Abstract
Study design
Laboratory based experiment using a pre/post-test design.
Objectives
To determine the effects of neuromuscular fatigue on quadriceps strength and activation and sagittal and frontal plane knee biomechanics during dynamic landing following anterior cruciate ligament reconstruction (ACLr).
Background
Impaired quadriceps central activation occurs post-ACLr, likely altering lower extremity biomechanics. Neuromuscular fatigue similarly reduces volitional muscle activation and impairs neuromuscular control. Upon return to full activity post-ACLr, individuals likely concurrently experience quadriceps central activation deficits and neuromuscular fatigue, though the effects of fatigue on muscle strength and activation and biomechanics post-ACLr are unknown.
Methods
Seventeen individuals 7–10 months post-ACLr and 16 controls participated. Quadriceps strength and central activation ratio were recorded pre-/post-fatigue, which was induced via sets of double-leg squats. Knee biomechanics were recorded during a dynamic landing activity pre-/post-fatigue.
Results
Both groups demonstrated smaller knee flexion (initial contact:P=.017; peak:P=.004) and abduction (initial contact:P=.005; peak:P=.009) angles post-fatigue. The ACLr group had smaller peak knee flexion angles (P<.001) pre- and post-fatigue than controls. Knee flexion moment was smaller in ACLr than controls pre- (P<.001), but not post-fatigue (P=.103). Controls had smaller knee flexion moments post-fatigue (P=.001). Knee abduction moment was smaller in both groups post-fatigue (P=.003). All participants demonstrated significantly lower strength (P<.001) and activation (P=.003) post-fatigue.
Conclusion
Impaired strength, central activation, and biomechanics presented post-fatigue in both groups, confirming that neuromuscular fatigue may increase non-contact ACL injury risk. However, these changes were not exaggerated in ACLr participants, likely because they already demonstrated a stiff-legged landing strategy pre-fatigue.
Keywords: ACL, muscle inhibition, quadriceps weakness, return to play
INTRODUCTION
Emerging evidence suggests that individuals who sustain an anterior cruciate ligament (ACL) injury are 6 times more likely to sustain a second ACL injury within 24 months after returning to activity compared to healthy individuals.35 The factors that precipitate the initial injury, such as reduced knee flexion2 and increased knee abduction11 angles and moments1 during stance, likely contribute to second ACL injury risk. Understanding how the injury, surgery, and rehabilitation processes influence the biomechanical risk factors for injury is important to developing better rehabilitation strategies to reduce subsequent injury risk.
Neuromuscular fatigue has been suggested to exacerbate poor landing biomechanics, likely further increasing ACL injury risk.3,6,31,41 Epidemiological data support this notion, with more injuries occurring as athletes become fatigued secondary to the physiological demands of their sports.4,15 Combined central and peripheral processes contribute to neuromuscular fatigue. Peripheral contributions arise from changes in the contractile elements of the muscle.13 Centrally, motor cortical output to the motoneuron pool becomes impaired, resulting in a progressive reduction in voluntary activation.13 In healthy individuals, neuromuscular fatigue has been demonstrated to impair quadriceps central activation as measured using the burst superimposition technique.38 The authors of this previous study observed that activation impairments were exacerbated in the presence of strength deficits. Stackhouse and colleagues38 compared healthy older and younger adults pre- and post-fatigue. Before fatigue, older adults were weaker and had greater deficits in quadriceps central activation than younger adults. This difference increased post-fatigue.
Following ACL reconstruction (ACLr), individuals may be particularly vulnerable to the effects of neuromuscular fatigue. These individuals often return to activities that place high demands on the knee joint. Further, these individuals often return to activity despite still experiencing quadriceps strength5 and central activation deficits42 as well as impaired biomechanics during activity. Notably, following ACLr, individuals continue to land with reduced hip12 and knee10 flexion angles and knee flexion moments,10 the same biomechanics that are implicated in the ACL injury mechanism.
Considering the influence of neuromuscular fatigue on landing biomechanics in healthy adults, it seems logical that individuals after ACLr who already have impaired quadriceps strength and activation, would also have altered landing patterns secondary to the influences of neuromuscular fatigue. However, little is known regarding the influence of neuromuscular fatigue on landing biomechanics in individuals following ACLr.
Therefore, this study examined the effects of neuromuscular fatigue on quadriceps strength and central activation as well as knee biomechanics in individuals following ACLr compared to healthy persons. We hypothesized that participants would reduce knee flexion angles and moments while increasing knee abduction angles and moments post-fatigue compared to pre-fatigue, with all biomechanical changes being greater in the ACLr group. Additionally, we postulated that the participants with ACLr would demonstrate greater quadriceps strength and central activation deficits before fatigue and reach maximal neuromuscular fatigue faster (ie, in less repetitions of the fatiguing exercise) than healthy participants. This knowledge will drive improvements in current rehabilitation strategies to better prepare individuals for return to full activity following ACLr.
METHODS
Participants
Seventeen individuals 7 to 10 months post-ACLr and 16 controls participated (TABLE 1). A sample size calculation conducted on pilot data revealed that to demonstrate differences in knee flexion angle 15 participants per group were needed (alpha level: .05; power: 0.8; effect size: 0.8). A large effect size was chosen to reflect the strong effect of fatigue on biomechanics observed in previous literature.3,31 Exclusion criteria for the ACLr group included: history of lower extremity surgery other than their recent ACLr, sustaining a lower extremity injury since undergoing ACLr, current pain in either knee, meniscectomy, grade 2+ collateral ligament injury concurrent with their ACL injury, known heart condition, or not receiving physician clearance for return to full activity following ACLr. The participants in the control group were recruited on the basis of being recreationally active and could not have a history of lower limb surgery or ACL injury or have sustained a leg injury in the previous 6-months. Pregnant females were excluded.
TABLE 1.
Participant demographics.
| ACLr (n=17; 7 female) | Control (n=16; 11 female) | |
|---|---|---|
| Age (years) | 21.41 ± 4.73 | 23.38 ± 4.11 |
| Height (m) | 1.75 ± 0.08 | 1.71 ± 0.08 |
| Mass (kg) | 76.52 ± 11.85 | 68.21 ± 10.17 |
| Graft Type (n) | ||
| - Patellar tendon | 10 | |
| - Hamstrings | 7 | |
| Meniscal injuries (n) | ||
| - Medial | 3 | |
| - Lateral | 3 | |
| - Medial & lateral | 1 | |
| - None | 10 | |
| Collateral ligament injuries (n) | ||
| - Medial | 5 | |
| - Lateral | 1 | |
| - Medial & lateral | 2 | |
| - None | 9 | |
| IKDC (0–100) | 83.84±11.78 | 98.99±2.05 |
| Tegner Activity Scale† (0–10) | 7.00±2.32 | 5.75±0.93 |
Abbreviations: ACLr, anterior cruciate ligament reconstruction; IKDC, International Knee Documentation Committee
Values are mean ± standard deviation unless otherwise indicated.
Reported at the time of testing
Five surgeons from a single sports medicine clinic performed all ACLr procedures using standard patellar tendon and semitendinosus/gracilis autograft procedures. All participants with ACL injury completed rehabilitation at the same outpatient clinic. Rehabilitation followed a standard protocol consisting of 2 to 3 treatment sessions per week. Rehabilitation took place from post-operative week 1 to week 12 or 16, depending on the individual’s progression. The rehabilitation protocol emphasized knee range of motion, muscle strengthening, and functional exercises. To be cleared to return to activity all patients had to pass a leg press test. This required at least 15 repetitions of the exercise with the knee being moved from neutral to 90° of flexion with the affected limb pressing a load equivalent to 100% of body weight. Institutional review board approval was obtained from the University of Michigan Medical School prior to the start of the investigation.
Participants completed the 2000 International Knee Documentation Committee (IKDC) subjective form to evaluate their knee symptoms and functional abilities.18 IKDC scores range from 0–100. Higher scores represent fewer symptoms/impairments. The IKDC scores were used to ensure that the control participants were healthy at the time of testing. The participants additionally completed the Tegner activity scale to determine current activity participation.39 Tegner scores range from 0–10, with 0 indicating disability/bed ridden and 10 indicating someone who regularly competes in elite level competitive sports.
Strength testing
Quadriceps strength was assessed via knee extension maximal voluntary isometric contractions (MVICs) with participants seated in 85° of hip and 90° of knee flexion (Biodex System 3, Biodex Medical Systems, Shirley, NY). A minimum of 3 knee extension MVICs were performed, with at least 2 minutes of rest between each repetition, until no further improvements in torque were observed. MVIC testing was administered and data were recorded using a custom-written program (Labview 8.5, National Instruments, Austin, TX).
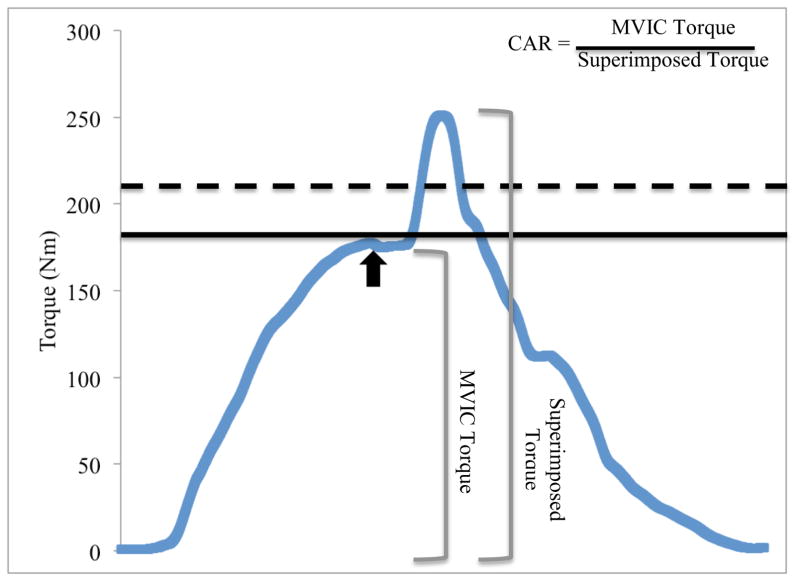
During the MVIC trials, 2 lines were shown on a computer screen in front of the participant (FIGURE 1). The first line (solid line in FIGURE 1) represented peak torque attained during previous MVIC trials. For the first MVIC trial, the peak torque represented that reached during warm-up repetitions. As torque increased during any subsequent trial, the height of the line was adjusted accordingly. To encourage maximal effort, a second, target line (dashed line in FIGURE 1) was shown on the computer screen. This target value was set 10% above the peak torque line.
FIGURE 1.
Screenshot from strength and central activation testing. The curved line represents the participant’s real-time torque output. The solid, straight line corresponds to the participant’s peak value from the maximal voluntary isometric contraction (MVIC) trials and also served as a threshold for central activation testing. Real-time torque output must cross this threshold for the electrical stimulus to be delivered. The dotted line represents the participant’s target value, which was set 10% above maximal strength. The black arrow corresponds to delivery of the electrical stimulus. The observed increase in torque following the plateau in MVIC is considered to be elicited by the electrical stimulus. The central activation ratio (CAR) is calculated as the MVIC value divided by the torque elicited when the stimulus is superimposed on the MVIC.
Central activation testing
For quadriceps central activation testing, self-adhesive, stimulating electrodes (Dura-Stick II [5×9cm] Chattanooga Group, Hixson, TN) were applied over the proximal rectus femoris and distal vastus medialis, through which the electrical stimuli were delivered (S88/SIU8T, GRASS Technologies, West Warwick, RI; train length:100ms; pulse duration: 600μs; delivery rate:100pps; maximum voltage:130V). The highest peak torque value obtained during the MVIC trials was displayed on the computer screen. This peak torque value served as the threshold for our automated triggering system and ensured the stimulus was not delivered if torque levels were below the participant’s maximal effort. A stimulus was delivered when a participant’s torque value crossed threshold (solid line), peaked, and then fell 1Nm.22 If threshold was not reached, a stimulus was not delivered; the participant rested for 2 minutes, and repeated the trial. The dashed target line was intended to be unreachable; however, if a participant reached the target value, all values were reset and central activation testing was reinitiated. Three repetitions of CAR testing were performed with 2 minutes of rest provided between repetitions.
Quadriceps central activation was quantified using the central activation ratio (CAR) (FIGURE 1).19 Three repetitions were performed before and after fatigue and the average for each was used to quantify quadriceps central activation. Knee extension strength was determined from these repetitions by dividing the torque value immediately before delivery of the electrical stimulus by the participant’s body mass (kg) and averaging across trials. All measurements were recorded bilaterally. Intrarater reliability for MVIC and CAR assessment was high (ICC=0.996 [95% confidence interval (CI) 0.991, 0.998; n=16] and ICC=0.978 [95% CI 0.949, 0.991; n=16], respectively).
Landing task
For dynamic landings, participants jumped forward off both legs over a 17cm box40 and landed on 1 limb on a force platform (OR 6–7; Advanced Medical Technology, Inc, Watertown, MA) located 1m away (FIGURE 2). Upon landing, participants performed a lateral hop as quickly as possible. Before fatigue, participants performed 3 good dynamic landings, defined as the proper limb landing completely on the force platform, to each side (FIGURE 3). The limb on which participants landed was randomly determined before each trial using a custom-written program displayed on a computer screen in front of the participant. Participants performed adequate practice trials until they were comfortable with the task.
FIGURE 2.
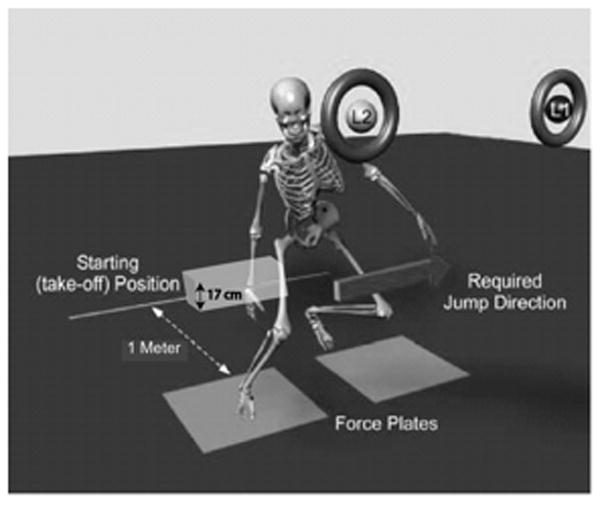
Dynamic landing activity. Participants took off from the starting position, jumping over a 17 cm high box and landing on one of the force platforms. L1 and L2 represent the computer screen indicating on which limb the participant was to land. L1 necessitated landing on the right leg and laterally jumping to the left. L2 necessitated landing on the left leg and laterally jumping to the right. Adapted from Borotikar BS, Newcomer R, Koppes R, McLean SG. Combined effects of fatigue and decision making on female lower limb landing postures: Central and peripheral contributions to ACL injury risk. Clin Biomech 2008; 23:81–92. Copyright © 2007 Elsevier Ltd. Used with permission.
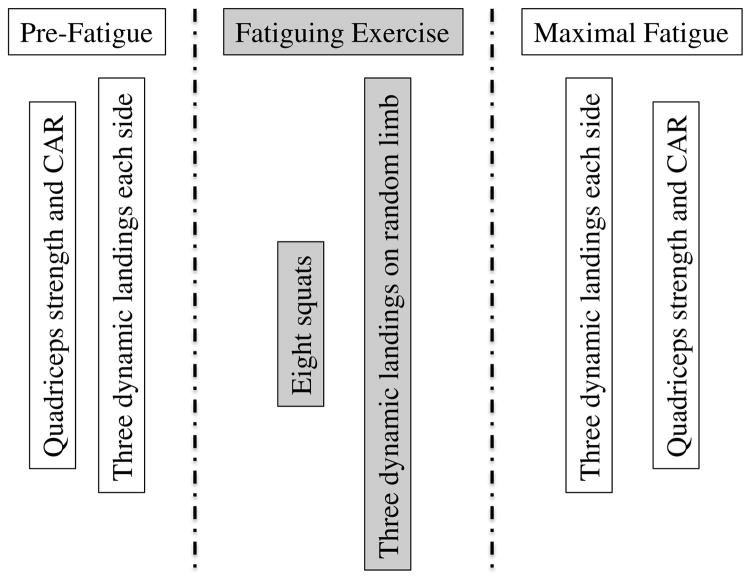
FIGURE 3.
Time course of strength, activation, and fatigue tasks. Abbreviation: CAR, central activation ratio.
Fatigue protocol
Following the pre-fatigue trials, participants began the fatiguing exercise. This consisted of sets of 8 double-leg squats to at least 90° of knee flexion followed by 3 dynamic landings.3 Knee joint angle was visually determined by the investigator based on the relative positions of the tibia and femur. The limb on which participants landed was randomly determined. Verbal encouragement3 and feedback regarding knee joint angle (ie, did the participant reach 90° of knee flexion during the squatting task) were provided.
Participants performed sets of squats and dynamic landings until maximal neuromuscular fatigue, which occurred when the investigator observed that participants could no longer perform 5 consecutive repetitions to 90° of knee flexion without assistance nor consistently reach the force platform during landing. The fatiguing exercise was stopped if participants reported pain in the ACLr knee. There was no limit on the number of squats a participant could perform. Intrarater reliability for determining participant fatigue was high (ICC=0.926; 95% CI −0.257, 1.00) when evaluating healthy adults over 2 sessions separated by a week.
Once maximal neuromuscular fatigue was reached, participants performed 3 dynamic landings to each side. In the event participants could no longer consistently reach the force platform, the 3 trials immediately before this occurrence were utilized. Immediately following the completion of 3 post-fatigue dynamic landings, participants underwent strength and CAR assessment.
Kinematics and kinetics
Participants wore 32 retro-reflective markers tracked via an 8-camera (240 Hz) motion capture system (Vicon, Oxford Metrics, London, England) from which kinematics were quantified. A static recording was captured29 to generate a kinematic model in Visual 3D (C-Motion; Rockville, MD). Joint rotations were calculated within Visual 3D using a Cardan rotation sequence7 and expressed relative to each participant’s stationary position.31 Three-dimensional ground reaction force (GRF) data were synchronized with the kinematic data and filtered using a zero-lag, Butterworth filter with a 12-Hz cut-off frequency31 and submitted to standard inverse dynamics analysis.36 Kinetic outputs were normalized to participant body mass and height44 and represented as external moments. Biomechanical data were time normalized to 100% of the stance phase for graphical purposes, with initial contact and toe-off representing the time when the vertical GRF first exceeded and fell below 10N, respectively.3,31
Sagittal and frontal plane knee angles were determined at initial contact (IC) and at peak knee flexion (PKF). Joint moments were determined at PKF only. Biomechanical data were averaged across trials pre- (PRE) and post-fatigue (POST) and submitted to statistical analysis. Intrarater reliability of these methods in our laboratory was established using 15 healthy adults tested during 2 sessions separated by 1 week. Reliability ranged from moderate to high for sagittal (rotation: ICC=0.828 [95% CI 0.562, 0.939]; moment: ICC=0.883 [95% CI 0.688, 0.959]) and frontal (rotation: ICC=0.891 [95% CI 0.707, 0.962]; moment: ICC=0.581 [95% CI 0.118, 0.837]) plane knee biomechanics obtained at PKF during landing (n=15; unpublished data).
Statistical analysis
Group (ACLr; control) by time (PRE; POST), mixed-model, repeated measures ANOVAs were performed to detect interaction as well as main effects of group and fatigue state on sagittal plane knee joint angles and moments, CAR, and MVIC. Independent samples t-tests were performed to determine if the ACLr group required fewer squats to reach maximal, neuromuscular fatigue than the control group. T-tests were used to determine group and time differences in the presence of significant interactions. Only data collected on the ACLr limb or randomly-determined test limb for the control group were analyzed statistically. The α-level was set at P ≤.05. Analyses were performed SPSS version 18.0 (SPSS, Inc., Chicago, IL).
RESULTS
Participant demographics are located in Table 1. Participants were involved in the following activities at the time of injury: basketball (n=4), football (n=2), skiing (n=2), sledding (n=1), soccer (n=5), softball (n=2), and ultimate Frisbee (n=1).
Muscle Strength and Activation
There was a significant group by time interaction for MVIC (P=.007). Participants with ACLr demonstrated weaker quadriceps PRE (P=.031) but not POST (P=.886) compared to controls. Quadriceps strength was significantly lower in both groups POST (ACLr and controls P<.001) compared to PRE. There was no group main effect for CAR (P=.166). All participants, regardless of group, demonstrated lower CAR (ACLr: P=.002; control: P=.047; TABLE 2) following fatigue.
TABLE 2.
Quadriceps strength and central activation ratio data.
| ACLr | Control | |||
|---|---|---|---|---|
| PRE | POST | PRE | POST | |
|
| ||||
| MVIC (Nm/kg) | 2.03±0.57* | 1.58±0.46† | 2.63±0.92 | 1.61±0.54† |
| Central activation ratio | 0.82±0.11 | 0.78±0.13† | 0.89±0.10 | 0.82±0.14† |
Abbreviations: ACLr, anterior cruciate ligament reconstruction; MVIC, maximal voluntary isometric contraction; POST, post-fatigue; PRE, pre-fatigue
Values are mean±standard deviation
Significantly different from Control group (P<.05)
Significant difference pre- to post-fatigue (P<.005)
The number of squats to reach fatigue was not significantly different between groups (mean ± standard deviation [range]: ACLr: 441±235 [128–1152]; control: 543±308 [176–1408]; P=.29).
Knee Biomechanics
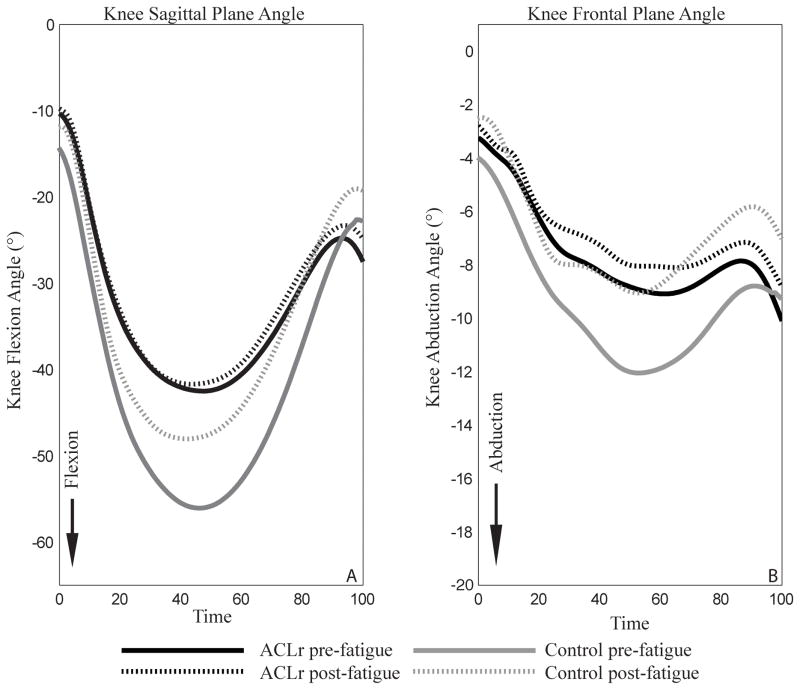
There were no significant group by time interactions for knee flexion angles at IC (P=.08) or PKF (P=.088). All participants, regardless of group assignment, demonstrated smaller knee flexion angles PRE compared to POST at IC (P=.017) and PKF (P=.004; FIGURE 4A). Similarly, there were no significant interactions for knee abduction angle at IC (P=.096) or PKF (P=.452). However, both groups demonstrated smaller knee abduction angles PRE compared to POST at IC (P=.005) and PKF (P=.009; FIGURE 4B). Regardless of time, participants in the control group demonstrated greater knee flexion angles than that with ACLr at PKF (P=.004). There were no group main effects for knee flexion angle (IC: P=0.110) or knee abduction angle (IC: P=.841; PKF: P=.544).
FIGURE 4.
Knee rotation results. Time is expressed as a percentage of the stance phase. Panel A represents sagittal plane knee joint rotations. Panel B represents frontal plane knee joint rotations.
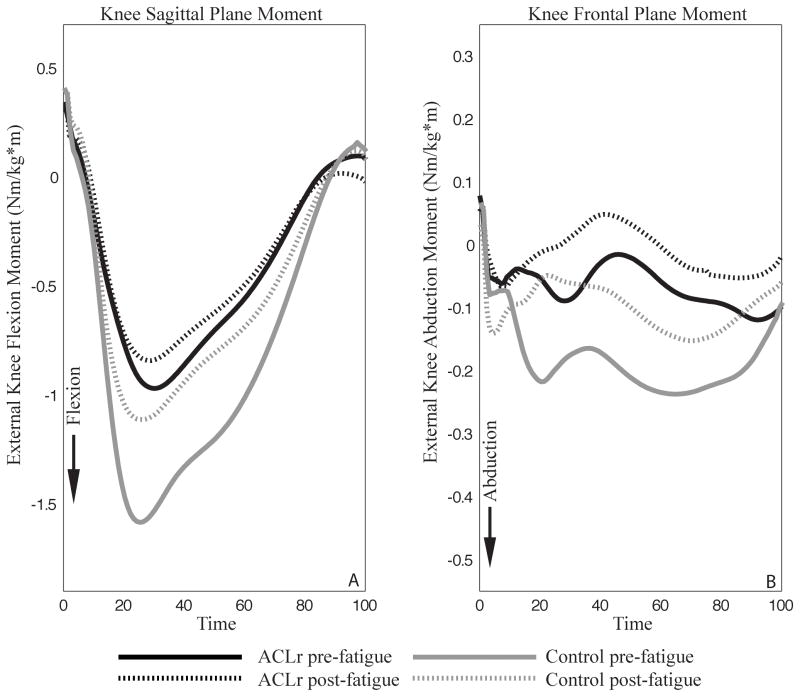
There was a significant group by time interaction for knee flexion moment (P=.005). Those with ACLr demonstrated smaller external knee flexion moments than controls PRE (P<.001), though this difference was not present POST (P=.103; FIGURE 5A). Further, participants with ACLr did not demonstrate any within-group differences in knee flexion moment between the PRE and POST time points (P=.562). However, those in the control group demonstrated greater knee flexion moments PRE compared to POST (P=.001). Regarding knee abduction moments, there were no significant interaction (P=.688). Both groups demonstrated smaller abduction moments POST (P=.003; FIGURE 5B). There was no group main effect for knee abduction moment (P=.155).
FIGURE 5.
Knee moment results. Time is expressed as a percentage of the stance phase. Panel A represents sagittal plane knee joint moments. Panel B represents frontal plane knee joint moments.
DISCUSSION
This study sought to determine the influence of neuromuscular fatigue on quadriceps strength and central activation as well as knee joint biomechanics in participants post-ACLr and healthy individuals. While both groups demonstrated reductions in quadriceps strength and central activation, only the control group demonstrated altered biomechanics as a result of fatigue. Importantly, ACLr participants demonstrated aberrant sagittal plane biomechanics prior to fatigue that remained unchanged following fatigue.
Participants in both the ACLr and control groups demonstrated less knee flexion following fatigue. Interestingly, those in the ACLr group landed with smaller peak knee flexion angles than controls before and after fatigue. While the control group experienced a reduction in the external knee flexion moment following fatigue, the moment observed in the ACLr group did not differ pre- to post-fatigue. A less flexed knee posture was demonstrated previously in healthy adults following global lower extremity neuromuscular fatigue3,28,31 and isolated quadriceps and hamstrings fatigue.41 However, previous researchers have also demonstrated that neuromuscular fatigue does not influence sagittal plane knee biomechanics.46 Less knee flexion may protect against collapse of the lower extremity on landing, while also increasing non-contact ACL injury risk.2 This adaptive strategy may be hazardous, though concomitant frontal and transverse plane biomechanical alterations may be necessary to induce injury.30
Participants in both the ACLr and control groups demonstrated smaller knee abduction angles and abduction moments following fatigue. It seems that participants could not exert the same effort during the cutting portion of the task as they did prior to fatigue, thereby becoming less aggressive with their movement. Consequently, they may not have pushed off as hard as they did pre-fatigue, resulting in them still successfully completing the task but doing so in a more neutral frontal plane limb alignment post-fatigue. Previous studies have reported increases in both the knee abduction angle3,6,31,34 and moment31 post-fatigue as well as a lack of change in knee frontal plane biomechanics as a result of fatigue.20 Differences in the fatigue protocol employed between studies may account for these discrepancies. Several of these previous studies6,31 incorporated a change of direction task into their fatiguing exercises which may have allowed fatigue of the out of plane hip stabilizers (eg, tensor fascia latae) and, therefore, increased knee abduction.25 However, our fatiguing exercise consisted of a primarily sagittal plane motion, thus fatigue was likely limited to the quadriceps, hamstrings, and gluteus maximus muscles.
In the current study, in spite of a 22% reduction in quadriceps strength following fatigue, those with ACLr demonstrated similar knee joint biomechanics pre- and post-fatigue. This finding, in conjunction with different pre- to post-fatigue biomechanics in the control group, suggests neuromuscular fatigue did not similarly influence both groups. This finding disagrees with those of Webster et al,43 suggesting fatigue reduces knee flexion angle in the ACLr limb. These authors43 utilized a single-leg drop-landing task 15–19 months post-operatively, which may help to account for the discrepancy in findings. Why knee flexion angles did not change post-fatigue in our group of participants with ACLr is unclear. The participants with ACLr may have relied on the healthy limb to perform the majority of the work during the squatting task, possibly resulting in greater healthy limb neuromuscular fatigue and relatively unchanged reconstructed limb biomechanics. However, bilateral biomechanical adaptations arise following unilateral neuromuscular fatigue,28 indicating that even if someone favored one limb during the fatiguing exercise, biomechanics could have been altered in the contralateral limb. Given the significant decline in involved limb quadriceps strength following fatigue, however, we do not believe that those with ACLr favored their healthy limb. Regardless, future investigations should utilize force platforms and vertical GRF to verify symmetry between limbs during the fatiguing tasks.
Contrary to our hypothesis, the effects of fatigue on biomechanics were not exaggerated in those with ACLr compared to healthy controls. This may be due to the participants with ACLr demonstrating smaller peak knee flexion angles than controls before fatigue. Thus, those with ACLr may have been using compensatory movement patterns prior to fatigue, leaving no need for further compensation in response to neuromuscular fatigue or no ability to alter biomechanics and still complete the dynamic landing task. ACL injury prevention programs17,32 may increase knee flexion angles during landing, purportedly reducing injury risk. Incorporating these biomechanics-modifying strategies into post-operative rehabilitation may be beneficial.
Before fatigue, participants with ACLr demonstrated impaired quadriceps strength but not central activation deficits compared to controls. Rehabilitation likely restored some quadriceps activation in the ACLr group, bringing the ACLr pre-fatigue CAR values closer to those of the control group. Additionally, the control group demonstrated an average pre-fatigue CAR of 0.89, which is below the previously accepted standard of 0.95 for full activation in healthy individuals.24 However, voluntary activation in young, healthy individuals may be slightly lower than previously believed with a recent study indicating CAR of 94% and 90% in the stronger and weaker limbs, respectively.21 Finally, as current knee injury and pain influence central activation assessment, control participants were carefully screened to be free of injury and pain (average IKDC score: 98.25/100), making it unlikely that these influenced our results.
Post-fatigue, both groups demonstrated reductions in quadriceps strength and central activation, though no differences were observed between groups. Previous studies have demonstrated greater central activation deficits (lower CAR values) following neuromuscular fatigue,8,33,45 suggesting neuromuscular fatigue is, in part, centrally mediated.13 That the control group demonstrated a larger, though not statistically significant, change in pre- to post-fatigue quadriceps strength in spite of similar changes in CAR suggests that the contributions to neuromuscular fatigue may differ between healthy and those post-ACLr. Future studies should investigate the central and peripheral contributions to neuromuscular fatigue in both populations so that strategies to counter fatigue may be developed.
The ACLr group required fewer squats to reach fatigue, though this difference did not reach statistical significance. It should be noted that 2 participants with ACLr requested to stop the fatiguing exercise due to pain in the reconstructed limb. This may account for the difference in number of squats to fatigue. That those with ACLr did not require statistically fewer squats to fatigue seems counter-intuitive considering previous research has demonstrated that weaker muscles fatigue more quickly than stronger muscles.38 However, Snyder-Mackler and colleagues37 have reported that the quadriceps are more fatigue resistant in the ACLr side compared to the contralateral limb of an individual. Selective Type IIb fiber atrophy in the ACLr limb quadriceps may have contributed to this finding.37 The results of morphological studies following ACLr are conflicting, however, with studies demonstrating both selective Type I atrophy,9 selective Type II atrophy,26 as well as a relative predominance of both.27 As we did not consider quadriceps morphology, we cannot determine if selective fiber type atrophy contributed to our findings.
This investigation is not without limitations. This study utilized individuals who underwent both patellar tendon and semitendinosus/gracilis autograft ACLr, which may have influenced our results. It is unknown how graft type influences the response to neuromuscular fatigue. Future studies may be needed to elucidate any differential responses of both graft types to neuromuscular fatigue so that graft type-specific rehabilitation strategies can be developed if necessary. Further, despite those with ACLr undergoing rehabilitation at a single sports medicine clinic and following a standard protocol for rehabilitation, it remains possible that attempts to individualize treatment led to differences in the treatment that each individual received. These differences could have influenced strength, activation, and/or biomechanics measured at baseline in these individuals and, therefore, influenced our outcomes. Also, both males and females were included within the present investigation. Considering potential sex differences in the response to exercise after ACLr may have influenced our outcomes. Our results may have been further influenced by a small sample size and unequal numbers of males and females in each group. Recent research suggests that females post-ACLr experience greater reductions in quadriceps function following exercise than males post-ACLr.14,23 Additionally, it is possible that some recovery occurred in the time between the post-fatigue dynamic landings and the post-fatigue strength and central activation testing. However, pilot testing indicated that strength remained below baseline levels 10 minutes after the fatiguing protocol was stopped. All strength and central activation testing was completed in this time frame. Further, it is possible that a more objective measure of fatigue may have changed our outcomes. However, quadriceps strength decreased by similar amounts in both groups, suggesting similar magnitudes of fatigue in those post-ACLr and control using the present fatiguing protocol. Finally, the participants in the control group reported lower Tegner scores than those in the ACLr group. Previous research has suggested that skill level and training influence lower extremity neuromuscular control.16 Though this difference in Tegner score was not significantly different between groups, differences in current activity level could have influenced our results. Despite this, the participants in the control group demonstrated similar changes in knee joint angles to those reported in healthy people,3,28,31,41 while those following ACLr did not change their biomechanics as a result of fatigue.
CONCLUSION
Decreased quadriceps strength and voluntary activation were observed in both those with ACLr and healthy controls following a neuromuscular fatigue protocol. Knee flexion and abduction angles were reduced in both groups following fatigue, though it should be noted that the ACLr group demonstrated smaller peak knee flexion angles than the control group at both time points. The less flexed knee posture has been linked to non-contact ACL injury risk and, thus, may prove injurious. Post-operative rehabilitation may benefit from the inclusion of strategies to increase knee flexion angles and moments during dynamic activity.
KEY POINTS.
Findings
History of ACLr does not exacerbate the influence of neuromuscular fatigue on quadriceps strength and central activation or knee biomechanics.
Importantly, ACLr participants land with reduced knee flexion prior to fatigue, suggesting strategies to improve knee joint biomechanics need to be better emphasized in post-operative rehabilitation.
Implications
Neuromuscular fatigue negatively impacts quadriceps function and knee joint biomechanics, thus fatigue-resistance training should be incorporated into ACL prevention and re-injury prevention strategies.
Caution
Before the combined effects of ACLr and neuromuscular fatigue on knee joint biomechanics can be fully understood, further research utilizing more participants and a variety of fatiguing exercises and measurement techniques needs to be conducted.
Acknowledgments
The project described was supported by Grant Number 1 K08 AR05315201A2 from NIAMS/NIH to Riann Palmieri-Smith. Additional funding was provided by the University of Michigan Rackham Graduate School to Abbey Thomas.
Footnotes
The protocol for this study was approved by the Institutional review board of the University of Michigan Medical School.
The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article.
References
- 1.Besier TF, Lloyd DG, Cochrane JL, Ackland TR. External loading of the knee joint during running and cutting maneuvers. Med Sci Sports Exerc. 2001 Jul;33(7):1168–1175. doi: 10.1097/00005768-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000 Jun;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 3.Borotikar BS, Newcomer R, Koppes R, McLean SG. Combined effects of fatigue and decision making on female lower limb landing postures: central and peripheral contributions to ACL injury risk. Clin Biomech (Bristol, Avon) 2008 Jan;23(1):81–92. doi: 10.1016/j.clinbiomech.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002 May-Jun;18(5):502–509. doi: 10.1053/jars.2002.30649. [DOI] [PubMed] [Google Scholar]
- 5.Bryant AL, Kelly J, Hohmann E. Neuromuscular adaptations and correlates of knee functionality following ACL reconstruction. J Orthop Res. 2008 Jan;26(1):126–135. doi: 10.1002/jor.20472. [DOI] [PubMed] [Google Scholar]
- 6.Chappell JD, Herman DC, Knight BS, Kirkendall DT, Garrett WE, Yu B. Effect of fatigue on knee kinetics and kinematics in stop-jump tasks. Am J Sports Med. 2005 Jul;33(7):1022–1029. doi: 10.1177/0363546504273047. [DOI] [PubMed] [Google Scholar]
- 7.Cole GK, Nigg BM, Ronsky JL, Yeadon MR. Application of the joint coordinate system to three-dimensional joint attitude and movement representation: a standardization proposal. J Biomech Eng. 1993 Nov;115(4A):344–349. doi: 10.1115/1.2895496. [DOI] [PubMed] [Google Scholar]
- 8.De Serres SJ, Enoka RM. Older adults can maximally activate the biceps brachii muscle by voluntary command. J Appl Physiol. 1998 Jan;84(1):284–291. doi: 10.1152/jappl.1998.84.1.284. [DOI] [PubMed] [Google Scholar]
- 9.Edstrom L. Selective atrophy of red muscle fibres in the quadriceps in long-standing knee-joint dysfunction. Injuries to the anterior cruciate ligament. J Neurol Sci. 1970 Dec;11(6):551–558. doi: 10.1016/0022-510x(70)90105-x. [DOI] [PubMed] [Google Scholar]
- 10.Ernst GP, Saliba E, Diduch DR, Hurwitz SR, Ball DW. Lower extremity compensations following anterior cruciate ligament reconstruction. Phys Ther. 2000 Mar;80(3):251–260. [PubMed] [Google Scholar]
- 11.Ford KR, Myer GD, Toms HE, Hewett TE. Gender differences in the kinematics of unanticipated cutting in young athletes. Med Sci Sports Exerc. 2005 Jan;37(1):124–129. [PubMed] [Google Scholar]
- 12.Frank BS, Gilsdorf CM, Goerger BM, Prentice WE, Padua DA. Neuromuscular fatigue alters postural control and sagittal plane hip biomechanics in active females with anterior cruciate ligament reconstruction. Sports Health. 2014 Jul;6(4):301–308. doi: 10.1177/1941738114530950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001 Oct;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 14.Goetschius J, Kuenze CM, Hart JM. Knee extension torque variability after exercise in ACL reconstructed knees. J Orthop Res. 2015 May 20; doi: 10.1002/jor.22858. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins RD, Fuller CW. A prospective epidemiological study of injuries in four English professional football clubs. Br J Sports Med. 1999 Jun;33(3):196–203. doi: 10.1136/bjsm.33.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. The Journal of bone and joint surgery. 2004 Aug;86–A(8):1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999 Nov-Dec;27(6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 18.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. The American journal of sports medicine. 2001 Sep-Oct;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 19.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996 Jul;19(7):861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Kernozek TW, Torry MR, Iwasaki M. Gender differences in lower extremity landing mechanics caused by neuromuscular fatigue. Am J Sports Med. 2008 Mar;36(3):554–565. doi: 10.1177/0363546507308934. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan C, Williams GN. Evoked tetanic torque and activation level explain strength differences by side. Eur J Appl Physiol. 2009 Jul;106(5):769–774. doi: 10.1007/s00421-009-1057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan C, Allen EJ, Williams GN. Torque-based triggering improves stimulus timing precision in activation tests. Muscle Nerve. 2009 Jul;40(1):130–133. doi: 10.1002/mus.21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuenze CM, Hertel J, Hart JM. Quadriceps muscle function after exercise in men and women with a history of anterior cruciate ligament reconstruction. J Athl Train. 2014 Nov-Dec;49(6):740–746. doi: 10.4085/1062-6050-49.3.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004 Jan;22(1):110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd DG, Buchanan TS. Strategies of muscular support of varus and valgus isometric loads at the human knee. J Biomech. 2001 Oct;34(10):1257–1267. doi: 10.1016/s0021-9290(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 26.Lopresti C, Kirkendall DT, Street GM, Dudley AW. Quadriceps Insufficiency following Repair of the Anterior Cruciate Ligament*. J Orthop Sports Phys Ther. 1988;9(7):245–249. doi: 10.2519/jospt.1988.9.7.245. [DOI] [PubMed] [Google Scholar]
- 27.Lorentzon R, Elmqvist LG, Sjostrom M, Fagerlund M, Fuglmeyer AR. Thigh musculature in relation to chronic anterior cruciate ligament tear: muscle size, morphology, and mechanical output before reconstruction. Am J Sports Med. 1989 May-Jun;17(3):423–429. doi: 10.1177/036354658901700318. [DOI] [PubMed] [Google Scholar]
- 28.McLean SG, Samorezov JE. Fatigue-induced ACL injury risk stems from a degradation in central control. Med Sci Sports Exerc. 2009 Aug;41(8):1661–1672. doi: 10.1249/MSS.0b013e31819ca07b. [DOI] [PubMed] [Google Scholar]
- 29.McLean SG, Lipfert SW, van den Bogert AJ. Effect of gender and defensive opponent on the biomechanics of sidestep cutting. Med Sci Sports Exerc. 2004 Jun;36(6):1008–1016. doi: 10.1249/01.mss.0000128180.51443.83. [DOI] [PubMed] [Google Scholar]
- 30.McLean SG, Huang X, Su A, Van Den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clinical biomechanics. 2004 Oct;19(8):828–838. doi: 10.1016/j.clinbiomech.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 31.McLean SG, Felin R, Suedekum N, Calabrese G, Passerallo A, Joy S. Impact of fatigue on gender-based high-risk landing strategies. Med Sci Sports Exerc. 2007;39:502–514. doi: 10.1249/mss.0b013e3180d47f0. [DOI] [PubMed] [Google Scholar]
- 32.Myers CA, Hawkins D. Alterations to movement mechanics can greatly reduce anterior cruciate ligament loading without reducing performance. J Biomech. Oct 19;43(14):2657–2664. doi: 10.1016/j.jbiomech.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Newham DJ, McCarthy T, Turner J. Voluntary activation of human quadriceps during and after isokinetic exercise. J Appl Physiol. 1991 Dec;71(6):2122–2126. doi: 10.1152/jappl.1991.71.6.2122. [DOI] [PubMed] [Google Scholar]
- 34.Nyland JA, Shapiro R, Caborn DN, Nitz AJ, Malone TR. The effect of quadriceps femoris, hamstring, and placebo eccentric fatigue on knee and ankle dynamics during crossover cutting. J Orthop Sports Phys Ther. 1997 Mar;25(3):171–184. doi: 10.2519/jospt.1997.25.3.171. [DOI] [PubMed] [Google Scholar]
- 35.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am J Sports Med. 2014 Apr 21;42(7):1567–1573. doi: 10.1177/0363546514530088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plowman-Prine EK, Triggs WJ, Malcolm MP, Rosenbek JC. Reliability of transcranial magnetic stimulation for mapping swallowing musculature in the human motor cortex. Clin Neurophysiol. 2008 Oct;119(10):2298–2303. doi: 10.1016/j.clinph.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Snyder-Mackler L, Binder-Macleod SA, Williams PR. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 1993 Jul;25(7):783–789. doi: 10.1249/00005768-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Stackhouse SK, Stevens JE, Lee SC, Pearce KM, Snyder-Mackler L, Binder-Macleod SA. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001 May;81(5):1102–1109. [PubMed] [Google Scholar]
- 39.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985 Sep;(198):43–49. [PubMed] [Google Scholar]
- 40.Thomas AC, Palmieri-Smith RM, McLean SG. Isolated hip and ankle fatigue are unlikely risk factors for anterior cruciate ligament injury. Scand J Med Sci Sports. 2011 Jun;21(3):359–368. doi: 10.1111/j.1600-0838.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 41.Thomas AC, McLean SG, Palmieri-Smith RM. Quadriceps and hamstrings fatigue alters hip and knee mechanics. J Appl Biomech. 2010;26(2):159–170. doi: 10.1123/jab.26.2.159. [DOI] [PubMed] [Google Scholar]
- 42.Urbach D, Nebelung W, Weiler HT, Awiszus F. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc. 1999 Dec;31(12):1691–1696. doi: 10.1097/00005768-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Webster KE, Santamaria LJ, McClelland JA, Feller JA. Effect of Fatigue on Landing Biomechanics following ACL Reconstruction Surgery. Med Sci Sports Exerc. 2012 Nov 5;44(5):910–916. doi: 10.1249/MSS.0b013e31823fe28d. [DOI] [PubMed] [Google Scholar]
- 44.Willson JD, Davis IS. Lower extremity mechanics of females with and without patellofemoral pain across activities with progressively greater task demands. Clin Biomech (Bristol, Avon) 2008 Feb;23(2):203–211. doi: 10.1016/j.clinbiomech.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 45.Yue GH, Ranganathan VK, Siemionow V, Liu JZ, Sahgal V. Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J Gerontol A Biol Sci Med Sci. 1999 May;54(5):M249–253. doi: 10.1093/gerona/54.5.m249. [DOI] [PubMed] [Google Scholar]
- 46.Zebis MK, Bencke J, Andersen LL, et al. Acute fatigue impairs neuromuscular activity of anterior cruciate ligament-agonist muscles in female team handball players. Scandinavian journal of medicine & science in sports. 2011 Dec;21(6):833–840. doi: 10.1111/j.1600-0838.2010.01052.x. [DOI] [PubMed] [Google Scholar]