Abstract
CYP11A1, found only in vertebrates, catalyzes the first step of steroidogenesis where cholesterol is converted to pregnenolone. The purified enzyme, also converts desmosterol and plant sterols including campesterol and β-sitosterol, to pregnenolone. Studies, initially with purified enzyme, reveal that 7-dehydrocholesterol (7DHC), ergosterol, lumisterol 3, and vitamins D3 and D2 also serve as substrates for CYP11A1, with 7DHC being better and vitamins D3 and D2 being poorer substrates than cholesterol. Adrenal glands, placenta, and epidermal keratinocytes can also carry out these conversions and 7-dehydropregnenolone has been detected in the epidermis, adrenal glands, and serum, and 20-hydroxyvitamin D3 was detected in human serum and the epidermis. Thus, this metabolism does appear to occur in vivo, although its quantitative importance and physiological role remain to be established. CYP11A1 action on 7DHC in vivo is further supported by detection of Δ7steroids in Smith-Lemli-Opitz syndrome patients. The activity of CYP11A1 is affected by the structure of the substrate with sterols having steroidal or Δ7-steroidal structures undergoing side chain cleavage following hydroxylations at C22 and C20. In contrast, metabolism of vitamin D involves sequential hydroxylations that start at C20 but do not lead to cleavage. Molecular modeling using the crystal structure of CYP11A1 predicts that other intermediates of cholesterol synthesis could also serve as substrates for CYP11A1. Finally, CYP11A1-derived secosteroidal hydroxy-derivatives and Δ7steroids are biologically active when administered in vitro in a manner dependent on the structure of the compound and the lineage of the target cells, suggesting physiological roles for these metabolites. This article is part of a special issue entitled ‘SI: Steroid/Sterol signaling’.
Keywords: CYP11A1, Cholesterol, Plant sterols, Vitamin D, 7-Dehydrocholesterol, Ergosterol
1. Overview of the function and phylogeny of CYP11A1
1.1. Biochemical characterization
CYP11A1, also known as cytochrome P450 side chain cleavage (P450scc), is a mitochondrial enzyme best characterized for its catalysis of the cleavage of the side chain of cholesterol to produce pregnenolone, the common precursor of steroid hormones [1,2]. The reaction involves initial hydroxylation at C22 producing 22R-hydroxycholesterol, then subsequent repositioning of the side chain in the active site resulting in a second hydroxylation at C20, producing 20R,22R-dihydroxycholesterol. This is followed by oxidative cleavage of the C20—C22 bond producing pregnenolone [1–3].
Electrons for these reactions are provided by NADPH via a short electron transport chain comprising adrenodoxin reductase and adrenodoxin [1,2,4,5]. The final product, pregnenolone, can leave the mitochondria and be converted to steroid hormones by cell- and gland-specific pathways [2]. The products of these pathways are dependent on cell type- or tissue-dependent expression of the different steroidogenic enzymes.
Recently, the crystal structures of bovine [6] and human CYP11A1 [7] have been reported with either cholesterol, 22R-hydroxycholesterol (bovine) or 20R,22R-dihydroxycholesterol in the active site. These structures reveal that the tight binding of the first intermediate, 22R-hydroxycholesterol, appears to be mediated, at least in part, by direct co-ordination of the C22-oxygen to the heme-iron. Comparison of the structures with the different intermediates bound reveals that subtle shifts occur in the positioning of the side-chain in the active site following each hydroxylation, enabling the three sequential oxidations required for side chain cleavage. These crystal structures have also proved useful for docking studies with other substrates of the purified enzyme [7–10] (see later).
In classical steroidogenic tissues such as the adrenal cortex, corpus luteum, and testis, and in non-classical tissues with low or very low steroidogenic activity including brain, skin, gastrointestinal tract, and the immune system, steroid synthesis is regulated by the action of the steroidogenic acute regulatory (StAR) protein or its equivalents (STARD3) that control cholesterol transport to the inner mitochondrial membrane site of CYP11A1 action [2,11–15]. In the classical pathway of cholesterol side chain cleavage, CYP11A1 activity is substrate limited and increased rates of cholesterol delivery cause a corresponding increase in the rate of pregnenolone synthesis. The activity of the StAR protein or STARD3 is elevated by both increased protein synthesis and its posttranslational modifications, predominantly mediated by cAMP-dependent pathways activated by ACTH, angiotensin II or LH [2,11,15].
Studies carried out over a number of years reveal that purified CYP11A1 and/or steroidogenic mitochondria that contain CYP11A1, can act on a range of steroids or secosteroids, other than cholesterol. Arthur et al. [16], reported that bovine, rat, and pig adrenal mitochondria could metabolize analogues of cholesterol with shorter 5C and 7C hydrophobic side chains at slightly higher or comparable rates to cholesterol. However, analogues with polar side chains such as 20α-hydroxycholesterol, 24-hydroxycholesterol, 25-hydroxycholesterol, or 26-hydroxycholesterol were metabolized to pregnenolone at appreciably higher rates than cholesterol. Mason et al. [17] and Craig et al. [18] subsequently attributed these differences to a slower rate of transfer of cholesterol into the mitochondrion by a cycloheximide-sensitive step (later found to be due to inhibition of StAR protein synthesis), compared to the cycloheximide-insensitive transfer of side-chain hydroxy analogues. Morisaki et al. [19] reported that in a CYP11A1 reconstituted system, cholesterol analogues with a 3C or 4C side chains were poorly metabolized to pregnenolone. Activity then increased with side chain length to a maximum with 7C, then decreased with further increases in side chain length. It has also been reported that adrenal mitochondria or a reconstituted CYP11A1 system can act on halogenated side chain analogues of cholesterol, such as 26-bromocholesterol or 25-fluorocholesterol, converting them to pregnenolone [19,20].
In 1986, Lambeth [21] reviewed the substrate specificity of CYP11A1 with respect to both binding and catalysis, with much of the data coming from synthetic analogues of cholesterol. It was concluded that there was high specificity towards a planar structure near C5 (as provided by a C5=C6 or C4=C5 double bond), and that there was not a great deal of specificity with respect to the side chain. Since that review, a number of new substrates for the purified enzyme have been identified that may be of physiological significance, and it is these substrates that we primarily focus on in this review with a critical analysis of their potential to be of physiological relevance. These new substrates include 7-dehydrocholesterol (7DHC) [8,14,22,23], vitamin D3 [22,24–27], vitamin D2 [9,28,29], ergosterol [10,30], and lumisterol [31]. For both cholesterol and its precursor, 7DHC, CYP11A1 catalyzes three oxidative reactions on their side chain, resulting in cleavage occurring between C20 and C22, producing pregnenolone and 7-dehydropregnenolone (7DHP), respectively, as well as isocaproic aldehyde [1,14,22]. Importantly, catalytic activity on non-traditional substrates such as vitamin D and ergosterol does not result in side chain cleavage, but involves sequential hydroxylations of the side chain including at C17 [[10,24–30], and see below].
1.2. Molecular biology and phylogenesis
The CYP11A1 gene is only found in vertebrates. The first cloning of the CYP11A1 gene was reported in 1984 by John et al. [32] and involved differential hybridization screening of bovine cDNA libraries. This facilitated the cloning and sequencing of the gene from other species including a number of mammals as well as species of birds, amphibians, and fish. Phylogenetic analysis of CYP11A1 reveals segregation into three broad groups comprising teleosts, mammals, and the stingray which split early in vertebrate evolution [33,34]. Stingray is a primitive vertebrate of the elasmobranch subclass and its CYP11A1 is highly divergent from both mammalian and teleost forms of the enzyme, with the southern stingray (Dasyatis americana) form displaying 48% identity with teleost species of CYP11A1 and 39–40% with mammals [33,34]. Identity between these three major subclasses is higher in known functional regions of the enzyme. For example, stingray CYP11A1 shows greater than 60% identity with the other forms in the putative adrenodoxin binding domain and the heme binding domain [34]. Of the 4 basic residues implicated in adrenodoxin binding to bovine CYP11A1(Lys423, Lys425, Arg465, and Arg466), the two arginine residues are found in similar positions in all CYP11A1 proteins, but the two lysines are not well conserved [33,35–37]. The putative steroid binding domain and heme binding domain of CYP11A1 is well conserved from teleosts to the frog, chick, and mouse forms of CYP11A1 [38]. The N-terminal mitochondrial target sequence is moderately conserved among mammalian species (approximately 50% identity) but poorly conserved when rainbow trout (Oncorhynchus mykiss) and stingray proteins are compared [34].
As might be expected, there is high amino acid sequence similarity in CYP11A1 between closely related mammalian species. For example, in primates baboon (Papio ursinus) CYP11A1 displays 98% identity with human CYP11A1 [39]. For more distantly related mammalian species, identities are typically above 70%. For example, sheep CYP11A1 shares 73% identity with human CYP11A1 and 72% with the rat enzyme [40]. Human CYP11A1 shares 80% identity with hamster (Mesocricetus auratus) CYP11A1 and 72% with the bovine enzyme [41,42].
Two CYP11A genes have been identified in zebrafish (Danio rerio), CYP11A1 and CYP11A2 [43,44]. This appears to have arisen from a relatively recent genome duplication, with up to 30% of the zebrafish genome being duplicated, including other cytochromes P450 involved in steroid biosynthesis [44]. Zebrafish CYP11A1 and CYP11A2 share 80% amino acid sequence identity. Zebrafish CYP11A2 has higher amino acid sequence homology than CYP11A1 to other vertebrates, including higher homology to other teleosts (72–73% identity versus 64–66% identity for zebrafish CYP11A1). Zebrafish CYP11A2 appears to be the functional equivalent of CYP11A1 in other vertebrates, being expressed in the interrenal gland, gonads, and brain. Zebrafish CYP11A1 is expressed in the early embryo and only in the gonads of the adult [44].
Despite the ever increasing number of CYP11A1 sequences from different vertebrate species being reported, little is known about any differences in function of the enzyme between species, particularly catalytic properties. All are assumed to convert cholesterol to pregnenolone to initiate de novo steroidogenesis. Non-mammalian forms of the enzyme are particularly poorly characterized. The conversion of cholesterol to pregnenolone by mitochondria isolated from the testis of brook trout (Salvelinus fontinalis) has been reported [45]. Rainbow trout (O. mykiss), Japanese eel (Anguilla japonica), and chicken CYP11A1 have been expressed in COS cells and shown to convert 25-hydroxycholesterol to pregnenolone [33,46,47]. Knockdown of CYP11A2 expression in zebrafish was shown to reduce both pregnenolone and cortisol concentrations. Only human and bovine CYP11A1 have been purified, from both steroidogenic tissues and following expression in Escherichia coli, and their catalytic properties fully compared [1,14,48,49]. These forms share 72% amino acid identity [42] and do show some differences in catalytic properties. They display similar Km values with cholesterol as substrate. The values of Kcat vary depending on the source of the enzyme and the purification procedure employed making it difficult to accurately compare these parameters [14,48,49]. A clear difference between these species is that human CYP11A1 shows poor catalytic efficiency for the metabolism of 25-hydroxycholesterol with its Kcat/Km value being 6.6-fold lower than that for the bovine enzyme [48].
2. 7DHC as a substrate for CYP11A1
2.1. Overview of cholesterol synthesis
Cholesterol balance is maintained by biosynthesis in the liver and extrahepatic tissues (the endogenous pathway) (Fig. 1) [50,51] and through absorption of dietary and biliary cholesterol (the exogenous pathway). Cholesterol biosynthesis can be divided into pre- and post-squalene pathways.
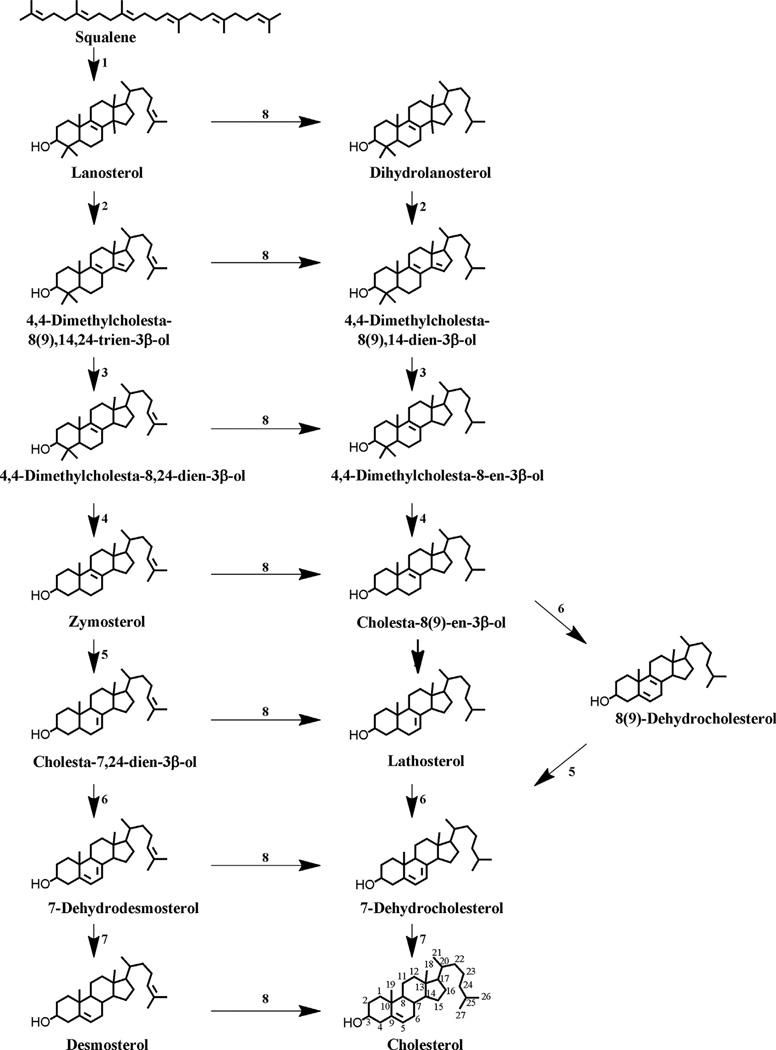
Fig. 1. Cholesterol synthesis: the later steps in the pathway.
Enzymes: 1, squalene monooxygenase, and squalene cyclase; 2, lanosterol 14-α-demethylase; 3, 3β-hydroxysterol Δ14-reductase; 4, C4 demethylation complex (C4-sterol methyloxidase, C4-sterol decarboxylase [NSDHL], and 3-ketoreductase); 5, 3β-hydroxysterol Δs,Δ7-isomerase; 6, lathosterol 5-desaturase; 7, 3β-hydroxysterol Δ7-reductase; 8, 3β-hydroxysterol Δ24-reductase. See [50,51] for additional detail.
2.1.1. Pre-squalene pathway of cholesterol synthesis
Synthesis of the 27 carbon cholesterol molecule includes approximately 30 enzymatic reactions with all of the carbon atoms originally derived from the two-carbon acetate group of acetyl-CoA. Synthesis of 3-hydroxy-3-methylglutaryl–CoA (HMG–CoA) is catalyzed by HMG-CoA synthase with acetyl-CoA and acetoacetyl-CoA serving as substrates. HMG–CoA is converted to mevalonate by membrane-bound HMG–CoA reductase, which is the rate-limiting and irreversible step of cholesterol biosynthesis. Mevalonate is then activated by two successive phosphorylations, yielding 5-pyrophosphomevalonate. Mevalonate is decarboxylated to isopentenyl pyrophosphate, an activated isoprenoid molecule which is in equilibrium with its isomer, dimethylallyl pyrophosphate. One molecule of isopentenyl pyrophosphate condenses with one molecule of dimethylallyl pyrophosphate to form geranyl pyrophosphate. This further condenses with another isopentenyl pyrophosphate molecule to yield farnesyl pyrophosphate. Two molecules of farnesyl pyrophosphate then condense to generate squalene through the action of squalene synthase in the endoplasmic reticulum.
2.1.2. Post-squalene pathway of cholesterol synthesis
Squalene is converted into the first sterol, lanosterol (4,4,14α-trimethylcholesta-8(9), 24-dien-3β-ol), by squalene epoxidase and oxidosqualene cyclase. Lanosterol is converted to cholesterol through a multistep process including the oxidative removal of three methyl groups at C4α, C4β, and C14, which converts the C30 molecule lanosterol to C27 cholesterol; isomerization of the Δ8(9) double bond to a Δ7 double bond; desaturation to form a Δ5 double bond; and finally, reduction of Δ14, Δ24, and Δ7 double bonds [52].
The saturation of the C-24 double bond of lanosterol can occur at multiple points in the pathway, creating two immediate precursors for cholesterol, desmosterol [cholesta-5(6), 24-dien-3β-ol] and 7-dehydrocholesterol [cholesta-5,7-dien-3β-ol)], (7DHC) [50,53,54]. The final steps in cholesterol biosynthesis might be tissue specific [55]. In both alternative pathways, lanosterol is demethylated to form zymosterol (5α-cholesta-8,24-dien-3β-ol) in a series of enzymatic reactions catalyzed by lanosterol 14-α-demethylase [CYP51A1] (2 in Fig. 1); 3β-hydroxysterol Δ14-reductase (3 in Fig. 1) and C4 demethylation complex (4 in Fig. 1). This complex consists of threeenzymes (C4-sterol methyloxidase, C4-sterol decarboxylase (3β-hydroxysterol dehydrogenase) [NSDHL], and 3-ketoreductase) that catalyse the demethylation of 4,4-dimethylcholesta-8-en-3β-ol and 4,4-dimethylcholesta-8,24-dien-3β-ol (Fig. 1). If reduction of the Δ24-bond occurs early, cholesterol is synthesized via the Kandutsch–Russel pathway (favored in most tissues) using 7-dehydrocholesterol as the immediate precursor of cholesterol. In this pathway zymosterol is metabolized sequentially by a 3β-hydroxysterol Δ24-reductase (8 in Fig. 1), 3β-hydroxysterol Δ8, Δ7-isomerase (5 in Fig. 1), and lathosterol 5-desaturase (6 in Fig. 1) to yield 7-dehydrocholesterol, which is reduced by the 3β-hydroxysterol Δ7-reductase (7 in Fig. 1) at the Δ7 position to form cholesterol (Fig. 1).
In the Bloch pathway reduction of the Δ24-bond occurs as the last enzymatic step that forms cholesterol from desmosterol. Zymosterol is converted to 7-dehydrodesmosterol by the 3β-hydroxysterol Δ8, Δ7-isomerase (5 in Fig. 1) and lathosterol 5-desaturase (6 in Fig. 1). 7-Dehydrodesmosterol is metabolized to desmosterol by the 3β-hydroxysterol Δ7-reductase (7 in Fig. 1) and then by the 3β-hydroxysterol Δ24-reductase (8 in Fig. 1) to form cholesterol (Fig. 1).
2.2. In vitro and in vivo models of 7DHC metabolism by CYP11A1
7DHC was first identified as a substrate for purified bovine CYP11A1 by Guryev et al. [22]. Subsequent studies performed with purified bovine and human recombinant CYP11A1 and isolated mitochondria from the adrenal glands and placentae have demonstrated the following pathway for the metabolism of 7DHC: 7DHC→22-hydroxy-7DHC[22(OH)7DHC]→20,22-dihydroxy-7DHC[20,22(OH)27DHC→7DHP] [8,14,22,23]. In fact, human CYP11A1 has a slightly higher catalytic efficiency with 7DHC as a substrate (higher Kcat/Km) than with cholesterol, indicating that 7DHC can be used preferentially by the enzyme depending on its availability [8,14]. The rate of 7DHC transfer to the inner mitochondrial membrane site of CYP11A1 action by the StAR protein is also slightly higher than that for cholesterol [8]. The definitive chemical structures of the intermediates of 7DHC metabolism by CYP11A1 (22(OH)7DHC and 20,22(OH)27DHC), and the final product (7DHP), were established by NMR spectrometry, UV spectra, mass spectrometry, and chemical synthesis of the standards [8,14,22,56,57].
Further support for the above pattern of 7DHC conversion to 7DHP came from molecular modeling using the crystal structure of human CYP11A1 bound to 20R,22R-dihydroxycholesterol [8]. 7DHC was docked to the crystal structure of CYP11A1 in place of the 20R,22R-dihydroxycholesterol. It showed that the C22 hydrogen was closer to the heme iron than the hydrogen at C20, consistent with initial hydroxylation at C22 being favored. Consistent with the above sequence of reactions, docking scores were highest for the 22-hydroxyderivatives of both 7DHC and cholesterol, with the 20,22-dihydroxyderivatives having intermediate docking scores, however, higher than for cholesterol or 7DHC [8]. This modeling is consistent with the known relative binding strengths for cholesterol, 7DHC and their intermediates, and with the direct coordination of the 22R-hydroxyl group to the heme iron [6,7].
It should be noted that we also detected production of two other hydroxy-7DHC metabolites by human CYP11A1 of which the identity remains to be established [8]. Neither of them corresponded to 20 (OH)7DHC as demonstrated by their different retention times. It is expected that the hydroxyl groups are added to the side chain, most likely at positions C23 and C17 since these represent known sites of vitamin D3 hydroxylation by CYP11A1 [25].
Experiments performed with adrenal glands isolated from both males and females of rats, pigs, rabbits, and from male dogs showed that adrenal fragments incubated ex-vivo with 7DHC transform it in a sequential manner to 7DHP, with 22(OH)7DHC and 20,22 (OH)27DHC serving as consecutive intermediates of the pathway [23]. The involvement of CYP11A1 in this transformation was confirmed by the inhibitory effect of dl-aminoglutethimide. The metabolism of 7DHC was stimulated by forskolin indicating the involvement of cAMP dependent pathways, as seen for cholesterol [23]. Studies with purified mitochondria from rat skin also revealed the transformation of exogenous 7DHC to 7DHP, consistent with the known expression of CYP11A1 in skin [23]. The most recent and extensive studies performed on human placentae and bovine adrenal glands, clearly confirmed the above studies and provided additional evidence that CYP11A1-dependent 7DHC metabolism is possible in the human placenta in vivo [8]. Final in vitro evidence that 7DHC is metabolized to 7DHP in the skin was obtained using cultured human (HaCaT) epidermal keratinocytes, and primary pig epidermal keratinocytes where products were detected without the addition of exogenous 7DHC [8].
The above findings are consistent with a well described phenomenon of increased accumulation of 7DHC, and production of 7DHP and its downstream steroid metabolites in Smith-Lemli-Opitz syndrome (SLOS), characterized by a deficiency of 7DHC Δ-reductase [58–61]. Similarly, in vivo production of 7DHP occurs during synthesis of equilin (Δ7-estrone) in horses [62]. The production of 7DHP in SLOS and during the synthesis of equilin is attributed to CYP11A1 action on 7DHC [61]. 7DHP may then undergo sequential transformation to the hydroxy-derivativesof 5,7-steroidal dienes or 7-dehydroprogesterone by classical steroidogenic enzymes, as demonstrated by accumulation of 7DHP, its hydroxy-derivatives(including 21-hydroxy-, 17-hydroxy-, 20-hydroxy-, and 17,20-dihydroxy-7DHP) and 7-dehydroprogesterone in body fluids of SLOS patients [60,63,64]. Further support for 7DHP serving as a substrate for CYP17A1 is the production of 17(OH)7DHP and Δ7-dehydroepiandrosterone [65]. CYP21A2 does not appear to use 7DHP as a substrate, similar to its inability to act on pregnenolone [65]. In accordance with this, our studies with pig adrenal glands showed the transformation of 7DHP to 17(OH)7DHP during ex-vivo incubations, but 21(OH)7DHP could not be detected [23]. Ex-utero incubations of human placentae [8] and ex-vivo incubations of pig adrenal glands [23] with 7DHP resulted in its transformation to 7-dehydroprogesterone. This finding was further supported by incubations with partially purified 3βHSD [23] and placental microsomes where 7-dehydroprogesterone was detected and sufficient of this product made to confirm its structure by NMR [8]. Additional products of the placental metabolism of 7DHP included 20-hydroxypregnenolone [8]. Incubation of 7DHP with skin microsomes generated two new products which likely had a 4,6-diene structure [23]. Details of the metabolism of 7DHP in skin cells represents an exciting area for future research since this organ shows steroidogenic activity and expresses the major classical steroidogenic enzymes [15,66] that can be regulated locally by endogenous hormonal signals [10,67]. Furthermore, Δ7-steroids produced in the skin may undergo photochemical transformation after exposure to the UVB, to the corresponding secosteroids as originally proposed [14], with further in vitro experiments and detailed discussion presented in [56,57,68,69].
3. Diversity in sterol substrates acted on by CYP11A1
3.1. Cholesterol esters
Cholesterol is stored esterified to fatty acids with some cholesterol also undergoing sulfation. Cholesterol sulphate is reported to have some regulatory activities, including promoting keratinocyte differentiation in the skin [70]. Human and bovine CYP11A1 have been shown to cleave the side chain of cholesterol sulfate, with lower catalytic efficiency than cholesterol for the purified enzymes [71], but at higher rates than cholesterol by CYP11A1 in ovarian mitochondria, due to its more rapid movement to the inner mitochondrial membrane [72]. Thus, pregnenolone sulfate which is an active neurosteroid in the brain [73], may be produced by side chain cleavage of cholesterol sulfate by CYP11A1 rather than by sulfation of pregnenolone. Purified CYP11A1 can also act on short chain cholesterol esters converting them to their corresponding pregnenolone esters, but activity is very low when the chain length exceeds 4 carbons [71].
3.2. Lumisterol 3
Recently, it has been reported that purified bovine and human CYP11A1 can metabolize lumisterol 3 with a catalytic efficiency approximately 20% of that for cholesterol [31]. Lumisterol 3 is produced in the skin by photochemical transformation of pre-vitamin D3 during prolonged periods of UV exposure (see Section 4.1). The reaction involves reformation of the intact B ring with C9 and C10 in a 9β,10α-configuration, making it a stereoisomer of 7DHC [74,75]. CYP11A1 initially hydroxylates lumisterol 3 at C22 or C24. 22-Hydroxylumisterol is then hydroxylated at C20 producing 20,22-dihydroxylumisterol 3 and some of this undergoes hydroxylation at other sites while some undergoes cleavage to pregnalumisterol [31]. Fragments of pig adrenal glands incubated with lumisterol also produce these products suggesting that this CYP11A1-catalysed reaction can occur in vivo. Pregnalumisterol and some synthetic hydroxylumisterol 3 derivatives exhibit biological activity [76,77] further suggesting that metabolism of lumisterol by CYP11A1 in the skin may produce metabolites with physiological activity.
3.3. Hydroxysterols and plant sterols
Purified bovine and human CYP11A1 and steroidogenic mitochondria can act on a number of other naturally occurring sterols besides cholesterol and its precursor, 7DHC. These include cleaving the side chains of 24-hydroxycholesterol, 25-hydroxycholesterol, and 27-hydroxycholesterol (discussed above) [16,17,19,48]. These sterols are produced from the metabolism of cholesterol by CYP46A1, cholesterol 25-hydroxylase, and CYP27A1, respectively, as intermediates in bile acid synthesis, but some are released into the bloodstream [78]. Desmosterol (5,24-cholestadien-3β-ol, see Fig. 1), which has recently come to prominence, is also converted to pregnenolone by bovine, rat, and human CYP11A1 [16,17,19], with similar catalytic efficiency to that seen for cholesterol [48]. Regulated accumulation of desmosterol appears to play a homeostatic and anti-inflammatory role in respect to macrophage activation in atherosclerotic lesions [79] and also plays a role in epidermal barrier function in the skin [80–82], an organ expressing CYP11A1 [14,83].
Human and bovine CYP11A1 can also cleave the side chain of the plant sterols, campesterol (24α-methylcholesterol), and β-sitosterol (24β-ethylcholesterol) producing pregnenolone [19], with catalytic efficiencies comparable to cholesterol for campesterol and 5–10 fold less for β-sitosterol [48]. These sterols are available through the diet of mammals although the quantitative importance of their metabolism in vivo remains to be established.
3.4. Ergosterol
Purified human CYP11A1 acts on the fungal membrane sterol, ergosterol, also available through the diet. No cleavage of the side chain occurs due to the presence of the double bond at C22. Rather, the C22=C23 double bond undergoes epoxidation to produce 20-hydroxy-22,23-epoxy-22,23-dihydroergosterol. The other major product is 22-keto-23-hydroxy-22,23-dihydroergosterol. The catalytic efficiency for the metabolism of ergosterol by human CYP11A1 is the same as that for cholesterol [10]. Bovine CYP11A1 also acts on ergosterol but the product profile varies compared to that seen for human CYP11A1, with major products including 24-hydroxyergosterol and 17α,24-dihydroxyergosterol [30]. 17α,24-Dihydroxyergosterol is biologically active on cultured human skin cells, inhibiting DNA synthesis, but the other products remain to be tested, as does any in vivo function.
4. Vitamins D3 and D2 as the substrates for CYP11A1
4.1. Photobiology of vitamin D
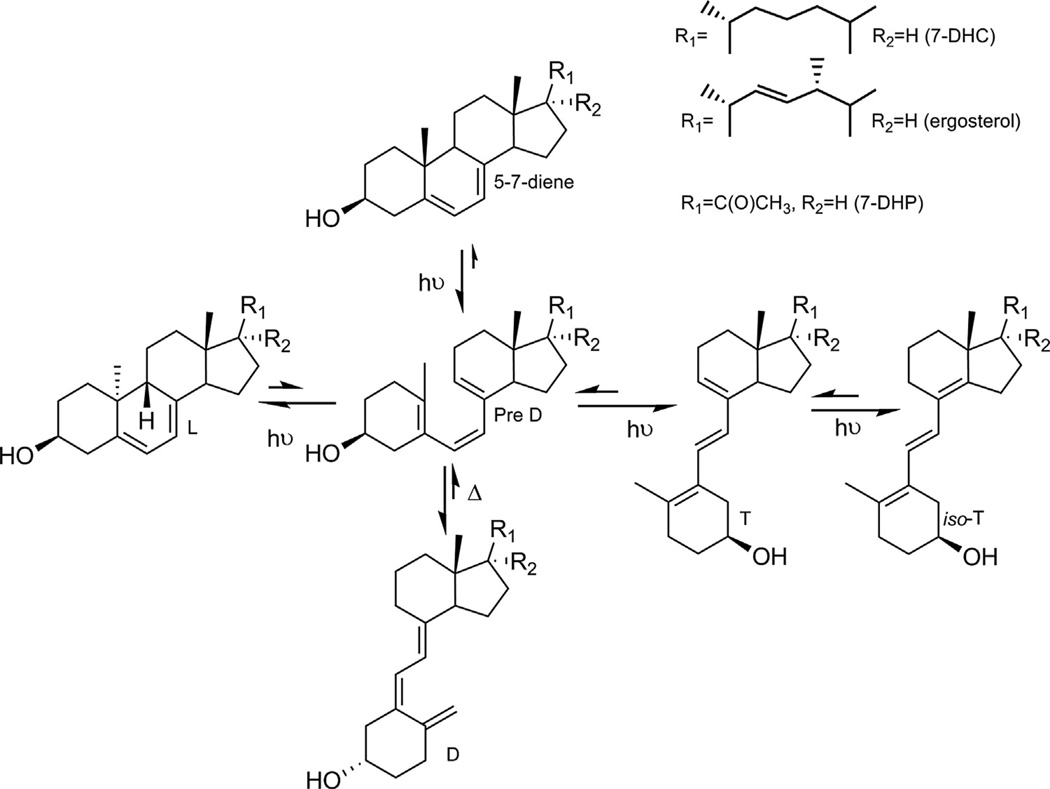
The vast majority of vitamin D3 (D3) in humans is produced in the skin after absorption of UVB energy by the B ring of 7DHC leading to its opening to produce the pre-vitamin D3 intermediate which then undergoes a temperature-dependent isomerization to vitamin D3. Further, UV irradiation of pre-vitamin D3 results in the formation lumisterol 3, tachysterol 3, and isotachysterol 3 [84–86] (Fig. 2). Similarly, vitamin D2 (D2), lumisterol 2, tachysterol 2, and isotachysterol 2 are produced by the action of UVB on ergosterol derived from fungi and phytoplankton [85,87] (Fig. 2). The relative photochemical production of the above steroids and secosteroids depends on UVB energy, temperature, and physicochemical environment.
Fig. 2. UVB-induced photolysis of 7-DHC, ergosterol and 7-DHP.
D, T, and L represent vitamin D, tachysterol or lumisterol compounds. Note that UVB transformation of 7DHC is similar to that described for 7DHP [56,124].
Until recently, it was widely believed that D3 and D2 were solely activated as follows:
D3/D2 → 25(OH)D3/D2 → 1,25(OH)2D3/D2
through the sequential hydroxylations by CYP27A1 or CYP2R1 on C25, and by CYP27B1 on C1 [85,88–97]. At the systemic level, 25 and 1α-hydroxylations take place in liver and kidney, respectively. However, both reactions occur in the epidermis to produce fully active 1,25-dihydroxyvitamin D (1,25 (OH)2D) [87,98,99]. 1,25(OH)2D is inactivated by CYP24A1 by hydroxylation at C24 with further oxidation to calcitrioic acid [100–102].
Recently, we discovered that the CYP11A1 hydroxylates the side chain of D3 and D2 in a sequential fashion:
D3/D2 → 20S(OH)D3/D2 → (OH)nD3/D2
The products can be further hydroxylated by CYP27B1, CYP27A1, and CYP24A1 (reviewed in [103], and see below), which is an alternative to the classical pathway (see above), although its physiological importance remains to be established.
4.2. Hydroxylation of vitamin D3 by CYP11A1
4.2.1. In vitro models
Initial analyses using purified bovine CYP11A1 demonstrated that it could hydroxylate the side chain of D3 producing 20(OH) D3 and 20,22(OH)2D3 without cleavage of the side chain occurring [22,24]. Adrenal mitochondria were also shown to produce 20(OH) D3 as the major product and several additional hydroxyderivatives of which 3 of the major ones apparently resulted from the action of CYP11A1 [24]. Subsequent enzymatic analysis revealed that CYP11A1 favors initial hydroxylation of D3 at C20 over C22 (or C23), with 20(OH)D3 representing the first and major metabolite. The absolute configuration of this compound was defined as 20S(OH)D3 by chemical synthesis and NMR analysis [10,22,24,25,103,104]. Support for the initial hydroxylation occurring at C20 was provided by docking of vitamin D3 into the catalytic site of the crystal structure of human CYP11A1 which predicted that C20 is located closer to the he me iron than C22 [7]. However, the conversion of vitamin D3 to 20(OH)D3 by CYP11A1 is less efficient (lower Kcat/Km) than hydroxylation of cholesterol by this enzyme [25], supported by molecular modeling [7], with bovine CYP11A1 metabolizing vitamin D3 more efficiently than the human enzyme [7,25,27]. The structural differences that mediate these differences in catalytic efficiency remain to be established. Nevertheless, CYP11A1 can hydroxylate D3 at C22 to produce 22S (OH)D3 as a minor product, with the structure of this metabolite being confirmed by NMR [26]. The preferred second site of hydroxylation of 20(OH)D3 is at C23 producing 20,23(OH)2D3 as the second major product of the reaction [25]. 20(OH)D3 is also hydroxylated at C22 and C17 to produce 20,22(OH)2D3, 17,20 (OH)2D3, and 17,20,23(OH)3D3 as metabolites [26]. These studies defined the conversions:
D3→20(OH)D3→20,23(OH)2D3→17,20,23(OH)3D3
as the major pathway of vitamin D3 metabolism by CYP11A1 with
D3 → 20(OH)D3 + 22(OH)D3 → 20,22(OH)2D3
as a minor pathway [103]. There is some flexibility in the order of the observed hydroxylations at C17, C20, C22, and C23 catalyzed by CYP11A1 creating other minor pathways [24,25,103,105].
Importantly, CYP11A1 does not metabolize 25-hydroxyvitamin D3 indicating that hydroxylation at C25 protects the secosteroid against hydroxylation of the side chain by CYP11A1 [24] and therefore, that this enzyme does not compete with CYP27B1 for activation of 25(OH)D3 [103]. Interestingly, CYP11A1 can hydroxylate the biologically inert prodrug, l(OH)D3, to the biologically active 1,20(OH)2D3, which could serve as an alternative route to 25-hydroxylation for activation of l(OH)D3 [106]. The same reaction is mediated by placental mitochondria [27].
Interestingly, 20(OH)D3 can be metabolized by recombinant human CYP27A1 and rat CY24A1 [107,108], with production of 20,25(OH)2D3 and 20,26(OH)2D3 by CYP27A1 and production of 20,24(OH)2D3 (major product) and 20,25(OH)2D3 (minor product) by CYP24A1. Structures of these compounds were determined by NMR and MS. Other minor dihydroxy-derivatives of which structures remain to be determined were also produced. 20,24(OH)2D3, 20,25(OH)2D3, and 20,26(OH)2D3 can also be 1α-hydroxylated by CYP27B1 with a catalytic efficiency much higher than for the parent 20(OH)D3 [109]. In addition, CYP27B1 can 1α-hydroxylate 20,23(OH)2D3 to produce 1,20,23(OH)2D3 and 20(OH)D3 to produce 1,20 (OH)2D3 [109,110].
4.2.2. In vivo models
Most importantly, the in vivo studies represented by ex-vivo and ex-utero incubations of vitamin D3 with fragments of adrenal glands and human placentae, respectively, clearly demonstrate the CYP11A1-catalyzed metabolism of vitamin D3 with 20(OH) D3 being the major metabolite. Less abundant products included 22(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3, and 17,20,23(OH)3D3 [27]. Both tissues were also shown to express CYP27A1, CYP2R1, and CYP27B1, and to convert vitamin D3 to 25(OH)D3, and 25(OH) D3 to 1,25(OH)2D3 [27]. This allowed us to conclude that the adrenal gland and placenta (tissues demonstrating high activity of CYP11A1) metabolize D3 by both the classical pathway:
D3→25(OH)D3→1,25(OH)2D3
and the CYP11A1-dependent pathway:
D3→20(OH)D3 + 22(OH)D3→20,22(OH)2D3 + 20,23(OH)2D3→17,20,23(OH)32D3.
Interestingly, the adrenal gland and placenta supplied with exogenous vitamin D3 showed higher production of 20(OH) D3 than 25(OH)D3. Detection of a monohydroxyvitamin D3 compound with a retention time corresponding to 20(OH) D3 in human serum, albeit at a level 20 times lower than that of 25 (OH)D3, indicates that 20(OH)D3 produced in these organs can enter the systemic circulation [27]. It must be noted that CYP27B1-dependent 1α-hydroxylation of 20(OH)D3 and 20,23 (OH)2D3 was observed in the placenta and adrenals producing 1,20 (OH)2D3 and 1,20,23(OH)3D3, respectively [27]. However, we did not detect 1,17,20(OH)3D3 or 1,17,20,23(OH)4D3 [27], consistent with enzymatic analyses showing that products with a 17α-hydroxyl group such as 17,20(OH)2D3 and 17,20,23(OH)3D3 are not substrates for CYP27B1 [109].
We also detected CYP11A1-dependent metabolism of D3 in cells with a relatively low expression of this enzyme such as human and pig epidermal keratinocytes [27], and colonic Caco-2 cells [103]. Thus, keratinocytes transformed D3 to 20(OH)D3, 22(OH) D3, 20,23(OH)2D3, 20,22(OH)2D3, and 17,20,23(OH)3D3, with CYP27B1-dependent production of 1,20(OH)2D3 and 1,20,23 (OH)3D3 also being observed [27]. Interestingly, skin cells showed slightly higher production of 22(OH)D3 and similar or higher production of 1,25(OH)2D3 in comparison to 20,22(OH)2D3 and 20,23(OH)2D3. Also, endogenous production of 20(OH)D3, 25(OH) D3, 22(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3, 1,25(OH)2D3, and 17,20,23(OH)3D3 was detected in HaCaT keratinocytes cultured in the presence of 5% serum without additional supplements of D3 [27]. Caco-2 cells also metabolized exogenous vitamin D3 to 20 (OH)D3 as the predominant metabolite, with lesser production of 22(OH)D3 and 25(OH)D3. These were further metabolized to 20,23 (OH)2D3, 1,20(OH)2D3, and 1,25(OH)2D3 [103]. These data indicate that organs expressing lower levels of CYP11A1 such as skin [15,66] and gastrointestinal (GI) tract [111] can metabolize D3 through the novel CYP11A1-initiated pathway, as an additional and alternative pathway to the classical one.
4.3. Metabolism of vitamin D2 by CYP11A1
4.3.1. In vitro models of D2 metabolism
Purified bovine CYP11A1 and adrenal mitochondria hydroxylate the side chain of vitamin D2 without its cleavage, which starts with hydroxylation at C20:
A similar sequence of reactions was seen for recombinant human CYP11A1 [9]. The major product was 20(OH)D2 with lower production of 17,20(OH)2D2 and 17,20,24 (OH)3D2 and another unidentified D2 metabolite [28,29]. The structure of the above products was defined by NMR. Consistent with the above data were molecular modeling studies using the crystal structure of human CYP11A1 bound to cholesterol [7], which predicted that D2, 20(OH)D2, and 17,20 (OH)2D2 were able to bind to the enzyme with similar docking scores (binding affinity) to cholesterol [9]. In addition, the distances from C22 and C20 to the heme iron for D2 and 20(OH) D2 were similar, and this plus the presence of the C22=C23 double bond predicted that the first hydroxylation would occur at C20 [9].
Although the side chains of D2 and ergosterol are identical, no epoxidation of the double bond between C22 and C23 occurs for D2, indicating its different positioning in the active site. CYP27B1 also 1α-hydroxylates 20(OH)D2 producing 1,20 (OH)2D2, with an efficiency much lower than for the conversion of 25(OH)D2 to 1,25(OH)2D2 [112]. In addition, the prodrug, 1α (OH)D2, could be hydroxylated by recombinant human CYP11A1 to 1,20(OH)2D2 [9].
4.3.2. In vivo models of D2 metabolism
Importantly, using human placentas and bovine adrenals which express high levels of CYP11A1 [1,2], and human epithelial cells (epidermal keratinocytes and colonic Caco-2 cells) which express relatively low levels of this enzyme [14,15,111,113] we were able to demonstrate the in vivo (ex-vivo, ex-utero, and in cultured cells) transformation of D2 to 20(OH)D2,17,20(OH)2D2, and 1,20(OH)2D2 [9]. The involvement of CYP11A1 in the conversion of D2 to 20(OH) D2, 20(OH)D2 to 17,20(OH)2D2, and the prodrug 1α(OH)D2 to 1,20 (OH)2D2 was demonstrated by its almost complete inhibition by 22R-hydroxycholesterol when placental and adrenal mitochondria were used to run the reaction [9]. Placentae ex-utero, as well as metabolizing vitamin D2 via CYP11A1, also converted D2 to 25(OH) D2 [9]. Placentae ex-utero and mitochondria isolated from adrenal glands not only 1α-hydroxylated 25(OH)D2 to 1,25(OH)2D2, but also 20(OH)D2 to 1,20(OH)2D2 [9].
Notably, the amount of 20(OH)D2 produced from D2 was higher than that of the initial product of the classical activation pathway, 25(OH)D2, in placenta and Caco-2 cells, but similar or lower than for 25(OH)D3 in human epidermal keratinocytes. The present data clearly show that after reaching these cells or tissues, D2 can be transformed in a sequential manner to 20(OH)D2 and 17,20 (OH)2D2 through the action of CYP11A1. Similarly the prodrug, 1α (OH)D2, is transformed to 1,20(OH)2D2. The latter is consistent with the ability of purified recombinant human CYP27B1 to 1α-hydroxylate 20(OH)D2 [112].
Epithelial cells expressing low CYP11A1 activity such as epidermal keratinocytes [14,83] and Caco-2 cells [111,113] metabolized D2 through the classical (25-hydroxylase-dependent) and CYP11A1-dependent pathways in a cell type dependent manner [9]. There was significantly higher production of 20(OH)D2 than 25(OH)D2 in Caco-2 cells that was opposite to what was observed in epidermal keratinocytes [9]. Also, production of 20(OH)D2 and 17,20(OH)2D2 was higher in Caco-2 cells than in HaCaT keratinocytes [9], but higher production of 1,20(OH)2D2 was seen in HaCaT keratinocytes [9]. In summary, ex-vivo, ex-utero, and cell culture studies indicate that D2 can be metabolized in vivo in tissues expressing CYP11A1, with further modification of products by CYP27B1, with metabolite profiles that are tissues- and cell-type specific [9]. The physiological role of these new pathways remain to be established, but as for the corresponding D3 metabolites, products display biological activity when applied under in vitro or in vivo conditions (discussed in Section 5).
4.4. Other potential substrates for CYPUA1
Analysis of the cholesterol biosynthetic pathway suggests that many other of its later intermediates besides 7DHC and desmosterol that have a steroidal ring, could serve as substrates for CYP11A1 (Fig. 1). Also, other phototransformation products of 7-DHC (Fig. 2) such as tachysterol could serve as the substrates for CYP11A1. Therefore, we carried out molecular modeling studies using the crystal structures of bovine and human CYP11A1 [6,7]. We observed excellent docking scores for several compounds listed in Table 1 that were comparable to cholesterol or hydroxycholesterol substrates. The docking scores for ergosterol, 7DHC, desmosterol, lumisterol, and vitamins D3 and D2 are consistent with experimental data presented earlier showing that they are substrates for CYP11A1. An excellent docking score for 8-dehydrocholesterol in conjunction with the presence of Δ8-steroids in body fluids of SLOS patients [60,61,63,64], predicts that it is also a substrate for CYP11A1 that undergoes cleavage of its side chain similar to 7-DHC and cholesterol. Interestingly, 7-dehydrodesmosterol and lathosterol intermediates of the cholesterol biosynthetic pathway, as well as tachysterol which is a product of UVB transformation of pre-D, have excellent docking scores suggesting that they could also serve as substrates for CYP11A1 in tissues expressing this enzyme and producing sufficient concentrations of these compounds. The challenge ahead is to test experimentally whether compounds listed in Table 1 or additional intermediates of the cholesterol biosynthetic pathway or other photoproducts of UV induced transformation of 7DHC (Fig. 2) are indeed metabolized by CYP11A1.
Table 1.
Docking scores of compounds binding to the catalyst site of human or bovine CYP11A1 crystal structures.
| Compound name | Chemical structure | Docking score |
|
|---|---|---|---|
| Human CYP11A1 (PDB entry: 3N9Y) |
Bovine CYP11A1 (PDB entry: 3MZS) |
||
| Ergosterol |  |
−11.97 | −11.61 |
| 22-Hydroxy cholesterol |  |
−11.94 | −10.95 |
| 20-Hydroxy cholesterol | 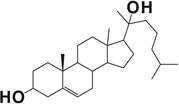 |
−11.68 | −10.00 |
| Cholesterol | 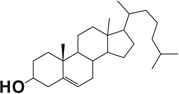 |
−11.67 | −11.12 |
| 8(9)-Dehydrocholesterol | 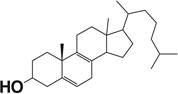 |
−11.59 | −11.02 |
| Desmosterol | 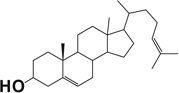 |
−11.54 | −10.95 |
| 7-Dehydrodesmosterol | 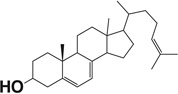 |
−11.41 | −11.13 |
| Lumisterol 3 | 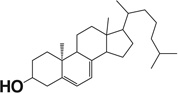 |
−11.20 | −10.85 |
| Lathosterol | 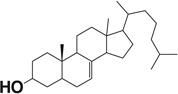 |
−11.18 | −11.04 |
| Vitamin D3 | 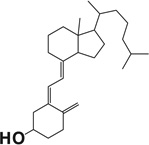 |
−11.06 | −10.59 |
| Vitamin D2 | 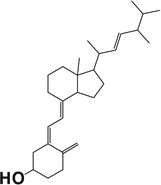 |
−10.94 | −11.00 |
| 7-Dehydrocholesterol | 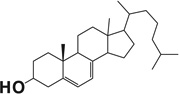 |
−10.67 | −10.18 |
| Tachysterol 3 | 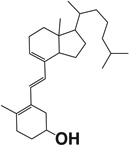 |
−10.39 | −10.67 |
Docking methodology description.
The molecular modeling studies were performed with Schrodinger Molecular Modeling Suite 2014 (Schrodinger LLC, New York, NY) as previously described [8,9]. All the compounds were built and prepared by the Ligprep module to generate at most 20 conformations of each ligand. The crystal structures of human CYP11A1 (PDB entry: 3N9Y) or bovine CYP11A1 (PDB entry: 3MZS) were imported into the Protein Preparation Wizard to correct problems including the missed hydrogen atoms and incomplete side chain and loops in the original PDB files. Next, the grids of catalytic sites (where the native ligand binds with enzyme) in the prepared CYP11A1 structures were generated. Then the compounds were docked into the CYP11A1 catalytic site by the Glide module. The best docking complexes were subject to restricted molecular dynamics to release any strains under OPLS-2005 force field.
5. Concluding remarks
The data reviewed above clearly show that purified CYP11A1, or CYP11A1 in isolated mitochondria or tissue fragments supplied with exogenous substrate, can act on a range of steroids other than cholesterol. Alternative substrates include cholesterol precursors (7DHC and desmosterol), hydroxycholesterols, plant sterols, ergosterol, lumisterol, and vitamins D3 and D2. Thus, the active site of CYP11A1 can accommodate a range of steroids but it should be emphasized that they are all closely related to cholesterol in structure, with the biggest structural variation being for the secosteroids which contain a broken B-ring. The structure of the substrate affects both the regio-selectivity of hydroxylation and the catalytic efficiency. Cleavage of the side chain usually occurs when only the side chain is modified from that found in cholesterol and the ring system is not, as seen for desmosterol, campesterol (24α-methylcholesterol), β-sitosterol and various side chain hydroxylated cholesterol derivatives. However, 7DHC with an unmodified side chain and a Δ7 double bond in the B-ring is a clear exception to this as it undergoes cleavage with high catalytic efficiency. Despite the presence of a Δ7 double bond in ergosterol it does not undergo cleavage due to the C22=C23 double bond in the side chain making it unfavorable to undergo hydroxylation at C22. Photochemical opening of the B-ring of 7DHC, as seen in D3 (Fig. 3), prevents side chain cleavage from occurring, even when the pattern of hydroxylation would seem to make it possible as no cleavage of 20,22(OH)2D3 is observed.
Importantly, in vitro studies with exogenously added compounds reveal that novel CYP11A1-derived secosteroids and Δ7steroids exerts anti-proliferative, pro-differentiation, and anti-inflammatory effects on cultured skin cells, which are dependent on the structure of the compound and the cell lineage [57,104,112,114–119]. Pharmacologically administered 20(OH) D3 shows antifibrotic activities both in vitro [57,117,118] and in vivo in bleomycin induced scleroderma [118]. It suppresses in vivo collagen-induced arthritis (CIA) in a mouse model [103]. It also shows anti-cancer and anti-tumor activities that are dependent on the cell-type lineage [57,77,112,119–121]. Importantly, the novel secosteroids in addition to acting as biased agonists on the vitamin D receptor (showing selectivity for certain receptor mediated effects) [103,112,122], can also interact with RORα and γ, presumably acting as inverse agonists [123].
A future challenge in this field is a quantitative assessment of the degree of in vivo metabolism of endogenous sources of these alternative substrates for CYP11A1, and to determine whether their metabolites are produced at sufficient levels to exert physiological effects. Studies of SLOS patients clearly show that under pathological conditions 7DHC can serve as a substrate for CYP11A1, with downstream Δ7 steroidal products possibly being responsible for some of the disease symptoms [58–61,63,64]. Tissue specific knockouts of CYP11A1, particularly in skin, are required to define whether metabolism of 7DHC and vitamin D are of physiological importance.
In summary, CYP11A1 shows considerable flexibility with respect to substrate specificity acting on a range of naturally occurring steroid molecules other than cholesterol. It can generate several intermediates and products that are biologically active in a context dependent manner when administered exogenously, but for which physiological roles remain to be defined.
Acknowledgments
This work was supported by NIH grants R01AR052190, R02AR052190, R21AR066505-01A1 and 1R01AR056666-01A2 to AS, 1R21AR063242-01A1, 1S10RR026377-01, and 1S10OD010678-01 to WL, by the University of Western Australia and by the College of Pharmacy at the University of Tennessee Health Science Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest
The authors declare no conflict of interest
References
- 1.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein S, Middleditch BS, Gut M. Mass spectrometric study of the enzymatic conversion of cholesterol to (22R)-22-hydroxycholesterol, (20R,22R)-20,22-dihydroxycholesterol, and pregnenolone, and of (22R)-22-hydroxycholesterol to the lgycol and pregnenolone in bovine adrenocortical preparations. Mode of oxygen incorporation. J. Biol. Chem. 1975;250:9028–9037. [PubMed] [Google Scholar]
- 4.Miller WL. Molecular biology of steroid hormone synthesis. Endocr. Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 5.Lambeth JD, Seybert DW, Lancaster JR, Jr, Salerno JC, Kamin H. Steroidogenic electron transport in adrenal cortex mitochondria. Mol. Cell. Biochem. 1982;45:13–31. doi: 10.1007/BF01283159. [DOI] [PubMed] [Google Scholar]
- 6.Mast N, Annalora AJ, Lodowski DT, Palczewski K, Stout CD, Pikuleva LA. Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1. J. Biol. Chem. 2011;286:5607–5613. doi: 10.1074/jbc.M110.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10139–10143. doi: 10.1073/pnas.1019441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, Sweatman T, Janjetovic Z, Tuckey RC. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int. J. Biochem. Cell Biol. 2012;44:2003–2018. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, Lin Z, Yates CR, Wang J, W Li, Tuckey RC. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes Caco-2 colon cells and the adrenal gland. Mol. Cell. Endocrinol. 2014;383:181–192. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuckey RC, Nguyen MN, Chen J, Slominski AT, Baldisseri DM, Tieu EW, Zjawiony JK, W Li. Human cytochrome P450scc (CYP11A1) catalyzes epoxide formation with ergosterol. Drug Metab. Dispos. 2012;40:436–444. doi: 10.1124/dmd.111.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller WL, Boser HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J. Lipid Res. 2011;52:2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim. Biophys. Acta. 2007;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. (Lond.) 2010;7:47. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur. J. Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, W Li, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arthur JR, Blair HA, Boyd GS, Mason JI, Suckling KE. Oxidation of cholesterol and cholesterol analogues by mitochondrial preparations of steroid-hormone-producing tissue. Biochem. J. 1976;158:47–51. doi: 10.1042/bj1580047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason JI, Arthur JR, Boyd GS. Control of sterol metabolism in rat adrenal mitochondria. Biochem. J. 1978;174:1045–1051. doi: 10.1042/bj1741045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig IF, Mason JI, Suckling KE, Boyd GS. The influence of the side chain on sterol side-chain cleavage in rat adrenal glands. Biochim. Biophys. Acta. 1982;711:123–127. doi: 10.1016/0005-2760(82)90017-0. [DOI] [PubMed] [Google Scholar]
- 19.Morisaki M, Duque C, Ikekawa N, Shikita M. Substrate specificity of adrenocortical cytochrome P-450scc-I. Effect of structural modification of cholesterol side-chain on pregnenolone production. J. Steroid Biochem. 1980;13:545–550. doi: 10.1016/0022-4731(80)90211-3. [DOI] [PubMed] [Google Scholar]
- 20.Mason JI, Arunachalam T, Caspi E. In vitro studies of the adrenal metabolism of halogenated side-chain analogues of cholesterol. Biochim. Biophys. Acta. 1983;752:265–276. doi: 10.1016/0005-2760(83)90122-4. [DOI] [PubMed] [Google Scholar]
- 21.Lambeth JD. Cytochrome P-450scc a review of the specificity and properties of the cholesterol binding site. Endocr. Res. 1986;12:371–392. doi: 10.3109/07435808609035446. [DOI] [PubMed] [Google Scholar]
- 22.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc. Natl. Acad. Sci. U. S. A. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS ONE. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski A, Semak I, Zjawiony J, Wortsman J, W Li, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuckey RC, W Li, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–2596. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Chen TK, Kim J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy-metabolites of vitamin D3 by cytochrome P450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab. Dispos. 2011;39:1577–1588. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen MN, Slominski A, W Li, Ng YR, Tuckey RC. Metabolism of vitamin D2-17,20,24-trihydroxyvitamin D2 by cytochrome p450scc (CYPHA1) Drug Metab. Dispos. 2009;37:761–767. doi: 10.1124/dmd.108.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, Tuckey RC. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–2901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, Zbytek B, W Li, Tuckey RC. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem. Biol. 2005;12:931–939. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Tuckey RC, Slominski AT, Y Cheng C, Chen J, K Kim T, Xiao M, W Li. Lumisterol is metabolized by CYP11A1: discovery of a new pathway. Int. J. Biochem. Cell Biol. 2014;55:24–34. doi: 10.1016/j.biocel.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.John ME, John MC, Ashley P, MacDonald RJ, Simpson ER, Waterman MR. Identification and characterization of cDNA clones specific for cholesterol side-chain cleavage cytochrome P-450. Proc. Natl. Acad. Sci. U. S. A. 1984;81:5628–5632. doi: 10.1073/pnas.81.18.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazeto Y, Ijiri S, Adachi S, Yamauchi K. Cloning and characterization of a cDNA encoding cholesterol side-chain cleavage cytochrome P450 (CYP11A1): tissue-distribution and changes in the transcript abundance in ovarian tissue of Japanese eel Anguilla japonica, during artificially induced sexual development. J. Steroid Biochem. Mol. Biol. 2006;99:121–128. doi: 10.1016/j.jsbmb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Nunez S, Trant JM. Isolation of the putative cDNA encoding cholesterol side chain cleavage cytochrome P450 (CYP11A) of the southern stingray (Dasyatis americana) Gene. 1997;187:123–129. doi: 10.1016/s0378-1119(96)00734-2. [DOI] [PubMed] [Google Scholar]
- 35.Usanov SA, Graham SE, Lepesheva GI, Azeva TN, Strushkevich NV, Gilep AA, Estabrook RW, Peterson JA. Probing the interaction of bovine cytochrome P450scc (CYP11A1) with adrenodoxin: evaluating site-directed mutations by molecular modeling. Biochemistry. 2002;41:8310–8320. doi: 10.1021/bi0255928. [DOI] [PubMed] [Google Scholar]
- 36.Tuls J, Geren L, Millett F. Fluorescein isothiocyanate specifically modifies lysine 338 of cytochrome P-450scc and inhibits adrenodoxin binding. J. Biol. Chem. 1989;264:16421–16425. [PubMed] [Google Scholar]
- 37.Wada A, Waterman MR. Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding. J. Biol. Chem. 1992;267:22877–22882. [PubMed] [Google Scholar]
- 38.Nakamoto M, Fukasawa M, Orii S, Shimamori K, Maeda T, Suzuki A, Matsuda M, Kobayashi T, Nagahama Y, Shibata N. Cloning and expression of medaka cholesterol side chain cleavage cytochrome P450 during gonadal development. Dev. Growth Differ. 2010;52:385–395. doi: 10.1111/j.1440-169X.2010.01178.x. [DOI] [PubMed] [Google Scholar]
- 39.Storbeck KH, Swart P, Graham S, Swart AC. The influence of the amino acid substitution I98K on the catalytic activity of baboon cytochrome P450 side-chain cleavage (CYP11A1) Endocr. Res. 2004;30:761–767. doi: 10.1081/erc-200044031. [DOI] [PubMed] [Google Scholar]
- 40.Okuyama E, Okazaki T, Furukawa A, Wu RF, Ichikawa Y. Molecular cloning and nucleotide sequences of cDNA clones of sheep and goat adrenocortical cytochromes P450scc (CYP11A1) J. Steroid Biochem. Mol. Biol. 1996;57:179–18 5. doi: 10.1016/0960-0760(95)00263-4. [DOI] [PubMed] [Google Scholar]
- 41.Vilchis F, Chavez B, Larrea F, Timossi C, Montiel F. The cDNA cloning and tissue expression of the cytochrome P450scc from Syrian hamster (Mesocricetus auratus) Gen. Comp. Endocrinol. 2002;126:279–286. doi: 10.1016/s0016-6480(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 42.Chung BC, Matteson KJ, Voutilainen R, Mohandas TK, Miller WL. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15 and expression in the placenta. Proc. Natl. Acad. Sci. U. S. A. 1986;83:8962–8966. doi: 10.1073/pnas.83.23.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai WW, Hsiao PH, Guiguen Y, Chung BC. Cloning of zebrafish cDNA for 3beta-hydroxysteroid dehydrogenase and P450scc. Endocr. Res. 1998;24:927–931. doi: 10.3109/07435809809032708. [DOI] [PubMed] [Google Scholar]
- 44.Parajes S, Griffin A, Taylor AE, Rose IT, Miguel-Escalada I, Hadzhiev Y, Arlt W, Shackleton C, Muller F, Krone N. Redefining the initiation and maintenance of zebrafish interrenal steroidogenesis by characterizing the key enzyme cyp11a2. Endocrinology. 2013;154:2702–2711. doi: 10.1210/en.2013-1145. [DOI] [PubMed] [Google Scholar]
- 45.Gilman CI, Leusch FD, Breckenridge WC, MacLatchy DL. Effects of a phytosterol mixture on male fish plasma lipoprotein fractions and testis P450scc activity. Gen. Comp. Endocrinol. 2003;130:172–184. doi: 10.1016/s0016-6480(02)00590-7. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi M, Tanaka M, Sakai N, Adachi S, Miller WL, Nagahama Y. Rainbow trout ovarian cholesterol side-chain cleavage cytochrome P450 (P450scc). cDNA cloning and mRNA expression during oogenesis. FEBS Lett. 1993;319:45–48. doi: 10.1016/0014-5793(93)80034-r. [DOI] [PubMed] [Google Scholar]
- 47.Nomura O, Nakabayashi O, Nishimori K, Mizuno S. The cDNA cloning and transient expression of a chicken gene encoding cytochrome P-450scc. Gene. 1997;185:217–222. doi: 10.1016/s0378-1119(96)00645-2. [DOI] [PubMed] [Google Scholar]
- 48.Tuckey RC, Cameron KJ. Side-chain specificities of human and bovine cytochromes P-450scc. Eur. J. Biochem. 1993;217:209–215. doi: 10.1111/j.1432-1033.1993.tb18235.x. [DOI] [PubMed] [Google Scholar]
- 49.Tuckey RC, Cameron KJ. Catalytic properties of cytochrome P-450scc purified from the human placenta: comparison to bovine cytochrome P-450scc. Biochim. Biophys. Acta. 1993;1163:185–194. doi: 10.1016/0167-4838(93)90180-y. [DOI] [PubMed] [Google Scholar]
- 50.Frantz DI, Jr, Schroepfer GJ. Sterol biosynthesis. Annu. Rev. Biochem. 1967;36:691–726. doi: 10.1146/annurev.bi.36.070167.003355. [DOI] [PubMed] [Google Scholar]
- 51.Porter FD. Malformation syndromes due to inborn errors of cholesterol synthesis. J. Clin. Invest. 2002;110:715–724. doi: 10.1172/JCI16386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaylor JL. Membrane-bound enzymes of cholesterol synthesis from lanosterol. Biochem. Biophys. Res. Commun. 2002;292:1139–1146. doi: 10.1006/bbrc.2001.2008. [DOI] [PubMed] [Google Scholar]
- 53.Waterham HR, Wanders RJ. Biochemical and genetic aspects of 7-dehydrocholesterol reductase and Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta. 2000;1529:340–356. doi: 10.1016/s1388-1981(00)00159-1. [DOI] [PubMed] [Google Scholar]
- 54.Bae SH, Paik YK. Cholesterol biosynthesis from lanosterol: development of a novel assay method and characterization of rat liver microsomal lanosterol delta 24-reductase. Biochem. J. 1997;326(Pt 2):609–616. doi: 10.1042/bj3260609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 2011;52:6–34. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem. Photobiol. Sci. 2008;7:1570–1576. doi: 10.1039/b809005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller DD, Zjawiony JK, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol. 2013;5:7–19. doi: 10.4161/derm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nowaczyk MJ, Waye JS. The Smith-Lemli-Opitz syndrome: a novel metabolic wayofunderstanding developmental biology, embryogenesis, and dysmorphology. Clin. Genet. 2001;59:375–386. doi: 10.1034/j.1399-0004.2001.590601.x. [DOI] [PubMed] [Google Scholar]
- 59.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Engl. J. Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 60.Shackleton CH, Roitman E, Kelley R. Neonatal urinary steroids in Smith-Lemli-Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids. 1999;64:481–490. doi: 10.1016/s0039-128x(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 61.Shackleton CH. Role of a disordered steroid metabolome in the elucidation of sterol and steroid biosynthesis. Lipids. 2012;47:1–12. doi: 10.1007/s11745-011-3605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tait AD, Hodge LC, Allen WR. Biosynthesis of 3 beta-hydroxy-5,7-pregnadien-20-one by the horse fetal gonad. FEBS Lett. 1983;153:161–164. doi: 10.1016/0014-5793(83)80139-2. [DOI] [PubMed] [Google Scholar]
- 63.Marcos J, Guo LW, Wilson WK, Porter FD, Shackleton C. The implications of 7-dehydrosterol-7-reductase deficiency (Smith-Lemli-Opitz syndrome) to neurosteroid production. Steroids. 2004;69:51–60. doi: 10.1016/j.steroids.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 64.Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD. Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome) J. Steroid Biochem. Mol. Biol. 2002;82:225–232. doi: 10.1016/s0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 65.Sushko TA, Gilep AA, Yantsevich AV, Usanov SA. Role of microsomal steroid hydroxylases in Delta7-steroid biosynthesis. Biochemistry (Mosc.) 2013;78:282–289. doi: 10.1134/S0006297913030103. [DOI] [PubMed] [Google Scholar]
- 66.Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp. Dermatol. 2014;23:369–374. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3 beta, 17 alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–228. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, Zjawiony J, Tuckey RC. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med. Chem. 2014;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strott CA, Higashi Y. Cholesterol sulfate in human physiology: what’s it all about? J. Lipid Res. 2003;44:1268–1278. doi: 10.1194/jlr.R300005-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Tuckey RC, Lawrence J, Cameron KJ. Side-chain cleavage of cholesterol esters by human cytochrome P-450(scc) J. Steroid Biochem. Mol. Biol. 1996;58:605–610. doi: 10.1016/0960-0760(96)00071-4. [DOI] [PubMed] [Google Scholar]
- 72.Tuckey RC. Side-chain cleavage of cholesterol sulfate by ovarian mitochondria. J. Steroid Biochem. Mol. Biol. 1990;37:121–127. doi: 10.1016/0960-0760(90)90380-4. [DOI] [PubMed] [Google Scholar]
- 73.Harteneck C. Pregnenolone sulfate: from steroid metabolite to TRP channel ligand. Molecules. 2013;18:12012–12028. doi: 10.3390/molecules181012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 75.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 76.Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, Halliday GM, Mason RS. 1alpha 25(OH)(2)-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev. Res. (Phila.) 2011;4:1485–1494. doi: 10.1158/1940-6207.CAPR-11-0165. [DOI] [PubMed] [Google Scholar]
- 77.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS ONE. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 79.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox Shaked I JN, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Menon GK, Feingold KR, Moser AH, Brown BE, Elias PM. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J. Lipid Res. 1985;26:418–427. [PubMed] [Google Scholar]
- 81.Elias PM, Crumrine D, Paller A, Rodriguez-Martin M, Williams ML. Pathogenesis of the cutaneous phenotype in inherited disorders of cholesterol metabolism: therapeutic implications for topical treatment of these disorders. Dermatoendocrinol. 2011;3:100–106. doi: 10.4161/derm.3.2.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elias PM, Williams ML, Feingold KR. Abnormal barrier function in the pathogenesis of ichthyosis: therapeutic implications for lipid metabolic disorders. Clin. Dermatol. 2012;30:311–322. doi: 10.1016/j.clindermatol.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1CYP17 and CYP21A2 genes are expressed in skin. J. Clin. Endocrinol. Metab. 1996;81:2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 84.Holick MF, Clark MB. The photobiogenesis and metabolism of vitamin D. Fed. Proc. 1978;37:2567–2574. [PubMed] [Google Scholar]
- 85.Holick MF. Vitamin D: a millenium perspective. J. Cell. Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 86.Holick MF, Tian XQ, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3124–3126. doi: 10.1073/pnas.92.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bikle DD. Vitamin D: an ancient hormone. Exp. Dermatol. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhu J, DeLuca HF. Vitamin D25-hydroxylase- four decades of searching, are we there yet? Arch. Biochem. Biophys. 2012;523:30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 89.Holick MF, Holick SA, Tavela T, Gallagher B, Schnoes HK, DeLuca HF. Synthesis of (6-3H)-1alpha-hydroxyvitamin D3 and its metabolism in vivo to (3H)-1alpha,25-dihydroxyvitamin D3. Science. 1975;190:576–578. doi: 10.1126/science.1188356. [DOI] [PubMed] [Google Scholar]
- 90.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu J, DeLuca HF. Vitamin D25-hydroxylase- four decades of searching, are we there yet? Arch. Biochem. Biophys. 2012;523:30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 92.Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC, Metabolism of cholesterol. vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J. Steroid Biochem. 2012;129:163–171. doi: 10.1016/j.jsbmb.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawada N, Sakaki T, Ohta M, Inouye K. Metabolism of Vitamin D3 by Human CYP27A1. Biochem. Biophys. Res. Commun. 2000;273:977–984. doi: 10.1006/bbrc.2000.3050. [DOI] [PubMed] [Google Scholar]
- 94.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 95.Flanagan JN, Wang L, Tangpricha V, Reichrath J, Chen TC, Holick MF. Regulation of the 25-hydroxyvitamin D-1alpha-hydroxylase gene and its splice variant. Recent Results Cancer Res. 2003;164:157–167. doi: 10.1007/978-3-642-55580-0_12. [DOI] [PubMed] [Google Scholar]
- 96.Wang L, Whitlatch LW, Flanagan JN, Holick MF, Chen TC. Vitamin D autocrine system and prostate cancer. Recent Results Cancer Res. 2003;164:223–237. doi: 10.1007/978-3-642-55580-0_16. [DOI] [PubMed] [Google Scholar]
- 97.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J. Biol. Chem. 2003;278:38084–38093. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Invest. 1986;78:557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lehmann B, Sauter W, Knuschke P, Dressler S, Meurer M. Demonstration of UVB-induced synthesis of 1 alpha,25-dihydroxyvitamin D3 (calcitriol) in human skin by microdialysis. Arch. Dermatol. Res. 2003;295:24–28. doi: 10.1007/s00403-003-0387-6. [DOI] [PubMed] [Google Scholar]
- 100.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front. Biosci. 2005;10:119–134. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 101.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 102.Tieu EW, Tang EK, Tuckey RC. Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. FEBS J. 2014;281:3280–3296. doi: 10.1111/febs.12862. [DOI] [PubMed] [Google Scholar]
- 103.Slominski AT, Kim Li TKW, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014;144PA:28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–935. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy metabolites of vitamin D3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab. Dispos. 2011;39:1577–1588. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, Slominski A. Metabolism of 1 alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 2008;112:213–219. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC, Metabolism of cholesterol. vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J. Steroid Biochem. Mol. Biol. 2012;129:163–171. doi: 10.1016/j.jsbmb.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tieu EW, Tang EK, Chen J, Li W, Nguyen MN, Janjetovic Z, Slominski A, Tuckey RC. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem. Pharmacol. 2012;84:1696–1704. doi: 10.1016/j.bcp.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang EK, Chen J, Janjetovic Z, Tieu EW, Slominski AT, Li W, Tuckey RC. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab. Dispos. 2013;41:1112–1124. doi: 10.1124/dmd.113.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang EK, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, Tuckey RC. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab. Dispos. 2010;38:1553–1559. doi: 10.1124/dmd.110.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sidler D, Renzulli P, Schnoz C, Berger B, Schneider-Jakob S, Fluck C, Inderbitzin D, Corazza N, Candinas D, Brunner T. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene. 2011;30:2411–2419. doi: 10.1038/onc.2010.629. [DOI] [PubMed] [Google Scholar]
- 112.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol. Cell Physiol. 2011;300:C526–C541. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am. J. Physiol. Endocrinol. Metab. 2011;301:E11–E24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT. 20-Hydroxyvitamin D3 a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J. Invest. Dermatol. 2008;128:2271–2280. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS ONE. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-Dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J. Cell. Phys. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, Nelson KE, Tuckey RC, Miller D, Jiao Y, Gu W, Postlethwaite AE. Vitamin D analogs 17,20S(OH) 2pD and 17,20R(OH) 2pD are noncalcemic and exhibit antifibrotic activity. J. Invest. Dermatol. 2011;131:1167–1169. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, Postlethwaite AE. 20S-Hydroxyvitamin D3 noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J. Clin. Endocrinol. Metab. 2013;98:E298–E303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, Nguyen MN, Tuckey RC. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effectson normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 120.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, Li W. 20-hydroxyvitamin D inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746. [PMC free article] [PubMed] [Google Scholar]
- 121.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM, Slominski AT. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Brit. J. Cancer. 2011;105:1874–1884. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim T-K, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, Tang EKY, Miller D, Li W, Slominski AT. Correlation between secosteroid induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol. Cell. Endocrinol. 2012;361:143–152. doi: 10.1016/j.mce.2012.04.001. doi: http://dx.doi.org/10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Slominski AT, Kim Takeda TKY, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, Jetten AM. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen J, Slominski AT, Miller DD, Li W. Effects of sidechain length and composition on the kinetic conversion and product distribution of vitamin D analogs determined by real-time NMR. Dermatoendocrinol. 2013;5:142–149. doi: 10.4161/derm.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]