Abstract
BACKGROUND
Household air pollution (HAP) resulting from the use of solid cooking fuels is a leading contributor to the burden of disease in India. Advanced combustion cookstoves that reduce emissions from biomass fuels have been considered potential interventions to reduce this burden. Relatively little effort has been directed, however, to assessing the concentration and exposure changes associated with the introduction of such devices in households.
OBJECTIVES
The aim of this study was to describe HAP exposure patterns in pregnant women receiving a forced-draft advanced combustion cookstove (Philips model HD 4012) in the SOMAARTH Demographic Development & Environmental Surveillance Site (DDESS) Palwal District, Haryana, India. The monitoring was performed as part of a feasibility study to inform a potential large-scale HAP intervention (Newborn Stove trial) directed at pregnant women and newborns.
METHODS
This was a paired comparison exercise study with measurements of 24-hour personal exposures and kitchen area concentrations of carbon monoxide (CO) and particulate matter less than 2.5 μm in aerodynamic diameter (PM2.5), before and after the cookstove intervention. Women (N = 65) were recruited from 4 villages of SOMAARTH DDESS. Measurements were performed between December 2011 and March 2013. Ambient measurements of PM2.5 were also performed throughout the study period.
FINDINGS
Measurements showed modest improvements in 24-hour average concentrations and exposures for PM2.5 and CO (ranging from 16% to 57%) with the use of the new stoves. Only those for CO showed statistically significant reductions.
CONCLUSION
Results from the present study did not support the widespread use of this type of stove in this population as a means to reliably provide health-relevant reductions in HAP exposures for pregnant women compared with open biomass cookstoves. The feasibility assessment identified multiple factors related to user requirements and scale of adoption within communities that affect the field efficacy of advanced combustion cookstoves as well as their potential performance in HAP intervention studies.
Keywords: biomass fuel, carbon monoxide, Haryana, indoor air pollution, Newborn Stove Trial, Philips gasifier stove, PM2.5
INTRODUCTION
Household air pollution (HAP) resulting from the use of solid cooking fuels is a leading contributor to the burden of disease in India, accounting for about 1 million premature deaths and approximately 31 million disability-adjusted life years annually, approximately 6% of the national burden of disease.1,2 Nearly 74% of India’s population continues to rely on solid fuels (such as biomass, dung, and coal) for their everyday household energy needs3, experiencing HAP exposures greatly in excess of the current World Health Organization air quality guideline (WHO-AQG) values.4,5 Additional environmental effects from biomass fuel use include black carbon emissions,6,7 unsustainable harvesting of fuelwood,8 and regional air pollution.9 The emissions, exposures, and disease burden estimates together argue for strenuous and targeted intervention efforts to address HAP in India.
The launch of the National Biomass Cookstove Initiative by the Ministry of New and Renewable Energy, Government of India represents an important step in this direction.10,11 The initiative has catalyzed the availability of a newer generation of “advanced combustion” biomass cookstoves (ACS) that meet the more stringent cookstove emission standards developed in 2013 by the Bureau of Indian Standards. Results from laboratory emissions testing for the newer ACS have been reported and have shown reductions ranging from 50% to 90% in emissions of particulate matter less than 2.5 μm in aerodynamic diameter (PM2.5) and carbon monoxide (CO).12-15 More recent studies also are beginning to provide an understanding of the determinants of community-level uptake and adoption of ACS.16-18
However, relatively little effort has been directed at assessing the concentration and exposure changes associated with the introduction of a new ACS. Results from field measurements in households using commercially available ACS models recently were reported from the states of Uttar Pradesh, Tamil Nadu, and Maharashtra.19,20 Reported reductions in 24-hour kitchen concentrations of PM2.5 and CO ranged from 2% to 71% and 10% to 66%, respectively compared with traditional cookstoves. Even with these reductions, however, resulting exposures and concentrations exceeded values recommended by the WHO-AQGs. Continued use of traditional stoves, infiltration of ambient air pollution, and perhaps other factors, appear to attenuate the reductions that are achieved by the ACSs within households, even when they perform as measured in the laboratory.
Laboratory testing of the Philips (Model HD 4012) forced-draft gasifier stoves consistently shows them to be among the best from an emissions standpoint.21 User acceptance and sustained adoption, however, has been less consistent.19,20,22-24 Additional field evaluations of the Philips stove, including personal and ambient monitoring are needed. Evidence from these evaluations could both improve the design of community-based intervention trials as well as inform the potential for intervention effectiveness in programs deploying ACS.
In this study, we describe the results of personal exposure and household and ambient monitoring after the dissemination of Philips ACS within the International Clinical Epidemiological Network (INCLEN) SOMAARTH Demographic Development & Environmental Surveillance Site (DDESS) located in Palwal, Haryana. These HAP monitoring results are part of a feasibility study to inform a potential large-scale HAP intervention (the NBS [National Newborn Stove] trial) directed at pregnant women and newborns. The details of the feasibility study and results from stove-use monitoring were published previously.23,24 We also discuss the broader implications of study results for intervention effectiveness in the context of national and global initiatives to address HAP.
The study described here was jointly undertaken by teams from Sri Ramachandra University (SRU), Chennai, the INCLEN Trust International, University of California, Berkeley, and Columbia University.
METHODS
Study protocols were jointly developed by investigators from collaborating institutions and approved by the Health Ministry Screening Committee of the government of India and the Institutional Ethics committee of SRU and the INCLEN Trust International.
Study Location and Participant Recruitment
The study was conducted between December 2011 and March 2013 in 4 villages (Manpur, Gahlab, Banchari, Rahrana) that are within the INCLEN SOMAARTH DDESS. The surveillance site had a population of approximately 200,000 in 51 villages across 3 administrative blocks of Palwal district, encompassing 308 km2. The site is supported by an extensive field infrastructure for research and surveillance activities pertaining to assessment of environmental and nutritional risk factors for children’s health and noncommunicable diseases in adults.
As part of the feasibility study for the NBS trial, the INCLEN team recruited 200 pregnant women who each received the Philips HD 4012 ACS after pilot testing the stove for user acceptability.23 Subsequently, the INCLEN team distributed the Philips stove through the network of trained female community accredited social health activists working at the SOMAARTH site. Eligibility for recruitment was based on the use of biomass as the primary household fuel and being less than 15 weeks pregnant at the time of recruitment into the study. Air pollution monitoring was planned in a subset (~25%; n = 50) of these households. Participants were enrolled after securing additional informed consents for air sampling.
The study was designed as a paired before–after comparison for detecting a desired level of improvement in HAP (ie, percent reduction between groups). Sample-size calculations were based on estimates of the expected differences between mean concentrations and the paired coefficient of variation for sample-size calculations as found in previous studies conducted.20,25 Because some monitoring protocols (ie, personal sampling for PM) could not be performed on pregnant women due to local cultural preferences, the INCLEN team identified additional households from the same villages from which nonpregnant women in similar households were recruited for air sampling. Fifty pregnant women and 15 nonpregnant women were enrolled for air sampling after securing additional informed consents for household and personal monitoring. Training on use and maintenance of the stove was provided to each study participant by the field staff of the INCLEN team. Although participants were asked not to use the traditional stove during the intervention-phase monitoring days, many households followed their usual cooking routines. Households were not specifically requested to ensure similar cooking behavior or meal type between phases.
Air Pollution Measurements
Sampling plan
We designed a sampling strategy to both assess the exposure reductions accompanying the use of the Philips ACS for pregnant women and to inform the feasibility of performing such exposure measurements in a larger cluster-randomized intervention trial. Because standard sampling instrumentation for PM2.5 is bulky, noisy, and inconvenient to wear, personal exposures for pregnant women were limited to CO measurements. Correlations between personal PM2.5 and CO exposures were assessed through personal exposure measurements on non-pregnant women together with area and ambient measurements to estimate the correlations with personal exposures.
Exposure was assessed over a single 24-hour period at baseline and then after the stove was installed. Paired baseline and postintervention phase measurements of personal CO exposures and ambient measurements of PM2.5 were performed in both winter (December-February) and summer (June-July) seasons, whereas other paired measurements were performed in one or both seasons depending on equipment availability and field logistics. Winter measurements were performed within 1 to 3 months of stove distribution; summer measurements were performed 9 to 12 months after stove distribution.
Air sampling methods
Area concentrations and personal exposures for PM2.5 were measured as integrated 24-hour samples, collected using low-volume air sampling pumps (supplied by SKC Inc., or Casella Measurement Inc.). Pumps were operated at a flow rate of 1.5 L/minute. PM2.5 was collected on 37-mm Teflon™ filters (Pall Corporation, Port Washington, NY, USA), backed with cellulose support pads placed in a filter cassette connected to a BGI cyclone (BGI Inc., Waltham, MA, USA). Using a laboratory-calibrated rotameter, flow rates were measured before and after initiation of the sampling in the field. Filters were weighed before and after sampling, using an electronic microbalance with a sensitivity of ±1 μg (supplied by Sartorius Inc.) in a temperature- and humidity-controlled room at SRU. Filters were conditioned for 24 hours before to weighing. Twenty percent of the gravimetric samples were paired with field blanks (n = 13); none of the pre- and post-field blank weights differed by more than 0.003 mg.
Kitchen area PM2.5 concentrations also were assessed continuously using the UCB-PATS (University of California Berkeley Particle and Temperature Sensor, Berkeley Air Monitoring Group; Berkeley, CA, USA) as described previously.20,26,27 Briefly, monitors were calibrated with combustion aerosols (eg, wood and charcoal) and against temperature in the laboratory before being used in the field. Particle coefficients were derived for each instrument in the field through colocation of UCB-PATs monitors and gravimetric samplers. All UCB-PATs were zeroed in a Ziploc bag for a period of 30 to 60 minutes before and after deployment. Particle and temperature coefficients, along with the results from zeroing, were subsequently used in the data-processing algorithm. After monitoring, all data files were batch-processed using a customized software package developed for this device. UCB measurements were performed primarily to allow estimation of the ratio of cooking to noncooking period concentrations of PM2.5.
CO concentrations and exposures were measured using the portable, battery-operated, data-logging Drager Pac 7000 (SKC, Inc; Eighty Four, PA, USA) instrument, that was calibrated with span gas as per manufacturer specifications. The Pac 7000 recorded and logged the peak concentration that occurred within each minute during the monitoring period.
For kitchen area measurements, PM2.5 and CO monitors were placed 1.5 m above the ground and 1 m from the primary stove. For personal exposure measurements, CO and PM monitors were attached to the participant’s clothing with the inlets close to her breathing zone. Women were asked to place the personal monitor next to them when sleeping and bathing.
Ambient measurements for PM2.5 were made using a MiniVol™ sampler placed in the center of a cluster of households, usually on a household roof top, at about 8 to 10 m height from the ground. Samplers were operated at a flow rate of 5 L/minute. Air was drawn through size selective PM2.5 impactors. PM2.5 was collected on 47-mm Teflon™ filters (Pall Corporation) and weighed in a microbalance following the same procedures used for area and personal samples. Six (20%) samples were paired with field blanks.
The full range of measurements thus included the following:
Paired 24-hour personal exposure measurements of CO for 50 pregnant women;
Paired 24-hour personal exposure measurements of PM2.5 in 15 nonpregnant women;
Paired 24-hour kitchen area measurements of PM2.5 and CO in 22 and 3 households, respectively; and
-
28 measurements of 24- to 72-hour ambient PM2.5
in 4 villages.
Questionnaire Administration
A postmonitoring questionnaire was administered to all participants by the research field staff immediately after completion of 24-hour monitoring. The questionnaire was printed in Hindi and English, but administered in Hindi. Time-activity recalls and information related to cooking activities (such as quantity and types of food prepared; quantity and types of fuel used; number of meals prepared in a day; cooking duration and ventilation) were collected. Information on other sources of HAP such as use of kerosene lamps, cigarettes, and incense was also recorded.
Statistical Analysis
All data analysis was performed using R (Version 3.02). Baseline and postintervention measurements were compared using 2-tailed nonparametric tests (Wilcoxon signed-rank test) to accommodate both positive and negative percent changes.
RESULTS
Household Characteristics
General characteristics of study households are summarized in Table 1. Biomass (a mixture of dung and wood) was the primary fuel used on account of the inclusion criteria specified. Except for 2 households that reported using liquid petroleum gas or electricity, virtually no other additional fuels were reported as used (during times that monitoring was performed). Of the households in the study, 96% cooked in outdoor kitchens (open on 3 sides, often with a short wall or a sheet of insulating material shielding the stove on the back). This feature had important implications for the exposure measurement results as explained later. Households typically cooked a meal that included breads (roti), vegetables (sabzi), lentils (dal), and rice. On average, households (median size >4) cooked 2 meals daily, spending around 3.5 hours daily on cooking per day. Fieldworkers observed that women spend much of this time near the stove, preparing rotis. Animal fodder and milk were simmered on “haroo” stoves that burned only dung cakes and were usually operational outdoors throughout the day (generally within 5 to 10 m of the kitchen).
Table 1.
General characteristics of participant households*
| Variable | Description | N (%) |
|---|---|---|
| Kitchen type | Indoor kitchen with partition |
1 (2) |
| Separate outdoor kitchen |
1 (2) | |
| Open-air kitchen | 48 (96) | |
| Fuel type | Biomass (dung + wood) |
49 (98) |
| Biomass + LPG/electricity |
1 (2) | |
| Doors in living room | 1 | 21 (42) |
| >1 | 29 (68) | |
| Windows in living room | 0 | 16 (32) |
| 1 | 20 (40) | |
| >1ne | 14 (28) | |
| Ventilator/open eve in living room |
Yes | 17 (34) |
| No | 33 (66) | |
| Wall material of living room |
Pucca | 44 (88) |
| Kutcha | 6 (12) | |
| Roof material of living room |
Concrete | 14 (28) |
| Nonconcrete | 36 (72) | |
| Floor material of living room |
Concrete | 34 (68) |
| Clay | 16 (32) | |
| Number of family members |
≤4 | 8 (16.3) |
| >4 | 41 (83.7) | |
| Quantity of fuel used kg/d |
≤2 | 39 (78) |
| >2 | 11 (22) |
LPG, liquefied petroleum gas.
Collected from enrolled pregnant women during the baseline phase of the study. Pucca houses are defined as those built using bricks, stones (packed with lime or cement), cement concrete, timber as wall materials and tiles, sheets (galvanized corrugated iron, asbestos cement, reinforced brick concrete and reinforced cement concrete). Kutcha houses have walls and/or roof made of material other than those just mentioned, such as unburnt bricks, bamboo, mud, grass, reeds, thatch, and loosely packed stones.
Comparison of Personal Exposures and Area Concentrations in Baseline and Intervention Phases
Table 2 and Figures 1 and 2 present results from personal exposure and area measurements for CO and PM2.5 recorded during the baseline and postintervention phases. As personal sampling for PM2.5 was not feasible on pregnant women, these measurements were performed only on nonpregnant women residing in nearby households from the same villages. The small sample sizes for other measurements on nonpregnant women, however, precluded reliable differential comparisons between household measurements for pregnant and non-pregnant women and hence the results are reported together. Valid paired measurements for personal CO exposures were obtained from 51 women; from 8 women for personal PM 2.5 exposures, from 3 households for kitchen CO concentrations, and from 22 households for kitchen PM 2.5 concentrations. Measurements could not be equally distributed across locations or seasons on account of equipment unavailability.
Table 2.
Distribution of 24-h personal exposures and area concentrations for PM2.5 and CO during baseline and postintervention
| Season of monitoring |
Monitoring period | N | Median | Mean (SD) | % change in median |
% change in mean |
P value (Wilcoxon signed-rank test*) |
|---|---|---|---|---|---|---|---|
| Personal CO exposure (ppm) | |||||||
| Summer | Baseline | 17 | 1.9 | 4.6 (7.2) | −6 | 16 | 0.89 |
| Postintervention | 17 | 2.1 | 3.9 (8.4) | ||||
| Winter | Baseline | 34 | 3.1 | 6.2 (7.7) | 45 | 57 | 0.001 |
| Postintervention | 34 | 1.7 | 2.7 (3.1) | ||||
| Pooled | Baseline | 51 | 2.8 | 5.7 (7.5) | 37 | 46 | 0.009 |
| Postintervention | 51 | 1.8 | 3.1 (5.4) | ||||
| Personal PM2.5 exposure (μg/m3) | |||||||
| Winter | Baseline | 8 | 148 | 184 (168) | −11 | −13 | 0.844 |
| Postintervention | 8 | 165 | 207 (172) | ||||
| Kitchen area CO concentration (ppm) | |||||||
| Summer | Baseline | 3 | 8 | 9.1 (4.7) | 54 | 58 | 0.25 |
| Postintervention | 3 | 3.7 | 3.9 (0.8) | ||||
| Kitchen area PM2.5 concentration (μg/m3) | |||||||
| Summer | Baseline | 11 | 46 | 92 (71) | −154 | −130 | 0.054 |
| Postintervention | 11 | 117 | 213 (237) | ||||
| Winter | Baseline | 11 | 372 | 670 (767) | 37 | 43 | 0.365 |
| Postintervention | 11 | 235 | 381 (352) | ||||
| Pooled | Baseline | 22 | 161 | 381 (608) | −16 | 22 | 0.750 |
| Postintervention | 22 | 186 | 297 (305) | ||||
PM concentrations reported in this table were measured using gravimetric samplers. The data include measurements from pregnant and nonpregnant women. The pool for personal CO measurements included 46 pregnant and 5 nonpregnant women, whereas the pool for PM2.5 measurements included only nonpregnant women.
CO, carbon monoxide; PM, particulate matter.
For paired comparison of median reductions.
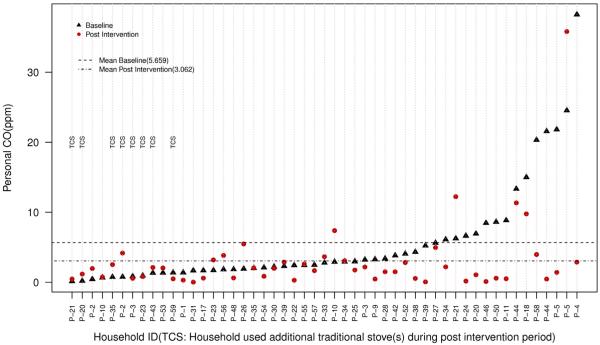
Figure 1.
Paired comparisons of personal CO exposures for pregnant (n = 46) and nonpregnant (n = 5) women across baseline and postintervention phases. CO, carbon monoxide; TCS, traditional cookstove.
In the baseline phase, the mean 24-hour personal exposures for PM2.5 and CO ranged from 72 to 297 μg/m3 and 3.9 to 7.5 ppm, respectively, whereas the mean 24-hour kitchen area concentrations for PM2.5 and CO ranged from 158 to 600 μg/m3 and 1.2 to 17 ppm, respectively. In the postintervention phase the mean 24-hour personal exposures for PM2.5 and CO ranged from 92 to 323 μg/m3 and 1.7 to 4.3 ppm, respectively, with the corresponding mean 24-hour kitchen area concentrations for PM2.5 and CO ranging from 185 to 410 μg/m3 and 2.5 to 5.2 ppm, respectively.
Reductions in paired mean 24-hour personal CO exposures ranged from 16% to 57% with a statistically significant reduction in median concentrations of 37% in the postintervention phase (P < 0.05). The changes in median 24-hour PM2.5 exposures and 24-hour kitchen area concentrations of CO and PM2.5 were not statistically significant (with several households/participants recording increases from baseline to postintervention phase measurements).
Although households were requested to refrain from using additional traditional cookstoves including the haroo during the postintervention monitoring period, some households reported using additional stoves (information on which was collected through the postmonitoring questionnaire). Comparison of reductions in paired measurements after exclusion of these households (n = 15), however, did not affect the observed changes significantly (Table 3).
Table 3.
Distribution of 24-h personal exposures and area concentrations for PM2.5 and CO during baseline and postintervention phases*
| Season of monitoring |
Monitoring period | N | Median | Mean (SD) | % change in median |
% change in mean |
P value (Wilcoxon signed-rank†) |
|---|---|---|---|---|---|---|---|
| Personal CO exposure (ppm) | |||||||
| Summer | Baseline | 13 | 1.7 | 2.2 (1.8) | 55 | 28 | 0.542 |
| Postintervention | 13 | 0.8 | 1.6 (1.4) | ||||
| Winter | Baseline | 30 | 2.9 | 5.4 (7.3) | 49 | 61 | 0.002 |
| Postintervention | 30 | 1.5 | 2.1 (2.6) | ||||
| Pooled | Baseline | 43 | 2.8 | 4.4 (6.3) | 46 | 56 | 0.002 |
| Postintervention | 43 | 1.5 | 1.9 (2.3) | ||||
| Personal PM2.5 exposure (mg/m3) | |||||||
| Winter | Baseline | 7 | 99 | 133 (96) | −48 | −54 | 0.469 |
| Postintervention | 7 | 147 | 206 (186) | ||||
| Kitchen area CO concentration (ppm) | |||||||
| Summer | Baseline | 2 | 9.7 | 9.7 (6.5) | 56 | 56 | 0.500 |
| Postintervention | 2 | 4.2 | 4.2 (0.7) | ||||
| Kitchen area PM2.5 concentration (μg/m3) | |||||||
| Summer | Baseline | 7 | 45 | 66 (52) | −82 | −32 | 0.688 |
| Postintervention | 7 | 82 | 87 (58) | ||||
| Winter | Baseline | 10 | 325 | 489 (503) | 37 | 23 | 0.625 |
| Postintervention | 10 | 206 | 374 (371) | ||||
| Pooled | Baseline | 17 | 174 | 315 (435) | 33 | 19 | 0.747 |
| Postintervention | 17 | 117 | 256 (316) | ||||
PM concentrations reported in this table were measured using gravimetric samplers. The data include measurements from pregnant and nonpregnant women. The pool for personal CO measurements reported in this table included only pregnant women, whereas the pool for PM2.5 measurements included only nonpregnant women.
CO, carbon monoxide; PM, particulate matter.
Excludes households reporting the use of an additional traditional cookstove during the postintervention phase.
For paired comparisons of median reductions.
Comparison of Real-time Concentrations of PM2.5 and CO During Cooking Periods Between Baseline and Intervention Phases
Previous studies have shown that multiple factors affect measured 24-hour concentrations and exposures, including the number of meals cooked, cooking duration, type of meal, type of fuel, ventilation parameters, and contributions from ambient concentrations.20 Although it was not feasible to control for these variables across phases, we compared paired cooking-period concentrations (Table 4), as these are more likely to be influenced by direct emissions from the stove. For PM measurements, this was possible only for households monitored using the real-time UCB-PATS monitors. The cooking period comparisons (Table 4) resulted in greater reductions being observed across baseline and postintervention phases, although (similar to 24-hour measurements) only reductions in CO personal exposures were statistically significant.
Table 4.
Distribution of cooking period personal exposures and area concentrations for PM2.5 and CO during baseline and postintervention phases
| Season of monitoring |
Monitoring period | N | Median | Mean (SD) | % change in median |
% change in mean |
P value (Wilcoxon signed-rank test*) |
|---|---|---|---|---|---|---|---|
| Personal CO exposure (ppm) | |||||||
| Summer | Baseline | 17 | 13.4 | 22.9 (25.5) | 9 | 30 | 0.329 |
| Postintervention | 17 | 12.2 | 16.1 (16.4) | ||||
| Winter | Baseline | 32 | 13.7 | 25.5 (26.7) | 46 | 62 | <0.001 |
| Postintervention | 32 | 7.4 | 9.6 (8.1) | ||||
| Pooled | Baseline | 49 | 13.4 | 24.6 (26) | 27 | 52 | <0.001 |
| Postintervention | 49 | 9.8 | 11.9 (11.9) | ||||
| Kitchen area CO concentration (ppm) | |||||||
| Summer | Baseline | 3 | 57.6 | 56.2 (13.8) | 65 | 62 | 0.250 |
| Postintervention | 3 | 20 | 21.5 (4.8) | ||||
| Kitchen area PM2.5 concentration (mg/m3) | |||||||
| Summer | Baseline | 11 | 689 | 1090 (1130) | e2 | −11 | 0.898 |
| Postintervention | 11 | 702 | 1206 (1201) | ||||
| Winter | Baseline | 9 | 2801 | 3092 (2451) | 60 | 17 | 0.426 |
| Postintervention | 9 | 1117 | 2555 (3770) | ||||
| Pooled | Baseline | 20 | 1451 | 1991 (2060) | 28 | 9 | 0.546 |
| Postintervention | 20 | 1046 | 1813 (2686) | ||||
PM concentrations reported in this table were measured using the UCB-PATS monitors. The data include measurements from pregnant and nonpregnant women. The pool for personal CO measurements reported in this table included 46 pregnant and 3 nonpregnant women, whereas the pool for PM2.5 measurements included only nonpregnant women
CO, carbon monoxide; PM, particulate matter; UCB-PATS, University of California-Berkeley Particle and Temperature Sensor.
For paired comparisons of median reductions.
Addressing Contributions of Seasonality Across Baseline and Intervention Phases
Because the field site was located in an area subject to temperature inversions in winter, considerable seasonal variations could be expected in background ambient air pollution levels. We addressed this through a limited set of 24- to 72-hour ambient measurements of PM2.5 performed using MiniVol® samplers. The levels in winter (n = 17; median: 175μg/m3; mean ± SD 177 [50] μg/m3) mean were nearly twice as high as recorded in summer (n = 11; median: 69μg/m3; mean ± SD 75 [22] μg/m3), indicating the potential for differential contributions to area concentrations and personal exposures across seasons.
DISCUSSION
In each season, measurements showed inconsistent improvements in 24-hour average concentrations and exposures for PM2.5 and CO with the use of the Philips stoves, and only those for CO showed statistically significant reductions. There was, however, considerable heterogeneity in the reductions obtained across households under conditions of actual use. Furthermore, the PM2.5 concentrations/exposures recorded in the postintervention phase consistently exceeded the highest of the recommended by the WHO-AQGs (ie, WHO interim target-1 (IT-1) values of 35 μg/m3 and 75μg/m3, respectively for annual mean and 24-hour concentrations for PM2.5) and frequently exceeded the 24-hour guideline value for CO of 6 ppm. We explored the reasons for the observed variability under actual field conditions and the implications of such variability for long-term exposure and elaborate here on the likely health relevance of exposure reductions achieved by the Philips model used in the study.
Variability in Household Concentrations and Personal Exposures: Role of Household-level Determinants and Ambient Concentrations
In pregnant women, reduction in the median 24-hour personal exposure and cooking period exposure to CO following the use of the Philips stove was found to be statistically significant. Reductions in kitchen concentrations of CO and PM2.5 were, however, not statistically significant and neither were personal exposures to CO and PM2.5 in nonpregnant women.
Emissions testing of the Philips stove demonstrated a greater than 90% reduction in emissions of PM2.5 and CO under controlled laboratory conditions.14 Several observations offer plausible explanations for the observed modest exposure reductions in the field.
Households reported requiring multiple stoves to satisfy household cooking needs. Fifteen (23%) of the 65 households reported using traditional stoves along with the Philips stoves during the post-intervention phases of monitoring. This was supported by quantitative measurements of stove use.24 Excluding households that simultaneously used the traditional and Philips cookstoves during the postintervention measurement phase limited the sample size for reliable comparisons.
The location of the kitchen in this setting may have limited the exposure reduction potential of a household-level cookstove intervention. Most women cooked in semienclosed outdoor spaces within their household compounds. With less than 5% of households within the village targeted with the ACS, neighborhood contributions to background ambient concentrations are likely to have influenced both kitchen area and personal exposure estimates in all households. This coupled with the observations from stove-use monitor measurements (that suggest only modest levels of displacement of traditional stoves within households that received the intervention), make it difficult to detect a consistent signal for exposure reduction within this setting. The observed reductions in area and personal exposure concentrations may not thus be representative of the potential exposure reductions achievable with the Philips stove in other populations. Use in communities with predominantly indoor kitchens and/or replacement of a high enough percentage of traditional stoves with cleaner stoves may produce substantial exposure reductions.
Ambient PM2.5 concentrations were significantly higher in winter than in summer. This necessitated making same-season-paired measurements to prevent biases introduced by contributions from background concentrations across the baseline and post-intervention phases. Some of the same-season-paired measurements were performed a full year after the installation of the ACS (ie, in the same season). Independent assessments by the INCLEN team (results not shown) and stove-use monitoring results have recorded households experiencing considerable difficulties in maintaining the Philips stove for a full year. Thus, although same-season-paired measurements were made, it may not have represented the optimal comparison for such a measurement, given stove failures.
The women in these villages commonly used dung as a major fuel. Because dung is a low energy-density fuel, women reported having to refuel the ACS more often, potentially leading to additional exposures during lighting as well as some inconvenience. This can tempt women to overfill the ACS combustion chamber with dung fuel, leading to higher emissions and exposures than would be the case with optimal fueling.28
Finally, 16 (25%) of the women reported staying indoors during the monitoring period on account of having to wear the personal monitoring equipment. Without serial time-activity records to assess their behavior, it was not possible to discern how this shift in behavior may have affected exposure estimates.
Implications of User Acceptability for Long-term Exposure Reductions
In studies after our choice of the Philips because of its good laboratory performance, user acceptance and sustained adoption have been less consistent.17,19,20,23,24 One factor was that the model we used was available only in a single-pot configuration. The need for an additional cooking pot often was reported and thus resulted in concurrent traditional stove use to fulfill specific meal requirements (stacking). The current configuration of the Philips stove model, therefore, appears to be poorly suited for exclusive use by households in the study area.20 Although emissions reductions are a prerequisite for achieving reductions in exposures, without appropriate levels of user acceptance and reduction in use of the traditional stove, any gains observed in the laboratory may be lost under actual field-use conditions.
Health Relevance of Observed Reductions in PM2.5 Concentrations
The present study was designed to assess how close an ACS would lower PM2.5 exposures in relation to annual average WHO-IT-1 values for PM2.5 of 35 μg/m3. Recent progress in the development of integrated exposure response curves29 over a continuous range of PM2.5 concentrations in relation to ambient air pollution, HAP, and passive and active tobacco smoking have allowed comparisons of excess risks for a range of health endpoints, including ischemic heart disease, stroke, chronic obstructive pulmonary disease, lung cancer, and child acute lower respiratory infections (ALRIs). The exposure-response functions for all of these except lung cancer are strikingly nonlinear, with the most discernible risk reductions beginning to appear well below 100 μg/m3 for ALRI.2 Here, as shown in Table 2, mean exposures for the female cooks both before and after stove introduction were well above 100 μg/m3. Although, there are as yet no accepted exposure-response functions for birth outcomes, the apparent inability of the stove to bring exposures of women below 100 μg/m3 was disappointing.
Although, measurements of personal exposures to PM2.5 were limited, they were significantly (P < 0.05) correlated with kitchen area concentrations (r = 0.65). With the mean 24-hour kitchen area concentrations ranging from to 150 to 650 μg/m3 and limited evidence of sustained use of the Philips stove in this population, the results from this study indicated that the stove is unlikely to accomplish health-relevant exposure reductions under current conditions of field use and expected level of adoption within a community.
Study Limitations
Considerable efforts were devoted to planning the air sampling strategy to adequately capture the exposure reductions accompanying the use of the ACS for pregnant women. However, field constraints related to both personal and ambient monitoring precluded several planned measurements, thus posing limitations for generalizability of the conclusions.
Personal monitoring for PM2.5 was limited by the reluctance of both pregnant and nonpregnant women to wear the UCB-PATs monitor. The available gravimetric samplers could not be worn by pregnant women because they were too bulky. Limitations of available gravimetric equipment necessitated restricting the number of nonpregnant women to 15 for personal PM2.5 measurements. This limited the sample size for comparing the reductions in PM2.5 exposures. Equipment availability also limited the ability to perform sufficient number of simultaneous (paired same season) area and exposure measurements for PM2.5 and CO, making it difficult to assess correlations across these measures.
The significant role of ambient pollution in influencing both personal exposures and kitchen area concentrations was not realized until direct observation of the kitchen locations in the field and winter time ambient measurements were analyzed. Although same-season measurements attempted to address some of this limitation, the time since distribution was significantly longer for the summer season measurements. The timings of measurements also may be important according to the woman’s stage of pregnancy. Although all women were enrolled at less than 15 weeks pregnant, the cooking habits of someone who is heavily pregnant may well be different to that of someone in early pregnancy. The potential influence of these factors could not be examined within the limited number of samples available.
We could not perform paired ambient measure-ments in baseline and intervention phases as household receiving the interventions were staggered across both seasons. Although the density of intervention households was fairly low (<15 out of >200 households) in each village, the differential effect in the 2 phases could not be discerned in ambient measurements.
CONCLUSION
The results of this study did not support the widespread use of this stove in this population as a means to reliably reduce CO and PM2.5 exposures of pregnant women cooks, compared with open biomass cookstoves, the original focus of this feasibility study. Although we observed reductions in CO exposures, more field evidence is needed to indicate that both PM2.5 and CO exposures are significantly lowered for both women and children over time so as to increase the likelihood of seeing the benefits for both birth outcomes and early child health in intervention studies and create sufficient exposure gradients to be able to determine an exposure–response relationship for birth outcomes, as has been done for other health conditions.
The HAP measurements performed in this feasibility assessment revealed multiple factors that need to be considered while assessing the efficacy of household cookstove interventions. In particular, quantifying and monitoring the continuing use of all available stoves is crucial for understanding the dynamics of the stove adoption process, as well as estimating long-term exposure reductions that are achievable with household-level interventions targeted at replacing individual traditional stoves.24 The exposure-monitoring protocols provided insights for sampling strategies to address multiple sources of variability and to avoid exposure misclassification in intervention trials concerning HAP.
Finally, although the study represents only an initial assessment of one specific advanced biomass cookstove, the results support the need for alternative ways of exploring cleaner fuel choices that could provide far greater exposure reductions and reduce the overall public health burden from HAP effects.
Figure 2.
Paired comparisons of kitchen PM2.5 concentrations in pregnant (n = 11) and nonpregnant (n = 11) women households across baseline and postintervention phases. PM, particulate matter; TCS, traditional cookstove.
Acknowledgments
This work was supported by funds received from the World Bank, The Earth Institute, Columbia University, The World Lung Foundation, and the US Centers for Disease Control.
Footnotes
The authors declare they have no conflicts of interest.
REFERENCES
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith KR, Bruce N, Balakrishnan K, et al. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- 3.Office of the Registrar General & Census Commissioner . India Population and Housing Census 2011. Office of the Registrar General & Census Commissioner; New Delhi, India: 2011. [Google Scholar]
- 4.Balakrishnan K, Ghosh S, Ganguli B, et al. State and national household concentrations of PM2.5 from solid cookfuel use: results from measurements and modeling in India for estimation of the global burden of disease. Environ Health. 2013;12(1):77. doi: 10.1186/1476-069X-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide; Global update 2005; Summary of risk assessment. WHO; Geneva, Switzerland: 2006. [Google Scholar]
- 6.Bond TC, Doherty SJ, Fahey DW, et al. Bounding the role of black car bon in the climate system: a scientific assessment. J Geophys Res. 2013;118(11):5380–552. [Google Scholar]
- 7.Kar A, Rehman IH, Burney J, et al. Real-time assessment of black carbon pollution in Indian households due to traditional and improved biomass cookstoves. Environ Sci Technol. 2012;46(5):2993–3000. doi: 10.1021/es203388g. [DOI] [PubMed] [Google Scholar]
- 8.Pattanayak SK, Sills EO, Kramer RA. Seeing the forest for the fuel. Environ Dev Econom. 2004;9:155–79. [Google Scholar]
- 9.Rehman IH, Ahmed T, Praveen PS, Kar A, Ramanathan V. Black carbon emissions from biomass and fossil fuels in rural India. Atmos Chem Phys. 2011;11(14):7289–99. [Google Scholar]
- 10.NRE . A new initiative on improved biomass cookstoves. Ministry of New and Renewable Energy; New Delhi, India: 2009. [Google Scholar]
- 11.Venkataraman C, Sagar A, Habib G, Smith K. The Indian National Initiative for Advanced Biomass Cookstoves: the benefits of clean combustion. Energy Sustain Dev. 2010;14:63–72. [Google Scholar]
- 12.Bhattacharya S, Albino D, Salam A. Emission factors for wood and charcoal fired cookstoves. Biomass Bioenerg. 2002;23:453–69. [Google Scholar]
- 13.Bhattacharya S, Albino DO, Khaing DM. Effect of selected parameters on perfromance and emission of biomass-fired cookstoves. Biomass Bioenerg. 2002;23:387–95. [Google Scholar]
- 14.Jetter J, Zhao Y, Smith KR, et al. Pollutant emissions and energy efficiency under controlled conditions for household biomass cookstoves and implications for metrics useful in setting international test standards. Environ Sci Technol. 2012;46(19):10827–34. doi: 10.1021/es301693f. [DOI] [PubMed] [Google Scholar]
- 15.Mukunda HS, Dasappa S, Paul PJ, et al. Gasifier stoves: Science, technology and field outreach. Curr Sci. 2011;98:630–8. [Google Scholar]
- 16.Bhojvaid V, Jeuland M, Kar A, et al. How do people in rural India perceive improved stoves and clean fuel? Evidence from Uttar Pradesh and Uttarakhand. Int J Environ Res Public Health. 2014;11(2):1341–58. doi: 10.3390/ijerph110201341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis JJ, Pattanayak SK. Who adopts improved fuels and cookstoves? A systematic review. Environ Health Perspect. 2012;120(5):637–45. doi: 10.1289/ehp.1104194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehfuess EA, Puzzolo E, Stanistreet D, Pope D, Bruce NG. Enablers and barriers to large-scale uptake of improved solid fuel stoves: a systematic review. Environ Health Perspect. 2014;122(2):120–30. doi: 10.1289/ehp.1306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muralidharan V, Sussan TE, Limaye S, et al. Field testing of alternative cookstove performance in a rural setting of western India. Int J Environ Res Public Health. 2015;12(2):1773–87. doi: 10.3390/ijerph120201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambandam S, Balakrishnan K, Ghosh S, et al. Can currently available advanced combustion biomass cookstoves provide health relevant exposure reductions? Results from initial assessment of select commercial models in India. Ecohealth. 2015;12(1):25–41. doi: 10.1007/s10393-014-0976-1. [DOI] [PubMed] [Google Scholar]
- 21.Jetter JJ, Kariher P. Solid-fuel household cookstoves: characterization of performance and emissions. Biomass Bioenergy. 2009;33:294–305. [Google Scholar]
- 22.Lewis JJ, Bhojvaid V, Brooks N, et al. Piloting improved cookstoves in India. J Health Commun. 2015;20(Suppl 1):28–42. doi: 10.1080/10810730.2014.994243. [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay R, Sambandam S, Pillarisetti A, et al. Cooking practices, air quality, and the acceptability of advanced cookstoves in Haryana, India: an exploratory study to inform large-scale interventions. Glob Health Action. 2012;5:1–13. doi: 10.3402/gha.v5i0.19016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillarisetti A, Vaswani M, Jack D, et al. Patterns of stove usage after introduction of an advanced cookstove: the long-term application of household sensors. Environ Sci Technol. 2014;48(24):14525–33. doi: 10.1021/es504624c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards R, Hubbard A, Khalakdina A, Pennise D, Smith K. Design considerations for field studies of changes in indoor air pollution due to improved stoves. Energy Sustain Dev. 2007;11(2) [Google Scholar]
- 26.Chowdhury Z, Edwards RD, Johnson M, et al. An inexpensive light-scattering particle monitor: field validation. J Environ Monit. 2007;9(10):1099–106. doi: 10.1039/b709329m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumoff K. Quantitative metrics of exposure and health for indoor air pollution from household biomass fuels in Guatemala and India. University of California, Berkeley; Berkeley: 2007. Unpublished doctoral thesis. [Google Scholar]
- 28.Edwards RD. Personal Communication. 2015.
- 29.Burnett RT, Pope CS, 3rd, Ezzati M, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014;122(4):397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]