Abstract
Drug induced kidney disease is a frequent cause of renal dysfunction; however, there are no standards to identify and characterize the spectrum of these disorders. We convened a panel of international, adult and pediatric, nephrologists and pharmacists to develop standardized phenotypes for drug induced kidney disease as part of the phenotype standardization project initiated by the International Serious Adverse Events Consortium. We propose four phenotypes of drug induced kidney disease based on clinical presentation: acute kidney injury, glomerular, tubular and nephrolithiasis, along with primary and secondary clinical criteria to support the phenotype definition, and a time course based on the KDIGO/AKIN definitions of acute kidney injury, acute kidney disease and chronic kidney disease. Establishing causality in drug induced kidney disease is challenging and requires knowledge of the biological plausibility for the specific drug, mechanism of injury, time course and assessment of competing risk factors. These phenotypes provide a consistent framework for clinicians, investigators, industry and regulatory agencies to evaluate drug nephrotoxicity across various settings. We believe that this is first step to recognizing drug induced kidney disease and developing strategies to prevent and manage this condition.
Keywords: Nephrotoxicity, acute kidney injury; drugs; hypersensitivity; adverse reaction; tubular toxicity; nephrolithiasis; glomerulonephritis; crystalluria
Introduction
Drug induced kidney disease (DIKD) accounts for approximately 19-26% of cases of acute kidney injury (AKI) in hospitalized patients [1]. There are no standards to identify drug induced nephrotoxicity and as a result - DIKD is often unrecognized. In recent years, the International Serious Adverse Event Consortium (iSAEC) has initiated a phenotype standardization project for drug induced adverse events[2]. In conjunction with the iSAEC, we have developed consensus definitions for DIKD, taking into account its wide spectrum and the need for balancing practicality with reliability of the classifications across different settings.
Consensus process
With the support of the iSAEC, we organized a series of eight teleconferences followed by two face-to-face meeting of international, adult and pediatric, nephrologists and pharmacists. The panel developed phenotypic criteria using a modified Delphi process to allow identification of patients across 4 categories representing the spectrum of DIKD, for subject recruitment into a genetic study of DIKD (DIRECT). The panel was divided into subgroups and researched specific phenotypes. Criteria were summarized and presented to the larger group for consensus. Criteria were considered in the context of using electronic medical records to screen for patients with DIKD in both hospitalized and ambulatory settings. Panelists were asked to consider the known mechanisms of nephrotoxicity, time course of drug exposure and the setting as discussed in more detail below. For the acute kidney injury (AKI) phenotype, established definitions were considered as the starting point and adapted for DIKD (e.g. AKIN/KDIGO criteria for AKI) [3].
Description of Phenotype
We propose that DIKD presents in one of four phenotypes: AKI, glomerular disorder, tubular disorder, or nephrolithiasis/crystalluria. The clinical presentation of each phenotype is based on a change in biomarkers and other evidence: Scr (AKI), proteinuria or hematuria (glomerular), electrolyte abnormalities (tubular), ultrasound findings (nephrolithiasis). To standardize the initial phenotype, we developed primary and secondary criteria. We suggest that at least one primary criterion must be met for all drugs suspected of causing DIKD (Table 1).
Table 1. Primary and Secondary Criteria for Individual Phenotypes.
| Phenotype | Acute kidney Injury | Glomerular Disorder | Nephrolithiasis | Tubular Dysfunction |
|---|---|---|---|---|
| Characteristics |
|
|
|
|
| Primary Criteria |
OR
|
AND Proteinuria as defined by:
Hematuria
|
|
Tubular: Hypophosphatemia OR Glucosuria
OR Hyperchloremi c metabolic acidosis AND Hypokalemia or hyperkalemia Diabetes insipidus:
|
| Secondary criteria |
|
|
|
Phosphaturia
Hypomagnesemia
Hypouricemia
Tubular Proteinuria
Diabetes insipidus
|
Hemodynamic changes may contribute to ATN, however, in the absence of any specific features are not considered individual criteria for the AKI phenotype.
SIADH does not reflect direct tubular damage but rather the impact of a drug on ADH secretion and subsequent impaired water handling.
AIN = acute interstitial nephritis, ATN = acute tubular necrosis, DM = diabetes mellitus, FeNa= fractional excretion of sodium, FePO4 = fractional excretion of phosphorus, GN = glomerulonephritis, HPF = high powered field, LDH = lactate dehydrogenase, RBC= red blood cell, SIADH= syndrome of inappropriate antidiuretic hormone, UPC = urine protein to creatinine ratio, UACR= urine albumin to creatinine ratio, WBC = white blood cell.
Mechanisms
Adverse drug reactions can be classified into type A and B reactions. Type A reactions are dose-dependent toxicities that are predictable based on the known pharmacology of the drug and alleviated by reducing drug exposure (i.e. dose reduction) or withdrawal of the drug (e.g. aminoglycoside toxicity). Type B reactions are unpredictable based on the known pharmacology of the drug. Toxicity is not dose-dependent and usually requires drug withdrawal for resolution (e.g. acute interstitial nephritis from proton pump inhibitors).
Often, the same drug may present as different DIKD phenotypes. For instance, NSAIDS can result in AKI due to hemodynamic changes or acute interstitial nephritis (AIN), or nephrotic range proteinuria from glomerular injury. The risk factors that predispose individuals to develop an adverse reaction from an individual drug are unknown in most cases. Genetic risk factors are emerging for the development of serious drug induced adverse reactions [4]. In type A reactions, genetic variation in drug elimination may determine overall drug exposure and pharmacological effect. For example, alterations in the expression of organic anion transporters (OAT) in the kidney could lead to increased intracellular concentrations of certain antimicrobials with increased toxicity to the renal tubules. The mechanisms underlying type B reactions are more complex and variable than type A reactions. In many instances of organ-directed toxicity, drug-induced disease may mimic other diseases. For example, hydralazine associated glomerulonephritis (GN) is immune mediated, may mimic a lupus or ANCA positive GN and may be categorized as a type B reaction. Based on our current understanding we classified common drugs associated with DIKD in relation to Type A and B reactions (Table 2&3).
Table 2. Drug toxicity mechanism and timing for AKI and Glomerular phenotypes.
| Drug | AKI | Glomerular | Time Course | Genetic Mechanism | ||
|---|---|---|---|---|---|---|
| Type A | Type B | Type A | Type B | |||
| Abacavir | X | Acute | HLA | |||
| Aminoglycosides | X | Acute/SA | Megalin/Cathepsins/Caspases | |||
| Amoxicillin | X | SA | HLA | |||
| Ampicillin | X | SA | HLA | |||
| Amphotericin | X | Acute/SA | ||||
| Bevacizumab | X | SA | VEGF | |||
| Cefazolin | X | SA | HLA | |||
| Ceftazidime | X | SA | HLA | |||
| Cidofovir | X | Acute | OAT | |||
| Ciprofloxacin | X | SA | HLA | |||
| Colistin | X | SA | OCT | |||
| Cyclosporine | X | X | Acute/SA | CYP 3A/PGP | ||
| Foscarnet | X | Acute/SA | ||||
| Hydralazine | X | SA/Chronic | HLA | |||
| Levofloxacin | X | SA | HLA | |||
| Lithium | X | SA/Chronic | VA receptors | |||
| Nafcillin | X | SA | HLA | |||
| NSAIDs | X | X | Acute/SA | HLA | ||
| Oxacillin | X | SA | HLA | |||
| Pamidronate | X | X | SA | |||
| Penicillin | X | SA | HLA | |||
| Piperacillin/tazobactam | X | Acute/SA | HLA | |||
| Propylthiouracil | X | SA/Chronic | HLA | |||
| Rifampin | X | X | SA | HLA | ||
| SMX/TMP | X | Acute/SA | HLA | |||
| Tacrolimus | X | X | Acute/SA | CYP 3A | ||
| Vancomycin | X | X | Acute/SA | Oxidative stress, HLA | ||
Type A= dose dependent toxicity, Type B= idiosyncratic, acute = within 7 days of drug initiation, SA = sub-acute, occurs within 4 weeks of drug exposure and may take up to 90 days to resolve, chronic = injury persisting beyond 90 days, OAT=organic anion transporter, HLA = human leukocyte antigen, CYP = cytochrome P450, PGP = p-glycoprotein, MRP = multi-drug resistance associated protein
Table 3. Drug toxicity mechanism and timing for Tubular and Nephrolithiasis phenotypes.
| Drug | Tubular | Nephrolithiasis | Time Course | Genetic Mechanism | ||
|---|---|---|---|---|---|---|
| Type A | Type B | Type A | Type B | |||
| Acyclovir | X | SA | OAT | |||
| Atazanavir | X | SA | ||||
| Cisplatin | X | SA/Chronic | OAT | |||
| Didanosine | X | SA | OAT/Mitochondria | |||
| Foscarnet | X | SA | NaPO4 transport | |||
| Ifosfamide | X | SA/Chronic | OAT | |||
| Indinavir | X | SA | OCT | |||
| Lamivudine | X | SA | OAT | |||
| Lithium | X | SA/Chronic | VA receptors | |||
| Ritonavir | X | SA | MRP 2,4 PGP | |||
| Tenofovir | X | SA | OAT | |||
Type A= dose dependent toxicity, Type B= idiosyncratic, acute = within 7 days of drug initiation, SA = sub-acute, occurs within 4 weeks of drug exposure and may take up to 90 days to resolve, chronic = injury persisting beyond 90 days, OAT=organic anion transporter, HLA = human leukocyte antigen, CYP = cytochrome P450, PGP = p-glycoprotein, MRP = multi-drug resistance associated protein
Time Course
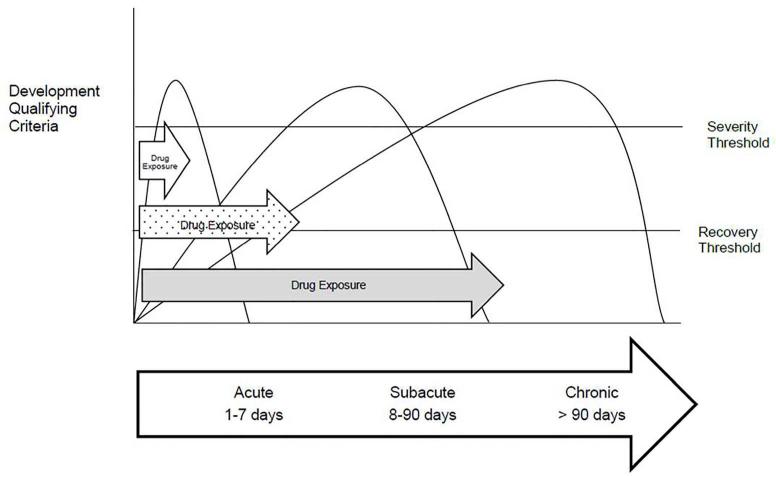
Several factors affect the presentation of DIKD, including the drug exposure duration, time course for biomarker change, identification of renal abnormalities and the duration of the DIKD event. Drugs vary widely in the presentation of nephrotoxicity within each mechanistic type with some causing acute injury (examples: aminoglycosides (Type A) and cephalosporins (Type B)) while others are associated with a slower insidious insult (example: lithium (Type A)). Recognition of DIKD depends on the frequency with which the diagnostic tests are obtained and reviewed and will differ based on the setting (discussed further below). The interplay of factors including the mechanism of toxicity, duration of drug exposure and frequency of biomarker testing influence the recognition, management and outcomes of DIKD. Based on these observations we propose categorizing DIKD into three broad subsets reflecting the time course of events. These categories build on conceptual models proposed by KDIGO for AKI (considered if the injury develops within 7 days) and CKD (persistence of injury for >90 days). Injury to the kidney beyond 7 days but less than 90 days reflects sub-acute injury similar conceptually to acute kidney disease proposed in KDIGO guidelines. The development of DIKD can similarly be divided into acute (1-7 days), sub-acute (8-90 days) and chronic (>90 days) post drug exposure (Figure 1). We propose utilizing this framework as a practical approach for applying the primary criteria for all 4 phenotypes of DIKD as discussed further below. Based on this conceptual model, for each phenotype, thresholds could be established to detect DIKD, define its severity and ascertain recovery.
Figure 1. Onset and Duration of Drug Induced Kidney Injury.
This figure conceptualizes the varying clinical presentations of drug induced nephrotoxicity drawing similarities to the KIDIGO definitions of kidney injury. Some antimicrobials can cause an acute rise in serum creatinine in relation to the start of the medication (e.g. aminoglycosides, amphotericin). Chemotherapeutic agents, such as cisplatin, cause a rise in serum creatinine that can occur beyond 7 days. Other medications have a slower onset of injury and can take months or years to be recognized clinically (e.g. tenofovir or lithium).
Setting
Nephrotoxicity is common in both hospitalized and ambulatory care settings, but its reported frequency varies based on several factors. Hospitalized patients are generally sicker, have a higher risk of exposure to nephrotoxins, contrast agents and procedures, and are more frequently monitored than ambulatory care patients. Recognizing DIKD in ambulatory care is more difficult, more likely to be missed and not reported. Additionally, establishing causality is more difficult when biomarker values are lacking and history of drug exposure is incomplete. This is particularly important for the AKI phenotype, where our current definitions evaluate changes in Scr over a set period of time in relation to a specific reference. Often, this reference creatinine is unavailable. Chronic forms of DIKD (e.g. tubular disorders, nephrolithiasis and glomerular disorders) are similarly more likely to be recognized in clinic settings but it may be more difficult to establish causality to a specific drug.
In order to account for these factors, we propose that DIKD cases meet the minimal criteria, as follows:
The drug exposure must be at least 24 hours preceding the event.
Reasonable evidence for biological plausibility for the causal drug, based on known mechanism of drug effect; metabolism and immunogenicity.
Complete data (including the medication history, biomarker concentrations, co-morbid diseases, concurrent risk factors) is required to account for concomitant risks and exposures to other nephrotoxic agents.
The strength of the relationship between the attributable drug and phenotype should be based on drug exposure duration, extent of primary and secondary criteria met and the time course of the injury.
Acute Kidney Injury Phenotype
Key Features
The AKI phenotype was based on KDIGO criteria with modifications to account for the presence of underlying CKD, time course and setting [3]. Since changes in serum creatinine are the hallmark of this phenotype, it includes acute tubular necrosis and acute interstitial nephritis. Although hemodynamic alterations are recognized for specific drugs e.g. angiotensin converting enzyme inhibitors (ACE), angiotensin receptor blockers (ARB), and non-steroidal anti-inflammatory drugs (NSAIDS), there are currently no consensus definitions of hemodynamic injury. Since transient changes in creatinine (usually Stage 1 AKI) can occur from other factors e.g. dehydration and hypotension in the setting of drug exposure and resolve when these factors are corrected, it is often difficult to distinguish primary drug induced effects from other factors. Recent studies from cardiac surgery patients exposed to ACE/ARB suggest that these changes are generally mild (Stage 1 criteria), resolve with dose reduction or withdrawal, and may not represent a clinically significant injury [5]. Consequently, in order to increase specificity, we did not include hemodynamic changes as distinct criteria and we proposed that the primary criteria must meet a minimum of KDIGO Stage 2, to be considered a potential DIKD event (Table 1). The overall severity of AKI is to be based on KIDGO staging criteria.
To further characterize the AKI phenotype based on initial presentation, we propose the secondary criteria shown in Table 1. Secondary criteria are used to further distinguish phenotypes (i.e. positive gallium scan for AIN), permit stratification and analysis within pre-specified subgroups (e.g. oliguric vs non-oliguric presentations). The AKI phenotype itself comprises several mechanisms. For instance, renal functional change can reflect a direct nephrotoxic effect (i.e. ATN from aminoglycoside or cisplatin), or an idiosyncratic effect (i.e. AIN from a proton pump inhibitor). In both instances, changes in Scr and urine output would define the phenotype but secondary criteria such as urine and peripheral eosinophilia and a positive gallium scan could provide additional classification (Table 1). We suggest that whenever feasible, kidney biopsy data would be used to confirm the underlying mechanisms (e.g. AIN vs ATN) and characterize the phenotype. A patient case is presented in Figure 2, demonstrating the application of the AKI criteria for DIKD. In this AKI case, the patient presents with a significant rise in serum creatinine secondary to drug injury. In this case, there is a reference serum creatinine prior to drug initiation and repeated during the course of treatment establishing a clear timeline of injury. In addition to renal injury, the patient has a pruritic rash suggesting this may be a type B reaction. Based on the timeline of drug exposure and plausible mechanisms of toxicity, it is likely she has vancomycin induced AKI but the sub-classification of acute tubular necrosis versus acute interstitial nephritis is difficult to establish without histologic evidence. Subsequently, a kidney biopsy confirms acute tubulointerstitial injury and the treatment plan includes changing antibiotics and a course of steroids. Emerging biomarkers of kidney damage (e.g. NGAL, KIM-1< IL18) combined with functional markers (e.g. serum creatinine and urine output) may permit further delineation of these events including transient hemodynamic alterations and could aid in defining the AKI phenotype.
Figure 2. Case Vignettes of Drug Induced Kidney Injury.
This figure contains two patient cases demonstrating the application of the phenotype criteria for acute kidney injury and glomerular phenotypes.
Influence of CKD
To permit a clear assessment of underlying CKD status, and to establish a standardized approach to determine the onset and duration of AKI, we standardized the definitions of “baseline” and “reference” Scr values (Appendix 1). We recognized that in some instances, patients may present with an elevated Scr without a preceding reference value. In these instances, we propose accepting an absolute or relative decline in Scr equivalent to a Stage 1 over 48 hours (following drug dose change) or 7 days (drug discontinuation), respectively. In these instances, the decline in Scr would need to meet the criteria within 2 weeks of stopping the drug to ensure specificity. Some forms of AKI may take longer to resolve after discontinuation of the drug (in the case of AIN) and would be categorized as a sub-acute injury. The development of AKI in the setting of pre-existing CKD requires a similar level of change in Scr or urine output; however, patients must meet criteria for CKD (Appendix 1).
Effect of Time Course and Setting
Since many drugs manifest biomarker changes outside the time frame of the acute time period, we propose a sub-acute phenotype that requires a similar severity of Scr change as in the AKI phenotype but permits a Scr elevation within 4 weeks from initiation of drug and in the setting of continued drug exposure or within a maximum of two weeks of drug discontinuation. For patients where a decline in Scr was to be considered as evidence of kidney injury, the criteria would need to be met within 90 days of a change in drug dosing or discontinuation. This approach permits classification and tracking of injuries for duration and outcomes.
Glomerular Phenotype
Although several drugs have been associated with the development of glomerular injury, this is a relatively infrequent form of DIKD. Significant proteinuria, hematuria and associated urinary sediment abnormalities are the key hallmarks of this phenotype; however, this must be distinguished from a primary (e.g. idiopathic minimal change disease) or secondary (e.g. diabetes) glomerular process. Consequently, we proposed that the phenotype requires a kidney biopsy during continued drug exposure or within 4 weeks after stopping the drug, showing specific features previously associated with drug toxicity (e.g. collapsing focal segmental glomerulosclerosis with pamidronate). We recognized that often the biopsy features would be confounded by other factors (e.g. concurrent diseases) and would need to be consistent with the drug exposure period. We selected a urine protein to creatinine (UPCR) and urine albumin to creatinine (UACR) ratios > 0.8 or a 24 hour protein excretion > 1 gram per day as evidence for significant proteinuria [6]. We additionally proposed that a urinalysis with greater than 50 red blood cells per high powered field or dysmorphic red blood cells (such as acanthocytes) or RBC casts as evidence for significant hematuria and glomerular involvement. These definitions reflect clinical situations where most clinicians would consider a kidney biopsy. We recognized that UPCR and UACR are not equivalent however for practical purposes we suggest that either test be used in the absence of a timed collection to identify patients with suspected glomerular lesions. We opted for more specificity in defining this syndrome given its rarity in contrast to idiopathic glomerular disorders and other causes of asymptomatic proteinuria. Figure 2 presents a glomerular disorder case highlighting the application of the above criteria with confirmatory evidence of DIKD on renal biopsy.
Tubular disorder
Drug induced tubular disorders have been described with several medications that are handled through tubular transport mechanisms and it is possible that mutations in renal transporters could give rise to tubular toxicity. Several different mechanisms have been implicated depending on the site of drug handling, drug exposure and duration of treatment. In most instances, these are dose-related and usually seen with chronic, continued exposure. Several patterns have been described ranging from isolated abnormalities (e.g. phosphate leak) to more generalized lesions contributing to a proximal renal tubular acidosis (RTA) or an acquired Fanconi’s syndrome. Recognizing the wide spectrum of tubular dysfunction, we proposed classifying this phenotype to include abnormalities in urinary losses of phosphate, glucose, magnesium, potassium, and tubular proteins or water handling. These would be associated with secondary changes in serum electrolytes, bicarbonate, and pH (Table 1). A key issue is to distinguish DIKD from congenital defects, other diseases (e.g. sarcoid) or toxin mediated tubular dysfunction. In the case of tubular disorders, the primary criteria alone may be used for electronic surveillance and detection of possible injury, but secondary criteria are essential to confirm diagnosis and improve specificity.
Nephrolithiasis
Medications may precipitate into crystals depending on their urinary solubility. The precipitation of medications spans the spectrum of asymptomatic, isolated crystalluria to obstructive stones. Crystalluria may also lead to AIN. This has been well described with anti-retrovirals such as indinavir which commonly causes isolated crystalluria but less commonly obstructive nephropathy. Drug induced renal calculi have also been described with sulfa antibiotics and triamterene. Additionally, nephrolithiasis can be associated with RTA syndromes related to tubular disorders. Imaging is often the only method to detect nephrolithiasis but may not be available in all cases. However, given the high incidence of nephrolithiasis in the general population, it is important to demonstrate the temporal relationship to the drug and analyze the stone composition, if available.
Combination Phenotypes
Although the phenotypes have distinct features, a patient may develop more than one phenotype. For example, drug induced crystalluria and nephrolithiasis could lead to AKI from obstruction or AIN. We considered that these combination phenotypes are possible. In such cases, each of the phenotypes will need to be evaluated independently, to establish a relationship to the drug exposure.
Causality assessment and Adjudication
Causality assessment tools such as the Naranjo scale have been used to attribute drug adverse reactions and have been modified to improve sensitivity for specific types of adverse reactions [7]. Causality assessment tools for DIKD have not been developed or reported. Challenges in causality assessment include multi-drug exposures and concurrent AKI risks. For instance, the risk of DIKD from antibiotics in the setting of sepsis would be enhanced by hypotensive episodes and exposure to contrast agents. In these situations, we propose that each drug be evaluated individually with respect to its possible contribution to the phenotype and underlying risk factors assessed. With multi-drug exposure, each causal agent should be rank classified (i.e. primary, secondary) based on the temporal relationship, magnitude and duration of effect, and knowledge of the underlying mechanism.
Anticipated Uses and Limitations
There is no current systematic way of identifying DIKD given the variability in the presentation with each individual drug. Phenotype standardization provides framework guidance to pharmaceutical industry for drug development, to regulatory agencies for safety surveillance, physicians and patients for recognition. The proposed classification requires validation but could be utilized by regulatory agencies to standardize the documentation of kidney toxicity in clinical trials. If these criteria are validated in clinical trials, physicians would have a uniform method to describe and record adverse drug events and to inform patients of the potential risk and consequences of specific drug toxicities. Researchers would build on these initial phenotypes with new tools e.g. damage biomarkers to further characterize the component toxicities. Electronic medical records (EMR’s) could be trained to identify the 4 broad phenotypes and build alert systems for pharmacists and physicians to recognize drug nephrotoxicity and develop quality metrics to prevent drug nephrotoxicity. This approach has been successfully implemented by recent studies in pediatrics and adults demonstrating the incidence of drug toxicity and the efficacy of an alert system to correct it [8]. The phenotype standardization is anticipated to improve recognition of DIKD. Once these criteria have been validated, they can also be used for quality measurement. This will enhance the description of the epidemiology of DIKD, as was demonstrated by Selby and colleagues with the implementation of KDIGO criteria for screening and recognition of AKI [9]. We recognize that the phenotype categorization is broad. However, a hierarchical categorization with primary and secondary criteria brings forward commonalities of the injuries to allow for enhanced recognition. The structured secondary criteria further distinguish the injury. For example, patients recognized as exhibiting tubular dysfunction can be further categorized as disordered water handling or acid base disorders. Broad categorization addresses the multi-mechanisms of injury since definition is based on biomarker presentation. This facilitates the standard detection and alerting of injury when a recognized nephrotoxic drug is being administered. We recognize these proposed phenotypes do not include every possible mechanism of DIKD. We have deliberately excluded hemodynamic injury because there is no consensus definition on hemodynamic changes and transient AKI. The current KDIGO definitions require adequate consideration and correction of pre-renal factors affecting recognition. In the absence of these standardized definitions, we have opted for greater specificity. We recognize this may lead to misclassification, if the effect is mild, transient and limited. We anticipate over time, with emerging biomarkers for pre-renal conditions, the identification of hemodynamic alterations secondary to DIKD will improve, leading to refinement of the AKI phenotype. In addition, if the phenotype is too sensitive in definition, the risk of prematurely stopping a drug for patient care or halting drug development for a candidate drug increases.
We recognize several limitations of the proposed framework. The AKI phenotype encompasses different pathologic injuries and the absence of specific mechanistic biomarkers makes the differentiation of AKI clinically challenging. We have proposed the KDIGO criteria as a unifying definition to identify patients and using the secondary criteria to provide further specificity of the nature, site and extent of injury. We anticipate that emerging biomarkers of kidney damage can identify site specificity and coupled with functional assessments, we can further refine the phenotype. The proposed approach is one of practicality and raises the need for biomarkers to distinguish injury. In addition, the proposed biomarker cut-offs were chosen for specificity, however, these cut-offs should not replace clinical judgment as there may be patients who develop DIKD but do not meet these thresholds for biomarker changes. For example, a patient who develops an increase in serum creatinine due to aminoglycosides but does not meet the criteria for Stage 2 AKI could still be diagnosed with DIKD based on the physician’s assessment. The current phenotypes do not address the multi-mechanism injury as an entity which may have a different prognosis from single mechanism injuries. Additionally, as mentioned previously, these definitions do not ascertain causality and often the patient may be exposed to multiple drugs. Recognizing the phenotype is limited by how often biomarker measurements are taken so there is inherently uncertainty on the exact time course of DIKD. The practicality for utilizing the primary and secondary criteria for EMR screening is yet to be determined. Although in broad categorization of DIKD loses some granularity, it enhances the feasibility of EMR detection strategies.
Conclusions
We have utilized a consensus based approach to establish 4 specific phenotypes to characterize DIKD based on existing knowledge of disease mechanisms, time course and setting. We acknowledge the inherent limitations of a consensus approach and the absence of any prospective validation. We recognize that the phenotypes would be subject to further revision based on their performance in prospective studies. However, we are confident that these phenotypes provide a consistent framework for clinicians, investigators and industry and regulatory agencies to evaluate drug toxicity across various settings. We believe that this is first step to recognizing DIKD and developing strategies to prevent and manage DIKD.
Appendix 1: Definitions
Acute kidney injury (AKI): is a process that causes an abrupt reduction in kidney function, and will be defined by meeting any of the following criteria[3]:
an absolute increase in Scr (≥ 0.3 mg/dl or ≥ 26.4 μmol/l) (within 48 hours’ time window) from the reference Scr
percentage increase in Scr of ≥50% (1.5-fold from reference) within 7 days
Reduction in urine output (documented oliguria of < 0.5 ml/kg/hr for >6 hours) despite adequate fluid resuscitation when applicable.
Absolute decrease in Scr of (≥ 0.3 mg/dl or ≥ 26.4 μmol/l) (within 48 hours’ time window) from the reference Scr
Relative decrease in Scr of ≥50% (1.5-fold from reference) within 7 days.
Chronic Kidney Disease (CKD): Prior evidence of markers of kidney damage for ≥ 3 months (microalbuminuria, proteinuria >300mg/24 hrs or abnormalities in imaging tests) or the presence of glomerular filtration rate (GFR) <60 mL/min/1.73 m2 for ≥3 months calculated with MDRD (Modification of Diet in Renal Disease) equation, with or without other signs of kidney damage as described above. Chronic kidney disease should be staged from stage 1 to 5 based on the calculated CKD-EPI/Ckid GFR.
Reference creatinine to determine timing of AKI: The following criteria should be used in order of preference depending on available values
-
a)Lowest Scr immediately prior to index event. Must meet following criteria
- Precede drug exposure
- Within 90 days of index event
- Closest value to index event
- Lowest value prior to drug exposure
- If no Scr measurement within 90 days of index use the hospital admission Scr
-
b)
For declining Scr criteria with no prior reference label lowest value post drug reduction or stoppage as reference
-
c)For AKI phenotype will have two reference Scr values:
- Reference 1:
- Lowest value within 90 days of initiation of primary drug
- Reference 2:
- Lowest value closest to initiation of drug
Baseline Scr to determine CKD status: Creatinine values > 90 days from index event
-
a)
Lowest values within 90 days to 12 months to establish eGFR stage based on CKD-EPI or Ckid (pediatrics)
-
b)
Historical evidence of CKD based on standard criteria: proteinuria, biopsy, ultrasound size
-
c)
Imaging studies consistent with CKD
-
d)
For chronic drug exposure need values prior to drug initiation e.g. lithium
New onset AKI: Evidence of AKI without prior evidence of kidney damage (normal urinalysis, normal imaging tests and calculated MDRD (Modification of Diet in Renal Disease) GFR is ≥90 ml/min/1.73m2).
AKI on CKD: Evidence of AKI with criteria of kidney damage as stated with CKD definition will be considered as AKI on CKD.
Footnotes
Disclosures:
Ravindra Mehta, Linda Awdishu, Andrew Davenport, Patrick Murray, Etienne Macedo, Jorge Cerda, Raj Chakaravarthi and Stuart Goldstein have received research funding from the International Serious Adverse Events Consortium. Arthur Holden is the Chief Executive Officer for the International Serious Adverse Events Consortium.
References
- 1.Mehta RL, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66(4):1613–21. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 2.Aithal GP, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806–15. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 3.Group., K.D.I.G.O.K.A.K.I.W. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter. 2012;2:1–138. [Google Scholar]
- 4.McCormack M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364(12):1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koyner JL, et al. Adjudication of etiology of acute kidney injury: experience from the TRIBE-AKI multi-center study. BMC Nephrol. 2014;15:105. doi: 10.1186/1471-2369-15-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park YH, et al. Hematuria and proteinuria in a mass school urine screening test. Pediatr Nephrol. 2005;20(8):1126–30. doi: 10.1007/s00467-005-1915-8. [DOI] [PubMed] [Google Scholar]
- 7.Naranjo CA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 8.Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011;6(4):856–63. doi: 10.2215/CJN.08110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selby NM, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol. 2012;7(4):533–40. doi: 10.2215/CJN.08970911. [DOI] [PubMed] [Google Scholar]