Abstract
The membrane co-factor protein CD46 is the cellular receptor for a number of pathogens including the human herpesvirus 6 (HHV-6). In addition to its function as an inhibitory complement receptor, engagement of CD46 in the context of T-cell receptor (TCR) signaling influences T-cell activation. Simultaneous cross-linking of the CD3/ CD46 molecules led to differentiation of a unique population of CD4+ T-cell subset characterized by enhanced expressions of IFN-γ, IL-10, granzyme B, adhesion molecule MAdCAM-1 (alpha-4-beta-7), surface-bound cytokine LIGHT, and chemokine receptor CCR9. Multiple sclerosis is a chronic inflammatory neurodegenerative disorder of the central nervous system (CNS) with unknown etiology. The HHV-6 is a candidate pathogen in MS and uses the CD46 molecule as its receptor. We hypothesize that binding of the HHV-6 glycoprotein to CD46 may trigger a pro-inflammatory response that could contribute to CNS tissue damage. To address this question, we examined immunological parameters such as proliferation, cytokine production and cytotoxic functions in CD4+ T cells of healthy individuals and MS patients following CD3/CD46 co-engagement by using anti-CD3 and anti-CD46 monoclonal antibodies as surrogates to mimic T-cell receptor and CD46 signaling. Our results demonstrated that CD3/CD46 cross-linking induced expression of IL-1β and IL-17A in multiple sclerosis patient T cells. Additionally, increase in transient surface expression of lysosomal associated protein CD107a suggested enhanced CD4+ T-cell cytotoxic functions following CD3/CD46 co-stimulation. Collectively, this study demonstrated evidence to suggest a potential mechanism of virus-induced neuroinflammation that may be involved in MS disease pathogenesis.
Keywords: CD46, HHV-6, IL-17, multiple sclerosis
Introduction
The CD46 protein is a ubiquitously expressed cell-surface molecule that has been identified as the binding receptor of at least seven different pathogenic bacteria and viruses including the Edmonston strain of measles virus, human herpesvirus 6 (HHV-6), and type IV pili of pathogenic Neisseria (Cattaneo 2004). Furthermore, CD46 is also involved in regulating a number processes in both the innate and adaptive immune responses in human (Astier et al. 2000; Zaffran et al. 2001; Marie et al. 2002; Kemper et al. 2003; Grossman et al. 2004; Russell 2004; Barchet et al. 2006; Alford et al. 2008). As a member of the regulator of complement activation proteins family, CD46 prevents spontaneous complement attack on host tissues in vivo by binding to complement components C3b and C4b (Riley-Vargas et al. 2004; Russell 2004). In the adaptive immunity, CD46 engagement has been shown to influence inflammation by functioning as a co-stimulatory molecule in CD4+ T-lymphocyte activation (Astier et al. 2000). Several additional studies also demonstrated that cross-linking of the CD3 and CD46 molecules on healthy individual T lymphocytes with monoclonal antibodies led to the differentiation of a unique population of T-regulatory cells characterized by enhanced IL-10 production as well as high granzyme B expression (Kemper et al. 2003; Grossman et al. 2004; Barchet et al. 2006). Furthermore, CD3/CD46-activated CD4+ lymphocytes up-regulated the expressions of adhesion molecule MAdCAM-1 (alpha-4-beta-7), surface-bound cytokine LIGHT—a herpes virus entry mediator on lymphocytes, and chemokine receptor CCR9. Increase in expression of cell migration molecules was suggested to alter the ability of these effector T cells to home to specific tissue sites during an inflammatory response (Alford et al. 2008). However, in MS patient CD4+ T cells, over-expression of the intracellular cytoplasmic tail-2 of the CD46 molecule was associated with diminished IL-10 production, suggesting a possible dysregulation in the CD46 receptor mediated signal transduction pathway in MS patients (Astier et al. 2006). Additionally, engagement of the CD46 molecule on myeloid-derived dendritic cells obtained from multiple sclerosis (MS) patients induced pronounced secretion of the IL-23 cytokine (Vaknin-Dembinsky et al. 2008). IL-23 is a cytokine produced by antigen presenting cells (APC) that has recently been determined to play a critical role in differentiation and maintenance of the highly pro-inflammatory TH-17 CD4+ T lymphocytes that are hypothesized to drive development of autoimmunity (Park et al. 2005; Chen et al. 2006; Bettelli et al. 2007; Awasthi et al. 2009). Studies on experimental autoimmune encephalomyelitis (EAE; an animal model of MS) have identified this TH-17 CD4+ T cell subset to be encephalitogenic, causing CNS demyelination in mice (Ogura et al. 2008; Stromnes et al. 2008). In addition, evidence supporting the role of IL-17 in MS pathogenesis has also been described. Immunohistochemistry and in situ analysis of MS patient brain tissues demonstrated increased IL-17 mRNA and protein expressions in infiltrating perivascular lymphocytes within areas of MS plaques compared with unaffected normal appearing white matters (Tzartos et al. 2008). Furthermore, enhanced IL-17 mRNA expression was observed in cerebrospinal fluid from MS patients (Graber et al. 2008). Collectively, these studies suggest that CD46-mediated signaling is involved in the regulation of a wide spectrum of immunological functions. Therefore, it is possible that interactions of CD46 with the various in vivo binding ligands such as complement molecules (C3b and C4b), bacterial and viral glycoproteins, in part, could contribute to development of the complex inflammatory activities in MS pathophysiology.
A neurodegenerative demyelinating inflammatory disorder of the CNS, MS is often associated with infections of ubiquitous viral agents that are suggested to act as triggers in disease development (Cermelli and Jacobson 2000). Among a diverse repertoire of pathogens that have been linked to MS, measles virus and the HHV-6 virus are two distinct agents that have been implicated in the disease and are also known to use the CD46 molecule as their cell-surface binding receptor (Cattaneo 2004; Astier 2008). In particular, HHV-6 has been identified in brains of subjects with MS including within plaques (Cermelli and Jacobson 2000; Challoner et al. 1995). Binding of these pathogen glycoproteins with CD46 triggered down-modulation of its cell-surface expression (Santoro et al. 1999; Crimeen-Irwin et al. 2003). Concurrently, aberrant T cell immunities against viral antigens of these two infectious agents have also been demonstrated in MS patients. Exposure of MS patient T cells to antigens of the Edmonston strain of the measles virus resulted in functional suppression of cyto-toxic CD4+ lymphocytes (Jacobson et al. 1985). In contrast, infected HHV-6 strain U1102 cell lysate preparation induced proliferation and IFN-γ production in T cells of a subset of the MS patients (Soldan et al. 2000). Interestingly, Oliaro and colleagues demonstrated that while cross-linking of CD46 on cytotoxic T lymphocytes separately from CD3 stimulation-inhibited TCR signaling, co-ligation of naïve T cells with antibodies to CD3 and CD46 caused an increase in T cell activation and IFN-γ production. Additionally, it was further demonstrated that ligation with measles hemagglutinin protein expressed on LH cells without CD3 stimulation led to a decrease in IFN-γ production suggesting a mechanism of T cell function subversion by the virus (Oliaro et al. 2006). On the contrary, binding of another pathogen glycoprotein from Streptococcus pyogene to CD46 in the presence of CD3 was shown to induce lymphocyte activation marked by high level of IL-10 production and increased expression of cytotoxic granzyme B granules that correlated with cytotoxic function (Barchet et al. 2006). These studies indicate that interactions of CD46 with the various biological binding ligands in conjunction with activation of T cells through CD3-mediated signaling cascade could contribute to divergent immune responses.
Using an immunoaffinity column comprised of mono-clonal antibodies to CD46, Fogdell-Hahn and colleagues demonstrated that HHV-6 viral particles were co-purified with a soluble form of CD46 in 4 of 42 MS sera but not from healthy individuals' sera (Ahlqvist et al. 2005). This study provided evidence to suggest a physical association between HHV-6 and the CD46 receptor that could occur in vivo in a subset of MS patients. We had previously demonstrated that CNS glial cells are susceptible to HHV-6 infection (Ahlqvist et al. 2005; Donati et al. 2005; Yao et al. 2006), and evidence of HHV-6 reactivation specifically confined to the CNS compartments was observed in inflammatory neurologic conditions including encephalitis and MS patients (Suen et al. 2008; Yao et al. 2009). Here, we hypothesize that interaction of HHV-6 glycoproteins expressed on infected CNS cells with CD46 expressed on responding CD4+ T lymphocytes could potentially trigger an aberrant pro-inflammatory response directed against CNS tissues. Given these divergent immunological functions CD46 participates in (Russell 2004), and the frequent association of MS with HHV-6, understanding the cellular and functional consequences following CD46 binding with HHV-6 envelope glycoprotein may shed light on possible mechanisms of virus-induced CNS inflammation. To address this issue, we used the J4.48 anti-CD46 monoclonal antibody that was shown to abrogate HHV-6 infection (Donati et al. 2005) as a surrogate to simulate the binding between the CD46 receptor and HHV-6 glycoprotein on lymphocytes in order to investigate changes in lymphocyte proliferation, cytokine production and cytotoxicity function in CD4+ T cells from relapsing-remitting MS (RRMS) patients and healthy controls.
Results and discussion
CD46 provides T-cell co-stimulation
The activation of naïve T cells requires signals from the TCR and a second, co-stimulatory signal. The engagement of a co-stimulatory molecule such as CD28 prevents energy in naïve T cells following TCR stimulation, and supports T-cell proliferation and the production of cytokines. Studies have shown that other molecules such as CD46 and OX40 are also capable of providing T-cell co-stimulatory signals (Zaffran et al. 2001; Nakae et al. 2003). Cross-linking of CD46 molecules in the context of TCR stimulation results in signaling that supports T-cell activation, as evidenced by phosphorylation of linker for activation of T cells and extracellular-signal related kinase/mitogen-activated protein kinase (Astier et al. 2000; Zaffran et al. 2001).
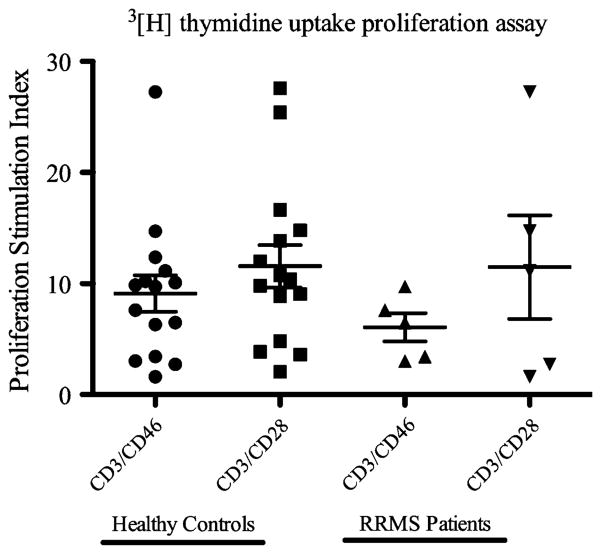
CD46 co-stimulation can elicit proliferative response in T cells comparable to CD28 co-stimulation. Purified CD4+ T cells from healthy donors stimulated with anti-CD3 and anti-CD46 antibodies showed proliferative responses comparable to that of anti-CD3 and anti-CD28 stimulation (Fig. 1). In subjects with MS, although the proliferative response to CD46 co-stimulation appeared more restricted compared with CD28 co-stimulation, the difference was not statistically significant (Fig. 1). Thus, proliferative response to CD46 co-stimulation did not distinguish healthy donors and subjects with MS.
Fig. 1.
Comparison of proliferative responses between healthy controls and RRMS patients CD4+ T cells after 72 h of stimulation. CD4+ T cells purified by magnetic beads were stimulated with soluble IgG isotype control, anti-CD3 and anti-CD46, or anti-CD3 and anti-CD28 and pulsed with 3[H] thymidine for the last 6 h. Proliferation stimulation index=proliferation due to soluble anti-CD3 and anti-CD46 or anti-CD3 and anti-CD28/proliferation due to soluble IgG isotype alone. Statistical analysis was performed using ANOVA, and no significance was detected between groups
Differential cytokine profile induced by CD46 co-stimulation
Response to CD46 co-stimulation may differ from CD28 co-stimulation with respect to cytokine production. Cytokines reportedly induced by CD46 co-stimulation include IL-10. Kemper and colleagues showed that co-stimulation through CD3 and CD46 induced a T-regulatory 1 (Tr1)-specific cytokine profile in CD4+ T cells distinguished by high level of IL-10 production (Kemper et al. 2003). However, increased IL-10 production in the setting of CD46 co-stimulation was not consistently observed, and may be influenced by underlying disease, as subjects with renal failure did not show increased IL-10 production with CD46 co-stimulation (Brinkkoetter et al. 2005).
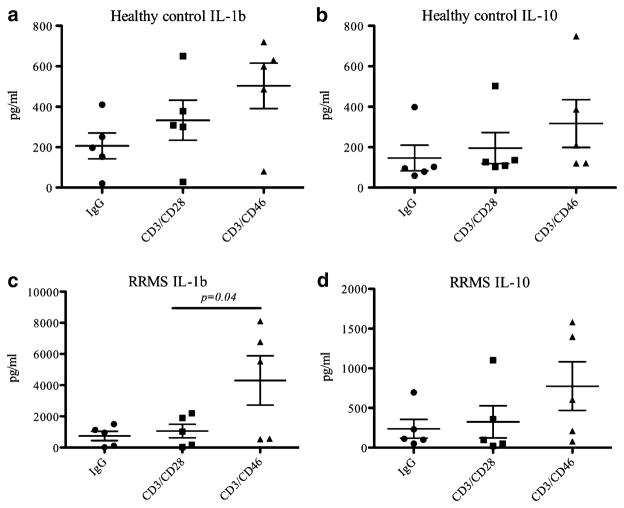
We have found that IL-1β may also be preferentially induced by CD46 co-stimulation. We tested the culture supernatant of lymphocytes from healthy donors and subjects with MS patients stimulated with anti-CD3 and anti-CD28 or anti-CD3 and anti-CD46 antibodies for production of inflammatory cytokines including IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IFN-γ, TNF-α, and MCP-1. CD46 co-stimulation resulted in increased IL-1β production in subjects with MS. IL-1β production was significantly higher in subjects with MS in the setting of CD46 co-stimulation compared to CD28. A trend towards increased IL-1β production with CD46 co-stimulation was also observed in healthy donors, but did not reach statistically significant difference. Compared to healthy donors, IL-1β production was several-fold higher in MS following CD46 co-stimulation (Fig. 2). IL-10 appeared increased with CD46 co-stimulation in MS, but the difference was not statistically significant. None of the other cytokines tested showed preferential production with CD46 co-stimulation.
Fig. 2.
Differential expression of IL-1b and IL-10 in healthy control and RRM patient CD4+ T cells. Purified CD4+ T cells from healthy controls or RRMS patients were stimulated with IgG isotype control, anti-CD3 and anti-CD28, or anti-CD3 and anti-CD46 antibodies for 3 days. Cytokine production in the culture supernatant was measured with the Raybio pro-inflammatory array as described in “Methods” Levels of IL-1β produced by a healthy control; c RRMS patients. IL-10 production in b healthy controls; d RRMS patients. Statistical analysis was performed with Student's t test (**p<0.05; ***p<0.005)
The finding of elevated IL-1β in response to CD3/CD46 stimulation may have relevance in MS disease pathogenesis. IL-1β has recently been shown to play an important role in the differentiation of TH-17 cells, an important pro-inflammatory T helper cell subset implicated in development of various autoimmune diseases including MS, rheumatoid arthritis, and systematic lupus erythematosis (Nakae et al. 2003; Ben-Sasson et al. 2009). Additionally, in vitro culture of astrocytes in the presence of IL-1β induced the expression of genes favoring vessel plasticity, including HIF-1α and its target, vascular endothelial growth factor-A. Consequently, changes in expressions of these proteins could alter the blood–brain barrier permeability and set the stage for the tissue damage in neurodegenerative disorders (Argaw et al. 2006). In an animal model of arthritis, IL-1β induced expression of a co-stimulatory molecule OX-40 that promoted differentiation of TH-17 cells (Nakae et al. 2003). Similarly, on human articular chondroctyes, exposure to IL-1β induced up-regulation of CD46 mRNA transcription (Hyc et al. 2003). Collectively, these studies support the role of IL-1β in influencing T cells activation by modulating expression levels of co-stimulatory molecules.
CD46 co-stimulation enhances IL-17A production in MS
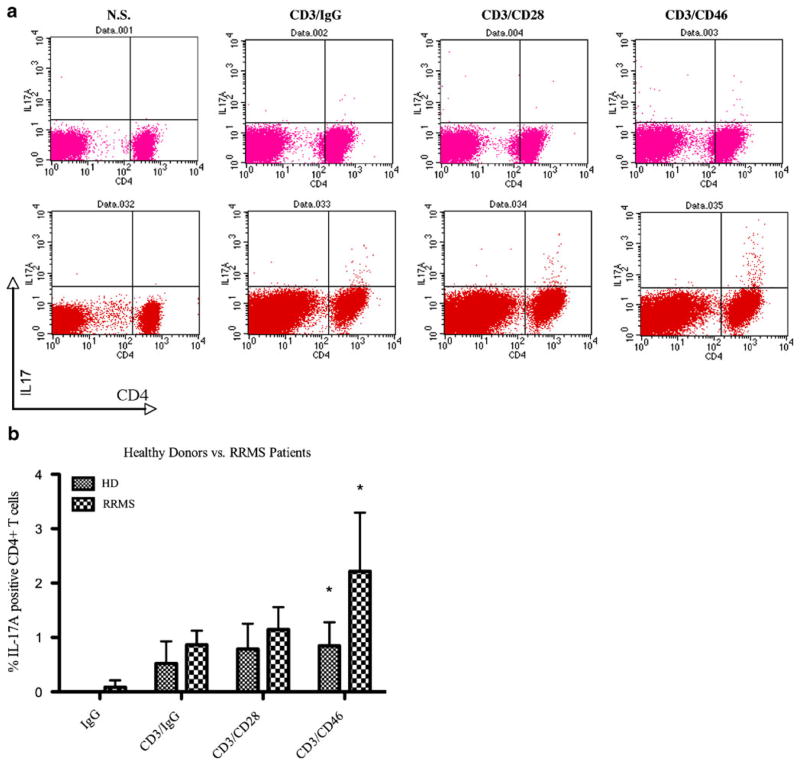
The work of Kemper and colleagues showed that CD46 co-stimulation could bias T-cell lineage differentiation to favor the development of Tr1 cells in healthy donors (Kemper et al. 2003). However, the cytokine production profile elicited by CD46 co-stimulation in MS showed a shift towards IL-1β over IL-10 production, suggesting a differential T-cell fate in MS compared to healthy volunteers in the setting of CD46 co-stimulation. Recently, a distinct subset of IL-17A producing T helper cells, TH-17, has been shown to contribute to autoimmune disorders including multiple sclerosis (Bettelli et al. 2007,2008). Although the conditions that drive differentiation of TH-17 cells are still being investigated, it has been shown that IL-1β, along with IL-21, IL-6, and TGF-β are essential cytokines that are required in differentiation of IL-17A producing cells (Nakae et al. 2003; Chen et al. 2006; Ogura et al. 2008; Yang et al. 2008). In addition, a recent study by Vaknin-Dembinsky and colleagues showed that the incubation of purified CD4+ T cells with culture supernatant of CD46 stimulated myeloid-derived dendritic cells led to heightened IL-17A expression (Vaknin-Dembinsky et al. 2008). Given the increased IL-1β production in subjects with MS following CD46 co-stimulation, we asked whether CD3/CD46 co-stimulation of lymphocytes in MS could induce the development of TH-17 cells. We stimulated peripheral blood mononuclear cells from healthy donors and subjects with MS with either CD46 or CD28 co-stimulation and assessed the development of TH-17 cells by detection of intracellular IL-17A production in CD4+-gated T cells. We found that whereas CD28 co-stimulation had negligible effect on IL-17A production, CD46 co-stimulation led to significantly greater frequency of IL-17A producing cells in MS. In contrast, CD46 co-stimulation had little effect on healthy donor peripheral blood mononuclear cells (PBMC) with respect to the differentiation of TH-17 cells (Fig. 3). These results suggest that a distinguishing feature of the response to CD46 co-stimulation in MS is the shift towards the development of TH-17 cells.
Fig. 3.
Intracellular flow cytometric analysis of IL-17A expression. PBMC from healthy donors (HD) and relapsing-remitting multiple sclerosis (RRMS) patients were stimulated with soluble IgG isotype control alone, anti-CD3 and IgG isotype control, anti-CD3 and anti-CD28 antibodies, or anti-CD3 and anti-CD46 for 72 h and analyzed by intracellular staining. a Representative FACS staining profile for healthy donor (top panels) and RRMS patient (bottom panels). b Quantification of percent IL-17A expressing CD4+ T cells in HD (n= 5) and RRMS patients (n=5) PBMC following the indicated in vitro stimulations. Statistical analysis was performed using 2-way ANOVA with Bonferroni correction (*p<0.001) during immune activation. Additionally, Kebir and colleagues
The role TH-17 cells in various autoimmune disorders such as RA, SLE, and MS are currently being investigated (Bettelli et al. 2007). Based on a series of studies with patient samples and various animal models, TH-17 cells are believed to be potentially pathogenic. TH-17 lymphocytes are thought to mediate inflammation by up-regulating expression of pro-inflammatory cytokines and chemokines demonstrated that TH-17 cells were also capable of eliciting cytotoxicity against human neuronal cultures in vitro through a granzyme B mediated mechanism (Kebir et al. 2007). Consistent with the proposed putative role of TH-17 cells in MS pathogenesis, IL-17-deficient animals exhibited delayed disease onset with diminished severity in the study of EAE, an animal model of MS (Komiyama et al. 2006). Taken together, these studies suggest a possible strategy of virus-triggered inflammation subsequent to cross-linking of the CD46 molecule with CD3 stimulation on CD4+ T lymphocytes.
Distinct binding domain of CD46 functions to mediate enhanced IL-17A production
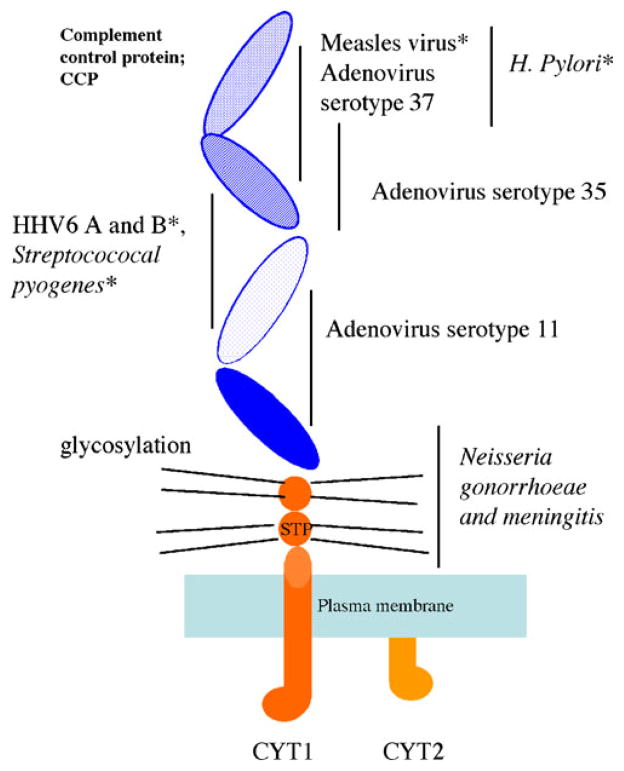
The CD46 molecule is comprised of four extracellular short-consensus-repeat domains (SCR, sushi domains), a heavily glycosylated region and the cytoplasmic domains (Fig. 4). Different pathogens are known to engage distinct domains on CD46. Greenstone and colleagues used a cell-fusion system to show that measles virus glycoprotein bound SCR 1 and 2, while blocking the SCR 2 and 3 domains with the J4.48 anti-CD46 monoclonal antibody abrogated HHV-6 infectivity. Type IV pili of the pathogenic Neisseria, on the other hand, likely interface with the heavily glycosylated region of CD46 (Kallstrom et al. 1997; Gill et al. 2003). Furthermore, the bound CD46 molecule is subject to different fates depending on the binding domain. In measles and HHV-6 infection, CD46 is down-modulated from the membrane surface by receptor internalization (Santoro et al. 1999; Crimeen-Irwin et al. 2003). By contrast, the CD46 molecule is lost by receptor shedding during infection with Neisseria gonorrhea (Gill et al. 2003).
Fig. 4.
Structure of CD46 and known binding sites for pathogens. STP serine, threonine, and proline rich region. CYT1 cytoplasmic tail domain 1. CYT2 cytoplasmic tail domain 2. Asterisk indicates pathogens associated with autoimmune diseases
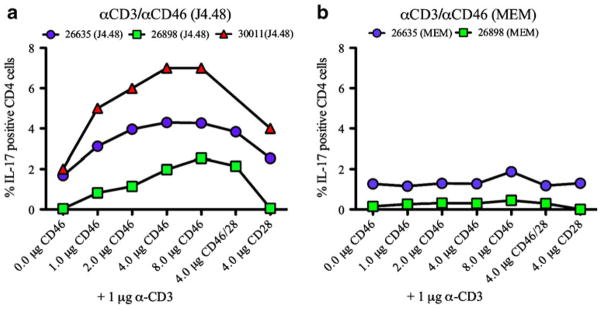
Although different pathogens are known to bind distinct domains on CD46, it is unknown whether the engagement of distinct domains mediates differential T-cell responses. To address this question, we stimulated healthy donor PBMC with two different commercially available anti-CD46 antibodies that are known to interact with separate regions of the molecule and assessed the resulting IL-17A expression by intracellular cytokine staining (Fig. 5). The monoclonal antibody clone J4.48 binds SCR 2 and 3, while the antibody clone MEM binds the glycosylated region of the CD46 molecule. As shown in Fig. 5, a dose-dependent increase in percent CD4+ T cell expressing IL-17A was observed in CD4+ T cells activated with the J4.48 clone of anti-CD46 antibody. By contrast, engagement with the MEM clone of anti-CD46 antibody had no effect on IL-17A expression in the CD4+ T cell subset (Fig. 5). These results indicate that the induction of IL-17A expressing cells was mediated by binding to the SCR 2 and 3, but not the glycosylated domain, and support to the prediction that engagement of CD46 by the viral glycoproteins (measles or HHV-6) is more likely than type IV pili of Neisseria to result in induction of TH17 responses.
Fig. 5.
Binding of distinct CD46 domains triggered differential IL-17A production. PBMC from healthy controls were cultured with anti-CD3 and anti-CD46 (clone J4.48) or anti-CD3 and anti-CD46 (clone MEM) for 72 h and IL-17A expression in CD4+ T cells were assessed by FACS analysis. a PBMC co-stimulated with indicated concentrations of anti-CD46 (J4.48) elicited IL-17A expression in a dose-dependent manner. b Co-stimulation of CD46 receptor with anti-CD46 antibody (MEM) did not demonstrate significant level of IL-17A
CD46 co-stimulation leads to enhanced CD4+ T-cell cytotoxicity
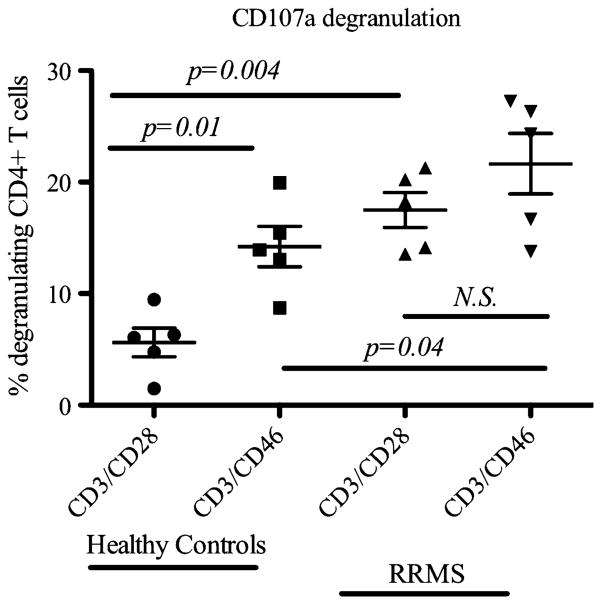
A striking pathology characteristic of MS lesions is destruction of the myelin producing oligodendrocytes that surround axonal projections (Lassmann et al. 2007). Cross-linking of CD46 on healthy donor CD4+ T cells with CD3 simulation resulted in elevated expression of the cytotoxic granzyme B molecule (Grossman et al. 2004). In addition, binding of the pathogen Streptococcus pyogenes M protein together with CD3 activation led to differentiation of CD4+ T cells with cytotoxic function against both allogeneic and autologous target cells (Barchet et al. 2006). Furthermore, in vitro study of MS patient lymphocytes suggested that TH-17 cells may contribute to neural tissue damage through exocytosis of cytotoxic granules (Kebir et al. 2007), and IL-17 was shown to be involved in destruction of bone cartilage in arthritic patients (Moseley et al. 2003). Given these observations, it was of interest to investigate the cytotoxic effector function of CD4+ T cells between healthy individuals and MS patients following CD3/CD46 co-engagement. To address this issue, we measured the expression of CD107a, a molecule that is transiently expressed on the surface of T cells after release of cytotoxic granules from the lysosomal compartments (Betts et al. 2004; Casazza et al. 2006). FACS analysis of purified healthy donor CD4+ T cells stimulated with CD3/CD46 was associated with augmented expression of CD107a. More importantly, in comparison to levels detected in healthy controls, CD107a levels were significantly higher in purified MS patient CD4+ T cells stimulated with CD3/CD28 and CD3/CD46. Although the difference between CD107a expression in MS patient CD4+ T cells stimulated with CD3/CD28 or CD3/CD46 was not statistically significant, a trend of enhanced CD107a expressed was observed in CD3/ CD46 activated cells (Fig. 6).
Fig. 6.
Enhanced cytotoxicity following anti-CD3 and anti-CD46 stimulation. To evaluate the potential of cytotoxicity associated with CD46 co-stimulation, purified CD4+ T cells from healthy controls (n= 5) and RRMS patients (n=5) were co-cultured with anti-CD3 and anti-CD46 antibodies or anti-CD3 and anti-CD28 antibodies for 72 h and CD107a degranulation was assessed by flow cytometric analysis. Statistical analysis was performed with Student's t test
The precise mechanism for the augmented cytotoxicity function in MS patients CD4+ T cells is unclear. Kemper and colleagues demonstrated that this unique population of CD4+ T cells retains its phenotypic and functional characteristic following cross-linking of CD3/CD46 in primary activation. Moreover, proliferation and IL-10 production was even more pronounced upon secondary stimulations even in the absence of co-stimulation signal (Kemper et al. 2003). Based on a large body of associative studies that suggest MS disease development is often linked to infection by HHV-6 that utilize CD46 as the cellular receptor (Cermelli and Jacobson 2000; Cattaneo 2004; Russell 2004), it is possible that periodic reactivation of latent HHV-6 in vivo and may contribute to differentiation and maintenance of this unique subset of cytotoxic CD4+ T cells. Collectively, this study indicated that engagement of CD3/CD46 on MS patient T cells gave rise to a population of pro-inflammatory cytotoxic CD4+ T cells. Future experiments that examine the immunological consequences associated with binding of native or recombinant HHV-6 viral glycoprotein with CD46 may help clarify the precise relationship of HHV-6 infection with development of neuroinflammation.
Methods
Proliferation assay
CD4+ T cells from either healthy donors or untreated RRMS patients with expanded disability status scale scores less than 2 were isolated from cryoperserved PBMC by negative selection with magnetic beads according to instructions provided by the human CD4+ T Cell Isolation Kit II (Miltenyi Biotec, CA). Based on stimulation conditions described in previous studies (Kemper et al. 2003; Astier et al. 2006), purified CD4+ T cells (1×106 cells/ml) were activated for 72 h with 1 μg/ml of anti-CD3 (Hit3a; BD Biosciences, CA) and 2 μg/ml of anti-CD28 (CD28.2, BD Biosciences) or 1 μg/ml of anti-CD3 (Hit3a) and 2 μg/ml of anti-CD46 (J4.48; US Biologics) plate-bound antibodies in 96-well format round bottom tissue culture plates. Cells were maintained in a 37°C incubator with 5% CO2 in complete RPMI containing 10% fetal bovine serum (FBS), 1% antibiotics and 1% glutamine. During the last 6 h of the experiment, activated cells were pulsed with 50 μl of 3[H]-thymidine. A cell harvester (Wallac Instruments, OH) was used to transfer cultured cells onto a fiberglass filter for enumeration of radioactivity incorporation by activated cells with a β-scintillation counter (Wallac Instruments, OH).
Cytokine analysis in cultured supernatant
Cryopreserved PBMC from healthy donors and RRMS patients were quick thawed in a 37°C water bath and DMSO was removed by centrifugation. PBMC at a concentration of 2×106 cells/ml were stimulated with 1 μg/ml of anti-CD3 and 2 μg/ml of anti-CD28 or 1 μg/ml of anti-CD3 and 2 μg/ml of anti-CD46 plate-bound antibodies in 96-well format round bottom tissue culture plates and maintained in a 37°C incubator with 5% CO2 for 72 h. Stimulations with isotype antibodies were included as negative controls. At the end of 72 h, 100 μl of culture supernatant was collected and cytokine levels were analyzed with Raybio pro-inflammatory array (Raybio, CA) according to recommended protocol by the manufacture.
Briefly, cultured supernatant was diluted 1:4 with sample diluent provided and incubated with pre-coated captured antibodies on the array for 2 h in room temperature. Samples were then decanted and the array was washed 5 times with wash buffer and a cocktail of fluorescently labeled detection antibodies were added and incubated again at room temperature for 2 h. The array was washed at the end of incubation and dried by centrifugation. An Axon 4000B microarray scanner was used to measure signal intensity and Genepix analysis software was used to quantify cytokine levels.
FACS analysis of IL-17A expression
CD4+ T cells were purified by negative selection with magnetic beads as described above from freshly isolated PBMC from apheresis of 3 independent healthy donors. Healthy donor CD4+ T (1×106 cells/ml) cells and PBMC (2×106 cells/ml) were stimulated with 1 μg/ml of anti-CD3 antibodies and increasing concentrations of anti-CD46 antibodies (1 μg/ml, 2 μg/ml, 4 μg/ml and 8 μg/ml; J4.48 or MEM, US Biologics). Negative control was activated with 1 μg/ml of anti-CD3 antibodies and 4 μg/ml of isotype controls. Stimulations with 1 μg/ml of anti-CD3 antibodies and 4 μg/ml of anti-CD28 antibodies were also included for comparison. Cells were activated in 96-well round bottom plates for 72 h.
For FACS analysis, cells were harvested and suspended in 30 μl of FACS buffer (0.01% sodium azide and 2% FBS in PBS) and incubated with 2 μl of anti-human CD4-APC (BD Biosciences) or isotype control for 30 min protected from light at 4°C. Prior to intracellular IL-17A staining, cells were washed two times with FACS buffer followed by fixation and permeabilization with BD Fix/Perm kit (BD Biosciences, CA). In 50 μl of permeabilization solution, 2 μl of anti-human IL-17A-PE antibodies or isotype control was added and incubated for an additional 30 min at 4°C. After incubation, cells were washed twice with permeabilization buffer and suspended in FACS buffer prior to analysis with the BD FACS Calibur.
Stimulation of cryopreserved healthy donor and RRMS patient PBMC
Cryopreserved PBMC (2×106 cells/ml) from healthy donors and RRMS patients were activated for 72 h with 1 μg/ml of anti-CD3 antibodies and 4 μg/ml of isotype controls, 1 μg/ml of anti-CD3 antibodies and 4 μg/ml of anti-CD46 antibodies, and 1 μg/ml of anti-CD3 antibodies and 4 μg/ml of anti-CD28 antibodies for 72 h in 96-well round bottom plates. IL-17A staining and analysis was performed as described above.
CD107a mobilization assay
Magnetic beads purified CD4+ T cells from either healthy donors or RRMS patients were first stimulated with 1 μg/ml of anti-CD3 antibodies and 4 μg/ml of anti-CD46 antibodies, and 1 μg/ml of anti-CD3 antibodies and 4 μg/ml of anti-CD28 antibodies for 72 h in 96-well round bottom plates. For CD107a analysis, cells were re-stimulated with 0.5 μg/ml of anti-CD3 antibodies for 12 h. Five-microliter aliquot of anti-human CD107a-FITC antibody (BD Biosciences) was added for the last 6 h of the experiment in the presence of brefeldin A (1 μg/ml) and GolgiStop (0.7 μg/ml). CD107a mobilization was assessed with the BD FACS Calibur.
Contributor Information
Karen Yao, Viral Immunology Section, NINDS, NIH, Bethesda, MD 20892, USA, Department of Biology, Johns Hopkins University, Baltimore, MD 21218, USA.
Jhanelle Graham, Viral Immunology Section, NINDS, NIH, Bethesda, MD 20892, USA.
Yoshimi Akahata, Viral Immunology Section, NINDS, NIH, Bethesda, MD 20892, USA.
Unsong Oh, Viral Immunology Section, NINDS, NIH, Bethesda, MD 20892, USA.
Steven Jacobson, Email: jacobsons@ninds.nih.gov, Viral Immunology Section, NINDS, NIH, Bethesda, MD 20892, USA.
References
- Ahlqvist J, Fotheringham J, Akhyani N, Yao K, Fogdell-Hahn A, Jacobson S. Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes. J Neurovirol. 2005;11:384–394. doi: 10.1080/13550280591002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford SK, Longmore GD, Stenson WF, Kemper C. CD46-induced immunomodulatory CD4+ T cells express the adhesion molecule and chemokine receptor pattern of intestinal T cells. J Immunol. 2008;181:2544–2555. doi: 10.4049/jimmunol.181.4.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- Astier AL. T-cell regulation by CD46 and its relevance in multiple sclerosis. Immunology. 2008;124:149–154. doi: 10.1111/j.1365-2567.2008.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchet W, Price JD, Cella M, Colonna M, MacMillan SK, Cobb JP, Thompson PA, Murphy KM, Atkinson JP, Kemper C. Complement-induced regulatory T cells suppress T-cell responses but allow for dendritic-cell maturation. Blood. 2006;107:1497–1504. doi: 10.1182/blood-2005-07-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- Brinkkoetter PT, Marinaki S, Gottmann U, Fleckenstein S, Stump C, Van Der Woude FJ, Braun C, Yard BA. Altered CD46-mediated T cell co-stimulation in haemodialysis patients. Clin Exp Immunol. 2005;139:534–541. doi: 10.1111/j.1365-2249.2005.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens' magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermelli C, Jacobson S. Viruses and multiple sclerosis. Viral Immunol. 2000;13:255–267. doi: 10.1089/08828240050144590. [DOI] [PubMed] [Google Scholar]
- Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates auto-immune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimeen-Irwin B, Ellis S, Christiansen D, Ludford-Menting MJ, Milland J, Lanteri M, Loveland BE, Gerlier D, Russell SM. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J Biol Chem. 2003;278:46927–46937. doi: 10.1074/jbc.M308261200. [DOI] [PubMed] [Google Scholar]
- Donati D, Martinelli E, Cassiani-Ingoni R, Ahlqvist J, Hou J, Major EO, Jacobson S. Variant-specific tropism of human herpesvirus 6 in human astrocytes. J Virol. 2005;79:9439–9448. doi: 10.1128/JVI.79.15.9439-9448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DB, Koomey M, Cannon JG, Atkinson JP. Down-regulation of CD46 by piliated Neisseria gonorrhoeae. J Exp Med. 2003;198:1313–1322. doi: 10.1084/jem.20031159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JJ, Allie SR, Mullen KM, Jones MV, Wang T, Krishnan C, Kaplin AI, Nath A, Kerr DA, Calabresi PA. Interleukin-17 in transverse myelitis and multiple sclerosis. J Neuroimmunol. 2008;196:124–132. doi: 10.1016/j.jneuroim.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- Hyc A, Osiecka-Iwan A, Strzelczyk P, Moskalewski S. Effect of IL-1beta, TNF-alpha and IL-4 on complement regulatory protein mRNA expression in human articular chondrocytes. Int J Mol Med. 2003;11:91–94. [PubMed] [Google Scholar]
- Jacobson S, Flerlage ML, McFarland HF. Impaired measles virus-specific cytotoxic T cell responses in multiple sclerosis. J Exp Med. 1985;162:839–850. doi: 10.1084/jem.162.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallstrom H, Liszewski MK, Atkinson JP, Jonsson AB. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Oliaro J, Pasam A, Waterhouse NJ, Browne KA, Ludford-Menting MJ, Trapani JA, Russell SM. Ligation of the cell surface receptor, CD46, alters T cell polarity and response to antigen presentation. Proc Natl Acad Sci U S A. 2006;103:18685–18690. doi: 10.1073/pnas.0602458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25:496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Russell S. CD46: a complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens. 2004;64:111–118. doi: 10.1111/j.1399-0039.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Leist TP, Juhng KN, McFarland HF, Jacobson S. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann Neurol. 2000;47:306–313. [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen PM, Zou C, Zhang YA, Lau TK, Chan J, Yao KM, Leung PS. PDZ-domain containing-2 (PDZD2) is a novel factor that affects the growth and differentiation of human fetal pancreatic progenitor cells. Int J Biochem Cell Biol. 2008;40:789–803. doi: 10.1016/j.biocel.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Murugaiyan G, Hafler DA, Astier AL, Weiner HL. Increased IL-23 secretion and altered chemokine production by dendritic cells upon CD46 activation in patients with multiple sclerosis. J Neuroimmunol. 2008;195:140–145. doi: 10.1016/j.jneuroim.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Mandel M, Akyani N, Maynard K, Sengamalay N, Fotheringham J, Ghedin E, Kashanchi F, Jacobson S. Differential HHV-6A gene expression in T cells and primary human astrocytes based on multi-virus array analysis. Glia. 2006;53:789–798. doi: 10.1002/glia.20333. [DOI] [PubMed] [Google Scholar]
- Yao K, Honarmand S, Espinosa A, Akhyani N, Glaser C, Jacobson S. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65:257–267. doi: 10.1002/ana.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffran Y, Destaing O, Roux A, Ory S, Nheu T, Jurdic P, Rabourdin-Combe C, Astier AL. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J Immunol. 2001;167:6780–6785. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]