Synopsis
Bipolar depression and cognitive impairment are pervasive and highly disabling aspects of the illness. Although cognitive impairment is partially independent from mood episodes, depressive symptoms may increase the risk of cognitive impairment in bipolar disorder through inflammatory processes as well as health risks such as obesity and sedentary behavior. Novel treatment avenues at the intersection of bipolar depression and cognitive impairment target inflammation and/or health behaviors such as diet, physical activity and sleep hygiene.
Keywords: Neuropsychology, mood disorders, aging, brain function, health behavior
Introduction
For many years, BD was not believed to be associated with durable cognitive problems. Indeed, a return to normal cognitive function was believed to be one of the main distinguishing features of BD when compared to schizophrenia. However, over the past 15 years, scores of studies have accumulated to indicate that BD is associated with clinically significant cognitive impairments evident both during mood episodes and during clinically euthymic periods. Collectively, depressive symptoms and cognitive impairment account for the majority of the immense disability produced by BD. Unfortunately, there remains a great need for efficacious treatments targeting either bipolar depression (as documented elsewhere in this volume) or cognitive dysfunction. Emerging research has shifted away from efforts to parse cognitive deficits from mood symptoms towards examining their shared neurobiological mechanisms, behavioral determinants, and treatment avenues.
Prevalence and Distribution of Cognitive Impairments in Euthymic Patients
The distribution of cognitive deficits in BD, as with most aspects of this illness, is heterogeneous and complex. Approximately 40 to 60% of patients with BD evidence clinically significant cognitive impairment1. Thus, while cognitive impairment may enact a marked impact on functioning at the population level in BD, global cognitive impairment is not evident in approximately half of patients. Within the BD spectrum, there is some evidence that risk of cognitive impairment varies by diagnostic subtype and clinical features. Some studies have found that cognitive deficits are more prominent in patients with bipolar I versus bipolar II disorders2, although this finding is not consistent3. A history of psychotic features, more common in bipolar I compared to bipolar II, is also associated with a greater likelihood of cognitive impairment4.
Some cognitive abilities appear more impacted by BD. In meta-analyses that have examined cognitive performance in euthymic patients compared to performance in healthy controls, deficits are apparent at medium to large effect sizes in the areas of verbal memory, executive function, processing speed and sustained attention5,6. In contrast, vocabulary, naming, and verbal fluency abilities are generally observed to be comparable to that of healthy comparators. A recent study by Burdick and colleagues employed cluster analysis to indicate that roughly 40% of patients exhibited normal cognition, 30% exhibited selective deficits in verbal memory, processing speed, attention and social cognition yet with normal functioning, and 30% were globally cognitively and functionally impaired7.
A growing number of studies have investigated domains of cognition that extend beyond traditional neuropsychological foci, such as social cognitive abilities. In a recent meta-analysis in euthymic patients across a range of social cognition measures, deficits were present in theory of mind and emotional reasoning tasks, whereas basic emotion recognition tasks were preserved8.
Comparison of Cognitive Deficits in BD to that in Other Psychiatric Illnesses
Compared to schizophrenia, the neuropsychological deficits of BD appear to be less severe and somewhat more selective. The rate of global cognitive impairment in schizophrenia is 90%, and a meta-analyses of cognitive function comparing schizophrenia to BD revealed a mean difference between these disorders at about a half of a standard deviation, with BD individuals performing better9. Severity distinctions compared to unipolar depression are less clear, and seem to vary by clinical state and medication status. For example, one study indicated greater impairment in unmedicated unipolar depressed patients compared to bipolar II disorder10. Another longitudinal study found evidence of increased dysfunction in bipolar I after treatment for acute depression but similar cognitive test performance during acute depression11, and, in another study, similarities in brain activation between bipolar and unipolar acutely depressed patients were seen12. Thus, the balance of evidence indicates that on a spectrum of cognitive impairment, BD is intermediate between unipolar depression and schizophrenia, particularly when comparing across mood disorders in euthymic states.
Studies of the Shorter-Term Effect of Bipolar Depression on Cognitive Function
Several studies have evaluated cognition in samples of patients with BD during the presence of active depressive symptoms versus euthymic or hypo/manic states. There is considerable variability, with a meta-analysis finding some evidence that bipolar depression was associated with poorer fluency and verbal learning13. Additional work found comparable performance in depressed versus euthymic patients 14,15, although others have found depressed patients were more likely to exhibit deficits in verbal recall and fine motor skills than euthymic patients16. Over the short-term (1 year) in first episode patients, a prospective study found no association between recurrence of depression and change in cognitive functioning17. Of note, patients with a verbal memory deficit in this study were more at risk for mood relapse. Overall, there appears to be some exaggeration of specific deficits by depression, but the impact of the depressed state appears much smaller than that associated with the presence of the bipolar diagnosis compared to healthy controls.
Studies of the Long-Term Effect of Bipolar Depression on Cognitive Function
Some authors have recently proposed that BD may involve a progressive neurodegenerative decline in cognitive function and that BD is associated with ‘neuroprogression’.18,19 Although there is some neurobiological evidence in favor of this deterioration in cognition hypothesis, the small number of longer-term follow up studies (3 to 10 years) have failed to consistently support that cognitive abilities decline at a faster rate in BD than in normal controls.
Nonetheless, a seminal review paper by Robinson et al. found some evidence that patients with a history of more hospitalizations, longer duration of illness and more severe manic symptoms were more likely to exhibit cognitive impairments20. Little evidence suggested that more frequent or severe depressive episodes were associated with deteriorating trajectories of cognitive function in the context of BD. Of course, cross-sectional studies cannot confirm causal effects of mood symptoms on longer-term cognitive impairment, as patients with more severe courses of affective symptoms may also have more severe cognitive deficits. More generally, however, recent longitudinal research has suggested that depressive symptoms precede and predict memory decline in non-demented elderly adults, leaving open the possibility that depressive symptoms could contribute to declines in BD if measured longitudinally21.
As such, over the long-term, there is some suggestion that cumulative effects of mood episodes may foster cognitive deterioration.. These life course findings have led to discussion of ‘stage’ models of BD in which earlier stages are associated with more episodic depression and latter stages more cognitive impairment, disability and persistent depression22.
Impact of Cognitive Impairment and Depression on Psychosocial Functioning
A sizable body of research has shown that cognitive ability is associated with psychosocial disability in BD. In a recent meta-analysis, the strength of this association was identical to that in a separate meta-analysis in studies examining the same question in schizophrenia23. This may indicate that even if the level of disability experienced by people with BD is lower than that experienced in schizophrenia, the averaged impact of cognitive impairment is comparable. An even larger body of literature, including the seminal prospective studies of Judd and colleagues24, has indicated that increases bipolar depression even at the subsyndromal level, account for a remarkable proportion of disability. As such, cognitive impairment and depression collectively produce the majority of disability in this illness.
There are emerging data that suggest nuances in the combined impact of cognitive impairment and bipolar depression on psychosocial function. Bowie et al. found that bipolar depression and cognitive ability were independently associated with occupational performance and interpersonal function, mediated through measures of capacity to perform these functional tasks25. However, cognitive ability, but not depression, was associated with performance of instrumental activities of daily living. Over a one year period, Bonnin et al found that verbal memory deficits exacerbated the impact of subsyndromal depression on functional outcome26. As such, cognitive impairment and depression likely amplify each other in producing disability, and the relative impact of each may differ by domain of disability. Depp et al. found that cognitive impairment was associated with whether or not patients were engaged in competitive employment, and depression was associated with the number of hours worked among those employed. The authors speculated that cognitive impairment could be seen as ‘rate-limiting’ in the acquisition of employment, whereas depression was more relevant to producing disability among the comparatively less cognitively impaired patients who were employed27.
Further understanding of the confluence of depression and cognitive ability may come from studies on self-assessment of performance. Emerging research indicates that bipolar depression influences self-assessment of performance on objective cognitive and functional tasks, and patients who evaluate their performance as poor are less likely to seek and attain functional milestones such as employment irrespective of their capacity to engage in such tasks28. In this way, depression may exacerbate the impact of mild cognitive deficits on functioning by introducing self-defeating biases.
Potential Pathways Between Depression and Cognitive impairment
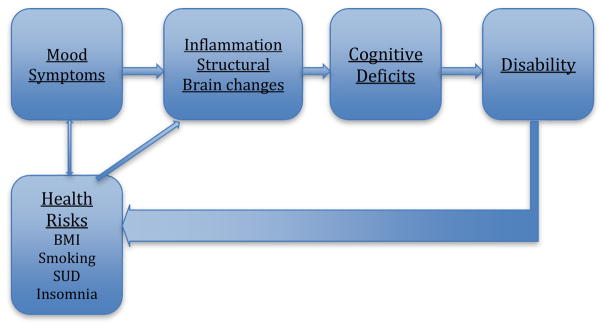
Research has pointed to at least three possible pathways between cognitive impairment and bipolar depression:
Sustained and recurrent mood symptoms may directly impact neurobiological pathways that diminish cognitive ability,
Mood symptoms may indirectly contribute to and exacerbate negative health behaviors that impact cognitive ability, and
Disability may reciprocally impact the determinants of cognitive deficits and depressive symptoms. See Figure 1 for a summary.
Figure 1.
Pathways between Bipolar Depression and Cognitive Impairment
These pathways are speculative and not yet confirmed by longitudinal study; they are also not mutually exclusive from one another, yet produce somewhat unique implications for treatment. We review the evidence for these possibilities below.
Evidence for a Direct Pathway from Bipolar Depression to Cognitive Impairment
One direct neurobiological pathway from cumulative exposure to affective symptoms to cognition may be chronic stress-induced dysregulation of the immune system. Individuals with BD display an abnormal inflammatory profile, characterized by altered levels of both pro- and anti- inflammatory cytokines. Studies that have investigated the effects of mood state on inflammatory cytokines and have reported both up- and down-regulation in these markers during depression, including C-Reactive Protein (CRP), Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α)29–32. Interestingly, there is some evidence to suggest that patterns of inflammation differ between mood states. Mania appears to produce more pronounced elevation across many inflammatory markers compared to patients in depressed or euthymic phases of the disorder. Ortiz-Dominguez (2007) reported that depressed BD patients in their sample exhibited increased IL-6 and TNF-α and decreased IL-2. In contrast, manic patients were associated with increases in TNF-α and IL-4 and decreases in IL-1 and IL-233. Thus, although BD appears to dysregulate inflammation across mood states, there is also some literature to suggest potential phasic differences in specific inflammatory cytokines.
Some preliminary investigations have highlighted the deleterious effect of a dysregulated inflammatory state on cognition. One study reported negative correlations between CRP expression and immediate memory, language and attention in BD 34. Another group reported that worse performance on a task of delayed auditory verbal memory was associated with higher levels of TNF-α in a sample of BD patients35. Associations between cognition and inflammation have also been reported in psychiatrically healthy adults36,37, and levels of IL-6 predicted future cognitive decline, particularly among those with elevated genetic risk for cognitive impairment. Taken together, these largely cross-sectional studies indicate that alterations in specific inflammatory cytokines, particularly those shown to be dysregulated during the depressive phase of the disorder, may contribute to cognitive impairment in BD.
Neuroimaging findings have elucidated abnormal neural activity as well as altered white and grey matter organization in BD. Of these, compromised white matter (WM) appears to be the most robust finding, and studies have demonstrated widespread alterations in WM integrity38. Importantly, these alterations are associated with deficits in processing speed, executive functioning, verbal fluency and emotion regulation39. A recent diffusion tensor imaging study reported that alterations in white matter integrity were associated with poorer verbal fluency in a sample of depressed bipolar patients40. Inflammation may contribute to white matter changes by inducing changes in myelin integrity and vascular permeability. Indeed, some studies have demonstrated a negative relationship between pro-inflammatory cytokines and white matter integrity in healthy older adults 41 and patients with schizophrenia42. A review of the current evidence, though limited, suggests that the prefrontal cortex, corpus callosum and temporal lobe may be particularly vulnerable to inflammation in patients diagnosed with major depressive disorder 43.
There is some evidence to support the role of the number and severity of depressive episodes in development of white matter pathology. Studies have demonstrated associations between more symptoms of depression and greater white matter compromise in the anterior thalamic radiation and the corpus callosum44. Depressed BD individuals also have greater compromise in the left cingulate sub-gyrus, the posterior limb of the right internal capsule and the right parietal white matter tracts compared to euthymic BD participants 45. There is also some evidence to suggest a larger degree of white matter alteration in bipolar depression compared to unipolar depression, particularly in the corpus callosum46,47, indicating individuals with BD may experience a similar, yet more severe pathophysiological disease course.
Evidence for an Indirect Pathway from Bipolar Depression to Health Risks to Cognitive Impairment
The alarmingly high prevalence of medical comorbidities in BD led many authors to conclude that BD is a “multi-system” disease impacting a diverse array of organs beyond the brain. In addition to the direct impacts of the illness on immune function and other systems that influence brain function, indirect pathways that interact with mood symptoms include poor health behaviors and other risk factors may impact cognition. These include diet and physical activity, smoking and substance use, and potential iatrogenic effects of medication used to treat symptoms.
Obesity
Approximately 60% of people with BD are overweight (defined as a Body Mass Index, or BMI, over 25) and 20 to 40% obese (defined as a BMI greater than 3048). These figures are somewhat elevated compared to that in the general U.S. adult population (http://www.cdc.gov/nchs/fastats/obesity-overweight.htm). There are a number of risk factors for obesity, with patients with BD less likely to report recommended levels of physical activity and intake of fruits and vegetables. In addition, a number of the medications used to treat BD are associated with weight gain and adiposity.
Data on the impact of obesity on cognition in BD is emerging, and studies have indicated obesity’s impact on both brain structural and functional changes as well as inflammatory markers. Depp et al.49 examined the association of overweight and obesity with cognition in a sample of outpatients with BD and, adjusting for sociodemographic and clinical covariates, found a step-wise association with increasing evidence for impairment in overweight and obese patients. Indeed, the difference in cognitive functioning between obese and normal weight patients was substantial (Cohen’s d = 0.43) and greater than observed in a comparison sample of obese, overweight and normal weight patients with schizophrenia.
Work has begun to explore the link between diet, inflammation and cognition in BD. Several studies have demonstrated a higher prevalence of a western diet (e.g. red meat, refined carbohydrates, and processed foods) among depressed individuals 50–53, including those diagnosed with BD54. Western diets have been linked to increased concentration of IL-6 and CRP inflammatory cytokines50,55, and a reduction of glucose and metabolic transport in hippocampal regions in rats56. Conversely, middle-aged individuals adherent to a Mediterranean diet were associated with reduced levels of these same cytokines57 and lower prevalence of metabolic syndrome58 and cardiovascular disorders59. Thus, there is some preliminary evidence that suggests dietary patterns may alter inflammatory profiles.
Physical activity
Several epidemiological studies indicate that people with BD are less likely than healthy comparators to engage in recommended levels of physical activity60, including as measured by objective ambulatory monitoring devices61,62. There is substantial evidence for an association between diminished physical activity and incidence of depressive symptoms, and reciprocal relationships have been observed in longitudinal study of adolescents, with increases in depression predicting later diminished physical activity63. Self-reported physical activity studies have indicated that less frequent exercise is associated with greater depression and lower quality of life, while increased activity predicts greater manic symptoms64.
Substance use
Rates of co-morbid substance use in BD range from 20% to 60% across different samples65. Although the self-medication hypothesis of substance use in BD does not explain all of the comorbidity between affective and substance-related symptoms, there is research to suggest that patients report use of substances to attempt to ameliorate depressive symptoms66. Chronic substance use, particularly alcohol, has been shown to negatively impact several cognitive domains in BD, including executive functioning and memory67. Many of the deficits linked to chronic alcohol use have also been shown to be present in substance free BD patients, and some investigators suggest that co-morbid SUD and BD may have an additive effect on cognitive impairment. For example, one study demonstrated that euthymic BD patients with a history of alcohol dependence demonstrate greater executive dysfunction compared to BD patients without a history of use68,69. Although a number of translational models have indicated the potential benefits of nicotine on brain function, there is mounting evidence that cigarette smoking is associated with cognitive deterioration. Depp et al. examined the association of current smoking and lifetime smoking patterns with cognition in BD70. Current smokers exhibited worse cognitive performance, and there was a modest negative association with between pack-years and global cognitive performance. Of note, patients who had ceased smoking did not differ in cognitive performance from never smokers.
Sleep
As many as 70% of patients with BD exhibit sleep problems even when euthymic. During bipolar depression, a study found 100% of patients experienced insomnia and 78% experienced hypersomnia71. Eidelman found that a greater number of past depressive episodes was associated with greater sleep disturbance72. Conversely, euthymic patients culled from the large STEP-BD trial who had evidence of sleep disturbance were more at risk for mood episode recurrence73. To date, few studies have examined the association between sleep and cognitive function in BD, although many studies have linked insomnia and worse cognition in other populations. Thus, strong connections between mood symptoms, sleep problems, and cognitive impairment in BD seem plausible. As with all of the risk factors described above, it is unclear if sleep mediates the associations between cognitive impairment, symptoms and function, or if aberrant sleep and cognitive impairments derive from the same neurobiological diathesis (e.g., disrupted circadian rhythms)74. Moreover, sleep problems also likely heavily exacerbate other health risk factors listed above; patients frequently use alcohol, prescription medications, and illicit substances to self-medicate sleep problems and subsequently further alter sleep architecture. Diminished physical activity may occur among patients with insomnia due to diminished energy, and poorer diet and obesity may be exacerbated through alterations to appetitive hormones associated with sleep problems.
Psychosocial Disabilities May Reciprocally Increase Depression and Cognitive Impairment
A lesser discussed possibility in models of the predictors of disability in BD is that disability may reciprocally diminish cognitive function and increase depression. For example, although little studied in BD, engagement in cognitively stimulating activities has been studied extensively as a protective factor in cognitive functioning in healthy aging. Higher educational attainment and engagement in cognitively complex occupations appear to forestall cognitive decline, and greater engagement in cognitively sedentary activities such as watching television appear to be associated with increased risk of cognitive decline75. These findings have led to the concept of such behaviors as contributing to ‘cognitive reserve’ and as protection against the age-associated deterioration in brain structure and function. It is evident that depressive symptoms predict diminished engagement in cognitively stimulating activities and vice versa76 and among patients with BD, participation in the workforce and potentially mentally challenging activity are both reduced.
In the general population, people with more robust and supportive social networks experience diminished risk of cognitive decline when followed prospectively77. These effects have been examined in studies that account for potential reciprocal effects of cognition on social functioning. Mechanisms of this association are unclear but may include that interpersonal relationships may provide cognitive challenges that enhance cognitive reserve and that strong social relations may buffer neurochemical effects of stress on cognitive deterioration. Numerous studies have indicated that the social networks of people with BD are depleted compared to healthy controls, and social strain and negative interactions are increased78.
Finally, disability may influence depression and cognition in BD through the deleterious effects of poverty. BD is associated with higher rates of poverty and reliance on disability income, and socioeconomic factors may contribute substantially to the excess mortality experienced by people with BD by diminishing access to healthy diets, safe places for physical activity and means of cognitive stimulation.
Interventions to Remediate Cognitive Impairment in Bipolar Depression
The optimization of therapies for bipolar depression remains a critical challenge for the field and is discussed in depth in this volume. Moreover, there have been few randomized controlled trials of any pharmacologic or non-pharmacological therapies designed to enhance cognitive abilities in BD. Nonetheless, there are a number of potential intervention strategies that are relevant to discuss when considering the junction of cognitive impairment and depression, examining the broader literature outside of BD and early stage studies. We review below treatments that have evidence for at least one of the following: 1) positive impact on both bipolar depression and cognitive ability, 2) mitigation of hypothesized biological mechanisms (e.g., inflammation) that undergird depression and cognitive impairment, and/or 3) successful targeting of a risk factor associated with increased risk for cognitive impairment and more severe depression.
Pharmacological Treatments
There are no currently FDA approved agents for cognitive enhancement in BD. Given the emerging focus on inflammation in the pathophysiology of life course models BD, an increasing number of anti-inflammatory agents have been examined as potential adjunctive treatments for cognitive enhancement79. These include lithium and valproate, which suppress IL-6. A variety of other agents that have been either evaluated or proposed as adjuvant therapies to diminish inflammation, and include Omega-3 fatty acids, aspirin and NSAIDs, NAC, as well as tetracyclic antibiotics (e.g., minocycline), monoclonal antibodies (e.g., TNF-alpha inhibitors), and phytochemicals (e.g., cumin). A recent excellent review by Rosenblat describes the mechanism and current status of trials of these agents in targeting unipolar and bipolar depression79. Tempering enthusiasm somewhat is that several therapies have been evaluated as targets to prevent cognitive decline in normal aging, and despite strong observational associations (e.g., Omega-3 fatty acids), randomized trials have proven disappointing80. Nonetheless, in contrast to normal aging, patients with BD are a substantially more “enriched” sample in which to demonstrate the impact of anti-inflammatory agents for both bipolar depression and cognitive function for the reasons detailed above.
Other agents that have been used to target cognition may also have anti-depressant effects and vice versa. For example, Burdick reported results of a trial of pramipexole targeting cognitive functioning in Bipolar I and found in post-hoc analyses a benefit among patients with cognitive impairment at baseline81. Pramipexole has also been examined as a treatment for bipolar depression. At this stage, trials typically attempt to isolate the impact of agents on cognitive enhancement by restriction to euthymic patients and/or examining whether (subsyndromal) depressive symptoms moderated or mediated the impact of the agent. However, it may be possible to consider agents with broad spectrum impact on both cognitive impairment and bipolar depression.
Cognitive Training and Remediation
Two recent randomized trials suggest limited impact of broadly targeted cognitive remediation on objective cognitive performance in euthymic patients with BD, despite earlier open trials indicating some enhancement of cognitive ability82,83. Nonetheless, in the larger trial reported by Torrent involving an intervention that provided psychoeducation and behavioral practice of cognitive compensatory techniques, psychosocial functioning was enhanced to a significant degree when compared to a symptom focused psychoeducation group82.
More targeted cognitive training, not yet tested in BD, has shown some ability to enhance both cognitive function and depressive symptoms. In the large ACTIVE study, a clinical trial of several cognitive training interventions evaluated in older adults, speed-of-processing training was associated with a 30% reduction in the likelihood of clinically significant increases in depressive symptoms over 5 years of follow up84. Emerging arenas at the boundary of cognitive training include cognitive bias modification, social cognitive skills training and emotion regulation focused interventions. These typically target aberrant or extreme pre-conscious cognitive phenomena (e.g., overgeneral memory biases in depression) and have been associated with typically short-term term improvements in biases and some impact on depressive symptoms. Given the emerging understanding of the neural basis of emotion in BD, these interventions hold promise as potential adjunctive treatments85.
Physical Activity and Weight Loss Interventions
Comprehensive weight loss interventions targeting energy balance and dietary intake have been effective in reducing weight in samples of people with serious mental illness86. Physical activity is frequently mentioned as a generally low risk broad spectrum intervention that could impact inflammatory processes, metabolism, depressive symptoms and cognitive function. Several reviews have described the status of the literature on physical activity interventions in BD, and most have concluded promise but need for more well-designed trials87. There are trials that have indicated positive impact of physical activity interventions on cognition in patients with depression and schizophrenia88.
Treatment for Insomnia
Recent preliminary work has shown impressive effects of adapted CBT for Insomnia (CBT-I) in BD. Harvey et al. (2015) reported pilot data from an open trial of an 8 session intervention targeting insomnia in BD, and in addition to improving sleep function at 6 months, reduced rates of mood relapse and diminished functional impairment were observed. It would be important to determine if such an approach might also reduce cognitive impairment.
Compensating for Cognitive Impairment in Learning Based Therapies
Cognitive impairments likely limit the degree to which patients retain information and subsequently benefit from learning based psychotherapies like CBT that target depressive symptoms. In the study by Harvey regarding CBT I described above, an interesting sub-analysis revealed that patients with insomnia recalled <20% of CBT material89. Mobile devices may provide a novel means of augmenting psychotherapies to enhance recall and community engagement in therapeutically-relevant activities. Depp et al.90 evaluated a mobile augmented intervention called Personalized Real-Time Intervention for Stabilizing Mood (PRISM), which involved brief psychoeducation combined with automated frequent assessments and linked personalized reminders to engage in coping strategies identified during in-person training. Compared to a condition in which patients only participated in face-to-face training and completed paper-and-pencil mood charts, patients who received PRISM experienced a significantly greater reduction in bipolar depression at post-study.
Conclusions
In summary, there is both remarkable challenge and opportunity at the intersection of bipolar depression and cognitive impairment. A pessimistic viewpoint would emphasize that, despite contributing to most of the personal and economic burden of BD, providers currently have very few treatment options to offer their patients. Remarkably, only three agents are approved by the FDA for acute bipolar depression and no agents are FDA-approved for cognitive enhancement.
An optimistic view would be that research has now identified shared determinants and treatment avenues for biological mechanisms that undergird bipolar depression and cognition, such as chronic inflammation. Work in this area has opened a host of novel pharmacologic and non-pharmacologic treatment possibilities that may simultaneously target these two pernicious aspects of the illness, and point to preventative approaches to forestall cognitive decline. Available to clinicians now are efficacious interventions that reduce health risks associated with both depression and cognitive impairment, such as physical activity, cognitive behavioral treatment for insomnia and weight loss intervention. Novel psychotherapeutic treatments may circumvent or compensate for cognitive deficits through technology, and, as such could provide more substantial impact on bipolar depression. These opportunities make BD a model condition in which to enhance understanding and develop treatments for co-occurring cognitive impairment and depression.
KEY POINTS.
Depressive symptoms and cognitive impairment together account for the majority of disability experienced by people with bipolar disorder
Bipolar depression and cognitive impairment seem to share neurobiological determinants, such as inflammation, as well has behavioral risks, such as sedentary lifestyles
Novel treatment avenues that may jointly target depression and cognitive impairment include pharmacological agents and health behavior modification
Acknowledgments
Role of the Funding Source: The study was funded in part by the National Institute of Mental Health Grants MH100417 and MH100318
Footnotes
Disclosures: None of the authors has any conflicts of interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burdick KE, Goldberg TE, Cornblatt BA, et al. The MATRICS Consensus Cognitive Battery in Patients with Bipolar I Disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011 Mar 30; doi: 10.1038/npp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonsen C, Sundet K, Vaskinn A, et al. Neurocognitive profiles in bipolar I and bipolar II disorder: differences in pattern and magnitude of dysfunction. Bipolar Disorders. 2008 Mar;10(2):245–255. doi: 10.1111/j.1399-5618.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- 3.Dittmann S, Hennig-Fast K, Gerber S, et al. Cognitive functioning in euthymic bipolar I and bipolar II patients. Bipolar Disorders. 2008;10(8):877–887. doi: 10.1111/j.1399-5618.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Aran A, Torrent C, Tabares-Seisdedos R, et al. Neurocognitive impairment in bipolar patients with and without history of psychosis. Journal of Clinical Psychiatry. 2008 doi: 10.4088/jcp.v69n0209. [DOI] [PubMed] [Google Scholar]
- 5.Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatrica Scandinavica. Supplementum. 2007;(434):17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 6.Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological Medicine. 2008;38(06):771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- 7.Burdick K, Russo M, Frangou S, et al. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychological medicine. 2014;44(14):3083–3096. doi: 10.1017/S0033291714000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samame C, Martino DJ, Strejilevich SA. An individual task meta-analysis of social cognition in euthymic bipolar disorders. Journal of Affective Disorders. 2015 Mar 1;173(0):146–153. doi: 10.1016/j.jad.2014.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Krabbendam L, Arts BM, Van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: A quantitative review. Schizophr Res. 2005 Sep; doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct Profiles of Neurocognitive Function in Unmedicated Unipolar Depression and Bipolar II Depression. Biological Psychiatry. 2007 Oct 15;62(8):917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Xu G, Lin K, Rao D, et al. Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: A longitudinal, naturalistic study. Journal of Affective Disorders. 2012 Feb;136(3):328–339. doi: 10.1016/j.jad.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Cerullo MA, Eliassen JC, Smith CT, et al. Bipolar I disorder and major depressive disorder show similar brain activation during depression. Bipolar Disorders. 2014;16(7):703–712. doi: 10.1111/bdi.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23(5):551. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Aran A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 15.Van Rheenen TE, Rossell SL. An empirical evaluation of the MATRICS Consensus Cognitive Battery in bipolar disorder. Bipolar Disorders. 2014;16(3):318–325. doi: 10.1111/bdi.12134. [DOI] [PubMed] [Google Scholar]
- 16.Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disorders. 2007;9(1–2):114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 17.Muralidharan K, Torres IJ, Silveira LE, et al. Impact of depressive episodes on cognitive deficits in early bipolar disorder: data from the Systematic Treatment Optimization Programme for Early Mania (STOP-EM) 2052014 doi: 10.1192/bjp.bp.113.135525. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin GM, Martinez-Aran A, Glahn DC, Vieta E. Cognitive impairment in bipolar disorder: neurodevelopment or neurodegeneration? An ECNP expert meeting report. European Neuropsychopharmacology. 2008 Nov;18(11):787–793. doi: 10.1016/j.euroneuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience & Biobehavioral Reviews. 2011 Jan;35(3):804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006 Apr;8(2):103–116. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 21.Zahodne LB, Stern Y, Manly JJ. Depressive Symptoms Precede Memory Decline, but Not Vice Versa, in Non-Demented Older Adults. Journal of the American Geriatrics Society. 2014 Jan 17;62(1):130–134. doi: 10.1111/jgs.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berk M, Berk L, Dodd S, et al. Stage managing bipolar disorder. Bipolar Disorders. 2014;16(5):471–477. doi: 10.1111/bdi.12099. [DOI] [PubMed] [Google Scholar]
- 23.Depp CA, Mausbach BT, Harmell AL, et al. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disorders. 2012 May;14(3):217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005 Dec;62(12):1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- 25.Bowie CR, Depp C, McGrath JA, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010 Sep;167(9):1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnin CM, Martinez-Aran A, Torrent C, et al. Clinical and neurocognitive predictors of functional outcome in bipolar euthymic patients: A long-term, follow-up study. Journal of Affective Disorders. 2010 Feb;121(1–2):156–160. doi: 10.1016/j.jad.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Depp CA, Mausbach BT, Bowie C, et al. Determinants of occupational and residential functioning in bipolar disorder. Journal of Affective Disorders. 2012;136(3):812–818. doi: 10.1016/j.jad.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey PD, Paschall G, Depp C. Factors influencing self-assessment of cognition and functioning in bipolar disorder: a preliminary study. Cognitive Neuropsychiatry 2015. 2015 Jul 04;20(4):361–371. doi: 10.1080/13546805.2015.1044510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brietzke E, Stertz L, Fernandes BS, et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. Journal of affective disorders. 2009 Aug;116(3):214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 30.De Berardis D, Conti CM, Campanella D, et al. Evaluation of C-reactive protein and total serum cholesterol in adult patients with bipolar disorder. International journal of immunopathology and pharmacology. 2008 Apr-Jun;21(2):319–324. doi: 10.1177/039463200802100208. [DOI] [PubMed] [Google Scholar]
- 31.Kapczinski F, Dal-Pizzol F, Teixeira AL, et al. Peripheral biomarkers and illness activity in bipolar disorder. Journal of psychiatric research. 2011 Feb;45(2):156–161. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. Journal of affective disorders. 2006 Feb;90(2–3):263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz-Dominguez A, Hernandez ME, Berlanga C, et al. Immune variations in bipolar disorder: phasic differences. Bipolar disorders. 2007 Sep;9(6):596–602. doi: 10.1111/j.1399-5618.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 34.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. Journal of affective disorders. 2013 Sep 5;150(2):456–459. doi: 10.1016/j.jad.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Doganavsargil-Baysal O, Cinemre B, Aksoy UM, et al. Levels of TNF-alpha, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Human psychopharmacology. 2013 Mar;28(2):160–167. doi: 10.1002/hup.2301. [DOI] [PubMed] [Google Scholar]
- 36.Teunissen CE, van Boxtel MP, Bosma H, et al. Inflammation markers in relation to cognition in a healthy aging population. Journal of neuroimmunology. 2003 Jan;134(1–2):142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 37.Wright CB, Sacco RL, Rundek T, Delman J, Rabbani L, Elkind M. Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2006 Jan-Feb;15(1):34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. Journal of affective disorders. 2013 Sep 5;150(2):192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 39.Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001 Jun;3(3):106–150. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151-103. [DOI] [PubMed] [Google Scholar]
- 40.Bauer IE, Ouyang A, Mwangi B, et al. Reduced white matter integrity and verbal fluency impairment in young adults with bipolar disorder: A diffusion tensor imaging study. Journal of psychiatric research. 2015 Mar;62:115–122. doi: 10.1016/j.jpsychires.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bettcher BM, Watson CL, Walsh CM, et al. Interleukin-6, age, and corpus callosum integrity. PloS one. 2014;9(9):e106521. doi: 10.1371/journal.pone.0106521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasad KM, Upton CH, Nimgaonkar VL, Keshavan MS. Differential susceptibility of white matter tracts to inflammatory mediators in schizophrenia: an integrated DTI study. Schizophrenia research. 2015 Jan;161(1):119–125. doi: 10.1016/j.schres.2014.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Progress in neuro-psychopharmacology & biological psychiatry. 2014 Jan 3;48:295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Sussmann JE, Lymer GKS, McKirdy J, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar disorders. 2009;11(1):11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 45.Zanetti M, Jackowski M, Versace A, et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci 2009. 2009 Sep 01;259(6):316–328. doi: 10.1007/s00406-009-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D. Voxel-Based Meta-Analytical Evidence of Structural Disconnectivity in Major Depression and Bipolar Disorder. Biological psychiatry. 2015 Mar 12; doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Yamada S, Takahashi S, Ukai S, et al. Microstructural abnormalities in anterior callosal fibers and their relationship with cognitive function in major depressive disorder and bipolar disorder: a tract-specific analysis study. Journal of affective disorders. 2015 Mar 15;174:542–548. doi: 10.1016/j.jad.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 48.McElroy SL, Keck PE. Obesity in Bipolar Disorder: An Overview. Current Psychiatry Reports. 2012:1–9. doi: 10.1007/s11920-012-0313-8. [DOI] [PubMed] [Google Scholar]
- 49.Depp CA, Strassnig M, Mausbach BT, et al. Association of obesity and treated hypertension and diabetes with cognitive ability in bipolar disorder and schizophrenia. Bipolar Disorders. 2014;16(4):422–431. doi: 10.1111/bdi.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC medicine. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacka FN, Pasco JA, Mykletun A, et al. Association of Western and traditional diets with depression and anxiety in women. The American journal of psychiatry. 2010 Mar;167(3):305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- 52.Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health study. Psychosomatic medicine. 2011 Jul-Aug;73(6):483–490. doi: 10.1097/PSY.0b013e318222831a. [DOI] [PubMed] [Google Scholar]
- 53.Nanri A, Kimura Y, Matsushita Y, et al. Dietary patterns and depressive symptoms among Japanese men and women. European journal of clinical nutrition. 2010 Aug;64(8):832–839. doi: 10.1038/ejcn.2010.86. [DOI] [PubMed] [Google Scholar]
- 54.Jacka FN, Pasco JA, Mykletun A, et al. Diet quality in bipolar disorder in a population-based sample of women. Journal of affective disorders. 2011 Mar;129(1–3):332–337. doi: 10.1016/j.jad.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. The American journal of clinical nutrition. 2004 Oct;80(4):1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 56.Hargrave SL, Davidson TL, Lee TJ, Kinzig KP. Brain and behavioral perturbations in rats following Western diet access. Appetite. 2015 Apr 8; doi: 10.1016/j.appet.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai J, Miller AH, Bremner JD, et al. Adherence to the Mediterranean Diet Is Inversely Associated With Circulating Interleukin-6 Among Middle-Aged Men: A Twin Study. Circulation. 2008 Dec 17;117(2):169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viscogliosi G, Cipriani E, Liguori ML, et al. Mediterranean Dietary Pattern Adherence: Associations with Prediabetes, Metabolic Syndrome, and Related Microinflammation. Metabolic Syndrome and Related Disorders. 2013;11(3):210–216. doi: 10.1089/met.2012.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ginter E, Simko V. Recent data on Mediterranean diet, cardiovascular disease, cancer, diabetes and life expectancy. Bratislavske lekarske listy. 2015;116(6):346–348. doi: 10.4149/bll_2015_065. [DOI] [PubMed] [Google Scholar]
- 60.Vancampfort D, Correll CU, Probst M, et al. A review of physical activity correlates in patients with bipolar disorder. Journal of Affective Disorders. 2013 Mar 5;145(3):285–291. doi: 10.1016/j.jad.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Krane-Gartiser K, Henriksen TE, Morken G, Vaaler A, Fasmer OB. Actigraphic assessment of motor activity in acutely admitted inpatients with bipolar disorder. PloS one. 2014;9(2):e89574. doi: 10.1371/journal.pone.0089574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janney CA, Fagiolini A, Swartz HA, Jakicic JM, Holleman RG, Richardson CR. Are adults with bipolar disorder active? Objectively measured physical activity and sedentary behavior using accelerometry. Journal of affective disorders. 2014 Jan;152–154:498–504. doi: 10.1016/j.jad.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jerstad SJ, Boutelle KN, Ness KK, Stice E. Prospective reciprocal relations between physical activity and depression in female adolescents. Journal of consulting and clinical psychology. 2010;78(2):268. doi: 10.1037/a0018793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sylvia LG, Friedman ES, Kocsis JH, et al. Association of exercise with quality of life and mood symptoms in a comparative effectiveness study of bipolar disorder. Journal of affective disorders. 2013 Nov;151(2):722–727. doi: 10.1016/j.jad.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 65.Nesvag R, Knudsen GP, Bakken IJ, et al. Substance use disorders in schizophrenia, bipolar disorder, and depressive illness: a registry-based study. Social psychiatry and psychiatric epidemiology. 2015 Feb 14; doi: 10.1007/s00127-015-1025-2. [DOI] [PubMed] [Google Scholar]
- 66.Bizzarri JV, Sbrana A, Rucci P, et al. The spectrum of substance abuse in bipolar disorder: reasons for use, sensation seeking and substance sensitivity. Bipolar Disorders. 2007;9(3):213–220. doi: 10.1111/j.1399-5618.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- 67.Balanza-Martinez V, Crespo-Facorro B, Gonzalez-Pinto A, Vieta E. Bipolar disorder comorbid with alcohol use disorder: focus on neurocognitive correlates. Frontiers in physiology. 2015;6:108. doi: 10.3389/fphys.2015.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levy B, Weiss RD. Neurocognitive impairment and psychosis in bipolar disorder during early remission from an acute episode of mood disturbance. The Journal of clinical psychiatry. 2010 Nov 17;71(2):201–206. doi: 10.4088/JCP.08m04663yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levy B, Weiss RD. Cognitive functioning in bipolar and co-occurring substance use disorders: a missing piece in the puzzle. Harvard review of psychiatry. 2009;17(3):226–230. doi: 10.1080/10673220902979870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Depp CA, Bowie CR, Mausbach BT, et al. Current smoking is associated with worse cognitive and adaptive functioning in serious mental illness. Acta Psychiatrica Scandinavica. 2015;131(5):333–341. doi: 10.1111/acps.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harvey AG, Talbot LS, Gershon A. Sleep Disturbance in Bipolar Disorder Across the Lifespan. Clinical Psychology: Science and Practice. 2009;16(2):256–277. doi: 10.1111/j.1468-2850.2009.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eidelman P, Talbot LS, Gruber J, Harvey AG. Sleep, illness course, and concurrent symptoms in inter-episode bipolar disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2010 Jun;41(2):145–149. doi: 10.1016/j.jbtep.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gruber J, Harvey AG, Wang PW, et al. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Journal of Affective Disorders. 2009 Apr;114(1–3):41–49. doi: 10.1016/j.jad.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boland EM, Alloy LB. Sleep disturbance and cognitive deficits in bipolar disorder: Toward an integrated examination of disorder maintenance and functional impairment. Clinical Psychology Review. 2013 Feb;33(1):33–44. doi: 10.1016/j.cpr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology. 2012 Nov;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhai L, Zhang Y, Zhang D. Sedentary behaviour and the risk of depression: a meta-analysis. British Journal of Sports Medicine. 2015 Jun 1;49(11):705–709. doi: 10.1136/bjsports-2014-093613. [DOI] [PubMed] [Google Scholar]
- 77.James BD, Wilson RS, Barnes LL, Bennett DA. Late-Life Social Activity and Cognitive Decline in Old Age. Journal of the International Neuropsychological Society. 2011;17(06):998–1005. doi: 10.1017/S1355617711000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eidelman P, Gershon A, Kaplan K, McGlinchey E, Harvey AG. Social support and social strain in inter-episode bipolar disorder. Bipolar Disorders. 2012;14(6):628–640. doi: 10.1111/j.1399-5618.2012.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. Inflamed moods: A review of the interactions between inflammation and mood disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014 Aug 4;53(0):23–34. doi: 10.1016/j.pnpbp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 80.Cederholm T, Salem N, Palmblad J. ω-3 Fatty Acids in the Prevention of Cognitive Decline in Humans. Advances in Nutrition: An International Review Journal. 2013 Nov 1;4(6):672–676. doi: 10.3945/an.113.004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burdick KE, Braga RJ, Nnadi CU, Shaya Y, Stearns WH, Malhotra AK. Placebo-controlled adjunctive trial of pramipexole in patients with bipolar disorder: targeting cognitive dysfunction. The Journal of clinical psychiatry. 2012 Jan;73(1):103–112. doi: 10.4088/JCP.11m07299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Torrent C, Bonnin CdM, Martinez-Aran A, et al. Efficacy of Functional Remediation in Bipolar Disorder: A Multicenter Randomized Controlled Study. American Journal of Psychiatry. 2013;170(8):852–859. doi: 10.1176/appi.ajp.2012.12070971. [DOI] [PubMed] [Google Scholar]
- 83.Demant KM, Vinberg M, Kessing LV, Miskowiak KW. Effects of Short-Term Cognitive Remediation on Cognitive Dysfunction in Partially or Fully Remitted Individuals with Bipolar Disorder: Results of a Randomised Controlled Trial. PLoS ONE. 2015;10(6):e0127955. doi: 10.1371/journal.pone.0127955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolinsky FD, Vander Weg MW, Martin R, et al. The Effect of Speed-of-Processing Training on Depressive Symptoms in ACTIVE. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009 Jan 1;2009 doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joormann J, Quinn ME. COGNITIVE PROCESSES AND EMOTION REGULATION IN DEPRESSION. Depression and Anxiety. 2014;31(4):308–315. doi: 10.1002/da.22264. [DOI] [PubMed] [Google Scholar]
- 86.Daumit GL, Dickerson FB, Wang N-Y, et al. A Behavioral Weight-Loss Intervention in Persons with Serious Mental Illness. New England Journal of Medicine. 2013;368(17):1594–1602. doi: 10.1056/NEJMoa1214530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malchow B, Reich-Erkelenz D, Oertel-Knochel V, et al. The effects of physical exercise in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci 2013. 2013 Sep 01;263(6):451–467. doi: 10.1007/s00406-013-0423-2. [DOI] [PubMed] [Google Scholar]
- 88.Kucyi A, Alsuwaidan MT, Liauw SS, McIntyre RS. Aerobic physical exercise as a possible treatment for neurocognitive dysfunction in bipolar disorder. Postgraduate medicine. 2010 Nov;122(6):107–116. doi: 10.3810/pgm.2010.11.2228. [DOI] [PubMed] [Google Scholar]
- 89.Lee JY, Harvey AG. Memory for therapy in bipolar disorder and comorbid insomnia. Journal of consulting and clinical psychology. 2015;83(1):92. doi: 10.1037/a0037911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Depp CA, Ceglowski J, Wang VC, et al. Augmenting psychoeducation with a mobile intervention for bipolar disorder: A randomized controlled trial. Journal of Affective Disorders. 2015 Mar 15;174(0):23–30. doi: 10.1016/j.jad.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]