Abstract
Introduction:
Absolute and comparative risk perceptions, worry, perceived severity, perceived benefits, and self-efficacy are important theoretical determinants of tobacco use, but no measures have been validated to ensure the discriminant validity as well as test-retest reliability of these measures in the tobacco context. The purpose of the current study is to examine the reliability and factor structure of a measure assessing smoking-related health cognitions and emotions in a national sample of current and former heavy smokers in the National Lung Screening Trial.
Methods:
A sub-study of the National Lung Screening Trial assessed current and former smokers’ (age 55–74; N = 4379) self-reported health cognitions and emotions at trial enrollment and at 12-month follow-up. Items were derived from the Health Belief Model and Self-Regulation Model.
Results:
An exploratory factor analysis of baseline responses revealed a five-factor structure for former smokers (risk perceptions, worry, perceived severity, perceived benefits, and self-efficacy) and a six-factor structure for current smokers, such that absolute risk and comparative risk perceptions emerged as separate factors. A confirmatory factor analysis of 12-month follow-up responses revealed a good fit for the five latent constructs for former smokers and six latent constructs for current smokers. Longitudinal stability of these constructs was also demonstrated.
Conclusions:
This is the first study to examine tobacco-related health cognition and emotional constructs over time in current and former heavy smokers undergoing lung screening. This study found that the theoretical constructs were stable across time and that the factor structure differed based on smoking status (current vs. former).
Introduction
Health cognitions have long been recognized as important constructs that influence tobacco use and smoking cessation,1 and they play an important role in several health behavior models. Numerous health behavior models have been developed to identify cognitive and affective factors that influence behavior change. The Health Belief Model (HBM) posits that behavior depends primarily on the value placed by individuals on a particular goal and their estimate of the likelihood that a given action will achieve that goal.2 HBM suggests that before taking health-relevant actions (to avoid or prevent illness), individuals assess disease severity, perceived susceptibility, the perceived benefits of enacting change to reduce the risk, and level of self-efficacy.2 Perceived susceptibility is an assessment of one’s chances of being afflicted with a certain disease, which can be further separated into estimates of one’s absolute (own) risk and comparative risk (one’s risk relative to that of other people). Perceived severity refers to one’s belief about how serious the consequences of this disease might be for oneself. Perceived benefits are one’s beliefs about the positive outcomes associated with a health-relevant action to reduce disease risk. Lastly, self-efficacy is the confidence one has to execute the health-relevant action.3
Another factor that may influence behavior change, but is not considered in the HBM, is affect. The Self-Regulation Model4 posits that behavioral choices derive from an interplay of personal cognitive and emotional evaluations of an illness threat and behavioral response. For example, a smoker might experience a cough that triggers both cognitive evaluations (eg, “perhaps I am coming down with a cold, but maybe it is something worse because I smoke”) and emotional evaluations (eg, “I am worried about what the cough might represent because I smoke”). Adding an emotional component such as worry (frequency and intensity) to the measurement of HBM cognitive constructs of behavior change affords a more comprehensive examination of the predictors of behavior change. Research has shown that affect related to risk, including worry, is distinguishable from commonly used cognitive measures such as perceived susceptibility.5,6 Moreover, affective responses such as worry are often stronger predictors of health behavior than risk perception alone, and can interact with risk, making both cognitive and affective evaluations important for consideration in any health context.7–9
Research relating the HBM constructs to smoking behavior has primarily focused on cessation, but with mixed results,10–16 which may be attributable to inconsistencies in how these constructs were measured. For example, Warnecke et al.16 found that perceived susceptibility predicted smoking cessation, whereas Aho10 only observed effects of perceived severity. Mallaghan and Pemberton12 found that perceived susceptibility, operationalized as risk perceptions of smoking harm, was significantly related to cessation, yet Croog and Richards11 found no HBM constructs related to smoking cessation.
Absolute and comparative risk perceptions of smoking have been treated with little consistency in measurement approach.17 Only one study to date, conducted with smokers recruited for a smoking cessation trial, has demonstrated cross-sectionally that comparative and absolute risk perceptions are distinct constructs as supported by an exploratory factor analysis (EFA).18 Moreover, research in the context of lung screening showed that current smokers with a long-term, heavy smoking history have distinct cognitions and emotions compared to those of former smokers with a long-term, heavy smoking history.19 For example, current smokers held significantly higher perceptions of worry about lung cancer and smoking related disease compared to former smokers.19
This study developed a smoking-related health cognition and emotion inventory focused on lung cancer and smoking-related disease (SRD) for long-term, older, current or former heavy smokers (30 pack-years or more). Older former and current smokers are both at increased risk for negative health outcomes.20 A better understanding of the underlying cognitions and emotions of smoking behavior may prove particularly useful in the development of future research and intervention strategies. In particular, longitudinal assessments are needed to determine the consistency and stability of these constructs. These constructs were examined as part of a longitudinal risk perception sub-study conducted with the National Lung Screening Trial (NLST). The NLST is a national clinical trial which determined the effectiveness of low-dose computed tomography compared to traditional chest x-ray screening in decreasing lung cancer-related morbidity and mortality. Clinical trial results have been reported elsewhere.21
The current study sought to examine participant responses to an NLST risk perception sub-study questionnaire in order to inform the measurement of smoking-relevant health beliefs. Despite empirical research demonstrating the importance of these constructs in tobacco research,1,22–27 no prior research has sought to demonstrate both the discriminant validity as well as the test-retest reliability of these measures.
Methods
Participants and Procedure
At the time of recruitment into NLST, participants were 55–74 years of age, current or former (quit within the past 15 years) smokers with a history of 30 pack-years or more, had no history of lung cancer, had not been treated for any cancer within the past 5 years other than a non-melanoma skin cancer, and were not participating in any other screening or cancer prevention trial. The American College of Radiology Imaging Network (ACRIN) NLST executive committee granted permission to administer the risk perception questionnaire as a sub-study within the ACRIN arm of the trial. Eight of the 23 ACRIN sites participated in the sub-study. From December 2003 to March 2004, all trial enrollees at these sites were invited to complete the sub-study questionnaire as part of the trial enrollment and 12-month follow-up screen. The risk perception sub-study began at the end of ACRIN trial enrollment, limiting the number of participants available to complete the sub-study questionnaire at baseline. Consequently, anyone entering the trial was invited to complete the sub-study questionnaire at baseline and 12-month follow-up, while those who had already entered the trial were invited to complete just the 12-month follow-up questionnaire. Nearly 90% of participants who completed the follow-up also completed the risk perception questionnaire.28 Baseline smoking status and recruitment site differed significantly for non-completers versus completers, and significantly more non-completers were current smokers.28 Prior to trial administration, the study questionnaire was cognitively tested with 15 participants from the Brown University ACRIN site (For additional details on the trial and risk perception sub-study methods, see NLST Research Team21; Park et al.19).
Data were categorized into baseline or 12-month follow-up categories. This resulted in 625 baseline participants (342 current and 283 former smokers; 214 participants had baseline data only), and 4165 follow-up participants (2094 current and 2070 former smokers; 410 longitudinal participants had both baseline and 12-month data). Thus, in total, 4379 participants were included in analyses. Participant characteristics and smoking history appear in Table 1.
Table 1.
Participant Characteristics at Baseline and Psychosocial Construct Means at Baseline and Follow-up
| Longitudinal sample | Total sample | |||
|---|---|---|---|---|
| Current smokers, n = 199 | Former smokers, n = 211 | Current smokers, n = 2237 | Former smokers, n = 2142 | |
| Sociodemographic characteristics | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 60.3 (4.5) | 61.8 (5.3) | 60.8 (4.8) | 62.2 (5.2) |
| N (%) | N (%) | N (%) | N (%) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 97 (48.7) | 133 (63.0) | 1242 (55.5) | 1252 (58.5) |
| Female | 102 (51.3) | 78 (37.0) | 996 (45.5) | 889 (41.5) |
| Race | ||||
| White | 178 (89.4) | 202 (95.7) | 2002 (89.5) | 2050 (95.7) |
| Black | 20 (10.1) | 7 (3.3) | 220 (9.8) | 77 (3.6) |
| Other | 1 (0.5) | 2 (0.9) | 17 (0.7) | 14 (0.7) |
| Ethnicity | ||||
| Hispanic/Latino | 0 (0.0) | 1 (0.5) | 12 (0.5) | 8 (0.4) |
| Non-Hispanic/Latino | 199 (100) | 210 (99.5) | 2225 (99.5) | 2132 (99.6) |
| Smoking characteristics | ||||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age started smoking | 16.6 (3.9) | 16.1 (2.9) | 16.5 (3.9) | 16.2 (3.4) |
| Number of years smoking | 43.2 (5.3) | 38.8 (7.6) | 43.8 (5.8) | 39.0 (8.8) |
| Pack-years | 56.5 (20.1) | 57.0 (24.0) | 56.1 (21.6) | 59.0 (26.7) |
| Cigarettes smoked per day | 26.3 (9.0) | 29.9 (12.3) | 25.7 (9.1) | 30.6 (12.7) |
| Psychosocial constructs | ||||
|---|---|---|---|---|
| Baseline constructs | Follow-up constructs | |||
| Current smokers, n = 342 | Former smokers, n = 283 | Current smokers, n = 2082 | Former smokers, n = 2057 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Absolute risk perceptions | 3.75 (0.82) | 3.43 (0.85) | 3.73 (0.79) | 3.43 (0.88) |
| Comparative risk perceptions | 3.64 (0.79) | 3.28 (0.78) | 3.66 (0.76) | 3.29 (0.81) |
| Overall risk perceptions | 3.68 (0.73) | 3.34 (0.75) | 3.69 (0.71) | 3.35 (0.79) |
| Worry | 2.49 (071) | 2.24 (0.68) | 2.53 (0.70) | 2.22 (0.66) |
| Perceived benefits | 3.00 (0.74) | 3.27 (0.67) | 2.99 (0.71) | 3.36 (0.61) |
| Perceived severity | 4.54 (0.57) | 4.65 (0.42) | 4.49 (0.54) | 4.59 (0.45) |
| Self-efficacy | 2.69 (1.12) | 4.63 (0.75) | 2.85 (1.13) | 4.65 (0.77) |
Only a subset of participants at baseline completed the risk perception questionnaire resulting in a smaller sample size (baseline sample). Additionally, of the subset of participants at baseline who completed the sub-study questionnaire, only a subset of those also completed the follow-up questionnaire (longitudinal sample). Chi-squares and t tests were used to compare the longitudinal sample to all other participants on baseline sociodemographic and smoking characteristics. None of these comparisons were statistically significant (all chi-squares < .45, all t tests < 1.88, all Ps > .06).
Measures
The current analysis focuses on 22 items included in the sub-study questionnaire. Items were developed based on constructs from the HBM and Self-Regulation Model.19 Questions appeared in the following order: risk perceptions, worry, self-efficacy to quit, perceived benefits of quitting, and perceived severity of smoking (Supplementary Table 1). As part of the trial, sociodemographic and smoking behavior items were collected (see www.acrin.org/Default.aspx?tabid=282 for the main NLST data forms).
Sociodemographic Information
Participants’ age, gender, race, and ethnicity were collected at baseline.
Smoking Behavior
Participants responded to open-ended questions: the age at which they began smoking daily, how many cigarettes they smoked per day at their highest smoking volume, and at what age they had quit (former smokers). Using this information, number of years spent smoking and pack years were calculated by multiplying the number of years spent smoking by the number of packs smoked per day.
Smoking Risk Perceptions
The sub-study risk perception questionnaire captured individuals’ perceptions of absolute and comparative risk first of lung cancer and then of other SRDs. Risk perceptions were assessed with 10 items29–31 (See Supplementary Table 1 for all items and alphas for each construct by smoking status and time point).
Absolute risk perception was assessed with four items. Comparative risk perception was assessed with six items. Comparative risk perceptions can differ based on the nature of the comparison group32; thus the questionnaire assessed comparative risk using three different referent groups; participants were asked if they were in danger of developing lung cancer and other SRDs compared to (1) the average person, (2) others of the same age and sex, and (3) other former/current smokers.
Self-Efficacy to Quit Smoking/Remain Quit
Self-efficacy was measured with a single item.33
Worry
Worry about lung cancer and other SRDs was assessed with four items about intensity and frequency of worry.29,34
Perceived Benefits of Quitting Smoking
Perceived benefits were assessed with three items that asked about the benefits of quitting in terms of reducing risk for lung cancer and SRDs.29,35
Perceived Severity
Perceived severity was measured with four items assessing the health consequences and severity of lung cancer and SRDs.36
Data Analysis
As it was determined that former and current smokers had distinct risk perceptions,19 all analyses were conducted separately for former and current smokers. An EFA using baseline data examined the smoking-relevant factors that emerged from the set of 22 items. Theorized constructs included perceived risk, worry, self-efficacy to quit, perceived benefits of quitting, and perceived severity of lung cancer and SRDs. We used confirmatory factor analysis (CFA) using the 12-month follow-up data to validate the exploratory factor structures identified. Finally, for participants with data available at both time points, we computed correlations between constructs at baseline and 12-month follow-up to assess the coefficient of stability, or reliability, separately for former and current smokers. Guided by the EFA and CFA results, we also created composite variables for each construct and assessed test-retest reliability across baseline and follow-up data.
We used SPSS v19 to conduct EFA of the baseline risk perception questionnaire data using principal components extraction and varimax rotation of factors (n = 625). EFA was used to explore the latent structure of the dataset, extract the factor structure, and examine internal reliability. We used MPlus version 637 to perform CFA on the follow-up sample (n = 3263) using a structural equation modeling approach with maximum likelihood estimation. CFA examines the extent to which a hypothesized factor structure is consistent with observed covariances.38 Our hypothesized factor structures follow theoretically from the HBM and were informed by the EFA. Self-efficacy was included as a measured variable rather than a construct, given that a self-efficacy construct would have only one indicator due to the single-item measure. Raw data were then imported into MPlus for analysis. CFA model fit was assessed using a number of methods: standardized root means square residual (SRMR; acceptable fit ≤ .08), root mean square error of approximation (RMSEA; acceptable fit confidence interval [CI] < .06 to .08), Tucker-Lewis Index (TLI; acceptable fit ≥ .95), and the comparative fit index (CFI, acceptable fit ≥ .95). We assessed modification indices to improve model fit, correlating error terms of indicators if the modification index for the correlation was above 50. Lastly, we computed correlation coefficients between baseline and follow-up risk perception questionnaire constructs to assess stability over time (n = 410).
Results
Exploratory Factor Analysis
A principal components analysis with varimax rotation was used to reduce the 22 baseline items to a set of factors. Five factors emerged for former smokers: risk perceptions, worry, perceived benefits, perceived severity, and self-efficacy. Communalities were all relatively high (no values < 0.52) which implied the variables were well defined by the five-factor structure. With a loading cut of 0.45, all variables loaded onto at least one factor, and there was one complex variable (loaded on more than one factor). The item capturing chance of getting a SRD compared to other smokers also loaded on the self-efficacy item (see online Supplementary Table 2 for rotated factor loadings and percent of variance explained by each factor).
Six factors emerged for current smokers: absolute risk perceptions, comparative risk perceptions, worry, perceived benefits, perceived severity, and self-efficacy. Communalities were all relatively high (no values < 0.67), implying the variables were well-defined by the six-factor structure. With a loading cut of 0.45, all variables loaded onto at least one factor, and there were no complex variables (See online Supplementary Table 2 for rotated factor loadings and percent of variance explained by each factor).
Confirmatory Factor Analysis
Next, two CFAs were conducted to validate the exploratory factor structures identified using 12-month follow-up data (former smoker n = 1624; current smoker n = 1639). Figures 1 and 2 present the final CFA results with standardized parameter estimates from the former and current smoker samples respectively, fit indexes, and squared multiple correlations indicating variance accounted for by the latent constructs for the observed variables.
Figure 1.
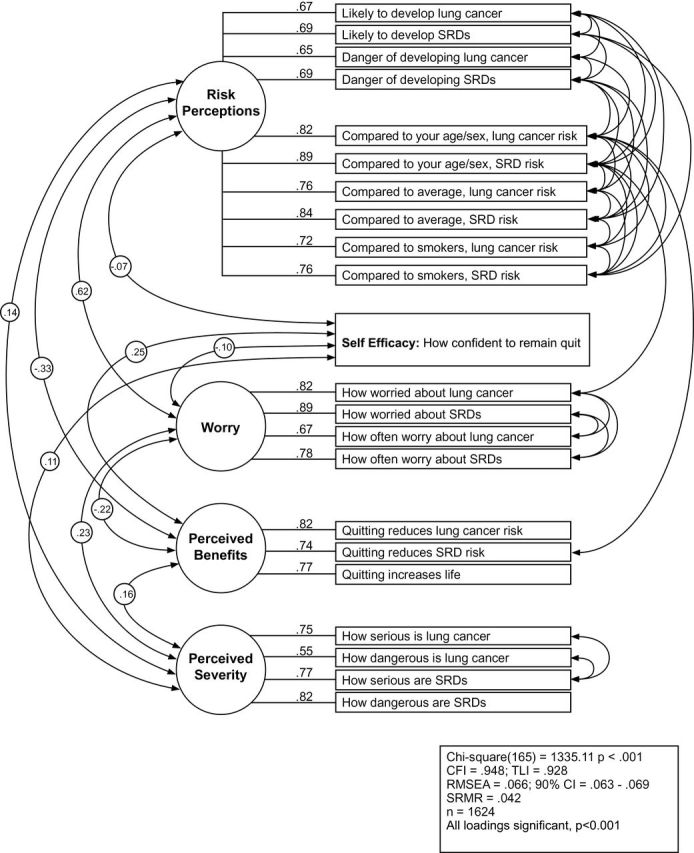
Confirmatory factor analysis for former cigarette smokers.
Figure 2.
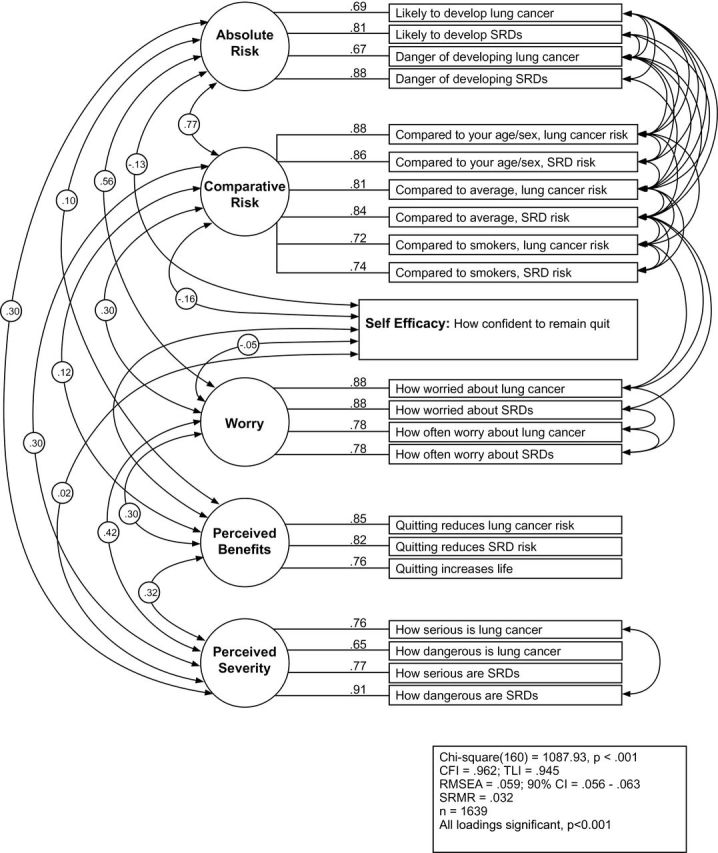
Confirmatory factor analysis for current cigarette smokers.
For former smokers, standardized factor loadings of items to five latent constructs ranged between 0.55 and 0.89 after the application of CFA (Figure 1). All factor loadings were significant. The fit indices for the original model (without correlations among error terms in accordance with modification indices) were χ2 (200) = 5818.45, P < .001, CFI = .752, TLI = .714, RMSEA = .132, CI = .129, 134, and SRMR = .057. Thirty-five correlations between error terms were incorporated into the final model in response to modification indices resulting in a final model fit of χ2 (165) = 1335.11, P < .001, CFI = .948, TLI = .928, RMSEA = .066, CI = .063, .069, and SRMR = .042.
For current smokers, factor loadings of items to six latent constructs ranged between 0.65 and 0.92 after the application of CFA (Figure 2). All factor loadings were significant. The fit indices for the original model were χ2 (195) = 5060.75, P < .001, CFI = .800, TLI = .763, RMSEA = .123, CI = .120, .126, and SRMR = .046. Thirty-five correlations between error terms were incorporated into the final model in response to modification indices, resulting in a final model fit of χ2 (160) = 1087.93, P < .001, CFI = .962, TLI = .945, RMSEA = .059, CI = .056, .063, and SRMR = .032.
Coefficient of Stability
Among participants with data at both baseline and 12-month follow-up (n = 410), we computed correlations between the constructs to assess the coefficient of stability, or reliability, separately for former and current smokers. For former smokers, correlations ranged from .40 to .68 (see the diagonals of Table 2 for former and current smoker correlations), indicating a moderate to high degree of continuity over the follow-up period.41 For current smokers, correlations ranged from .47 to .69, also indicating moderate to high degrees of continuity for constructs over the follow-up period.
Table 2.
Coefficient of Stability Analysis (Total n = 410)
| Former smokers (n = 211) | |||||
|---|---|---|---|---|---|
| Overall risk follow-up | Worry follow-up | Perceived benefits follow-up | Perceived severity follow-up | Self-efficacy follow-up | |
| Overall risk baseline | .68*** | .41*** | −.14**** | .17* | −.01 |
| Worry baseline | .50*** | .65*** | −.04 | .27*** | .004 |
| Perceived benefits baseline | −.15* | .01 | .58*** | .21** | .32*** |
| Perceived severity baseline | .18* | .29*** | .20** | .55** | .10 |
| Self-efficacy baseline | −.04 | −.11 | .18* | −.001 | .40*** |
| Current smokers (n = 199) | ||||||
|---|---|---|---|---|---|---|
| Absolute risk follow-up | Comparative risk follow-up | Worry follow-up | Perceived benefits follow-up | Perceived severity follow-up | Self-efficacy follow-up | |
| Absolute risk baseline | .49*** | .46*** | .35*** | .15**** | .34*** | −.03 |
| Comparative risk baseline | .56*** | .63*** | .46*** | .16* | .40*** | −.07 |
| Worry baseline | .30*** | .43*** | .69*** | .22** | .42*** | −.09 |
| Perceived benefits baseline | .28*** | .14**** | .26** | .49*** | .26** | .08 |
| Perceived severity baseline | .32*** | .41*** | .40*** | .22** | .59*** | −.03 |
| Self-efficacy baseline | −.11 | −.26** | −.03 | .02 | −.15**** | .47*** |
*P < .05; **P < .01; ***P < .001; ****P < .10.
Discussion
This study provides psychometric data on an inventory of smoking-related health cognitions and emotions, based on the HBM and Self-Regulation Model, which were included in a sub-study of the National Lung Screening Trial. An EFA with 22 items yielded a five-factor solution for former smokers and a six-factor solution for current smokers. The same pattern emerged in a CFA. This study also demonstrated that these smoking-related constructs remained stable across time among both current and former heavy smokers. Longitudinal stability in smokers’ risk perceptions has not been shown previously and, as found here, is particularly notable given that participants were undergoing screening that provided information on the presence or absence of lung abnormalities.28 This is also the first study to develop a smoking-related health belief and emotion inventory focused on lung cancer and SRD for a national sample of current and former smokers.
Health beliefs are the focus of a large amount of empirical research on tobacco control interventions.1,22–27 However, despite the centrality of these constructs in tobacco research, little work has been conducted to ensure the discriminant validity of these measures, a critical step in ensuring the validity of any measure.40 In a sample of heavy lifetime current and former smokers, we found evidence for the discriminability of health cognition and emotion constructs derived from major health behavior theories.2,4 Consistent results emerged from an EFA conducted on baseline items and a CFA from a 1-year follow-up assessment, increasing our confidence in the observed factor structure.
One important finding was that the factor structure differed for current versus former smokers, such that risk perception split into two distinct factors (absolute and comparative risk perceptions) for current but not former smokers. There are several potential explanations for this difference. Comparative risk evaluations require a deeper, more complex level of processing because they require both an assessment of personal risk and a comparison of that risk with that of a comparison target (eg, the average smoker). Thus, one possibility is that former smokers may engage in more in-depth cognitive processing of risk information than current smokers, leading to greater consistency in their absolute and comparative perceptions. Indeed, qualitative interviews with a subset of participants in the current sub-study suggested a deeper level of processing of personal risk by former compared to current smokers who might not engage in this level of processing.41 When asked about their risk of lung cancer, current smokers often gave “uncertain and vague” responses (eg, “I don’t know… I have no idea.”), whereas former smokers “were likely to give a definitive response, percentage, or a justification for their response” (Park et al.41, p. 169).
A related possibility is that current smokers may distinguish between absolute and comparative risk as a method of dissonance reduction. For instance, putting oneself at high perceived absolute risk of lung cancer by continuing to smoke may be easier to accept as long as one does not think the risk is high compared to others. Much work has demonstrated that current smokers engage in cognitive strategies to downplay their perceptions of risk and vulnerability, a type of dissonance reduction that discourages quitting.23,41–43 In theory, absolute and comparative risk perceptions may emerge as distinct constructs if current smokers prefer to minimize perceptions of both types of risk but are more constrained in their evaluations of one type than the other.44 Former smokers, on the other hand, have already completed behavior change with respect to smoking and have less reason to engage in motivated reasoning and biased information processing.45
Limitations
The sub-study questionnaire was administered to a subset of participants enrolled in the National Lung Screening Trial. Thus, it is limited in its scope and applicability given that the participants were current or former heavy (30 pack-years or more) smokers engaged in a clinical trial. The psychometrics of this instrument should be examined in other populations. Although smoking behavior was based on self-reports, research suggests this is a reliable measure of smoking behavior.46,47 The items were developed based on the HBM and Self-Regulation Model and thus do not include other constructs (eg, outcome expectancies, social norms, fear) that may be important. In addition, self-efficacy was only assessed using a single item of quitting self efficacy. A greater number of former smokers than current smokers completed follow-up, which may have had an impact on the stability coefficient; however given that the CFA replicated the exploratory factor analysis this is a minor concern. Despite these limitations, our findings are useful for furthering knowledge about health belief models and measurement of smoking-related health beliefs among current and former smokers.
Implications
Understanding the cognitions and emotions that influence smoking-related behavior change and maintenance is important to reducing tobacco related morbidity and mortality. By providing evidence that this inventory has sound psychometric properties with distinct factors maintained across time, this study provides researchers and clinicians with a framework for understanding and evaluating the constructs underlying smoking-related behavior change. Given the recently released national US Preventive Services Task Force guideline for annual lung cancer screening among people aged 55 to 80 with a 30 pack-year history of smoking,48 there will be many opportunities for patients and clinicians to discuss perceived risk, worry, perceived severity, perceived benefits of quitting, and self-efficacy to quit. Research reported here and elsewhere41 suggests that current smokers may benefit from opportunities to actively consider their risk, such as by asking them to generate their own list of consequences of smoking.24,49 The inventory developed and validated in this study can guide clinicians in assessing the smoking-related health cognitions and emotions of their lung screening patients and those considering undergoing lung screening.
Future research should explore whether current smokers’ distinction between absolute and comparative risk is associated with lower quit intentions or reduced rates of quitting. In other health domains such as cancer screening, consistency in health beliefs has been found to be critical in motivating and sustaining behavior change.50 A similar effect for smoking would suggest a greater need to address the interrelations among various health cognitions and emotions—rather than simply their absolute levels—in health communication and cessation counseling. Further understanding of how health beliefs change over time and how this relates to smoking behavior (eg, smoking cessation and initiation) will also help us to better understand intervention points and strategies.
Supplementary Material
Supplementary Tables 1 and 2 can be found online at http://www.ntr.oxfordjournals.org
Funding
This work was funded by an American Cancer Society Mentored Research Scholar Award (MRSG-005-05-CPPB) to ERP, and the American College of Radiology Imaging Network (ACRIN) received funding from the National Cancer Institute through the grants U01 CA079778 and U01 CA080098.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy, or position of the US Department of Health and Human Services or any of its affiliated institutions or agencies.
References
- 1. Borrelli B, Hayes RB, Dunsiger S, Fava JL. Risk perception and smoking behavior in medically ill smokers: a prospective study. Addiction. 2010;105 (6):1100–1108. 10.1111/j.1360-0443.2010.02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr. 1974;2 (4):328–335. [DOI] [PubMed] [Google Scholar]
- 3. Glanz K, Rimer BK, Lewis FM. Health Behavior and Health Education: Theory, Research and Practice. 3rd ed. San Francisco, CA: Wiley & Sons; 2002. [Google Scholar]
- 4. Leventhal H. Findings and theory in the study of fear communications. Adv Exp Soc Psychol. 1970;5:119–186. 10.1016/S0065-2601(08)60091-X. [Google Scholar]
- 5. Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychol Bull. 2014;140(2):511–543. 10.1037/a0033065. [DOI] [PubMed] [Google Scholar]
- 6. Portnoy DB, Kaufman AR, Klein WM, Doyle TA, de Groot M. Cognitive and affective perceptions of vulnerability as predictors of exercise intentions among people with type 2 diabetes. J Risk Res. 2014;17(2)177–193. 10.1080/13669877.2013.794153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrer RA, Portnoy DB, Klein WM. Worry and risk perceptions as independent and interacting predictors of health protective behaviors. J Health Commun. 2013;18 (4):397–409. 10.1080/10810730.2012. 727954. [DOI] [PubMed] [Google Scholar]
- 8. Klein WM, Zajac LE, Monin MM. Worry as a moderator of the association between risk perceptions and quitting intentions in young adult and adult smokers. Ann Behav Med. 2009;38 (3):256–261. 10.1007/s12160-009-9143-2. [DOI] [PubMed] [Google Scholar]
- 9. Portnoy DB, Ferrer RA, Bergman HE, Klein WM. Changing deliberative and affective responses to health risk: a meta-analysis. Health Psychol Rev. 2014;8 (3):296–318. 10.1080/17437199.2013.798829. [DOI] [PubMed] [Google Scholar]
- 10. Aho WR. Smoking, dieting, and exercise: age differences in attitudes and behavior relevant to selected health belief model variables. The perceived seriousness is an important factor in influencing behavior. R I Med J. 1979;62 (3):85–92. [PubMed] [Google Scholar]
- 11. Croog SH, Richards NP. Health beliefs and smoking patterns in heart patients and their wives: a longitudinal study. Am J Public Health. 1977;67 (10):921–930. 10.2105/AJPH.67.10.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mallaghan M, Pemberton J. Some behavioural changes in 493 patients after an acute myocardial infarction. Br J Prev Soc Med. 1977;31 (2):86–90. 10.1136/jech.31.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullens AB, McCaul KD, Erickson SC, Sandgren AK. Coping after cancer: risk perceptions, worry, and health behaviors among colorectal cancer survivors. Psycho-oncology. 2004;13 (6):367–376. 10.1002/pon.751. [DOI] [PubMed] [Google Scholar]
- 14. Pederson L, Wanklin J, Baskerville J. The role of health beliefs in compliance with physician advice to quit smoking. Soc Sci Med. 1984;19 (5):573–580. 10.1016/0277-9536(84)90052-2. [DOI] [PubMed] [Google Scholar]
- 15. Romer D, Jamieson P. The role of perceived risk in starting and stopping smoking. In: Slovic P. ed. Smoking: Risk, Perception, and Policy. Thousand Oaks, CA: Sage; 2001:64–80. [Google Scholar]
- 16. Warnecke R, Graham S, Rosenthal S, Manfredi C. Social and psychological correlates of smoking behavior among black women. J Health Soc Behav. 1978;19 (4):397–410. www.jstor.org/stable/2136587. Accessed May 7, 2015. [PubMed] [Google Scholar]
- 17. Ranby KW, Aiken LS, Gerend MA, Erchull MJ. Perceived susceptibility measures are not interchangeable: absolute, direct comparative, and indirect comparative risk. Health Psychol. 2010;29 (1):20–28. 10.1037/a0016623. [DOI] [PubMed] [Google Scholar]
- 18. Hayaki J, Anderson BJ, Stein MD. Perceptions of health risk susceptibility in methadone maintained smokers. J Addict Dis. 2005;24 (1):73–84. 10.1300/J069v24n01_07. [DOI] [PubMed] [Google Scholar]
- 19. Park ER, Ostroff JS, Rakowski W, et al. Risk perceptions among participants undergoing lung cancer screening: baseline results from the National Lung Screening Trial. Ann Behav Med. 2009;37 (3):268–279. 10.1007/s12160-009-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 21. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dosecomputed tomographic screening. N Engl J Med. 2011;365 (5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chapman S, Liberman J. Ensuring smokers are adequately informed: Reflections on consumer rights, manufacturer responsibilities, and policy implications. Tob Control. 2005;14 (suppl 2):ii8–ii13. 10.1136/tc.2005.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gibbons FX, McGovern PG, Lando HA. Relapse and risk perception among members of a smoking cessation clinic. Health Psychol. 1991;10 (1):42–45. 10.1037/0278-6133.10.1.42. [DOI] [PubMed] [Google Scholar]
- 24. Glock S, Müller BCN, Ritter SM. Warning labels formulated as questions positively influence smoking-related risk perception. J Health Psychol. 2013;18 (2):252–262. 10.1177/1359105312439734. [DOI] [PubMed] [Google Scholar]
- 25. Persoskie A, Mao Q, Chou WY, et al. Absolute and comparative cancer risk perceptions among smokers in two cities in China. Nicotine Tob Res. 2014;16 (6):899–903. 10.1093/ntr/ntu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slovic P. Cigarette smokers: rational actors or rational fools? In: Slovic P. ed. Smoking: Risk, Perception, and Policy. Thousand Oaks, CA: Sage; 2001:97–124. [Google Scholar]
- 27. Viscusi WK. Smoking: Making the Risky Decision. New York, NY: Oxford University Press; 1992. [Google Scholar]
- 28. Park ER, Gareen IF, Jain A, et al. Examining whether lung screening changes risk perceptions: National Lung Screening Trial participants at 1-year follow-up. Cancer. 2013;119 (7):1306–1313. 10.1002/cncr.27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lyna P, McBride C, Samsa G, Pollak KI. Exploring the association between perceived risks of smoking and benefits to quitting: who does not see the link? Addict Behav. 2002;27 (2):293–307. 10.1016/S0306-4603(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 30. Diefenbach MA, Weinstein ND, O’Reilly J. Scales for assessing perceptions of health hazard susceptibility. Health Educ Res. 1993;8 (2):181–192. 10.1093/her/8.2.181. [DOI] [PubMed] [Google Scholar]
- 31. Weinstein N. Smokers’ recognition of their vulnerability to harm. In: Slovic P, ed. Smoking: Risk, Perception, and Policy. Thousand Oaks, CA: Sage; 2001:81–96. 10.4135/9781452232652. [Google Scholar]
- 32. Klein WMP. Comparative risk estimates relative to the average peer predict behavioral intentions and concern about absolute risk. Risk, Decision and Policy. 2002;7 (2):193–202. 10.1017/S1357530902000613. [Google Scholar]
- 33. Rigotti NA, Park ER, Regan S, et al. Efficacy of telephone counseling for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2006;108 (1):83–92. 10.1097/01.AOG.0000218100.05601.f8. [DOI] [PubMed] [Google Scholar]
- 34. Lerman C, Schwartz M. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Res Treat. 1993;28(2):145–155. 10.1007/BF00666427. [DOI] [PubMed] [Google Scholar]
- 35. Price JH, Everett SA. Perceptions of lung cancer and smoking in an economically disadvantaged population. J Community Health. 1994;19 (5):361–375. 10.1007/BF02260405. [DOI] [PubMed] [Google Scholar]
- 36. Aiken LS, West SG, Woodward CK, Reno RR, Reynolds KD. Increasing screening mammography in asymptomatic women: evaluation of a second-generation, theory-based program. Health Psychol. 1994;13 (6):526–538. 10.1037//0278-6133.13.6.526. [DOI] [PubMed] [Google Scholar]
- 37. Muthén LK, Muthén BO. MPlus User’s Guide. 6th ed. Los Angeles, CA: Muthén & Muthén; 1998. –2010. [Google Scholar]
- 38. Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88 (3):588–606. 10.1037/0033-2909.88.3.588. [Google Scholar]
- 39. Rousson V, Gasser T, Seifert B. Assessing intrarater, interrater and test-retest reliability of continuous measurements. Stat Med. 2002;21 (22):3431–3446. 10.1002/sim.1253. [DOI] [PubMed] [Google Scholar]
- 40. Clark LA, Watson D. Constructing validity: basic issues in objective scale development. Psychol Assess. 1995;7 (3):309–319. 10.1037/1040-3590.7.3.309. [Google Scholar]
- 41. Park ER, Streck JM, Gareen IF, et al. A qualitative study of lung cancer risk perceptions and smoking beliefs among national lung screening trial participants. Nicotine Tob Res. 2014;16 (2):166–173. 10.1093/ntr/ntt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Festinger L. A Theory of Cognitive Dissonance. Oxford, UK: Stanford University Press; 1957. [Google Scholar]
- 43. Oakes W, Chapman S, Borland R, Balmford J, Trotter L. “Bulletproof skeptics in life’s jungle”: which self-exempting beliefs about smoking most predict lack of progression towards quitting? Prev Med. 2004;39 (4):776–782. 10.1016/j.ypmed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 44. Kunda Z. The case for motivated reasoning. Psychol Bull. 1990;108 (3):480–498. 10.1037/0033-2909.108.3.480. [DOI] [PubMed] [Google Scholar]
- 45. Liberman A, Chaiken S. Defensive processing of personally relevant health messages. Pers Soc Psychol B. 1992;18 (6):669–679. 10.1177/0146167292186002 [Google Scholar]
- 46. Persoskie A, Nelson WL. Just blowing smoke? Social desirability and reporting of intentions to quit smoking. Nicotine Tob Res. 2013;15 (12):2088–2093. 10.1093/ntr/ntt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeager DS, Krosnick JA. The validity of self-reported nicotine product use in the 2001–2008 National Health and Nutrition Examination Survey. Med Care. 2010;48 (12):1128–1132. 10.1097/MLR.0b013e3181ef9948. [DOI] [PubMed] [Google Scholar]
- 48. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 49. Müller BC, van Baaren RB, Ritter SM, et al. Tell me why. The influence of self-involvement on short term smoking behaviour. Addict Behav. 2009;34 (5):427–431. 10.1016/j.addbeh.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 50. Ferrer RA, Hall KL, Portnoy DB, Ling BS, Han PK, Klein WMP. Relationships among health perceptions vary depending on stage of readiness for colorectal cancer screening. Health Psychol. 2011;30 (5):525–535. 10.1037/a0023583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


