Abstract
While there is agreement that overt maternal hypothyroidism (serum thyroid stimulating hormone (TSH) >10 mIU/L) should be treated immediately, the evidence is mixed regarding the harm associated with subclinical hypothyroidism and the benefits of thyroxine replacement. The diagnosis of subclinical hypothyroidism rests on the recognition of an increased serum concentration of TSH which may be affected by many factors including gestational age, analytical method, the antibody status of the mother, ethnicity, iodine nutrition and even the time of day when the blood is collected. The 97.5th percentile of TSH at the end of the first trimester is commonly used as the upper boundary of normal in early pregnancy with a default value of 2.5 mIU/L specified in a number of recent clinical guidelines. There have now been numerous papers showing that a more realistic figure is between 3.0 and 4.0 mIU/L depending on the analytical method that is used. There are suggestions that ethnicity may also have a significant effect on TSH and FT4 reference limits in pregnancy.
Introduction
Thyroid function testing in pregnancy is an area of concern for pregnant women, doctors and laboratories. Most anxiety relates to the diagnosis of hypothyroidism, the most common thyroid disease in our community and the focus of this review. Approximately 15–20% of young Australian women have thyroid autoantibodies and 2–3% have subclinical hypothyroidism in pregnancy.1–3
Some women are known to have thyroid disease before pregnancy and require monitoring to ensure no harm comes to them or their baby. Others may have unrecognised disease and there has been debate about the merits of screening, the potential harm caused by mild hypothyroidism, and how women should be tested. Two excellent recent reviews cover many of these areas.4,5 We will examine the factors that should be kept in mind when assessing the literature in this area and discuss the things that laboratories should consider when deciding how to report thyroid function tests in pregnancy.
Physiological Changes in Pregnancy and Effects on Thyroid Function
There are several physiological changes during pregnancy that affect maternal thyroid function and thyroid hormone levels. Most important is that human chorionic gonadotropin (hCG) is structurally similar to TSH, and has a direct stimulating effect on the thyroid gland mediated through the TSH receptor. During pregnancy hCG peaks towards the end of the first trimester followed by a decrease to a plateau in second and third trimesters. The thyrotrophic effect of hCG causes increased thyroid hormone production resulting in a transient increase in free thyroxine (FT4) towards the end of the first trimester.6,7 This in turn leads to a concomitant lowering of TSH concentrations. With the decline in hCG as pregnancy progresses there is a trend towards an increase in TSH.8
Thyroxine binding globulin (TBG) increases by 2–3 times compared with the pre-pregnancy level by the 20th week of gestation.9 This is a result of both increased production stimulated by oestrogens and the reduced clearance of the more heavily sialylated forms that are more common in pregnancy. This elevation causes an increase of total triiodothyronine (TT3) and thyroxine (TT4) by an average of 1.5 times by the 16th gestational week.
Maternal iodine requirements increase in pregnancy for a number of reasons.7,10 One postulated mechanism is increased renal iodide loss although its significance has been subject to debate.11 Iodine is transported across the placenta to the growing baby. It is also needed to supply the increased production of maternal thyroid hormone which rises to match the increased concentration of TBG. Extra thyroid hormone may also be required to counter losses through placental deiodination.12
A number of authors have stated that pregnancy is a “stress test” for the thyroid where the maintenance of adequate thyroid hormones levels for the mother and foetus requires an intact thyroid gland and an adequate supply of iodine.13 Patients with mild underlying thyroid disease or inadequate dietary iodine may fail the test and become hypothyroid. Those with known thyroid disease will also need to have their treatment reviewed.
What do the Guidelines say about Thyroid function Testing in Pregnancy?
Three guidelines have been recently published by expert groups in North America and Europe regarding the diagnosis and management of thyroid disease in pregnancy.10,13,14 These addressed a number of aspects and were in broad general agreement as discussed in accompanying editorials.15–17 One commentator lamented the fact that there were so many guidelines in this area and another pointed out that the two North American guidelines had four authors in common.
There were mixed views about screening women for thyroid disease. The experts agreed that high risk women (for example, older women or those with a personal or family history of autoimmune thyroid disease) should be screened. There was no consensus about those at low risk however. Screening is favoured by the frequency of disease, the difficulty of making a clinical diagnosis and the relatively ease of measuring TSH. Arguing against screening are the uncertainties about the harm caused by untreated subclinical hypothyroidism and the lack of evidence that early thyroxine treatment makes any difference. The optimal timing of testing is probably toward the end of the first trimester or before pregnancy in those at high risk.10
Regarding the interpretation of thyroid function tests in pregnancy the guidelines were similar with all recommending 2.5 mIU/L as the upper limit of normal for TSH in the first trimester if locally-derived reference intervals were not available. The 2011 American Thyroid Association (ATA) guidelines recommended that the interpretation of thyroid function in pregnancy be based on trimester specific reference ranges as defined in populations with optimal iodine intake. In recognition of the fact that there may not be appropriate trimester-specific reference intervals for individual populations, they stated that the default TSH values should be 0.1–2.5 mIU/L (first trimester), 0.2–3.0 mIU/L (second trimester), and 0.3–3.5 mIU/L (third trimester).13 The American Endocrine Society also quoted 0.1–2.5 mIU/L as the “normal range” for TSH in the first trimester and recommended thyroxine treatment for women with TSH >2.5 mIU/L in the first trimester or >3.0 mIU/L in the second and third.10 The European Thyroid Association had similar recommendations.14
The Endocrine Society reference intervals were based on six studies, amongst which there was significant variation.18–23 The first trimester upper limits of TSH ranged from 2.30 to 3.61 mIU/L, with one significantly higher at 5.00 mIU/L and similar variation in the second and third trimesters. The studies were heterogeneous in terms of the populations studied, number of subjects, and the calculation of the reference intervals. Only four reported the 2.5th – 97.5th percentiles as recommended by National Academy of Clinical Biochemistry (NACB).24 Two of the studies had fewer than 100 participants 20,21 and none measured urinary iodine.
All of the guidelines warned against the uncritical use of FT4 results in pregnancy. The ATA recommended the measurement of TT4 and calculation of FT4 index (FTI) as preferable to FT4 immunoassays although some have argued that this is misguided and regressive.17 The Endocrine Society guidelines also suggested either FTI or TT4 (multiplying the non-pregnant range by 1.5 for the second and third trimesters), while the European guidelines recommended either TT4 or FT4 measurement with locally established trimester-specific reference ranges.
The guidelines varied in their recommendations on the way that subclinical hypothyroidism (defined as an increased TSH and normal FT4) should be managed. The Endocrine Society and European Thyroid Association recommended treatment based on the raised TSH alone while the Endocrine Society required positive anti-thyroid peroxidase (ATPO) antibodies as well.
While the guidelines were in agreement in many regards it is not known whether this is what doctors actually do. A recent publication from Sweden indicated that thyroid testing and management in pregnancy in that country was often suboptimal.25 One editorial also pointed to the paradox that most of the guidelines were published by endocrinologists while most of the patients were treated by obstetricians, another possible cause of an evidence-practice gap.15
What is the Evidence that Hypothyroidism causes Harm?
There is general agreement that overt hypothyroidism can cause harm to the mother and baby although this condition is uncommon today and much of the evidence is from times when the epidemiology thyroid disease and diagnostic methods were very different. Regarding subclinical hypothyroidism and adverse obstetric and neonatal effects, the Endocrine Society guidelines grade the evidence as “fair or poor” with the rationale for the recommended treatment being that “the potential benefits outweigh the potential harms”.10 A recent review stated that whilst “trials of levothyroxine replacement for mild hypothyroidism in pregnancy have not indicated definite evidence of improvements in these outcomes, professional guidelines recommend treatment”.26 Current professional opinion is that maternal hypothyroidism probably contributes to some complications in pregnancy and may have adverse effects on foetal neurological development. There is no clear evidence to date that either of these adverse events can be prevented with thyroxine or any other treatment.
There are several preliminary observations about the research in this area. Many early reports were small series from high risk clinics and the findings were not replicated in large population studies. Severe iodine deficiency was more common in the past and laboratory methods were primitive. Studies are still quoted that used butanol extractable iodine to measure thyroid hormones, a method that was abandoned long ago.27–29 It is not clear whether obstetric complications have been defined in a uniform way and studies of neurocognitive development in children are intrinsically difficult requiring large numbers of subjects, long follow up and careful correction for confounding factors.
Regarding obstetric complications, there is evidence linking subclinical hypothyroidism with selected adverse events. A recent review tabulated a summary of 16 studies, mostly from the last five years.5 It highlighted the variation in size of the studies, the definition of subclinical hypothyroidism and the results for eight pregnancy outcomes. Preterm delivery was the most commonly measured endpoint although only 4/10 studies showed a positive association. A recent meta-analysis of 14 studies of women with subclinical hypothyroidism demonstrated a significant increased risk of pregnancy loss (odds ratio 1.93), preterm delivery (odds ratio 1.30), placental abruption (odds ratio 2.16), and breech presentation at birth (odds ratio 2.30).26 The studies that this was based on however, had varied definitions for subclinical hypothyroidism, some used a percentile cut off for TSH (including 95th, 97.5th or 98th percentile) while others used arbitrary cut points ranging from 2–6 mIU/L. The number of subjects varied from 204 to 16,609 and the proportion with hypothyroidism from 1 to 14%. It is interesting that the unusual complication of placental abruption was only demonstrated in one study 30 but not in four others.31–34 Even in the one positive study the numbers were small with 1.0% (4/404) of hypothyroid pregnancies affected compared with 0.3% (52/15689) of euthyroid pregnancies (p=0.026). Whilst placental abruption is often quoted as a potential complication of subclinical hypothyroidism it is obvious that getting good data on uncommon events like this is very difficult.
The ATA guidelines highlighted two studies from southern Italy, a region with mild iodine deficiency similar to Australia.13 The first of these showed that pregnancy loss was significantly higher in a group of more than 4000 antibody-negative women who had TSH in the range 2.5–5.0 mIU/L compared with those with TSH less than 2.5 mIU/L (6.1 versus 3.6%, p=0.006). The TSH was measured with the Roche assay.35
In a second paper the same group showed that screening was needed to detect all women with thyroid disease in pregnancy and that thyroxine treatment reduced obstetric complications in women with TSH >2.5 mIU/L and positive thyroid antibodies.36
At the same time there were two large, well-organised studies that came to the opposite conclusion. The first examined women with subclinical hypothyroidism (n=240, 2.2%) or isolated hypothyroxinaemia (n=232, 2.1%) from 10,990 enrolled in the multicentre FASTER trial.33 There were no increased adverse events in the group with subclinical hypothyroidism compared with controls. The second study involved a birth cohort of 5805 women in Northern Finland followed for 20 years.32 Thyroid dysfunction and antibodies during pregnancy were associated with subsequent maternal thyroid disease but not with any adverse outcomes during pregnancy.34
Other studies have looked at the association of thyroid antibodies rather than hypothyroidism with adverse pregnancy outcomes. A meta-analysis of eight case-control and 10 longitudinal studies found an association between thyroid autoimmunity and miscarriage (odds ratios 2.73, 95% confidence interval 2.20–3.40 and 2.30, 1.80–2.95 respectively).37 Whether this is the result of subtle thyroid dysfunction, heightened autoimmunity or another factor is unclear although a study in 2006 showed that thyroxine treatment of ATPO-positive women reduced the miscarriage rate.38 A recent Melbourne study found that ATPO concentrations were higher in nulliparous women with miscarriages compared with those who had had two or more live births (median 0.3 versus 0.2 IU/mL, p<0.0001). The concentrations were low in both groups however and the numbers above the upper reference limit were no different.39
The evidence that mild maternal hypothyroidism can cause neurological injury in the developing foetus is even less certain than the evidence regarding obstetric complications. Some of this relates to the difficulty studying this area where the timing and type of assessment of the child are critical along with correction for confounding factors. One of the subtleties is that neurological injuries at different times of gestation may have different effects requiring specific tests later in childhood. Lazarus stated that the idea that subclinical hypothyroidism might cause neurocognitive deficits is “biologically plausible, but not clearly proven”.14
Two studies are most often quoted in this area, one positive and one negative. The first is a paper from 1999 in which the children of 62 women with raised TSH in pregnancy were evaluated at 7–9 years of age with a battery of psychometric tests.40 The study group was tiny given the original cohort comprised 25,216 women. It was found that the children’s IQ scores were seven points lower on average than 124 children from matched euthyroid pregnancies with the proportion with IQ scores less than 86% being significantly higher (15 versus 5%, p=0.08). Apart from the small numbers it is also noteworthy that the hypothyroidism was often severe with many women having TSH results above 10 mIU/L.
The second paper of interest described an intervention study in 2012 in which 21,846 pregnant women were screened for hypothyroidism before 16 weeks.41 Of this group 390 were treated with thyroxine whilst 404 controls were not. The median time for starting thyroxine was 13 weeks three days and the target TSH was less than 1.1 mIU/L. The primary endpoint was IQ testing at three years of age which showed no difference between the two groups. A follow up study of the same children now aged 7–10 years is underway.42 This will give valuable additional information although critics of this study have pointed out that the thyroxine treatment may have been started too late in pregnancy to have a beneficial effect.
There are numerous other studies in this area which have reached different conclusions. Two separate Chinese studies of approximately 1000 women each found a link between maternal hypothyroidism and developmental problems in children tested at six months or two years of age.43,44 Even though the findings were statistically significant they should be treated with caution however, as the numbers in different subgroups ranged from only nine to forty-three children. For example, the conclusion that maternal subclinical hypothyroidism might cause “poor visual development” in children is tenuous when it was based on 2/41 children of hypothyroid mothers being affected compared with 8/845 euthyroid controls. There was inadequate detail of the visual defects that were found given the potential seriousness of this complication and the fact that they have not been described before.41
There has been vigorous debate about the relative importance of hypothyroidism (i.e. high TSH) and hypothyroxinaemia (i.e. low FT4) as the more important predictor of adverse events in pregnancy. The argument for the precedence of hypothyroxinaemia is that the mother is the only source of thyroid hormones for the foetus until at least 12 weeks gestation. This proposition has been supported by a number of Dutch studies which found an association between euthyroid hypothyroxinaemia and delayed cognitive development at different ages.45–49 Some people have argued in support of this theory50 whilst others have found no association51 or have argued that there is insufficient evidence.52 The important practical message for laboratories is that FT4 assays need to be sorted out because they may come to have an equal or greater role than TSH in the management of thyroid disease in pregnancy.
Thyroid Function Tests in Pregnancy
The diagnosis of hypothyroidism in pregnancy is largely dependent on blood tests, in particularly an increased serum TSH concentration and, with “overt hypothyroidism”, a reduced FT4. The following discussion covers the different factors that must be considered when interpreting thyroid function tests in pregnancy.
Pre-analytical factors
1. Gestational age
Gestational age will have a major effect on thyroid function tests through the first trimester as hCG concentrations rise and fall. This can present practical problems in laboratories that do not record gestational age or cannot adjust reference intervals to match different stages of pregnancy.
The changes in thyroid function tests with gestational age were elegantly shown in a study of 13,599 singleton pregnancies assessed at one week intervals from week 6 to term where serum TSH fell to a trough at week 10 followed by a progressive increase to term.53 The 2.5th percentile was lowest at weeks 11–14. The 97.5th percentile was lower than the non-pregnant range from week 10 and remained low until a rise in the third trimester.
A study of almost 6,000 Finnish women also demonstrated a decline in TSH from very early pregnancy to a low point at 11 weeks54 and a similar pattern was found in 4,800 pregnant Chinese women studied between 4 to 12 weeks gestation.55 TSH concentrations decreased significantly from the seventh week of pregnancy to their lowest point between gestational weeks 10 and 11. In the latter study hCG was also measured. It reached a peak at 9–11 weeks which coincided with the trough in TSH. TSH concentrations at 4–6 weeks of gestation were the same as women who were not pregnant.55
Similar results were found with local data through the analysis of 6,706 TSH results from pregnant women tested in a Victorian private pathology laboratory over a 12 month period using the Roche Cobas e 602. Test numbers and median TSH concentrations were plotted against weeks of gestation (Figure). The notable features were the popularity of week 10 as a collection time, presumably coinciding with other antenatal testing, and the decrease in TSH to its lowest point toward the end of the first trimester. The median TSH concentration at week 10 was almost half of the value at the beginning of pregnancy. An important limitation of this data is that it was collected as part of clinical care rather than as part of an epidemiological survey.
Figure.
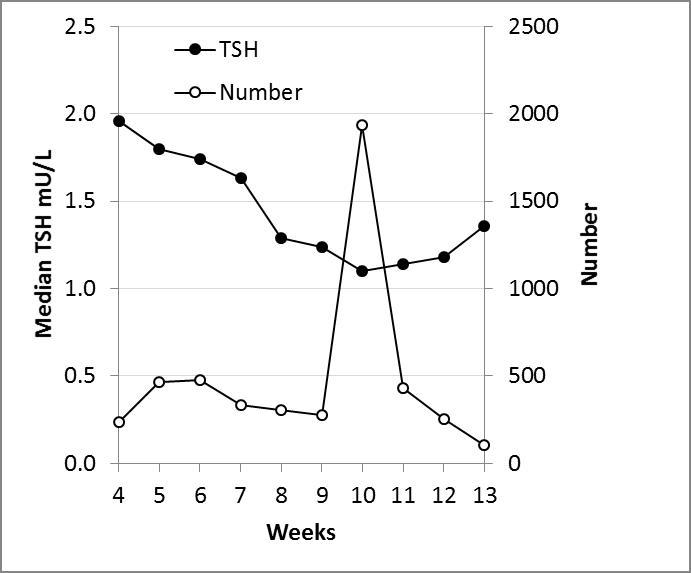
Median thyroid stimulating hormone (TSH) concentrations and test numbers for 6706 specimens tested in a Victorian private pathology laboratory over one year.
These observations indicate that the correct interpretation of thyroid function tests require knowledge of a woman’s gestational age. For laboratories that choose to establish their own first trimester reference intervals it is important for them to specify whether their subjects come from the beginning, middle or end of this period.
2. Antibody status
Anti-thyroid peroxidase (ATPO) and anti-thyroglobulin (ATG) antibodies are markers of thyroid autoimmunity. There is consistent evidence that women with increased serum concentrations of these antibodies tend to have increased TSH concentrations suggesting they have mild, asymptomatic impairment of thyroid function.22,56,57 Surveys in Australia at 10–13 weeks gestation found that 15–20% of pregnant women are positive for one or other antibody.1,2
Median first trimester TSH concentrations were significantly higher in one study which compared 1,211 antibody-positive women with 8,351 antibody-negative controls (1.64 versus 1.00 mIU/L, p<0.0001).58 Amongst the antibody-positive women, the median TSH levels were highest in those who were positive for both antibodies rather than just one (both negative 1.43 mIU/L, ATG positive 1.43 mIU/L, ATPO positive 1.75 mIU/L and both positive 1.94 mIU/L, p<0.0001).
Another study showed that removing women with ATPO antibodies from a reference population reduced the upper limit for TSH from 3.17 mIU/L to 2.70 mIU/L.18 Analysis of a cohort of 4800 women in China the first half of pregnancy showed positive ATPO was a risk factor for increased gestational age-specific TSH concentrations.56 The authors also showed that ATPO positivity was significantly more common in women with subclinical hypothyroidism compared with those who were euthyroid.
The fact that TSH concentrations tend to be higher in antibody-positive women indicates that they should not be included in reference interval studies as healthy controls. Even though this advice has been widely publicised it has not been followed in a number of studies raising the concern that their reference intervals may be falsely high.4
3. Iodine status
Iodine is an essential substrate for thyroid hormone synthesis and iodine deficiency is the most common cause of hypothyroidism worldwide. Low dietary intake of iodine is a problem in Australia and New Zealand where the soil iodine content is low, non-iodised salt is widely used and changes in dairy practices have decreased the iodine content of milk. Given the increased requirements for iodine in pregnancy it is recommended that iodine intake should be at least 250 ug per day.11 This would give urine iodine concentrations greater than 150 ug/L in most women compared with the usual non-pregnant target of 100 ug/L.59,60
Iodine deficiency and excess can have different effects on thyroid function. Some papers have claimed that iodine deficiency, particularly when severe, causes increased TSH in pregnancy although this has not been found in all studies.8,61–63 As with thyroid autoantibodies, it is recommended that reference populations used in studies of thyroid function in pregnancy are iodine replete. Ideally this would be confirmed with urine iodine measurement, particularly in places like Australia where low iodine intake is common.
4. Multiple pregnancy
Serum hCG concentrations tend to be higher and TSH concentrations lower in women with multiple pregnancies. One large study found that first trimester TSH concentrations were lower in 132 women with twin pregnancies by approximately 0.04 mIU/L compared with 13,599 singleton controls (p<0.001).53 The practical implications of these observations are that TSH concentrations might be expected to be slightly lower in women with twins and that women with multiple pregnancies should be excluded from reference populations to determine TSH reference intervals.
5. Ethnicity
A number of studies have shown differences in thyroid hormone concentrations based on ethnicity although there has been discussion about the relative contribution of genetic factors, hCG concentrations, iodine nutrition, thyroid antibodies and maternal age. The magnitude of the reported TSH differences between ethnic groups ranged from 0.1664 to 0.40 mIU/L.65
Two large studies from the Netherlands showed significant differences in TSH, thyroid hormone concentrations and thyroid antibodies between Dutch, Turkish, Surinamese and Moroccan women.64,65 Four different studies found that TSH concentrations were lower in black compared with white women.57,65–67 The first found lower TSH and higher FT4 concentrations in African-American women in a study of 589 women in Florida.65 The African-Americans had higher hCG concentrations in the first trimester but there were no differences in urine iodine concentrations or antibody positivity. The second study also found lower TSH concentrations in the second trimester with no difference in FT4.66 A later paper from the same group reported first trimester reference ranges by ethnic background using antibody-negative samples. Iodine status and medical histories were not known but black women had the lowest and Asian women the highest TSH concentrations.67 The final study of a large, antibody-negative UK cohort found TSH, FT4 and FT3 were all lower at 11–13 weeks in black compared with white women.57
These data suggest that black women may have lower, and Asian women higher TSH concentrations in pregnancy compared with white women. While more information is needed, these findings indicate that care is required when extrapolating thyroid reference intervals from one ethnic group to another. There are no publications comparing thyroid function in pregnancy in different ethnic groups in Australia and New Zealand but these would be worthwhile to reduce the risk of misdiagnosis.
6. Time of blood collection
Studies in adults and children have shown that there is significant diurnal variation in serum TSH concentrations.68,69 TSH is lowest in the afternoon, rises in the evening with a peak in the early half of the night.68 The peak to trough difference may exceed 100% although variation during daylight hours is considerably less.68,70
The diurnal rhythm of TSH has also been demonstrated in pregnant women. In a study of eight women in the first trimester, hourly blood samples revealed a pattern of TSH similar to non-pregnant adults, with trough in the afternoon and peak between 10 pm and 2 am.71 In this study, the TSH levels between 10 pm and 2 am were 112% above levels between 12 midday and 4 pm, and during the day there was approximately a 30% decrease in TSH between 8 am and 12 midday to 3 pm. Circadian variation was also shown to be maintained in the second and third trimesters.72 In this study the mean TSH at 2 pm was almost half the concentration measured at 8 am in the third trimester, although the difference was less marked in the second trimester.
As a result of this diurnal variation, failure to standardise the time of collection has the potential to introduce unhelpful noise into both the generation of reference intervals and the interpretation of patient results.
Analytical factors
1. Thyroid hormones
Accurate serum FT4 measurements are needed to differentiate overt hypothyroidism from subclinical hypothyroidism and for the diagnosis of euthyroid hypothyroxinaemia, the controversial diagnostic group that some claim is associated with adverse pregnancy outcomes.50
If pregnancy is a stress test for the maternal thyroid, it is also a stress test for the laboratory’s immunoassays. In a paper with the arresting title “Free T4 immunoassays are flawed during pregnancy” it was stated that current FT4 immunoassays are actually “FT4 estimate tests”.73 Immunoassays may be affected by changes in binding proteins in pregnancy, in particular increased serum TBG, increased free fatty acids and decreased albumin. Whilst some authors have found good agreement between immunoassays and a reference method74 others have not.73,75 In the latter paper they found that FT4 results from two immunoassays changed in opposite directions in the first trimester.
All groups recommend taking care with FT4 assays in pregnancy and using method-specific reference intervals where they are available. Some favour the time-honoured North American approach of measuring total T4 and calculating the free thyroxine index.73 They claim that the simplest way is to measure serum total T4 and use a reference interval increased by 50% to account for increased TBG. One problem with this approach is that few laboratories measure TT4. Another is that one study found that TT4 did not increase by 50% in their subjects, suggesting that this formula might be an over-simplification.66
2. TSH
TSH is a small molecular weight heterodimeric glycoprotein that is secreted by the anterior pituitary. It is not affected by the binding protein changes that increase thyroxine concentrations in pregnancy. There is natural variation in TSH glycosylation that can affect its biological activity although this does not appear to change during pregnancy.76 Different immunoassays may give different TSH results as discussed in the final section of this paper.
3. Thyroid antibodies
The measurement of both ATPO and ATG antibodies has the greatest sensitivity for detecting thyroid autoimmunity although many studies have only measured ATPO.14,77 Two analytical factors are important. The first is that the results of different assays may not be concordant meaning that some women may appear to have thyroid autoimmunity with one blood test but not with another. The second is that antibody concentrations tend to fall through pregnancy meaning that the apparent proportion of women with autoimmunity decreases with increasing gestational age.18,78,79
Post-analytical factors
Assay differences, gestational age, twins or triplets, ethnicity and the time of blood collection should be accounted for in deriving thyroid function test reference intervals as discussed above. Other factors such as iodine insufficiency, positive antibody status, goitre or a past history of thyroid disease represent an increased risk of underlying thyroid pathology. Individuals with these conditions should not be included in a “healthy” reference population.13,24 Failure to exclude them will tend to increase the apparent upper limit of normal for TSH.
The NACB guidelines recommend that reference intervals for TSH be established from the central 95% (between 2.5th and 97.5th percentiles) of the log-transformed values of at least 120 rigorously screened normal euthyroid volunteers.24 Bigger numbers are generally better and some argue that at least 400 subjects are needed to account for the skewed distribution of TSH.4
The Problems with using Non-pregnant TSH Reference Intervals
It follows from the discussion above that standard thyroid function test reference intervals should not be used for pregnant women with the most worrying problem being the misclassification of women with subclinical hypothyroidism as “normal” in the first trimester. This was illustrated in a Western Australian study where the use of non-pregnant TSH intervals would result in 98 of 2159 (4.5%) of women being classified as “normal” when they in fact had increased TSH and subclinical hypothyroidism.2 Other studies have made similar findings.19,55,56,80,81
What Reference Intervals should you use?
There have been at least 50 pregnancy thyroid function test reference interval studies published in the last 20 years. Some were large and well planned but many were not. Small numbers, failure to exclude women with thyroid antibodies and the use of old laboratory methods were common problems.
In sorting through this information laboratories should be looking for large studies with analytical methods and populations that match their own. Ideally the reference populations would have been carefully screened to eliminate women with thyroid disease or iodine deficiency.
The following section summarises studies of thyroid function test reference intervals in pregnancy for the five instrument groups that are commonly used in Australia and New Zealand. Studies with fewer than 120 subjects have been excluded.
1. Abbott Architect
Three studies were rejected because of small numbers.82–84 Seven of the eight studies that were included screened for autoimmunity using both ATPO and ATG. Two studies were from China, four from Europe, one from North America and one from Australia. None measured the iodine status of their subjects although three stated their populations were known to be iodine sufficient. Only one study collected all of the specimens in the morning (Table 1).
Table 1.
Details of pregnancy thyroid function test studies using the Abbott Architect. Study results in Table 2.
| Study | Year | Author | Country | Number | Ab | Ethnicity | Iodine sufficiency | Time | Ref |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 2011 | La’ulu | USA | 2172 | Both | Mixed (34% white) | Not known | Not specified | 67 |
| A2 | 2008 | Gilbert | Australia | 1817 | Both | Not specified | Not known | Not specified | 2 |
| A3 | 2007 | Stricker | Switzerland | 783 | Both | Not specified | Not known | Not specified | 19 |
| A4 | 2011 | Mannisto | Finland | 667 | Both | Not specified | Inferred from population surveys | Not specified | 54 |
| A5 | 2014 | Shen | China | 365 | Both | Asian | Inferred from population surveys | Not specified | 90 |
| A6 | 2009 | Bocos-Terraz | Spain | 330 | Both | Mixed (85% white) | Not known | Not specified | 22 |
| A7 | 2014 | Springer | Czech Republic | 216 | ATPO | Not specified | Inferred from population surveys | Not specified | 94 |
| A8 | 2013 | Fan | China | 140 | Both | Asian | Not known | Morning | 89 |
Abbreviations: Ab - anti-thyroid antibodies, ATPO - anti-thyroid peroxidase, ATG – anti-thyroglobulin.
There was relatively close agreement between TSH limits which tended to be lowest in early pregnancy (Table 2). The mean first trimester 97.5th percentile was 3.00 mIU/L (range 2.15 – 3.78 mIU/L). FT4 limits tended to be highest in the first trimester with a mean value 18.5 pmol/L (range 17.7–21.6 pmol/L). Interestingly the two Chinese studies had the highest TSH cut-offs while the Australian study had the lowest. It is not clear if this was a chance event or related to ethnicity, iodine status (not measured in any of these studies) or other factors such as the gestational ages of the subjects.
Table 2.
TSH 2.5th and 97.5th percentiles for each trimester in healthy women using the Abbott Architect. Study details in Table 1.
| Study | TSH mIU/L | FT4 pmol/L | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | First | Second | Third | |||||||
| 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | |
| A1 | 0.02 | 2.69 | 0.15 | 3.11 | 11.4 | 18.6 | 9.3 | 15.2 | ||||
| A2 | 0.02 | 2.15 | 10.4 | 17.8 | ||||||||
| A3 | 0.09 | 2.83 | 0.20 | 2.79 | 0.31 | 2.90 | 10.5 | 18.3 | 9.5 | 15.7 | 8.6 | 13.6 |
| A4 | 0.12 | 3.04 | 0.35 | 3.32 | 11.7 | 21.6 | 11.2 | 18.9 | ||||
| A5 | 0.16 | 3.78 | 0.34 | 3.51 | 0.34 | 4.30 | 10.9 | 17.7 | 9.3 | 15.2 | 7.9 | 14.1 |
| A6 | 0.41 | 2.63 | 0.15 | 2.59 | 0.28 | 3.48 | 10.8 | 17.8 | 9.0 | 14.9 | 8.0 | 15.1 |
| A7 | 0.22 | 3.27 | 11.8 | 17.7 | ||||||||
| A8 | 0.03 | 3.60 | 0.14 | 3.86 | 0.54 | 3.26 | 11.5 | 18.8 | 10.3 | 17.7 | 10.0 | 15.5 |
| Mean | 0.13 | 3.00 | 0.22 | 3.20 | 0.37 | 3.49 | 11.1 | 18.5 | 9.8 | 16.3 | 8.6 | 14.6 |
| SD | 0.13 | 0.54 | 0.10 | 0.47 | 0.12 | 0.59 | 0.53 | 1.32 | 0.83 | 1.63 | 0.97 | 0.87 |
2. Beckman Access and DxI
There were four Beckman studies with more than 120 subjects (Table 3). Two were European, one was from China and one was Australian. ATPO and ATG antibodies were used to identify autoimmunity in one study and ATPO alone in three. None measured urine iodine and one collected fasting blood, presumably in the morning. The first trimester TSH 97.5th percentiles were similar in the three studies with an average value of 3.12 mIU/L (range 2.96–3.33 mIU/L). Beckman FT4 values tended to be lower than all other methods at all time points (Table 4).
Table 3.
Details of pregnancy thyroid function test studies using the Beckman Access or DxI. Study results in Table 4.
| Study | Year | Author | Country | Number | Ab | Ethnicity | Iodine sufficiency | Time | Ref |
|---|---|---|---|---|---|---|---|---|---|
| B1 | 2007 | Benhadi | Netherlands | 2475 | ATPO | White | Inferred from population surveys | Not specified | 64 |
| B2 | 2015 | Zhang | China | 1521 | Both | Asian | Inferred from population surveys | Morning | 99 |
| B3 | 2014 | Springer | Czech Republic | 216 | ATPO | Not specified | Inferred from population surveys | Not specified | 94 |
| B4 | 2013 | Ekinci | Australia | 129 | ATPO | Not specified | Urine iodine measured but women with concentrations < 100 ug/L were not excluded | Not specified | 80 |
Abbreviations: Ab - anti-thyroid antibodies, ATPO - anti-thyroid peroxidase, ATG – anti-thyroglobulin.
Table 4.
TSH 2.5th and 97.5th percentiles for each trimester in healthy women using the Beckman Access or DxI. Study details in Table 3.
| Study | TSH mIU/L | FT4 pmol/L | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | First | Second | Third | |||||||
| 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | |
| B1 | 0.27 | 2.96 | 0.38 | 3.04 | ||||||||
| B2 | 0.06 | 3.13 | 0.07 | 4.13 | 0.15 | 5.02 | 8.7 | 15.2 | 7.1 | 13.6 | 6.2 | 12.0 |
| B3 | 3.33 | 8.1 | 13.2 | |||||||||
| B4 | 0.03 | 3.05 | 0.42 | 3.36 | 0.34 | 2.83 | 5.9 | 15.6 | 4.9 | 11.3 | 4.4 | 11.2 |
| Mean | 0.12 | 3.12 | 0.29 | 3.51 | 0.25 | 3.93 | 7.6 | 14.7 | 6.0 | 12.5 | 5.3 | 11.6 |
| SD | 0.13 | 0.16 | 0.19 | 0.56 | 0.13 | 1.55 | 1.5 | 1.3 | 1.6 | 1.6 | 1.3 | 0.6 |
3. Siemens Centaur
One study was excluded because of small numbers85 and another that had unusually high TSH cut-offs, possibly the result of the statistical tools that were used.86 Of the six studies that were included, there was one each from Australia, the United Kingdom, North America and China and two from the same group in the Czech Republic (Table 5). Data from a local unpublished study in Australia was also included in which TSH was measured in 261 women at ten weeks gestation after the exclusion of those with ATPO or ATG antibodies or known thyroid disease. The UK study was by far the largest but there were no details about excluding women who were antibody positive or iodine deficient.87 The Chinese study was the smallest but also the most careful.88 Women with past thyroid disease or palpable goitre were excluded along with those with positive ATPO or ATG. Iodine was measured in urine, table salt and drinking water. They also excluded 22/827 antibody-negative women who had TSH greater than 5.0 mIU/L.
Table 5.
Details of pregnancy thyroid function test studies using the Siemens Centaur. Study results in Table 6.
| Study | Year | Author | Country | Number | Ab | Ethnicity | Iodine sufficiency | Time | Ref |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 2014 | Bestwick | UK | 16334 | None | Not specified | Not known | Not specified | 87 |
| C2 | 2009 | Springer | Czech Republic | 4337 | ATPO | Not specified | Inferred from population surveys | Not specified | 95 |
| C3 | 2008 | Pearce | USA | 585 | ATPO | Mixed (77% white) | Not known | Not specified | 96 |
| C4 | 2015 | McNeil | Australia | 261 | Both | Not specified | Not known | Not specified | |
| C5 | 2014 | Springer | Czech Republic | 216 | ATPO | Not specified | Inferred from population surveys | Not specified | 94 |
| C6 | 2011 | Yan | China | 168 | Both | Asian | Urine iodine 150–200 ug/L | Not specified | 88 |
Abbreviations: Ab - anti-thyroid antibodies, ATPO - anti-thyroid peroxidase, ATG – anti-thyroglobulin.
It was interesting that the first trimester TSH cut-offs were much higher in the Chinese study than the others, similar to the findings with two Abbott Architect studies.89,90
4. Siemens Immulite
Five studies were included, three from North America and two from Europe (Table 7). Exclusion according to antibody status differed between the studies and none measured urine iodine or had a fixed blood collection time. Despite these differences the TSH and FT4 cut-offs were close in the different studies with the average for the first trimester 97.5th percentile 3.09 mIU/L (range 2.53–3.61 mIU/L) (Table 8). No information on third trimester limits was provided.
Table 7.
Details of pregnancy thyroid function test studies using the Siemens Immulite. Study results in Table 8.
| Study | Year | Author | Country | Nuamber | Ab | Ethnicity | Iodine sufficiency | Time | Ref |
|---|---|---|---|---|---|---|---|---|---|
| I1 | 2008 | Lambert-Messerlian | USA | 8351 | Both | Not specified | Not known | Not specified | 58 |
| I2 | 2004 | Haddow | USA | 1005 | ATPO | White | Not known | Not specified | 18 |
| I3 | 2005 | Dashe | USA | 982 | None | Mixed (84% Hispanic) | Not known | Not specified | 53 |
| I4 | 2014 | Springer | Czech Republic | 216 | ATPO | Not specified | Inferred from population surveys | Not specified | 94 |
| I5 | 2011 | Karakosta | Greece | 143 | Both | Not specified | Inferred from population surveys | Not specified | 81 |
Abbreviations: Ab - anti-thyroid antibodies, ATPO - anti-thyroid peroxidase, ATG – anti-thyroglobulin.
Table 8.
TSH 2.5th and 97.5th percentiles for each trimester in healthy women using the Siemens Immulite. Study details in Table 7.
| Study | TSH mIU/L | FT4 pmol/L | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | First | Second | Third | |||||||
| 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | |
| I1 | 0.12 | 3.37 | 0.35 | 3.35 | 10.4 | 17.8 | 9.3 | 16.2 | ||||
| I2 | 0.08 | 3.61 | 0.39 | 3.71 | ||||||||
| I3 | 0.02 | 3.12 | 0.28 | 3.04 | ||||||||
| I4 | 0.17 | 2.83 | 10.4 | 16.4 | ||||||||
| I5 | 0.05 | 2.53 | 0.18 | 2.73 | 12.2 | 19.7 | 11.2 | 18.7 | ||||
| Mean | 0.09 | 3.09 | 0.30 | 3.21 | 11.0 | 18.0 | 10.3 | 17.4 | ||||
| SD | 0.06 | 0.43 | 0.09 | 0.42 | 1.06 | 1.65 | 1.34 | 1.74 | ||||
5. Roche Cobas, E170 and Modular
Three Roche studies were not included because of small numbers.23,91,92 Three of the remaining studies were from China, three from Europe and one was unpublished local data. In the latter TSH was measured in 331 women at ten weeks gestation after the exclusion of those with anti-thyroid antibodies or thyroid disease.
There were different policies for excluding women with thyroid antibodies in the studies (none, ATPO only, or ATPO and ATG). Two studies collected blood in the morning and two measured urine iodine concentrations (Table 9).
Table 9.
Details of pregnancy thyroid function test studies using the Roche Cobas, E170 and Modular. Study results in Table 10.
| Study | Year | Author | Country | Number | Ab | Ethnicity | Iodine sufficiency | Time | Ref |
|---|---|---|---|---|---|---|---|---|---|
| R1 | 2014 | Li | China | 640 | Both | Asian | Median urine iodine 162 ug/L | Morning | 55 |
| R2 | 2004 | Roche | Germany | 418 | None | Not specified | Not known | Not specified | 97 |
| R3 | 2014 | Khalid | Ireland | 341 | ATPO | Not specified | Iodine deficient population | Not specified | 98 |
| R4 | 2015 | McNeil | Australia | 331 | Both | Not specified | Not known | Not specified | |
| R5 | 2010 | Yu | China | 301 | ATPO | Asian | Urine tested | Not specified | 93 |
| R6 | 2014 | Springer | Czech Republic | 216 | ATPO | Not specified | Inferred from population surveys | Not specified | 94 |
| R7 | 2013 | Fan | China | 140 | Both | Asian | Not known | Morning | 89 |
Abbreviations: Ab - anti-thyroid antibodies, ATPO - anti-thyroid peroxidase, ATG – anti-thyroglobulin.
The average first trimester 97.5th percentile for TSH with the Roche analysers was the highest of all five instrument groups at 4.00 mIU/L although the range of results was large (Table 10). The Chinese study with the highest result (5.17 mIU/L) was the same one that gave the highest value amongst the Abbott Architect studies.89 Next highest (4.59 mIU/L) was a Roche study which had limited details about whether women with thyroid antibodies or thyroid disease had been excluded.97 The results from another large and detailed Chinese study55 were high (4.34 mIU/L) but those of another were unremarkable.93
Table 10.
TSH 2.5th and 97.5th percentiles for each trimester in healthy women using the Roche Cobas, E170 and Modular. Study details in Table 9.
| Study | TSH mIU/L | FT4 pmol/L | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | First | Second | Third | |||||||
| 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | |
| R1 | 0.10 | 4.34 | 12.3 | 20.9 | ||||||||
| R2 | 0.33 | 4.59 | 0.35 | 4.10 | 0.21 | 3.15 | 12.1 | 19.6 | 9.6 | 17.0 | 8.4 | 15.6 |
| R3 | 0.20 | 3.00 | 0.30 | 3.10 | 0.60 | 3.30 | 12.1 | 18.7 | 9.9 | 16.7 | 8.6 | 14.9 |
| R4 | 0.07 | 3.45 | 12.4 | 21.0 | ||||||||
| R5 | 0.02 | 3.65 | 0.36 | 3.46 | 0.44 | 5.04 | 11.9 | 21.5 | 9.5 | 12.3 | 9.3 | 17.1 |
| R6 | 0.25 | 3.81 | 11.5 | 18.6 | ||||||||
| R7 | 0.05 | 5.17 | 0.21 | 6.00 | 0.25 | 5.11 | 12.9 | 22.4 | 10.2 | 17.6 | 9.6 | 16.0 |
| Mean | 0.15 | 4.00 | 0.31 | 4.17 | 0.38 | 4.15 | 12.2 | 20.4 | 9.8 | 15.9 | 9.0 | 15.9 |
| SD | 0.12 | 0.74 | 0.07 | 1.29 | 0.18 | 1.07 | 0.43 | 1.44 | 0.31 | 2.44 | 0.55 | 0.92 |
Given the wide variation in results it is interesting to consider that a study of non-pregnant individuals in Germany using the Roche Elecsys and strict exclusions according to NACB criteria gave a 97.5th percentile of 3.77 mIU/L.100 Almost 50% of the subjects in this study were excluded which raises the question of how much of the variation we have seen is the result of occult disease and how much the result of true biological differences.
6. Overall
The cut-offs for all methods are summarised in Table 11. Care is required with those method groups in which there was large variation but it appears that the all-important first trimester TSH 97.5th percentiles fell into two groups – Architect, Beckman and Immulite were around 3.0 mIU/L whilst Centaur and Roche were closer to 4.0 mIU/L. Of the 27 studies there are only four, two Abbott Architect,2,22 one Immulite81 and one Centaur,63 that were close to, or below the publicised 2.5 mIU/L cut-off.
Table 11.
Summary of pregnancy thyroid function test reference interval studies from Tables 1–10. Each number is the mean value for all studies for a given method group.
| TSH mIU/L | FT4 pmol/L | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trimester: | First | Second | Third | First | Second | Third | |||||||
| Group | Studies | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th |
| Architect | 8 | 0.13 | 3.00 | 0.22 | 3.20 | 0.37 | 3.49 | 11.1 | 18.5 | 9.8 | 16.3 | 8.6 | 14.6 |
| Beckman | 4 | 0.12 | 3.12 | 0.29 | 3.51 | 0.25 | 3.93 | 7.6 | 14.7 | 6.0 | 12.5 | 5.3 | 11.6 |
| Centaur | 6 | 0.08 | 3.55 | 0.05 | 4.50 | 0.47 | 4.54 | 11.8 | 19.4 | 10.6 | 17.6 | 9.2 | 16.7 |
| Immulite | 5 | 0.09 | 3.09 | 0.30 | 3.21 | 11.0 | 18.0 | 10.3 | 17.4 | ||||
| Roche | 7 | 0.15 | 4.00 | 0.31 | 4.17 | 0.38 | 4.15 | 12.2 | 20.4 | 9.8 | 15.9 | 9.0 | 15.9 |
While TSH appeared to fall into two broad groups, FT4 seemed more consistent across methods with an interval of approximately 11.5–19 pmol/L in the first trimester and slightly lower values later in pregnancy. The exception was the Beckman Access DxI groups where the concentrations were considerably lower.
Conclusions
Whilst overt hypothyroidism (TSH greater than 10 mIU/L and/or low FT4) should be treated without hesitation in pregnant women, the approach to subclinical hypothyroidism is more complicated because the harms and benefits are not well established. These uncertainties have led to disagreement on whether women should be screened for thyroid disease in pregnancy or not.
If subclinical hypothyroidism is going to be diagnosed on the basis of a raised serum TSH concentration, the immediate question is what is a normal TSH? Numerous studies have now shown that the figure depends on the gestational age of the subjects, the analytical method and the care with which the reference population was selected. Given the variation in published studies it can be hard to know which figures can be reliably used in your laboratory.
Many laboratories use non-pregnant reference intervals during pregnancy because of limitations in their computer system although this is undesirable because it risks missing women with early hypothyroidism. The nomination of 2.5 mIU/L as the universal cut-off has also been unhelpful as it is unrealistically low in many settings and gives the false impression that a complex situation is very simple.
The 97.5th percentile of TSH at the end of the first trimester, when TSH concentrations are lowest has received most attention as the cut-off to trigger further investigation and treatment. Whilst agreeing on this point has been difficult enough it is worth considering that this time might be too late. If future studies find that subclinical hypothyroidism poses a significant threat to the baby, the optimal time for treatment may be much earlier when he/she is completely dependent on the mother’s thyroxine. Further research is required in this important period of foetal development.
Table 6.
TSH 2.5th and 97.5th percentiles for each trimester in healthy women using the Siemens Centaur. Study details in Table 5.
| Study | TSH mIU/L | FT4 pmol/L | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | First | Second | Third | |||||||
| 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | |
| C1 | 0.06 | 3.50 | 10.9 | 17.9 | ||||||||
| C2 | 0.06 | 3.67 | ||||||||||
| C3 | 0.04 | 3.60 | ||||||||||
| C4 | 0.08 | 2.67 | 12.4 | 20.3 | ||||||||
| C5 | 0.22 | 3.34 | 11.8 | 18.4 | ||||||||
| C6 | 0.03 | 4.51 | 0.05 | 4.50 | 0.47 | 4.54 | 11.8 | 21.0 | 10.6 | 17.6 | 9.2 | 16.7 |
| Mean | 0.08 | 3.55 | 0.05 | 4.50 | 0.47 | 4.54 | 11.8 | 19.4 | 10.6 | 17.6 | 9.2 | 16.7 |
| SD | 0.08 | 0.46 | 0.52 | 1.7 | ||||||||
Footnotes
Competing Interests: None declared.
References
- 1.McElduff A, Morris J. Thyroid function tests and thyroid autoantibodies in an unselected population of women undergoing first trimester screening for aneuploidy. Aust N Z J Obstet Gynaecol. 2008;48:478–80. doi: 10.1111/j.1479-828X.2008.00903.x. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert RM, Hadlow NC, Walsh JP, Fletcher SJ, Brown SJ, Stuckey BG, et al. Assessment of thyroid function during pregnancy: first-trimester (weeks 9–13) reference intervals derived from Western Australian women. Med J Aust. 2008;189:250–3. doi: 10.5694/j.1326-5377.2008.tb02015.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison S, et al. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203–7. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 4.Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. Thyroid function in pregnancy: what is normal? Clin Chem. 2015;61:704–13. doi: 10.1373/clinchem.2014.236646. [DOI] [PubMed] [Google Scholar]
- 5.Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ. 2014;349:g4929. doi: 10.1136/bmj.g4929. [DOI] [PubMed] [Google Scholar]
- 6.Hershman JM. The role of human chorionic gonadotropin as a thyroid stimulator in normal pregnancy. J Clin Endocrinol Metab. 2008;93:3305–6. doi: 10.1210/jc.2008-1461. [DOI] [PubMed] [Google Scholar]
- 7.Moleti M, Trimarchi F, Vermiglio F. Thyroid physiology in pregnancy. Endocr Pract. 2014;20:589–96. doi: 10.4158/EP13341.RA. [DOI] [PubMed] [Google Scholar]
- 8.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–33. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 9.Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–87. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 10.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2012;97:2543–65. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 11.Delange F. Optimal iodine nutrition during pregnancy, lactation and the neonatal period. Int J Endocrinol Metab. 2004;2:1–12. [Google Scholar]
- 12.Roti E, Fang SL, Green K, Emerson CH, Braverman LE. Human placenta is an active site of thyroxine and 3,3′5-triiodothyronine tyrosyl ring deiodination. J Clin Endocrinol Metab. 1981;53:498–501. doi: 10.1210/jcem-53-3-498. [DOI] [PubMed] [Google Scholar]
- 13.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3:76–94. doi: 10.1159/000362597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagnaro-Green A. Optimal care of the pregnant woman with thyroid disease. J Clin Endocrinol Metab. 2012;97:2619–22. doi: 10.1210/jc.2012-2380. [DOI] [PubMed] [Google Scholar]
- 16.Negro R. Thyroid dysfunction and pregnancy: where are we five years later? J Clin Endocrinol Metab. 2012;97:2629–31. doi: 10.1210/jc.2012-2440. [DOI] [PubMed] [Google Scholar]
- 17.Soldin OP. When thyroidologists agree to disagree: comments on the 2012 Endocrine Society pregnancy and thyroid disease clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2632–5. doi: 10.1210/jc.2012-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddow JE, Knight GJ, Palomaki GE, McClain MR, Pulkkinen AJ. The reference range and within-person variability of thyroid stimulating hormone during the first and second trimesters of pregnancy. J Med Screen. 2004;11:170–4. doi: 10.1258/0969141042467340. [DOI] [PubMed] [Google Scholar]
- 19.Stricker R, Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, et al. Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. Eur J Endocrinol. 2007;157:509–14. doi: 10.1530/EJE-07-0249. [DOI] [PubMed] [Google Scholar]
- 20.Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001;38:329–32. doi: 10.1258/0004563011900830. [DOI] [PubMed] [Google Scholar]
- 21.Soldin OP, Soldin D, Sastoque M. Gestation-specific thyroxine and thyroid stimulating hormone levels in the United States and worldwide. Ther Drug Monit. 2007;29:553–9. doi: 10.1097/FTD.0b013e31815709ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bocos-Terraz JP, Izquierdo-Alvarez S, Bancalero-Flores JL, Alvarez-Lahuerta R, Aznar-Sauca A, Real-López E, et al. Thyroid hormones according to gestational age in pregnant Spanish women. BMC Res Notes. 2009;2:237. doi: 10.1186/1756-0500-2-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008;115:602–6. doi: 10.1111/j.1471-0528.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 24.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Guidelines Committee, National Academy of Clinical Biochemistry Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 25.Granfors M. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1081. Uppsala: Acta Universitatis Upsaliensis; Hypothyroidism and Pregnancy; p. 69. ISBN 978-91-554-9201-4. [Google Scholar]
- 26.Chan S, Boelaert K. Optimal management of hypothyroidism, hypothyroxinaemia and euthyroid TPO antibody positivity preconception and in pregnancy. Clin Endocrinol (Oxf) 2015;82:313–26. doi: 10.1111/cen.12605. [DOI] [PubMed] [Google Scholar]
- 27.Man EB, Jones WS, Holden RH, Mellits ED. Thyroid function in human pregnancy. 8. Retardation of progeny aged 7 years; relationships to maternal age and maternal thyroid function. Am J Obstet Gynecol. 1971;111:905–16. [PubMed] [Google Scholar]
- 28.Greenman GW, Gabrielson MO, Howard-Flanders J, Wessel MA. Thyroid dysfunction in pregnancy. Fetal loss and follow-up evaluation of surviving infants. N Engl J Med. 1962;267:426–31. doi: 10.1056/NEJM196208302670902. [DOI] [PubMed] [Google Scholar]
- 29.Jones WS, Man EB. Thyroid function in human pregnancy. VI. Premature deliveries and reproductive failures of pregnant women with low serum butanol-extractable iodines. Maternal serum TBG and TBPA capacities. Am J Obstet Gynecol. 1969;104:909–14. [PubMed] [Google Scholar]
- 30.Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–45. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 31.Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7:127–30. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 32.Matalon S, Sheiner E, Levy A, Mazor M, Wiznitzer A. Relationship of treated maternal hypothyroidism and perinatal outcome. J Reprod Med. 2006;51:59–63. [PubMed] [Google Scholar]
- 33.Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112:85–92. doi: 10.1097/AOG.0b013e3181788dd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Männistö T, Vääräsmäki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, et al. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metab. 2010;95:1084–94. doi: 10.1210/jc.2009-1904. [DOI] [PubMed] [Google Scholar]
- 35.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. 2010;95:E44–8. doi: 10.1210/jc.2010-0340. [DOI] [PubMed] [Google Scholar]
- 36.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010;95:1699–707. doi: 10.1210/jc.2009-2009. [DOI] [PubMed] [Google Scholar]
- 37.Prummel MF, Wiersinga WM. Thyroid autoimmunity and miscarriage. Eur J Endocrinol. 2004;150:751–5. doi: 10.1530/eje.0.1500751. [DOI] [PubMed] [Google Scholar]
- 38.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab. 2006;91:2587–91. doi: 10.1210/jc.2005-1603. [DOI] [PubMed] [Google Scholar]
- 39.Grossmann M, Hoermann R, Francis C, Hamilton EJ, Tint A, Kaitu’u-Lino T, et al. Measuring thyroid peroxidase antibodies on the day nulliparous women present for management of miscarriage: a descriptive cohort study. Reprod Biol Endocrinol. 2013;11:40. doi: 10.1186/1477-7827-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 41.Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493–501. doi: 10.1056/NEJMoa1106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hales C, Channon S, Taylor PN, Draman MS, Muller I, Lazarus J, et al. The second wave of the Controlled Antenatal Thyroid Screening (CATS II) study: the cognitive assessment protocol. BMC Endocr Disord. 2014;14:95. doi: 10.1186/1472-6823-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;96:3234–41. doi: 10.1210/jc.2011-0274. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 2010;72:825–9. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 45.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 46.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 47.Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. 2010;95:4227–34. doi: 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- 48.Finken MJ, van Eijsden M, Loomans EM, Vrijkotte TG, Rotteveel J. Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J Clin Endocrinol Metab. 2013;98:1417–26. doi: 10.1210/jc.2012-3389. [DOI] [PubMed] [Google Scholar]
- 49.Ghassabian A, El Marroun H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, et al. Downstream effects of maternal hypothyroxinemia in early pregnancy: nonverbal IQ and brain morphology in school-age children. J Clin Endocrinol Metab. 2014;99:2383–90. doi: 10.1210/jc.2013-4281. [DOI] [PubMed] [Google Scholar]
- 50.Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 2013;79:152–62. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- 51.Craig WY, Allan WC, Kloza EM, Pulkkinen AJ, Waisbren S, Spratt DI, et al. Mid-gestational maternal free thyroxine concentration and offspring neurocognitive development at age two years. J Clin Endocrinol Metab. 2012;97:E22–8. doi: 10.1210/jc.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Negro R, Soldin OP, Obregon MJ, Stagnaro-Green A. Hypothyroxinemia and pregnancy. Endocr Pract. 2011;17:422–9. doi: 10.4158/EP10309.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dashe JS, Casey BM, Wells CE, McIntire DD, Byrd EW, Leveno KJ, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753–7. doi: 10.1097/01.AOG.0000175836.41390.73. [DOI] [PubMed] [Google Scholar]
- 54.Männistö T, Surcel HM, Ruokonen A, Vääräsmäki M, Pouta A, Bloigu A, et al. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid. 2011;21:291–8. doi: 10.1089/thy.2010.0337. [DOI] [PubMed] [Google Scholar]
- 55.Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. 2014;99:73–9. doi: 10.1210/jc.2013-1674. [DOI] [PubMed] [Google Scholar]
- 56.Shan ZY, Chen YY, Teng WP, Yu XH, Li CY, Zhou WW, et al. A study for maternal thyroid hormone deficiency during the first half of pregnancy in China. Eur J Clin Invest. 2009;39:37–42. doi: 10.1111/j.1365-2362.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- 57.Ashoor G, Kametas NA, Akolekar R, Guisado J, Nicolaides KH. Maternal thyroid function at 11–13 weeks of gestation. Fetal Diagn Ther. 2010;27:156–63. doi: 10.1159/000313301. [DOI] [PubMed] [Google Scholar]
- 58.Lambert-Messerlian G, McClain M, Haddow JE, Palomaki GE, Canick JA, Cleary-Goldman J, et al. FaSTER Research Consortium First- and second-trimester thyroid hormone reference data in pregnant women: a FaSTER (First- and Second-Trimester Evaluation of Risk for aneuploidy) Research Consortium study. Am J Obstet Gynecol. 2008;199:62.e1–6. doi: 10.1016/j.ajog.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersson M, de Benoist B, Delange F, Zupan J, WHO Secretariat Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10(12A):1606–11. doi: 10.1017/S1368980007361004. [DOI] [PubMed] [Google Scholar]
- 60.National Health and Medical Research Council . Iodine supplementation during pregnancy and lactation. Canberra: NHMRC; 2009. [Google Scholar]
- 61.Silva JE, Silva S. Interrelationships among serum thyroxine, triiodothyronine, reverse triiodothyronine, and thyroid-stimulating hormone in iodine-deficient pregnant women and their offspring: effects of iodine supplementation. J Clin Endocrinol Metab. 1981;52:671–7. doi: 10.1210/jcem-52-4-671. [DOI] [PubMed] [Google Scholar]
- 62.Rebagliato M, Murcia M, Espada M, Alvarez-Pedrerol M, Bolúmar F, Vioque J, et al. Iodine intake and maternal thyroid function during pregnancy. Epidemiology. 2010;21:62–9. doi: 10.1097/EDE.0b013e3181c1592b. [DOI] [PubMed] [Google Scholar]
- 63.Glinoer D. The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. Best Pract Res Clin Endocrinol Metab. 2004;18:133–52. doi: 10.1016/j.beem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, van der Wal MF, Bonsel GJ. Ethnic differences in TSH but not in free T4 concentrations or TPO antibodies during pregnancy. Clin Endocrinol (Oxf) 2007;66:765–70. doi: 10.1111/j.1365-2265.2007.02803.x. [DOI] [PubMed] [Google Scholar]
- 65.Walker JA, Illions EH, Huddleston JF, Smallridge RC. Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstet Gynecol. 2005;106:1365–71. doi: 10.1097/01.AOG.0000185475.61612.ea. [DOI] [PubMed] [Google Scholar]
- 66.La’ulu SL, Roberts WL. Second-trimester reference intervals for thyroid tests: the role of ethnicity. Clin Chem. 2007;53:1658–64. doi: 10.1373/clinchem.2007.089680. [DOI] [PubMed] [Google Scholar]
- 67.La’ulu SL, Roberts WL. Ethnic differences in first-trimester thyroid reference intervals. Clin Chem. 2011;57:913–5. doi: 10.1373/clinchem.2010.161240. [DOI] [PubMed] [Google Scholar]
- 68.Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocr Rev. 2013;34:619–57. doi: 10.1210/er.2012-1076. [DOI] [PubMed] [Google Scholar]
- 69.Fisher DA. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clin Chem. 1996;42:135–9. [PubMed] [Google Scholar]
- 70.Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13:1069–78. doi: 10.1089/105072503770867237. [DOI] [PubMed] [Google Scholar]
- 71.Pekonen F, Alfthan H, Stenman UH, Ylikorkala O. Human chorionic gonadotropin (hCG) and thyroid function in early human pregnancy: circadian variation and evidence for intrinsic thyrotropic activity of hCG. J Clin Endocrinol Metab. 1988;66:853–6. doi: 10.1210/jcem-66-4-853. [DOI] [PubMed] [Google Scholar]
- 72.Roti E, Bartalena L, Minelli R, Salvi M, Gardini E, Pistolesi A, et al. Circadian thyrotropin variations are preserved in normal pregnant women. Eur J Endocrinol. 1995;133:71–4. doi: 10.1530/eje.0.1330071. [DOI] [PubMed] [Google Scholar]
- 73.Lee RH, Spencer CA, Mestman JH, Miller EA, Petrovic I, Braverman LE, et al. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol. 2009;200:260.e1–6. doi: 10.1016/j.ajog.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 74.Sapin R, d’Herbomez M. Free thyroxine measured by equilibrium dialysis and nine immunoassays in sera with various serum thyroxine-binding capacities. Clin Chem. 2003;49:1531–5. doi: 10.1373/49.9.1531. [DOI] [PubMed] [Google Scholar]
- 75.Anckaert E, Poppe K, Van Uytfanghe K, Schiettecatte J, Foulon W, Thienpont LM. FT4 immunoassays may display a pattern during pregnancy similar to the equilibrium dialysis ID-LC/tandem MS candidate reference measurement procedure in spite of susceptibility towards binding protein alterations. Clin Chim Acta. 2010;411:1348–53. doi: 10.1016/j.cca.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 76.Estrada JM, Soldin D, Buckey TM, Burman KD, Soldin OP. Thyrotropin isoforms: implications for thyrotropin analysis and clinical practice. Thyroid. 2014;24:411–23. doi: 10.1089/thy.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Unuane D, Velkeniers B, Anckaert E, Schiettecatte J, Tournaye H, Haentjens P, et al. Thyroglobulin autoantibodies: is there any added value in the detection of thyroid autoimmunity in women consulting for fertility treatment? Thyroid. 2013;23:1022–8. doi: 10.1089/thy.2012.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glinoer D, Riahi M, Grün J-P, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J Clin Endocrinol Metab. 1994;79:197–204. doi: 10.1210/jcem.79.1.8027226. [DOI] [PubMed] [Google Scholar]
- 79.Ekinci EI, Chiu WL, Lu ZX, Sikaris K, Churilov L, Bittar I, et al. A longitudinal study of thyroid autoantibodies in pregnancy: the importance of test timing. Clin Endocrinol (Oxf) 2015;82:604–10. doi: 10.1111/cen.12571. [DOI] [PubMed] [Google Scholar]
- 80.Ekinci EI, Lu ZX, Sikaris K, Bittar I, Cheong KY, Lam Q, et al. Longitudinal assessment of thyroid function in pregnancy. Ann Clin Biochem. 2013;50:595–602. doi: 10.1177/0004563213486450. [DOI] [PubMed] [Google Scholar]
- 81.Karakosta P, Chatzi L, Bagkeris E, Daraki V, Alegakis D, Castanas E, et al. First- and second-trimester reference intervals for thyroid hormones during pregnancy in “Rhea” mother-child cohort, Crete, Greece. J Thyroid Res. 2011;2011:490783. doi: 10.4061/2011/490783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhatt GS, Jayasundaram R, Wareth LA, Nagelkerke N, Jayasundaram K, Darwish EA, et al. Thyrotrophin and free thyroxine trimester-specific reference intervals in a mixed ethnic pregnant population in the United Arab Emirates. Clin Chim Acta. 2006;370:147–51. doi: 10.1016/j.cca.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Han SM, Han JH, Park JA, Quinn FA, Park J, Oh E. Longitudinal evaluation of thyroid autoimmunity and function in pregnant Korean women. Clin Chem Lab Med. 2013;51:2295–301. doi: 10.1515/cclm-2013-0598. [DOI] [PubMed] [Google Scholar]
- 84.Larsson A, Palm M, Hansson LO, Axelsson O. Reference values for clinical chemistry tests during normal pregnancy. BJOG. 2008;115:874–81. doi: 10.1111/j.1471-0528.2008.01709.x. [DOI] [PubMed] [Google Scholar]
- 85.Vila L, Serra-Prat M, Palomera E, Casamitjana R, de Castro A, Legaz G, et al. Reference values for thyroid function tests in pregnant women living in Catalonia, Spain. Thyroid. 2010;20:221–5. doi: 10.1089/thy.2008.0264. [DOI] [PubMed] [Google Scholar]
- 86.Cotzias C, Wong SJ, Taylor E, Seed P, Girling J. A study to establish gestation-specific reference intervals for thyroid function tests in normal singleton pregnancy. Eur J Obstet Gynecol Reprod Biol. 2008;137:61–6. doi: 10.1016/j.ejogrb.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 87.Bestwick JP, John R, Maina A, Guaraldo V, Joomun M, Wald NJ, et al. Thyroid stimulating hormone and free thyroxine in pregnancy: expressing concentrations as multiples of the median (MoMs) Clin Chim Acta. 2014;430:33–7. doi: 10.1016/j.cca.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 88.Yan YQ, Dong ZL, Dong L, Wang FR, Yang XM, Jin XY, et al. Trimester- and method-specific reference intervals for thyroid tests in pregnant Chinese women: methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clin Endocrinol (Oxf) 2011;74:262–9. doi: 10.1111/j.1365-2265.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 89.Fan JX, Han M, Tao J, Luo J, Song MF, Yang S, et al. Reference intervals for common thyroid function tests, during different stages of pregnancy in Chinese women. Chin Med J (Engl) 2013;126:2710–4. [PubMed] [Google Scholar]
- 90.Shen FX, Xie ZW, Lu SM, Aw TC, Zhu B. Gestational thyroid reference intervals in antibody-negative Chinese women. Clin Biochem. 2014;47:673–5. doi: 10.1016/j.clinbiochem.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 91.Kurioka H, Takahashi K, Miyazaki K. Maternal thyroid function during pregnancy and puerperal period. Endocr J. 2005;52:587–9. doi: 10.1507/endocrj.52.587. [DOI] [PubMed] [Google Scholar]
- 92.Boas M, Forman JL, Juul A, Feldt-Rasmussen U, Skakkebaek NE, Hilsted L, et al. Narrow intra-individual variation of maternal thyroid function in pregnancy based on a longitudinal study on 132 women. Eur J Endocrinol. 2009;161:903–10. doi: 10.1530/EJE-09-0579. [DOI] [PubMed] [Google Scholar]
- 93.Yu B, Wang Q-W, Huang R-P, Cao F, Zhu Z-Q, Sun D-C, et al. Establishment of self-sequential longitudinal reference intervals of maternal thyroid function during pregnancy. Exp Biol Med (Maywood) 2010;235:1212–5. doi: 10.1258/ebm.2010.010136. [DOI] [PubMed] [Google Scholar]
- 94.Springer D, Bartos V, Zima T. Reference intervals for thyroid markers in early pregnancy determined by 7 different analytical systems. Scand J Clin Lab Invest. 2014;74:95–101. doi: 10.3109/00365513.2013.860617. [DOI] [PubMed] [Google Scholar]
- 95.Springer D, Zima T, Limanova Z. Reference intervals in evaluation of maternal thyroid function during the first trimester of pregnancy. Eur J Endocrinol. 2009;160:791–7. doi: 10.1530/EJE-08-0890. [DOI] [PubMed] [Google Scholar]
- 96.Pearce EN, Oken E, Gillman MW, Lee SL, Magnani B, Platek D, et al. Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr Pract. 2008;14:33–9. doi: 10.4158/EP.14.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elecsys thyroid tests Mannheim. Germany: Roche Diagnostics Gmbh; 2004. Reference intervals for children and adults. [Google Scholar]
- 98.Khalid AS, Marchocki Z, Hayes K, Lutomski JE, Joyce C, Stapleton M, et al. Establishing trimester-specific maternal thyroid function reference intervals. Ann Clin Biochem. 2014;51:277–83. doi: 10.1177/0004563213496394. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J, Li W, Chen Q-B, Liu L-Y, Zhang W, Liu M-Y, et al. Establishment of trimester-specific thyroid stimulating hormone and free thyroxine reference interval in pregnant Chinese women using the Beckman Coulter UniCel™ DxI 600. Clin Chem Lab Med. 2015;53:1409–14. doi: 10.1515/cclm-2014-0615. [DOI] [PubMed] [Google Scholar]
- 100.Kratzsch J, Fiedler GM, Leichtle A, Brügel M, Buchbinder S, Otto L, et al. New reference intervals for thyrotropin and thyroid hormones based on National Academy of Clinical Biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem. 2005;51:1480–6. doi: 10.1373/clinchem.2004.047399. [DOI] [PubMed] [Google Scholar]


