Summary
Recent studies linked aberrant B cell activation in the context of aberrant immune responses to infectious pathogens to malignant transformation and development of leukemia and lymphoma. A new study in this issue demonstrated that common infections can be drivers of clonal evolution of pre-malignant B cell precursors towards childhood leukemia.
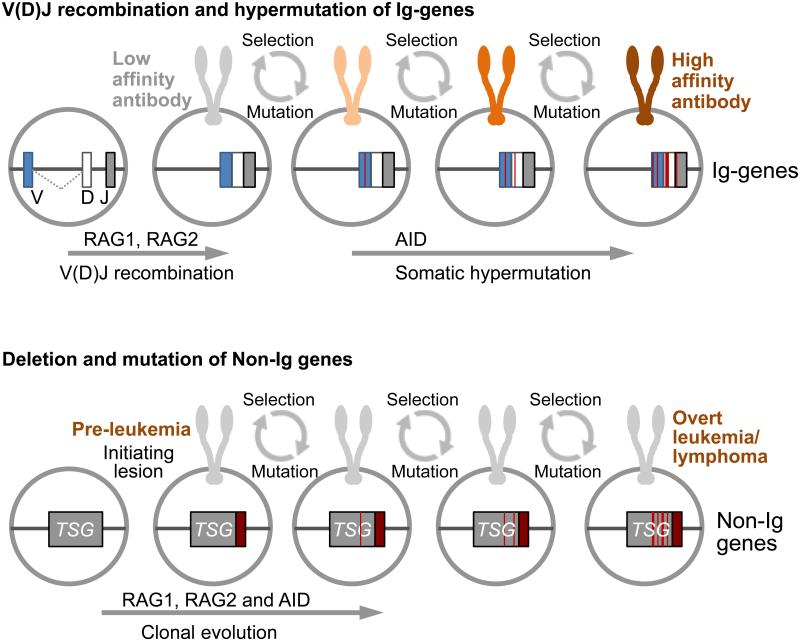
B cells are unique in their ability to generate antibodies against infectious pathogens and refine their specificity in multiple rounds of mutation and clonal selection (1). Through genetic mutation and recombination events, activated B cells can continuously adapt to antigen. Thereby, B cells undergo a Darwinian selection process, which favors clones that have evolved antibodies with the highest affinity to antigen derived from infectious pathogens. While this process is essential for adaptive immune responses, the same mechanisms of genetic diversification and Darwinian selection can also drive clonal evolution in cancer (2) (Figure 1). The propensity of B cell affinity maturation to potentially deleterious genetic lesions led to the concept that repetitive or chronic infections will drive mutation and recombination events of immunoglobulin (Ig) genes in B cells to diversify the antibody repertoire but also increase the risk of malignant transformation. RAG genes mediate V(D)J recombination and AID drives somatic hypermutation and class-switch recombination of Ig genes. However, both RAG and AID can accidentally act outside of Ig loci and potentially target oncogenes and tumor suppressors. The activity of RAG and AID are typically segregated to early and late stages of B cell development, respectively. However, the two enzymes can be concurrently expressed under conditions of abnormal B cell activation by bacterial lipopolysaccharides (LPS) and potentiate the risk of acquiring genetic lesions when acting together (3, 4).
Figure 1.
A schematic to illustrate common mechanisms of normal B cell affinity maturation (top) and clonal evolution towards leukemia and lymphoma (bottom). The activity of RAG1 and RAG2 enzymes in V(D)J recombination and AID in somatic hypermutation of immunoglobulin (Ig) genes is depicted. Multiple rounds of mutation and selection lead to expression of high-affinity antibodies (top). Chroni and repetitive B cell activation (e.g. through aberrant immune responses to infection) can also lead to accidental targeting of RAG1, RAG2 and AID to Non-Ig genes, including tumor suppressor genes (TSG; bottom). Multiple rounds of mutation and selection favor the outgrowth of individual clones based on competitive fitness and drives clonal evolution towards overt leukemia or lymphoma. Recent studies identified Helicobacter pylori, HCV and Plasmodium falciparum as specific infectious pathogens driving of progressive B cell transformation. Here, Borkhardt and Sanchez-Garcia demonstrated that common infections can be drivers of clonal evolution of pre-malignant B cell precursors towards childhood leukemia.
Indeed, infection by Helicobacter pylori has been recognized as critical driver of mucosa-associated lymphoid tissue (MALT) B cell lymphoma, which is often reversible by antibiotic therapy. Likewise, chronic B cell activation in the context of hepatitis C virus (HCV) infection was identified as causative agent in the etiology of HCV-associated diffuse-large B cell lymphomas. Key evidence for this concept comes from a recent study on the role of Plasmodium falciparum in the etiology of endemic Burkitt’s lymphoma (5). In a genetic mouse model for chronic recurrent malaria infection, this study demonstrated that chronic B cell activation in this model causes aberrant activation of AID and AID-mediated genomic instability leading to B cell lymphoma. Of note, development of B cell lymphoma in this model was critically dependent on protracted AID activity (somatic hypermutation and class-switch recombination) in the presence of plasmodium infection.
Initial genetic lesions spawning pre-leukemic clones in ALL usually arise in utero (e.g. the common ETV6-RUNX1 fusion) (6). However, as evidenced from studies with unselected new born cord bloods and monozygotic twins with ALL, additional genetic changes, including recurrent copy number changes (mostly deletions), are required for development of clinical ALL and these are post-natal in origin (6). Epidemiological evidence supports a ‘delayed infection’ hypothesis predicting that common infections promote those latter secondary genetic events, but only the context of prior deficits in infectious exposure of infants (7). A meta-analysis of 15 studies indicated that infectious exposures in the first year of life significantly reduces the risk of ALL (8). This cancer has a major peak in incidence in developed societies of 3-5 years concomitant with the period when children are commonly exposed to infections via peer group social contacts. In addition, newborns with low levels of IL10, a cytokine that limits duration and intensity of B cell activation, was recently identified as predictor of an increased risk to develop leukemia (9). Other data on the impact of certain vaccinations in infancy also accords with a role for infection-associated immune dysregulation in the pathogenesis of childhood ALL (7).
Recent genetic studies in a mouse model provides additional support for this model and implicates cooperative activity of RAG and AID enzymes (3, 4). Mouse B cell precursors carrying the ETV6-RUNX1 fusion alone failed to initiate leukemia in transplant recipient mice. However, when ETV6-RUNX1 B cells underwent repetitive hyperactivation with bacterial LPS, they acquired secondary lesions that enabled development of fatal leukemia in transplant recipients (4). Acquisition of these lesions was dependent on both RAG and AID4. Genetic lesions in patient-derived childhood ALL samples revealed a strong bias for known hypermutation target genes of AID. In addition, deletions and gene rearrangements in these patient samples were often targeted to known RAG-specific recombination signal sequences (RSS) motifs that randomly occur in the genome and showed junctional insertions at break points that are characteristic of RAG-mediated recombination events (3, 4).
While previous work focused on RAG and AID as mechanistic drivers of secondary lesions, a new elegant study by the groups of Borkhardt and Sanchez-Garcia in this issue (10), directly demonstrate, in a model system, that common infections can be drivers of clonal evolution of pre-leukemic B cell clones. Previous work revealed PAX5 haploinsufficiency as predisposing factor in childhood ALL. This study was based on Pax5+/− heterozygous mice to model partial loss of PAX5-function. Importantly, Pax5+/− mice did not develop leukemia unless they were exposed to common infectious pathogens. Under pathogen-free conditions neither wildtype (0/15) nor Pax5+/− (0/14) mice acquired leukemia. In the presence of common infectious pathogens, 9 of 41 Pax5+/− mice developed fatal pre-B ALL, while none of 20 wildtype littermates did. Transplantation experiments and whole exome sequencing demonstrated that leukemia was initiated in a B cell-autonomous manner. Strikingly, the developing leukemia clones not only recapitulated typical aspects of human pre-B ALL phenotype (surface markers and gene expression pattern) but also acquired the same genetic lesions that were previously identified in human pre-B ALL: These lesions included activating JAK3 mutations in 6 of 9 mice carrying the mouse homolog of the human JAK3R657Q mutation. In addition, two mice acquired a mutation of the remaining wildtype PAX5 allele, resulting in further reduction of PAX5 activity.
Helicobacter pylori, HCV and Plasmodium falciparum were identified as specific infectious pathogens driving of progressive B cell transformation towards lymphoma. The identity of infectious pathogens responsible for the acquisition of postnatal genetic lesions leading towards childhood ALL, however, are still elusive. This current study did not focus on specific subclasses of infectious pathogens that are responsible for clonal evolution of Pax5+/− B cell precursors towards leukemia. However, for future extensions of this work, it would be of great interest to pinpoint the relevant infectious agents, which may help to develop new preventive strategies for childhood ALL. In addition, only 9 of 41 Pax5+/− mice exposed to infectious pathogens developed leukemia, which raises the question whether development of leukemia in this cohort correlated with an abnormal immune response status in the mice that developed leukemia. Testable predictions in this model would be a negative correlation with IL10 serum levels, higher levels of B cell activation markers and higher expression and activity of AID and RAG. As it stands, Borkhardt, Sanchez-Garcia and colleagues present compelling evidence for exposure to infection during early childhood years as a driver of clonal evolution of pre-leukemic clones towards ALL. Given the ongoing debate about potential usefulness of government-mandated vaccination programs during early childhood in the US and elsewhere, further studies building on the work by Borkhardt, Sanchez-Garcia et al. will be relevant and timely. Together with previous work elucidating the role of Helicobacter pylori, HCV and Plasmodium falciparum as infectious drivers in B cell lymphoma, the new findings support a broader scenario in which chronic B cell activation increases propensity to malignant transformation. AID and RAG, mutagenic enzymes, that beneficially promote a diverse antibody repertoire, accidentally orchestrate this process.
Acknowledgements
We thank Drs. Michael Lieber (Los Angeles, CA) and Joseph Wiemels (San Francisco, CA) for discussions and advice. M.M. is a Wellcome Trust Senior Investigator and a Scholar of the Leukemia and Lymphoma Society.
Financial support: The work is supported by NIH grants R01CA139032, R01CA137060, R01CA157644, R01CA169458 and R01CA172558. M.M. is a Scholar of the Leukemia and Lymphoma Society and a Senior Investigator of the Wellcome Trust.
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–8. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 2.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–13. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai AG, Lu H, Raghavan SC, Muschen M, Hsieh CL, Lieber MR. Human chromosomal translocations at CpG sites and a theoretical basis for their lineage and stage specificity. Cell. 2008;135:1130–42. doi: 10.1016/j.cell.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaminathan S, Klemm L, Park E, Papaemmanuil E, Ford A, Kweon SM, et al. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nat Immunol. 2015;16:766–74. doi: 10.1038/ni.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbiani DF, Deroubaix S, Feldhahn N, Oliveira TY, Callen E, Wang Q, et al. Plasmodium Infection Promotes Genomic Instability and AID-Dependent B Cell Lymphoma. Cell. 2015;162:727–37. doi: 10.1016/j.cell.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman CM, Colman SM, Chaplin T, Young BD, Eden TO, Bhakta M, et al. Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood. 2010;115:3553–3558. doi: 10.1182/blood-2009-10-251413. [DOI] [PubMed] [Google Scholar]
- 7.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6:193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 8.Urayama KY, Buffler PA, Gallagher ER, Ayoob JM, Ma X. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. Int J Epidemiol. 2010;39:718–732. doi: 10.1093/ije/dyp378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JS, Zhou M, Buffler PA, Chokkalingam AP, Metayer C, Wiemels JL. Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2011;20:1736–40. doi: 10.1158/1055-9965.EPI-11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Lorenzo A, Hauer J, Vicente-Duenas C, Auer F, Gonzalez-Herrero I, Garcia-Ramirez I, et al. Infection exposure is a causal factor in B-precursor acute lymphoblastic leukemia as a result of Pax5 inherited susceptibility. Cancer Discov. 2015 doi: 10.1158/2159-8290.CD-15-0892. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]