Abstract
Background.
Loss of muscle mass occurs with aging and in lower limbs it may be accelerated by foot problems. In this cross-sectional analysis, we evaluated the relationship of leg muscle mass to foot symptoms (presence or absence of pain, aching, or stiffness), structure while standing (high arch or low arch), and function while walking (pronated or supinated) in a community-based study of Caucasian and African American men and women who were 50–95 years old.
Methods.
In the Johnston County Osteoarthritis Project, leg muscle mass was measured with whole body dual-energy x-ray absorptiometry, and plantar foot pressure data, using predetermined values, were used to classify foot structure and function. Sex-specific crude and adjusted (age, body mass index, and race) linear regression models examined associations of leg muscle mass index (Leg muscle mass [kg] / Height [m]2) with foot symptoms, structure, and function.
Results.
Complete data were available for 1,037 participants (mean age 68 years, mean body mass index 31kg/m2, 68% women, 29% African American). In women, pronated foot function was associated with lower leg muscle mass in crude (p = .02), but not adjusted (p = .22), models. A low arch was associated with a higher leg muscle mass in adjusted models for both men and women (p < .01).
Conclusions.
Leg muscle mass was associated with foot structure in our biracial sample, whereas relations between leg muscle mass and foot function were attenuated by age, body mass index, and race. Future longitudinal analyses are needed to explain the temporal relationship between these conditions and how they relate to other aspects of impairment and physical function.
Key Words: Foot, Pain, Muscle, Gait, Cohort study
Poor physical function, disability, and falls have been linked to foot pain (1–5), high toe pressures of the foot during walking (6), and decreased plantar flexor strength (4,5). Theoretically, a pathway to disability and falls may begin with foot problems. Structural or functional disorders of the foot or pain in the foot may contribute to weakness or loss of the musculature of the foot and lower leg. Over time, particularly in combination with age-related muscle loss, the resulting muscle weakness attributed to these foot problems may lead to functional limitations and overall physical decline. Leg muscle mass is a marker of mechanical loading and has been shown to be associated with physical function (ie, higher lean mass is associated with better function) (7,8), but little is known about how foot pain or the structure and dynamic function of the foot might affect leg muscle mass.
In clinical populations, poorer muscle function of the lower extremity has been linked with flatfoot deformity (9), and among runners, overpronation of the foot is seen among individuals with lower extremity muscle weakness (10). Recently, McLean and colleagues (11) examined the cross-sectional associations between leg muscle mass and foot pain, posture, and function in the first large community-based study. In the Framingham Foot Study cohort (average age 67 years, 43% men, all Caucasian), reduced leg muscle mass was associated with foot pain and pronation, with some results suggesting potential differences by sex. However, the time difference between assessment of leg muscle mass and foot exam (on average 6.7 years) was a limitation that may have underestimated the associations between the two measures; the authors adjusted for potential age-related loss in their analyses by assuming a constant rate across all participants, but leg muscle mass loss over time likely varied.
Our prior work has shown differences in foot type and disorders between African Americans and Caucasians (12,13), and no prior population-based study has reported associations of leg muscle mass and the foot among African Americans. The purpose of this cross-sectional analysis was to examine the association of foot symptoms, foot structure, and foot function with leg muscle mass in a community-based study of Caucasian and African American men and women 50 years of age or older. Along with the inclusion of African Americans, this investigation benefited from the acquisition of leg muscle mass and foot exam data during the same study visit. We hypothesized that foot symptoms and more extreme foot structures (eg, high arch or low arch foot types) and poorer foot function (eg, supination or pronation, correspondingly described as excessive outward or inward rolling of the foot while walking) would be linked to lower leg muscle mass, even after controlling for other potential confounders of sex, race, age, and obesity.
Methods
Study Participants
Participants were from the community-based Johnston County Osteoarthritis Project, an ongoing, prospective study of osteoarthritis in African Americans and Caucasians living in a rural county in North Carolina. Civilian, noninstitutionalized residents aged 45 and older from six townships in Johnston County were enrolled between 1991 and 1997 (14), and additional Johnston County residents were enrolled during 2003–2004. Clinical examinations were completed by 1,695 participants during a follow-up visit conducted from 2006 to 2010, and participants were at least 50 years of age at this visit. Cross-sectional analyses for the present study were based on data collected during this follow-up visit. Participants completed an examination that included dual-energy x-ray absorptiometry, a walking plantar pressure assessment, and collection of demographic and clinical variables.
Leg Muscle Mass
Whole body dual-energy x-ray absorptiometry (Hologic QDR Delphi A, New Rochelle, NY) scans were obtained for eligible participants. These scans provide reliable measures of body composition, including lean body mass, total body fat mass, and leg lean mass (7,15–18). Individuals were considered ineligible to complete this scan if they had metal anywhere in their body. Participants were asked to lie supine and remain still during the exam, which took approximately 5 minutes while the scanner completed three passes over the body. Lean muscle mass of the leg (includes whole lower extremity below the pelvis) was measured in kilograms.
Foot Symptoms
Participants were asked to respond “yes” or “no” to the questions: “On most days, do you have pain, aching or stiffness in your [right/left] foot?”
Foot Structure and Foot Function
Plantar pressure data were collected at a sampling frequency of 40 Hz using a Tekscan Matscan System (Tekscan Inc, Boston, MA). This system has a 5-mm-thick floor mat (432×368mm) with 2,288 resistive sensors (1.4/cm2), and reliability for the MatScan system has been shown to be moderate to good (19). Scans were completed during standing (bipedal relaxed stance) and walking. For the walking scans, participants walked at a self-selected pace and scans were collected using the two-step method (ie, striking the foot mat on the second step) (20). Two trials were collected for each foot.
Foot structure was assessed using the Modified Arch Index (21). Using the maximum peak pressure image from the standing scan, the Modified Arch Index was calculated as the ratio of the area of the middle third of the foot to the whole foot area, excluding the toes. Modified Arch Index cutoff values were set a priori based on data from the Framingham Foot Study (3,100 participants with data available for 6,153 feet) (11) in which values in the lowest 20% for that cohort were considered to represent a high arch foot structure and those in the highest 20% represented a flat arch foot structure (22,23). Based on these values, we created a three-category foot structure variable in the present study in which high arch was defined as values ≤0.030, low arch as values ≥0.164, and values >0.030 to <0.164 were considered the referent category.
Foot function was determined by calculating the Center of Pressure Excursion Index (CPEI) from the walking trials data. The CPEI measures dynamic foot pronation and supination and is sensitive to changes in foot structure (24). To determine the CPEI, a line was drawn to connect the first and last center of pressure data points of the foot and the distance between this line and the center of pressure in the anterior third of the foot was measured. The CPEI was calculated as the distance between the lines and the center of pressure divided by the forefoot width. A lower CPEI suggested a more pronated foot, whereas a higher CPEI designated a more supinated foot during gait (24). The mean CPEI value for the two walking trials was used in the present study. CPEI cutoff values were set a priori based on values from the Framingham Foot Study (22,23). In the Framingham Cohort, values in the lowest 20% were considered to represent a pronated foot function (inward rolling of the foot during walking) and those in the highest 20% represented a supinated foot function (outward rolling). A three-category foot function variable was created for the present study based on these cutpoints, with CPEI values ≤7.3% for pronated foot function, values ≥21.0% for supinated foot function, and values >7.3% to <21.0% were considered the referent category.
Demographic and Clinical Characteristics
Age in years, female/male sex, body mass index (BMI; calculated as Weight in kilograms / Height in meters squared [kg/m2]), and race (African American and Caucasian) were collected at the time of the exam. Weight was measured in pounds using a balance beam scale and converted to kilograms, and height without shoes was measured using a calibrated stadiometer in inches and converted to meters.
Analysis
Because leg muscle mass, foot symptoms, foot structure, and foot function may vary by sex, sex-specific analyses were conducted. Distributions of age, BMI, race, sex, leg muscle mass, foot symptoms, foot structure, and foot function were calculated. Sex-specific crude and adjusted (controlling first for age and BMI, then adding race (with Caucasian as referent) linear regression models were used to determine the association between leg muscle mass (response variable) and foot symptoms, structure, and function (explanatory variables). BMI was included as a covariate to account for adiposity, which is related to foot pain (25,26), foot function (13), and leg lean mass (27). For these models, a leg muscle mass index was created by dividing leg muscle mass (kg) by height (m) squared to create a measure of relative muscle mass compared with body size to account for the high correlation between absolute body mass and body height (28). Analyses were foot based rather than person based to examine associations within a given limb, and generalized estimating equations were used to adjust for correlations between right and left feet. Interactions of foot symptoms, foot structure, or foot function by age, BMI, or race were examined, and a p < .10 for interaction was considered statistically significant.
Results
Characteristics of Participants
Of the 1,695 Johnston County Osteoarthritis Project participants who attended the follow-up clinical exam visit, 643 participants were removed from analyses because of missing leg muscle mass data, followed by 12 for missing foot pain data, and 3 participants missing foot structure data on both feet, leaving 1,037 participants with complete data for this study. Additionally, 2 feet were deleted for missing foot structure data, and 29 feet for missing foot function data (Figure 1). Thirty-one of the 1,037 participants contributed only one foot to the analysis because values for the other foot were missing or improbable, and a total of 2,043 feet were analyzed (1,006 participants contributing both feet for a total of 2,012 feet and 31 participants contributing one foot).
Figure 1.
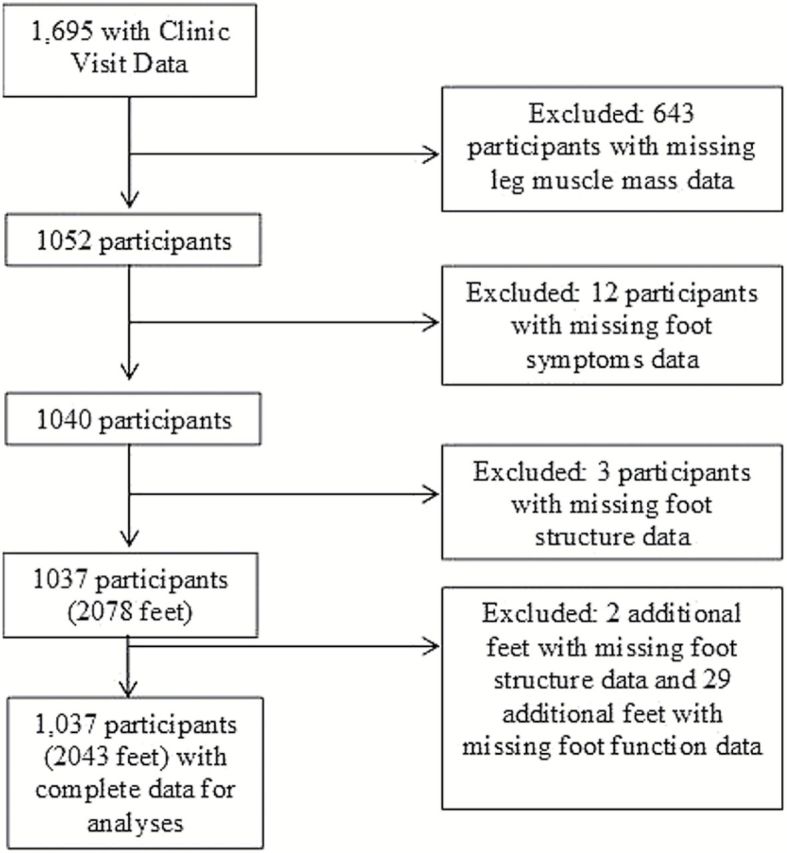
Johnston County Osteoarthritis Project participants included in analyses.
Table 1 shows characteristics for the study sample and by men and women. Participants were 68% women and 29% African American with a mean age of 68 years (SD 8.7, range 50–95) and mean BMI of 30.5kg/m2 (SD 6.0). Eighteen percent of feet had symptoms. Seventeen percent of feet had a high arch foot structure, 27% had a low arch foot, 14% had a pronated foot function, and 19% had a supinated function. Leg muscle mass index was greater on average for men’s than for women’s legs (3.4 vs 2.9kg/ m2). Foot symptoms (20% vs 14%) and pronated foot function (16% vs 10%) were more common in women’s than in men’s feet.
Table 1.
Characteristics of Sample, by Sex
| Study Sample | Men | Women | |
|---|---|---|---|
| N = 1037 | n = 329 (31.7%) | n = 708 (68.3%) | |
| Mean age, y (SD) | 68 (8.7) | 67 (8.7) | 68 (8.8) |
| Mean BMI, kg/m2 (SD) | 30.5 (6.0) | 30.2 (4.8) | 30.6 (6.5) |
| Mean height, m (SD) | 1.64 (0.09) | 1.73 (0.07) | 1.59 (0.06) |
| African American, n (%) | 306 (30) | 86 (26) | 220 (31) |
| N (feet) = 2043 | n (feet) = 652 (31.9%) | n (feet) = 1391 (68.1%) | |
| Mean leg muscle mass, kg (SD) | 8.31 (2.01) | 10.25 (1.66) | 7.40 (1.44) |
| Mean leg muscle mass/height2, kg/m2 (SD) | 3.08 (0.56) | 3.41 (0.47) | 2.92 (0.53) |
| Foot symptoms, n (%) | 365 (18) | 92 (14) | 273 (20) |
| High arch foot structure, n (%) | 353 (17) | 115 (18) | 238 (17) |
| Flat arch foot structure, n (%) | 548 (27) | 160 (25) | 388 (28) |
| Pronated foot function, n (%) | 284 (14) | 68 (10) | 216 (16) |
| Supinated foot function, n (%) | 398 (19) | 139 (21) | 259 (19) |
Note: BMI = body mass index.
Associations Among Men
Results of linear regression models are summarized in Table 2. In men, a high arch foot structure was associated with a lower leg muscle mass (p < .001) and a flat arch foot structure was associated with a higher leg muscle mass (p < .001) in crude models. After adjusting for age, BMI, and race, the association between a high arch foot structure and leg muscle mass was no longer statistically significant (p = .63) whereas the relationship between a flat arch foot structure and higher leg muscle mass maintained significance (p < .04). Foot function and foot symptoms were not associated with leg muscle mass in men.
Table 2.
Associations of Foot Symptoms, Foot Structure, and Foot Function with Leg Muscle Mass / Height2
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Model | Category | Beta (SE) | p Value | Beta (SE) | p Value | |
| Foot symptoms | Crude | Symptoms present | 0.056 (0.075) | .45 | 0.091 (0.048) | .06 |
| + Age, BMI | Symptoms present | −0.010 (0.054) | .85 | −0.038 (0.024) | .11 | |
| + Race | Symptoms present | −0.012 (0.044) | .79 | −0.001 (0.022) | .98 | |
| Foot structure | Crude | High arch | −0.184 (0.047) | <.001 | −0.336 (0.031) | <.001 |
| Flat arch | 0.383 (0.056) | <.001 | 0.449 (0.037) | <.001 | ||
| + Age, BMI | High arch | −0.038 (0.030) | .20 | −0.071 (0.020) | .001 | |
| Flat arch | 0.211 (0.041) | <.001 | 0.116 (0.021) | <.001 | ||
| + Race | High arch | 0.014 (0.030) | .63 | −0.030 (0.020) | .12 | |
| Flat arch | 0.079 (0.038) | .04 | 0.044 (0.019) | .02 | ||
| Foot function | Crude | Pronated | −0.042 (0.066) | .52 | −0.099 (0.042) | .02 |
| Supinated | −0.025 (0.055) | .65 | −0.030 (0.042) | .47 | ||
| + Age, BMI | Pronated | −0.042 (0.054) | .44 | −0.001 (0.024) | .98 | |
| Supinated | −0.061 (0.033) | .07 | 0.017 (0.020) | .38 | ||
| + Race | Pronated | −0.058 (0.046) | .20 | −0.025 (0.021) | .22 | |
| Supinated | −0.052 (0.030) | .08 | −0.001 (0.018) | .98 | ||
Note: BMI = body mass index.
Associations Among Women
Among women, a high arch foot structure was associated with lower leg muscle mass (p < .001) and a flat arch foot structure with higher leg muscle mass (p < .001), but after adjustment, relationships for the high arch foot structure were not statistically significant whereas the linkage between flat arch foot structure and leg muscle mass maintained significance (p = .02). A pronated foot function was associated with lower leg muscle mass (p = .02), but these associations were attenuated in adjusted models. Foot symptoms were not associated with leg muscle mass in women.
Assessment of Statistical Interactions
Interactions were statistically significant for foot symptoms and race (p = .02) and foot function and BMI (BMI < 30 vs BMI ≥ 30kg/m2, p = .09), but no other interactions were significant. When associations between foot symptoms and leg muscle mass were stratified by race, foot symptoms were associated with lower leg mass among Caucasians (men: beta = −0.0502, p = .30; women: beta = −0.0264, p = .24) and with higher leg mass among African Americans (men: beta = 0.1042, p = .21; women: beta = 0.0888, p = .13), but these results were not statistically significant. When associations between foot function and leg muscle mass were stratified by BMI, a supinated foot function was associated with lower leg mass among those with BMI <30kg/m2 (men: beta = −0.0978, p = .04; women: beta = −0.0600, p = .04), whereas a pronated foot function was associated with lower leg mass among those with BMI ≥30kg/m2 (men: beta = −0.1210, p = .02; women: beta = −0.1075, p = .02).
Discussion
In women, foot structure and foot function were associated with leg muscle mass in this biracial sample, but age, BMI, and race attenuated relationships for foot function. In men and women, foot structure was associated with leg muscle mass, even after adjustment, suggesting a link between a low arch foot structure and a higher leg muscle mass. Estimates of associations for foot symptoms, foot structure, and foot function with leg muscle mass for men and women were generally similar in direction and magnitude. Our findings for associations between leg muscle mass and foot structure are consistent with those reported in the Framingham Foot Study (11), providing confirmation of their results in a different population that included African Americans and with simultaneous attainment of leg muscle mass and foot data.
The key result of a relationship between low arch foot structure and higher leg muscle mass countered our hypothesis of poorer foot structure with lower muscle mass, which we based on an assumption that foot problems may lead to less muscle mass in the legs, particularly with a loss of muscle function with aging. In our cross-sectional analyses, however, we could not account for temporality, and perhaps our results demonstrate either a nonsequential pairing of these two factors or that higher leg muscle mass may occur in response to a low arch foot structure in an attempt to provide greater muscular control to a dysfunctional medial longitudinal arch. Compared with individuals with normal-arched feet, Murley and colleagues (29) reported greater tibialis posterior and peroneus longus activation on electromyography during ambulation (ie, mid- and late-stance) in adults with low arch feet, suggesting that a low arch foot structure places “a greater demand” on the lower leg musculature that controls arch movement. Furthermore, Angin and colleagues (30) recently reported that greater cross-sectional area and extrinsic (ie, leg) muscle thickness were observed among 49 people with flat feet compared with 49 with normal foot posture. The authors suggest that the function of the intrinsic foot muscles may be limited by the flat foot structure, and the extrinsic muscles compensate for this deficiency.
Another interesting result of this study was the difference in foot function and leg muscle mass by obesity where a supinated foot function was associated with lower muscle mass among nonobese participants and a pronated foot function was associated with lower muscle mass among obese individuals. Interpretation of these results is challenging because of the cross-sectional nature of this study and the lack of knowledge about the sequence of events for obesity, foot function, and leg muscle mass either over the short term or over trajectories in an adult life span. Future longitudinal studies may clarify these findings.
Strengths of the present study are that it is community based, consists of African American and Caucasian men and women, includes a reliable measure of leg muscle mass, and comprises quantitative, biomechanical measures of foot structure and foot function. These foot pressure measures were captured twice and averaged to approximate representative foot structure and foot function measures for each participant. An additional strength is that the analyses were foot based and included left and right feet for an individual (when data were available), and statistical methods were used to account for correlated data within a participant.
A limitation of the present study is that a temporal relationship between foot symptoms, foot structure, foot function, and leg muscle mass cannot be inferred due to the cross-sectional study design. Potentially, associations were attenuated in the present study because of the cross-sectional nature, and a longitudinal analysis would assist in determining how they may be linked and whether there is an order of these components on the pathway to functional decline. The current study is a starting point. Repeat measures comparable with those used in the present analysis currently are being collected during a new follow-up visit for the Johnston County Osteoarthritis Project, and these data will allow for assessment of associations over time. Another limitation is that leg muscle mass may not be directly associated with muscle strength, and leg strength (31) or muscle quality (32) may have been more suitable measures when examining foot symptoms, structure, and function. However, muscle strength and quality variables for the lower extremity were not available for this secondary analysis.
Overall, the results of this study suggest that foot symptoms and foot function may not be associated with leg muscle mass when examined at a single time point and when controlling for individual characteristics. However, a flat arch foot structure may be associated with higher leg muscle mass, and longitudinal analyses may help elucidate this relationship. Given the lack of publications concerning foot symptoms, structure, and function in the community, these data are provocative and open the door for future work, especially research focusing on longitudinal changes. An essential aspect of future research will be how these conditions relate to other aspects of impairment and physical function.
Funding
This work was supported by the National Center for Advancing Translational Sciences, the National Institutes of Health(KL2TR000084 / UL1 TR000083); the Arthritis Foundation (Postdoctoral Fellowship), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR047853); and the Centers for Disease and Prevention Control / Association of Schools of Public Health (S043, S3486). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the CDC, or the Arthritis Foundation.
Conflict of Interest
None
References
- 1. Benvenuti F, Ferrucci L, Guralnik JM, et al. Foot pain and disability in older persons: an epidemiologic survey. J Am Geriatri Soc 1995;43:479–484. doi:10.1111/j.1532-5415.1995.tb06092.x [DOI] [PubMed] [Google Scholar]
- 2. Leveille SG, Guralnik JM, Ferrucci L, et al. Foot pain and disability in older women. Am J Epidemiol. 1998;148:657–665. doi:10.1093/aje/148.7.657 [DOI] [PubMed] [Google Scholar]
- 3. Menz HB, Lord SR. The contribution of foot problems to mobility impairment and falls in community-dwelling older people. J Am Geriatr Soc. 2001;49:1651–1656. doi:10.1111/j.1532-5415.2001.49275.x [PubMed] [Google Scholar]
- 4. Menz HB, Morris ME, Lord SR. Foot and ankle risk factors for falls in older people: a prospective study. J Gerontol Med Sci. 2006;61:866–870. [DOI] [PubMed] [Google Scholar]
- 5. Mickle KJ, Munro BJ, Lord SR, et al. ISB Clinical Biomechanics Award 2009: toe weakness and deformity increase the risk of falls in older people. Clin Biomech (Bristol, Avon). 2009;24:787–791. doi:10.1016/j.clinbiomech.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 6. Mickle KJ, Munro BJ, Lord SR, et al. Foot pain, plantar pressures, and falls in older people: a prospective study. J Am Geriatr Soc. 2010;58:1936–1940. doi:10.1111/j.1532-5415.2010.03061.x [DOI] [PubMed] [Google Scholar]
- 7. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi:10.1046/j.1532-5415.2002.50217.x [DOI] [PubMed] [Google Scholar]
- 8. Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49:1641–1645. doi:10.1111/j.1532-5415.2001.49273.x [DOI] [PubMed] [Google Scholar]
- 9. Neville C, Flemister AS, Houck JR. Deep posterior compartment strength and foot kinematics in subjects with stage II posterior tibial tendon dysfunction. Foot Ankle Int. 2010;31:320–328. doi:10.3113/FAI.2010.0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hintermann B, Nigg BM. Pronation in runners. Implications for injuries. Sports Med. 1998;26:169–176. [DOI] [PubMed] [Google Scholar]
- 11. McLean RR, Dufour AB, Katz PP, et al. The associations of leg lean mass with foot pain, posture and function in the Framingham foot study. J Foot Ankle Res. 2014;7:46 doi:10.1186/s13047-014-0046-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golightly YM, Hannan MT, Dufour AB, et al. Racial differences in foot disorders and foot type. Arthritis Care Res (Hoboken). 2012;4:1756–1759. doi:10.1002/acr.21752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golightly YM, Hannan MT, Dufour AB, et al. Foot disorders associated with overpronated and oversupinated foot function: the Johnston County Osteoarthritis Project. Foot Ankle Int. 2014;35:1159–1165. doi:10.1177/1071100714543907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 15. Visser M, Kiel DP, Langlois J, et al. Muscle mass and fat mass in relation to bone mineral density in very old men and women: the Framingham Heart Study. Appl Radiat Isot. 1998;49:745–747. doi:10.1016/S0969-8043(97)00101-2 [DOI] [PubMed] [Google Scholar]
- 16. Visser M, Pahor M, Tylavsky F, et al. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol (1985). 2003;94:2368–2374. doi:10.1152/japplphysiol.00124.2002 [DOI] [PubMed] [Google Scholar]
- 17. Wang ZM, Visser M, Ma R, et al. Skeletal muscle mass: evaluation of neutron activation and dual-energy x-ray absorptiometry methods. J Appl Physiol (1985). 1996;80:824–831. [DOI] [PubMed] [Google Scholar]
- 18. Visser M, Harris TB, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–M221. doi:10.1093/gerona/53A.3.M214 [DOI] [PubMed] [Google Scholar]
- 19. Zammit GV, Menz HB, Munteanu SE. Reliability of the TekScan MatScan® system for the measurement of plantar forces and pressures during barefoot level walking in healthy adults. J Foot Ankle Res. 2010;3:11 doi:10.1186/1757-1146-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McPoil TG, Cornwall MW, Dupuis L, et al. Variability of plantar pressure data. A comparison of the two-step and midgait methods. J Am Podiatr Med Assoc. 1999;89:495–501. doi:10.7547/87507315-89-10-495 [DOI] [PubMed] [Google Scholar]
- 21. Chu WC, Lee SH, Chu W, Wang TJ, Lee MC. The use of arch index to characterize arch height: a digital image processing approach. IEEE Trans Biomed Eng. 1995;42:1088–1093. doi:10.1109/10.469375 [DOI] [PubMed] [Google Scholar]
- 22. Hagedorn TJ, Dufour AB, Riskowski JL, et al. Foot disorders, foot posture, and foot function: the Framingham foot study. PLoS One. 2013;8:e74364 doi:10.1371/journal.pone.0074364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riskowski JL, Dufour AB, Hagedorn TJ, et al. Associations of foot posture and function to lower extremity pain: results from a population-based foot study. Arthritis Care Res. 2013;65:1804–1812. doi:10.1002/acr.22049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song J, Hillstrom HJ, Secord D, et al. Foot type biomechanics. comparison of planus and rectus foot types. J Am Podiatr Med Assoc. 1996;86:16–23. doi:10.7547/87507315-86-1-16 [DOI] [PubMed] [Google Scholar]
- 25. Golightly YM, Hannan MT, Shi XA, et al. Association of foot symptoms with self-reported and performance-based measures of physical function: the Johnston County Osteoarthritis Project. Arthritis Care Res. 2011;63:654–659. doi:10.1002/acr.20432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gay A, Culliford D, Leyland K, et al. Associations between body mass index and foot joint pain in middle-aged and older women: a longitudinal population-based cohort study. Arthritis Care Res 2014;66:1873–1879. doi:10.1002/acr.22408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi:10.1046/j.1532-5415.2003.51534.x [DOI] [PubMed] [Google Scholar]
- 28. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 29. Murley GS, Menz HB, Landorf KB. Foot posture influences the electromyographic activity of selected lower limb muscles during gait. J Foot Ankle Res. 2009;2:35 doi:10.1186/1757-1146-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angin S, Crofts G, Mickle KJ, et al. Ultrasound evaluation of foot muscles and plantar fascia in pes planus. Gait Posture. 2014;40:48–52. doi:10.1016/j.gaitpost.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 31. Stenholm S, Alley D, Bandinelli S, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes (Lond). 2009;33:635–644. doi:10.1038/ijo.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ivey FM, Tracy BL, Lemmer JT, et al. Effects of strength training and detraining on muscle quality: age and gender comparisons. J Gerontol A Biol Sci Med Sci. 2000;55:B152–B157. doi:10.1093/gerona/55.3.B152 [DOI] [PubMed] [Google Scholar]


