Abstract
Patients with chronic heart failure (CHF) have an insufficient perfusion to the peripheral tissues due to decreased cardiac output. The compensatory mechanisms are triggered even prior to the occurrence of clinical symptoms, which include activation of the sympathetic nervous system (SNS) and other neurohumoral factors. However, the long-term activation of the SNS contributes to progressive cardiac dysfunction and has toxic effects on the cardiomyocytes. The mechanisms leading to the activation of SNS include changes in peripheral baroreceptor and chemoreceptor reflexes and the abnormal regulation of sympathetic nerve activity (SNA) in the central nervous system (CNS). Recent studies have focused on the role of brain mechanisms in the regulation of SNA and the progression of CHF. The renin-angiotensin system, nitric oxide and pro-inflammatory cytokines were shown to be involved in the abnormal regulation of SNA in the CNS. The alteration of these neurohumoral factors during CHF influences the activity of neurons in the autonomic regions and finally increase the sympathetic outflow. The present review summarizes the brain mechanisms contributing to sympathoexcitation in CHF.
Keywords: chronic heart failure, central nervous system, sympathetic nervous system, renin-angiotensin system, nitric oxide, pro-inflammatory cytokines
1. Introduction
With the increasing morbidity of coronary heart disease and hypertension, the prevalence of chronic heart failure (CHF) has been increasing over the past several decades (1). CHF is the terminal state of various cardiovascular diseases and the mortality rate remains high in spite of the large number of studies which have been performed to clarify its underlying mechanisms and to improve its treatment (2). The most significant hallmark of CHF is the continuous interaction between the underlying myocardial dysfunction and the compensatory neurohumoral mechanisms (3). Activation of the sympathetic nervous system (SNS) is a major compensatory mechanism in the development of CHF, which may be due to the changes in peripheral baroreceptor and chemoreceptor reflexes, chemical mediators that control sympathetic outflow and central integrative sites (4). Recent studies have indicated that a variety of agents, including angiotensin II (AngII), nitric oxide (NO) and pro-inflammatory cytokines are involved in the regulation of sympathetic nerve activity (SNA) in the central nervous system (CNS) during CHF (3). The interaction between these agents and neurotransmitters can influence the neuronal activity via central autonomic pathways in different autonomic regions in the CNS. The abnormal sympathoexcitation in the CNS deteriorates the cardiac function during CHF and contributes to disease progression.
2. Main sympathetic activity-regulating nuclei in the CNS
The paraventricular nucleus (PVN) in the hypothalamus, the rostral ventrolateral medulla (RVLM) and the nucleus tractus solitarius (NTS) are three important regions within the CNS controlling SNA (5). The NTS receives direct input from the cardiopulmonary afferents, such as baroreceptors and chemo-receptors, and the area postrema (AP), and has an indirect effect on the neuronal activity of the RVLM through modulating the caudal ventrolateral medulla (CVLM) region (6,7). In addition, the NTS has neural connections to the PVN, a major integrative nucleus that can influence SNA and extracellular fluid volume, and the intermediolateral (IML) column of the spinal cord (5,8). The PVN receives signals from the sub-fornical organ (SFO), which positively influences the activity of the PVN (9). The RVLM has a key role in determining the tonic and reflex control of SNA (10). It receives input signals from the PVN and subsequently communicates with the IML, which is also under direct influence of the PVN (11–13). Finally, the IML receives input signals from these sympathetic activity-regulating nuclei and stimulates the sympathetic ganglionic neurons projecting to target organs.
3. RAS
Previous studies have demonstrated that the renin-angiotensin system (RAS), whose main effective molecule is AngII, is a key regulator of cardiovascular system function and fluid homeostasis. RAS is activated upon decreases and inhibited upon increases of the blood pressure, increases of sodium delivery to the macula densa and volume overload. AngII has two types of receptor: AngII type 1 (AT1) receptor is responsible for water and sodium retention, aldosterone secretion and vasoconstriction, while AT2 receptor is able to dilate the blood vessels.
According to the classic mechanism, AngII exerts its effects via the circulating system. For example, the concentration of AngII in plasma is increased under CHF conditions, which can inhibit the sympathoinhibitory baroreflex and enhance the sympathoexcitatory chemoreflex (14,15). Apart from these actions, circulating AngII can also increase SNA in the CNS through binding to its AT1 receptors in certain autonomic regions, which have no blood-brain barrier, such as AP and SFO (16,17). However, a large number of studies have demonstrated the presence of an endogenous brain RAS within the CNS (18). This is supported by the fact that angiotensinogen, renin and angiotensin-converting enzyme (ACE) are generated by glial cells and neurons in a number of nuclei (19–21).
Sympathoexcitatory effect of AngII in CHF
Microinjection of AngII into the PVN was shown to increase plasma norepinephrine, renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP) and the heart rate (HR) to a greater extent in rats with ischemia-induced CHF compared with those in sham rats, whereas inhibition of AT1 receptors produced a significant decrease of RSNA, MAP and HR (22). Administration of AngII into the RVLM evoked sympathoexcitation in sham rats as well as rats with CHF, with a significantly larger response in the animals with CHF (23). In addition, basal SNA and the cardiac sympathetic afferent reflex were shown to be decreased by bilateral NTS injection of the AT1 receptor antagonist losartan in rats with CHF but not in sham rats, suggesting that activation of the AT1 receptor in the NTS can increase SNA during CHF (21). Over-activity of the RAS of the endogenous brain may be partly attributed to the increased expression of AT1 receptor and ACE in the CNS. In CHF, the activity of ACE in the PVN is increased and the expression of AT1 receptor is also increased in the neurons of the PVN, RVLM and NTS (23–26). Further investigation showed that AngII-triggered mitogen-activated protein kinases (MAPKs) have an important role in the upregulation of AT1 receptor in the PVN in rats with CHF (26). Under normal conditions, AT1 receptor is weakly expressed in astrocytes, while it is upregulated in brainstem astrocytes and has a key role in enhancing sympathetic outflow under CHF conditions. However, the expression of AT1 receptor in astrocytes of other sympathetic activity-regulating nuclei during CHF requires further study (27). In an ovine model of CHF, central infusion of losartan significantly decreased the elevated levels of cardiac sympathetic nerve activity (CSNA), while RSNA was neither elevated nor affected by losartan. Furthermore, the expression of AT1 receptor was increased in the PVN but decreased in the NTS (28). These results were not completely consistent with the observations made in rodent models of CHF and the reasons for these discrepancies remain elusive. In spite of these discrepancies, the abovementioned studies suggested that increased AngII signaling, via activating the AT1 receptor, is responsible for the enhanced SNA during CHF.
Sympathoinhibitory effects of the AT2 receptor
In contrast to the AT1 receptor, the AT2 receptor has an opposite role in regulating SNA within the CNS. Intracerebroventricular injection of AngII in AT2 receptor gene knock-out mice resulted in a greater increase in blood pressure compared to that in wild-type mice (29). Intracerebroventricular infusion of Compound 21, a non-peptide AT2 receptor agonist, decreased norepinephrine excretion and blood pressure via the NO signaling pathway in the PVN of normal rats and Compound 21 also suppressed the sympathetic outflow by improving baroreflex sensitivity in rats with CHF (30,31). Overexpression of AT2 receptor in the RVLM led to a significant decrease in MAP and urinary noradrenaline excretion in normal rats (32). However, the expression of AT2 receptor in the RVLM of CHF rats was significantly lower than that in sham rats. These studies indicated that AT2 receptor activation within the CNS causes a decrease in SNA and that impairment of AT2 receptor signaling is involved in the sympathoexcitation in CHF (23). While the exact underlying mechanisms for the beneficial effects of AT2 receptor activation have yet to be elucidated, it has been indicated that activation of the AT2 receptor may inhibit the neuronal excitability and decrease the sympathetic outflow through increasing the potassium current in neurons (33). However, direct evidence for the sympathoinhibitory effects of AT2 receptor activation in the NTS and PVN on the modulation of SNA during CHF is currently lacking and the mechanisms of AT2 receptor downregulation in CHF state require further investigation.
Interaction between AngII and neurotransmitters in the regulation of SNA
Glutamate and γ-aminobutyric acid (GABA) were observed to have a role in the regulation of SNA within the CNS. The PVN contains excitatory and inhibitory neurons, which use glutamate and GABA as neurotransmitters. Microinjection of AngII into the PVN resulted in an increase in glutamatergic and a decrease in GABAergic transmission (34,35). Glutamatergic neurons also exist in the RVLM and project to the IML. Microinjection of AngII into the RVLM increased the release of glutamate in the IML, which then enhanced the sympathetic outflow to target organs (36). In addition, microinjection of AngII into the NTS potentiated GABA release through the effect of endothelial-derived NO. Elevated levels of GABA inhibit the activity of glutamatergic neurons within the NTS, which can further inhibit the GABAergic neurons in the CVLM. As the GABAergic neurons in the CVLM have an inhibitory effect on the glutamatergic neurons in the RVLM, the activity of these glutamatergic neurons subsequently increases and potentiates the sympathetic outflow (2,37,38). Thus, the interaction between AngII, glutamate and GABA has an important role in controlling SNA.
Interaction between AngII and reactive oxygen species (ROS) in the regulation of SNA
ROS, which include oxygen ions, free radicals and peroxides, are generated during the normal metabolism of enzymes such as NADPH oxidase. They are converted by superoxide dismutase (SOD) into hydrogen peroxide and then rapidly degraded by enzymes. Accumulating evidence indicated that ROS are important mediators of AngII signaling within the CNS and are involved in the regulation of SNA (39–41). Intracerebroventricular infusion of AngII significantly increased RSNA in rabbits with CHF accompanied by increased levels of NADPH-dependent ROS in the RVLM. In addition, pre-treatment with an intra-cerebroventricular infusion of SOD mimetic tempol and the inhibitor of NADPH oxidase apocynin abolished the increased RSNA induced by AngII, suggesting that ROS mediates the sympathoexcitation of AngII (42). Although the exact mechanisms by which AngII induces ROS generation have remained elusive, it has been reported that application of AngII caused NADPH-dependent ROS production in NTS neurons, which was dependent on intracellular Ca2+ and protein kinase C (43). ROS also have an important role in AngII-induced AT1 receptor upregulation in CHF. Intracerebroventricular infusion of tempol significantly decreased AT1 receptor protein expression in the RVLM of rabbits with CHF; furthermore, tempol reversed the AngII-induced increases in AT1 receptor mRNA expression in a neuronal cell line through inhibition of the c-Jun N-terminal kinase and AP1 signaling pathways (44). Application of AngII decreases the GABAergic currents in PVN neurons, which can be abolished by treatment with SOD; in addition, ROS enhances excitatory inputs from the PVN to the RVLM through increasing glutamatergic and decreasing GABAergic transmission (Fig. 1) (35,45). Furthermore, physical exercise decreased SNA via enhancing anti-oxidant pathways and suppressing pro-oxidant mechanisms in the RVLM of rabbits with CHF (46). These studies indicated that NADPH-dependent ROS are involved in the AngII signaling pathway and contribute to the increased sympathetic outflow.
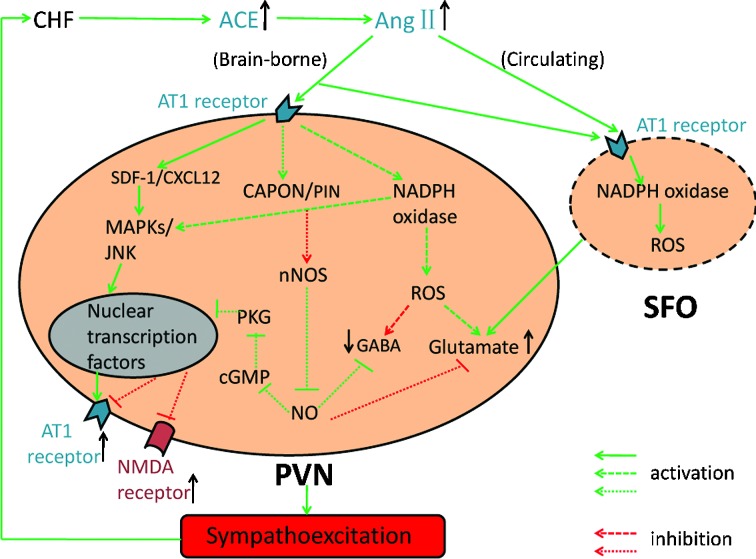
Figure 1.
Effects of AngII and NO within the PVN in the regulation of SNA under CHF conditions. AngII is elevated in both peripheral tissues and CNS during CHF. The circulating AngII can bind to its AT1 receptors in the autonomic regions which have no blood-brain barrier, such as AP and SFO. The SFO has a positive influence on the activity of the PVN and activation of the SFO by AngII can increase the glutamate levels in the PVN. The brain-borne AngII is produced by multiple cell types in autonomic regions, including SFO. In the PVN, AngII increases the MAPKs, decreases the expression of NO and increases the levels of ROS. As a result, the expression of AT1 receptor is upregulated and the balance between the glutamatergic and GABAergic systems is disturbed. Finally, the action of AngII in the different autonomic regions produces sympathoexcitation under CHF conditions. AngII, angiotensinII; AT1 receptor, AngII type 1 receptor; SDF-1/CXCL12, chemokine stromal cell-derived factor-1; MAPKs, mitogen-activated protein kinases; JNK, c-Jun N-terminal kinase; CAPON, carboxy-terminal PDZ ligand of nNOS; PIN, protein inhibitor of nNOS; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; cGMP, 3′,5′ guanosine monophosphate; PKG, protein kinase G; NMDA receptor, N-methyl-d-aspartate receptor; ROS, reactive oxygen species; GABA, γ-aminobutyric acid; PVN, paraventricular nucleus; AP, area postrema; SFO, subfornical organ; CHF, chronic heart failure; ACE, angiotensin-converting enzyme.
4. NO
NO, a metabolic product of the arginine metabolism catalyzed by nitric oxide synthase (NOS), is recognized as another modulator of SNA in the CNS. NOS occurs in three isoforms: Neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS). nNOS has been found to be involved in the control of sympathetic outflow within all nuclei in the PVN, NTS and RVLM, while eNOS and iNOS are mainly expressed in neurons in the brainstem (47–50). Most of the previous studies demonstrated that NO generated by these NOS isoforms exerts inhibitory effects on SNA (51).
Impaired sympathoinhibitory effect of NO in CHF
As mentioned above, the baroreflex is impaired in CHF, which contributes to sympathoexcitation. Gene transfer of nNOS into the RVLM was shown to normalize the impaired baro-reflex function (52). Similarly, overexpression of eNOS in the NTS reduced urinary norepinephrine excretion in mice with CHF (53). Microinjection of nNOS anti-sense into the PVN elicited a significant pressor in normal rats, while rats with CHF were less affected, suggesting that the sympathoexcitatory effect of nNOS anti-sense is blunted in CHF. This effect of nNOS anti-sense was accompanied by decreased nNOS protein levels in the PVN and a decreased responsiveness to NO in animals with CHF (54,55). The expression of nNOS protein was demonstrated to be downregulated in the NTS and the RVLM in rats with CHF (56). Chronic administration of AT1 receptor antagonist losartan prevented the downregulation of nNOS gene expression in the PVN, NTS and RVLM during CHF (57). Thus, it is indicated that enhanced AngII signaling in CHF decreases the expression of nNOS; however, the underlying mechanisms remain elusive. It has been demonstrated that the expression of carboxy-terminal PDZ ligand of nNOS (CAPON), a protein which can interact with nNOS and prevent NO production (58), was increased, whereas the expression of nNOS was decreased in the PVN of rats with CHF (59). Furthermore, treatment of losartan abrogated the changes in expression of CAPON and nNOS in animals with CHF, but also abolished AngII-induced increases in CAPON in neuronal cells (59). These results indicated that CAPON may act as a downstream mediator in AngII-induced downregulation of nNOS during CHF (59). In addition, the expression of protein inhibitor of nNOS (PIN) is upregulated due to increased AngII levels in the PVN under CHF conditions. Binding of PIN to nNOS leads to the de-stabilization of nNOS. As a result, the inactive monomeric state of nNOS is susceptible to ubiquitination and proteasomal degradation (Fig. 1) (60). These results suggested that the sympathoinhibitory effect of NO is impaired by decreases of nNOS, which thereby contribute to sympathoexcitation during CHF.
Mechanisms of the regulation of SNA by NO
Excitatory and inhibitory neurotransmitters have been demonstrated to be involved in the mechanism by which NO regulates the SNA. Administration of NO into the PVN has been shown to increase local levels of GABA (61). NO-induced decreases in RSNA, blood pressure and HR were inhibited by blocking of the GABAergic system, which indicated that the inhibitory effects of NO within the PVN are mediated by GABA (62). However, administration of N-methyl-d-aspartate (NMDA) into the PVN increased RSNA, blood pressure and HR. These responses were enhanced by prior microinjection of NOS inhibitor NG-monomethyl-l-arginine (L-NMMA), indicating that NO inhibits NMDA-mediated increases in SNA in the PVN (63). Gene transfer of nNOS into the PVN of rats with CHF significantly enhanced the blunted changes in RSNA, blood pressure and HR in response to L-NMMA, while the response to NMDA was significantly decreased. This effect was due to gene transfer of nNOS reducing the increased NMDA receptor sub-unit NR(1) mRNA and protein expression in the PVN of rats with CHF (64). Similarly, gene transfer of eNOS into the RVLM significantly decreased MAP and HR in normal rats, coupled with an increased expression of GABA. By contrast, microinjection of GABA receptor antagonist following gene transfer enhanced the increases in MAP (65). In addition, inhibition of iNOS in the RVLM enhanced the pressor response caused by glutamate, suggesting that NO derived from iNOS inhibited the action of glutamate in the RVLM (66). In vitro, NO negatively regulated AT1 receptor expression via the protein kinase G pathway in primary cultures of hypothalamic and neuronal cell lines (67), indicating that reduced NO production within the CNS during CHF may enhance the activity of the RAS (Fig. 1).
However, due to conflicting results, no consensus has been reached regarding the role of NO in the regulation of SNA in the RVLM and NTS. It has been reported that nNOS inhibition in the RVLM decreased MAP and the HR. Microinjection of nNOS inhibitor attenuated the pressor response caused by glutamate, indicating that NO derived from nNOS produces sympathoexcitation through enhancing the effects of glutamate in the RVLM (66). In addition, microinjection of L-NMMA or an nNOS inhibitor into the RVLM attenuated the cardiac sympathetic afferent reflex elicited by epicardial application of bradykinin (68). In the NTS, NO was shown to be able to potentiate GABAergic as well as glutamatergic transmission (69). However, the activation of glutamatergic transmission in the NTS evoked a decrease in blood pressure and HR (70).
It is therefore indicated that through interacting with RAS and neurotransmitters, NO produced by various isoforms of NOS is able to decrease the SNA, while the sympathoinhibitory effect of NO is impaired in CHF. Of note, in the RVLM and NTS, NO can also increase SNA. Recently, it has been reported that endogenous NO in the CNS exerted excitatory effects to increase resting CSNA in healthy subjects, while exogenously administrated NO inhibited CSNA under normal and CHF conditions (71). Thus, the sympathoexcitatory and sympathoinhibitory effects of NO in the CNS may partly depend on the balance of glutamatergic and GABAergic activity, the specific sympathetic activity-regulating nuclei on which it acts and the method of NO administration.
5. Pro-inflammatory cytokines
Pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), are elevated in CHF and contribute to the progression of the disease (72). In analogy to AngII, peripheral pro-inflammatory cytokines can penetrate into the CNS at the nuclei where no blood-brain barrier exists; furthermore, cytokines can also be generated by multiple cell types in the CNS (73,74). Pro-inflammatory cytokines within the CNS can increase the sympathetic outflow during CHF (75).
Sympathoexcitatory effects of pro-inflammatory cytokines
Through either acting on the SFO to produce multiple neurohumoral factors or activating the perivascular macrophages to generate prostaglandin E2 (PGE2), circulating pro-inflammatory cytokines can further stimulate the activity of the PVN and increase the sympathetic outflow (74,76). The central inflammation-induced PGE2 generation depends on the NADPH-oxidase and the p38 MAPK pathway under pathophysiological conditions (77). Furthermore, activated cardiac sympathetic afferent nerves transmit signals to the CNS to increase cytokine production, which contributes to sympathoexcitation by upregulation of the activity of the RAS and the hypothalamic-pituitary-adrenal axis in the PVN under CHF conditions (78,79). It has been indicated that nuclear factor (NF)-κB may mediate the interaction between the RAS and pro-inflammatory cytokines, as inhibition of the synthesis of NF-κB reduced cytokine and AT1 receptor expression in the PVN of rats with CHF and in turn, blockade of the AT1 receptor decreased the expression of cytokines and NF-κB (80). In addition, central TNF increased the NADPH oxidase sub-unit and ROS production in the PVN and RVLM under CHF conditions, which further activated the RAS and NF-κB (81,82). Toll-like receptor (TLR) signaling has an important role in mediating the inflammation cascade. Stimulation of TLR4 led to the activation of NF-κB via the myeloid differentiation primary-response protein 88 (MyD88)-dependent pathway, which induced pro-inflammatory cytokine production (83). Intracerebroventricular infusion of an AT1 receptor blocker decreased the elevated SNA and reduced the increased expression of TLR4, MyD88 and NF-κB in the brainstem of mice with CHF. Therefore, it is indicated that TLR4 is involved in AT1 receptor-induced pro-inflammatory cytokine production in CHF (84). Cytokines were also indicated to upregulate SNA via increasing excitatory neurotransmitters and decreasing inhibitory neurotransmitters, as intracerebroventricular infusion of cytokine blockers attenuated HF-induced increases in glutamate and decreases in GABA in the PVN of rats with CHF, probably through an NO-associated mechanism (Fig. 2) (85). In addition, chemokine stromal cell-derived factor-1 may mediate the sympathoexcitation of TNF-α and AngII in the PVN through activating p44/42 MAPK signaling, which further activates the transcription factors and upregulates the expression of AT1 receptor (Fig. 1) (86).
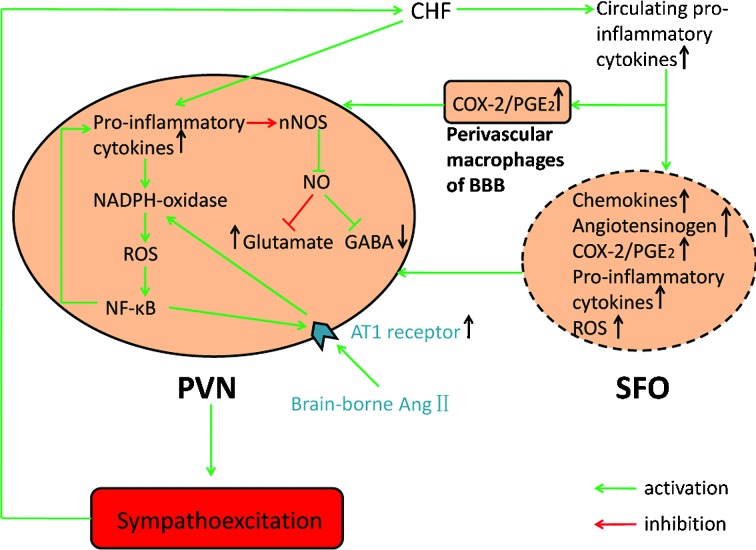
Figure 2.
Effects of pro-inflammatory cytokines within the PVN in the regulation of SNA under CHF conditions. Circulating pro-inflammatory cytokines are increased during CHF, either through activation of the SFO to produce multiple neurohumoral factors or activation of perivascular macrophages to generate PGE2. These circulating pro-inflammatory cytokines can further stimulate the activity of PVN and increase the sympathetic outflow. In the PVN, the increased pro-inflammatory cytokines and AngII upregulate the expression of ROS and NF-κB via enhancing the activity of NADPH-oxidase, which in turn elevates the levels of pro-inflammatory cytokines and AT1 receptor. In addition, pro-inflammatory cytokines may also improve glutamate levels and decrease GABA levels through reducing the synthesis of NO. These alterations in the PVN finally produce sympathoexcitation during CHF. COX-2, cyclooxygenase 2; PGE2, prostaglandin E2; BBB, blood-brain barrier; ROS, reactive oxygen species; NF-κB, nuclear factor-kappaB; AT1 receptor, angiotensinII type 1 receptor; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; GABA, γ-aminobutyric acid; PVN, paraventricular nucleus; SFO, subfornical organ; CHF, chronic heart failure.
6. Conclusion
CHF is a clinical syndrome characterized by continuous interaction between the underlying myocardial dysfunction and compensatory neurohumoral mechanisms. Activation of neurohumoral mechanisms in the CNS contributes to the sympathoexcitation in CHF. Numerous studies have explored the role of AngII, NO and pro-inflammatory cytokines in the regulation of SNA in the CNS. By acting on sympathetic activity-regulating nuclei at various levels, these neurohumoral factors influence the activity of neurons and finally produce a sympathoexcitatory or sympathoinhibitory effect. However, the underlying mechanisms by which these factors regulate SNA remain to be fully elucidated and the interaction between these agents within the CNS also remains to be clarified. Finally, although blockade of the abnormal neurohumoral axis in the CNS has shown beneficial effects in animal experiments, further study is required to determine whether the central neurohumoral axis may represent a novel target for the treatment of patients with CHF.
References
- 1.Azad N, Lemay G. Management of chronic heart failure in the older population. J Geriatr Cardiol. 2014;11:329–337. doi: 10.11909/j.issn.1671-5411.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansen H, Strauss B, Arnold JM, Moe G, Liu P. On the rise: The current and projected future burden of congestive heart failure hospitalization in Canada. Can J Cardiol. 2003;19:430–435. [PubMed] [Google Scholar]
- 3.Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117–122. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 6.Potts JT, Paton JF, Mitchell JH, Garry MG, Kline G, Anguelov PT, Lee SM. Contraction-sensitive skeletal muscle afferents inhibit arterial baroreceptor signalling in the nucleus of the solitary tract: Role of intrinsic GABA interneurons. Neuroscience. 2003;119:201–214. doi: 10.1016/S0306-4522(02)00953-3. [DOI] [PubMed] [Google Scholar]
- 7.Schreihofer AM, Guyenet PG. The baroreflex and beyond: Control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29:514–521. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- 8.Affleck VS, Coote JH, Pyner S. The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience. 2012;219:48–61. doi: 10.1016/j.neuroscience.2012.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braga VA, Medeiros IA, Ribeiro TP, França-Silva MS, Botelho-Ono MS, Guimarães DD. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: Implications in neurogenic hypertension. Braz J Med Biol Res. 2011;44:871–876. doi: 10.1590/S0100-879X2011007500088. [DOI] [PubMed] [Google Scholar]
- 10.Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, Sakata K, Osaka M, Onami T, Takimoto C, et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res. 2012;35:132–141. doi: 10.1038/hr.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagawa T, Dampney RA. AT(1) receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.HYP.34.6.1301. [DOI] [PubMed] [Google Scholar]
- 12.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/S0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 13.Nunn N, Womack M, Dart C, Barrett-Jolley R. Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Curr Neuropharmacol. 2011;9:262–277. doi: 10.2174/157015911795596531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol (1985) 1999;86:1264–1272. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- 15.Reid IA. Interactions between ANG II, sympathetic nervous system and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992;262:E763–E778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- 16.Liu JL, Murakami H, Sanderford M, Bishop VS, Zucker IH. ANG II and baroreflex function in rabbits with CHF and lesions of the area postrema. Am J Physiol. 1999;277:H342–H350. doi: 10.1152/ajpheart.1999.277.1.H342. [DOI] [PubMed] [Google Scholar]
- 17.Llewellyn TL, Sharma NM, Zheng H, Patel KP. Effects of exercise training on SFO-mediated sympathoexcitation during chronic heart failure. Am J Physiol Heart Circ Physiol. 2014;306:H121–H131. doi: 10.1152/ajpheart.00534.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons KK, Coffman TM. The reninangiotensin system: It's all in your head. J Clin Invest. 2007;117:873–876. doi: 10.1172/JCI31856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of renin and angiotensinogen in the rostral ventro-lateral medulla using a dual-reporter transgenic model. Hypertension. 2004;43:1116–1119. doi: 10.1161/01.HYP.0000125143.73301.94. [DOI] [PubMed] [Google Scholar]
- 20.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of renin expressing cells in the brain, by use of a REN-eGFP transgenic model. Physiol Genomics. 2004;16:240–246. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- 21.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: Recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1364–R1374. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: Potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H1216–H1226. doi: 10.1152/ajpheart.00557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan J, Wang H, Leenen FH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1665–H1671. doi: 10.1152/ajpheart.00858.2003. [DOI] [PubMed] [Google Scholar]
- 26.Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension. 2008;52:679–686. doi: 10.1161/HYPERTENSIONAHA.108.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isegawa K, Hirooka Y, Katsuki M, Kishi T, Sunagawa K. Angiotensin II type 1 receptor expression in astrocytes is upregulated leading to increased mortality in mice with myocardial infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2014;307:H1448–H1455. doi: 10.1152/ajpheart.00462.2014. [DOI] [PubMed] [Google Scholar]
- 28.Ramchandra R, Hood SG, Watson AM, Allen AM, May CN. Central angiotensin type 1 receptor blockade decreases cardiac but not renal sympathetic nerve activity in heart failure. Hypertension. 2012;59:634–641. doi: 10.1161/HYPERTENSIONAHA.111.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui TX, Horiuchi M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Physiol Heart Circ Physiol. 2003;284:H116–H121. doi: 10.1152/ajpheart.00515.2002. [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens. 2011;24:724–730. doi: 10.1038/ajh.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Zucker IH, Gao L. Activation of central angiotensin type 2 receptors by compound 21 improves arterial baroreflex sensitivity in rats with heart failure. Am J Hypertens. 2014;27:1248–1256. doi: 10.1093/ajh/hpu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension. 2008;51:521–527. doi: 10.1161/HYPERTENSIONAHA.107.101717. [DOI] [PubMed] [Google Scholar]
- 33.Kang J, Posner P, Sumners C. Angiotensin II type 2 receptor stimulation of neuronal K+ currents involves an inhibitory GTP binding protein. Am J Physiol. 1994;267:C1389–C1397. doi: 10.1152/ajpcell.1994.267.5.C1389. [DOI] [PubMed] [Google Scholar]
- 34.Qi J, Zhang DM, Suo YP, Song XA, Yu XJ, Elks C, Lin YX, Xu YY, Zang WJ, Zhu Z, Kang YM. Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol. 2013;13:48–54. doi: 10.1007/s12012-012-9184-9. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q, Pan HL. Signaling mechanisms of angiotensin II-induced attenuation of GABAergic input to hypothalamic presympathetic neurons. J Neurophysiol. 2007;97:3279–3287. doi: 10.1152/jn.01329.2006. [DOI] [PubMed] [Google Scholar]
- 36.Hu L, Zhu DN, Yu Z, Wang JQ, Sun ZJ, Yao T. Expression of angiotensin II type 1 (AT(1)) receptor in the rostral ventrolateral medulla in rats. J Appl Physiol (1985) 2002;92:2153–2161. doi: 10.1152/japplphysiol.00261.2001. [DOI] [PubMed] [Google Scholar]
- 37.Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paton JF, Boscan P, Murphy D, Kasparov S. Unravelling mechanisms of action of angiotensin II on cardiorespiratory function using in vivo gene transfer. Acta Physiol Scand. 2001;173:127–137. doi: 10.1046/j.1365-201X.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- 39.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 40.Gao L, Li Y, Schultz HD, Wang WZ, Wang W, Finch M, Smith LM, Zucker IH. Downregulated Kv4.3 expression in the RVLM as a potential mechanism for sympathoexcitation in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H945–H955. doi: 10.1152/ajpheart.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: Roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 43.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Ladecola C. Nox2, Ca2+ and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–489. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res. 2008;103:186–193. doi: 10.1161/CIRCRESAHA.108.179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishihara M, Hirooka Y, Matsukawa R, Kishi T, Sunagawa K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens. 2012;30:97–106. doi: 10.1097/HJH.0b013e32834e1df4. [DOI] [PubMed] [Google Scholar]
- 46.Gao L, Wang W, Liu DM, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115:3095–3102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: Role of GABA. Neuroscience. 2003;118:585–601. doi: 10.1016/S0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 48.Krukoff TL, Khalili P. Stress-induced activation of nitric oxide-producing neurons in the rat brain. J Comp Neurol. 1997;377:509–519. doi: 10.1002/(SICI)1096-9861(19970127)377:4<509::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 49.Lin LH, Taktakishvili O, Talman WT. Identification and localization of cell types that express endothelial and neuronal nitric oxide synthase in the rat nucleus tractus solitarii. Brain Res. 2007;1171:42–51. doi: 10.1016/j.brainres.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan SH, Wang LL, Chan JY. Differential engagements of glutamate and GABA receptors in cardiovascular actions of endogenous nNOS or iNOS at rostral ventrolateral medulla of rats. Br J Pharmacol. 2003;138:584–593. doi: 10.1038/sj.bjp.0705081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med (Maywood) 2001;226:814–824. doi: 10.1177/153537020122600902. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Patel KP, Cornish KG, Channon KM, Zucker IH. nNOS gene transfer to RVLM improves baroreflex function in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2003;285:H1660–H1667. doi: 10.1152/ajpheart.00239.2003. [DOI] [PubMed] [Google Scholar]
- 53.Sakai K, Hirooka Y, Shigematsu H, Kishi T, Ito K, Shimokawa H, Takeshita A, Sunagawa K. Overexpression of eNOS in brain stem reduces enhanced sympathetic drive in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289:H2159–H2166. doi: 10.1152/ajpheart.00408.2005. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Liu XF, Cornish KG, Zucker IH, Patel KP. Effects of nNOS antisense in the paraventricular nucleus on blood pressure and heart rate in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H205–H213. doi: 10.1152/ajpheart.00497.2004. [DOI] [PubMed] [Google Scholar]
- 55.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 56.Hirooka Y, Shigematsu H, Kishi T, Kimura Y, Ueta Y, Takeshita A. Reduced nitric oxide synthase in the brainstem contributes to enhanced sympathetic drive in rats with heart failure. J Cardiovasc Pharmacol. 2003;42(Suppl 1):S111–S115. doi: 10.1097/00005344-200312001-00023. [DOI] [PubMed] [Google Scholar]
- 57.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: The roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 58.Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: A protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron. 1998;20:115–124. doi: 10.1016/S0896-6273(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 59.Sharma NM, Zheng H, Mehta PP, Li YF, Patel KP. Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: Role for CAPON and Ang II. Cardiovasc Res. 2011;92:348–357. doi: 10.1093/cvr/cvr217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma NM, Llewellyn TL, Zheng H, Patel KP. Angiotensin II-mediated posttranslational modification of nNOS in the PVN of rats with CHF: Role for PIN. Am J Physiol Heart Circ Physiol. 2013;305:H843–H855. doi: 10.1152/ajpheart.00170.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paravenstricular nucleus: Cardiovascular and neurochemical implications. Am J Physiol. 1994;266:R306–R313. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- 62.Zhang K, Patel KP. Effect of nitric oxide within the para-ventricular nucleus on renal sympathetic nerve discharge: Role of GABA. Am J Physiol. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 63.Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: Role of nitric oxide. Am J Physiol Heart Circ Physiol. 2001;281:H2328–H2336. doi: 10.1152/ajpheart.2001.281.6.H2328. [DOI] [PubMed] [Google Scholar]
- 64.Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension. 2011;58:966–973. doi: 10.1161/HYPERTENSIONAHA.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901. [PubMed] [Google Scholar]
- 66.Martins-Pinge MC, Garcia MR, Zoccal DB, Crestani CC, Pinge-Filho P. Differential influence of iNOS and nNOS inhibitors on rostral ventrolateral medullary mediated cardiovascular control in conscious rats. Auton Neurosci. 2007;131:65–69. doi: 10.1016/j.autneu.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Sharma NM, Zheng H, Li YF, Patel KP. Nitric oxide inhibits the expression of AT1 receptors in neurons. Am J Physiol Cell Physiol. 2012;302:C1162–C1173. doi: 10.1152/ajpcell.00258.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo ZL, Tjen-A-Looi SC, Fu LW, Longhurst JC. Nitric oxide in rostral ventrolateral medulla regulates cardiac-sympathetic reflexes: Role of synthase isoforms. Am J Physiol Heart Circ Physiol. 2009;297:H1478–H1486. doi: 10.1152/ajpheart.00209.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S, Paton JF, Kasparov S. Differential sensitivity of excitatory and inhibitory synaptic transmission to modulation by nitric oxide in rat nucleus tractus solitarii. Exp Physiol. 2007;92:371–382. doi: 10.1113/expphysiol.2006.036103. [DOI] [PubMed] [Google Scholar]
- 70.Dias AC, Vitela M, Colombari E, Mifflin SW. Nitric oxide modulation of glutamatergic, baroreflex and cardiopulmonary transmission in the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2005;288:H256–H262. doi: 10.1152/ajpheart.01149.2003. [DOI] [PubMed] [Google Scholar]
- 71.Ramchandra R, Hood SG, May CN. Central exogenous nitric oxide decreases cardiac sympathetic drive and improves baroreflex control of heart rate in ovine heart failure. Am J Physiol Regul Integr Comp Physiol. 2014;307:R271–R280. doi: 10.1152/ajpregu.00057.2014. [DOI] [PubMed] [Google Scholar]
- 72.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.CIR.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 73.Utsuyama M, Hirokawa K. Differential expression of various cytokine receports in the brain after stimulation with LPS in young and old mice. Exp Gerontol. 2002;37:411–420. doi: 10.1016/S0531-5565(01)00208-X. [DOI] [PubMed] [Google Scholar]
- 74.Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemo-dynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–125. doi: 10.1161/HYPERTENSIONAHA.113.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Felder RB, Yu Y, Zhang ZH, Wei SG. Pharmacological treatment for heart failure: A view from the brain. Clin Pharmacol Ther. 2009;86:216–220. doi: 10.1038/clpt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang ZH, Yu Y, Wei SG, Felder RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens. 2010;28:806–816. doi: 10.1097/HJH.0b013e3283358b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H791–H797. doi: 10.1152/ajpheart.00099.2004. [DOI] [PubMed] [Google Scholar]
- 79.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–H236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypo-thalamic paraventricular nucleus in heart failure: Role of nuclear factor-kappaB. Cardiovasc Res. 2008;79:671–678. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M, Francis J. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: A role for superoxide and nitric oxide. Basic Res Cardiol. 2011;106:273–286. doi: 10.1007/s00395-010-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/S0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 83.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 84.Ogawa K, Hirooka Y, Kishi T, Sunagawa K. Brain AT1 receptor activates the sympathetic nervous system through toll-like receptor 4 in mice with heart failure. J Cardiovasc Pharmacol. 2011;58:543–549. doi: 10.1097/FJC.0b013e31822e6b40. [DOI] [PubMed] [Google Scholar]
- 85.Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res. 2009;83:737–746. doi: 10.1093/cvr/cvp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei SG, Zhang ZH, Yu Y, Felder RB. Central SDF-1/CXCL12 expression and its cardiovascular and sympathetic effects: The role of angiotensin II, TNF-α and MAPK signaling. Am J Physiol Heart Circ Physiol. 2014;307:H1643–H1654. doi: 10.1152/ajpheart.00432.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]