INTRODUCTION
Human herpesvirus-6 (HHV-6) has been implicated in the development of a diverse array of neurologic conditions, including seizures, encephalitis, mesial temporal lobe epilepsy (MTLE), and multiple sclerosis (MS) [Dewhurst et al., 1997; Dewhurst, 2004; Birnbaum et al., 2005; Fotheringham and Jacobson, 2005; Isaacson et al., 2005; Fotheringham et al., 2007a,b]. HHV-6 infection is ubiquitous in the general population. Numerous studies have demonstrated HHV-6 DNA sequences in non-pathological brain tissues obtained from autopsies or by surgeries [Challoner et al., 1995; Cermelli and Jacobson, 2000; Donati et al., 2003; Opsahl and Kennedy, 2005], suggesting that it can be a commensal virus of the brain. Thus, attributing a pathological role to the virus in diseases of the central nervous system (CNS) can be challenging.
At the same time, as discussed later, HHV-6 has in vitro tropism for various cells of the CNS. Higher frequencies of HHV-6 DNA detection have often been reported in samples taken from patients with neurological diseases [Challoner et al., 1995; Cermelli and Jacobson, 2000; Donati et al., 2003; Opsahl and Kennedy, 2005; Fotheringham et al., 2007b] implicating its role in diseases. Additionally, HHV-6 viral protein expression has been observed in pathological specimens [Challoner et al., 1995; Opsahl and Kennedy, 2005] but not in healthy tissues, suggesting that active viral replication may in part contribute to manifestation of clinical symptoms.
Primary childhood infection of HHV-6 is often asymptomatic and self-limiting. However, it also commonly produces febrile illnesses [Hall et al., 1994]. Furthermore, many studies suggest that the association of HHV-6 with neurological disorders may be related to its ability to enter a state of latency following primary exposure: reactivation later in life could plausibly cause neurologic symptoms.
One of the most intriguing characteristics of HHV-6 is that it may be an etiological agent for multiple and quite different pathological conditions of the CNS. Inherent viral properties such as sequence variations and differences in antigenic specificity between the A and B variants of HHV-6 may be responsible for the diverse pathology, as may various host factors. The evidence associating HHV-6 with various neurological diseases and the associated neuroimaging features in HHV-6 encephalitis is reviewed as well as information on the ability of HHV-6 to infect glial cells, and the potential viral mechanisms that may influence clinical manifestations.
ASSOCIATION OF HHV-6 WITH MULTIPLE SCLEROSIS
HHV-6 has been implicated repeatedly as a possible infectious trigger for MS. The possible role of HHV-6 in MS was first raised by Challoner et al. [1995]. Using an unbiased search method known as representation differential analysis (RDA), which allowed for rapid enrichment of non-human DNA from clinical materials by successive PCR amplifications, the MDBP gene of HHV-6 variant B was observed in MS plaques. Although PCR analysis in this study showed comparable frequencies of HHV-6 DNA in MS and control brain material, several subsequent studies demonstrated significantly higher prevalence of HHV-6 DNA detected specifically in lesions in MS brains compared to normal non-pathological areas [Cermelli and Jacobson, 2000; Opsahl and Kennedy, 2005]. Using monoclonal antibodies against the HHV-6 p41 and 101K molecules, Challoner et al. [1995] demonstrated that viral protein expression was observed in MS but not in control brain samples. Importantly, HHV-6 protein expression was localized to oligodendrocytes, the myelin-producing glial cells of the CNS.
The association of HHV-6 with MS is supported further by immunological and molecular studies. Cermelli and Jacobson [2000] investigated the frequency of HHV-6 DNA detection in MS plaques and the surrounding normal appearing white matter. PCR analysis revealed that HHV-6 DNA was detected more often in MS lesions, compared to normal appearing white matter from MS patients or in the brains of individuals without MS. Consistent with this earlier report, a subsequent study demonstrated in MS plaques both the expression of HHV-6 DNA transcripts by in situ PCR as well as greater expression of HHV-6 mRNA expression, compared to normal appearing white matter from MS brains or to the brains of individuals without MS [Opsahl and Kennedy, 2005]. Finding replication of HHV-6 and expression of its mRNA in diseased areas of MS brains suggests an etiologic association of HHV-6 with MS. Lending further support to the potential role of HHV-6 in MS is a study that showed lack of viral transcripts of other closely related herpesviruses such as EBV, HHV-7, and HHV-8 in autopsy brain tissues of MS patients using in situ detection [Opsahl and Kennedy, 2006, 2007].
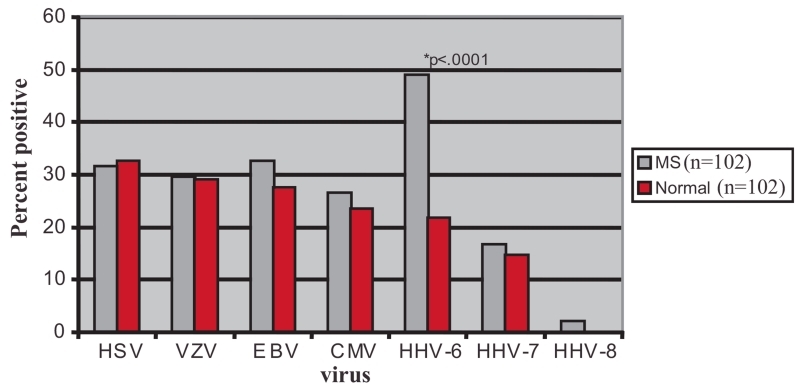
In addition to CNS tissue-based investigations, the association of HHV-6 with MS is also supported by viral expression in bodily fluids outside of the CNS. A cohort study including 42 MS patients and 34 healthy controls examined the presence of HHV-6 in saliva, urine, sera and peripheral blood mononuclear cells (PBMCs). While HHV-6 DNA was detected readily in saliva and PBMCs of both MS patients and healthy controls, it was found in sera and urine in 23% of MS patients but in none of the controls. Further sub-typing of PCR products by restriction digest indicated that the HHV-6A variant was present more frequently in MS patient samples [Akhyani et al., 2000]. A subsequent study with a larger number of MS and control cohorts described similar findings [Alvarez-Lafuente et al., 2002]. Among the various human herpesviruses, only HHV-6 DNA was found more frequently in the PBMCs of MS patients (Fig. 1).
Fig. 1.
Prevalence of herpesvirus DNA in PBMC.
HHV-6 is normally a cell-associated virus and shedding of viral particles occurs only during active replication. Therefore, detection of viral DNA in cell-free compartments of MS patients suggests of active viral replication. To determine if exacerbations of MS were associated with active replication of HHV-6, Berti et al. [2002] carried out a longitudinal study over 5 months in 59 MS patients. Although HHV-6 DNA could be detected in the sera of MS patients both during remissions and relapses, it was found more often during relapses. In the same study, longitudinal samples from two MS patients over a period of 19 months revealed that HHV-6 DNA was detected more often in the serum at times when pathological exacerbations were noted using gadolinium-enhanced brain magnetic resonance imaging (MRI) scans [Berti et al., 2002]. Together, these studies indicate an association between active replication of HHV-6 and clinical disease activity in patients with MS.
Serological studies of HHV-6 also support an association of HHV-6 with MS. Elevated antibody reactivity against HHV-6 has been demonstrated in sera of MS patients [Soldan et al., 1997; Villoslada et al., 2003]. In particular, it has been shown that the levels of IgG and IgM antibodies against HHV-6 are more prominent during early phases of the disease, suggesting that the virus may serve as a “trigger” in the pathogenesis of MS [Villoslada et al., 2003]. Soldan et al. [1997] demonstrated that levels of IgM antibody to HHV-6 early antigen (p41/38) is higher in patients with relapsing-remitting MS (RRMS), compared to patients with chronic progressive MS (CPMS), other neurological diseases, other autoimmune diseases, and healthy controls [Soldan et al., 1997]. Higher levels of IgM antibody to viral early antigens indicate that recent exposure or reactivation of HHV-6 is associated with RRMS. As expected, HHV-6 IgG reactivity was not significantly different among the different patient groups, probably because latent HHV-6 infection is so ubiquitous.
If HHV-6 is a CNS pathogen, antibodies would be expected against the virus in the cerebrospinal fluid (CSF). Our laboratory has developed an electrochemiluminescence assay (ECL) which was demonstrated to be both sensitive and reproducible for measuring HHV-6 antibody reactivity in CSF as well as serum specimens [Yao et al., 2008, 2009]. Using this ECL assay, the levels of HHV-6 IgG in CSF of RRMS patients and other neurological diseases patients were studied. Consistent with the findings reported by Soldan et al. [1997], levels of HHV-6 IgG antibody in the CSF of RRMS patients was comparable to that in other neurological disease patients.
Using the ECL assay, however, it was found that antibody levels were elevated in RRMS patients treated with natalizumab, an agonist of alpha-4-beta-integrin. Natalizumab is an FDA-approved therapy for MS patients and has demonstrated efficacy in reducing CNS inflammation in clinical trials. However, natalizumab can trigger a rare condition known as progressive multi-leukoencephalopathy (PML), a demyelinating disorder caused by reactivation of the JC virus within the CNS [Steinman, 2005]. It has been hypothesized that reactivation of JC virus in some MS patients treated with natalizumab is related to reduced CNS immune surveillance [McFarland and Jacobson, 2006]. Interestingly, HHV-6 expression also has been demonstrated in PML brain lesions resulting from JC virus reactivation [Mock et al., 1999; Blumberg et al., 2000]. The reduced immune trafficking to the CNS caused by natalizumab thus may allow for opportunistic reactivation of latent CNS infection with both JC virus and HHV-6 [Yao et al., 2008].
MS is an inflammatory neurodegenerative disease. Virus infections have been suggested as one possible trigger of an immune response that becomes misdirected against CNS self-antigens [Soldan et al., 2000; Tejada-Simon et al., 2002]. Indeed, when HHV-6A or HHV-6B infected cell lines were used as antigens for assessment of HHV-6-specific T cell precursor frequencies, elevated T cell reactivity towards HHV-6A antigens was observed specifically. In contrast, no significant difference was detected in terms of T cell reactivity towards HHV-6B antigens between MS patients and healthy controls.
Despite Challoner et al.’s [1995] initial finding that HHV-6B might be a trigger for MS, current evidence favors a greater role for HHV-6A. Elevated T cell reactivity against HHV-6A antigens, increased serum antibody to HHV-6A p31/48 protein, and detection of HHV-6A DNA in sera of MS patients collectively suggest a possible predominant role of HHV-6A with MS disease activity [Soldan et al., 1997, 2000; Akhyani et al., 2000]. One plausible mechanism is molecular mimicry. Tejada-Simon et al. [2002] found that the U27 gene of HHV-6 shares a stretch of seven amino acids with the human myelin basic protein, one of the putative auto-antigens in MS. T cell lines with specificity for myelin basic protein harvested from MS patients also exhibited increased reactivity toward the HHV-6 U27 peptide, and vice versa. The frequencies of T cells with specificities against either the U27 peptide or MBP were also elevated significantly in MS patients compared with healthy controls. Collectively, these results suggest a potential mechanism of HHV-6 triggered autoimmunity against CNS auto-antigens.
HHV-6 AND MESIAL TEMPORAL LOBE EPILEPSY
Primary infection with HHV-6B infection may be the cause of approximately 30% of the febrile convulsions in children under the age of 2 years [Hall et al., 1994]. Primary or reactivated HHV-6 also may be an important cause of more severe seizures in children [Epstein et al., 2006]. HHV-6, like all herpes viruses, often enters a state of latency following primary exposure, with various factors (e.g., stress or transient immunosuppression) that could trigger reactivation.
Several recent studies suggest a potential pathophysiological role of HHV-6 in MTLE, in both children and adults. MTLE is one of the most common and intractable forms of seizure disorder. It begins typically in childhood, particularly in children with a history of prolonged febrile seizures. In MTLE, astrogliosis and neuronal loss are common pathological characteristics, but CNS inflammation is observed rarely [Theodore et al., 2008]. Temporal lobectomy is an effective surgical treatment for MTLE.
The etiology of MTLE is unknown, but evidence suggests HHV-6 infection may be one cause. HHV-6 DNA was detected by PCR in 6 of 17 (35%) of patients with MTLE, studying brain tissue removed during surgery. All six of the HHV-6 positive patients were shown to have anterior hippocampal sclerosis, a common pathological finding in MTLE [Uesugi et al., 2000]. In a subsequent study, Donati et al. [2003] used virus-specific real-time TaqMan PCR assay, Western blot analysis, and in situ immunohistochemistry to detect and characterize HHV-6 infection in a series of epilepsy surgery specimens. High levels of HHV-6 DNA were detected in the hippocampal and temporal lobe regions of a subset of patients, by real-time quantitative PCR. In addition, Western blot analysis and immunohistochemistry (IHC) staining also demonstrated expression of an early viral protein (p41) in hippocampal surgical materials indicating active viral replication.
Donati et al. [2003] then isolated primary human astrocytes from the hippocampus of MTLE patients. Following in vitro culture of the astrocytes, expression of HHV-6 protein was demonstrated, presumably reflecting reactivation in these cells of latent HHV-6 infection, by unknown mechanisms. This observation suggests that in MTLE, reactivation of active replication could be a trigger for seizures. Alternatively, persistent low level of infection might also lead to seizures as the result of cumulative neurological injury. Collectively, these tissue-based and molecular studies provide evidence to support an etiological link between a ubiquitous herpevirus infection in a non-inflammatory neurological condition.
HHV-6 AND ENCEPHALITIS IN IMMUNOCOMPROMISED AND IMMUNOCOMPETENT PATIENTS
HHV-6 reactivation is common in immunosuppressed individuals, as first reported in a case of fatal HHV-6B encephalitis following bone marrow transplantation confirmed by dot blot hybridization and IHC [Drobyski et al., 1994]. Approximately 50% of the patients undergoing myeloablative hematopoietic stem-cell transplantation develop reactivated HHV-6 infection. HHV-6 related neurological complications are rare following bone marrow transplantation, occurring in less than 1% (11/1,148) in one series [Fujimaki et al., 2006]. However, in these small subset of patients, a syndrome of limbic encephalitis with anterograde amnesia can develop after hematopoietic stem-cell transplantation [Singh and Paterson, 2000; Wainwright et al., 2001; Fujimaki et al., 2006; Gorniak et al., 2006; Noguchi et al., 2006; Seeley et al., 2007; Vu et al., 2007; Provenzale et al., 2008, 2010]. Fotheringham et al. [2007a] found active HHV-6 infection in four post-hematopoietic stem-cell transplantation patients who developed neurologic complications. In one patient where autopsy brain specimens were available, high HHV-6 viral loads were detected throughout the brain—including in the hippocampus, basal ganglia, insular cortex, temporal lobe, and cingular gyrus. Furthermore, IHC revealed astrogliosis and neuronal loss in regions of the hippocampus where the HHV-6 protein expression was detected, suggesting a possible relationship between viral expression and CNS pathology [Fotheringham et al., 2007a]. Surprisingly, while high levels of HHV-6 viral load could be detected in autopsy brain samples of this patient, only a small amount of HHV-6 DNA could be amplified from the CSF specimens. This finding underscores the cell-associated nature of the virus, and suggests that even low levels of cell-free HHV-6 detected in CSF may signify an active infection within the CNS.
In pediatric patients, there have been many reports of exanthem subitum associated with neurological complications such as meningoencephalitis [Irving et al., 1990; Ishiguro et al., 1990; Asano et al., 1992; Inagaki et al., 1992; Sato et al., 1992; Yoshikawa et al., 1992; Sloots et al., 1993; Suga et al., 1993; Tan et al., 1993; Itokazu et al., 1994; Jones et al., 1994; Oki et al., 1995; Yanagihara et al., 1995; Fujiwara et al., 1996]. More recently, several reports of HHV-6 associated encephalitis of non-immunocompromised children [Kamei et al., 1997; Kimura and Nezu, 1998; Crawford et al., 2007, 2009; Nagasawa et al., 2007; Yoshinari et al., 2007] and adults [Birnbaum et al., 2005; Isaacson et al., 2005] in the absence of classical roseola have been described.
To explore the relationship of HHV-6 with encephalitis in immunocompetent patients, 35 patients were studied who had encephalitis of unknown origin, excluding other known causes [Yao et al., 2009]. CSF specimens were analyzed using a highly sensitive nested PCR technique. HHV-6 DNA was amplified from 37% of the samples tested. In contrast, no HHV-6 DNA was amplified from CSF samples of patients with early RRMS or other neurological diseases. The sensitive ECL assay developed in our laboratory found elevated levels of both anti-HHV-6 IgM as well as IgG in the CSF among the immunocompetent patients with encephalitis, compared to the other neurological cohorts examined. (The RRMS patients had elevated levels of IgG but not IgM antibody.) These findings suggest that the encephalitis patients had either a subacute exposure to HHV-6 or marked reactivation.
NEUROIMAGING FEATURES OF HHV-6 ENCEPHALITIS
The clinical features and laboratory diagnosis of HHV-6 encephalitis has led to a diverse neuroradiographic subset of disease characteristics in both immunocompetent and immunocompromised patients as shown in Table I. Initial case reports on pediatric patients with CNS complications of exanthem subitum revealed computerized tomography (CT) findings of cerebral edema and hypodensities in the cortex, thalami, cerebellum, and brainstem [Irving et al., 1990; Ishiguro et al., 1990; Asano et al., 1992; Inagaki et al., 1992; Sato et al., 1992; Yoshikawa et al., 1992; Sloots et al., 1993; Suga et al., 1993; Tan et al., 1993; Itokazu et al., 1994; Jones et al., 1994; Oki et al., 1995; Yanagihara et al., 1995; Fujiwara et al., 1996]. Larger case series in pediatric immunocompetent patients diagnosed with HHV-6 encephalitis using MRI, revealed signal abnormalities [Kamei et al., 1997; Kimura and Nezu, 1998; Crawford et al., 2007, 2009; Nagasawa et al., 2007; Yoshinari et al., 2007] of the frontal/temporal-parietal-occipital lobes, cerebellum, brainstem, and deep gray nuclei (Table I). Yoshinari et al. [2007] demonstrated restricted diffusion on MRI diffusion weighted imaging during the acute phases of illness in six pediatric patients, with frontal lobe hypoperfusion on single photon emission computerized tomography (SPECT) during the convalescent stage. In our series, acute MRI changes were found in the cerebellum/brainstem or thalami in six of seven children, with chronic necrotization of the affected regions several months after initial imaging [Crawford et al., 2007, 2009].
TABLE I.
MRI Abnormalities Associated With HHV-6 Encephalitis Immunocompetent and Immunocompromised Patients
| Number of patients (N) |
Immune status |
Age | Clinical features | HHV-6 diagnostic testing | MRI abnormalities (acute) | MRI abnormalities (chronic) |
HHV-6 disease outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| 11 | Compromised | 28–58yo | Headache (n = 1), short term memory loss (n = 5), seizures (n = 7), encephalopathy (n = 10) |
HHV-6 PCR CSF (n = 11), HHV-6 PCR serum (n = 4) |
Hypothalamus (n = 5) | ND | Death (n = 3) | Fujimaki et al. [2006] |
| 12 | Compromised | 13–49yo | Encephalopathy (n = 12), headache (n = 3), seizures (n = 3), movement disorder (n = 1), ataxia (n = 1), coma (n = 2) |
HHV-6 PCR CSF, HHV-6 viral culture (biopsy) |
Mesial temporal lobe (n = 1), fronto-temporal-parietal-occipital (n = 1) |
ND | Death (n = 5) | Singh and Paterson [2000] |
| 5 | Compromised | 9–22yo | Insomnia (n = 5), agitation (n = 2), short term memory loss (n = 4), hallucinations (n = 1), rigidity (n = 1) |
HHV-6 PCR CSF (n = 3), IHC (autopsy specimen) |
Hippocampus (n = 5), basal ganglia (n = 1), brainstem (n = 1), diencephalon (n = 1), parahippocampal gyri (n = 1) |
ND | Death (n = 1) | Wainright et al. [2001] |
| 6 | Compromised | 36–55yo | Encephalopathy (n = 4), short term memory loss (n = 4), coma (n = 3), seizures (n = 3), hypopnea (n = 3) |
HHV-6 PCR CSF (n = 5), HHV-6 PCR serum (n = 1) |
Hippocampus (n = 6), amygdale (n = 6) |
Atrophy | Death (n = 1) | Noguchi et al., [2006] |
| 4 | Compromised | 32–59yo | Short term memory loss (n = 4), encephalopathy (n = 4), seizures (n = 2) |
HHV-6 PCR CSF (n = 4) | Medial temporal lobes (n = 4) | ND | Short term memory loss (n = 3) | Gorniak et al. [2006] |
| 9 | Compromised | 22–59yo | Encephalopathy (n = 9), amnesia (n = 9), hallucinations (n = 1), movement disorder (n = 1), seizures (n = 2) |
HHV-6 PCR CSF | Amygdala (n = 5), hippocampus (n = 8), uncus (n = 7) |
Hippocampal atrophy (n = 1) |
Cognitive deficits (n = 6) | Seeley et al. [2007] |
| 5 | Compromised | 39–66yo | Encephalopathy (n = 5), amnesia (n = 3), seizures (n = 2) |
HHV-6 PCR CSF (n = 5) | Medial temporal lobe (n = 3), white matter (n = 2) |
ND | Death (n = 1) | Vu et al. [2007] |
| 9 | Compromised | 6–39yo | Encephalopathy (n = 9), seizures (n = 5), insomnia (n = 6), hallucinations (n = 3), short term memory loss (n = 4), tremor (n = 1) |
HHV-6 PCR CSF (n = 9) | Hippocampus (n = 7), entorhinal cortex (n = 6), other cortical regions (n = 11), amygdale (n = 6), hypothalamus (n = 8), other deep forebrain (n = 9), white matter (n = 6) |
ND | Unknown | Provenzale et al. [2008, 2010] |
| 10 | Normal | 8–15mo | Seizures (n = 10), hemiparesis (n = 4) |
HHV-6 PCR CSF (n = 5) | Hemispheric atrophy (n = 3) | ND | Hemiplegia (n = 3), Mental retardation (n = 2) |
Nagasawa et al. [2007] |
| 10 | Normal | 8–26mo | Seizures (n = 10) | HHV-6 IgM (n = 10), HHV-6 PCR CSF (n = 1), HHV-6 PCR serum (n = 2) |
Frontal lobes (n = 8), temporal lobes (n = 2) |
ND | Mental retardation (n = 4), spastic quadraparesis (n = 2) |
Yoshinari et al. [2007] |
| 16 | Normal | 6mo–37y | Seizures (n = 14), cranial neuropathy (n = 1), status epilepticus (n = 7) |
HHV-6 PCR CSF, HHV-6 serum IgM |
Parietal-occipital lobe (n = 2), thalami (n = 2), cerebellum (n = 1) |
Atrophy (n = 6) | Death (n = 4), mental retardation (n = 4), motor deficits (n = 4) |
Kamei et al. [1997] |
| 7 | Normal | 9mo–3yo | Encephalopathy (n = 7), seizures (n = 4), ataxia (n = 2) |
HHV-6 PCR CSF (n = 7), HHV-6 PCR serum (n = 6) |
Midbrain (n = 3), pons (n = 4), medulla (n = 2), cerebellum (n = 3), thalami (n = 4), frontal lobes (n = 1), temporal lobes (n = 1), white matter (n = 2), insular cortex (n = 1) |
Atrophy and Necrotization (n = 5) |
Developmental delay (n = 7), seizures (n = 6), motor deficits (n = 5), visual deficits (n = 1) |
Crawford et al. [2007] |
| 8 | Normal | 5–12mo | Fever (n = 9); seizures (n = 9), status epilepticus (n = 9), hemiplegia (n = 1), coma (n = 4) |
HHV-6 PCR CSF (5/9) HHV-6 IgM (3/9) |
Basal ganglia (n = 3), parieto-occipital lobe (n = 3), thalamus (n = 3) |
ND | Death (n = 2), hemiplegia (n = 2), chronic encephalopathy (n = 1) |
Kimura and Nezu [1998] |
| 3 | Normal | 9mo–3yo | Ataxia (n = 2), seizures (n = 3), encephalopathy (n = 3), cranial neuropathy (n = 3) |
HHV-6 PCR CSF (n = 3), HHV-6 PCR serum (n = 3) |
Cerebellum (n = 1), thalamus (n = 1), putamen (n = 1), insular cortex (n = 1) |
Atrophy (n = 2) | Developmental delay (n = 2), seizures (n = 2), opsoclonus myoclonus (n = 1) |
Crawford et al. [2009] |
HHV-6, human herpesvirus-6; CSF, cerebrospinal fluid; ND, not done.
Adult MRI imaging series of HHV-6 meningoencephalitis occur largely post-HSCT and involve both hippocampal and extrahippocampal structures as shown in Table I [Singh and Paterson, 2000; Wainwright et al., 2001; Fujimaki et al., 2006; Gorniak et al., 2006; Noguchi et al., 2006; Seeley et al., 2007; Vu et al., 2007; Provenzale et al., 2008, 2010]. Wainwright et al. [2001] first reported a series of HHV-6 limbic encephalitis following stem cell transplantation with bilateral, symmetric MRI signal changes in the hippocampal structures. Provenzale et al. [2008] later demonstrated that in addition to the hippocampi, a diverse set of extrahippocampal structures can also be affected following transplant including the amygdala, enterorhinal cortex, hypothalamus, and deep forebrain structures [Provenzale et al., 2008, 2010]. The multitude of MRI patterns associated with HHV-6 meningoencephalitis in both immunocompetent and immunocompromised patients should allow for more timely recognition and the possibility of prospective clinical trials aimed at disease management.
CHRONIC FATIGUE SYNDROME
Chronic fatigue syndrome (CFS) is a debilitating chronic illness [Fukuda et al., 1994] that often begins suddenly with a “flu-like” illness. Patients with CFS have great functional impairment [Komaroff et al., 1996]. The cost to the U.S. economy from lost productivity alone (not including medical care costs) is $9 billion annually [Reynolds et al., 2004].
While the pathogenesis of CFS is unknown, there is abundant evidence of an underlying biological process. In comparison to various health and disease control groups, patients with CFS have abnormal findings in the CNS and autonomic nervous system, evidence of chronic activation of various parts of the immune system, and disordered energy metabolism.
CNS abnormalities have been found using MRI [Buchwald et al., 1992; Schwartz et al., 1994a; Lange et al., 2001; de Lange et al., 2005], functional MRI [Tanaka et al., 2006], SPECT [Schwartz et al., 1994b; Schmaling et al., 2003], and positron-emission tomography (PET) [Yamamoto et al., 2004]. Neuroendocrine studies reveal hypofunction of corticotropin releasing hormone (CRH) neurons in the hypothalamus [Demitrack et al., 1991], disruption of both serotonergic and noradrenergic hypothalamic pathways [Demitrack et al., 1992; Cleare et al., 1995], and of growth hormone secretion [Moorkens et al., 2000]. Typically, these abnormalities are in patterns opposite to those seen in major depression. Cognitive testing has revealed abnormalities [Tiersky et al., 1997; Daly et al., 2001; Deluca et al., 2004] that are not explained by concomitant mood disorders [Marcel et al., 1996]. Autonomic nervous system testing has found abnormalities—particularly postural orthostatic tachycardia syndrome, neurally mediated hypotension, and heart rate variability during head-up tilt testing [Bou-Holaigah et al., 1995; Freeman and Komaroff, 1997; Stewart, 2000; Naschitz et al., 2002].
The immunological findings described most commonly in CFS are impaired function of natural killer cells, increased numbers of CD8+ cytotoxic T cells that bear antigenic markers of activation on their cell surface, and increased production of various pro-inflammatory and TH2 cytokines [Komaroff, 2006]. Many of these cytokines can produce symptoms characteristic of CFS: fatigue, fevers, adenopathy, myalgias, arthralgias, sleep disorders, cognitive impairment, and mood disorders.
Many recent studies of patients with CFS have identified disorders of energy metabolism [Myhill et al., 2009], increased allostatic load [Maloney et al., 2009], and increased oxidative and nitrosative stress [Maes and Leunis, 2008].
Cases of CFS can follow in the wake of well-documented infection with several infectious agents, and may be more likely when the symptoms of acute infection were most severe [Hickie et al., 2006]. The first large study on the possible role of HHV-6 in CFS included 259 patients with a “CFS-like” illness (the case definition had not yet been developed) and age- and gender-matched healthy control subjects. Primary culture of lymphocytes showed active replication of HHV-6 in 70% of the patients versus 20% of the control subjects (P < 10 −8) [Buchwald et al., 1992].
Some subsequent studies have employed only serological techniques that do not distinguish active from latent infection. The results have been mixed: a slight preponderance has showed an association between CFS and HHV-6 infection [Ablashi et al., 2000; Reeves et al., 2000; Hickie et al., 2006].
In contrast, other studies have employed assays that can detect active infection: PCR of serum or plasma, IgM early antigen antibodies, and primary cell culture. Most of these studies have shown an association between CFS and active HHV-6 infection [Patnaik et al., 1995; Secchiero et al., 1995; Wagner et al., 1996; Zorzenon et al., 1996; Ablashi et al., 2000; Nicolson et al., 2003], whereas a few have not [Koelle et al., 2002; Reeves et al., 2000]. The number of patients in the studies that have found an association between CFS and active HHV-6 infection (N = 717) is much larger than the number in studies that have failed to find an association (N = 48).
Several observations, summarized above, together suggest that active infection with HHV-6 may cause some cases of CFS. First, active infection with HHV-6 is present in a substantial fraction of patients with CFS. Second, HHV-6 is tropic for the nervous system and immune system cells, and CFS is characterized by neurological and immunological abnormalities. Clinical studies with antiviral drugs that have in vitro activity against HHV-6 could provide strong evidence in favor of, or against, the hypothesis that HHV-6 may trigger and perpetuate some cases of CFS.
INFECTION OF HHV-6 IN CNS GLIAL CELLS
IHC and in situ hybridization analysis of MS, MTLE and encephalitis patient brain tissues suggest that CNS glial cells are possible in vivo reservoirs for HHV-6 [Challoner et al., 1995; Donati et al., 2003; Fotheringham et al., 2007a]. The three types of glial cells in the CNS perform important maintenance and regulatory functions. Astrocytes have elaborate networks of processes that envelope neuronal synapses and express glial fibrillary acidic protein (GFAP). They are responsible primarily for biochemical support of endothelial cells that form the blood–brain barrier, provision of metabolites and nutrients to neurons, reuptake of neurotransmitters, and participate in repair and scarring process following neural injury. Oligodendrocytes are the myelin producing cells in the CNS (whereas Schwann cells perform this function in the peripheral nervous system). The myelin sheath is crucial for providing insulation to axon, thereby increasing the speed with which action potentials are propagated. Microglia are smaller in size than astrocytes and oligodendrocytes. As the immune cells of the CNS, the main function of microglia is to survey the brain for tissue damages or infectious agents [Araque, 2008; Benarroch, 2009; Kaur and Ling, 2009].
In vitro studies have provided evidence that HHV-6 can infect astroglial and oligodendroglial cells [He et al., 1996; Ahlqvist et al., 2005]. The two variants of HHV-6 appear to exhibit differential tropisms for glial cells with regards to infectivity and viral expression. The U1102 strain of HHV-6A variant produces productive infection of glial cells, whereas the Z29 strain of the HHV-6B variant causes persistent or abortive infections. HHV-6 infection in astrocytes can alter cellular functions and metabolic processes that are essential for maintaining a healthy CNS environment. For instance, Meeuwsen et al. [2005] have demonstrated changes in cellular gene expression, as measured by cDNA microarray, in cultured human adult astrocytes infected by HHV-6A. Modest upregulation of cytokines such as leukocyte interferon-inducible peptide (LIP), CCL5 (RANTES), insulin-like growth factor binding protein 6 (IGFBP6), vascular endothelial growth factor C (VEGF-C) was observed following infection with the virus. When these astrocytes infected by HHV-6 were exposed to a cocktail of pro-inflammatory cytokines including TNF-α, IL-1β and IFN-γ, marked induction of IL-10, IL-11, IL-1β, and IL-6 was observed. Additionally, chemotactic factors such as CCL3 (MIP-1α), CXCL2 (MIP-2α), CCL5 (RANTES), and CXCL6 (granulocyte chemotactic protein 2) were also elevated along with genes that play important roles in cell growth and differentiation such as IGFBP6, VEGF-C, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-7 receptor gamma chain. These results suggested that infection of CNS astroglial cells by HHV-6 has the potential to modulate and skew the immune responses within the CNS environment [Meeuwsen et al., 2005].
One of the many essential functions of astrocytes is regulation of neurotransmitters such as glutamate. Excess glutamate in the CNS can be toxic to neurons. Astrocytes express various glutamate transporters that ensure proper uptake of this metabolite, and prevent toxic concentrations of glutamate from accumulating near neurons. Dysfunction of these transporters has been implicated in a number of neurodegenerative pathologies such as amyotrophic lateral sclerosis, epilepsy, Huntington’s disease, Alzheimer’s disease, and ischemic stroke injury [Beart and O’Shea, 2007]. When human astrocytes are infected in vitro with HHV-6 there is decreased expression of the EAAT-2 high affinity glial glutamate transporter, and the up-take of excess glutamate is impaired [Fotheringham et al., 2008]. Dysregulation of EAAT-2 and glutamate excitotoxicity have been associated with seizures, epilepsy, MS, and other neurological conditions [Vallejo-Illarramendi et al., 2006; Vercellino et al., 2007; Pampliega et al., 2008].
HOW CAN HHV-6 BE ASSOCIATED WITH MULTIPLE CNS DISEASES?
Is it plausible that one virus might contribute to the pathogenesis of several different neurological conditions? HHV-6 a pleiotropic virus, capable of infecting different cell types of the CNS; therefore, it is possible that development of a particular neurologic condition may be related to infection of a specific cell type (i.e., infection of oligodendrocytes in MS whereas astrocytes are affected in disorders such as mesial temporal lobe epilepsy and encephalitis). Alternatively, host genetics and immune status may also, in part, influence the severity and clinical manifestations of virus-induced pathology [Oksenberg et al., 2008].
Studies using animal models infected with mouse hepatitis virus (MHV) or Theiler’s murine encephalitis virus (TMEV) have shed light on possible mechanisms by which one viral agent can induce either acute encephalitis or chronic inflammatory demyelination in genetically identical hosts [Wada and Fujinami, 1993; Sun and Perlman, 1995]. MHV and TMEV are both murine viruses that can cause acute encephalitis or chronic demyelination in mice and are commonly used to induce disease in animal models of MS. In a study by Sun and Perlman [1995], mice inoculated with the MHV–JHM developed rapidly acute encephalitis. However, spinal cord demyelination developed in mice infused with anti-MHV neutralizing antibodies following inoculation. Based on IHC analysis of viral distribution in the CNS between neutralizing antibody treated mice and non-treated mice, it was apparent that dampening of viral infection and spread by administering neutralizing antibody allowed the virus to survive and spread to the spinal cord [Sun and Perlman, 1995].
Similarly, Wada and Fujinami [1993] demonstrated that infection of the olfactory nerve by TMEV rapidly produced encephalitis in nude mice. By contrast, intracranial inoculation of this same virus that was associated with a slower viral spread throughout the CNS, resulting in demyelination of brain tissues. The use of nude mice allowed the investigators to dissect CNS transmission of TMEV by different routes of entry since inoculation of immunocompetent mice with TEMV results invariably in demyelination with strong antiviral immune response associated with viral persistence [Wada and Fujinami, 1993]. Collectively, these studies using animal models of CNS diseases suggest that: (1) the route of viral transmission may affect viral spread throughout the CNS; (2) the degree of viral infectivity could be an important factor that is associated with the disease outcomes; (3) immune reactivity in the CNS is critical in dampening viral infection but can also serve to promote viral survival. Furthermore, these animal studies also are in agreement with human clinical data that demonstrate the development of acute encephalitis occurs in mostly young children or the elderly, a time when the immune status may be somewhat compromised. MS, on the other hand, often strikes immunocompetent young adults and is a disease of chronic neuroinflammation.
CONCLUSION
HHV-6 infects most humans in the first years of life, and produces a lifelong infection thereafter. It is very difficult, therefore, to conclusively demonstrate that such a ubiquitous virus contributes to the pathogenesis of any particular condition. With that caveat, there is growing evidence associating HHV-6 infection with MS, MTLE, encephalitis in both immunocompromised and immunocompetent subjects, and with the CFS. The neuroimaging features of the various associations will lend themselves for earlier detection and prospective clinical trials. As more is learned about the cellular mechanisms of HHV-6 infections, it may be possible to establish more firmly a cause and effect relationship between HHV-6 and CNS disease.
REFERENCES
- Ablashi DV, Eastman HB, Owen CB, Roman MM, Friedman J, Zabriskie JB, Peterson DL, Pearson GR, Whitman JE. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J Clin Virol. 2000;16:179–191. doi: 10.1016/s1386-6532(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Ahlqvist J, Fotheringham J, Akhyani N, Yao K, Fogdell-Hahn A, Jacobson S. Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes. J Neurovirol. 2005;11:384–394. doi: 10.1080/13550280591002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhyani N, Berti R, Brennan MB, Soldan SS, Eaton JM, McFarland HF, Jacobson S. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: Increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis. 2000;182:1321–1325. doi: 10.1086/315893. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lafuente R, Martin-Estefania C, de Las Heras V, Castrillo C, Picazo JJ, Varela de Seijas E, Gonzalez RA. Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch Neurol. 2002;59:929–933. doi: 10.1001/archneur.59.6.929. [DOI] [PubMed] [Google Scholar]
- Araque A. Astrocytes process synaptic information. Neuron Glia Biol. 2008;4:3–10. doi: 10.1017/S1740925X09000064. [DOI] [PubMed] [Google Scholar]
- Asano Y, Yoshikawa T, Kajita Y, Ogura R, Suga S, Yazaki T, Nakashima T, Yamada A, Kurata T. Fatal encephalitis/encephalopathy in primary human herpesvirus-6 infection. Arch Dis Child. 1992;67:1484–1485. doi: 10.1136/adc.67.12.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beart PM, O’Shea RD. Transporters for L-glutamate: An update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Oligodendrocytes: Susceptibility to injury and involvement in neurologic disease. Neurology. 2009;72:1779–1785. doi: 10.1212/WNL.0b013e3181a6b123. [DOI] [PubMed] [Google Scholar]
- Berti R, Brennan MB, Soldan SS, Ohayon JM, Casareto L, McFarland HF, Jacobson S. Increased detection of serum HHV-6 DNA sequences during multiple sclerosis (MS) exacerbations and correlation with parameters of MS disease progression. J Neurovirol. 2002;8:250–256. doi: 10.1080/13550280290049615-1. [DOI] [PubMed] [Google Scholar]
- Birnbaum T, Padovan CS, Sporer B, Rupprecht TA, Ausserer H, Jaeger G, Pfister HW. Severe meningoencephalitis caused by human herpesvirus 6 type B in an immunocompetent woman treated with ganciclovir. Clin Infect Dis. 2005;40:887–889. doi: 10.1086/427943. [DOI] [PubMed] [Google Scholar]
- Blumberg BM, Mock DJ, Powers JM, Ito M, Assouline JG, Baker JV, Chen B, Goodman AD. The HHV6 paradox: Ubiquitous commensal or insidious pathogen? A two-step in situ PCR approach. J Clin Virol. 2000;16:159–178. doi: 10.1016/s1386-6532(99)00084-0. [DOI] [PubMed] [Google Scholar]
- Bou-Holaigah I, Rowe PC, Kan J, Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA. 1995;274:961–967. [PubMed] [Google Scholar]
- Buchwald D, Cheney PR, Peterson DL, Henry B, Wormsley SB, Geiger A, Ablashi DV, Salahuddin SZ, Saxinger C, Biddle R, Kikinis R, Jolesz FA, Folks T, Balachandran N, Peter JB, Gallo RC, Komaroff AL. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Ann Intern Med. 1992;116:103–113. doi: 10.7326/0003-4819-116-2-103. [DOI] [PubMed] [Google Scholar]
- Cermelli C, Jacobson S. Viruses and multiple sclerosis. Viral Immunol. 2000;13:255–267. doi: 10.1089/08828240050144590. [DOI] [PubMed] [Google Scholar]
- Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M, Schad PA, Stewart PM, Nowinski RC, Brown P, Burmer GC. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare AJ, Bearn J, Allain T, McGregor A, Wessely S, Murray RM, O’Keane V. Contrasting neuroendocrine responses in depression and chronic fatigue syndrome. J Affect Disord. 1995;34:283–289. doi: 10.1016/0165-0327(95)00026-j. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Kadom N, Santi MR, Mariani B, Lavenstein BL. Human herpesvirus 6 rhombencephalitis in immunocompetent children. J Child Neurol. 2007;22:1260–1268. doi: 10.1177/0883073807307086. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Chang T, Lavenstein BL, Mariani B. Acute and chronic magnetic resonance imaging of herpesvirus-6 associated encephalitis. J Pediatr Neurol. 2009;7:367–373. [Google Scholar]
- Daly E, Komaroff AL, Bloomingdale K, Wilson S, Albert MS. Neuropsychological function in patients with chronic fatigue syndrome, multiple sclerosis, and depression. Appl Neuropsychol. 2001;8:12–22. doi: 10.1207/S15324826AN0801_3. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JW, Toni I. Gray matter volume reduction in the chronic fatigue syndrome. Neuroimage. 2005;26:777–781. doi: 10.1016/j.neuroimage.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Deluca J, Christodoulou C, Diamond BJ, Rosenstein ED, Kramer N, Natelson BH. Working memory deficits in chronic fatigue syndrome: Differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. 2004;10:101–109. doi: 10.1017/S1355617704101124. [DOI] [PubMed] [Google Scholar]
- Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, Chrousos GP, Gold PW. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73:1224–1234. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- Demitrack MA, Gold PW, Dale JK, Krahn DD, Kling MA, Straus SE. Plasma and cerebrospinal fluid monoamine metabolism in patients with chronic fatigue syndrome: Preliminary findings. Biol Psychiatry. 1992;32:1065–1077. doi: 10.1016/0006-3223(92)90187-5. [DOI] [PubMed] [Google Scholar]
- Dewhurst S. Human herpesvirus type 6 and human herpesvirus type 7 infections of the central nervous system. Herpes. 2004;11:105A–111A. [PubMed] [Google Scholar]
- Dewhurst S, Skrincosky D, van Loon N. Human herpesvirus 6. Expert Rev Mol Med. 1997;1997:1–17. doi: 10.1017/S146239949700001X. [DOI] [PubMed] [Google Scholar]
- Donati D, Akhyani N, Fogdell-Hahn A, Cermelli C, Cassiani-Ingoni R, Vortmeyer A, Heiss JD, Cogen P, Gaillard WD, Sato S, Theodore WH, Jacobson S. Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections. Neurology. 2003;61:1405–1411. doi: 10.1212/01.wnl.0000094357.10782.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyski WR, Knox KK, Majewski D, Carrigan DR. Brief report: Fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med. 1994;330:1356–1360. doi: 10.1056/NEJM199405123301905. [DOI] [PubMed] [Google Scholar]
- Epstein LND, Hamidullah A, Pellock JM, Frank LM, Lewis DV, Hesdorffer DC, Marmarou A, O’Dell C, Shinnar S. The role of primary human herpesvirus 6, 7 (HHV-6, HHV-7) infection in febrile status epilepticus. J Clin Virol. 2006;37:S116. [Google Scholar]
- Fotheringham J, Jacobson S. Human herpesvirus 6 and multiple sclerosis: Potential mechanisms for virus-induced disease. Herpes. 2005;12:4–9. [PubMed] [Google Scholar]
- Fotheringham J, Akhyani N, Vortmeyer A, Donati D, Williams E, Oh U, Bishop M, Barrett J, Gea-Banacloche J, Jacobson S. Detection of active human herpesvirus-6 infection in the brain: Correlation with polymerase chain reaction detection in cerebrospinal fluid. J Infect Dis. 2007a;195:450–454. doi: 10.1086/510757. [DOI] [PubMed] [Google Scholar]
- Fotheringham J, Donati D, Akhyani N, Fogdell-Hahn A, Vortmeyer A, Heiss JD, Williams E, Weinstein S, Bruce DA, Gaillard WD, Sato S, Theodore WH, Jacobson S. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 2007b;4:e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotheringham J, Williams EL, Akhyani N, Jacobson S. Human herpesvirus 6 (HHV-6) induces dysregulation of glutamate uptake and transporter expression in astrocytes. J Neuroimmune Pharmacol. 2008;3:105–116. doi: 10.1007/s11481-007-9084-0. [DOI] [PubMed] [Google Scholar]
- Freeman R, Komaroff AL. Does the chronic fatigue syndrome involve the autonomic nervous system? Am J Med. 1997;102:357–364. doi: 10.1016/s0002-9343(97)00087-9. [DOI] [PubMed] [Google Scholar]
- Fujimaki K, Mori T, Kida A, Tanaka M, Kawai N, Matsushima T, Kishi K, Fujisawa S, Sakura T, Yokota A, Kanda Y, Taguchi J, Akiyama H, Kanamori H, Maruta A, Okamoto S, Sakamaki H. Human herpesvirus 6 meningoencephalitis in allogeneic hematopoietic stem cell transplant recipients. Int J Hematol. 2006;84:432–437. doi: 10.1532/IJH97.06072. [DOI] [PubMed] [Google Scholar]
- Fujiwara FTK, Wada N, Kihara M, Ohno K, Odani I, Ishizaki T, Yamada A, Sudo S. A case of human her-pesvirus-6 encephalitis with prolonged seizures followed byfocal spikes (in Japanese) Nippon Shonikagakkai Zasshi (Tokyo) 1996;100:1117–1122. [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A, International Chronic Fatigue Syndrome Study Group The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Gorniak RJ, Young GS, Wiese DE, Marty FM, Schwartz RB. MR imaging of human herpesvirus-6-associated encephalitis in 4 patients with anterograde amnesia after allogeneic hematopoietic stem-cell transplantation. AJNR Am J Neuroradiol. 2006;27:887–891. [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, Knott A, Dewhurst S, Insel RA, Epstein LG. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331:432–438. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- He J, McCarthy M, Zhou Y, Chandran B, Wood C. Infection of primary human fetal astrocytes by human herpesvirus 6. J Virol. 1996;70:1296–1300. doi: 10.1128/jvi.70.2.1296-1300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ. 2006;333:575. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HIH, Imaeda H, Mizutani F, Ando M, Matsubayashi T, Suzuki Y, Nishimura Y. A case of HHV-6 encephalitis (in Japanese) Shonika Rinsho (Tokyo) 1992;45:273–276. [Google Scholar]
- Irving WL, Chang J, Raymond DR, Dunstan R, Grattan-Smith P, Cunningham AL. Roseola infantum and other syndromes associated with acute HHV6 infection. Arch Dis Child. 1990;65:1297–1300. doi: 10.1136/adc.65.12.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson E, Glaser CA, Forghani B, Amad Z, Wallace M, Armstrong RW, Exner MM, Schmid S. Evidence of human herpesvirus 6 infection in 4 immunocompetent patients with encephalitis. Clin Infect Dis. 2005;40:890–893. doi: 10.1086/427944. [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Yamada S, Takahashi T, Takahashi Y, Togashi T, Okuno T, Yamanishi K. Meningo-encephalitis associated with HHV-6 related exanthem subitum. Acta Paediatr Scand. 1990;79:987–989. doi: 10.1111/j.1651-2227.1990.tb11369.x. [DOI] [PubMed] [Google Scholar]
- Itokazu NIS, Ooba K, Sonoda T, Sugimoto T. Chorea-like involuntary movements after encephalitis due tohuman herpesvirus-6 (in Japanese) Shonika Rinsho (Tokyo) 1994;47:1339–1344. [Google Scholar]
- Jones CM, Dunn HG, Thomas EE, Cone RW, Weber JM. Acute encephalopathy and status epilepticus associated with human herpes virus 6 infection. Dev Med Child Neurol. 1994;36:646–650. doi: 10.1111/j.1469-8749.1994.tb11903.x. [DOI] [PubMed] [Google Scholar]
- Kamei A, Ichinohe S, Onuma R, Hiraga S, Fujiwara T. Acute disseminated demyelination due to primary human herpesvirus-6 infection. Eur J Pediatr. 1997;156:709–712. doi: 10.1007/s004310050695. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA. Periventricular white matter damage in the hypoxic neonatal brain: Role of microglial cells. Prog Neurobiol. 2009;87:264–280. doi: 10.1016/j.pneurobio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Kimura S, Nezu A. Neuroradiologic findings of brain lesions related to exanthema subitum. Pediatr Neurol. 1998;19:343–346. doi: 10.1016/s0887-8994(98)00069-1. [DOI] [PubMed] [Google Scholar]
- Koelle DM, Barcy S, Huang ML, Ashley RL, Corey L, Zeh J, Ashton S, Buchwald D. Markers of viral infection in monozygotic twins discordant for chronic fatigue syndrome. Clin Infect Dis. 2002;35:518–525. doi: 10.1086/341774. [DOI] [PubMed] [Google Scholar]
- Komaroff AL. Is human herpesvirus-6 a trigger for chronic fatigue syndrome? J Clin Virol. 2006;37:S39–46. doi: 10.1016/S1386-6532(06)70010-5. [DOI] [PubMed] [Google Scholar]
- Komaroff AL, Fagioli LR, Doolittle TH, Gandek B, Gleit MA, Guerriero RT, Kornish RJ, II, Ware NC, Ware JE, Jr., Bates DW. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med. 1996;101:281–290. doi: 10.1016/S0002-9343(96)00174-X. [DOI] [PubMed] [Google Scholar]
- Lange G, Holodny AI, DeLuca J, Lee HJ, Yan XH, Steffener J, Natelson BH. Quantitative assessment of cerebral ventricular volumes in chronic fatigue syndrome. Appl Neuropsychol. 2001;8:23–30. doi: 10.1207/S15324826AN0801_4. [DOI] [PubMed] [Google Scholar]
- Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: Effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. 2008;29:902–910. [PubMed] [Google Scholar]
- Maloney EM, Boneva R, Nater UM, Reeves WC. Chronic fatigue syndrome and high allostatic load: Results from a population-based case-control study in Georgia. Psychosom Med. 2009;71:549–556. doi: 10.1097/PSY.0b013e3181a4fea8. [DOI] [PubMed] [Google Scholar]
- Marcel B, Komaroff AL, Fagioli LR, Kornish RJ, II, Albert MS. Cognitive deficits in patients with chronic fatigue syndrome. Biol Psychiatry. 1996;40:535–541. doi: 10.1016/0006-3223(95)00422-x. [DOI] [PubMed] [Google Scholar]
- McFarland HF, Jacobson S. Natalizumab and immune cells. Arch Neurol. 2006;63:1366–1367. doi: 10.1001/archneur.63.10.1366. [DOI] [PubMed] [Google Scholar]
- Meeuwsen S, Persoon-Deen C, Bsibsi M, Bajramovic JJ, Ravid R, De Bolle L, van Noort JM. Modulation of the cytokine network in human adult astrocytes by human herpesvirus-6A. J Neuroimmunol. 2005;164:37–47. doi: 10.1016/j.jneuroim.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Mock DJ, Powers JM, Goodman AD, Blumenthal SR, Ergin N, Baker JV, Mattson DH, Assouline JG, Bergey EJ, Chen B, Epstein LG, Blumberg BM. Association of human herpesvirus 6 with the demyelinative lesions of progressive multifocal leukoencephalopathy. J Neurovirol. 1999;5:363–373. doi: 10.3109/13550289909029477. [DOI] [PubMed] [Google Scholar]
- Moorkens G, Berwaerts J, Wynants H, Abs R. Characterization of pituitary function with emphasis on GH secretion in the chronic fatigue syndrome. Clin Endocrinol (Oxf) 2000;53:99–106. doi: 10.1046/j.1365-2265.2000.01049.x. [DOI] [PubMed] [Google Scholar]
- Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009;2:1–16. [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Kimura I, Abe Y, Oka A. HHV-6 encephalopathy with cluster of convulsions during eruptive stage. Pediatr Neurol. 2007;36:61–63. doi: 10.1016/j.pediatrneurol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Naschitz JE, Sabo E, Naschitz S, Rosner I, Rozenbaum M, Priselac RM, Gaitini L, Zukerman E, Yeshurun D. Fractal analysis and recurrence quantification analysis of heart rate and pulse transit time for diagnosing chronic fatigue syndrome. Clin Auton Res. 2002;12:264–272. doi: 10.1007/s10286-002-0044-8. [DOI] [PubMed] [Google Scholar]
- Nicolson GL, Gan R, Haier J. Multiple co-infections (Mycoplasma, Chlamydia, human herpes virus-6) in blood of chronic fatigue syndrome patients: Association with signs and symptoms. APMIS. 2003;111:557–566. doi: 10.1034/j.1600-0463.2003.1110504.x. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Mihara F, Yoshiura T, Togao O, Atsumi K, Matsuura T, Kuroiwa T, Honda H. MR imaging of human herpesvirus-6 encephalopathy after hematopoietic stem cell transplantation in adults. AJNR Am J Neuroradiol. 2006;27:2191–2195. [PMC free article] [PubMed] [Google Scholar]
- Oki J, Yoshida H, Tokumitsu A, Takahashi S, Miyamoto A, Yoda M, Miura J. Serial neuroimages of acute necrotizing encephalopathy associated with human herpesvirus 6 infection. Brain Dev. 1995;17:356–359. doi: 10.1016/0387-7604(95)00077-o. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Baranzini SE, Sawcer S, Hauser SL. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat Rev Genet. 2008;9:516–526. doi: 10.1038/nrg2395. [DOI] [PubMed] [Google Scholar]
- Opsahl ML, Kennedy PG. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain. 2005;128:516–527. doi: 10.1093/brain/awh390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsahl ML, Kennedy PG. Investigating the presence of human herpesvirus 7 and 8 in multiple sclerosis and normal control brain tissue. J Neurol Sci. 2006;240:37–44. doi: 10.1016/j.jns.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsahl ML, Kennedy PG. An attempt to investigate the presence of Epstein Barr virus in multiple sclerosis and normal control brain tissue. J Neurol. 2007;254:425–430. doi: 10.1007/s00415-006-0316-7. [DOI] [PubMed] [Google Scholar]
- Pampliega O, Domercq M, Villoslada P, Sepulcre J, Rodriguez-Antiguedad A, Matute C. Association of an EAAT2 polymorphism with higher glutamate concentration in relapsing multiple sclerosis. J Neuroimmunol. 2008;195:194–198. doi: 10.1016/j.jneuroim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Patnaik M, Komaroff AL, Conley E, Ojo-Amaize EA, Peter JB. Prevalence of IgM antibodies to human herpesvirus 6 early antigen (p41/38) in patients with chronic fatigue syndrome. J Infect Dis. 1995;172:1364–1367. doi: 10.1093/infdis/172.5.1364. [DOI] [PubMed] [Google Scholar]
- Provenzale JM, vanLandingham KE, Lewis DV, Mukundan S, Jr., White LE. Extrahippocampal involvement in human herpesvirus 6 encephalitis depicted at MR imaging. Radiology. 2008;249:955–963. doi: 10.1148/radiol.2492071917. [DOI] [PubMed] [Google Scholar]
- Provenzale JM, van Landingham K, White LE. Clinical and imaging findings suggesting human herpesvirus 6 encephalitis. Pediatr Neurol. 2010;42:32–39. doi: 10.1016/j.pediatrneurol.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Reeves WC, Stamey FR, Black JB, Mawle AC, Stewart JA, Pellett PE. Human herpesviruses 6 and 7 in chronic fatigue syndrome: A case-control study. Clin Infect Dis. 2000;31:48–52. doi: 10.1086/313908. [DOI] [PubMed] [Google Scholar]
- Reynolds KJ, Vernon SD, Bouchery E, Reeves WC. The economic impact of chronic fatigue syndrome. Cost Eff Resour Alloc. 2004;2:4. doi: 10.1186/1478-7547-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Inoue T, Kajiwara M, Miyazaki C, Kusunoki K, Ueda K. Acute encephalopathy following exanthem subitum caused by human herpesvirus-6. Kansenshogaku Zasshi. 1992;66:551–554. doi: 10.11150/kansenshogakuzasshi1970.66.551. [DOI] [PubMed] [Google Scholar]
- Schmaling KB, Lewis DH, Fiedelak JI, Mahurin R, Buchwald DS. Single-photon emission computerized tomography and neurocognitive function in patients with chronic fatigue syndrome. Psychosom Med. 2003;65:129–136. doi: 10.1097/01.psy.0000038942.33335.9b. [DOI] [PubMed] [Google Scholar]
- Schwartz RB, Garada BM, Komaroff AL, Tice HM, Gleit M, Jolesz FA, Holman BL. Detection of intracranial abnormalities in patients with chronic fatigue syndrome: Comparison of MR imaging and SPECT. AJR Am J Roentgenol. 1994a;162:935–941. doi: 10.2214/ajr.162.4.8141020. [DOI] [PubMed] [Google Scholar]
- Schwartz RB, Komaroff AL, Garada BM, Gleit M, Doolittle TH, Bates DW, Vasile RG, Holman BL. SPECT imaging of the brain: Comparison of findings in patients with chronic fatigue syndrome, AIDS dementia complex, and major unipolar depression. AJR Am J Roentgenol. 1994b;162:943–951. doi: 10.2214/ajr.162.4.8141022. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Carrigan DR, Asano Y, Benedetti L, Crowley RW, Komaroff AL, Gallo RC, Lusso P. Detection of human herpesvirus 6 in plasma of children with primary infection and immunosuppressed patients by polymerase chain reaction. J Infect Dis. 1995;171:273–280. doi: 10.1093/infdis/171.2.273. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH, Baden LR, Bromfield EB. Post-transplant acute limbic encephalitis: Clinical features and relationship to HHV6. Neurology. 2007;69:156–165. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- Singh N, Paterson DL. Encephalitis caused by human herpesvirus-6 in transplant recipients: Relevance of a novel neurotropic virus. Transplantation. 2000;69:2474–2479. doi: 10.1097/00007890-200006270-00002. [DOI] [PubMed] [Google Scholar]
- Sloots TP, Mackay IM, Carroll P. Meningoencephalitis in an adult with human herpesvirus-6 infection. Med J Aust. 1993;159:838. doi: 10.5694/j.1326-5377.1993.tb141389.x. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Berti R, Salem N, Secchiero P, Flamand L, Calabresi PA, Brennan MB, Maloni HW, McFarland HF, Lin HC, Patnaik M, Jacobson S. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: Increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Leist TP, Juhng KN, McFarland HF, Jacobson S. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann Neurol. 2000;47:306–313. [PubMed] [Google Scholar]
- Steinman L. Blocking adhesion molecules as therapy for multiple sclerosis: Natalizumab. Nat Rev Drug Discov. 2005;4:510–518. doi: 10.1038/nrd1752. [DOI] [PubMed] [Google Scholar]
- Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res. 2000;48:218–226. doi: 10.1203/00006450-200008000-00016. [DOI] [PubMed] [Google Scholar]
- Suga SKS, Kozawa T, Oonishi M, Asano Y. Electroencephalogram of a patient with status epilepticus due to exanthem subitum meningoencephalitis (in Japanese) Shonika Rinsho (Tokyo) 1993;46:133–138. [Google Scholar]
- Sun N, Perlman S. Spread of a neurotropic coronavirus to spinal cord white matter via neurons and astrocytes. J Virol. 1995;69:633–641. doi: 10.1128/jvi.69.2.633-641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HOS, Yamashita S, Miyake S, Yamada M, Iwa-moto H. A case of exanthem subitum and encephalitic illness resulting in severe sequelae (in Japanese) Nippon Shounikagakkai Zasshi (Tokyo) 1993;97:1647–1652. [Google Scholar]
- Tanaka M, Sadato N, Okada T, Mizuno K, Sasabe T, Tanabe HC, Saito DN, Onoe H, Kuratsune H, Watanabe Y. Reduced responsiveness is an essential feature of chronic fatigue syndrome: A fMRI study. BMC Neurol. 2006;6:9. doi: 10.1186/1471-2377-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Killian JM, Zhang JZ. Detection of viral DNA and immune responses to the human herpesvirus 6 101-kilodalton virion protein in patients with multiple sclerosis and in controls. J Virol. 2002;76:6147–6154. doi: 10.1128/JVI.76.12.6147-6154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore WH, Epstein L, Gaillard WD, Shinnar S, Wainwright MS, Jacobson S. Human herpes virus 6B: A possible role in epilepsy? Epilepsia. 2008;49:1828–1837. doi: 10.1111/j.1528-1167.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiersky LA, Johnson SK, Lange G, Natelson BH, DeLuca J. Neuropsychology of chronic fatigue syndrome: A critical review. J Clin Exp Neuropsychol. 1997;19:560–586. doi: 10.1080/01688639708403744. [DOI] [PubMed] [Google Scholar]
- Uesugi H, Shimizu H, Maehara T, Arai N, Nakayama H. Presence of human herpesvirus 6 and herpes simplex virus detected by polymerase chain reaction in surgical tissue from temporal lobe epileptic patients. Psychiatry Clin Neurosci. 2000;54:589–593. doi: 10.1046/j.1440-1819.2000.00758.x. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Perez-Cerda F, Ravid R, Matute C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol Dis. 2006;21:154–164. doi: 10.1016/j.nbd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Vercellino M, Merola A, Piacentino C, Votta B, Capello E, Mancardi GL, Mutani R, Giordana MT, Cavalla P. Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: Correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J Neuropathol Exp Neurol. 2007;66:732–739. doi: 10.1097/nen.0b013e31812571b0. [DOI] [PubMed] [Google Scholar]
- Villoslada P, Juste C, Tintore M, Llorenc V, Codina G, Pozo-Rosich P, Montalban X. The immune response against herpesvirus is more prominent in the early stages of MS. Neurology. 2003;60:1944–1948. doi: 10.1212/01.wnl.0000069461.53733.f7. [DOI] [PubMed] [Google Scholar]
- Vu T, Carrum G, Hutton G, Heslop HE, Brenner MK, Kamble R. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:705–709. doi: 10.1038/sj.bmt.1705666. [DOI] [PubMed] [Google Scholar]
- Wada Y, Fujinami RS. Viral infection and dissemination through the olfactory pathway and the limbic system by Theiler’s virus. Am J Pathol. 1993;143:221–229. [PMC free article] [PubMed] [Google Scholar]
- Wagner MKG, Ablashi DV, Whitman JE. Chronic fatigue syndrome (CFS): A critical evaluation of testing for active human herpesvirus-6 (HHV-6) infection: Review of data of 107 cases. J Chron Fatigue Syndr. 1996;2:3–16. [Google Scholar]
- Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Coleman RE, Morgan MA, Hulette C, Kurtzberg J, Bushnell C, Epstein L, Lewis DV. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50:612–619. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Ouchi Y, Onoe H, Yoshikawa E, Tsukada H, Takahashi H, Iwase M, Yamaguti K, Kuratsune H, Watanabe Y. Reduction of serotonin transporters of patients with chronic fatigue syndrome. Neuroreport. 2004;15:2571–2574. doi: 10.1097/00001756-200412030-00002. [DOI] [PubMed] [Google Scholar]
- Yanagihara K, Tanaka-Taya K, Itagaki Y, Toribe Y, Arita K, Yamanishi K, Okada S. Human herpesvirus 6 meningoencephalitis with sequelae. Pediatr Infect Dis J. 1995;14:240–242. [PubMed] [Google Scholar]
- Yao K, Gagnon S, Akhyani N, Williams E, Fotheringham J, Frohman E, Stuve O, Monson N, Racke MK, Jacobson S. Reactivation of human herpesvirus-6 in natalizumab treated multiple sclerosis patients. PLoS ONE. 2008;3:e2028. doi: 10.1371/journal.pone.0002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Honarmand S, Espinosa A, Akhyani N, Glaser C, Jacobson S. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65:257–267. doi: 10.1002/ana.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Nakashima T, Suga S, Asano Y, Yazaki T, Kimura H, Morishima T, Kondo K, Yamanishi K. Human herpesvirus-6 DNA in cerebrospinal fluid of a child with exanthem subitum and meningoencephalitis. Pediatrics. 1992;89:888–890. [PubMed] [Google Scholar]
- Yoshinari S, Hamano S, Minamitani M, Tanaka M, Eto Y. Human herpesvirus 6 encephalopathy predominantly affecting the frontal lobes. Pediatr Neurol. 2007;36:13–16. doi: 10.1016/j.pediatrneurol.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Zorzenon MRG, Botta GA, Colle R, Barsanti LA, Ceccherini-Nelli L. Active HHV-6 infection in chronic fatigue syndrome patients from Italy: New data. J Chron Fatigue. 1996;2:3–12. [Google Scholar]