Abstract
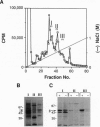
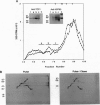
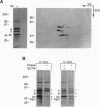
A role in folding newly translated cytoskeletal proteins in the cytosol of eukaryotes has been proposed for t-complex polypeptide 1 (TCP1). In this study, we investigated tubulin and actin biogenesis in Chinese hamster ovary (CHO) cells. When extracts of pulse-labeled cells were analyzed by anion-exchange and size-exclusion chromatography, newly synthesized alpha-tubulin, beta-tubulin, and actin were observed to enter a large molecular mass complex (approximately 900 kDa). These proteins were released from this complex capable, in the case of tubulin, of forming heterodimers. The large molecular mass complexes coeluted with TCP1 and could be immunoprecipitated by using an anti-TCP1 antibody. These findings demonstrate that there is a cytosolic pathway for folding tubulin and actin in vivo that involves the TCP1 complex.
Full text
PDF
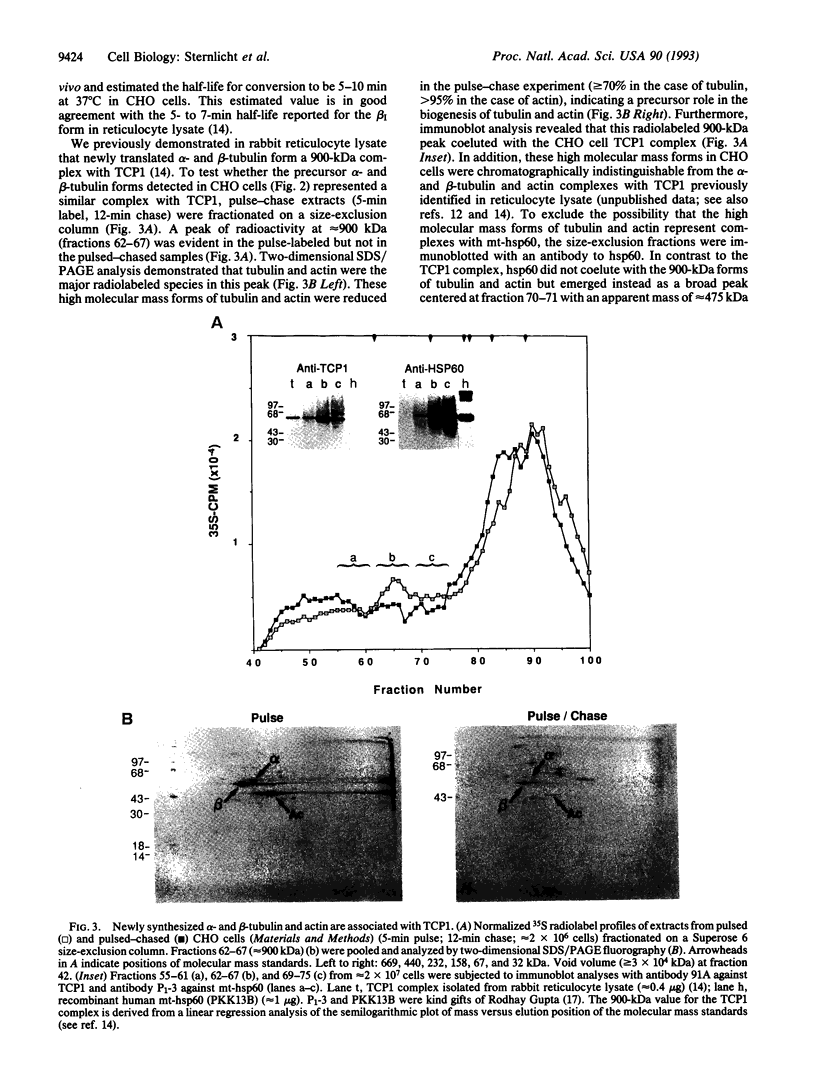
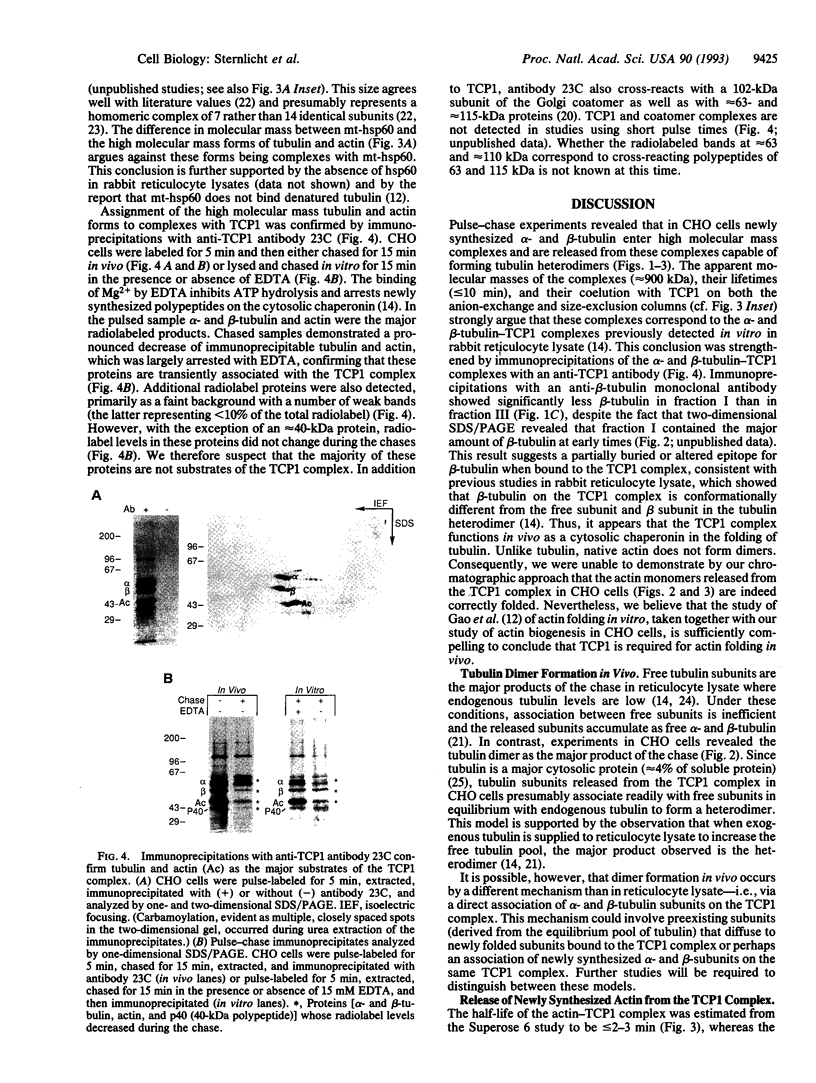

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellis R. J. Molecular chaperones: the plant connection. Science. 1990 Nov 16;250(4983):954–959. doi: 10.1126/science.250.4983.954. [DOI] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Erdjument-Bromage H., Wall J. S., Tempst P., Hartl F. U. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 1992 Dec;11(13):4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B. Assembly associated with the cytomatrix. J Cell Biol. 1984 Jul;99(1 Pt 2):209s–211s. doi: 10.1083/jcb.99.1.209s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Thomas J. O., Chow R. L., Lee G. H., Cowan N. J. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992 Jun 12;69(6):1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Gao Y., Vainberg I. E., Chow R. L., Cowan N. J. Two cofactors and cytoplasmic chaperonin are required for the folding of alpha- and beta-tubulin. Mol Cell Biol. 1993 Apr;13(4):2478–2485. doi: 10.1128/mcb.13.4.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Grasso J. A. Cytoplasmic microtubules in mammalian erythropoietic cells. Anat Rec. 1966 Dec;156(4):397–413. doi: 10.1002/ar.1091560404. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. Mitochondria, molecular chaperone proteins and the in vivo assembly of microtubules. Trends Biochem Sci. 1990 Nov;15(11):415–418. doi: 10.1016/0968-0004(90)90276-h. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. Sequence and structural homology between a mouse T-complex protein TCP-1 and the 'chaperonin' family of bacterial (GroEL, 60-65 kDa heat shock antigen) and eukaryotic proteins. Biochem Int. 1990;20(4):833–841. [PubMed] [Google Scholar]
- Gupta R. S., Venner T. J., Chopra A. Genetic and biochemical studies with mutants of mammalian cells affected in microtubule-related proteins other than tubulin: mitochondrial localization of a microtubule-related protein. Can J Biochem Cell Biol. 1985 Jun;63(6):489–502. doi: 10.1139/o85-068. [DOI] [PubMed] [Google Scholar]
- Isaacs W. B., Cook R. K., Van Atta J. C., Redmond C. M., Fulton A. B. Assembly of vimentin in cultured cells varies with cell type. J Biol Chem. 1989 Oct 25;264(30):17953–17960. [PubMed] [Google Scholar]
- Lewis V. A., Hynes G. M., Zheng D., Saibil H., Willison K. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature. 1992 Jul 16;358(6383):249–252. doi: 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- Nagle B. W., Doenges K. H., Bryan J. Assembly of tubulin from cultured cells and comparison with the neurotubulin model. Cell. 1977 Nov;12(3):573–586. doi: 10.1016/0092-8674(77)90258-6. [DOI] [PubMed] [Google Scholar]
- Picketts D. J., Mayanil C. S., Gupta R. S. Molecular cloning of a Chinese hamster mitochondrial protein related to the "chaperonin" family of bacterial and plant proteins. J Biol Chem. 1989 Jul 15;264(20):12001–12008. [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Rubinstein N., Chi J., Holtzer H. Coordinated synthesis and degradation of actin and myosin in a variety of myogenic and non-myogenic cells. Exp Cell Res. 1976 Feb;97(2):387–393. doi: 10.1016/0014-4827(76)90630-3. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Penningroth S. M., Kirschner M. W. Turnover of tubulin and the N site GTP in Chinese hamster ovary cells. Cell. 1977 Nov;12(3):587–600. doi: 10.1016/0092-8674(77)90259-8. [DOI] [PubMed] [Google Scholar]
- Ursic D., Culbertson M. R. The yeast homolog to mouse Tcp-1 affects microtubule-mediated processes. Mol Cell Biol. 1991 May;11(5):2629–2640. doi: 10.1128/mcb.11.5.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P. V., Lorimer G. H., Seetharam R., Gupta R. S., Oppenheim J., Thomas J. O., Cowan N. J. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J Biol Chem. 1992 Jan 15;267(2):695–698. [PubMed] [Google Scholar]
- Willison K., Lewis V., Zuckerman K. S., Cordell J., Dean C., Miller K., Lyon M. F., Marsh M. The t complex polypeptide 1 (TCP-1) is associated with the cytoplasmic aspect of Golgi membranes. Cell. 1989 May 19;57(4):621–632. doi: 10.1016/0092-8674(89)90131-1. [DOI] [PubMed] [Google Scholar]
- Yaffe M. B., Farr G. W., Miklos D., Horwich A. L., Sternlicht M. L., Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992 Jul 16;358(6383):245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- Yaffe M. B., Farr G. W., Sternlicht H. Translation of beta-tubulin mRNA in vitro generates multiple molecular forms. J Biol Chem. 1988 Nov 5;263(31):16023–16031. [PubMed] [Google Scholar]