Abstract
The conventional approach of double immunostaining to visualize more than one protein in tissues or cells using antibodies from two different host species is not always feasible due to limitations with antibody availability. Previously reported methodologies for performing multiple immunostains on the same tissue or cells with antibodies originating from the same species are varied in their complexity, sensitivity, and approach to prevent unwanted interactions between antibodies. In the ever-expanding field of macrophage biology, much more is known about mouse and human macrophages than their rat counterparts. The limited availability of validated and well-characterized monoclonal antibodies from different species is one factor responsible for preventing advances in rat macrophage biology. Here we describe an immunostaining method for identifying and examining rat macrophages that is sufficiently sensitive for use in formalin-fixed paraffin embedded tissue and that uses only commercially available reagents and antibodies. This method can be used to help characterize both physiological and pathophysiological processes in rat macrophages, and can be adapted for use with any two antibodies from the same species of origin as long as one of the antibodies is biotinylated.
Keywords: Biotinylated, IHC, macrophage, rat, Ki-67, double stain
INTRODUCTION
Visualizing more than one protein in the context of tissues or cells is often of great interest in the biomedical field. The conventional approach to double immunostaining, be it by immunohistochemistry or by immunofluorescence, is by targeting two proteins within a cell or tissue with two antibodies from two different host species (eg. mouse IgG and rabbit IgG), isotypes of the same species (eg. mouse IgG and IgM), or isotype subclasses from the same species (eg. mouse IgG1 and IgG3). These combinations facilitate the double immunostaining protocol because incubation steps with both primary antibodies, and subsequently both secondary antibodies, can be carried out simultaneously. Unfortunately, the aforesaid combinations are not always feasible because in some cases the antibodies commercially available for the two proteins of interest are frequently from the same species and isotype subclass. To overcome this limitation, several approaches have been developed for performing immunostaining with antibodies from the same host species.
Previously reported methodologies for performing multiple immunostains on the same tissue or cells with antibodies originating from the same species are varied in their complexity, sensitivity, and approach to prevent unwanted interactions between antibodies. One of the simplest but least sensitive approaches is to use direct detection (Boorsma 1984), where the primary antibodies are directly conjugated either to enzymes (horseradish peroxidase or alkaline phosphatase) for immunoenzymatic detection or to fluorophores for fluorescent detection. Another approach is to use sequential immunohistochemical detection performing the first round of staining with diaminobenzidene (DAB), which masks the first immune complex but also prevents detection of two proteins within the same cellular compartment (Valnes and Brandtzaeg 1982; Valnes and Brandtzaeg 1984). Other approaches rely on either eluting (Pirici et al. 2009; Ranjan et al. 2012) or denaturing (Lan et al. 1995; Wang and Larsson 1985) bound antibodies between rounds of staining. Others have resorted to prebinding primary and secondary antibody pairs in reaction tubes prior to adding them to tissues (Kroeber et al. 1998), to detecting the first primary antibody with conjugated Fab fragments (Negoescu et al. 1994) or with tyramide signal amplification (Shindler and Roth 1996), or to combining indirect detection methods with direct detection methods (van der Loos et al. 1987).
The characterization of rat macrophages in physiological and pathophysiological processes is one present day task for which methods like those described in the previous paragraph are necessary. In the ever-expanding field of macrophage biology, much more is known about mouse and human macrophages than their rat counterparts. The limited availability of monoclonal antibodies that are well-characterized and validated for use in rat tissue is one factor responsible for preventing advances in rat macrophage biology. Herein we describe a method for identifying and examining rat macrophages that is sufficiently sensitive for use in formalin-fixed paraffin embedded (FFPE) tissue and that uses only commercially available reagents and antibodies. Our method relies on detecting macrophages with a biotinylated mouse anti-rat CD68 IgG1 antibody and fluorescently-conjugated streptavidin, after staining other proteins within macrophages or elsewhere with an unconjugated mouse IgG1 antibody and fluorescently labeled anti-mouse IgG secondary antibody. This method can be adapted for use with any pair of antibodies originating from the same species as long as one of the antibodies is biotinylated.
MATERIALS & METHODS
Tissue collection & preparation
All animal studies were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at Ponce Health Sciences University and of the National Institutes of Health. Colonic, hepatic, and cerebral tissues from male Sprague-Dawley rats with or without experimental colitis were fixed overnight in 10% buffered formalin (0.075 M sodium phosphate buffer) at room temperature. Tissues were washed with running water for 30 minutes, dehydrated with graded alcohols (70%, 80%, 95% ×2, 100% ×2) for an hour each, cleared with methyl salicylate first for an hour and then for 30 minutes, and left overnight in a 1:1 solution of methyl salicylate and paraffin at 37°C. The following day, tissues were infiltrated with paraffin during two 30-minute baths and then embedded in paraffin. Tissue sections were cut at a thickness of 2–4 microns with a microtome (Microm, Walldorf, Germany) and mounted on charged glass slides (Thermo Scientifice, Waltham, Massachusetts; Azer Scientific, Morgantown, Pennsylvania; VWR, Radnor, Pennsylvania). The regions of interest sectioned were distal colon, a sagittal section through the anteromedial aspect of the right hepatic lobe, and the middle segment of brain in the rostro-caudal axis at the level of the hippocampus. Archived deidentified slides with human colonic biopsy samples were used for images shown in Supplemental Figure 3.2.
Double immunofluorescence
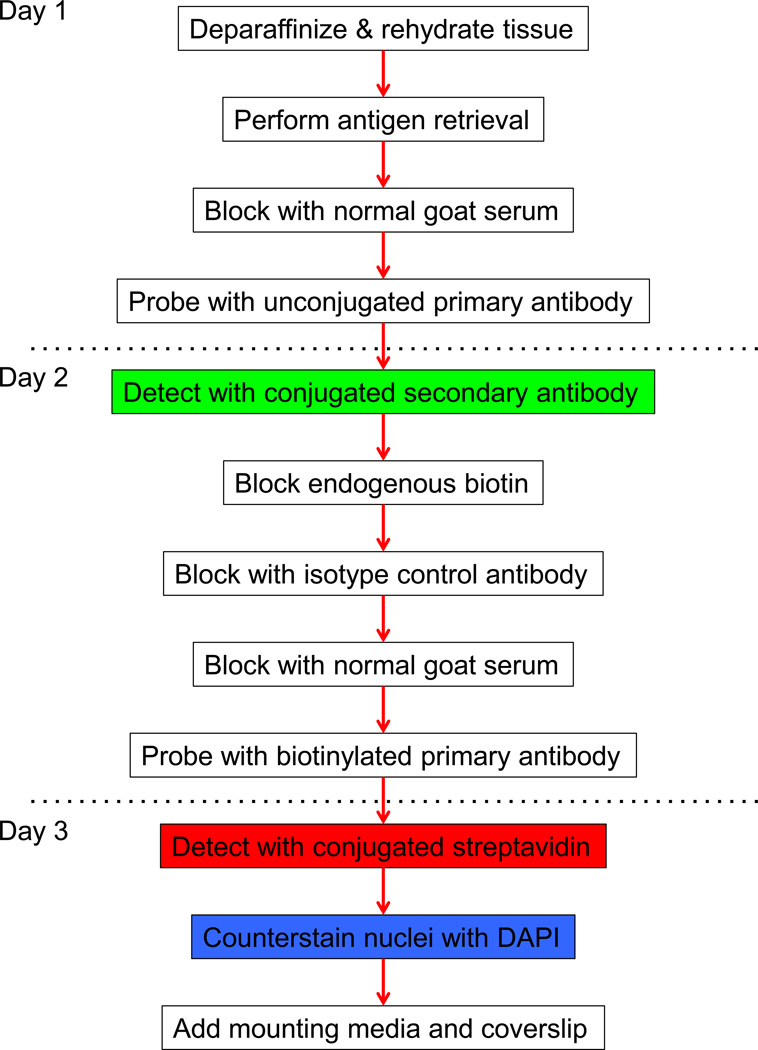
Slides were submerged in the xylene-substitute HemoDe for 30 minutes in order to deparaffinize the tissue section. Tissues were rehydrated by 3-minute washes in graded alcohols (ethanol 100% ×2, 95%, 80%, 70%) and a 1-minute wash in distilled water. After washing in phosphate-buffered saline (PBS) for 5 minutes, slides were submerged in a solution containing 10mM Citrate, 2mM ethylenediaminetetraacetic acid (EDTA), and 0.05% Tween 20 at pH 6.2, placed for 40 minutes in a water bath set to 98.5°C, and incubated for a further 20 minutes at room temperature in order to achieve antigen retrieval. Slides were then washed twice in distilled water for 2 minutes and once in PBS for 5 minutes. Upon drying the slides, each tissue was covered with normal goat serum (NGS, Biogenex, Fremont, California) and incubated at room temperature for 15 minutes to reduce non-specific staining. Afterwards, excess NGS was removed from the slides with absorbent paper. The unconjugated primary antibody targeting inducible nitric oxide synthase (iNOS, sc-7271, Santa Cruz Biotechnology, Dallas, TX) or Ki-67 (550609, BD Pharmingen, San Jose, CA) was diluted 1:50 in PBS, and enough volume of this solution was added to each tissue section to cover the entire tissue. Slides were placed in a humid chamber and incubated overnight at 4°C. From this point onward, washes consist of submerging slides for 5 minutes twice in PBS and drying excess PBS from slides with absorbent paper. The highly cross-adsorbed, Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (A11029, Life Technologies, Carlsbad, CA), diluted 1:100 in PBS, was then added to each tissue section after washing. The slides were again placed in a humid chamber and incubated for 30 minutes at room temperature and subsequently washed. Endogenous biotin was blocked after washing the tissue by incubating the slides with reagents A and B of the Endogenous Biotin Blocking Kit (E21390, Life Technologies) for 20 minutes each, and with each incubation followed by a round of washing. Free Fab portions on tissue-bound anti-mouse secondary antibody were saturated by incubating tissues for 20 minutes with a solution of mouse IgG1 isotype control (MCA-1209, AbD Serotec, Raleigh, NC) diluted 1:10 in PBS. After washing and blocking with NGS for 15 minutes, tissues were incubated overnight with the biotinylated primary antibody targeting CD68 (MCA-341B, AbD Serotec) diluted 1:50 in PBS. Bound biotinylated primary antibody was detected by incubating tissues at room temperature for 30 minutes with Alexa Fluor 594-conjugated streptavidin (S32356, Life Technologies), diluted 1:100 in PBS, after washing the slides. Nuclei were stained with DAPI (R37606, Life Technologies), prepared by adding four drops of DAPI per 1 mL of PBS, for five minutes between two rounds of washes. Slides were dried, coverslipped, and then sealed with nail polish. Prolong Gold Antifade reagent (P36934, Life Technologies) was used as the mounting medium. This protocol is summarized in Fig. 1. Details for all antibodies used are shown in Table 1.
Fig. 1.
Schematic representation of protocol for staining formalin-fixed paraffin-embedded tissue by double immuno uorescence with two mouse monoclonal antibodies
Table 1.
Antibodies and detection agents
| Target | Conjugation | Catalogue # | Company | Dilution | Origin Species |
|
|---|---|---|---|---|---|---|
| 1st Primary | iNOS | - | sc-7271 | Santa Cruz | 1:50 | Mouse |
| Ki-67 | - | 550609 | BD Pharm. | 1:10 | Mouse | |
| IgG1 Isotype Control | Human CD45RA | - | MCA1209 | Abd Serotec | 1:10 | Mouse |
| 1st Secondary | Mouse IgG | Alexa Fluor 488 | A11029 | Life Tech. | 1:100 | Goat |
| 2nd Primary | CD68 | Biotin | MCA341B | Abd Serotec | 1:50 | Mouse |
| Streptavidin | Biotin | Alexa Fluor 594 | S32356 | Life Tech. | 1:100 | - |
iNOS, inducible nitric oxide synthase. IgG, immunoglobulin G. All mouse antibodies are IgG1.
Controls
A total of three negative controls were used for each tissue stained. The first negative control consisted of substituting the primary antibody solutions with PBS in order to assess autoflourescence and non-specific interactions between the tissue and the fluorescently-conjugated detection reagents. The second and third negative controls consisted of single-stain controls in which the biotinylated primary antibody or the unconjugated primary antibody, respectively, was substituted with PBS. These two negative controls allowed for assessing the individual staining patterns of each antibody and for evaluating artifactual staining resulting from interaction between the goat anti-mouse secondary antibody and the biotinylated mouse primary antibody.
Fluorescent microscopy & imaging
Stained tissues were visualized using an Olympus BX60 microscope (Waltham, MA) equipped with an X-cite light source (EXFO Photonic Solutions, Mississauga, Ontario, Canada). Filter sets from Semrock, Inc. (Rochester, New York) with the following excitation/emission wavelengths were used for detecting the stated fluorescent stain: 482nm/536nm for Alexa Fluor 488-conjugated secondary antibodies, 562nm/624nm for Alexa Fluor 594-conjugated streptavidin, and 387nm/447nm for DAPI. Areas of interest were photographed sequentially under each of the aforementioned filter sets using a Digital Sight DS-Fi1 camera (Nikon, Melville, NY) and the NIS-Elements software package (Nikon), where the latter was used for overlaying single-channel images to create a merged image file.
RESULTS
Investigating macrophage biology often requires the use of at least one antibody to identify macrophages and a second antibody to probe for a protein of interest, whose expression might be indicative of phenotype, proliferative status, or other characteristics. To test whether rat colonic macrophages could be examined with two mouse IgG1 antibodies, we subjected FFPE tissue from rats with colitis to our sequential double immunofluorescence protocol (Fig. 1). This protocol involves a round of indirect detection, in which an unconjugated mouse IgG1 primary antibody is detected with a goat-anti-mouse IgG secondary antibody that is conjugated to the fluorophore Alexa Fluor 488, followed by a round of biotin-streptavidin mediated detection, in which a biotinylated mouse IgG1 is detected with streptavidin that is conjugated to the fluorophore Alexa Fluor 594. For our primary antibodies we used a biotinylated anti-CD68 antibody to identify macrophages and an unconjugated anti-iNOS antibody to probe these macrophages.
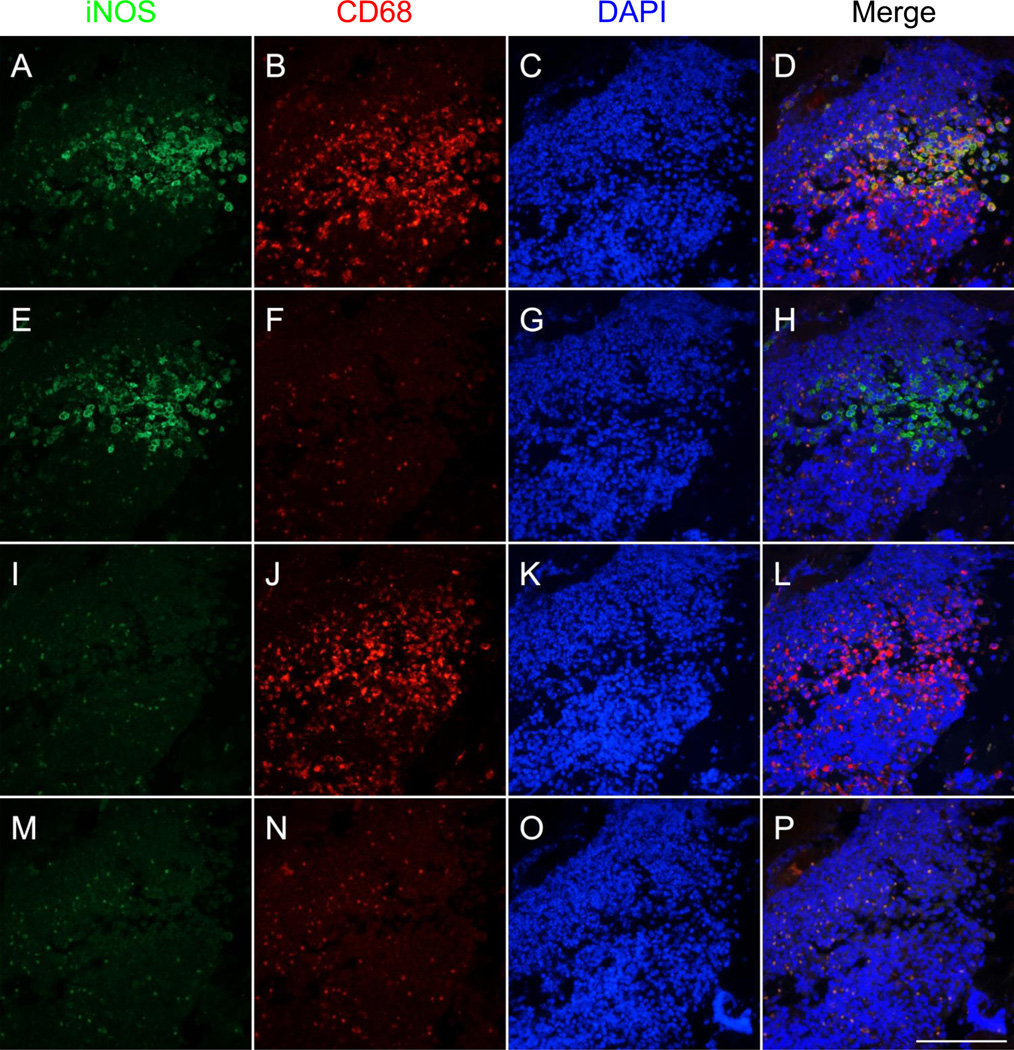
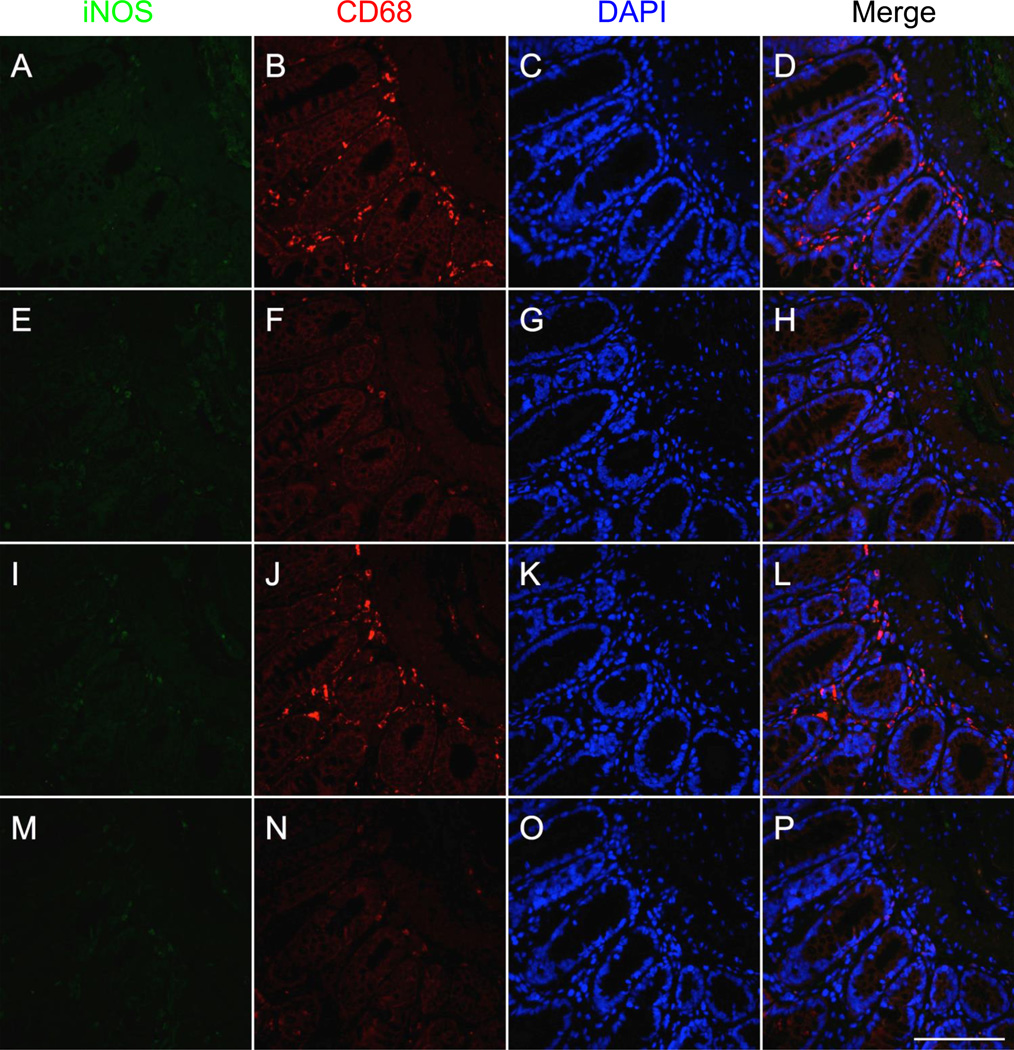
Our double immunofluorescence protocol successfully stained both macrophages and iNOS on the same tissue without interactions and allowed for the identification of cells co-expressing both markers in colonic tissue from rats undergoing experimental colitis (Fig. 2a–d). Areas of co-staining correspond to areas that stain positively for iNOS in the single-stain control for iNOS (Fig. 2e–h) and for CD68 in the CD68 single-stain control (Fig. 2i–l). Although autofluorescence is also detectable, it can be easily identified by examining the negative control (Fig. 2m–p). CD68 staining was prevalent in the mucosa, submucosa, and muscularis propria of the colon, whereas iNOS staining was mainly limited to the inflammatory infiltrate at the base of areas of damaged mucosa. Furthermore, iNOS staining was absent in the colon of normal rats (Fig. 3).
Fig. 2.
Double immunofluorescent staining of macrophages using two mouse monoclonal antibodies targeting CD68 and inducible nitric oxide synthase (iNOS) in formalin-fixed paraffin embedded colon from a colitic rat. Images depict serial sections inflammatory infiltrate within colonic mucosa damaged by colitis induction. Staining for iNOS (unconjugated primary antibody, green), CD68 (biotinylated primary antibody, red), and DAPI (blue) is shown in the first, second, and third columns, respectively. Overlays of the green, red, and blue channels are shown in the fourth column. a–d Staining for CD68, iNOS, and DAPI reveals the presence of several macrophages (CD68-positive cells) co-expressing iNOS. e–h The iNOS single-stain control (tissue did not receive CD68 antibody) reveals iNOS staining in areas of colocalization seen in a–d. i–l The CD68 single-stain control (tissue did not receive iNOS antibody) reveals CD68 staining in areas of colocalization as well as in areas negative for iNOS as seen in a–d. m–p Negative control tissue (did not receive either of the primary antibodies) demonstrates some autofluorescence that can be distinguished from areas of true positivity (orange color in p). Scale bar = 100µm
Fig. 3.
Double immunofluorescent staining of macrophages using two mouse monoclonal antibodies targeting CD68 and inducible nitric oxide synthase (iNOS) in formalin-fixed paraffin embedded colon from a normal rat. Images depict serial sections of normal colonic mucosa and submucosa. Staining for iNOS (unconjugated primary antibody, green), CD68 (biotinylated primary antibody, red), and DAPI (blue) is shown in the first, second, and third columns, respectively. Overlays of the green, red, and blue channels are shown in the fourth column. a–d Staining for CD68, iNOS, and DAPI reveals the presence of several macrophages (CD68-positive cells) lacking iNOS staining. e–h The iNOS single-stain control (tissue did not receive CD68 antibody) confirms the lack of iNOS staining observed in a–d. i–l The CD68 single-stain control (tissue did not receive iNOS antibody) shows CD68 staining consistent with that shown in a–d. m–p Negative control tissue (did not receive either of the primary antibodies) demonstrates some autofluorescence that can be distinguished from areas of true positivity (orange color in p). Scale bar = 100µm
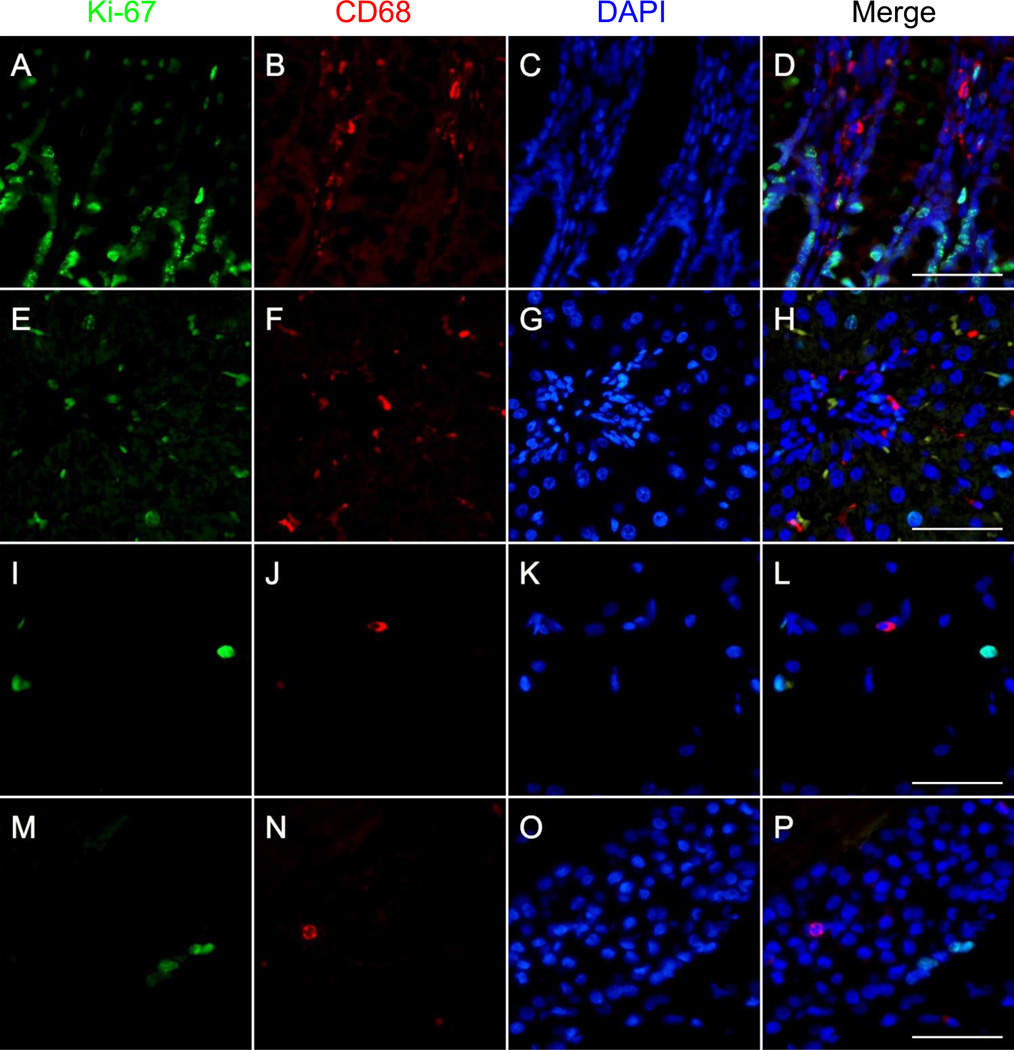
To further test our protocol, we performed double immunostaining with a different unconjugated mouse IgG1 monoclonal antibody. We chose to use an anti-Ki-67 primary antibody because Ki-67, a marker of proliferation, is present to different degrees in several normal tissues and because it is a nuclear antigen, which facilitates the identification of artifactual colocalization (ie. the presence of nuclear CD68 staining in Ki-67-positive cells would be indicative of artifactual colocalization). Using our protocol, we successfully stained for Ki-67 and CD68 in colonic, hepatic, and cerebral tissue from normal rats (Fig. 4). Staining for CD68 and Ki-67 did not colocalize within the same cellular compartment, even when staining for both proteins was present in the same cell, indicating that absence of unwanted interactions between the anti-mouse secondary antibody and the biotinylated anti-CD68 mouse antibody. A diagrammatic representation of the staining protocol is shown in Fig. 3.6a (see Online Resource 1 for an animated version of this diagram).
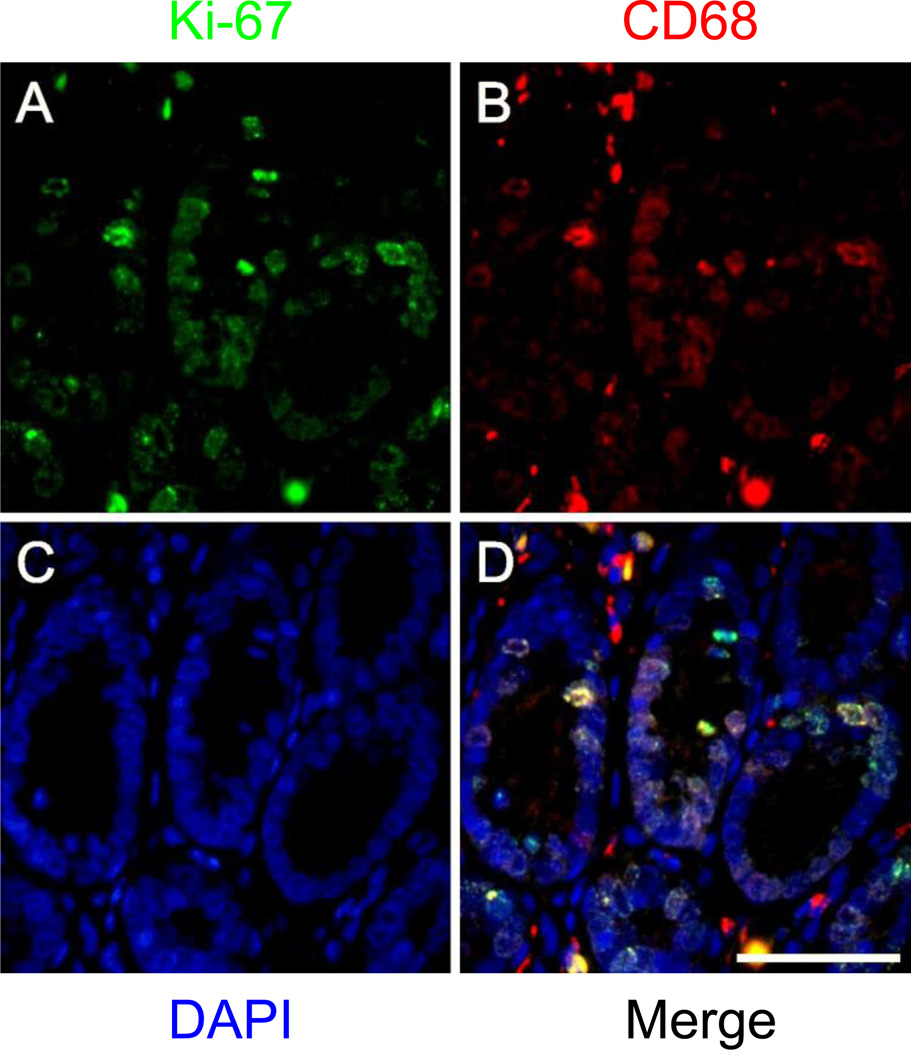
Fig. 4.
Double immunofluorescent staining of macrophages using two mouse monoclonal antibodies targeting CD68 and Ki-67 in formalin-fixed paraffin embedded colon, liver, and brain from normal rats. Staining for Ki-67 (unconjugated primary antibody, green), CD68 (biotinylated primary antibody, red), and DAPI (blue) is shown in the first, second, and third columns, respectively. Overlays of the green, red, and blue channels are shown in the fourth column. a–d Staining for CD68, Ki-67, and DAPI in colonic tissue reveals the presence of several macrophages (CD68+ cells) in the lamina propria and abundant proliferating (Ki-67+) epithelial cells in the bottom half of the colonic crypts. e–h Staining for CD68, Ki-67, and DAPI in hepatic tissue shows various macrophages (CD68+ cells) in the interstitium and several proliferating (Ki-67+) hepatocytes, including mitotic figures. i–l Staining for CD68, Ki-67, and DAPI in the cerebral cortex demonstrates occasional CD68+ cells and Ki-67+ cells. m–p Staining for CD68, Ki-67, and DAPI in the CA2 region of the hippocampus demonstrates the presence of some proliferating cells (Ki-67+) and the occasional macrophage (CD68+). Scale bars = 50µm
Performing our double immunostaining protocol without incubating the tissues with the mouse IgG1 isotype control after the first round of staining resulted in marked artifactual colocalization (Fig. 5). Nuclear CD68 staining is prevalent (Fig. 5b) and colocalizes with Ki-67-positive nuclei (Fig. 5a,d). Taken together, this data indicated that the artifactual colocalization was attributable to interactions between free Fab fragments on bound anti-mouse IgG secondary antibody with biotinylated mouse IgG1 primary antibody targeting CD68 (Fig. 6b, Online Resource 2).
Fig. 5.
Artifactual co-localization resulting from interaction between tissue-bound anti-mouse secondary antibody and biotinylated CD68 antibody in formalin-fixed paraffin embedded colon from a normal rat. Images depict crypts and lamina propria in normal colonic mucosa. Staining for Ki-67 (unconjugated primary antibody, green), CD68 (biotinylated primary antibody, red), and DAPI (blue) as well as an overlay of the green, red, and blue channels are shown in a–d, respectively. Nuclear CD68 staining in b coincides with Ki-67-positivity in crypt nuclei in a and indicates artifactual co-localization, which results when free binding sites on tissue bound anti-mouse secondary antibody are not saturated with isotype mouse IgG1. Scale bar = 50µm
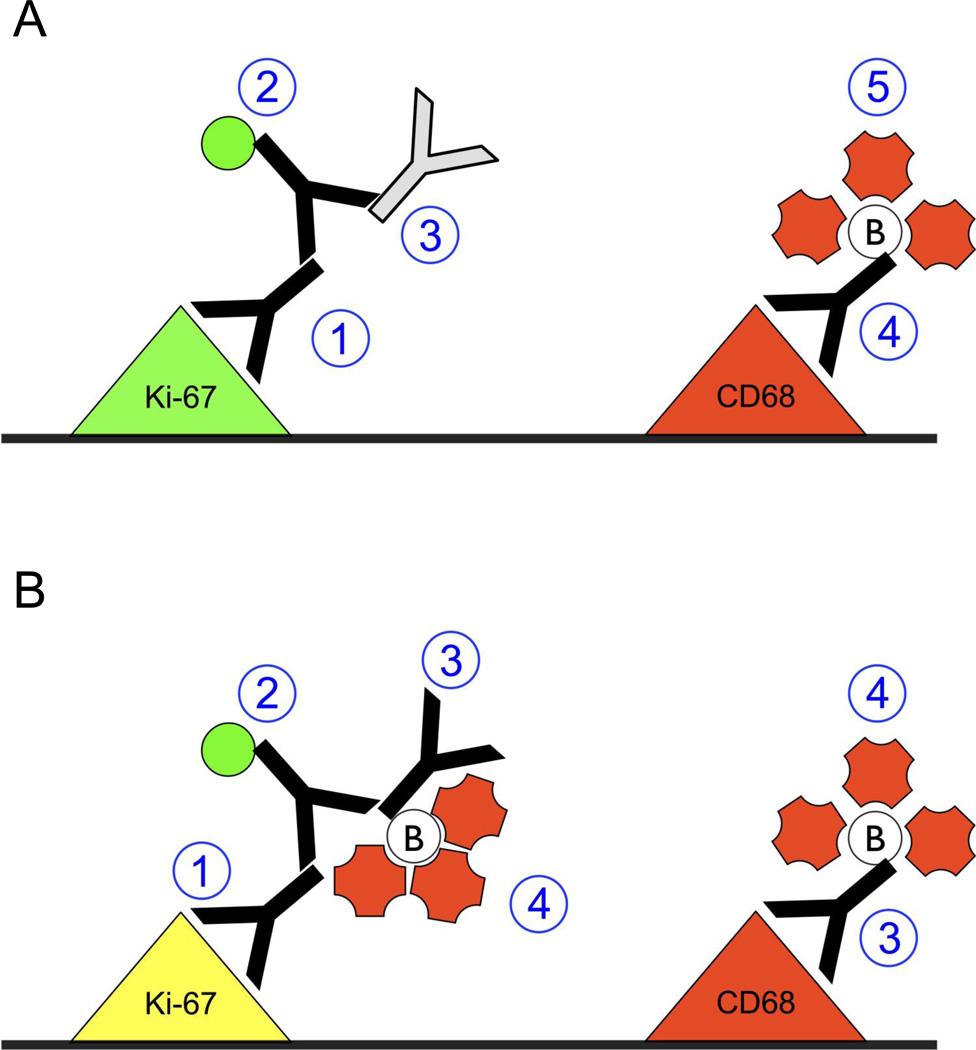
Fig. 6.
Diagrammatic representation of the steps employed for performing double immunofluorescent staining with two mouse IgG1 antibodies, with each step enumerated with blue circles. a Illustration of staining protocol in which an unconjugated isotype control antibody is used to prevent interactions between tissue-bound anti-mouse secondary antibody and the biotinylated mouse antibody. First, the unconjugated primary antibody is added to the tissue to detect the first protein of interest. Second, the conjugated secondary antibody is added to the tissue to detect the unconjugated primary antibody. Third, an unconjugated isotype control is added to saturate the free Fab fragments on tissue-bound anti-mouse secondary antibody. Fourth, biotinylated primary antibody is added to the tissue detect the second protein of interest. Fifth, conjugated streptavidin is added to the tissue to detect the biotinylated primary antibody. See Online Resource 1 for an animated version of this diagram. b Illustration of staining protocol that results in artifactual colocalization (as seen in Fig. 5) caused by interactions between tissue-bound anti-mouse secondary antibody and the biotinylated mouse antibody. First, the unconjugated primary antibody is added to the tissue to detect the first protein of interest. Second, the conjugated secondary antibody is added to the tissue to detect the unconjugated primary antibody. Third, the biotinylated primary antibody is added to the tissue, where it not only binds to its target protein but also to the free arms on the secondary antibody that is bound to the tissue. Fourth, conjugated streptavidin is added to detect the biotinylated primary antibody, resulting in red fluorescence in iNOS-positive areas as well as in CD68-positive areas. See Online Resource 2 for an animated version of this diagram
In addition to the examples discussed above, we have also successfully applied our protocol to staining colonic tissues from rats (Online Resource 3) and humans (Online Resource 4) with other pairs of mouse (Online Resource 3A–D, Online Resource 4A–D) and rabbit antibodies (Online Resource 3E–L, Online Resource 4E–L). Details on the antibodies used are listed in Online Resource 5. Lastly, we include in Online Resource 6 a list of biotinylated antibodies with which we have not been able to obtain single immunofluorescent staining.
DISCUSSION
In the present study we have described a method for staining two proteins on FFPE tissue using two primary antibodies of the same origin species and isotype. We have applied this method to the specific example of analyzing macrophages in tissue from the rat large intestine (colon), liver, and brain. The essence of our method consists of combining indirect detection, using an unconjugated primary antibody and fluorescently-conjugated secondary antibody, with biotin-streptavidin-mediated direct detection, using a biotinylated primary antibody and fluorescently-conjugated streptavidin.
Others have previously used this approach for double staining with antibodies originating from the same species. Campana and Janossy (1986) described a method for staining human leukemic blasts by using indirect detection with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody on cell suspensions followed by biotin-avidin mediated direct detection with tetramethylrhodamine (TRITC)-conjugated avidin on cytospins. In this case, the use of this particular approach was not essential as both primary antibodies were of different isotypes: Campana and Janossy used an unconjugated primary antibody of the IgM isotype and a biotinylated primary antibody of the IgG1 isotype. Würden and Homberg (1993) published a method with a similar approach for performing double immunofluorescence on paraformaldehyde-fixed brain tissues from Schistocerca gregaria. They also used an FITC-conjugated secondary antibody for the indirect detection, but used streptavidin, instead of avidin, that was conjugated to Texas Red, rather than TRITC. Most recently, Frisch and colleagues (2011) developed a method for performing triple immunofluorescence with primary antibodies from the same species. In addition to combining indirect detection with biotin-streptavidin-mediated direct detection, they added a digoxigenin-mediated indirect detection step to enable detection of a third protein. In contrast to the method by Frisch et al., we find that saturating the free Fab segments on tissue-bound secondary antibody after performing the indirect staining is essential, which is consistent with the methods described by Campana and Janossy and by Würden and Homberg.
Our described method differs from the aforementioned studies in three key aspects. First, we used a commercially available, biotinylated primary antibody, whereas the biotinylation of the primary antibodies described in the previously mentioned studies was performed by the authors themselves. In fact, all the materials and reagents used in our method are commercially available, which should facilitate the use of our protocol in other laboratories. Second, we used primary antibodies of mouse origin on rat tissue, whereas the other studies used antibodies from rabbit origin on rat or S. gregaria tissue, or from mouse origin on human tissue. Given the homology between mice and rats, performing indirect staining with mouse IgG antibodies on rat tissue requires the use of anti-mouse IgG secondary antibodies that are cross-adsorbed against rat IgG antibodies in order to avoid non-specific detection of endogenous antibodies. Third, we used tissues that were fixed in formalin and embedded in paraffin, a process that is known to mask tissue antigens (Rait et al. 2004; Shi et al. 1991; Sompuram et al. 2004). Therefore, enzymatic amplification, such as that provided by peroxidase- or alkaline phosphatase-mediated immunohistochemistry, is often preferred for immunostaining FFPE tissue even after performing antigen recovery steps. Nonetheless, our method demonstrated that it was sufficiently sensitive to detect both proteins of interest without the need for enzymatic amplification.
We have applied our method for double immunostaining with two mouse IgG1 antibodies to the analysis of macrophages in the rat. Specifically, we have coupled a monoclonal antibody against the panmacrophage marker CD68 with a monoclonal antibody against inducible nitric oxide synthase as a method of identifying proinflammatory macrophages (Mills et al. 2000). We have also used an antibody against Ki-67 as a means of investigating the proliferative potential of CD68-positive cells and the relation of CD68 positive cells with other proliferating cells. The former point is of interest given that certain mouse macrophages have been shown to be capable of proliferation (Jenkins et al. 2011). Given that the macrophage marker is commercially available in the biotinylated format, this method could be adapted to probe macrophages with any other mouse antibody. Therefore, our method allows for future studies to investigate the presence within macrophages of cytokines, transcription factors, other markers of proliferation, or any other protein for which a validated antibody exists. It is our hope that this method can be employed to further advance our knowledge of the biology of rat macrophages to a point comparable to our current knowledge of mouse and human macrophages. Lastly, this method can be adapted for use with any two antibodies from the same species of origin as long as one of the antibodies is biotinylated.
The following are a few recommendations and tips meant for those seeking to implement this protocol in their laboratories. First of all, we highly recommend that each antibody be optimized first by single immunofluorescence prior to performing the full protocol. Second, we have made an effort to select isotype controls that are as similar as possible to the biotinylated antibody to be used. Specifically, we select isotype controls that are of the same species, isotype, isotype subclass, and manufacturer as the biotinylated antibody to be used as the second primary antibody. In terms of specificity, we have successfully used isotype antibodies that are not known to detect any known antigen, that detect antigens from species other than that of the tissue being stained (isotype antibody targets a human antigen but is used on rat tissue, on which it does not target any antigens), and that detect antigens on the tissue being stained (isotype antibody targets a human antigen and is used on human tissue). Third, although we have found similar sensitivity when staining for the same antigen with either an unconjugated primary antibody and a fluorophore-conjugated secondary antibody or with a biotinylated primary antibody and fluorophore-conjugated streptavidin, staining antigens that are more prevalent with the biotinylated antibody as a second primary antibody may allow using lower concentrations of isotype antibody to saturate free Fab fragments on tissue bound secondary antibody.
Supplementary Material
Online Resource 1 Double immunofluorescent staining with two mouse IgG1 antibodies (video)
Online Resource 2 Staining protocol that results in artifactual colocalization caused by interactions between tissue-bound anti-mouse secondary antibody and the biotinylated mouse antibody (video)
Online Resource 3 Double immunofluorescent staining with antibodies from the same species in formalin-fixed paraffin-embedded colonic tissue from rats. Staining for the green (unconjugated primary antibody), red (biotinylated primary antibody), and blue channels is shown in the first, second, and third columns, respectively. Overlays of the three channels are shown in the fourth column. A–D, staining for CD68 (green), phosphorylated tyrosine (p-tyrosine, red), and DAPI (blue) in the inflamed colonic submucosa of a colitic rat shows several single- and double-positive cells for both CD68 and p-tyrosine. E–H, staining of matrix metalloprotease 9 (MMP9, green), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, red), and DAPI (blue) in the inflamed colonic submucosa of a colitic rat shows several MMP9 and GAPDH double-positive cells and many GAPDH single-positive cells. I–L, staining of vitamin D receptor (VDR, green), GAPDH (red), and DAPI (blue) in the colonic mucosa of a normal rat shows several VDR and GAPDH double-positive cells and GAPDH single-positive cells. Scale bars = 100 µm
Online Resource 4 Double immunofluorescent staining with antibodies from the same species in formalin-fixed paraffin-embedded colonic tissue from humans. Staining for the green (unconjugated primary antibody), red (biotinylated primary antibody), and blue channels is shown in the first, second, and third columns, respectively. Overlays of the three channels are shown in the fourth column. A–D, staining for CD68 (green), CD45RA (red), and DAPI (blue) in a mucosal lymphoid follicle of a normal human colon biopsy shows few CD68-positive cells (macrophages) in the center of the follicle surrounded by several CD45RA cells (lymphocytes). E–H, staining of vitamin D receptor (VDR, green), cleaved poly (ADP-ribose) polymerase (cPARP, red), and DAPI (blue) in the inflamed mucosa of a colonic biopsy from a patient with Crohn’s disease shows prevalent VDR staining in the lamina propria cells and crypt epithelium with scattered cPARP-positive cells. I–L, staining of VDR (green), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, red), and DAPI (blue) in the mucosa of a normal human colon biopsy shows several VDR and GAPDH double-positive cells. Scale bars = 100 µm
Online Resource 5 Antibodies used in Online Resource 3 and Online Resource 4
Online Resource 6 Biotinylated antibodies that did not stain in formalin-fixed paraffin-embedded tissue by single immunofluorescence with fluorophore-conjugated streptavidin (table)
Online Resource 7 Hematoxylin and eosin histochemical staining of areas of rat colon depicted in Figs. 2, 3, and 5. A, inflammatory infiltrate within colonic mucosa damaged by colitis induction as shown in Fig. 2. Scale bar = 100 µm. B, normal colonic mucosa and submucosa as shown in Fig. 3. Scale bar = 100 µm. C, crypts and lamina propria in normal colonic mucosa as shown in Fig. 5. Scale bar = 50 µm
Acknowledgments
The authors would like to thank Alcira Benitez Barros for histotechnical assistance. We also thank Dr. Pedro Santiago for comments on the manuscript. RA Isidro was supported by a William Townsend Porter Predoctoral Fellowship from the American Physiological Society. This study was also funded in part by the National Institute of General Medical Sciences (R25GM082406 to RAI, SH) and the National Cancer Institute (U54CA163071 to RAI, CBA) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors acknowledge the support of the PHSU Molecular and Genomics Core Laboratory (RR003050/MD007579).
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS:
All animal studies were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at Ponce Health Sciences University and of the National Institutes of Health. All human studies were performed in accordance with the guidelines of the Internal Review Board at Ponce Health Sciences University and of the National Institutes of Health.
CONFLICT OF INTEREST:
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS:
RAI, AAI, & CBA designed the study and analyzed the data
RAI, MLC, & SH performed the experiments and acquired the data
RAI drafted the manuscript
AAI, MLC, SH, & CBA revised manuscript for important intellectual content
RAI, AAI, MLC, SH, & CBA approved the final version of the manuscript for publication and agree to be accountable for all aspects of the work
ETHICAL APPROVAL:
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. Informed consent was obtained from all individual participants included in this study.
REFERENCES
- Boorsma DM. Direct immunoenzyme double staining applicable for monoclonal antibodies. Histochemistry. 1984;80:103–106. doi: 10.1007/BF00679982. [DOI] [PubMed] [Google Scholar]
- Campana D, Janossy G. Leukemia diagnosis and testing of complement-fixing antibodies for bone marrow purging in acute lymphoid leukemia. Blood. 1986;68:1264–1271. [PubMed] [Google Scholar]
- Frisch J, et al. Novel multicolor immunofluorescence technique using primary antibodies raised in the same host species. Methods in molecular biology (Clifton, NJ) 2011;717:233–244. doi: 10.1007/978-1-61779-024-9_13. [DOI] [PubMed] [Google Scholar]
- Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeber S, Schomerus C, Korf HW. A specific and sensitive double-immunofluorescence method for the demonstration of S-antigen and serotonin in trout and rat pinealocytes by means of primary antibodies from the same donor species. Histochemistry and cell biology. 1998;109:309–317. doi: 10.1007/s004180050231. [DOI] [PubMed] [Google Scholar]
- Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC. A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1995;43:97–102. doi: 10.1177/43.1.7822770. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. Journal of immunology (Baltimore, Md: 1950) 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Negoescu A, Labat-Moleur F, Lorimier P, Lamarcq L, Guillermet C, Chambaz E, Brambilla E. F(ab) secondary antibodies: a general method for double immunolabeling with primary antisera from the same species. Efficiency control by chemiluminescence. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1994;42:433–437. doi: 10.1177/42.3.7508473. [DOI] [PubMed] [Google Scholar]
- Pirici D, Mogoanta L, Kumar-Singh S, Pirici I, Margaritescu C, Simionescu C, Stanescu R. Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2009;57:567–575. doi: 10.1369/jhc.2009.953240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rait VK, Xu L, O'Leary TJ, Mason JT. Modeling formalin fixation and antigen retrieval with bovine pancreatic RNase A II. Interrelationship of cross-linking, immunoreactivity, and heat treatment Laboratory investigation; a journal of technical methods and pathology. 2004;84:300–306. doi: 10.1038/labinvest.3700041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan AK, Joglekar MV, Atre AN, Patole M, Bhonde RR, Hardikar AA. Cellular detection of multiple antigens at single cell resolution using antibodies generated from the same species. Journal of immunological methods. 2012;379:42–47. doi: 10.1016/j.jim.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Roth KA. Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1996;44:1331–1335. doi: 10.1177/44.11.8918908. [DOI] [PubMed] [Google Scholar]
- Sompuram SR, Vani K, Messana E, Bogen SA. A molecular mechanism of formalin fixation and antigen retrieval. American journal of clinical pathology. 2004;121:190–199. doi: 10.1309/BRN7-CTX1-E84N-WWPL. [DOI] [PubMed] [Google Scholar]
- Valnes K, Brandtzaeg P. Comparison of paired immunofluorescence and paired immunoenzyme staining methods based on primary antisera from the same species. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1982;30:518–524. doi: 10.1177/30.6.6178779. [DOI] [PubMed] [Google Scholar]
- Valnes K, Brandtzaeg P. Paired indirect immunoenzyme staining with primary antibodies from the same species. Application of horseradish peroxidase and alkaline phosphatase as sequential labels. The Histochemical journal. 1984;16:477–487. doi: 10.1007/BF01041348. [DOI] [PubMed] [Google Scholar]
- van der Loos CM, Das PK, Houthoff HJ. An immunoenzyme triple-staining method using both polyclonal and monoclonal antibodies from the same species. Application of combined direct, indirect, and avidin-biotin complex (ABC) technique. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1987;35:1199–1204. doi: 10.1177/35.11.2443555. [DOI] [PubMed] [Google Scholar]
- Wang BL, Larsson LI. Simultaneous demonstration of multiple antigens by indirect immunofluorescence or immunogold staining. Novel light and electron microscopical double and triple staining method employing primary antibodies from the same species. Histochemistry. 1985;83:47–56. doi: 10.1007/BF00495299. [DOI] [PubMed] [Google Scholar]
- Würden S, Homberg U. A simple method for immunofluorescent double staining with primary antisera from the same species. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1993;41:627–630. doi: 10.1177/41.4.8450202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1 Double immunofluorescent staining with two mouse IgG1 antibodies (video)
Online Resource 2 Staining protocol that results in artifactual colocalization caused by interactions between tissue-bound anti-mouse secondary antibody and the biotinylated mouse antibody (video)
Online Resource 3 Double immunofluorescent staining with antibodies from the same species in formalin-fixed paraffin-embedded colonic tissue from rats. Staining for the green (unconjugated primary antibody), red (biotinylated primary antibody), and blue channels is shown in the first, second, and third columns, respectively. Overlays of the three channels are shown in the fourth column. A–D, staining for CD68 (green), phosphorylated tyrosine (p-tyrosine, red), and DAPI (blue) in the inflamed colonic submucosa of a colitic rat shows several single- and double-positive cells for both CD68 and p-tyrosine. E–H, staining of matrix metalloprotease 9 (MMP9, green), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, red), and DAPI (blue) in the inflamed colonic submucosa of a colitic rat shows several MMP9 and GAPDH double-positive cells and many GAPDH single-positive cells. I–L, staining of vitamin D receptor (VDR, green), GAPDH (red), and DAPI (blue) in the colonic mucosa of a normal rat shows several VDR and GAPDH double-positive cells and GAPDH single-positive cells. Scale bars = 100 µm
Online Resource 4 Double immunofluorescent staining with antibodies from the same species in formalin-fixed paraffin-embedded colonic tissue from humans. Staining for the green (unconjugated primary antibody), red (biotinylated primary antibody), and blue channels is shown in the first, second, and third columns, respectively. Overlays of the three channels are shown in the fourth column. A–D, staining for CD68 (green), CD45RA (red), and DAPI (blue) in a mucosal lymphoid follicle of a normal human colon biopsy shows few CD68-positive cells (macrophages) in the center of the follicle surrounded by several CD45RA cells (lymphocytes). E–H, staining of vitamin D receptor (VDR, green), cleaved poly (ADP-ribose) polymerase (cPARP, red), and DAPI (blue) in the inflamed mucosa of a colonic biopsy from a patient with Crohn’s disease shows prevalent VDR staining in the lamina propria cells and crypt epithelium with scattered cPARP-positive cells. I–L, staining of VDR (green), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, red), and DAPI (blue) in the mucosa of a normal human colon biopsy shows several VDR and GAPDH double-positive cells. Scale bars = 100 µm
Online Resource 5 Antibodies used in Online Resource 3 and Online Resource 4
Online Resource 6 Biotinylated antibodies that did not stain in formalin-fixed paraffin-embedded tissue by single immunofluorescence with fluorophore-conjugated streptavidin (table)
Online Resource 7 Hematoxylin and eosin histochemical staining of areas of rat colon depicted in Figs. 2, 3, and 5. A, inflammatory infiltrate within colonic mucosa damaged by colitis induction as shown in Fig. 2. Scale bar = 100 µm. B, normal colonic mucosa and submucosa as shown in Fig. 3. Scale bar = 100 µm. C, crypts and lamina propria in normal colonic mucosa as shown in Fig. 5. Scale bar = 50 µm