP. Colocalisation of transferrin receptor and transferrin is represented by yellow fluorescence. (D) Western blot analysis and quantification (E) of AKT phosphorylation relative to total AKT, prior and subsequent to treatment of non-malignant (PNT1a and PNT2) and prostate cancer cells (22RV1 and LNCaP) with transferrin for 20 minutes. Asterisk represents (P ≤ 0.05).
Altered Akt signalling in prostate cancer cells
The total amount of Akt protein detected in non-malignant control cells was similar to that detected in prostate cancer cells (Figure 6D & E). There were, however, differences in the amount of phosphorylated Akt in the prostate cancer lines, with 22RV1 showing a marked reduction in the amount of phosphorylated Akt whereas LNCaP had an increased amount of phosphorylated Akt (Figure 6D), a phenomenon previously observed by Shukla et al. and related to mutations of PTEN in LNCaP (23). More importantly, following the addition of transferrin, there was a significant increase in the amount of phosphorylated Akt in the non-malignant control cell lines, but no change in the amount of phosphorylated Akt in either of the cancer cells (Figure 6E).
LAMP1and APPL1 mRNA expression in a prostate cancer microarray cohort and distribution of LAMP-1 and Appl1 in prostate tissue
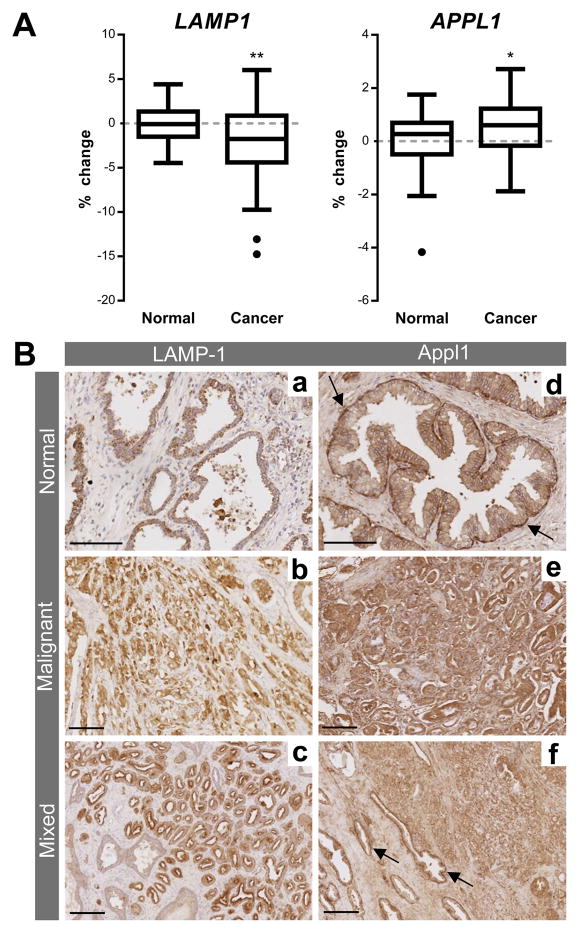
To support the hypothesis of altered endosome biogenesis in prostate cancer, the percentage change of mRNA expression for LAMP1 and APPL1 was analysed from the Taylor microarray cohort (Figure 7A). LAMP1 gene expression was significantly decreased (P ≤ 0.01) in prostate cancer tissue compared with non-malignant prostate tissue. APPL1 gene expression was significantly increased (P ≤ 0.05) in prostate cancer tissue compared to non-malignant tissue. Immunohistochemistry was used to investigate the distribution of LAMP-1 and Appl1 in prostate cancer patient tissue samples (Figure 7B). The lysosomal marker LAMP-1 showed tumour specific staining in some patient samples, but consistent with previous studies there were variable results with some patient samples having little or no LAMP-1 staining (data not shown for the latter). In non-malignant tissue Appl1 clearly delineated basement membranes, whereas in the malignant tissue there was no evidence of basement membrane staining (Figure 7B). In addition, Appl1 specifically delineated the cancer margins and showed increased staining within the tumour mass (Figure 7B).
Figure 7. Analysis of gene expression and protein distribution in prostate cancer compared to non-malignant tissue.
(A) Box-and-whisker graphs showing percentage change of LAMP1 and APPL1 mRNA expression in normal (n = 29) and prostate cancer tissue (n = 150) from metanalysis of the cohort by Taylor et al. (22). Box-and-whisker graphs were plotted with Tukey outliers (black points). Statistical significance is represented by an asterisk (P ≤ 0.05).
(B) LAMP-1 (a, b) and Appl1 (d, e) expression in matched human normal (a, d) and malignant (b; Gleason grade 3+3, e; Gleason grade 3+4) prostate tissue. Both normal and malignant mixed-tissue is stained for LAMP-1 (c; Gleason grade 3+3) and Appl1 (f; Gleason grade 3+4). The arrows in d & f show Appl1 staining the basement membrane in non-malignant prostate tissue. Scale bar represents 100 μM in a, b & d and 200 μM in c, e & f.
Discussion
Prostate cancer is one of the most frequently diagnosed cancers in men and a leading cause of cancer related deaths world-wide, particularly in the United States and Australasian populations (24, 25). The prostate specific antigen is still commonly used to detect prostate cancer, but has significant problems in terms of miss-diagnosis and prognostic prediction (see for example: 26). Some promising adjunct tests have recently been developed including prostate cancer antigen 3 (PCA3) (27), the analysis of cholesterol sulphate (28) and a novel sequence of the gene protein kinase C-zeta (PRKCZ), which is translated to the protein PRKC-ζ-PrC (29). However, these biomarkers do not provide early and accurate detection of prostate cancer, which is needed to enable the selection of the most appropriate therapeutic intervention and to avoid potential overtreatment (2). Based on our observations of altered LIMP-2 expression (14), we investigated altered endosomal biogenesis in prostate cancer to help provide more sensitive and specific markers for early detection and disease prediction.
There have been extensive protein and proteomic studies undertaken to identify potential new prostate cancer biomarkers (see for example: 26), however, the ideal marker with appropriate sensitivity and specificity is yet to be established. Interestingly, many of the early biomarkers investigated, and some of the recent proteins identified in proteomic studies, are either lysosomal hydrolases (e.g. lysosomal cathepsins, acid ceramidase and acid phosphatase), lysosomal membrane proteins (e.g. LAMP 1–3 also called CD107a, b and CD63) or proteins that are delivered from the cell surface into the endosome-lysosome system (e.g. sialomucin/CD164, CD1, CD47, CD75). Additional evidence supports the concept that endosomal-lysosomal biogenesis is altered in prostate cancer, including the altered distribution of lysosomes that has been reported in prostate cancer cells (30). Despite these indications on lysosomal biogenesis, a set of optimal prostate cancer biomarkers have yet to be defined. In a recent study of endosome and lysosome markers in prostate cancer cell lines we also found that lysosomal markers were unable to discriminate prostate cancer from non-malignant cell lines, but there was evidence suggesting that endosome biogenesis may be altered in prostate cancer cells (14).
Here we observed altered distribution of specific endosome subsets and lysosomes into the cellular periphery of prostate cancer cells, which could have important implications for cancer cell biomarker release and intracellular signalling. Acidic extracellular pH has been shown to enhance organelle re-distribution, stimulating the traffic of endosome-lysosome related organelles to the periphery of cancer cells (10, 31). This altered endosome-lysosome traffic has been linked with the release of cathepsin B and tumour invasiveness (32), presumably due to the hydrolysis of extracellular matrix after the exocytosis of this enzyme (33). However, cathepsin B has been reported to be more enriched in endosomes (34) rather than lysosomes, whereas the reverse is true for another proposed prostate cancer biomarker cathepsin D (35). The movement of lysosomal related vesicles to the periphery of prostate cancer cells has been shown to be dependent on GTPases (e.g. RhoA), microtubules, the molecular motor protein KIF5b, and to involve PI3K, Akt/Erk1/2 phosphorylation and MAPK signalling (32). Moreover, a component of the MAPK signalling pathway, the endosome-localised MAPK/Erk Kinase (MEK1) p14-MP1 scaffolding complex, has been shown to specifically interact with and regulate the distribution of endosomes via ERK signalling (36). Increases in Na+/H+ exchange activity (acidification), RhoA GTPase activity and PI3K activation have been shown to result in exocytosis from prostate cancer cells (31). The increased endosomal associated gene and protein expression observed here, together with the previously observed cathepsin B release, suggested that endosome related proteins may provide an important new focus for prostate cancer disease biomarker studies.
Increased expression of the endosomal protein LIMP-2 has been shown in oral squamous cell carcinoma and was associated with tumour metastasis (37). LIMP-2 has been reported to have a role in endosome biogenesis and its overexpression evoked the enlargement of both early and late endosomes (16). We observed increased gene and protein expression of the endosomal protein LIMP-2 in prostate cancer cell lines (14), prompting us to investigate other endosomal proteins in prostate cancer cells. The early endosome associated proteins Appl1, Appl2, EEA1 and recycling endosome protein Rab4 were significantly upregulated (gene and protein) in prostate cancer cells, supporting the hypothesis of altered endosome biogenesis in prostate cancer. APPL1 expression was significantly increased in the Taylor prostate cancer tissue microarray, supporting the expression profiles observed in cell lines. Furthermore, the EEA1 and Appl1 endosome sub-populations each displayed altered intracellular distribution consistent with altered endosome traffic and potentially function. Interestingly, whilst Rab7 expression was unaltered, Rab7-positive compartments displayed differential distribution in prostate cancer compared to non-malignant cells. Changes in subcellular localisation may affect signalling in a similar manner to that which transpires through downregulated gene/protein expression that affects prostate cancer progression through enhanced signalling (38). Thus, the analysis of compartment distribution may distinguish cancer cell phenotypes independently of altered gene and protein expression.
The significant changes that we observed in endosome associated gene and protein expression, together with the altered distribution of endosome populations prompted us to investigate transferrin receptor expression together with transferrin endocytosis, sorting and Akt signalling as measures of endosome function. Significant increases in the amount of transferrin receptor have previously been reported in prostate cancer cells (39), and this has been linked to c-Myc activation, which alters proliferation and tumourigenesis (40). Akt signalling is also essential for regulating cell growth and survival; and this controls the cell surface expression of transferrin and growth factor receptors (41). The transferrin receptor has previously been observed to co-localise with Rab5 and the motor protein myosin VI; the latter of which is involved in retrograde transport to the plasma membrane (42). This was consistent with our observations of endosome populations co-staining with labelled transferrin in the cellular periphery of prostate cancer cells. There also appeared to be a deregulation of Akt signalling in prostate cancer cells, with control cells being responsive to transferrin endocytosis, but prostate cancer cells being unresponsive, despite having variable high or low amounts of Phospho-Akt/Akt. This altered signalling may be related to the intracellular location of the transferrin receptor that can be disturbed through changes in localisation or depletion of PtdIns3P (43) or affected through variable internalisation resulting from altered Appl1 or Rab5 expression (as is the case with epidermal growth factor receptor (44)), affecting receptor trafficking and signal modulation.
Appl1 has been shown to be directly involved in insulin signalling and the translocation of the glucose transporter GLUT-4, which is mediated by direct binding of Appl1 to PI3K and Akt (45), inducing endosome re-localisation. In prostate cancer cells, Appl1 potentiated Akt activity has also been shown to suppress androgen receptor transactivation (46). The increased gene and protein expression of Appl1 that we observed in prostate cancer cells might be expected to cause increased glucose uptake, due to its effect on GLUT-4 and this could have implications for energy metabolism in these cancer cells. Indeed, Appl1 also regulates other aspects of both lipid and glucose metabolism, activating AMP-activated kinase, p38 MAP kinase (MAPK) and PPARα (see for example: 45). Appl2 has been shown to function as a negative regulator of adiponectin signalling, by competitive binding with Appl1 for interaction with the adiponectin receptor, again regulating energy metabolism. The increased expression of both Appl1 and Appl2 could therefore impact heavily on prostate cancer cell metabolism with direct significance for increased energy utilisation and prostate cancer cell survival. The altered Appl1 expression and effect on Akt signalling in prostate cancer cells would be expected to also have significant consequence for other aspects of prostate cancer biology, due to the importance of the Appl1/PI3K/Akt signalling pathway in leading cell adhesion and cell migration (47). Notably, Appl1 also acts as a mediator of other signalling pathways, by interaction with the cytosolic face of integral or membrane associated proteins either at the cell surface or in the endosome pathway; where it is directly involved in endosome traffic.
Rab GTPases are integrally involved in the control of endosome traffic, cycling between the cytoplasmic GDP bound state and the active membrane associated GTP bound state. Rab5 and Rab7 respectively define early and late endosome compartments and during endosome maturation Rab5 recruits the HOPS complex as a mechanism to activate and be replaced by Rab7. While mVps39 is known to be a guanine nucleotide exchange factor (GEF) that promotes the GTP bound state on endosomal Rabs, TBC-2/TBC1D2 is a Rab GTPase activating protein (GAP) that promotes the GDP bound state; and in combination is used to regulate the membrane localisation of Rab proteins. TBC-2/TBC1D2 is therefore thought to act as a regulator of endosome to lysosome traffic and is required to maintain the correct size and distribution of endosomes (48). The altered distribution of endosome populations that we observed in prostate cancer cells suggests that TBC-2/TBC1D2 (GAP) and or mVps39 (GEF) might be functionally impaired; particularly as the early endosomes were routed mainly toward the cell periphery, whereas late endosomes remained in a perinuclear location. Interestingly, microarray analysis has detected increased expression of TBC-2/TBC1D2 and reduced Vps39 mRNA in relation to altered endosomal-lysosomal traffic (49). This altered GEF and GAP function has been shown to be critical for endosomal traffic of integrins and there have been direct links established between altered Rab GTPase activity and cancer progression (50).
The expression of endosome markers has not previously been investigated thoroughly in prostate cancer, although some lysosomal related cell surface CD (cell differentiation) markers and LAMP-1/LAMP-2 have been utilised in tissue biopsy analysis. The Gleason grading system is used to define histological differentiation in conjunction with marker analysis to predict the course of disease in prostate cancer patients. The lysosomal membrane proteins LAMP 1–3 and CD markers CD164, CD1, CD47 and CD75 are often evident in primary and metastatic cancer biopsies (51), but their consistency and predictive capacity for disease progression is limited. We observed increased amounts of Appl1 protein in malignant tissue from biopsies of prostate cancer patients, confirming the increased gene and protein expression of Appl1 in prostate cancer cell lines. Appl1 appeared more concentrated in the basement membranes in non-malignant tissue, whereas in the malignant tissue there was no basement membrane staining, indicating diagnostic/prognostic potential for this biomarker. Further immunohistochemical and patient tissue analysis of Appl1 and other endosomal proteins is required to establish the validity and predictive value of these proteins as prognostic biomarkers in prostate cancer.
In summary, we have demonstrated increased expression of early endosome markers and altered localisation of endosome and lysosome compartments in prostate cancer cells, which was associated with altered endocytosis and recycling of the transferrin receptor and aberrant Akt signalling. The alterations to the endocytic machinery that we have observed here, may increase the amount of endocytosis in prostate cancer cells, which could increase nutrient uptake/availability, provide additional membrane for cell division (9) and alter intracellular signalling (10); which are all hallmarks of cancer cell biology. There appeared to be a specific disconnect between the cellular location of early endosomes (and lysosomes) in the cell periphery and late endosomes in the perinuclear region, which could impact on degradative and signalling processes in prostate cancer cells. We concluded that endosome biogenesis and function is altered in prostate cancer cells, opening a potentially new avenue to investigate biomarkers that aid in the diagnosis and prognosis of prostate cancer. Endosomes are directly involved in the processes of cellular secretion and exosome release, making these newly identified endosomal proteins potentially available for detection in patient samples, such as blood and urine.
Supplementary Material
Implications.
This discovery of altered endosome biogenesis in prostate cancer may lead to novel biomarkers for more precise cancer detection and patient prognosis.
Acknowledgments
This project was funded by a University of South Australia Presidents Scholarship and a University of South Australia Postgraduate Award, together with additional support from University of South Australia Research SA Seeding Funds. We thank Dr Shalini Jindal and Ms Marie Pickering (Dame Roma Mitchell Cancer Research Laboratories, University of Adelaide, South Australia) for expert assistance with the immunohistochemistry and pathology of human prostate tissue samples.
Footnotes
Disclosure statement:
The authors declare that they have no relevant financial interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. The New England journal of medicine. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Moyer VA. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 4.Parachoniak CA, Park M. Dynamics of receptor trafficking in tumorigenicity. Trends in cell biology. 2012;22:231–40. doi: 10.1016/j.tcb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Hurley JH, Odorizzi G. Get on the exosome bus with ALIX. Nature cell biology. 2012;14:654–5. doi: 10.1038/ncb2530. [DOI] [PubMed] [Google Scholar]
- 6.Hu CT, Wu JR, Wu WS. The role of endosomal signaling triggered by metastatic growth factors in tumor progression. Cellular signalling. 2013;25:1539–45. doi: 10.1016/j.cellsig.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nature reviews Molecular cell biology. 2013;14:283–96. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7939–44. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palfy M, Remenyi A, Korcsmaros T. Endosomal crosstalk: meeting points for signaling pathways. Trends in cell biology. 2012;22:447–56. doi: 10.1016/j.tcb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia. 2003;5:533–45. doi: 10.1016/s1476-5586(03)80037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stow JL, Murray RZ. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine & growth factor reviews. 2013;24:227–39. doi: 10.1016/j.cytogfr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Henneberry MO, Engel G, Grayhack JT. Acid phosphatase. Urol Clin North Am. 1979;6:629–41. [PubMed] [Google Scholar]
- 13.Quintero IB, Araujo CL, Pulkka AE, Wirkkala RS, Herrala AM, Eskelinen EL, et al. Prostatic acid phosphatase is not a prostate specific target. Cancer Res. 2007;67:6549–54. doi: 10.1158/0008-5472.CAN-07-1651. [DOI] [PubMed] [Google Scholar]
- 14.Johnson IR, Parkinson-Lawrence EJ, Butler LM, Brooks DA. Prostate cell lines as models for biomarker discovery: Performance of current markers and the search for new biomarkers. The Prostate. 2014;74:547–60. doi: 10.1002/pros.22777. [DOI] [PubMed] [Google Scholar]
- 15.Camacho L, Meca-Cortes O, Abad JL, Garcia S, Rubio N, Diaz A, et al. Acid ceramidase as a therapeutic target in metastatic prostate cancer. Journal of lipid research. 2013;54:1207–20. doi: 10.1194/jlr.M032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuronita T, Eskelinen EL, Fujita H, Saftig P, Himeno M, Tanaka Y. A role for the lysosomal membrane protein LGP85 in the biogenesis and maintenance of endosomal and lysosomal morphology. J Cell Sci. 2002;115:4117–31. doi: 10.1242/jcs.00075. [DOI] [PubMed] [Google Scholar]
- 17.Huotari J, Helenius A. Endosome maturation. The EMBO journal. 2011;30:3481–500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerial M, McBride H. Rab proteins as membrane organizers. Nature reviews Molecular cell biology. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 19.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–7. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 20.Capes-Davis A, Theodosopoulos G, Atkin I, Drexler HG, Kohara A, MacLeod RA, et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. International journal of cancer Journal international du cancer. 2010;127:1–8. doi: 10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 21.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–7. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 22.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. International journal of cancer Journal international du cancer. 2007;121:1424–32. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 24.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 25.AIHW. AIHW (Australian Institute of Health and Welfare) 2010. Cancer series no. 60. Cat. no. CAN 56. Canberra: AIHW; 2010. Cancer in Australia 2010: an overview. [Google Scholar]
- 26.Roobol MJ, Haese A, Bjartell A. Tumour markers in prostate cancer III: biomarkers in urine. Acta Oncol. 2011;50(Suppl 1):85–9. doi: 10.3109/0284186X.2010.524935. [DOI] [PubMed] [Google Scholar]
- 27.Ploussard G, de la Taille A. Urine biomarkers in prostate cancer. Nat Rev Urol. 2010;7:101–9. doi: 10.1038/nrurol.2009.261. [DOI] [PubMed] [Google Scholar]
- 28.Eberlin LS, Dill AL, Costa AB, Ifa DR, Cheng L, Masterson T, et al. Cholesterol sulfate imaging in human prostate cancer tissue by desorption electrospray ionization mass spectrometry. Analytical chemistry. 2010;82:3430–4. doi: 10.1021/ac9029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao S, Ireland SJ, Bee A, Beesley C, Forootan SS, Dodson A, et al. Splice variant PRKC-zeta(-PrC) is a novel biomarker of human prostate cancer. British journal of cancer. 2012;107:388–99. doi: 10.1038/bjc.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloane BF, Moin K, Sameni M, Tait LR, Rozhin J, Ziegler G. Membrane association of cathepsin B can be induced by transfection of human breast epithelial cells with c-Ha-ras oncogene. J Cell Sci. 1994;107:373–84. doi: 10.1242/jcs.107.2.373. [DOI] [PubMed] [Google Scholar]
- 31.Steffan JJ, Snider JL, Skalli O, Welbourne T, Cardelli JA. Na+/H+ Exchangers and RhoA Regulate Acidic Extracellular pH-Induced Lysosome Trafficking in Prostate Cancer Cells. Traffic. 2009;10:737–53. doi: 10.1111/j.1600-0854.2009.00904.x. [DOI] [PubMed] [Google Scholar]
- 32.Steffan JJ, Cardelli JA. Thiazolidinediones induce Rab7-RILP-MAPK-dependent juxtanuclear lysosome aggregation and reduce tumor cell invasion. Traffic. 2010;11:274–86. doi: 10.1111/j.1600-0854.2009.01012.x. [DOI] [PubMed] [Google Scholar]
- 33.Roshy S, Sloane BF, Moin K. Pericellular cathepsin B and malignant progression. Cancer and Metastasis Reviews. 2003;22:271–86. doi: 10.1023/a:1023007717757. [DOI] [PubMed] [Google Scholar]
- 34.Authier F, Kouach M, Briand G. Endosomal proteolysis of insulin-like growth factor-I at its C-terminal D-domain by cathepsin B. FEBS letters. 2005;579:4309–16. doi: 10.1016/j.febslet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 35.Zaidi N, Maurer A, Nieke S, Kalbacher H. Cathepsin D: A cellular roadmap. Biochemical and Biophysical Research Communications. 2008;376:5–9. doi: 10.1016/j.bbrc.2008.08.099. [DOI] [PubMed] [Google Scholar]
- 36.Deacon SW, Nascimento A, Serpinskaya AS, Gelfand VI. Regulation of bidirectional melanosome transport by organelle bound MAP kinase. Current biology : CB. 2005;15:459–63. doi: 10.1016/j.cub.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 37.Pasini FS, Maistro S, Snitcovsky I, Barbeta LP, Rotea Mangone FR, Lehn CN, et al. Four-gene expression model predictive of lymph node metastases in oral squamous cell carcinoma. Acta Oncol. 2012;51:77–85. doi: 10.3109/0284186X.2011.620619. [DOI] [PubMed] [Google Scholar]
- 38.Steffan JJ, Dykes SS, Coleman DT, Adams LK, Rogers D, Carroll JL, et al. Supporting a role for the GTPase Rab7 in prostate cancer progression. PloS one. 2014;9:e87882. doi: 10.1371/journal.pone.0087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keer HN, Kozlowski JM, Tsai YC, Lee C, McEwan RN, Grayhack JT. Elevated transferrin receptor content in human prostate cancer cell lines assessed in vitro and in vivo. The Journal of Urology. 1990;143:381–5. doi: 10.1016/s0022-5347(17)39970-6. [DOI] [PubMed] [Google Scholar]
- 40.O’Donnell KA, Yu D, Zeller KI, Kim JW, Racke F, Thomas-Tikhonenko A, et al. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Mol Cell Biol. 2006;26:2373–86. doi: 10.1128/MCB.26.6.2373-2386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Molecular biology of the cell. 2002;13:2276–88. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puri C, Chibalina MV, Arden SD, Kruppa AJ, Kendrick-Jones J, Buss F. Overexpression of myosin VI in prostate cancer cells enhances PSA and VEGF secretion, but has no effect on endocytosis. Oncogene. 2010;29:188–200. doi: 10.1038/onc.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fili N, Calleja V, Woscholski R, Parker PJ, Larijani B. Compartmental signal modulation: Endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15473–8. doi: 10.1073/pnas.0607040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JR, Hahn HS, Kim YH, Nguyen HH, Yang JM, Kang JS, et al. Adaptor protein containing PH domain, PTB domain and leucine zipper (APPL1) regulates the protein level of EGFR by modulating its trafficking. Biochemical and biophysical research communications. 2011;415:206–11. doi: 10.1016/j.bbrc.2011.10.064. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Xin X, Xiang R, Ramos FJ, Liu M, Lee HJ, et al. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. The Journal of biological chemistry. 2009;284:31608–15. doi: 10.1074/jbc.M109.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, Lin HK, Altuwaijri S, Xie S, Wang L, Chang C. APPL suppresses androgen receptor transactivation via potentiating Akt activity. The Journal of biological chemistry. 2003;278:16820–7. doi: 10.1074/jbc.M213163200. [DOI] [PubMed] [Google Scholar]
- 47.Broussard JA, Lin WH, Majumdar D, Anderson B, Eason B, Brown CM, et al. The endosomal adaptor protein APPL1 impairs the turnover of leading edge adhesions to regulate cell migration. Molecular biology of the cell. 2012;23:1486–99. doi: 10.1091/mbc.E11-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chotard L, Mishra AK, Sylvain MA, Tuck S, Lambright DG, Rocheleau CE. TBC-2 regulates RAB-5/RAB-7-mediated endosomal trafficking in Caenorhabditis elegans. Molecular biology of the cell. 2010;21:2285–96. doi: 10.1091/mbc.E09-11-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peralta ER, Martin BC, Edinger AL. Differential effects of TBC1D15 and mammalian Vps39 on Rab7 activation state, lysosomal morphology, and growth factor dependence. The Journal of biological chemistry. 2010;285:16814–21. doi: 10.1074/jbc.M110.111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramani D, Alahari SK. Integrin-mediated function of Rab GTPases in cancer progression. Mol Cancer. 2010;9:312. doi: 10.1186/1476-4598-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu AY, Roudier MP, True LD. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. The American journal of pathology. 2004;165:1543–56. doi: 10.1016/S0002-9440(10)63412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.