Abstract
We present the case of a patient who underwent cardiac transplantation with the diagnosis of idiopathic dilated cardiomyopathy. Once the explanted heart was examined, a type of granulomatous myocarditis compatible with cardiac sarcoidosis was observed. However, there was severe involvement of the right ventricle, with markedly reduced width of the muscular layer and extensive fibrofatty replacement, findings similar to the ones encountered in cases of arrhythmogenic right ventricular cardiomyopathy (ARVC). Confocal immunofluorescence analysis revealed a reduced signal for plakoglobin and desmoplakin at the cardiac intercalated disks. The immunoreactive signal for desmin showed the typical sarcomeric distribution but not a concentrated signal at the intercalated disks, a pattern previously seen in an 11-year-old girl with Carvajal syndrome bearing a C-terminal truncating mutation in the desmoplakin gene. This case illustrates the difficult and challenging work involved in performing a differential diagnosis among idiopathic dilated cardiomyopathy, isolated cardiac sarcoidosis, and ARVC, all of which are clinical entities known to masquerade as one another.
Keywords: Sarcoidosis, Arrhythmogenic right ventricular cardiomyopathy, Immunohistochemistry, Heart diseases
CASE REPORT
A 60-year-old woman presented with exertional dyspnea, with mild limitation during ordinary physical activities. An electrocardiogram revealed a complete left bundle branch block and sinus tachycardia. Doppler echocardiography showed a severely dilated left ventricle (LV; 61 and 71 mm in systole and diastole, respectively), and a mildly dilated left atrium (40 mm). The LV ejection fraction (LVEF) was 30%. Cardiac catheterization revealed dilatation of the LV, and the coronary arteries appeared normal. Chagas disease serology was negative. Carvedilol, losartan and furosemide were then prescribed, with clinical improvement.
The patient had no previous history of smoking or alcohol abuse. Her past surgical history included a cholecystectomy due to symptomatic biliary lithiasis complicated with pancreatitis. She had hypothyroidism and used levothyroxin 125 µg per day. Two siblings had been diagnosed with dilated cardiomyopathy, while another brother had died suddenly in adolescence.
One year after the onset of symptoms, the patient was admitted to the intensive care unit with cardiogenic shock, and received dobutamine, with temporary clinical improvement. Soon after discharge, she underwent cardiac resynchronization therapy due to refractory heart failure. Despite this, over the following months she presented with additional episodes of cardiogenic shock, and became dependent on dobutamine. Two years after the onset of symptoms, she was referred to a university hospital for cardiac transplantation. Upon clinical examination, she had cold extremities, slow capillary refilling, arterial hypotension, signs of increased central venous pressure, tachycardia, a third heart sound, and hepatomegaly. There was no lower limbs edema. Doppler echocardiography revealed a dilated LV (68 mm and 74 mm in systole and diastole, respectively) and an enlarged left atrium (44 mm); the LVEF was 18%. Biochemical and hematological tests were normal.
Under the presumed clinical diagnosis of idiopathic dilated cardiomyopathy, orthotopic cardiac transplantation was performed and the patient was put on immunosuppressive drugs: prednisone, cyclosporine, and mycophenolate mofetil. Post-operative thoracic computed tomography revealed bilateral hemothorax and pulmonary atelectasis but no lymphadenopathy or other pulmonary abnormalities.
The patient was regularly followed in the post-transplantation period and was doing well, with adequate function of the graft and no significant adverse events. Nevertheless, two and a half years after the transplantation, she died from acute heart failure in another hospital. No autopsy was performed.
Pathological Examination
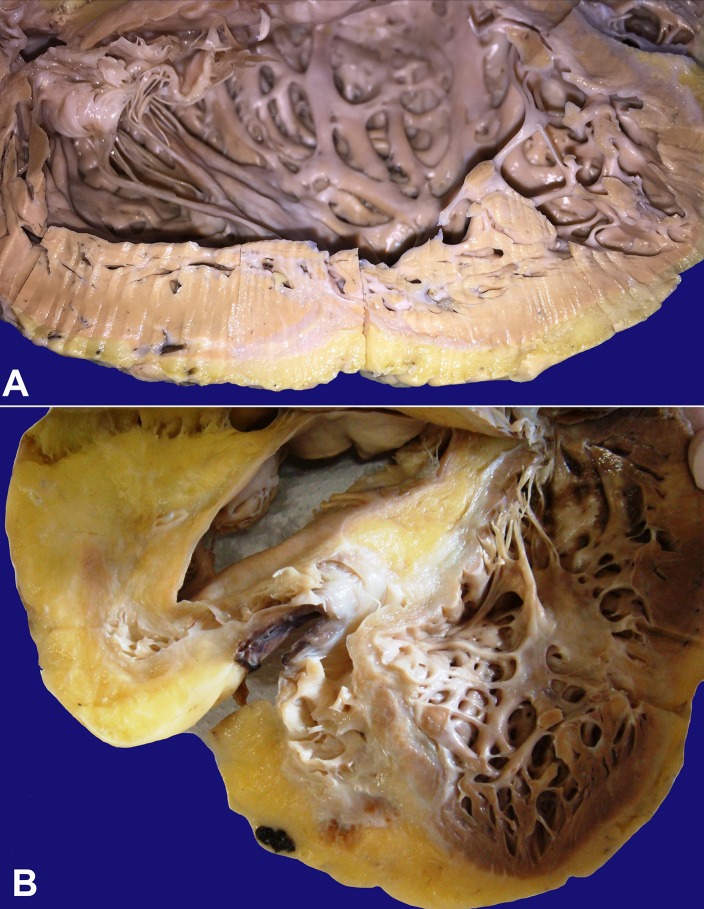
The explanted heart weighed 450 g (mean reference value for a 60 kg female = 262 g), had a round shape, and both ventricles formed the apex. On the epicardial surface there was a moderate amount of fat, mainly in the anterior right ventricle (RV). The coronary arteries showed no obstruction, and only mild atherosclerotic lesions were seen. The RV and the right atrium were dilated. The LV showed moderate dilatation and the septum was arched into the RV. No intracavitary thrombi were seen. There were light-colored areas in the free LV wall and septum suggesting fibrosis (Figure 1A). A great amount of fat was encountered in the RV wall, mainly in the anterior, inferior, and lateral regions, with a diffuse and severely reduced width of the muscular layer, but there was no aneurysmatic formation (Figure 1B). In the anterior RV wall, almost all the muscle was replaced by fat.
Figure 1. Gross pathology of the heart. A - LV showing wall thinning and a grey-whitish fibrous tissue spreading from the epicardium towards the myocardium; B - Severe fibrofatty replacement of the RV and the interventricular septum.
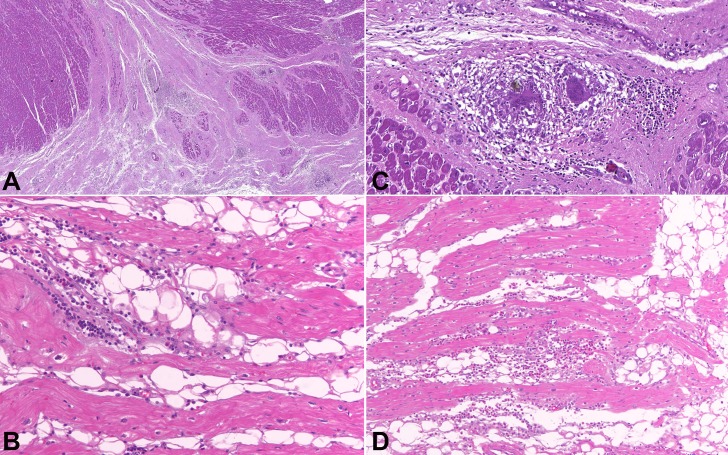
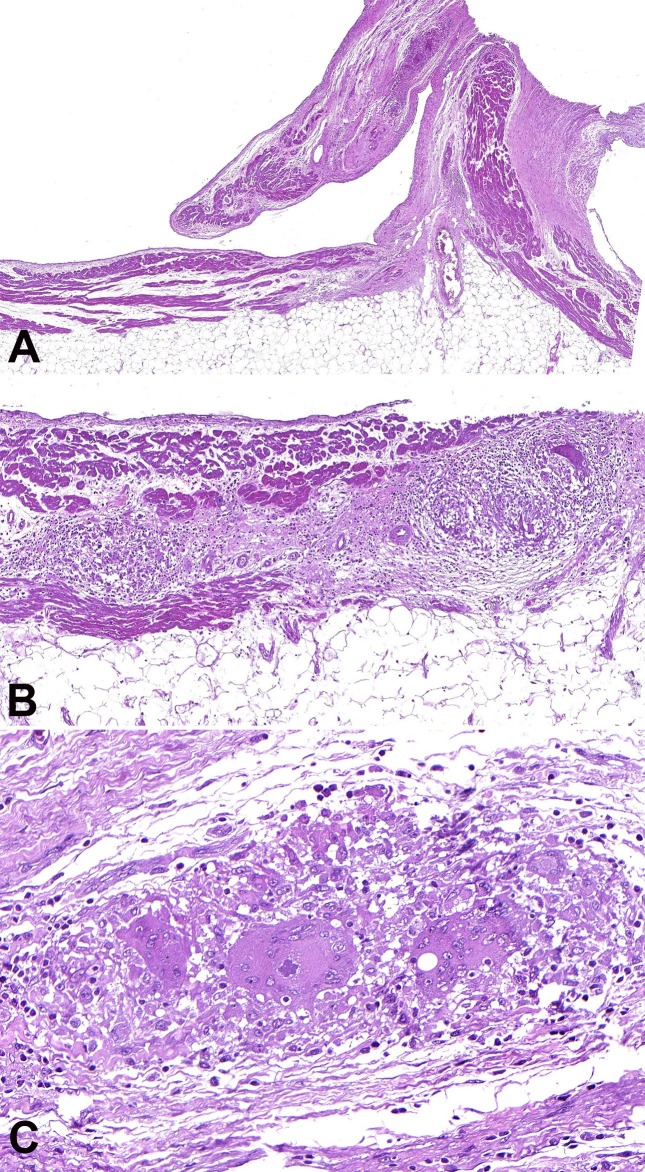
The histologic examination included hematoxylin and eosin stain (H&E), Masson trichrome, periodic acid-Schiff (PAS), and Wade stain for acid-alcohol resistant bacilli. In the LV wall, there were multiple areas of fibrosis (Figure 2A); fatty tissue separating myocardial fascicles (Figure 2B); numerous epithelioid granulomas with multinucleated giant cells and no central necrosis (Figure 2C); and scattered interstitial, predominantly mononuclear, inflammatory infiltrates, with a significant number of eosinophils in some areas (Figure 2D). No infectious agents were found. The RV wall was almost completely replaced with fibrofatty tissue (Figure 3A). In the remaining few layers of the RV myocardium, there were myocytes in degeneration along with inflammatory infiltrates and several well-formed granulomas with multinucleated giant cells and no central necrosis (Figure 3B). Some of the giant cells contained cytoplasmic basophilic structures of uncertain nature, surrounded by a clear halo (Figure 3C).
Figure 2. Photomicrography of the myocardium, LV histological analysis. A - Free wall with a great amount of subepicardial fibrous tissue penetrating muscular fascicles, and scattered, well-formed granulomas; B - Fatty tissue separating myocardial fascicles and moderate inflammatory infiltrate; C - Well-formed myocardial epithelioid granuloma with giant multinucleated cells and no necrosis; D - Inflammatory infiltrate with a significant number of eosinophils. H&E stain.
Figure 3. Photomicrography of the myocardium, RV histological analysis. A - Extensive fibrofatty replacement of the wall with conspicuous thinning of the muscular layer; B - Granulomatous inflammation; C - Basophilic structure with a polyhedral shape and a clear halo in the cytoplasm of a giant cell. H&E stain.
ANATOMIC DIAGNOSIS
Cardiac sarcoidosis with severe RV involvement.
Confocal Immunofluorescence Analysis
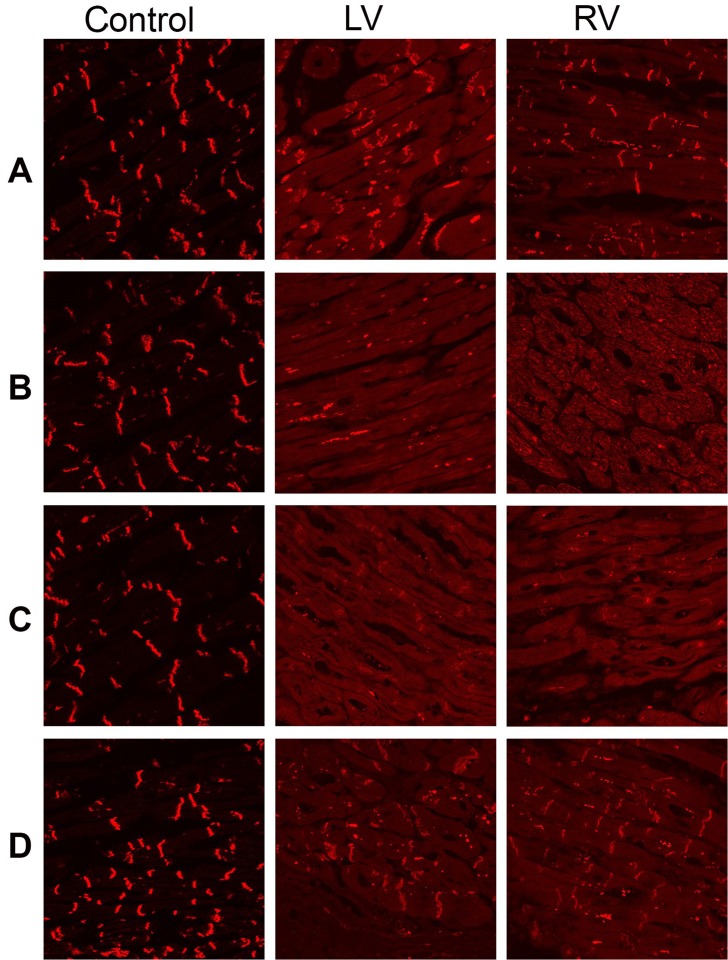
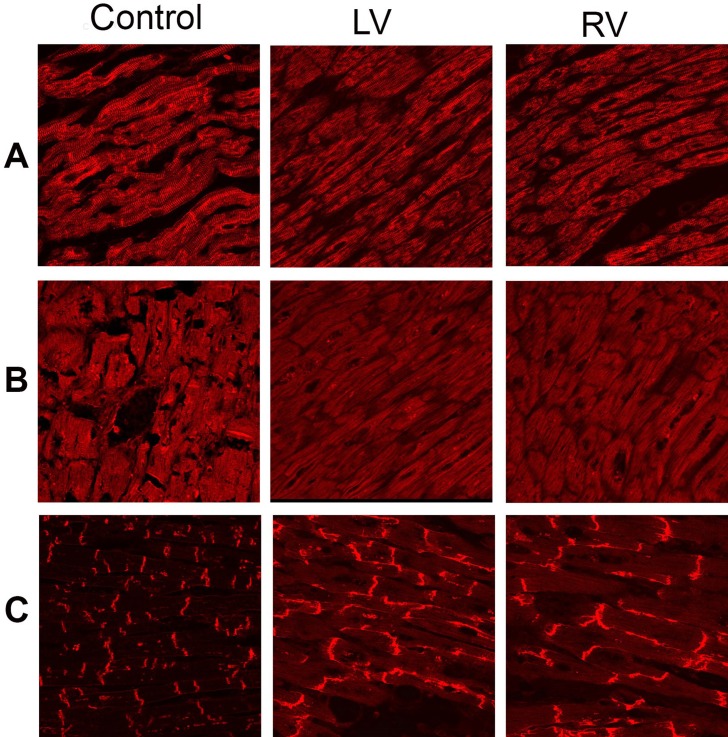
The immunoreactive signal for the non-desmosomal protein N-cadherin (control protein) was strong and indistinguishable from the controls, indicating that the tissue samples were well-preserved and suitable for analysis (Figure 4A). The immunoreactive signal for the desmosomal protein plakoglobin was severely depressed at the cardiac intercalated disks compared to the controls (Figure 4B); the same occurred for the immunoreactive signal for desmoplakin (Figure 4C). The signal for plakophillin-2 was strong and was not different from the controls (Figure 4D). The desmin (Figure 5A) and the SAP97 (Figure 5B) signals were strong in a sarcomeric distribution, but were severely reduced at the intercalated disks in relation to the controls. The signal for the major gap junction protein Cx43 was strong but showed profound lateralization (Figure 5C). Specimens from three hearts with no evidence of disease were used as the controls.
Figure 4. Representative confocal immunofluorescence images from the right and left ventricular myocardium of the patient and a control heart. A - Normal immunoreactive signal for the non-desmosomal protein N-cadherin (control used to judge the quality of the sample); B - Severely depressed immunoreactive signal for plakoglobin; C - Reduced immunoreactive signal for desmoplakin; D - Normal immunoreactive signal for plakophilin-2. LV = left ventricle; RV = right ventricle.
Figure 5. Representative confocal immunofluorescence images from the right and left ventricular myocardium of the patient and a control heart. A - Normal sarcomeric distribution, but severely reduced junctional immunoreactive signal for desmin; B - Normal sarcomeric distribution, but severely reduced junctional immunoreactive signal for SAP97; C - Strong but lateralized immunoreactive signal for Cx43. LV = left ventricle; RV = right ventricle.
DISCUSSION
Concerning the granulomatous myocarditis of uncertain origin, it is important to differentiate between two anatomoclinical conditions: sarcoidosis and idiopathic giant cell myocarditis. Usually a systemic disease, but occasionally restricted to the heart, sarcoidosis seems to result from an abnormal immune response to different pathogenic stimuli in genetically susceptible hosts.1 Among such stimuli, some infectious agents are presumed to play a role as a trigger. Rather than being a distinct disease, sarcoidosis seems to be a final common pathway of several separate entities. There is an unquestionable family clustering and an increased risk (~5-fold) in siblings of affected individuals.2,3
Regarding cardiac sarcoidosis, most patients had little or no clinical evidence of dysfunction of any organ system other than the heart in a review of 113 autopsies.4 In another study, in 84 consecutive autopsied patients with systemic disease, 23 (27%) had myocardial granulomas, which were clinically silent in 8 (35%), but in 15 (65%) there was a history of heart failure, arrhythmias and/or conduction defects. Arrhythmias and sudden cardiac death were markedly more common among those with gross and severe disease.5
There are no pathognomonic histopathologic findings that allow an unequivocal diagnosis of sarcoidosis, which makes the diagnosis, mainly in cases restricted to the heart, a clinical challenge – “an imperfect science, a hesitant art”.6,7 The asteroid bodies can be found in less than 10% of cases and are not specific, whereas the Schaumann bodies, although present in about 80% of cases, are also encountered in other granulomatous conditions, such as the infectious ones and berylliosis. However, the presence of these cytoplasmic inclusion bodies favors the diagnosis of sarcoidosis, since they occur more frequently in this disease. The granulomas of sarcoidosis are epithelioid, non-necrotizing, tight, and naked; there is more extensive fibrosis and a modest eosinophilic infiltrate. On the other hand, in idiopathic giant cell myocarditis – a disease that usually presents in a catastrophic way, with severe and subacute heart failure, and heralds a worse prognosis – the granulomas are not well-formed; myocyte necrosis is frequent; and there is widespread or serpiginous inflammation with giant cells, lymphocytes, and a remarkable number of eosinophils.8
Although LV is the affected chamber in the great majority of patients, predominant RV disease has been found in sarcoidosis as well. In such cases, the RV showed remarkable wall thinning, along with conspicuous muscular hypotrophy and fibrous or, eventually, fibrofatty replacement.9-11 Mainly in the latter situation, it is mandatory to perform the differential diagnosis with arrhythmogenic right ventricular cardiomyopathy (ARVC). Both conditions have very similar clinical aspects, so sarcoidosis might be considered a phenocopy of ARVC. Even the epsilon waves, a classical electrocardiographic sign of ARVC, were described in one case of sarcoidosis.12 Some characteristics should raise suspicion for cardiac sarcoidosis over ARVC: older age of symptoms onset, nonfamilial pattern of disease, PR interval prolongation, high-grade atrioventricular block, significant left ventricular dysfunction, myocardial delayed enhancement of the septum on magnetic resonance, and mediastinal lymphadenopathy.13 Clinical differentiation is important once sarcoidosis patients may benefit from treatment with glucocorticoids, but not ARVC patients.
ARVC, previously (and improperly) called arrhythmogenic right ventricular dysplasia, is an under-recognized entity with a broad clinical and pathological spectrum. It is a clinically and genetically heterogeneous progressive disorder of the heart muscle, which is associated with ventricular arrhythmias and the risk of sudden death, particularly in the young and in athletes.14 First described as a distinct heredofamilial disease by Giovanni Maria Lancisi in 1736, ARVC may be seen as a “paradigm of translational medicine”.15,16 In most cases, the RV is predominantly affected – hence the expression ARVC – but some degree of LV involvement, such as inflammation and myocyte degeneration, is present in up to 76% of cases, mainly in the posterior and lateral walls.17 In fact, the spectrum of the disease is wider than initially thought, with the identification of biventricular or even isolated LV forms in addition to the more frequent RV involvement. Based on these findings, the condition is increasingly being referred to simply as “arrhythmogenic cardiomyopathy”.14
In the early stage – the “concealed phase” – there is no gross structural abnormality, but the patients have a higher risk of sudden cardiac death from ventricular arrhythmias, mainly during exercise; histologically, there is inflammation and myocyte degeneration. Macroscopic structural abnormalities may develop sooner or later. They are characterized by ventricular wall thinning due to myocytes loss and fibrous or fibrofatty replacement, occasionally giving rise to aneurysms, and finally evolving to global ventricular dilatation. During this phase, there may be arrhythmic manifestations, intraventricular conduction abnormalities, repolarization delays and/or heart failure, and the clinical picture is indistinguishable from that found in other types of dilated cardiomyopathies.
The disease has a marked segmental pattern. Classically, it has been accepted that the RV inflow tract, the outflow tract, and the apex were the most affected areas, the so-called “triangle of dysplasia”. More recently, however, this concept has been challenged, and a different pattern of distribution – the epicardial subtricuspid region, the basal free RV wall, the LV lateral wall, but sparing the RV apex – was described as the “displacement of RV apex from the triangle of dysplasia”.18 This pattern would be useful in differentiating ARVC from other etiologies of heart disease, such as sarcoidosis and idiopathic dilated cardiomyopathy. The diagnosis of ARVC is not easy and there is no gold standard test or criterion, but a set of aspects should be considered. In 1994, an International Task Force proposed some criteria for the diagnosis of the disease; in 2010, those criteria were revised to improve sensitivity, mainly of the early forms, without reducing specificity.19
Although the pronounced fat deposition in ARVC is remarkable at heart examination, the amount of fat is the least reliable criterion for its diagnosis, and a thorough and detailed analysis is mandatory in order to avoid overdiagnosis. Inflammation, myocyte degeneration, and fibrofatty replacement are the distinguishing characteristics. Fat deposition in the heart is a well-known and common finding. The so-called adipositas cordis refers to fat accumulation in the epicardial surface, especially in the anterolateral surface of the RV and the interventricular furrows, mainly in obese women with hypertension and/or atherosclerotic coronary artery disease. There is a more or less clear distinction in the boundary between the fatty and the muscular layers, and the muscular wall thickness is preserved. In the so-called “fatty infiltration of the myocardium”, the RV is again the most commonly affected area, and three patterns of fat deposition have been described: (1) around vessels and nerves; (2) separating the myocardium into coarse bundles; and (3) separating the muscular fibers, which are then subject to regressive alterations. None of these conditions is usually associated with any clinical manifestations, and there are controversies about their pathological meaning. It is noteworthy that fatty tissue can reach up to 50% of the total heart weight; moreover, in normal hearts the thickness of the epicardial fat can reach up to 13.6 mm in the free wall of the RV.20,21
In our case, the presence of multiple well-formed granulomas with no central necrosis, in a patient who presented with heart failure progressing over a few years to the point of heart transplantation, suggested the diagnosis of cardiac sarcoidosis. No extracardiac manifestations of sarcoidosis were clinically identified, although an autopsy was not performed. Nevertheless, the severe involvement of the right ventricle, with conspicuous reduction in the muscular layer thickness and fibrofatty replacement, makes this a remarkable case of sarcoidosis masquerading as ARVC. The significant eosinophilic infiltrate observed in some areas of the myocardium was attributed to a presumptive hypersensitivity reaction to the pre-transplantation dobutamine infusion.22
ARVC is usually inherited as an autosomal dominant trait. Only 30-50% of patients have an abnormal gene that has been identified as causing the disease, but this percentage is variable and ranges from 26% to 58%, with the higher percentage in patients with clinical familial disease.23 Mutations in the plakophilin-2 gene are more common, but they also have been found in desmoglein-2, desmocollin-2, desmoplakin, and plakoglobin, among others. However, the interpretation of an “abnormal gene” for ARVC must also take into consideration the probability that the gene identified as abnormal is indeed causative. Disease manifestation and progression is known to be affected by additional mutations (digenic or compound heterozygosity), otherwise benign polymorphisms in desmosomal genes, mutations in genes yet to be identified, and environmental factors, particularly exercise.
According to some authors, changes in the plakophilin-2 gene sequence have been found in cases of sudden unexpected death with negative autopsy in the same frequency as in ARVC cases, highlighting the arrhythmogenic effect of these mutations, even in the absence of fibrofatty, inflammatory or degenerative myocardial alterations.24 Other authors have found desmosomal mutations typically related to ARVC in idiopathic familial dilated cardiomyopathy.25,26 These findings question the relationship between the morphological findings of ARVC and desmosomal mutations, and prompts a discussion of the basis on which the definition of ARVC should be made: classical morphological criteria or the presence of desmosomal protein gene mutations with different phenotypic manifestations?
The pathogenesis of ARVC is an evolving field, but the most accepted proposed mechanism is that impaired genetically determined desmosome functioning under conditions of mechanical stress (altered shear response without changes in cell-to-cell adhesion) is thought to cause myocyte detachment and death.27 The myocardial injury is usually accompanied by inflammation, with subsequent repair by fibrofatty replacement. It looks like disease progression might occur in bursts, the so-called “hot phases”, which are usually asymptomatic, but can sometimes present with arrhythmias and even chest pain.28 The desmosome has not only mechanical functions, responsible for maintaining the structural integrity of the myocardium by resisting shear forces, but also electrical and transcriptional functions. Because some of the desmosomal proteins (such as plakoglobin) play roles as structural proteins in cell-to-cell mechanical junctions and as signaling molecules, the pathogenesis of ARVC also might be related to altered nuclear signaling.29 In fact, there is evidence that plakoglobin translocation to the nucleus could cause a myogenic to adipogenic switch in cardiac progenitor cells, due to the ability of plakoglobin to sequester T-cell / lymphoid-enhancing binding (Tcf/Lef) transcription factors, thus suppressing the pro-myogenic canonical Wnt/β-catenin signaling pathway.30-33 To explain the electrical instability, that is brought about even before structural abnormalities appear, current hypotheses implicate acute cell death, gap-junction remodeling, and ion-channel crosstalk, in addition to re-entry arrhythmias caused by fibrofatty replacement.14
Naxos disease, which is caused by a mutation in the plakoglobin gene, and Carvajal syndrome, which is caused by a mutation in the desmoplakin gene, are autosomal recessive cardiocutaneous syndromes that present with woolly hair, palmoplantar keratosis, and cardiomyopathy. In Carvajal syndrome, it was observed, in a so far single studied case by confocal immunofluorescence analysis, a normal sarcomeric distribution of desmin, the major constituent of intermediate filaments that interact with the C-terminal region of desmoplakin, but its absence at intercalated disks. In that case, it was also identified a C-terminal desmoplakin gene truncating mutation, and it was speculated that it could impair the interaction between the desmoplakin and the intermediate filaments of the cytoskeleton, leading to a cardiomyopathy with biventricular involvement.34 As in Carvajal syndrome, in our case confocal immunofluorescence analysis revealed a pattern of sarcomeric distribution of desmin, which was not detected at the intercalated disks (as in normal subjects), raising the question of whether there would be a truncating mutation in the C-terminal region of the desmoplakin protein. Unfortunately, as the patient had died from acute heart failure in another institution and no blood samples remained stored, genetic analysis could not be performed.
Besides the sarcomeric distribution of desmin, other important confocal immunofluorescence findings were identified in our case. Like the immunoreactive signal for desmin, the SAP97 immunoreactive signal was strong in a sarcomeric distribution, but was severely reduced at the intercalated disks compared to the controls. SAP97 is a MAGUK protein known to regulate the forward trafficking of sodium and potassium channel subunits. More recently, it has been shown that SAP97 holds a key role in the forward trafficking of plakoglobin to the cell membrane and that SAP97-mediated trafficking is critical in the disease pathway in ARVC.35,36 We also found a severe depression of the immunoreactive signal for plakoglobin at the intercalated disks. This finding was once considered to be a distinguishing feature of ARVC, in order to be proposed as a diagnostic test.37 Thereafter, however, it was demonstrated that the depressed immunoreactive signal for plakoglobin can also be seen in sarcoidosis and giant cell myocarditis, but not in lymphocytic myocarditis. It was hypothesized that inflammatory mechanisms would be implicated in plakoglobin redistribution from junctional to intracellular sites in ARVC and in granulomatous myocarditis, suggesting potential mechanistic links in the pathogenesis of those entities.38 Finally, we found that the Cx43 signal was normal and strong, but intensely lateralized on confocal immunofluorescence analysis, whereas in the normal heart control the Cx43 signal was observed only at the intercalated disks. Translocation of the immunoreactive signal for Cx43 from the junctional to the lateral myocyte membranes (termed “lateralization”) is a sign of adverse remodeling to the stress faced by the myocardium. Previous studies have revealed a diminished or normal Cx43 signal in ARVC cases, but no mention has been made of lateralization. However, downregulation or redistribution of Cx43 is a common finding in several forms of cardiomyopathies, such as hypertrophic, ischemic and idiopathic dilated, in some cases presenting a lateralized pattern, which makes this a nonspecific finding.39
To the best of our knowledge, only one case of ARVC and sarcoidosis association has been described so far.40 In that case, there was evidence of extracardiac sarcoidosis manifestations, and the diagnosis of ARVC was based on the finding of extensive RV fibrofatty replacement, with no other ancillary tests, as confocal immunofluorescence analysis.
The clinical diagnosis in our case was idiopathic dilated cardiomyopathy. In a series of 314 patients having undergone cardiac transplantation, clinical and post-transplantation morphological diagnoses were congruent in 87% of cases. However, all clinical sarcoidosis and ARVC diagnoses were missed in this series, highlighting the difficulty of the identification of these cardiomyopathies.41
Some patients with sarcoidosis present with refractory heart failure and undergo cardiac transplantation. Besides the possible complications inherent to this procedure, there is a risk of sarcoidosis recurrence in the transplanted organ (2 out of 8 in a series of patients).42 However, patients with sarcoidosis undergoing orthotopic heart transplantation seem to have a better short- and intermediate-term survival than the majority of heart transplantation recipients.43 Our patient died from acute heart failure two and a half years after transplantation. The exact cause could not be determined due to the rapid and fatal evolution, but it could have been due to rejection of the graft or recurrence of the original heart disease, since recurrence of sarcoidosis in the cardiac graft has been reported to occur as early as 24 weeks after transplantation.43
In conclusion, herein we present the case of a patient who underwent heart transplantation for cardiac sarcoidosis with severe RV involvement characterized by a markedly reduced width of the muscular layer and fibrofatty replacement. Prior to pathologic evaluation of the explanted heart, a presumptive diagnosis of idiopathic dilated cardiomyopathy was made. Confocal immunofluorescence analysis revealed a desmin sarcomeric distribution pattern, with no signal at the intercalated disks, a finding that was previously described in an 11-year-old girl with Carvajal syndrome bearing a desmoplakin gene truncating mutation at the C-terminal region. Although we have not been able to search for a genetic component, this case highlights the importance of the differential diagnosis among isolated cardiac sarcoidosis, ARVC and idiopathic dilated cardiomyopathy, which are clinical entities that appear to have more overlap than originally believed.
Footnotes
Siqueira WC, Cruz SG, Asimaki A, et al. Cardiac sarcoidosis with severe involvement of the right ventricle: a case report. Autopsy Case Rep [Internet]. 2015;5(4):53-63. http://dx.doi.org/10.4322/acr.2015.030
REFERENCES
- 1.Spagnolo P, Grunewald J. Recent advances in the genetics of sarcoidosis. J Med Genet. 2013;50(5):290-7. http://dx.doi.org/10.1136/jmedgenet-2013-101532. PMid: [DOI] [PubMed] [Google Scholar]
- 2.McGrath DS, Daniil Z, Foley P, et al. Epidemiology of cardiac sarcoidosis in the UK. Thorax. 2000;55(9):751-4. http://dx.doi.org/10.1136/thorax.55.9.751. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iannuzzi MC. Genetics of sarcoidosis. Semin Respir Crit Care Med. 2007;28(1):15-21. http://dx.doi.org/10.1055/s-2007-970330. PMid: [DOI] [PubMed] [Google Scholar]
- 4.Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart: a clinicopathologic study of 35 necropsy patients (group I) and review of 78 previously described necropsy patients (group II). Am J Med. 1977;63(1):86-108. http://dx.doi.org/10.1016/0002-9343(77)90121-8. PMid: [DOI] [PubMed] [Google Scholar]
- 5.Silverman KJ, Hutchins JM, Bulkley BH. Cardiac sarcoid: a clinicopathological study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58(6):1204-11. http://dx.doi.org/10.1161/01.CIR.58.6.1204. PMid: [DOI] [PubMed] [Google Scholar]
- 6.Lagana SM, Parwani AV, Nichols LC. Cardiac sarcoidosis: a pathology-focused review. Arch Pathol Lab Med. 2010;134(7):1039-46. PMid: [DOI] [PubMed] [Google Scholar]
- 7.Sharma OP. Diagnosis of cardiac sarcoid: an imperfect science, a hesitant art. Chest. 2003;123(1):18-9. http://dx.doi.org/10.1378/chest.123.1.18. PMid: [DOI] [PubMed] [Google Scholar]
- 8.Okura Y, Dec GW, Hare JM, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41(2):322-9. http://dx.doi.org/10.1016/S0735-1097(02)02715-8. PMid: [DOI] [PubMed] [Google Scholar]
- 9.Halushka MK, Yuh DD, Russell SD. Right ventricle-dominant cardiac sarcoidosis with sparing of the left ventricle. J Heart Lung Transplant. 2006;25(4):479-82. http://dx.doi.org/10.1016/j.healun.2005.11.442. PMid: [DOI] [PubMed] [Google Scholar]
- 10.Vakil K, Minami E, Fishbein DP. Right ventricular sarcoidosis: Is it time for updated diagnostic criteria? Tex Heart Inst J. 2014;41(2):203-7. http://dx.doi.org/10.14503/THIJ-12-3086. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ott P, Marcus FI, Sobonya RE, Morady F, Knight BP, Fuenzalida CE. Cardiac sarcoidosis masquerading as right ventricular dysplasia. Pacing Clin Electrophysiol. 2003;26(7):1498-503. http://dx.doi.org/10.1046/j.1460-9592.2003.t01-1-00217.x. PMid: [DOI] [PubMed] [Google Scholar]
- 12.Khaji A, Zhang L, Kowey P, Martinez-Lage M, Kocovic D. Mega-epsilon waves on 12-lead ECG: just another case of arrhythmogenic right ventricular dysplasia/cardiomyopathy? J Electrocardiol. 2013;46(6):524-7. http://dx.doi.org/10.1016/j.jelectrocard.2013.08.007. PMid: [DOI] [PubMed] [Google Scholar]
- 13.Philips B, Madhavan S, James CA, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis: distinguishing features when the diagnosis is unclear. Circ Arrhythm Electrophysiol. 2014;7(2):230-6. http://dx.doi.org/10.1161/CIRCEP.113.000932. PMid: [DOI] [PubMed] [Google Scholar]
- 14.Basso C, Bauce B, Corrado D, Thiene G. Pathophysiology of arrhythmogenic cardiomyopathy. Nat Rev Cardiol. 2012;9(4):223-33. http://dx.doi.org/10.1038/nrcardio.2011.173. PMid: [DOI] [PubMed] [Google Scholar]
- 15.Thiene G. Arrhythmogenic cardiomyopathy: from autopsy to genes and transgenic mice. Cardiovasc Pathol. 2012;21(4):229-39. http://dx.doi.org/10.1016/j.carpath.2011.09.012. PMid: [DOI] [PubMed] [Google Scholar]
- 16.Thiene G. The research venture in arrhythmogenic right ventricular cardiomyopathy: a paradigm of translational medicine. Eur Heart J. 2015;36(14):837-46. http://dx.doi.org/10.1093/eurheartj/ehu493. PMid: [DOI] [PubMed] [Google Scholar]
- 17.Corrado D, Basso C, Thiene G, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30(6):1512-20. http://dx.doi.org/10.1016/S0735-1097(97)00332-X. PMid: [DOI] [PubMed] [Google Scholar]
- 18.Te Riele ASJM, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical challenges in a changing disease spectrum. Trends Cardiovasc Med. 2015;25(3):191-8. http://dx.doi.org/10.1016/j.tcm.2014.11.003. PMid: [DOI] [PubMed] [Google Scholar]
- 19.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force criteria. Circulation. 2010;121(13):1533-41. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.840827. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basso C, Thiene G. Adipositas cordis, fatty infiltration of the right ventricle, and arrhythmogenic right ventricular cardiomyopathy: just a matter of fat? Cardiovasc Pathol. 2005;14(1):37-41. http://dx.doi.org/10.1016/j.carpath.2004.12.001. PMid: [DOI] [PubMed] [Google Scholar]
- 21.Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol. 1995;76(5):414-8. http://dx.doi.org/10.1016/S0002-9149(99)80116-7. PMid: [DOI] [PubMed] [Google Scholar]
- 22.Spear GS. Eosinophilic explant carditis with eosinophilia: ?Hypersensitivity to dobutamine infusion. J Heart Lung Transplant. 1995;14(4):755-60. PMid: [PubMed] [Google Scholar]
- 23.Marcus FI, Edson S, Towbin JA. Genetics of arrhythmogenic right ventricular cardiomyopathy: a practical guide for physicians. J Am Coll Cardiol. 2013;61(19):1945-8. http://dx.doi.org/10.1016/j.jacc.2013.01.073. PMid: [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Tavora F, Oliveira JB, et al. PKP2 mutations in sudden death from arrhythmogenic right ventricular cardiomyopathy (ARVC) and sudden unexpected death with negative autopsy (SUDNA). Circ J. 2012;76(1):189-94. http://dx.doi.org/10.1253/circj.CJ-11-0747. PMid: [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Pavia P, Syrris P, Salas C, et al. Desmosomal protein gene mutations in patients with idiopathic dilated cardiomyopathy undergoing cardiac transplantation: a clinicopathological study. Heart. 2011;97(21):1744-52. http://dx.doi.org/10.1136/hrt.2011.227967. PMid: [DOI] [PubMed] [Google Scholar]
- 26.Elliott P, O’Mahony C, Syrris P, et al. Prevalence of desmosomal protein gene mutations in patients with dilated cardiomyopathy. Circ Cardiovasc Genet. 2010;3(4):314-22. http://dx.doi.org/10.1161/CIRCGENETICS.110.937805. PMid: [DOI] [PubMed] [Google Scholar]
- 27.Hariharan V, Asimaki A, Michaelson JE, et al. Arrhythmogenic right ventricular cardiomyopathy mutations alter shear response without changes in cell-cell adhesion. Cardiovasc Res. 2014;104(2):280-9. http://dx.doi.org/10.1093/cvr/cvu212. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrado D, Basso C, Pilichou K, Thiene G. Molecular biology and clinical management of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart. 2011;97(7):530-9. http://dx.doi.org/10.1136/hrt.2010.193276. PMid: [DOI] [PubMed] [Google Scholar]
- 29.Saffitz JE. Arrhythmogenic cardiomyopathy and abnormalities of cell-to-cell coupling. Heart Rhythm. 2009;6(8, Suppl):S62-5. http://dx.doi.org/10.1016/j.hrthm.2009.03.003. PMid: [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Gras E, Lombardi R, Giocondo MJ, et al. Suppression of canonical Wnt/β-catenin by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116(7):2012-21. http://dx.doi.org/10.1172/JCI27751. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lombardi R, Dong J, Rodriguez G, et al. Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res. 2009;104(9):1076-84. http://dx.doi.org/10.1161/CIRCRESAHA.109.196899. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardi R, Cabreira-Hansen MG, Bell A, Fromm RR, Willerson JT, Marian AJ. Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res. 2011;109(12):1342-53. http://dx.doi.org/10.1161/CIRCRESAHA.111.255075. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swope D, Li J, Radice GL. Beyond cell adhesion: the role of armadillo proteins in the heart. Cell Signal. 2013;25(1):93-100. http://dx.doi.org/10.1016/j.cellsig.2012.09.025. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan SR, Gard JJ, Carvajal-Huerta L, Ruiz-Cabezas JC, Thiene G, Saffitz JE. Structural and molecular pathology of the heart in Carvajal syndrome. Cardiovasc Pathol. 2004;13(1):26-32. http://dx.doi.org/10.1016/S1054-8807(03)00107-8. PMid: [DOI] [PubMed] [Google Scholar]
- 35.Asimaki A, Kapoor S, Plovie E, et al. . Abstract 15076: altered trafficking of junctional proteins mediated by SAP97 in arrhythmogenic cardiomyopathy. Circulation. 2013;128:A15076. [Google Scholar]
- 36.Asimaki A, Kapoor S, Plovie E, et al. Identification of a new modulator of the intercalated disk in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6(240):240ra74. http://dx.doi.org/10.1126/scitranslmed.3008008. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asimaki A, Tandri H, Huang H, et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360(11):1075-84. http://dx.doi.org/10.1056/NEJMoa0808138. PMid: [DOI] [PubMed] [Google Scholar]
- 38.Asimaki A, Tandri H, Duffy ER, et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4(5):743-52. http://dx.doi.org/10.1161/CIRCEP.111.964890. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fontes MSC, van Veen TAB, de Bakker JMT, , van Rijen HVM. Functional consequences of abnormal Cx43 expression in the heart. Biochim Biophys Act. 2012; 1818(8): 2020-9. http://dx.doi.org/10.1016/j.bbamem.2011.07.039. PMid: [DOI] [PubMed] [Google Scholar]
- 40.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises: case 34-1996: a 50-year-old woman with cardiac disease, an electronic pacemaker, and cardiac arrest in ventricular fibrillation. N Engl J Med. 1996;335(18):1378-86. http://dx.doi.org/10.1056/NEJM199610313351808. PMid: [DOI] [PubMed] [Google Scholar]
- 41.Roberts WC, Roberts CC, Ko JM, Filardo G, Capehart JE, Hall SA. Morphologic features of the recipient heart in patients having cardiac transplantation and analysis of the congruence and incongruence between the clinical and morphologic diagnoses. Medicine. 2014;93(5):211-35. http://dx.doi.org/10.1097/MD.0000000000000038. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yager JEE, Hernandez AF, Steenbergen C, et al. Recurrence of cardiac sarcoidosis in a heart transplant recipient. J Heart Lung Transplant. 2005;24(11):1988-90. http://dx.doi.org/10.1016/j.healun.2005.02.016. PMid: [DOI] [PubMed] [Google Scholar]
- 43.Zaidi AR, Zaidi A, Vaitkus PT. Outcome in heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant. 2007;26(7):714-7. http://dx.doi.org/10.1016/j.healun.2007.05.006. PMid: [DOI] [PubMed] [Google Scholar]