Abstract
Streptococcus agalactiae is a well-known pathogen during pregnancy and in neonates. Among non-pregnant adults, invasive infection, although rare, is showing increasing frequency, especially in chronically ill, immunosuppressed, or older patients. Although rare, the clinical features of meningeal infection caused by S. agalactiae are similar to other bacterial meningitis. The authors report the case of a middle-aged man previously diagnosed with hypertension, diabetes mellitus, and alcoholic liver cirrhosis, who was admitted at the emergency department with a Glasgow Coma Scale of 11/12, generalized spasticity, bilateral Babinski sign, and hypertension. The clinical outcome was bad, with refractory shock and death within 24 hours of hospitalization. The bacteriological work-up isolated S. agalactiae in the cerebral spinal fluid (CSF), blood, and urine. An autopsy revealed meningoencephalitis, acute myocardial infarction, and pyelonephritis due to septic emboli. The authors point out the atypical CSF findings, the rapid fatal outcome, and the importance of including this pathogen among the etiologic possibilities of invasive infections in this group of patients.
Keywords: Streptococcus agalactiae, Meningitis, Bacterial, Sepsis, Autopsy
CASE REPORT
A 56-year-old man was brought to the emergency unit having been found unconscious at home. His family member reported that the patient had showed recent hypoactivity and lethargy. On the day before, he self-medicated with 2 mg of cyclobenzaprine for lumbar pain. His past medical history included alcoholic cirrhosis of the liver (with bleeding esophageal varices), poorly controlled diabetes mellitus, and hypertension. He had been a heavy drinker since his twenties, but quit drinking four months prior to hospitalization. On admission, he was mentally confused (Glasgow Coma Scale of 11/12), icteric, tachypneic, with the blood pressure of 182/104 mmHg. There was generalized spasticity and bilateral Babinski sign, but the meningeal signs were non-assessable. Pulmonary examination revealed bilateral rhonchi. The remainder of the physical examination was unremarkable. Initial laboratory examination results are shown in Table 1.
Table 1. Initial laboratory work-up.
| Exam | Result | RV | Exam | Result | RV |
|---|---|---|---|---|---|
| Hemoglobin | 18.7 | 12.3-15.3 g/dL | Lactate* | 77.4 | 4.5–19.8 mg/dL |
| Hematocrit | 53.5 | 36.0-45.0% | ALT | 57 | 9-36 U/L |
| Leukocytes | 4.89 | 4.4-11.3 × 103/mm3 | AST | 97 | 10-31 U/L |
| Promyelocytes | 2 | 0% | AP | 144 | 10-100 U/L |
| Metamyelocytes | 4 | 0% | γGT | 280 | 2-30 U/L |
| Bands | 27 | 0-5% | Albumin | 3.0 | 3.0-5.0 g/dL |
| Segmented | 61 | 45-70% | TB | 9.08 | 0.3-1.2 mg/dL |
| Lymphocytes | 4% | 18-40% | DB | 7.46 | 30-118 U/L |
| Monocytes | 2 | 2-9% | CK | 92 | 20-200 U/L |
| Platelets | 34 | 150-400 × 103/mm3 | CK–MB | 17.6 | < 5.0 ng/mL |
| INR | 1.88 | 1 | Tropo I | 3.26 | < 0.06 ng/mL |
| Urea | 119 | 5-25 mg/dL | CRP | 111 | < 5 mg/L |
| Creatinine | 0.99 | 0.4-1.3 mg/dL | Anti-HIV | Negative | |
| Potassium | 4.3 | 3.5-5.0 mEq/L | Anti-HCV | Negative | |
| Sodium | 138 | 136-146 mEq/L | HB serology | Negative | |
| Glucose | 170 | < 99 mg/dL | VDRL | Negative |
arterial.
ALT = alanine aminotransferase; AP = alkaline phosphatase; AST = aspartate aminotransferase; CK = creatine phosphokinase; CK-MB = creatine phosphokinase MB fraction; CRP = C-reactive protein; DB = direct bilirubin; γGT= gamma-glutamyl transpeptidase; HCV = hepatitis C virus; HB = hepatitis B; HIV = human immunodeficiency virus; INR = international normalized ratio; LDH = lactate dehydrogenase; RV = reference value; TB = total bilirubin; Tropo I = troponin I; VDRL = venereal disease research laboratory.
Urinalysis showed leukocyturia of 28,750/mm3 (reference value [RV]: < 10,000/mm3) and granular casts of 1000/mm3 (RV: none). The cerebral spinal fluid (CSF) analysis revealed three cells (RV: < 5 cells); protein was 816.6 mg/dL (RV: 15-45 mg/dL); glucose was 19 mg/dL (RV: 2/3 of serum glucose); and lactate was 144.2 mg/dL (9-26 mg/dL). Blood culture samples were positive for Gram-positive cocci. The brain computed tomography was normal.
The clinical parameters deteriorated, with hypotension, atrial fibrillation, and respiratory failure, with the need for mechanical ventilatory support. The patient was hospitalized with the diagnosis of sepsis and hepatic encephalopathy. Volume resuscitation, vasoactive drugs, and empirically intravenous infusions of ceftriaxone and amiodarone (after unsuccessful electrical cardioversions) were initiated. A few hours after admission, the patient had a cardiac arrest and died within the first 24 hours of hospitalization. On subsequent days, blood samples, a urine culture, and a CSF culture turned positive for Streptococcus agalactiae.
AUTOPSY FINDINGS
The ectoscopy evidenced jaundice, facial plethora, cyanosis of the extremities, and some petechiae on the sternal region.
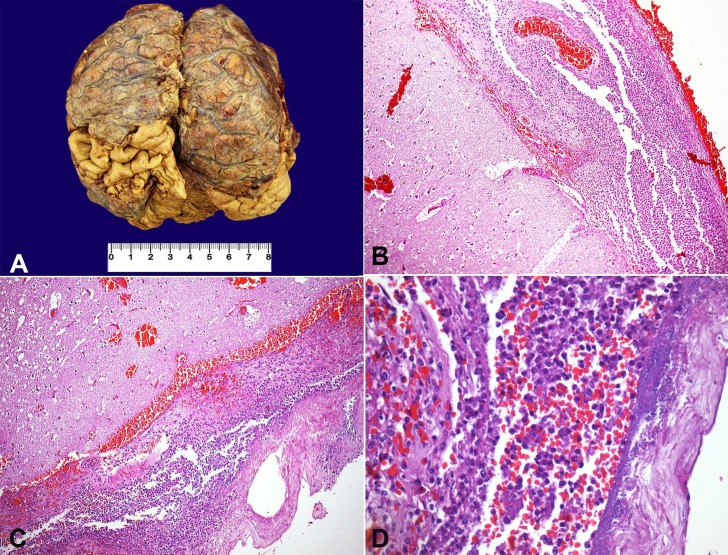
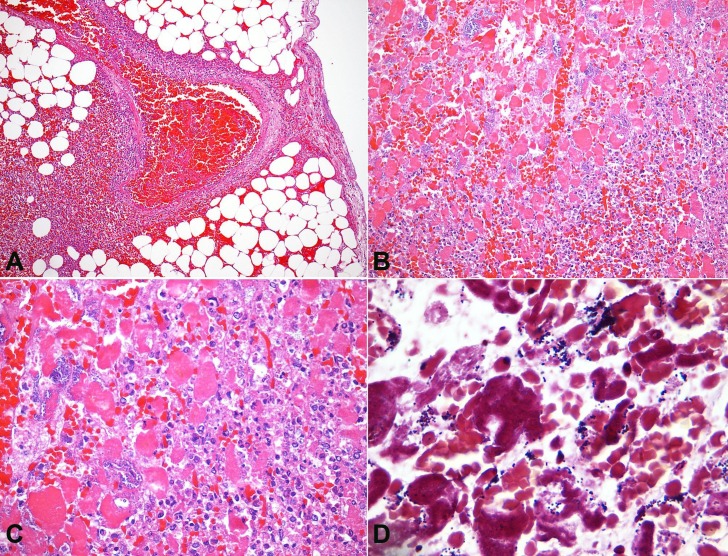
At the opening of the skull, a marked leptomeningeal purulent exudate with vascular congestion was observed (Figure 1A). The brain weighed 1223 g (RV: 1200-1600 g); the architecture was preserved, and neither focal lesions nor hemorrhage were found. Microscopically, a marked leptomeningeal polymorphonuclear leukocyte infiltrate, predominantly at the cerebral convexity and along the superficial vessels, with infiltration of the adjacent parenchyma, was present, which was consistent with the diagnosis of acute meningoencephalitis (Figure 1B, C). Several bacterial colonies (Gram-positive cocci) and ischemic neurons were identified (Figure 1D).
Figure 1. A - Gross appearance of the brain, showing thickened meninges with purulent exudate deposition. Photomicrography of the meninges; B - Thickened meninges with an intense acute inflammatory exudate (H&E, 100X); C - Marked acute inflammatory infiltrate with superficial involvement of the cerebral cortex, besides local hemorrhage (H&E, 100X); D - Presence of bacterial colonies intermingled with a meningeal acute inflammatory infiltrate (H&E, 400X).
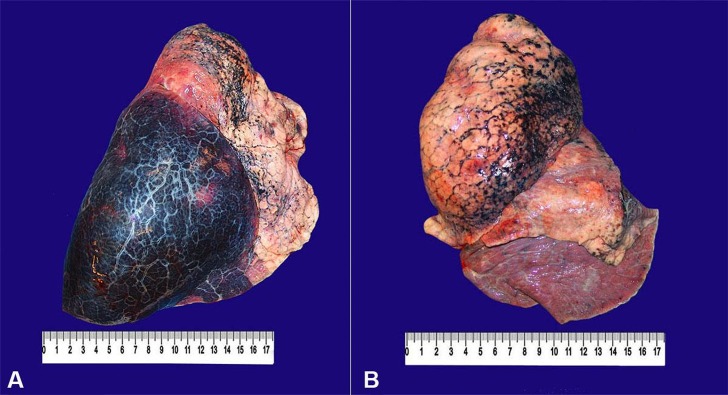
At the opening of the thoracic cavity, 500 mL of sero-hemorrhagic effusion was drained from the right pleural space. The right lung weighed 1085 g (RV: 400-800 g). Parenchymal hemorrhage involved the whole inferior lobe, but it was not friable at digital compression (Figure 2A).
Figure 2. Gross findings of the lungs. A - The right lung is enlarged, with a smooth pleural surface, anthracosis, and marked inferior lobe hemorrhage; B - The left lung shows a smooth pleural surface, anthracosis, and mild inferior lobe congestion.
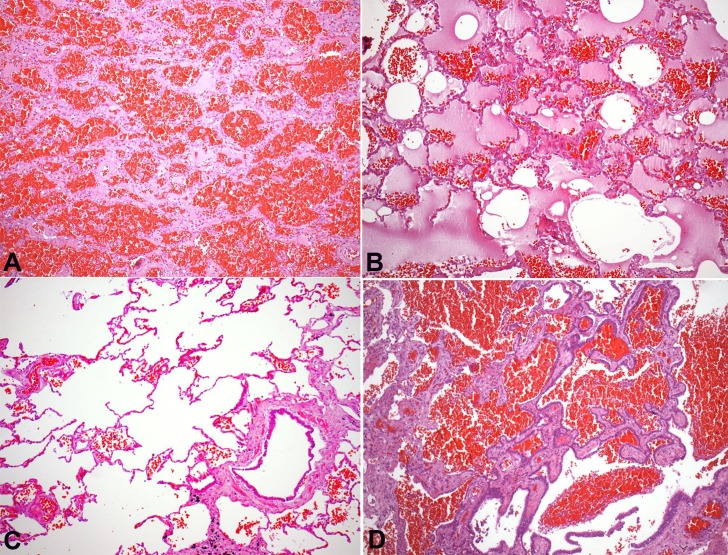
The left lung, in turn, weighed 477 g (RV: 400-800 g) and was congested, with anthracotic lymph nodes (Figure 2B). Microscopically, there was pulmonary emphysema and moderate anthracosis. An extensive parenchymal hemorrhage, with foci of alveolar edema and scattered fibrinous thrombi in arterioles, was found in the right lung. A focal area of parenchymal honeycombing, characterized by septal thickening, bronchiolization of alveolar spaces, and foreign body giant cells were present (Figure 3).
Figure 3. Photomicrography of the lung. A - Alveolar hemorrhage (H&E, 100X); B - Pulmonary parenchyma with edema and extravasated erythrocytes (H&E, 100X); C - Pulmonary emphysema, represented by distended alveolar spaces and rupture of the alveolar septa (H&E, 100X); D - Area of pulmonary honeycombing, with alveoli lined by bronchial epithelium, plus the presence of alveolar hemorrhage (H&E, 100X).
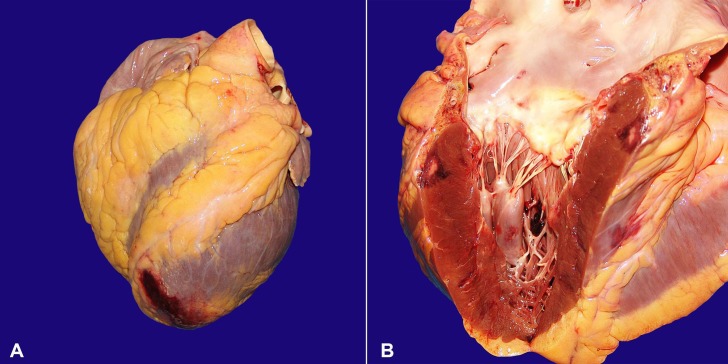
The heart weighed 482 g (RV: 200-350 g), and showed moderate left ventricular hypertrophy. There was coronary atherosclerosis, with obstruction of up to 90% of the arterial lumens. The valves were normal, without vegetations. At the cut surface, the left anterior septum showed a transmural infarction area, characterized by a pale core surrounded by a rim of hemorrhage, with extension to the epicardial surface (Figure 4). Another subepicardial infarction area was present on the posterior free wall of the left ventricle. Microscopically, there were septic emboli in epicardial vessels, with polymorphonuclear leukocytes infiltration (acute phlebitis), besides several bacterial colonies (Gram-positive cocci) within the myocardial infarction areas (Figure 5).
Figure 4. Gross findings of the heart. A - Note the hemorrhage in the epicardial surface of the apex; B - Presence of a subepicardial triangular infarction area in the left ventricular wall.
Figure 5. Photomicrography of the heart. A - Thrombophlebitis of a subepicardial vessel (H&E, 100X); B - Necrotic muscle fibers intermingled with marked acute inflammatory infiltrate, hemorrhage, and several bacterial colonies in an area of acute myocardial infarction (H&E, 200X); C - Detail of the bacterial colonies between the necrotic cardiomyocytes (H&E, 400X); D - Gram-positive cocci in detail, by Brown-Hopps staining (1000X).
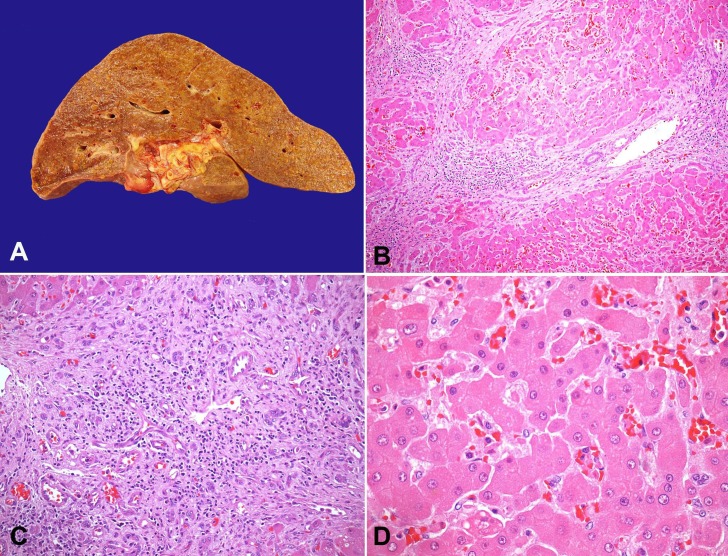
At the opening of the abdominal cavity, 800 mL of citrine fluid was drained. The peritoneal surface was smooth with no signs of peritonitis. The esophagus presented venous ectasias, but there were no signs of recent bleeding. Microscopically, submucous dilated vessels, consistent with varices, were observed. The liver weighed 2322 g (RV: 1400-1600 g), and presented a multinodular, yellowish-brown surface. At the cut surface, micronodular cirrhosis was evident, which was confirmed by microscopy (Figure 6A). Also, there were portal and septal lymphoid aggregates, marked ductular reaction, and sinusoidal congestion (Figure 6B–D). Liver interface activity was not observed.
Figure 6. A - Gross appearance of the liver, showing micronodular cirrhosis. Photomicrography of the liver; B - Multiple parenchymal regeneration nodules, with septal inflammatory infiltrates (H&E, 100X); C - Marked ductular reaction (H&E, 200X); D - Sinusoidal congestion, with no parenchymal inflammatory activity (H&E, 400X).
The spleen was markedly congested, and weighed 878 g (RV: 120-150 g). The bone marrow was hypercellular for the patient's age (60-70% of hematopoietic tissue), with relative granulocytic series hyperplasia.
The kidneys were congested and diffusely purplish, weighing nearly 290 g each (RV: 120-150g). Microscopically, there was acute pyelonephritis, with septic emboli, as well as acute tubular necrosis and intraluminal basophilic calcifications. The prostate was nodular and hardened. A Gleason 6 adenocarcinoma was incidentally found, as well as a schwannoma in the right adrenal gland.
DISCUSSION
S. agalactiae, a group B streptococcus (GBS), frequently colonizes the urinary and gastrointestinal tracts,1 and is known as one of the main etiological agents of neonatal meningitis.2 However, GBS invasive infections among adults are appearing with increasing frequency,3-5 and with a higher mortality compared with the pediatric population.6,7 In adults, these infections occur more often in (i) pregnant women and those who have just given birth; and (ii) non-pregnant adults with chronic and debilitating diseases.8 The most involved sites of infection are the skin, soft tissue, bone, joints, urinary tract, lungs, peritoneum, and the genital tract.9,10 Endocarditis is uncommon, but bacteremia of unknown origin is frequently documented.10 GBS meningitis among adults is rare, accounting for no more than 4% of the invasive infection cases involving this pathogen,3 and representing only 0.3-4.3% of all cases of bacterial meningitis.10 However, the disease burden has shown an increasing trend in recent years.3
Pregnancy doubles the risk for GBS infection, while the puerperium stage has a 20-fold risk for the development of invasive infection.11 The pathogenesis behind this higher susceptibility is still unknown, but it is presumed that the altered immune status related to pregnancy and the postpartum period may play a role.11 Half of the infections caused by GBS in pregnant women occur in association with urinary tract infections, infections of the placenta, and chorioamnionitis.3 However, for unknown reasons, meningitis during pregnancy and puerperium is very rare, although well reported.9,12-14 Intriguingly, a peripartum streptococcal infection is more common in puerperas after a cesarean section, but virtually all cases of GBS meningitis occur after vaginal delivery.13 It is speculated that the increased pelvic venous pressure during vaginal delivery is somehow responsible for this association.14
GBS meningitis among non-pregnant adults, like other invasive infections involving this pathogen, occurs more frequently among middle-aged patients, or patients older than 50 years.15 Ninety percent of patients with GBS invasive infection present at least one chronic debilitating disease, particularly (i) diabetes mellitus; (ii) cirrhosis of the liver; (iii) chronic renal failure; (iv) heart failure; (v) lung disease; (vi) malignancy; and (vi) immunosupression of any kind.7,15-17 However, diabetes mellitus, probably due to its associated leukocyte dysfunction, is the main chronic disease associated with invasive GBS infection.17-19
The majority of adult GBS meningitis cases are associated with ruptures of the mucosa or the skin.10 Some patients with GBS meningitis have a local predisposing factor, such as a previous liquoric fistula, a pharyngeal malignancy, or an ocular infection. Other cases may result from dissemination of a distant focus of infection, such as the endometrium, the respiratory tract, or the endocardium.5,20 Those predisposing factors or distant infectious foci were not present in the case reported herein.
The clinical features of S. agalactiae meningitis are similar to other bacterial etiologies.15 Fever (90%), an altered level of consciousness (67%), and nuchal rigidity (62%) are the most common findings.21 CSF analysis reveals neutrophilic pleocytosis, hyperproteinorachy, and hypoglucorachy15. The lethality of GBS meningitis in non-pregnant adults is high, similar to other bacterial miningitis related to chronically ill patients, like those caused by staphylococci and pneumococci.2,15 Similar to the case reported herein, hyperacute cases, with less than 24 hours of initial symptoms before hospitalization, are frequently reported.12
Despite the high sensitivity of GBS to penicillin and third-generation cephalosporins, early diagnosis should be considered the cornerstone for a better outcome. Permanent hearing loss is one of the more frequent sequelae among the survivers.4
Autopsy case report on GBS meningitis is scarce, as far as we know, this is the second report of in an adult in the English literature.22 Our patient's age, his predisposing chronic diseases, and the fatal course are all in accordance with earlier descriptions. However, he also presented some peculiar aspects: (i) the lack of alterations in body temperature (fever or hypothermia); (ii) the absence of unequivocal meningeal irritation signs on admission; and (iii) normal CSF cellularity. Though uncommon, similarly atypical cases of GBS meningitis have been reported.12,23,24 Finally, this case is also remarkable for its dissemination pattern, with septic emboli to coronary arteries and renal parenchyma. In this setting, septic emboli to the heart generally derive from infectious endocarditis, although other sources have been described.25,26 The explanation for the unexpected hypocellularity of the CSF observed in this case with marked meningeal inflammatory process should consider: i) a preanalytical error, ii) hyperacute inflammation of the meninges, or iii) the Froin's syndrome, already described in acute meningoencephalomyelitis.27 The cardiac valves of our patient were free of vegetations. In our case, the septic emboli to the heart caused myocardial infarction with raised myocardial necrosis markers on admission.
Despite the thorough examination during this autopsy, it was not possible to identify a primary site of infection that could explain the meningitis and the septic emboli. No skin lesions were found as portals of entry for microorganisms. The triple isolation of S. agalactiae in the blood, in the CSF, and in the urine is noteworthy in our case. Taking into account the diagnosis of benign prostatic hyperplasia and prostatic adenocarcinoma, it is fair to consider this urinary tract obstruction as a possible source of the initial infection. In a series of 64 cases of GBS infection, 21 presented a urinary tract infection, and half of them presented a subjacent urologic disease.7 However, in cases where the primary site of infection cannot be precisely ascertained, every possibility should be taken into account.
The immediate cause of death of our patient seems to be related to septicemia, but we should not forget to consider the acute myocardial infarction as a possible cause of hemodynamic collapse.
This case report highlights the importance of GBS, especially S. agalactiae, as a cause of severe infection in chronically ill patients, as well as the possibility of unusual clinical features and the high risk of fatal outcome in these cases.
Footnotes
Batista RP, Ferreira CR. Streptococcus agalactiae septicemia in a patient with diabetes and hepatic cirrhosis. Autopsy Case Rep [Internet]. 2015;5(4):35-43. http://dx.doi.org/10.4322/acr.2015.028
REFERENCES
- 1.Dutra VG, Alves VM, Olendzki AN, et al. Streptococcus agalactiae in Brazil: serotype distribution, virulence determinants and antimicrobial susceptibility. BMC Infect Dis. 2014;14(1):323. http://dx.doi.org/10.1186/1471-2334-14-323. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364(21):2016-25. http://dx.doi.org/10.1056/NEJMoa1005384. PMid: [DOI] [PubMed] [Google Scholar]
- 3.Kamaratos A, Kokkoris S, Tzanakari A, et al. Group B streptococcus (Streptococcus agalactiae) meningitis in a diabetic adult. Acta Diabetol. 2005;42(3):117-8. http://dx.doi.org/10.1007/s00592-005-0189-8. PMid: [DOI] [PubMed] [Google Scholar]
- 4.Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008;299(17):2056-65. http://dx.doi.org/10.1001/jama.299.17.2056. PMid: [DOI] [PubMed] [Google Scholar]
- 5.Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis. 2001;33(4):556-61. http://dx.doi.org/10.1086/322696. PMid: [DOI] [PubMed] [Google Scholar]
- 6.Skoff TH, Farley MM, Petit S, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin Infect Dis. 2009;49(1):85-92. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre N, Forestier E, Mohseni-Zadeh M, et al. Les infections invasives à Streptococcus agalactiae chez l’adulte (femme enceinte exclue). Med Mal Infect. 2007;37(12):796-801. http://dx.doi.org/10.1016/j.medmal.2007.04.003. PMid: [DOI] [PubMed] [Google Scholar]
- 8.Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine. 2013;31(Suppl 4):D7-12. http://dx.doi.org/10.1016/j.vaccine.2013.01.009. PMid: [DOI] [PubMed] [Google Scholar]
- 9.Vittorino R, Hui-Yuen J, Ratner AJ, Starr A, McCann T. Case report: group B Streptococcus meningitis in an adolescent. F1000 Res. 2014;3:167. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyanguren B, Esteban L, Guillán M, et al. Central nervous system involvement in adult patients with invasive infection caused by Streptococcus agalactiae. Neurologia. 2015;30(3):158-62. http://dx.doi.org/10.1016/j.nrl.2013.12.002. PMid: [DOI] [PubMed] [Google Scholar]
- 11.Deutscher M, Lewis M, Zell ER, et al. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clin Infect Dis. 2011;53(2):114-23. http://dx.doi.org/10.1093/cid/cir325. PMid: [DOI] [PubMed] [Google Scholar]
- 12.Gielchinsky Y, Cohen R, Revel A, Ezra Y. Postpartum maternal group B streptococcal meningitis. Acta Obstet Gynecol Scand. 2005;84(5):490-1. http://dx.doi.org/10.1111/j.0001-6349.2005.0243b.x. PMid: [DOI] [PubMed] [Google Scholar]
- 13.Ghani NA, Jaafar R, Ishak S, Zainuddin AA, Mukari SA, Mahdy ZA. Mother with post-partum group B Streptococcus meningitis and cerebellar abscess. J Obstet Gynaecol Res. 2007;33(2):195-8. http://dx.doi.org/10.1111/j.1447-0756.2007.00495.x. PMid: [DOI] [PubMed] [Google Scholar]
- 14.Guerin JM, Leibinger F, Mofredj A, Ekherian JM. Streptococcus B meningitis in post-partum. J Infect. 1997;34(2):151-3. http://dx.doi.org/10.1016/S0163-4453(97)92528-7. PMid: [DOI] [PubMed] [Google Scholar]
- 15.Domingo P, Barquet N, Alvarez M, Coll P, Nava J, Garau J. Group B streptococcal meningitis in adults: report of twelve cases and review. Clin Infect Dis. 1997;25(5):1180-7. http://dx.doi.org/10.1086/516094. PMid: [DOI] [PubMed] [Google Scholar]
- 16.Schwartz B, Schuchat A, Oxtoby MJ, Cochi SL, Hightower A, Broome CV. Invasive group B streptococcal disease in adults. A population-based study in metropolitan Atlanta. JAMA. 1991;266(8):1112-4. http://dx.doi.org/10.1001/jama.1991.03470080082034. PMid: [PubMed] [Google Scholar]
- 17.Blancas D, Santin M, Olmo M, Alcaide F, Carratala J, Gudiol F. Group B streptococcal disease in nonpregnant adults: incidence, clinical characteristics, and outcome. Eur J Clin Microbiol Infect Dis. 2004;23(3):168-73. http://dx.doi.org/10.1007/s10096-003-1098-9. PMid: [DOI] [PubMed] [Google Scholar]
- 18.Chaiwarith R, Jullaket W, Bunchoo M, Nuntachit N, Sirisanthana T, Supparatpinyo K. Streptococcus agalactiae in adults at Chiang Mai University Hospital: a retrospective study. BMC Infect Dis. 2011;11(1):149. http://dx.doi.org/10.1186/1471-2334-11-149. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsubara K, Hoshina K, Suzuki Y. Early-onset and late-onset group B streptococcal disease in Japan: a nationwide surveillance study, 2004-2010. Int J Infect Dis. 2013;17(6):e379-84. http://dx.doi.org/10.1016/j.ijid.2012.11.027. PMid: [DOI] [PubMed] [Google Scholar]
- 20.Bahloul H, Mofredj A, Rousselier P, Gineyt G. Méningite à streptocoque B rapidement fatale chez un adulte sain. Ann Fr Anesth Reanim. 2008;27(9):762-3. http://dx.doi.org/10.1016/j.annfar.2008.07.084. PMid: [DOI] [PubMed] [Google Scholar]
- 21.Gunasekera P, Asumang A. Group B streptococcal infection — an emerging cause of sepsis in fit non-peripartum adults. JICS. 2014;15(3):250-3. http://dx.doi.org/10.1177/175114371401500316. [Google Scholar]
- 22.Batalis NI, Caplan MJ, Schandl CA. Acute deaths in nonpregnant adults due to invasive streptococcal infections. Am J Forensic Med Pathol. 2007;28(1):63-8. http://dx.doi.org/10.1097/01.paf.0000248775.34108.da. PMid: [DOI] [PubMed] [Google Scholar]
- 23.Martins ER, Florindo C, Martins F, et al. Streptococcus agalactiae serotype Ib as an agent of meningitis in two adult nonpregnant women. J Clin Microbiol. 2007;45(11):3850-2. http://dx.doi.org/10.1128/JCM.01358-07. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfe RR Jr, Norwick ML, Bofill JA. Fatal maternal beta- hemolytic group B streptococcal meningitis: a case report. Am J Perinatol. 1998;15(11):597-600. http://dx.doi.org/10.1055/s-2007-994076. PMid: [DOI] [PubMed] [Google Scholar]
- 25.Stawicki SP, Firstenberg MS, Lyaker MR, et al. Septic embolism in the intensive care unit. Int J Crit Illn Inj Sci. 2013;3(1):58-63. http://dx.doi.org/10.4103/2229-5151.109423. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniike M, Nishino M, Egami Y, et al. Acute myocardial infarction caused by a septic coronary embolism diagnosed and treated with a thrombectomy catheter. Heart. 2005;91(5):e34. http://dx.doi.org/10.1136/hrt.2004.055046. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piaggio Blanco RA, Ferrar Forcade A, Grille A, Pinyero Ja, Froin Syndrome in acute meningoencephalomyelitis. An Fac Med Univ Repub Montev Urug 1952;37(7-8):359-65. [PubMed] [Google Scholar]