Abstract
IMPORTANCE
Among patients whose need for intensive care is uncertain, the relationship of intensive care unit (ICU) admission with mortality and costs is unknown.
OBJECTIVE
To estimate the relationship between ICU admission and outcomes for elderly patients with pneumonia.
DESIGN, SETTING, AND PATIENTS
Retrospective cohort study of Medicare beneficiaries (aged >64 years) admitted to 2988 acute care hospitals in the United States with pneumonia from 2010 to 2012.
EXPOSURES
ICU admission vs general ward admission.
MAIN OUTCOMES AND MEASURES
Primary outcome was 30-day all-cause mortality. Secondary outcomes included Medicare spending and hospital costs. Patient and hospital characteristics were adjusted to account for differences between patients with and without ICU admission. To account for unmeasured confounding, an instrumental variable was used—the differential distance to a hospital with high ICU admission (defined as any hospital in the upper 2 quintiles of ICU use).
RESULTS
Among 1 112 394 Medicare beneficiaries with pneumonia, 328 404 (30%) were admitted to the ICU. In unadjusted analyses, patients admitted to the ICU had significantly higher 30-day mortality, Medicare spending, and hospital costs than patients admitted to a general hospital ward. Patients (n = 553 597) living closer than the median differential distance (<3.3 miles) to a hospital with high ICU admission were significantly more likely to be admitted to the ICU than patients living farther away (n = 558 797) (36%for patients living closer vs 23%for patients living farther, P < .001). In adjusted analyses, for the 13%of patients whose ICU admission decision appeared to be discretionary (dependent only on distance), ICU admission was associated with a significantly lower adjusted 30-day mortality (14.8%for ICU admission vs 20.5%for general ward admission, P = .02; absolute decrease, −5.7%[95%CI, −10.6%, −0.9%]), yet there were no significant differences in Medicare spending or hospital costs for the hospitalization.
CONCLUSIONS AND RELEVANCE
Among Medicare beneficiaries hospitalized with pneumonia, ICU admission of patients for whom the decision appeared to be discretionary was associated with improved survival and no significant difference in costs. A randomized trial may be warranted to assess whether more liberal ICU admission policies improve mortality for patients with pneumonia.
The United States has seen considerable growth in intensive care unit (ICU) use over the last 3 decades.1 This growth may be an appropriate response to the aging population, the greater burden of comorbid illness, and the improvements in care for an increasingly complex array of patients in the outpatient setting.2 Alternatively, increasing ICU use may reflect “supply side” factors, such as expansion in critical care capacity and relatively generous reimbursement.3,4 This uncertainty underlies the concern that ICUs may be an important and expensive source of low-value care.
The value of ICU care, however, depends on the effectiveness of ICUs. Intensive care allows for greater attention to the patient, timelier delivery of treatments, and multidisciplinary expertise in the care of patients at risk for clinical deterioration. On the other hand, for some patients, the ICU may provide no additional benefit to care provided in the general ward while also increasing the risk for nosocomial infection and the likelihood that patients receive invasive, potentially harmful procedures.
Observational studies examining the relationship between ICU admission frequency and patient outcomes often suggest that greater ICU use does not achieve better outcomes.5–8 However, these results are likely subject to confounding by indication because sicker patients are more likely to be admitted to the ICU. With pneumonia as a leading reason for hospitalization,9 it is important to understand the implications of delivering intensive care to patients with pneumonia.
We sought to determine the association between ICU admission and outcomes, 30-day mortality and costs, among elderly Americans hospitalized for pneumonia. We hypothesized that ICU admission would not be associated with a survival benefit but would be associated with greater costs.
Methods
Data Source
The institutional review board for the University of Michigan approved the study and provided a waiver of consent (HUM00053488). A retrospective cohort study of all acute care hospitalizations from 2010 to 2012 was performed among fee-for-service Medicare beneficiaries 65 years and older. The Medicare Provider Analysis and Review file was linked to mortality data in the Medicare Beneficiary Summary File. Hospital characteristics were obtained from the 2010 to 2012 American Hospital Association’s Annual Surveys and the 2010 and 2011 Healthcare Cost Reporting Information Systems. Population and geographic information was obtained by linking the patient’s zip code of residence to 2010 US Census data.
Study Cohort
All patients with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) (1) primary diagnosis code for pneumonia or (2) primary diagnosis code for respiratory failure or sepsis and secondary diagnosis code of pneumonia were identified (eTable 1 in the Supplement). This method of identifying pneumonia through administrative claims data is commonly used.10–12 The analysis was limited to the first hospitalization for those with multiple eligible hospitalizations in the same year (eFigure 1 in the Supplement).
Treatment Variable and Covariate Definitions
The treatment variable was ICU admission, defined as the presence of an ICU or coronary care unit revenue center code in the administrative billing record.13 To account for differences between patients admitted to the ICU and those admitted to the wards, the analysis adjusted for demographics, comorbid illness, severity of illness, type of pneumonia, and year of admission. Income was defined by the patient’s zip code of residence using 2010 US Census data. Preexisting comorbid illness was measured according to Elixhauser et al,14 and severity of illness was captured by using secondary ICD-9-CM diagnosis and procedural codes for acute organ dysfunction,15 mechanical ventilation, respiratory failure, sepsis, shock, cardiac or respiratory arrest, and cardiopulmonary resuscitation.
The analysis adjusted for several additional hospital characteristics including hospital ownership (for profit, nonprofit, or government), medical school affiliation, teaching hospital status (resident-to–hospital bed ratio), hospital size by number of beds, ICU size by proportion of total hospital beds, annual hospital pneumonia case volume, nursing ratio (nursing full-time equivalent per 1000 patient-days), proportion of Medicaid patients admitted, geographic region, and technological index16 (weighted sum of hospital capabilities).
Outcome Measures
The primary outcome was 30-day all-cause mortality measured from the time of hospital admission. Secondary outcomes included Medicare reimbursements to the hospital and hospital costs, calculated as the patient’s hospital charges multiplied by the hospital-specific annual cost-to-charge ratio.
Instrumental Variable
In a properly executed instrumental variable analysis, the instrument approximates random assignment of patients to a treatment group analogous to a randomized clinical trial. In this study, the commonly used “differential distance”17,18 instrument was selected. Differential distance was calculated as the difference between (1) the distance from a patient’s residence to the nearest hospital with high ICU admission and (2) the distance from a patient’s residence to the nearest hospital of any type. In other words, the differential distance is the extra distance, if any, beyond the closest hospital a patient would have to travel to arrive at a hospital with high ICU admission. The distribution of ICU admission rates was examined across all hospitals, and hospitals with high ICU admission were empirically defined as those with an ICU admission rate for pneumonia in the top 2 quintiles of the included hospitals, which corresponded to an ICU admission rate for pneumonia of higher than 32%. Distances were calculated using the linear arc distance function, which measures the number of miles between the centroids of 2 zip codes.
An instrumental variable was confirmed to be necessary for the analysis as the Durbin-Wu-Hausman tests of endogenicity were significant for all instrumental variable models (eAppendix 1 in the Supplement), indicating that standard multivariable regression resulted in biased estimates when compared with the instrumental variable model.19 The instrument satisfied 3 conditions necessary to establish validity (eAppendix 1 in the Supplement). First, differential distance was highly correlated with ICU admission (partialF1,2986 = 245, P < .001); instruments with F statistics higher than 10 are considered strong20 (eTable 2 in the Supplement). Because most ill patients with pneumonia will seek care at the nearest hospital, patients who live close to a hospital with high ICU admission are more likely to be transported to that hospital, which increases their likelihood of being admitted to the ICU. Indeed, when stratified by the median differential distance (3.3 miles [interquartile range, 0–18.9]), ICU admission was substantially more likely among patients living near a hospital with high ICU admission than those living farther away (36%for patients living closer vs 23% for patients living farther) (eTable 3 in the Supplement). Second, differential distance was not associated with the outcomes, 30-day mortality, Medicare spending, or hospital costs, except through the instrument’s effect on ICU admission (eTables 4–6 in the Supplement). Third, there should not be any mutual confounders between the instrument and the outcome. This condition was evaluated by (1) the distribution of patient-level covariates across differential distance (eTable 3 in the Supplement) and (2) the distribution of hospital-level characteristics across quintiles of ICU use (eTable 7 in the Supplement). If observed confounders are comparable across levels of differential distance, it provides greater confidence that unobserved confounders are similar as well.21 For instruments defined by geography, differences in urbanity and associated variables (eg, race and socioeconomic status) are commonly observed.18,22 The recommended approach to address such imbalances in these and other variables is to perform analyses stratified by these variables and/or adjust for them in the instrumental variable model.18,21
Interpreting the Instrumental Variable Results
In contrast to standard multivariable regression in which the coefficient for ICU admission represents the adjusted treatment effect for the average patient, the coefficient in the instrumental variable analysis represents the adjusted treatment effect for the so-called marginal patient. Statistically, marginal patients are those that are admitted to the ICU solely due to their proximity to a hospital with high ICU admission.23 The instrumental variable analysis does not rely on defining the specific clinical characteristics of these patients—instead it relies on the fact that patients reside randomly around hospitals and some patients are treated differently in different hospitals. In this context, these marginal patients (referred to as borderline patients in this article) may be interpreted as those whose need for ICU admission is borderline or discretionary—that is, patients who might receive care on a general ward at one hospital and in the ICU at another because it is uncertain whether ICU admission would benefit the patient23 (eFigure 2 in the Supplement).
Statistical Analysis
χ2 and t tests were used to evaluate associations between ICU admission and patient characteristics. Unadjusted analyses without covariates were performed using logistic regression for 30-day mortality and linear regression for Medicare spending and hospital costs. To account for average differences between patients, the association between ICU admission and 30-day mortality, payments by Medicare, and hospital costs were evaluated by logistic and linear regression models adjusted for patient and hospital characteristics. All regression models estimated robust standard errors with clustering at the hospital level.
In the instrumental variable analyses, 2-stage least squares regressions24,25 were performed on all patients after adjusting for patient and hospital characteristics described above, and standard errors adjusted for clustering of patients in hospitals. The adjusted outcomes from the instrumental variable model represent the mean predicted difference in the probability of death at 30 days, Medicare payments, or hospital costs. Adjusted absolute rates of outcomes were estimated using predictive margins.
The method of Newhouse and McClellan21 was used to estimate the fraction of patients hospitalized with pneumonia who were admitted to the ICU because they presented to a hospital with high ICU admission. In this approach, the percentage of patients for which the instrumental variable analysis applies can be determined by subtracting the average rate of ICU admission in the 2 patient populations stratified by median differential distance.
Subgroup and Sensitivity Analyses
To test the robustness of the findings, several subgroup and sensitivity analyses were performed. First, to address the potential for unmeasured confounding due to correlates of race and urbanity, which demonstrated imbalance by median differential distance, instrumental variable analyses were stratified by race or the National Center for Health Statistics Urban-Rural Classification Scheme.26 Second, instrumental variable analyses were stratified by the proportion of total hospital beds that were ICU beds, an indirect measure of a hospital’s likelihood of ICU capacity constraint that may be associated with increased mortality.27 Third, to address observed differences in severity of illness by differential distance and to rule out the possibility that severely ill patients could be driving the association, the instrumental variable analysis was stratified by organ failure score and also repeated after excluding patients with ICD-9-CM codes for the following: mechanical ventilation, cardiopulmonary resuscitation, shock, or cardiac or respiratory arrest. Fourth, the instrumental variable analyses were repeated to assess the association of Medicare payments and hospital costs stratified by in-hospital mortality. Fifth, to assess the robustness of the results to the choice of modeling method, the average treatment effect of ICU admission on 30-day mortality was determined using inverse probability weighting (eAppendix 2 in the Supplement).
Data management and analysis was performed using SAS (SAS Institute), version 9.3, and Stata (StataCorp), version 13.1. All tests were 2-sided with a P value of less than .05 considered significant.
Results
From 2010 to 2012, 1 327 370 acute care hospitalizations of Medicare beneficiaries with pneumonia were identified. Admissions to hospitals without ICU capabilities (3%), transfers from other acute care hospitals (3.6%), patients with missing zip codes (1.6%), or hospitalizations in US territories (0.01%) were excluded. After applying exclusion criteria, the final sample included 1 112 394 patients admitted to 2988 hospitals (eFigure 1 in the Supplement). Among these patients, 328 404 patients (29.5%) were admitted to the ICU, with patient characteristics listed in Table 1 and eTable 8 in the Supplement. In the sample, 1193 hospitals (40%) were defined as hospitals with high ICU use. Hospital characteristics by ICU use and patient outcomes by ICU admission are listed in Table 2 and Table 3.
Table 1.
Patient Characteristics by Admission to the ICU vs General Ward
| Characteristics | Patients, No. (%) | |
|---|---|---|
| ICU | General Ward | |
| Patients, No.a | 328 404 | 783 990 |
| Age, mean (SD), y | 78 (8) | 80 (8) |
| 65–74 | 120 106 (36.6) | 223 187 (28.5) |
| 75–84 | 120 814 (36.8) | 276 773 (35.3) |
| ≥85 | 87 484 (26.6) | 284 030 (36.2) |
| Women | 169 078 (51.5) | 437 085 (55.8) |
| Race/ethnicity | ||
| White | 273 507 (83.3) | 688 644 (87.8) |
| Black | 35 696 (10.9) | 60 951 (7.8) |
| Other | 19 201 (5.9) | 34 395 (4.4) |
| Urbanitya | ||
| Large central metropolitan | 81 986 (25.0) | 145 992 (18.7) |
| Large suburban metropolitan | 77 100 (23.5) | 181 996 (23.3) |
| Medium metropolitan | 67 596 (20.6) | 172 286 (22.0) |
| Small metropolitan | 34 888 (10.7) | 100 057 (12.8) |
| Micropolitan | 37 660 (11.5) | 107 401 (13.7) |
| Noncore | 28 398 (8.7) | 74 604 (9.5) |
| Median household income by zip code, $ | ||
| <40 000 | 91 285 (27.8) | 203 080 (25.9) |
| 40 000–100 000 | 218 521 (66.5) | 535 203 (68.3) |
| >100 000 | 18 598 (5.7) | 45 707 (5.8) |
| Elixhauser comorbidities count, mean (SD)b | 2.6 (1.3) | 2.9 (1.3) |
| Admission source | ||
| Outpatient | 250 420 (76.3) | 619 027 (79.0) |
| Emergency department | 76 391 (23.3) | 162 503 (20.7) |
| Hospital diagnosesa | ||
| Pneumonia as primary diagnosis | 111 315 (33.9) | 643 237 (82.1) |
| Respiratory failure | 221 308 (67.4) | 126 661 (16.2) |
| Sepsis | 143 093 (43.6) | 102 998 (13.1) |
| Shock | 90 392 (27.5) | 9325 (1.2) |
| Cardiac or respiratory arrest | 9421 (2.9) | 1901 (0.2) |
| Type of pneumonia | ||
| Unspecified | 267 864 (81.6) | 695 069 (88.7) |
| Viral | 2904 (0.9) | 8348 (1.1) |
| Bacterial | 57 636 (17.6) | 80 573 (10.3) |
| Procedures performed during hospitalization | ||
| Mechanical ventilation | 159 346 (48.5) | 22 525 (2.9) |
| Cardiopulmonary resuscitation | 9229 (2.8) | 1843 (0.2) |
| Angus organ failure scorec | ||
| 0 | 98 547 (30.0) | 607 759 (77.5) |
| 1 | 107 979 (32.9) | 154 269 (19.7) |
| ≥2 | 121 878 (37.1) | 21 962 (2.8) |
| Year of admission | ||
| 2010 | 108 136 (29.4) | 260 229 (70.6) |
| 2011 | 112 625 (29.4) | 270 888 (70.6) |
| 2012 | 107 643 (29.9) | 252 873 (70.1) |
Abbreviation: ICU, intensive care unit.
There were 11 703 patients (1%) excluded from regression models due to missing differential distance (n = 5166), admission source (n = 4053), urban/rural (n = 2430), and pneumonia volume (n = 107).
All 29 Elixhauser comorbidities are listed in eTable 8 in the Supplement.
The Angus organ failure score identifies severity of illness by patient organ failures derived from the administrative record with a maximum score of 6, and higher scores indicating more organ failures.
Table 2.
Hospital Characteristics by ICU Utilization
| Characteristics | Hospitals, No. (%) | |
|---|---|---|
| High ICU Admissiona |
Low ICU Admission |
|
| Hospitals, No. | 1193 | 1795 |
| Hospital ownership | ||
| For profit | 361 (30.3) | 318 (17.7) |
| Nonprofit | 661 (55.4) | 1209 (67.4) |
| Government | 171 (14.3) | 268 (14.9) |
| Medical school affiliation | 453 (38.0) | 545 (30.4) |
| Teaching status | ||
| No residents | 891 (74.7) | 1448 (80.7) |
| Minor teaching program, <0.25 residents/bed | 202 (16.9) | 257 (14.3) |
| Major teaching program, ≥0.25 residents/bed | 100 (8.4) | 90 (5.0) |
| Hospital beds | ||
| <100 | 200 (16.8) | 568 (31.6) |
| 100–199 | 373 (31.3) | 527 (29.4) |
| ≥200 | 620 (52.0) | 700 (39.0) |
| ICU beds, %b | ||
| <5 | 166 (13.9) | 239 (13.3) |
| 5–10 | 461 (38.6) | 882 (49.1) |
| >10 | 566 (47.5) | 674 (37.5) |
| Hospital pneumonia annual case volume, mean (SD) | 359 (287) | 446 (348) |
| Nursing FTE per 1000 patient-days, mean (SD) | 4.0 (1.5) | 3.7 (1.5) |
| Technology index, mean (SD)c | 21.9 (14.5) | 20.9 (12.4) |
| Medicaid patients, % | ||
| <7 | 270 (22.6) | 734 (40.9) |
| 7–11 | 406 (34.0) | 668 (37.2) |
| >11 | 517 (43.3) | 393 (21.9) |
| Census regions | ||
| Northeast | 148 (12.4) | 346 (19.3) |
| Midwest | 438 (36.7) | 596 (33.2) |
| South | 319 (26.7) | 569 (31.7) |
| West | 288 (24.1) | 284 (15.8) |
Abbreviations; FTE, full-time equivalent; ICU, intensive care unit.
High ICU use hospitals were defined as hospitals with an ICU admission rate for pneumonia in the top 40% of all hospitals over the 3-year period with a minimum ICU rate of admission for pneumonia of 32%.
Percentage of hospital beds that are ICU beds.
The technology index is the weighted sum of the following hospital capabilities: obstetrics, medical/surgical ICU, cardiac ICU, emergency department, trauma center, open heart surgery, radiation therapy, computed tomography, diagnostic radiology, magnetic resonance imaging, positron-emission tomography, single-photon emission computed tomography, ultrasonography, and transplantation service.16
Table 3.
Patient Outcomes of Care by Admission to ICU vs General Ward
| Outcomes | Patients, No. (%) | |
|---|---|---|
| ICU | General | |
| Total patients | 328 404 | 783 990 |
| Length of stay, median (IQR), d | 7 (4–12) | 4 (3–6) |
| Quartiles ($ range) | ||
| Total Medicare payment per patient | ||
| 1 (0–4981) | 22 124 (6.7) | 256 169 (32.7) |
| 2 (4982–7639) | 36 511 (11.1) | 241 472 (30.8) |
| 3 (7640–11 162) | 88 292 (26.9) | 189 783 (24.2) |
| 4 (11 163–882 637) | 181 477 (55.3) | 96 566 (12.3) |
| Hospital costs per patienta | ||
| 1 (153–4614) | 16 003 (4.9) | 260 844 (33.4) |
| 2 (4615–7389) | 34 589 (10.6) | 242 305 (31.0) |
| 3 (7390–13 154) | 79 773 (24.4) | 197 112 (25.3) |
| 4 (13 155–1 375 266) | 196 387 (60.1) | 80 504 (10.3) |
| Discharge destination | ||
| Home | 94 961 (29.1) | 476 492 (61.2) |
| Rehabilitation or nursing facility | 114 466 (35.1) | 225 484 (28.9) |
| Dead | 79 382 (24.3) | 35 709 (4.6) |
| Other | 39 595 (11.5) | 46 305 (5.3) |
| 30-d Readmission | 61 414 (18.7) | 132 548 (16.9) |
| 30-d Mortality | 118 001 (35.9) | 92 059 (11.7) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
There were 4877 patients (0.4%) excluded from regression models due to missing hospital cost-to-charge ratios.
In unadjusted analyses, patients admitted to the ICU compared with patients admitted to a general ward had greater 30-day mortality (35.9% for ICU admission vs 11.7% for general ward admission; absolute difference, 24.2% [95% CI, 23.8%–24.6%]), Medicare spending ($19 279 for ICU admission vs $7308 for general ward admission; absolute difference, $11 971 [95% CI, $11 634–$12 307]), and hospital costs ($23 475 for ICU admission vs $7411 for general ward admission; absolute difference, $16 064 [95%CI, $15 658–$16 469]) (Table 4).
Table 4.
Association of ICU Admission on 30-Day Mortality, Medicare Spending, and Hospital Costs
| Model | ICU Patients | General Ward Patients |
Absolute Difference (95% CI) | P Value |
|---|---|---|---|---|
| 30-d Mortality, % | ||||
| Unadjusted regression | 35.9 | 11.7 | 24.2 (23.8 to 24.6) | <.001 |
| Adjusted regressiona | 21.5 | 17.8 | 3.7 (3.3 to 4.0) | <.001 |
| Instrumental variablea,b | 14.8 | 20.5 | −5.7 (−10.6 to −0.9) | .02 |
| Mean Medicare payments per patient, $ | ||||
| Unadjusted regression | 19 279 | 7308 | 11 971 (11 634 to 12 307) | <.001 |
| Adjusted regressiona | 12 711 | 10 052 | 2659 (2513 to 2805) | <.001 |
| Instrumental variablea,b | 9918 | 11 238 | −1320 (−3421 to 781) | .22 |
| Mean hospital costs per patient, $ | ||||
| Unadjusted regression | 23 475 | 7411 | 16 064 (15 658 to 16 469) | <.001 |
| Adjusted regressiona | 17 160 | 10 048 | 7112 (6874 to 7349) | <.001 |
| Instrumental variablea,b | 14 162 | 11 320 | 2842 (−168 to 5851) | .06 |
Abbreviation: ICU, intensive care unit.
Model adjusted for all variables in Table 1 and Table 2 in addition to all 29 individual Elixhauser comorbidities. Angus organ failure score, which identifies severity of illness by patient organ failures derived from the administrative record with a maximum score of 6, was defined to include all organ failures numbered 0 to 5 or higher. Higher scores indicate more organ failures. Hospital region included the 9 US census defined regions. All standard errors for models were adjusted for clustering of patients within hospitals.
Differences between patients admitted to the ICU and patients admitted to a general ward persisted in adjusted multivariable regression models. Though attenuated, average patients admitted to the ICU had significantly higher 30-day mortality compared with patients admitted to a general ward (21.5% for patients in the ICU vs 17.8% for patients in the general ward; absolute difference, 3.7% [95% CI, 3.3–4.0]) (Table 4). Risk-adjusted payments by Medicare ($12 711 for patients in the ICU vs $10 052 for patients in the general ward; absolute difference, $2659 [95% CI, $2513–$2805]) remained greater with ICU admission as did hospital costs ($17 160 for patients in the ICU vs $10 048 for patients in the general ward; absolute difference, $7112 [95% CI, $6874–$7349]).
The median differential distance to a hospital with high ICU admission was 3.3miles. Of the patients for whom the differential distance was less than 3.3 miles, one-third (201 144 of 553 597 patients; 36.3%) were admitted to the ICU compared with one-fourth (127 260 of 558 797 patients; 22.8%) of those patients with pneumonia whose differential distance was more than 3.3 miles (eTable 3 and eFigure 2 in the Supplement). Therefore, following the method of Newhouse et al,21 ICU admission appeared to depend only on distance for approximately 13% of patients.
In the instrumental variable analysis, which estimates the effect in this subset of borderline patients and which also controlled for patient and hospital characteristics, ICU admission was associated with significantly lower 30-day mortality when compared with general ward admission (14.8% for ICU admission vs 20.5%for general ward admission, P = .02) with an absolute reduction in 30-day mortality of 5.7% (95% CI, −10.6% to −0.9%) (Table 4 and eTable 9 in the Supplement). ICU admission was not associated with significant differences in payments by Medicare ($9918 for ICU admission vs $11 238 for general ward admission; absolute decrease, $1320 [95%CI, −$3421 to $781], P = .22) or hospital costs ($14 162 for ICU admission vs $11 320 for general ward admission; absolute increase, $2842 [95%CI, −$168 to $5851], P = .06) (Table 4 and eTables 10–11 in the Supplement).
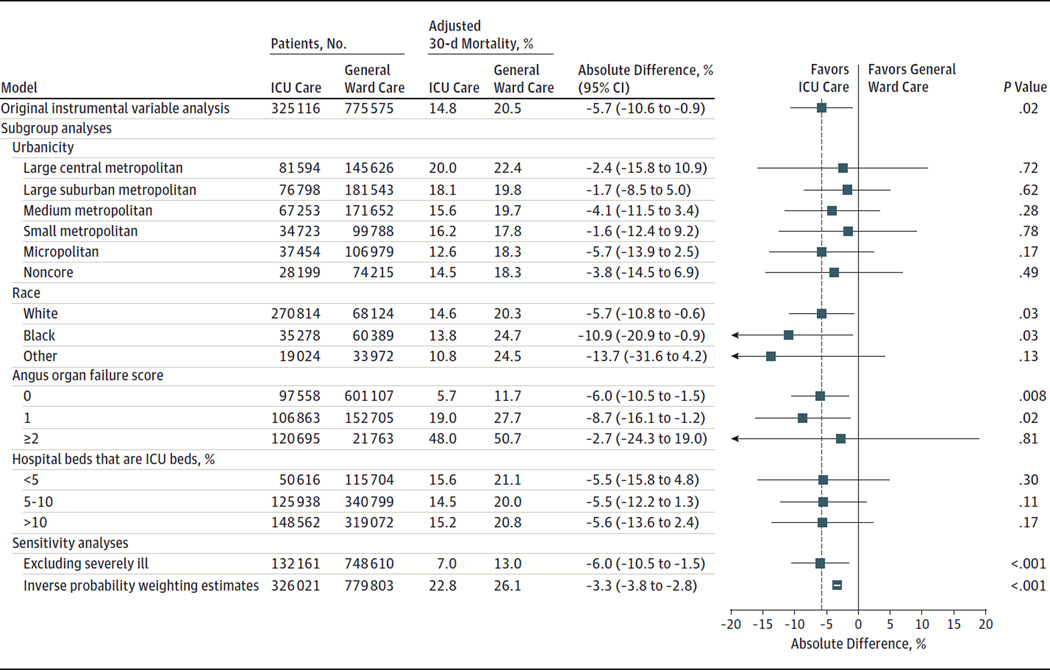
Sensitivity analyses demonstrated consistent results in the estimated benefit of ICU admission across urban and rural categories, strata of race, organ failures, ICU beds as a percentage of total hospital beds, after excluding severely ill patients, or when estimating the association of ICU admission using inverse probability weighting. None of these analyses yielded results that were substantially different from the pooled estimate (Figure). When stratified by in-hospital mortality, ICU admission was not associated with significant differences in Medicare spending or hospital costs (eTable 12 in the Supplement).
Figure.
Instrumental Variable Subgroup and Sensitivity Analyses for 30-Day Mortality Among Elderly Patients With Pneumonia Admitted to the ICU vs General Ward
ICU indicates intensive care unit; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification. With exception of the inverse probability weighting estimate, all models used an instrumental variable to adjust for all variables in Table 1 and Table 2 in addition to all 29 individual Elixhauser comorbidities and clustering of patients within hospitals. The regression models excluded 11 703 patients (1%) due to missing differential distance (n = 5166), admission source (n = 4053), urban/rural (n = 2430), pneumonia volume (n = 107). The Angus organ failure score identifies severity of illness by patient organ failures derived from the administrative record with a maximum score of 6. Higher scores indicate more organ failures. Details of the inverse probability weighting estimate can be found in eAppendix 2 in the Supplement. The severely ill subgroup excluded individuals with shock (ICD-9-CM: 458, 785.5–785.59, 958.4, 998.0), cardiac or respiratory arrest (ICD-9-CM: 427.5, 799.1), cardiopulmonary resuscitation (ICD-9-CM: 99.60, 99.63), or invasive or noninvasive mechanical ventilation (ICD-9-CM: 96.7, 96.70, 96.71, 96.72, 93.90). Error bars represent 95%CIs for absolute mortality differences (ICU vs general ward) for all models.
Discussion
Among hospitalized patients with pneumonia, ICU admission of patients for whom the decision appeared to be discretionary was associated with a 5.7% absolute survival advantage at 30 days compared with patients admitted to general wards. There were no significant differences in Medicare spending or hospital costs associated with ICU admission. Contrary to the prespecified hypothesis, these findings suggest that ICU admission for borderline patients (those for whom ICU admission depends on the hospital to which they present) is associated with reduced mortality without a considerable increase in costs.
When interpreting the results of this study, it is important to understand the population to which it applies. Whereas traditional regression models can be applied only to statistically average patients, the instrumental variable findings apply only to patients with pneumonia whose ICU admission decision varied depending on distance from a hospital with high ICU admission. This population of patients does not immediately translate into specific clinical criteria; however, it is likely that these are patients who would be admitted to the ICU in one hospital but not another. Such patients represent those with a borderline or uncertain need for the ICU. Instrumental variable analyses do not definitively identify the exact size of this population; however, our results suggest that the population of patients who might benefit from ICU admission is not trivial, particularly given the substantial number of Medicare patients with pneumonia each year. Our results should not, however, be extrapolated to patients whose ICU triage decision is straightforward—those who clearly benefit from ICU admission (eg, mechanically ventilated) and those for whom ICU admission is obviously not indicated (eg, low-risk admissions).23
There are several reasons why ICU admission may be beneficial for borderline patients with pneumonia. First, the ICU brings patients greater attention from nurses allowing for more timely recognition of decompensation.28,29 Late admission to an ICU for patients with pneumonia was associated with worse outcomes compared with patients with a similar disease severity admitted early to an ICU, at least in a 2-site study.30 Second, pneumonia is the most common cause of sepsis, a syndrome in which earlier, more aggressive care (more readily delivered in ICUs than the general ward) has been associated with reduced mortality.31 Third, many studies,32–34 but not all,35 suggest that ICU admission for pneumonia has been associated with increased rates of guideline-based treatment, which has been linked with improved mortality and reduced costs. Fourth, ICU admission increases the likelihood that a patient with pneumonia is managed by pulmonary or critical care specialists, clinicians whose case volume or expertise in pneumonia care may yield better outcomes.32,36 Further research is needed to elucidate these and other potential mechanisms underlying the ICU’s beneficial association with mortality for patients with pneumonia. This research could include randomized trials to provide a degree of causal evidence not possible even from instrumental variable analyses and other observational approaches.
The study results differ from several that have examined the association between ICU admission and patient outcomes, primarily because they sought to answer different questions. Previous studies assessed the outcomes of average patients admitted to the ICU with traditional risk adjustment and have shown increased overall mortality and costs.35,37 Yet, such studies fail to fully address the confounding by indication for ICU admission.38 For example, many individuals are denied admission to the ICU for reasons that cannot be measured by administrative data or because they do not require life-sustaining therapies, potentially gaining less additional benefit from ICU-level care that cannot fully be accounted for using severity of illness measures. This study addresses the potential for unmeasured confounding with instrumental variable analyses.
This study should be interpreted in the context of several limitations. First, administrative data were used, which may under-identify or improperly identify patients with pneumonia.13 However, patients with pneumonia were identified using a well-established definition from epidemiologic research, which may better identify patients with pneumonia than the definition employed by Medicare due to variations in hospital coding.10–12 Second, it cannot be proven that the instrument fully addresses unmeasured confounding.23,24 However, subgroup and sensitivity analyses performed to address this concern corroborated the primary results. Third, because the analysis includes only Medicare beneficiaries, it may not generalize to a younger population with pneumonia. Fourth, data was not available to identify either the timing of ICU admission within a hospitalization or the reason for ICU admission, preventing an exploration of the chain of events leading up to ICU admission or the mechanism through which the ICU may benefit borderline patients. Similarly, although there are many reasons for the variation in ICU use between hospitals, this study was unable to examine clinician-specific effects on ICU triage. Finally, although true economic costs were not examined and we could not examine physician, facility, or outpatient payments, hospital costs and Medicare payments represent the real-world transaction of money between hospitals and Medicare for patient care.39
These findings may have implications for health system leaders and policy makers seeking to improve the quality and efficiency of ICU care. In order to contain US health care costs, it has been suggested that reducing critical care bed supply would result in more efficient admission decisions and cost savings with minimal mortality decrements, particularly in certain possibly “oversupplied” regions of the country.3,40 This assertion presumes that ICU admission for more discretionary patients provides minimal benefit but substantially increases costs. The findings of this study conflict with such assertions and suggest that greater rates of ICU admissions for patients with pneumonia may not only improve survival, but might do so without significantly increasing hospital costs. Indeed, if replicated by others, these results could motivate a trial of increased access to ICU (or ICU-like) care for patients with pneumonia who might otherwise be cared for on the ward.
Conclusions
Among Medicare beneficiaries hospitalized with pneumonia, ICU admission of patients for whom the decision appeared to be discretionary was associated with improved survival and no significant difference in costs. A randomized trial may be warranted to assess whether more liberal ICU admission policies improve mortality for patients with pneumonia.
Acknowledgments
Funding/Support: This work was supported by grant T32HL007749 from the National Institutes of Health (Drs Valley and Sjoding), grant 11–109 from the Department of Veterans Affairs Health Services Research and Development Service (Dr Iwashyna), and grant K08HS020672 from Agency for Healthcare Research and Quality (Dr Cooke).
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Valley had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Valley, Ryan, Cooke.
Acquisition, analysis, or interpretation of data: Valley, Sjoding, Ryan, Iwashyna, Cooke.
Drafting of the manuscript: Valley, Cooke.
Critical revision of the manuscript for important intellectual content: Valley, Sjoding, Ryan, Iwashyna, Cooke.
Statistical analysis: Valley, Sjoding, Ryan, Iwashyna.
Obtained funding: Cooke.
Administrative, technical, or material support: Cooke.
Study supervision: Cooke.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: This article does not necessarily represent the view of the US government or the Department of Veterans Affairs.
REFERENCES
- 1.Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005. Crit Care Med. 2010;38(1):65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 2.Wunsch H. Is there a Starling curve for intensive care? Chest. 2012;141(6):1393–1399. doi: 10.1378/chest.11-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooch RA, Kahn JM. ICU bed supply, utilization, and health care spending. JAMA. 2014;311(6):567–568. doi: 10.1001/jama.2013.283800. [DOI] [PubMed] [Google Scholar]
- 4.Escher M, Perneger TV, Chevrolet JC. National questionnaire survey on what influences doctors’ decisions about admission to intensive care. BMJ. 2004;329(7463):425. doi: 10.1136/bmj.329.7463.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Admon AJ, Seymour CW, Gershengorn HB, Wunsch H, Cooke CR. Hospital-level variation in ICU admission and critical care procedures for patients hospitalized for pulmonary embolism. Chest. 2014;146(6):1452–1461. doi: 10.1378/chest.14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershengorn HB, Iwashyna TJ, Cooke CR, Scales DC, Kahn JM, Wunsch H. Variation in use of intensive care for adults with diabetic ketoacidosis. Crit Care Med. 2012;40(7):2009–2015. doi: 10.1097/CCM.0b013e31824e9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safavi KC, Dharmarajan K, Kim N, et al. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation. 2013;127(8):923–929. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun HK, Nicolau DP, Kuti JL. Resource utilization of adults admitted to a large urban hospital with community-acquired pneumonia caused by Streptococcus pneumoniae. Chest. 2006;130(3):807–814. doi: 10.1378/chest.130.3.807. [DOI] [PubMed] [Google Scholar]
- 9.Torio CM, Ph D, Andrews RM. National inpatient hospital costs. [Accessed August 25, 2015]; https://www.hcup-us.ahrq.gov/reports/statbriefs/sb160.jsp.
- 10.Lindenauer PK, Lagu T, Shieh M-S, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–1413. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 11.Rothberg MB, Pekow PS, Priya A, Lindenauer PK. Variation in diagnostic coding of patients with pneumonia and its association with hospital risk-standardized mortality rates. Ann Intern Med. 2014;160(6):380–388. doi: 10.7326/M13-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjoding MW, Iwashyna TJ, Dimick JB, Cooke CR. Gaming hospital-level pneumonia 30-day mortality and readmission measures by legitimate changes to diagnostic coding. Crit Care Med. 2015;43(5):989–995. doi: 10.1097/CCM.0000000000000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 14.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Landon BE, Normand S-LT, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511–2517. doi: 10.1001/archinte.166.22.2511. [DOI] [PubMed] [Google Scholar]
- 17.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? JAMA. 1994;272(11):859–866. [PubMed] [Google Scholar]
- 18.Garabedian LF, Chu P, Toh S, Zaslavsky AM, Soumerai SB. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann Intern Med. 2014;161(2):131–138. doi: 10.7326/M13-1887. [DOI] [PubMed] [Google Scholar]
- 19.Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33(13):2297–2340. doi: 10.1002/sim.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rassen JA, Brookhart MA, Glynn RJ, Mittleman MA, Schneeweiss S. Instrumental variables II. J Clin Epidemiol. 2009;62(12):1233–1241. doi: 10.1016/j.jclinepi.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newhouse JP, McClellan M. Econometrics in outcomes research. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Kahn JM, Ten Have TR, Iwashyna TJ. The relationship between hospital volume and mortality in mechanical ventilation. Health Serv Res. 2009;44(3):862–879. doi: 10.1111/j.1475-6773.2009.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris KM, Remler DK. Who is the marginal patient? Health Serv Res. 1998;33(5 Pt 1):1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 24.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358(8):771–783. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 25.Pirracchio R, Sprung C, Payen D, Chevret S. Benefits of ICU admission in critically ill patients. BMC Med Res Methodol. 2011;11(1):132. doi: 10.1186/1471-2288-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram DD, Franco SJ. 2013 NCHS urban-rural classification scheme for counties. Vital Health Stat 2. 2014;(166):1–73. [PubMed] [Google Scholar]
- 27.Gabler NB, Ratcliffe SJ, Wagner J, et al. Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med. 2013;188(7):800–806. doi: 10.1164/rccm.201304-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly DM, Kutney-Lee A, McHugh MD, Sloane DM, Aiken LH. Impact of critical care nursing on 30-day mortality of mechanically ventilated older adults. Crit Care Med. 2014;42(5):1089–1095. doi: 10.1097/CCM.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sales A, Sharp N, Li Y-F, et al. The association between nursing factors and patient mortality in the Veterans Health Administration. Med Care. 2008;46(9):938–945. doi: 10.1097/MLR.0b013e3181791a0a. [DOI] [PubMed] [Google Scholar]
- 30.Restrepo MI, Mortensen EM, Rello J, Brody J, Anzueto A. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010;137(3):552–557. doi: 10.1378/chest.09-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Ramar K, Park JG, Gajic O, Li G, Kashyap R. Increased fluid administration in the first 3 hours of sepsis resuscitation is associated with reduced mortality. Chest. 2014;146(4):908–915. doi: 10.1378/chest.13-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin HC, Xirasagar S, Chen CH, Hwang YT. Physician’s case volume of intensive care unit pneumonia admissions and in-hospital mortality. Am J Respir Crit Care Med. 2008;177(9):989–994. doi: 10.1164/rccm.200706-813OC. [DOI] [PubMed] [Google Scholar]
- 33.Merchant S, Mullins CD, Shih Y-CT. Factors associated with hospitalization costs for patients with community-acquired pneumonia. Clin Ther. 2003;25(2):593–610. doi: 10.1016/s0149-2918(03)80099-1. [DOI] [PubMed] [Google Scholar]
- 34.Oster G, Berger A, Edelsberg J, Weber DJ. Initial treatment failure in non-ICU community–acquired pneumonia. J Med Econ. 2013;16(6):809–819. doi: 10.3111/13696998.2013.794805. [DOI] [PubMed] [Google Scholar]
- 35.Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, Cooke CR. Hospitals with the highest intensive care utilization provide lower-quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186. doi: 10.1097/CCM.0000000000000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahn JM. What’s new in ICU volume-outcome relationships? Intensive Care Med. 2013;39(9):1635–1637. doi: 10.1007/s00134-013-2992-y. [DOI] [PubMed] [Google Scholar]
- 37.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Ann Intern Med. 2003;138(4):288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 38.Sjoding MW, Luo K, Miller MA, Iwashyna TJ. When do confounding by indication and inadequate risk adjustment bias critical care studies? Crit Care. 2015;19(1):195. doi: 10.1186/s13054-015-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahn JM. Understanding economic outcomes in critical care. Curr Opin Crit Care. 2006;12(5):399–404. doi: 10.1097/01.ccx.0000244117.08753.38. [DOI] [PubMed] [Google Scholar]
- 40.Wallace DJ, Angus DC, Seymour CW, Barnato AE, Kahn JM. Critical care bed growth in the United States. Am J Respir Crit Care Med. 2015;191(4):410–416. doi: 10.1164/rccm.201409-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]