Abstract
Large plasmids of some Bacillus species encode a distinct tubulin homolog, TubZ, implicated in maintenance of the host plasmid. A recent study has shown that TubZ polymers exhibit treadmilling behavior in vivo, suggesting that they are involved in mitotic activity.
It is now clear that bacteria harbor several families of tubulin and actin that have cytoskeletal functions [1,2]. The bacterial actin family includes: MreB and MreB-like proteins, which polymerize into helical structures on the cytoplasmic membrane that direct cell-wall biosynthesis in rod-shape bacteria; FtsA, which acts with FtsZ in cell division; MamK, which organizes magnetosomes in magnetotactic bacteria; and plasmid-encoded actin homologs such as ParM and AlfA, which form mitotic spindle-like polymers required for partitioning of the host plasmids into daughter cells. There is also good evidence that MreB polymers are involved in partitioning of chromosomes. Therefore, diverse homologs of actin function in various organizational tasks in the bacterial cell, including mitotic-like partitioning of DNA.
Bacterial tubulins also come in several varieties. The best-studied version is FtsZ, which assembles into protofilaments that form an organized array at the division plane, called the Z ring, required for cytokinesis [3]. Another type of bacterial tubulin, present in some Verrucomicrobia, is a family of two and much more like tubulin than FtsZ; its gene was probably acquired from a eukaryote by horizontal transfer [4]. Archaea also contain a family of divergent tubulins, some of which are clearly homologs of FtsZ, and others that are FtsZ-like but distinct from both FtsZ and tubulin [5]. Other than the Verrucomicrobia, the only other bacteria that have tubulin homologs apart from FtsZ consist of a group of Bacillus species. These bacteria have a typical ftsZ gene on their chromosome for cytokinesis, but harbor an additional, divergent ftsZ on a large plasmid.
One of these plasmids is pXO1, a large virulence plasmid of B. anthracis, the species that causes anthrax. This plasmid, which encodes the major toxins crucial in causing disease [6], is very stably inherited, indicating that it has a dedicated partitioning system. The divergent ftsZ on this plasmid, named repX, is required for proper maintenance of a pXO1 mini-plasmid, although its precise function is not known [7]. Similarly, B. thuringiensis subsp. israelensis contains a large plasmid called pBtoxis that encodes the well-known crystalline Bt toxin, and this plasmid also harbors a similar divergent ftsZ gene in addition to the familiar chromosomal ftsZ [8]. As with pXO1, a mini-pBtoxis plasmid containing the divergent ftsZ is capable of replicating [9]. A recent study by Larsen et al. [10] suggests that this FtsZ, renamed TubZ because of its similarity to both tubulin and FtsZ, represents a new class of bacterial cytoskeletal proteins.
Because it is related to FtsZ and tubulin, Larsen et al. [10] first tagged TubZ with green fluorescent protein (GFP) to test whether it forms polymers in B. thuringiensis cells. When produced at its native locus on pBtoxis downstream from another gene called tubR, TubZ–GFP formed long filamentous structures. Such structures are often found when FtsZ is overproduced, and the observations confirm that TubR is proficient in forming polymers in cells. The big surprise, however, was that individual filaments of TubZ–GFP moved rapidly along the cell membrane, giving the impression that an entire filament was being translocated around the cell. When not at a cell pole, a typical TubZ–GFP filament extended along one side of the cell, next to the cytoplasmic membrane. But once the leading edge of the traveling filament arrived at the bacterial cell pole, it bent around the curved pole and continued migrating back up the other side of the cell, where it straightened out again (Figure 1). This astonishing dynamic migration showed that the TubZ–GFP filament is highly flexible, yet seems to have a high affinity for the cytoplasmic membrane as it translocates.
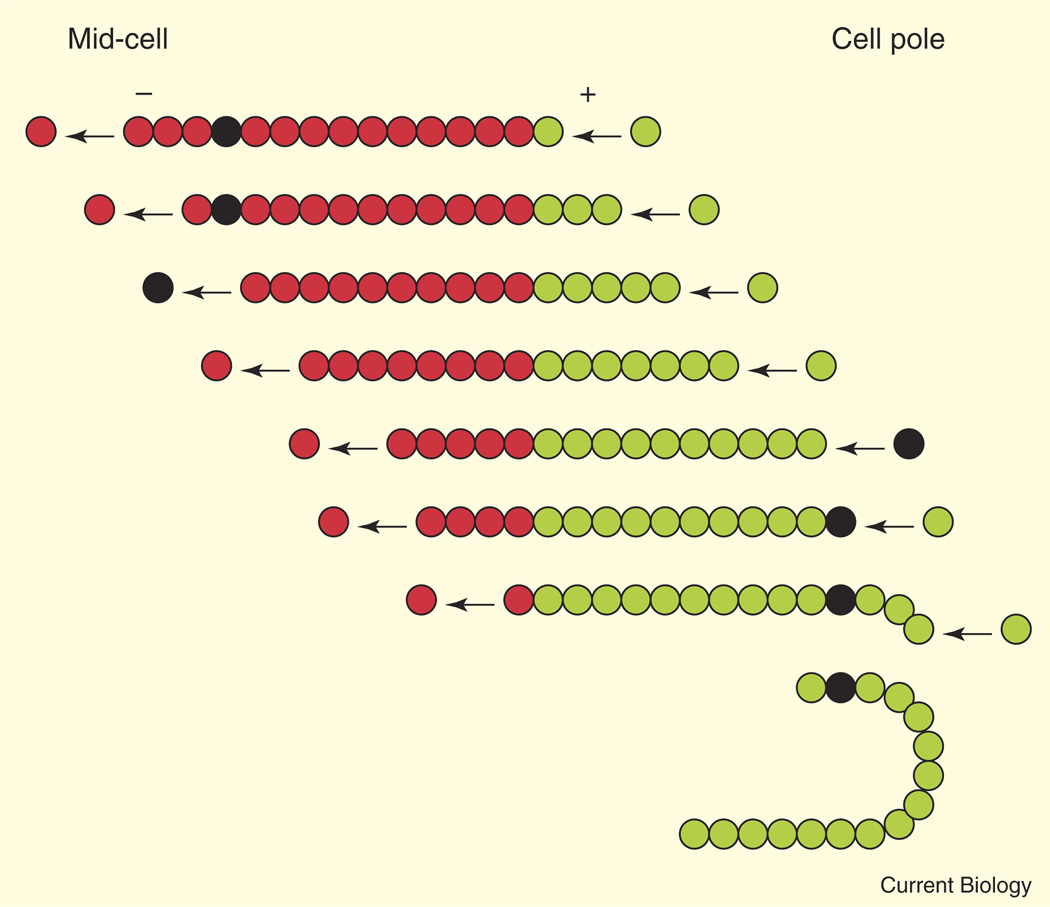
Figure 1. Treadmilling of a TubZ protofilament toward a cell pole and a model for mitotic-like transport of pBToxis plasmid DNA.
Each row shows a successive theoretical time point during the treadmilling process, with the leading (plus) end of the TubZ protofilament extending rightward down the cell membrane. The first time point shows the protofilament near the mid-cell position. At the last time point, the protofilament has migrated around the cell pole and continues leftward back to mid-cell. Circles show assembled subunits (red), newly added subunits (green), or a subunit (black) tethered to plasmid DNA via an adaptor complex (not shown). The release of the plasmid-tethered subunit from the minus end and eventual reincorporation at the plus end results in net movement of the attached plasmid. The black circle can also represent a photobleached subunit to illustrate how treadmilling is identified. For simplicity, protofilament subunits are not drawn to scale, and only one protofilament per treadmilling TubZ filament is depicted.
What is the mechanism behind this filament migration? One possibility is that a TubZ–GFP filament with fixed ends can slide along the membrane, perhaps propelled by anchored motor proteins. The other possibility is that new TubZ–GFP subunits are added at the leading end of the filament, while TubZ–GFP subunits are lost at the trailing end. The latter mechanism, which gives the impression of transport of a fixed filament but is actually achieved by polarized growth and shrinkage, is called treadmilling.
To distinguish between the two mechanisms, Larsen et al. [10] photobleached the middle of a traveling TubZ–GFP filament and monitored the movement of the bleached spot. They found that the spot did not move with the traveling filament but instead moved toward the trailing end of the filament, remaining at the same fixed location in the cell (Figure 1). This is strong evidence for treadmilling. Similar TubZ–GFP filament treadmilling was observed when TubR and TubZ–GFP were co-produced in Escherichia coli, which lacks a pBtoxis plasmid or TubZ, indicating that TubZ polymerization and treadmilling do not depend on a B. thuringiensis-specific factor. In some cases, the minus end of the TubZ–GFP filament remained stationary while the plus end grew, additional evidence for polarized filament growth consistent with a treadmilling mechanism. Interestingly, in E. coli cells starved for nutrients, TubZ–GFP filaments could be seen rapidly disassembling from both ends, suggesting that, like microtubules, TubZ filaments undergo catastrophe.
Treadmilling of TubZ can be uncoupled from its assembly into filaments. For example, a mutant TubZ lacking a critical residue for GTP hydrolysis assembled into long filaments, but these filaments were static and often displayed morphological abnormalities, such as branching. Excess levels of TubZ also inhibited treadmilling, as did co-production of the TubZ mutant with wild-type TubZ. Finally, TubZ assembly into filaments required a critical concentration, typical of tubulin and actin assembly. These results indicate that the assembly dynamics of TubZ are sensitive to TubZ concentration, and that GTP hydrolysis within the filament is a necessary switching mechanism to generate the asymmetry needed for a growing (plus) end and a shrinking (minus) end.
How common is treadmilling in vivo? Actin filaments involved in motility translocate by treadmilling, with growth at the barbed end and loss of subunits at the pointed end [11]. The bacterial actin homolog MreB also moves via treadmilling [12]. Microtubules treadmill in vivo under certain conditions [13,14], although this is not commonly observed in animal cells because most microtubules are anchored at their minus ends by centrosomes. In contrast, plants form ordered arrays of microtubules in interphase, despite the lack of centrosomes. Plant microtubules literally get around this problem by treadmilling to reorient themselves [15]. FtsZ has not yet been shown to treadmill, although the rapid movement of non-ring FtsZ in helical paths might occur via such a mechanism, which has also been suggested by the effects of mutations in FtsZ [16,17].
The velocity of TubZ filament growth in B. thuringiensis averages 1.8 mm per minute, remarkably similar to the rate of translocation of treadmilling plant microtubules. However, the latter change their total length by growing faster at their leading end than they shrink at their trailing end. TubZ filaments, in contrast, move their plus and minus ends on average at similar rates, thus maintaining fairly constant filament lengths. Therefore, at physiological concentrations of TubZ, the plus ends of TubZ filaments should have a net rate of assembly equivalent to the net rate of disassembly at the minus end.
This suggests that the cellular concentration of TubZ must be regulated to be higher than the critical concentration at the plus end of a TubZ filament but lower than the critical concentration at the minus end, creating an even balance of assembly and disassembly. This would explain why excess TubZ or inhibition of GTP hydrolysis blocks TubZ treadmilling. Such precise regulation of TubZ levels is probably achieved via the action of TubR, a DNA-binding protein that represses expression of the tubRZ operon. Not surprisingly, Larsen et al. showed that TubR was dispensable for TubZ filament assembly, although it is not yet clear whether TubR is required for TubZ filament dynamics.
The study by Larsen et al. [10] raises a number of interesting questions. First, what is the precise nature of the TubZ filament? One possibility is that it is a monomer-thick protofilament, although this is unlikely given the strong GFP fluorescence signal observed for the TubZ filament. It is more likely that the TubZ filament consists of a bundle of protofilaments. If so, then the unidirectional motion of the putative bundle requires that the individual protofilaments be polarized, like actin filaments. The possibility that TubZ forms a microtubule-like structure cannot be ruled out, although the TubZ filaments clearly have a high degree of flexibility to treadmill around the 180° curve of the bacterial cell poles and remain intact. It is not clear why TubZ usually assembles into one or two single long filaments per cell instead of many short filaments, but it is reasonable to assume that cooperative assembly limits new nucleation events.
The second key question is how dynamic TubZ filaments function to maintain and segregate the pBtoxis plasmid in B. thuringiensis. It is hard to imagine how TubZ could be involved in the process of DNA replication. It is more plausible that after the plasmids are replicated, they attach to dynamic TubZ filaments, which then transport each plasmid to opposite cell halves. As with other plasmid segregation systems involving bacterial actin filaments or polymers of other ATPases [18], the TubZ-pBtoxis system must have a way to tether the plasmid DNA to the filaments. It is possible that TubR is this tethering protein, although there is currently no evidence for this. If TubZ filaments of constant length move by treadmilling, then attachment of plasmid DNA via a tethering protein to an individual TubZ subunit within a TubZ protofilament would not immediately move the DNA toward the cell poles. However, once the carrier subunit was lost from the minus end, it would eventually be reincorporated at the plus end, which would have translocated a significant distance. This is a potential mechanism whereby a plasmid might hitch a ride on the TubZ tram (Figure 1), although it is not clear how the bidirectional transport and eventual polar anchoring of two daughter plasmids would be achieved. This would be especially critical during formation of the endospore at the extreme end of a B. thuringiensis cell.
Finally, how did the TubZ cytoskeleton arise and how is it related to the FtsZ cytoskeleton? Whereas other large Bacillus plasmids also encode TubZ homologs, nothing is known about whether they form dynamic polymers or where they localize. Interestingly, TubZ and FtsZ do not colocalize in B. thuringiensis or E. coli, probably because their subunit contacts are as divergent as their primary sequences. Indeed, several key residues of TubZ match those of tubulin and not FtsZ, supporting the idea that TubZ, tubulin and FtsZ diverged early in evolution of the tubulin family. This underscores the point that TubZ is a not a run of the mill tubulin, but instead is the first bacterial tubulin known to treadmill and to be implicated in a mitotic-like process.
References
- 1.Michie KA, Löwe J. Dynamic filaments of the bacterial cytoskeleton. Annu. Rev. Biochem. 2006;75:467–492. doi: 10.1146/annurev.biochem.75.103004.142452. [DOI] [PubMed] [Google Scholar]
- 2.Gitai Z. Diversification and specialization of the bacterial cytoskeleton. Curr. Opin. Cell. Biol. 2007;19:5–12. doi: 10.1016/j.ceb.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Margolin W. FtsZ and the division of prokaryotic cells and organelles. Nat. Rev. Mol. Cell. Biol. 2005;6:862–871. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlieper D, Oliva MA, Andreu JM, Löwe J. Structure of bacterial tubulin BtubA/B: evidence for horizontal gene transfer. Proc. Natl. Acad. Sci. USA. 2005;102:9170–9175. doi: 10.1073/pnas.0502859102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaughan S, Wickstead B, Gull K, Addinall SG. Molecular evolution of FtsZ protein sequences encoded within the genomes of archaea, bacteria, and eukaryota. J. Mol. Evol. 2004;58:19–29. doi: 10.1007/s00239-003-2523-5. [DOI] [PubMed] [Google Scholar]
- 6.Koehler TM. Bacillus anthracis genetics and virulence gene regulation. Curr. Top. Microbiol. Immunol. 2002;271:143–164. doi: 10.1007/978-3-662-05767-4_7. [DOI] [PubMed] [Google Scholar]
- 7.Tinsley E, Khan SA. A novel FtsZ-like protein is involved in replication of the anthrax toxin-encoding pXO1 plasmid in Bacillus anthracis. J. Bacteriol. 2006;188:2829–2835. doi: 10.1128/JB.188.8.2829-2835.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry C, O’Neil S, Ben-Dov E, Jones AF, Murphy L, Quail MA, Holden MT, Harris D, Zaritsky A, Parkhill J. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2002;68:5082–5095. doi: 10.1128/AEM.68.10.5082-5095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang M, Bideshi DK, Park HW, Federici BA. Minireplicon from pBtoxis of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2006;72:6948–6954. doi: 10.1128/AEM.00976-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen RA, Cusumano C, Fujioka A, Lim-Fong G, Patterson P, Pogliano J. Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis. Genes Dev. 2007;21:1340–1352. doi: 10.1101/gad.1546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theriot JA, Mitchison TJ. Actin microfilament dynamics in locomoting cells. Nature. 1991;352:126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Gitai Z, Kinkhabwala A, Shapiro L, Moerner WE. Single molecules of the bacterial actin MreB undergo directed treadmilling motion in Caulobacter crescentus. Proc. Natl. Acad. Sci. USA. 2006;103:10929–10934. doi: 10.1073/pnas.0604503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waterman-Storer CM, Salmon ED. Microtubule dynamics: treadmilling comes around again. Curr. Biol. 1997;7:369–372. doi: 10.1016/s0960-9822(06)00177-1. [DOI] [PubMed] [Google Scholar]
- 14.Rodionov VI, Borisy GG. Microtubule treadmilling in vivo. Science. 1997;275:215–218. doi: 10.1126/science.275.5297.215. [DOI] [PubMed] [Google Scholar]
- 15.Shaw SL, Kamyar R, Ehrhardt DW. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science. 2003;300:1715–1718. doi: 10.1126/science.1083529. [DOI] [PubMed] [Google Scholar]
- 16.Thanedar S, Margolin W. FtsZ exhibits rapid movement and oscillation waves in helix-like patterns in Escherichia coli. Curr. Biol. 2004;14:1167–1173. doi: 10.1016/j.cub.2004.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redick SD, Stricker J, Briscoe G, Erickson HP. Mutants of FtsZ targeting the protofilament interface: effects on cell division and GTPase activity. J. Bacteriol. 2005;187:2727–2736. doi: 10.1128/JB.187.8.2727-2736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes F, Barilla D. The bacterial segrosome: a dynamic nucleoprotein machine for DNA trafficking and segregation. Nat. Rev. Microbiol. 2006;4:133–143. doi: 10.1038/nrmicro1342. [DOI] [PubMed] [Google Scholar]