Abstract
Purpose
The current study aims to evaluate a porous silicon-based drug delivery system meant for sustained delivery of dexamethasone (Dex) to the vitreous and retina.
Methods
Dexamethasone was grafted covalently into the pore walls of fully oxidized porous silicon particles (pSiO2-COO-Dex), which then was evaluated for the pharmacological effect of the payload on cultured ARPE19 cells before intravitreal injection. The Dex release profile was investigated in a custom designed dynamic dissolution chamber to mimic the turnover of vitreous fluid in rabbit eyes. Ocular safety, in vivo release, and pharmacodynamics were evaluated in rabbit eyes, and the human VEGF-induced rabbit retinal vascular permeability model.
Results
Loading efficiency of Dex was 69 ± 9 μg per 1 mg of the pSiO2-COO-Dex particles. Dynamic in vitro release demonstrated a sustained mode when compared to free Dex, with the drug half-life extended by 5 times. The released Dex was unaltered and biologically active. In vivo drug release in rabbit eyes revealed a mode similar to the release seen in vitro, with a vitreous half-life of 11 days. At 2 and 4 weeks after a single intravitreal injection of pSiO2-COO-Dex particles (mean 2.71 ± 0.47 mg), intravitreal 500 ng of VEGF did not induce significant retinal vessel dilation or fluorescein leakage, while these events were observed in the eyes injected with empty pSiO2 particles or with free Dex. The retinal vessel score from fluorescein angiography for the control eyes was double the score for the eyes injected with pSiO2-COO-Dex. No adverse reaction was observed for the eyes injected with drug-loaded pSi particles during the course of the study.
Conclusions
The porous silicon-based Dex delivery system (pSiO2-COO-Dex) can be administered safely into vitreous without toxicity. Dex release from the porous silicon particles was sustained for 2 months and was effective against VEGF-induced retinal vessel reaction.
Keywords: porous silicon, mesopores, controlled drug release, intravitreal drug delivery, dexamethasone, rabbit eye
Retinal vasculature diseases have become more prevalent as a result of the aging population.1 The population over age 40 has increased by 20% from years 2000 to 2010 in the United States (data available in the public domain at http://www.visionproblemsus.org/introduction.html). Vision loss from macular edema or scarring associated with retinal neovascularization has become the leading cause of blindness in people aged 50 years or older. For example, incidences of diabetic macular edema2,3 and macular edema from retinal vein occlusions4,5 have been on the rise and the expenditure associated with their medical care is challenging for the medical community and the patients it serves.6 Even in a booming era of biological therapeutics, steroids still are a major player in the medical care of these refractory eye diseases due to their well-established efficacy at a fraction of the expense of anti-VEGF biological therapeutics.7–9 Triamcinolone acetonide (TA) is a naturally slow-release formulation of steroid and has been injected intravitreally for various retinal diseases.10 However, intravitreal TA is associated with a high incidence of elevation of IOP, which has limited its application. Dexamethasone (Dex) is a cortical steroid that has a very short vitreous half-life11 compared to the depository steroid TA.12,13 However, Dex is more potent than TA, and the recently Food and Drug Administration (FDA) approved long-acting Dex delivery system Ozurdex appears to cause less ocular hypertension and cataract formation than intravitreal TA or intravitreal implants of fluocinolone acetonide (Retisert).14 Dex has been used for treatment of posterior segment eye diseases including uveitis, proliferative vitreoretinopathy, diabetic macular edema, and wet form of age-related macular degeneration.15,16 There is considerable interest in delivering Dex to the back of the eye by sustained release systems.17–20 Poly(lactic-co-glycolic acid) (PLGA) microparticles and nanoparticles have been investigated for sustained delivery of Dex through intravitreal injection.17,21 The advantages of particulate formulations over implants, such as Ozurdex or Iluvien, are that the particles can be delivered through a traditional fine needle (27 gauge or smaller) to avoid invasive surgery or the use of a larger needle, such as 22 gauge for Ozurdex or 25 gauge for Iluvien. In addition, microparticulate formulations have less risk of migrating into the anterior chamber, mitigating the risk of corneal or IOP issues that are reported commonly with injectable implants, such as Ozurdex and Iluvien.22–24 Anterior migrated implant requires surgical repositioning or removal due to corneal complications.22–24 The Ozurdex or Iluvienis meant to treat macular diseases at the posterior segment but the implant localizes at the anterior segment, which not only decreases its therapeutic efficacy, but also potentially increases the risk of complications, such as cataract formation and IOP elevation in addition to the risk of migration into anterior chamber.
In the current study, we investigated pharmacokinetics and pharmacodynamics of the engineered oxidized porous silicon (pSiO2) particles loaded with Dex in vitro and in vivo. The porous silicon (pSi) we propose to use in this study differs from the most commonly used biomaterial, such as PLGA. The pore size distribution of pSi is very narrow and the pore diameter can be tuned between 10 and 100 nm. These features allow us to adjust the loading of different drug molecules and to study the ocular kinetics of drug release with high precision. In addition, pSi is more resistant to heat, pH, mechanical stress, and hydrolysis-induced degradations. The goal of the study was to characterize this novel intravitreal delivery system of Dex in a rabbit eye model.
Materials and Methods
Synthesis of pSiO2 Microparticles
Microparticles of pSiO2 were prepared by thermal oxidation of pSi microparticles. The pSi microparticles were prepared by anodic electrochemical etch of highly doped, (100)-oriented, p-type silicon wafers (boron-doped, 1.29 mΩ·cm resistivity; Siltronix, Inc., Archamps, France), as previously described.25 A silicon wafer with an exposed area of 8.04 cm2 was contacted on the backside with a strip of aluminum foil and mounted in a Teflon etching cell that was fitted with a platinum counter-electrode. The silicon wafers were etched in a 3:1 (vol/vol) solution of 48% aqueous hydrofluoric acid (HF) and absolute ethanol (Thermo Fisher Scientific, Pittsburgh, PA, USA) at a constant current density (112 mA/cm2) for 200 seconds. The porous layer resulting from each etch was removed from the silicon substrate by electropolishing in a 1:29 solution of 48% aqueous HF and absolute ethanol for 100 seconds. The films were harvested every 4 etches and the resulting porous layers were ultrasonicated in ethanol (FS5 dual action ultrasonic cleaner, Thermo Fisher Scientific) for 120 minutes to form pSi particles. After ultrasonic treatment, the supernatant was removed and the particles were resuspended in ethanol. This procedure was repeated three times until the supernatant was transparent. The pSi particles were isolated, dried at room temperature, and stored under vacuum in a desiccator. The pSi particles then were converted to pSiO2 particles by high temperature air oxidation. The pSi particles were heated from room temperature to 800°C inside a muffle furnace (Thermolyne FD1535M; Thermo Fisher Scientific), and maintained at 800°C for 2 hours.
The particle thickness and open porosity were calculated by optical measurements of the reflectivity spectrum on the pSi layer (before removal from the silicon substrate) as a function of liquid infiltration using the spectroscopic liquid infiltration method (SLIM).26 Average particle size was calculated from digital photographs of particles obtained under an optical microscope by measuring width (shorter dimension) and length (longer dimension) of 100 randomly selected particles (without overlap) using Image J (National Institutes of Health [NIH], Bethesda, MD, USA). Approximate average pore size of the particles was determined from scanning electron microscope (SEM) plan-view images of randomly selected particles (n = 30 ) using a Phillips XL30 field emission electron microscope operating at an accelerating voltage of 5 kV (FEI Phillips, Hillsboro, OR, USA).
Dex Loading Into pSiO2 Microparticles
Dex was loaded into the particles through covalent attachment by creating a chemical bond between the drug and functional groups placed on the particle surface, as previously described.27 Briefly, pSiO2 particles then were treated with an aqueous HCl solution (2% concentrated HCl by volume) for 1.5 hours, rinsed two times with water and ethanol, and dried. The particles then were vortexed in an ethanol solution 2% in 3-aminopropyltrimethoxysilane (Sigma-Aldrich Corp., St. Louis, MO, USA) for 2 hours, rinsed with ethanol, and dried, resulting in alkylamine-modified particles. The amine-functionalized pSiO2 particles were reacted with 0.2 M succinic anhydride (99%, Sigma-Aldrich Corp.) in N,N-dimethylformamide (DMF; Sigma-Aldrich Corp.) for 16 to 17 hours and rinsed with DMF and ethanol to obtain a carboxylic acid functional surface (pSiO2-CO2H). The surface carboxyl species then were activated by treatment with an aqueous solution containing 13 mg dicyclohexylcarbodiimide (DCC; Sigma-Aldrich Corp.), 3 mg 4-N,N-dimethylamminopyridine (DMAP; Sigma-Aldrich Corp.) and 1 mL dichloromethane (DCM; Thermo Fisher Scientific) for 20 minutes. Dex then was coupled to the activated surface by addition of 4 mg free Dex (Sigma-Aldrich Corp.) to the mixture. The tubes were sealed with paraffin film, protected from direct sunlight with aluminum foil wrapping, and rotated at room temperature for 7 days. After the loading procedure, the particles were pelletized by centrifugation and rinsed with DCM and ethanol five times to remove any unloaded drug and excess linker reagent. The presence of the functional linker on the particle surface and the successful covalent attachment of Dex to the microparticles were confirmed using attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy (Nicolet 6700 FTIR with a Smart iTR diamond ATR fixture; Thermo Fisher Scientific).
Drug loading efficiency was analyzed by thermogravimetry (TGA) as shown previously.25 The pSiO2-CO2H (pSiO2 with carboxylic acid functional surface) and pSiO2-COO-Dex (pSiO2 containing Dex covalently attached to the pore walls) samples (3 mg each) were placed in two 90 μL alumina sample cups, respectively. Samples then were heated at a constant rate of 10°C/minute up to 800°C in nitrogen atmosphere with a purge rate of 10 mL/min using a Q600 simultaneous TGA/DSC apparatus (TA Instruments, New Castle, DE, USA). Weight percent loading efficiency of Dex in the samples was determined by analyzing TGA curves of pSiO2-CO2H as well as pSiO2-COO-Dex.
In Vitro Drug Release Study
Dynamic drug release was simulated in vitro using a custom-designed flow chamber and a syringe pump as described previously.25 The dissolution chamber is made of poly methyl methacrylate. Inlet tubing connects from a syringe pump to the upper part of the chamber, and the outlet tubing leaves from the lower part of the chamber to the sample collector. In the center of the dissolution chamber, a titanium mesh circle is installed to support a 10-mm diameter filter paper (with 0.22-μm pore and disposable) to prevent large drug particles from occluding the outgoing tubing. The volume of this dissolution chamber is 1.5 mL to mimic rabbit vitreous volume28 and flow rate was set at 1 μL/min using an NE-1000 syringe pump (New Era Pump Systems, Farmingdale, NY, USA) to mimic rabbit vitreous fluid turnover.29 pSiO2-COO-Dex (6 mg) was weighed into a small tube and suspended in 100 μL HBSS (without calcium and magnesium) immediately before injection into the flow chamber. The entire setup was maintained at 37°C. After injection, leftover particles in tubes and syringes were carefully collected, rinsed with deionized water and ethanol, dried in vacuum, and weighed to determine the real dose in the release system. As a control, release of equivalent doses of free Dex was performed in parallel using the same release system of the flow chamber. During the 60-day release period, the infusate was collected at the same time every day. The samples were stored at −80°C until the analysis by high-pressure liquid chromatography with ultraviolet detector (HPLC-UV). Ketorolac (HPLC grade; Cayman Chemical Company, Ann Arbor, MI, USA) was spiked into a 100 μL sample as an internal standard. Pure HBSS was used as a blank control.
In Vitro Bioactivity Assay of the Dex Released From pSiO2-COO-Dex
To test the biological activity of the released Dex, the collected infusate containing Dex was used to inhibit VEGF expression in RPE cells that were stimulated by platelet-derived growth factor (PDGF). The infusate was stored under −80°C for 6 weeks before testing on the cell line. ARPE-19 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's medium: Nutrient Mixture F-12 (Corning, Corning, NY, USA) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Grand Island, NY, USA) and penicillin/streptomycin (Life Technologies). Cells were seeded at a density of 40,000 cells per well of 24-well tissue culture plates and grown to 80% confluence. At 24 hours ahead of test sample addition, FBS in the culture medium was reduced to 2%. The cells then were incubated in 2% FBS media containing 2.5 ng/mL PDGF-BB along with either commercial Dex (Sigma-Aldrich) or the sample from the in vitro release, at concentrations of 100, 40, and 10 ng/mL. After 15 hours of incubation, the supernatants were collected and assayed for VEGF concentrations using Human VEGF Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA).
In Vivo Pharmacokinetic Study
We used 21 New Zealand Red rabbits to study the intraocular pharmacokinetics of pSiO2-COO-Dex particles. All animal handlings were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the studies were approved by the institutional animal care and use committee of The University of California, San Diego, California, United States. Only one eye of each animal was used for pSiO2-COO-Dex intravitreal injections. For the intravitreal injection procedure, the rabbits were anesthetized with intramuscular injections of 20 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA) and 5 mg/kg xylazine (Akorn, Inc., Decatur, IL, USA). Slit-lamp and indirect ophthalmoscopy were performed on all animal eyes before injection. Baseline fundus images were recorded with a digital camera (Cannon T2i; Cannon, Inc., Tokyo, Japan) with camera setting of F-stop f/16, exposure time 1/30 seconds, ISO auto, no flash. After eye disinfection procedures, pSiO2-COO-Dex particles suspended in 150 μL sterile balanced salt solution (BSS) were injected into the mid vitreous cavity of right eyes using a 1-mL syringe and 27-gauge ½-inch needle under direct view of a surgical microscope. Considering the inherent variation of the pSi particle suspension, particles remaining in the tubes and syringes after injection in the corresponding rabbit were carefully collected, rinsed with deionized water, dried in vacuum, and weighed to calculate the actual injected dose. The target dose was 3 mg, and the average actual injected dose was 2.71 ± 0.47 mg. The eyes were examined at days 3, 7, 14, 21, 28, 35, and 56 after injection using slit-lamp biomicroscopy, a handheld tonometer (Tonopen; Medtronic, Jacksonville, FL, USA) for IOP, and indirect ophthalmoscopy. Color fundus photographs were obtained at each exam. Electroretinogram (ERG) was performed on each animal at days 7, 21, 35, and 56 before euthanized. Three rabbits were killed at each time point. After euthanization, eye globes were enucleated and dissected. After the cornea and lens were removed, vitreous was sampled using a 3 mL syringe as described previously.25 Whole vitreous was centrifuged for 20 minutes at 14,674g, and vitreous supernatant was subjected to HPLC-UV analysis as described above. Normal rabbit vitreous supernatant was used as a blank control.
Inhibition of VEGF-Induced Retinal Vascular Leakage in Rabbit Model
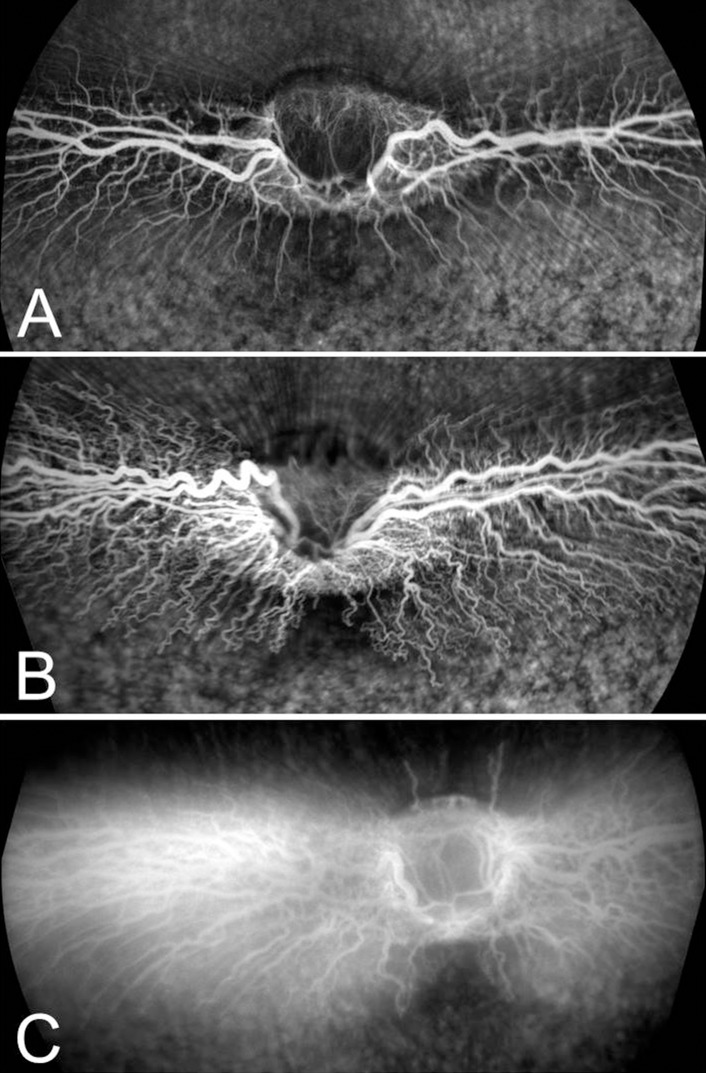
We used 24 New Zealand Red rabbits to induce retinal vascular response from external injection of VEGF to evaluate the efficacy of pSiO2-COO-Dex. Only one eye of each animal was used. Eyes of nine rabbits were injected with pSiO2-COO-Dex as the treatment group. The average actual injected dose was 2.31 ± 0.61 mg per eye. The other nine rabbits were injected with empty pSiO2 as a control group, and an additional six rabbits were injected with free Dex (0.16 mg/eye, equal to the quantity of Dex contained in 2.31 mg of pSiO2-COO-Dex) as the free-Dex control group. Clinical examination was performed every week. Two or four weeks after the initial injection, recombinant human VEGF165 (R&D Systems, Minneapolis, MN, USA) was injected intravitreally to challenge retinal vascular barriers.30 Six rabbits each for the pSiO2-COO-Dex and empty pSiO2 control groups and three rabbits for the free Dex group were used for the 2-week time point. For the 4-week time point, three rabbits each were used for the three study groups. After anesthesia and eye disinfection preparations, approximately 0.1 mL aqueous humor was removed by anterior chamber paracentesis before VEGF (500 ng in 0.1 mL) intravitreal injection. At 48 hours later, each rabbit was examined using slit-lamp biomicroscopy and indirect ophthalmoscopy. Color fundus photographs were obtained. Afterwards, rabbits were anesthetized and fundus fluorescein angiogram (FA) was performed to detect vascular abnormality. The 55° wide-field FA and ocular coherence tomography (OCT) images were obtained with an SD-OCT/SLO Spectralis imaging system (Heidelberg Engineering, Inc., Heidelberg, Germany). Angiograms were obtained at 1, 3, 5, and 10 minutes after intravenous injection (100 μL 12.5% sodium fluorescein). Two independent ophthalmologists reviewed FA images and late phase (10-minutes) images were used to score the abnormality of retinal vasculature. A score of 1 was assigned to eyes with normal presentation of the medullary ray vasculature, 2 was given to eyes with vessels showing obvious vasodilation and tortuosity, and 3 was assigned to eyes with vessels having obvious fluorescein leakage (Fig. 1).
Figure 1.
Grading standard of FA images. (A) Score 1, normal vasculature. (B) Score 2, vasodilation and tortuosity. (C) Score 3, fluorescein leakage from the retinal vessels.
After all the examinations, rabbits were killed and the eye globes were enucleated and fixed into cold 4% glutaraldehyde. Five minutes later, two 1-mm punches at 3 and 9 o'clock were made 2 mm behind the limbus to facilitate better fixative penetration into the eye globe. After 48 hours of fixation, the eye globes were dissected vertically for standard processing for paraffin embedding and H&E staining.
Statistical Analysis
For the in vivo pharmacokinetic study, drug concentration data from each rabbit was normalized using the median injection dose (2.7 mg). The pharmacokinetic data were analyzed using Phoenix WinNonlin 64 (Pharsight, Certara USA, Inc., Princeton, NJ, USA) and IOP was recorded multiple times at different time points from both eyes; hence, a paired t-test was used. The pooled vascular leakage grading scores from the different groups and different time points (2 and 4 weeks) were analyzed using the ordinal regression statistical method. All analyses were performed using JMP statistical software (version 10; SAS Institute, Inc., Cary, NC, USA) and P values smaller than 0.05 were considered significant.
Results
Characterization of the Particle Texture
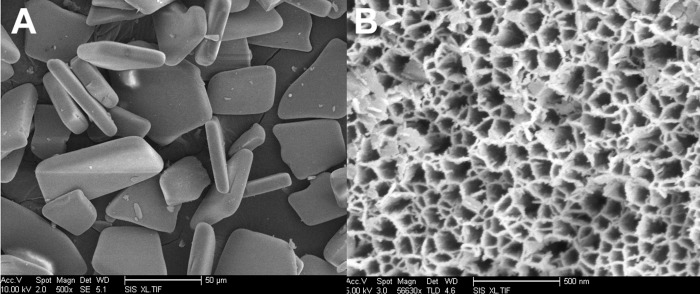
The average particle width was 20 ± 5 μm and length 36 ± 12 μm. The SLIM measurement indicated the thickness of the microparticles to be 14.2 ± 0. 3 μm, and the average porosity to be 50 ± 3%. Scanning electron microscopic (Fig. 2) measurements revealed that the average pore size was 25 ± 10 nm.
Figure 2.
Scanning electron microscopic images showing the overall shape and size of the microparticles (A) and the porous nanostructure (B).
Drug Loading by Covalent Attachment
Dex covalent attachment to the pSi microparticles was confirmed by FTIR. Characteristic vibrational bands for the surface linker and drug were observed at approximately 1721, 1663, 1632, and 1548 cm−1 as we reported previously.27 The Dex loading efficiency in pSiO2-COO-Dex particles as calculated by TGA was 69 ± 9 μg/mg.
In Vitro Release
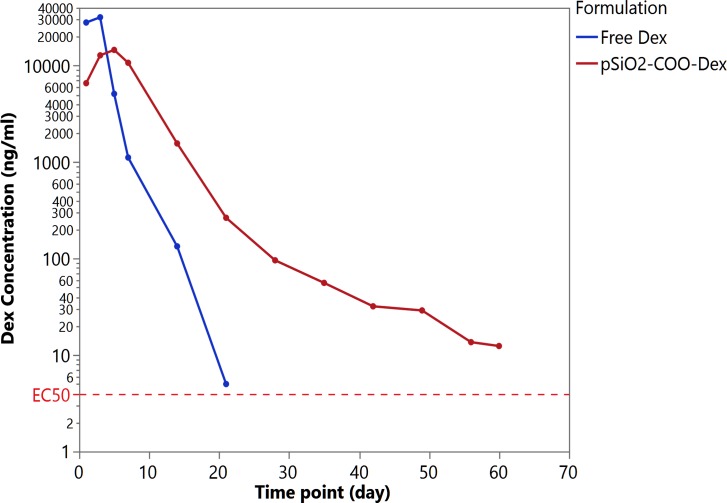
The actual doses of pSiO2-COO-Dex and free Dex for the dynamic in vitro release studies were 3.35 and 0.23 mg. After a 60-day in vitro release, the pSiO2-COO-Dex particles demonstrated a 92% decrease by weight (0.28 mg remaining), indicative of pSi degradation and Dex release in the dissolution chamber over time. The peak Dex concentration from the equivalent free Dex was 31,810 ng/mL and dropped below 1 ng/mL within 3 weeks. In contrast, the peak Dex concentration for pSiO2-COO-Dex was 14,594 ng/mL and still was at a therapeutic level (12.4 ng/mL) at the end of the 60-day release study. The pSiO2-COO-Dex formulation demonstrated a sustained release mode with a terminal half-life of 7.4 days, which is substantially longer than the half-life (1.5 days) of free Dex in the release chamber (Fig. 3).
Figure 3.
Dynamic in vitro release. For free Dex formulation (blue trace), release profile is first-order. For pSiO2-COO-Dex (red trace), a burst release is observed during days 1 to 3. Afterwards, the Dex release was sustained until the end of the 60-day observation period. EC50 = half-maximal effective concentrations in trans-activation assay of three cell lines.31
In Vitro Bioactivity Assay
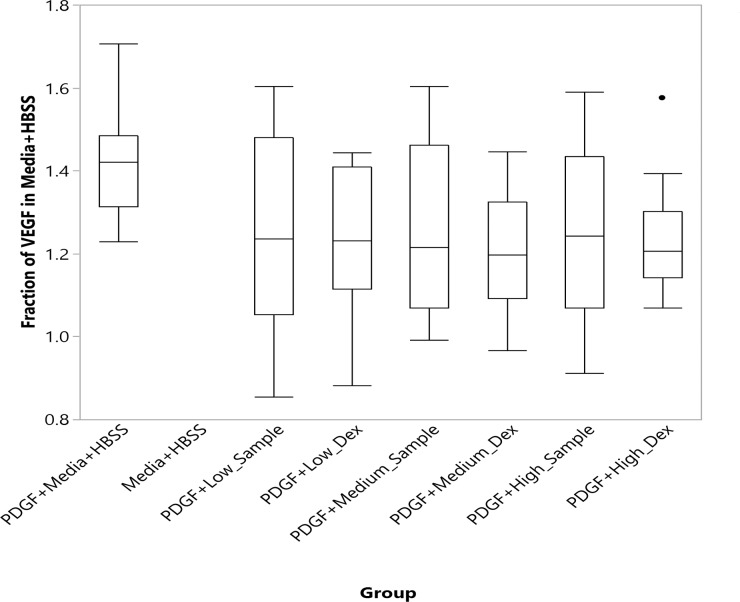
The VEGF level in each well of each dose- and sample-group was normalized using the mean VEGF level of the wells with media + HBSS. The VEGF expression level is quantified in Figure 4. The VEGF level in PDGF + Media + HBSS was significantly higher than all other groups with addition of Dex (P < 0.05, nonparametric comparisons for each pair using the Wilcoxon method). The groups with added Dex, either from in vitro release samples or from commercial Dex, were not statistically different from each other (Fig. 4).
Figure 4.
Vascular endothelial growth factor expression levels in the cultured ARPE19 cells stimulated with PDGF with absence or presence of dexamethasone addition.
Ocular Pharmacokinetics of Intravitreal Injected PSiO2-COO-Dex
For the pharmacokinetics study, the actual dose of PSiO2-COO-Dex injected (based on mass balance) was 2.71 ± 0.47 mg. During 56 days of clinical observation, no signs of cataracts, high IOP, or toxicity were observed. Compared to the noninjected fellow eyes, the injected eyes showed similar IOP and ERG readings (Treated Eye-Control Eye IOP = −0.56 mm Hg, P = 0.1, paired t-test; Dark-adapted ERG b wave amplitude Treated Eye-Control Eye = 8.05 μV, P = 0.1, paired t-test; Light adapted ERG b wave amplitude Treated Eye-Control Eye = 1 μV, P = 0.86, paired t-test). After the injection, fundus images revealed that the rabbit vitreous remained clear and the number of PSiO2-COO-Dex particles decreased gradually over the course of 56 days (Fig. 5).
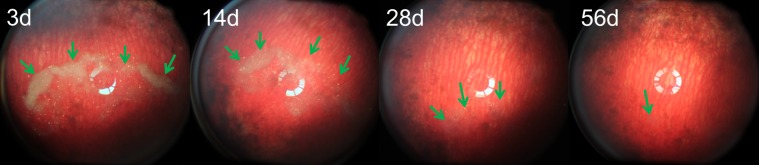
Figure 5.
Fundus images from a rabbit obtained at multiple time points, showing a decrease in the number of visible particles and a gradual decrease in particle opacity over time (arrows). The vitreous and retina appeared normal. At day 56, the pSiO2-COO-Dex particles still were visible at the bottom of the vitreous cavity by indirect ophthalmoscope.
The vitreous samples showed a sustained release of Dex over time, with the peak concentration being 543 ng/mL and the minimum concentration being 7 ng/mL at day 60. The in vivo drug release profile is shown in Figure 6; the profile was similar to the in vitro release data. A one-compartment pharmacokinetic model yielded a half-life of Dex in the rabbit vitreous of 10.98 days.
Figure 6.

Dexamethasone concentration detected by HPLC-UV in the rabbit vitreous supernatant. Dexamethasone release was sustained until the end of the 56-day observation period. By day 56, the Dex concentration in the vitreous supernatant was 7.01 ± 0.88 ng/mL.
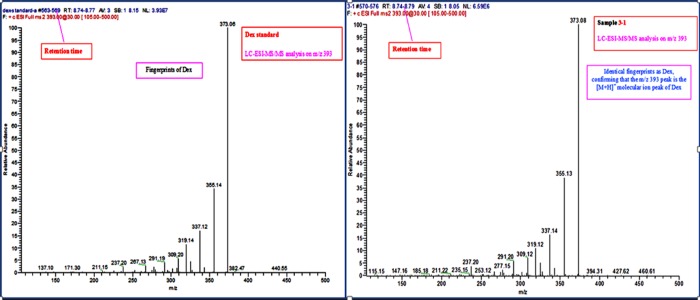
To confirm the identity of the Dex detected in rabbit vitreous, liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) was performed on randomly selected vitreous supernatant samples. The data revealed a single peak at a retention time of 8.74 to 8.79 minutes and a protonated molecular ion peak [M+H]+ at m/z 393. Analysis of LC-ESI-MS/MS on the peak revealed identical fingerprints to a Dex standard. Representative LC-ESI-MS/MS chromatograms of standard Dex (commercial Dex) and the Dex released from pSiO2-COO-Dex are shown in Figure 7.
Figure 7.
Analysis of LC-ESI-MS/MS showing identical signatures for the molecule derived from the in vivo sample (right) compared to a commercial Dex sample (left), confirming the identity of the Dex released from pSiO2-COO-Dex.
Inhibition of VEGF-Induced Vascular Leakage by pSiO2-COO-Dex
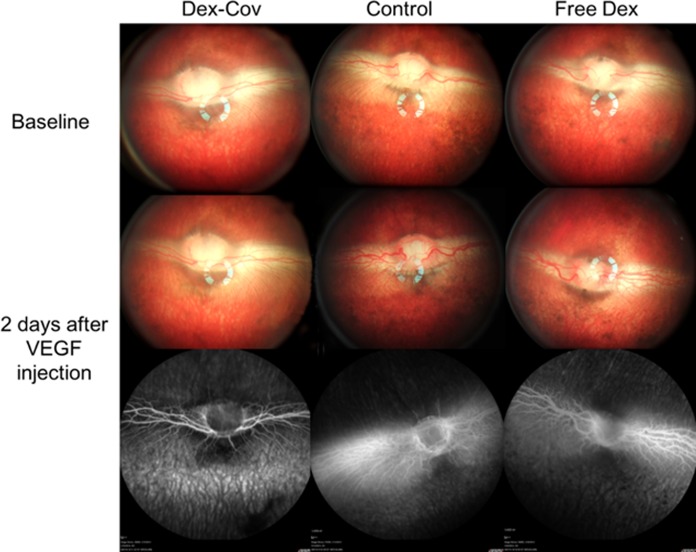
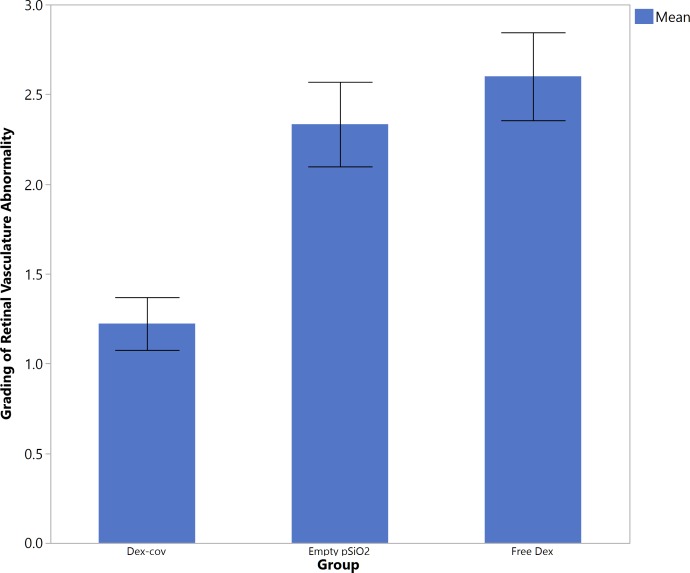
Clinical observation 48 hours after intravitreal injection of 500 ng recombinant human VEGF165 revealed pronounced vasodilation and vessel tortuosity in the medullary ray region relative to the baseline (Fig. 8) in all rabbits in the free Dex group and empty pSiO2 control group (Fig. 8, middle). Fluorescein angiography showed corresponding vascular changes in the early phase; and two-thirds of those eyes showed fluorescein leakage in the late phase (Fig. 8, bottom). In contrast, the eyes with intravitreal pSiO2-COO-Dex (Dex-Cov) showed normal retinal vasculature on color fundus photo and FA. Statistical analysis revealed pooled vascular scores (from 2- and 4-week) for the pSiO2-COO-Dex group were significantly smaller than that of the other two groups (Dex-cov 1.22 ± 0.44 versus Empty pSiO2 2.33 ± 071 or versus Free Dex 2.6 ± 0.55, P = 0.0023, Kruskal-Wallis Test; Fig. 9). The retinal vessel scores before injection of VEGF (2 vs. 4 weeks = 2 ± 0.85 vs. 1.88 ± 0.83) were not significantly different (P = 0.73, the Wilcoxon test).
Figure 8.
Color fundus and FA images of rabbits at 4-week time point showing the effect of added VEGF on treated and untreated eyes. Vascular endothelial growth factor was administered by intravitreal injection 4 weeks after the injection of therapeutic pSiO2-COO-Dex (“Dex-Cov”), empty pSiO2 (“Control”), or free dexamethasone (“Free Dex”), as indicated. Severe vessel dilation, tortuosity, and leakage were induced by VEGF in the control and free Dex groups, whereas the pSiO2-COO-Dex group appears normal. Top (“baseline”) corresponds to treated eyes before VEGF injection (4 weeks after therapeutic injection), and the bottom two panels are images obtained 2 days after VEGF injection (4 weeks + 2 days after therapeutic injection).
Figure 9.
The pooled average scores of the retinal vessels from 2- and 4-week time points (mean score of the medullary ray vasculature), read from FA of retina. Data obtained 2 days after VEGF injection, including data at 2 and 4 weeks following the intravitreal injection of pSiO2-COO-Dex (“Dex-Cov”), nondrug-loaded control particles (“empty pSiO2”), or free dexamethasone (“Free Dex”), as indicated. Error bars: indicate 1 SEM.
Discussion
In the current study, the pSiO2 particles were delivered through a 27-gauge needle through the pars plana and the Dex-loaded pSiO2 particles were deposited in the posterior vitreous. The vitreous was clear after injection and the particles were far from the optical axis. The size of the pSiO2 particles was on the order of several micrometers, and the particles did not remain suspended in the vitreous for long. The pSiO2 microparticles were not observed to be internalized by retinal cells and no retinal cell toxicity was observed. pSi or pSiO2 has demonstrated good biocompatibility in animal studies, including in the eye environment.32,33 The pSiO2 particles degrade into silicic acid in the vitreous and the silicic acid is cleared out of the eye through vitreous and aqueous pathways.34
Though PLGA microparticles or nanoparticles have been an active research topic for sustained delivery of Dex through the vitreous route,35–37 to date the only FDA-approved PLGA delivery device is Ozurdex, developed by Allergan, Inc. (available in the public domain at http://www.drugs.com/history/ozurdex.html). This product is a PLGA pellet loaded with Dex, designed for sustained treatment of diabetic or retinal vein occlusive macular edema and uveitis affecting the posterior segment. Though the regimen for Ozurdex reinjection is disease- and study-dependent,38,39 this device is reported to provide approximately 2 months of effective vitreous Dex.40 After 60 days the concentration of Dex in the vitreous quickly drops below the therapeutic level.40 Compared to Ozurdex, the current pSiO2 Dex delivery system provided a similar residence time and concentration of Dex in the vitreous. However, they differ in Tmax (the time following drug administration at which the peak concentration, Cmax, occurs) and in the tapering profile of free Dex in the vitreous. Ozurdex has a Tmax of 60 days and a rapid decrease in drug level thereafter.40 In contrast, the pSi-Dex delivery system displayed a gradual tapering of the Dex level and a Tmax of 3 days in the vitreous, which was the first sampling time point. Currently, it is unclear what the most favorable vitreous kinetics of Dex for treatment of a chorioretinal disease might be. However, free Dex in vitreous was between 200 and 300 ng/mL for 60 days following Ozurdex implant. In contrast, free Dex in vitreous of this study was higher at the beginning (first few days) but below 100 ng/mL for over 80% of the study period. We reasoned that a higher initial concentration will quickly suppress the disease as an induction therapy and subsequent lower drug levels will maintain the therapeutic effect as maintenance therapy. This type of treatment strategy may reduce steroid-related ocular side effects, such as cataract formation and incidence of high IOP associated with constant high level of steroid in vitreous (approximately 1000 ng/mL for a month or longer) as seen with conventional intravitreal injection TA.41
It is noteworthy that in the current study, the in vivo pharmacokinetics closely mirrored the dynamic in vitro pharmacokinetic simulation, though the Dex levels were higher in the first 3 weeks for the in vitro dynamic release study. Due to the high capacity of a living eye to eliminate externally introduced drugs, Dex levels in the first 3 weeks of the in vivo release study were much lower than that seen in the in vitro simulation. This study also validated our in vitro dynamic drug release system, including the custom dissolution chamber matching the volume of a rabbit vitreous, and the fluid turnover rate of 1 μL/min provided by a syringe pump. This flow chamber was custom-designed to mimic fluid turnover in rabbit eyes, the PSiO2-COO-Dex particles were exposed to constant shear forces from the circulating irrigation fluid at 37°C. In a human eye, fluid turnover may be different due to the volume difference (4.5 vs. 1.5 mL) and the species difference. In addition, the current study used HBSS instead of vitreous, which is more viscous. These difference or factors should be taken into consideration when generalizing these findings to human eye or different species. This pSiO2-based Dex delivery system provides a much needed, controlled release of Dex compared to the traditional single intravitreal injection of Dex. For example, in the study of Shen et al.,20 a single intravitreal injection of 400μg dexamethasone sodium phosphate (equivalent to 304 μg Dex) in a normal rabbit eye, the Dex level in the vitreous humor dropped to approximately 0.3 μg/mL within 24 hours. By contrast, in the current study, injection of 2.7 mg PSiO2-COO-Dex (equivalent to 188 μg Dex) resulted in a Dex concentration in the vitreous humor of 0.2 μg/mL 1 week later. Thus, it appears that we can achieve a therapeutic concentration of Dex for longer than commercial systems using lower amounts of drug. One of the dangers of a sustained drug delivery system is that prolonged maintenance of even a low level of drug outside the targeted area may cause side effects. Therefore, it is imperative to confine the therapeutics to the disease site as much as possible. We postulate that this pSiO2-based Dex delivery system will result in lower levels of Dex around the lens and in the aqueous humor than might be achieved from an Ozurdex implant, because pSiO2-COO-Dex particles are injected into the posterior vitreous cavity, while Ozurdex is injected in the vitreous base that is next to the lens. It has been reported that steroid-related cataract formation and high IOP is dependent on drug level.42 Ozurdex was reported to result in approximately 20% incidence of cataract formation and high IOP within a 12-month period following two injections.43 We are aware that steroid-induced high IOP and cataract formation do not reliably occur in animal models except for bovine and sheep eyes.44,45 In the current study, the lens and IOP were not monitored.
Dex is a synthetic steroid that is 5 times more potent than the commonly used triamcinolone for retinal diseases and 25 times more potent than the physiological steroid hormone cortisone in humans. However, it has been reported that Dex readily degrades with a half-life of approximately 10 days at room temperature (Bourne D, et al. IOVS 2015;56:ARVO E-Abstract 4154). In the current study, in vitro and in vivo samples for HPLC-MS analysis were frozen at −80°C soon after collection. The subsequent analysis confirmed the identity of Dex, and the in vitro bioactivity assay confirmed the equivalent bioactivity to commercial Dex controls in suppressing VEGF expression. There was no dose response in the in vitro bioactivity assay; presumably, all doses were sufficient to suppress the cultured cells, but the range of Dex concentrations studied was too narrow for a substantial dose response to manifest. For the in vivo eye disease model, we chose VEGF intravitreal injection because upregulation of VEGF is confirmed in several important chorioretinal diseases, such as age-related macular degeneration, diabetic retinopathy, and retinal vein occlusive macular edema. Intravitreal injection of 500 ng of VEGF caused retinal vessel dilation, increased vessel tortuosity, and fluorescein leakage in the control eyes, which were observed under color fundus photography and fundus FA, respectively. In contrast, pSiO2-COO-Dex injected eyes demonstrated minimal retinal vessel abnormalities. This suggests the controlled release of Dex with this delivery system is sufficient to suppress the deleterious effects of elevated VEGF levels in the eye.
In summary, the current study demonstrated an effective sustained intravitreal Dex delivery system using porous silicon dioxide as the carrier. The controlled release of Dex was sustained for at least 8 weeks without apparent ocular toxicity. We believe that by further engineering the porous silicon dioxide matrix the drug release course may be extended well beyond 2 months. Such an optimized delivery system may significantly impact the current treatment of chorioretinal diseases.
Acknowledgments
Supported by National Institutes of Health (NIH) Grant NIH EY020617.
Disclosure: H. Hou, None; C. Wang, None; K. Nan, None; W.R. Freeman, Spinnaker Biosciences (C, I), P; M.J. Sailor, Spinnaker Biosciences (I), P; L. Cheng, Spinnaker Biosciences (C, I), P
References
- 1. Klein R,, Klein BEK. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013; 54;ORSF5–ORSF13. [DOI] [PMC free article] [PubMed]
- 2. Ghanchi F,, Guidelines DR. The Royal College of Ophthalmologists' clinical guidelines for diabetic retinopathy: a summary Fast Track Paper. Eye. 2013; 27: 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ehrlich R,, Harris A,, Ciulla TA,, Kheradiya N,, Winston DM,, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol. 2010; 88; 279–291. [DOI] [PubMed] [Google Scholar]
- 4. Kiire CA,, Chong NV. Managing retinal vein occlusion. Brit Med J. 2012; 344: e499. [DOI] [PubMed] [Google Scholar]
- 5. Ford JA,, Clar C,, Lois N,, et al. Treatments for macular oedema following central retinal vein occlusion: systematic review. BMJ Open. 2014; 4: e004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen E,, Looman M,, Laouri M,, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin. 2010; 26: 1587–1597. [DOI] [PubMed] [Google Scholar]
- 7. Ip MS,, Scott IU,, VanVeldhuisen PC,, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 5. Arch Ophthalmol-Chic. 2009; 127: 1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gado AS,, Macky TA. Dexamethasone intravitreous implant versus bevacizumab for central retinal vein occlusion-related macular oedema: a prospective randomized comparison. Clin Exp Ophthalmol. 2014; 42: 650–655. [DOI] [PubMed] [Google Scholar]
- 9. Ciulla TA,, Harris A,, McIntyre N,, Jonescu-Cuypers C. Treatment of diabetic macular edema with sustained-release glucocorticoids: intravitreal triamcinolone acetonide, dexamethasone implant, and fluocinolone acetonide implant. Expert Opin Pharmaco. 2014; 15: 953–959. [DOI] [PubMed] [Google Scholar]
- 10. Bucolo C,, Grosso G,, Drago V,, Gagliano C. Intravitreal triamcinolone acetonide in the treatment of ophthalmic inflammatory diseases with macular edema: a meta-analysis study. J Ocul Pharmacol Th. 2015; 31: 228–240. [DOI] [PubMed] [Google Scholar]
- 11. Kwak HW,, D'Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol. 1992; 110: 259–266. [DOI] [PubMed] [Google Scholar]
- 12. Audren F,, Tod M,, Massin P,, et al. Pharmacokinetic-pharmacodynamic modeling of the effect of triamcinolone acetonide on central macular thickness in patients with diabetic macular edema. Invest Ophthalmol Vis Sci. 2004; 45: 3435–3441. [DOI] [PubMed] [Google Scholar]
- 13. Yilmaz T,, Cordero-Coma M,, Federici TJ. Pharmacokinetics of triamcinolone acetonide for the treatment of macular edema. Exp Opin Drug Metab Toxicol. 2011; 7: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 14. Arcinue CA, Ceron OM, Foster CS. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J Ocul Pharmacol Ther. 2013; 29: 501–507. [DOI] [PubMed] [Google Scholar]
- 15. Chennamaneni SR,, Mamalis C,, Archer B,, Oakey Z,, Ambati BK. Development of a novel bioerodible dexamethasone implant for uveitis and postoperative cataract inflammation. J Control Release. 2013; 167: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calvo P,, Ferreras A,, Al Adel F,, Wang Y,, Brent MH. Dexamethasone intravitreal implant as adjunct therapy for patients with wet age-related macular degeneration with incomplete response to ranibizumab. Brit J Ophthalmol. 2015; 99: 723–726. [DOI] [PubMed] [Google Scholar]
- 17. Gomez-Graete C,, Tsapis N,, Besnard M,, Bochot A,, Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int J Pharm. 2007; 331: 153–159. [DOI] [PubMed] [Google Scholar]
- 18. Barcia E Herrero-Vanrell R, Diez A, Alvarez-Santiago C, Lopez I, Calonge M. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp Eye Res. 2009; 89: 238–245. [DOI] [PubMed] [Google Scholar]
- 19. Xu J,, Wang Y,, Li Y,, et al. Inhibitory efficacy of intravitreal dexamethasone acetate-loaded PLGA nanoparticles on choroidal neovascularization in a laser-induced rat model. J Ocul Pharmacol Ther. 2007; 23: 527–540. [DOI] [PubMed] [Google Scholar]
- 20. Shen J,, Durairaj C,, Lin T,, Liu Y,, Burke J. Ocular pharmacokinetics of intravitreally administered brimonidine and dexamethasone in animal models with and without blood-retinal barrier breakdown. Invest Ophthalmol Vis Sci. 2014; 55: 1056–1066. [DOI] [PubMed] [Google Scholar]
- 21. Checa-Casalengua P,, Jiang CH,, Bravo-Osuna I,, et al. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J Control Release. 2011; 156: 92–100. [DOI] [PubMed] [Google Scholar]
- 22. Vela JI,, Crespí J,, Andreu D. Repositioning of dexamethasone intravitreal implant (Ozurdex®) migrated into the anterior chamber. Int Ophthalmol. 2012; 32: 583–584. [DOI] [PubMed] [Google Scholar]
- 23. Pardo-Lopez D,, Frances-Munoz E,, Gallego-Pinazo R,, Diaz-Llopis M. Anterior chamber migration of dexametasone intravitreal implant (Ozurdex®). Graefe's Arch Clin Exp Ophthalmol. 2012; 250: 1703–1704. [DOI] [PubMed] [Google Scholar]
- 24. El-Ghrably IA,, Saad A,, Dinah C. A novel technique for repositioning of a migrated ILUVIEN (fluocinolone acetonide) implant into the anterior chamber. Ophthalmol Ther. 2015; 4: 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hou H,, Nieto A,, Ma F,, Freeman WR,, Sailor MJ,, Cheng L. Tunable sustained intravitreal drug delivery system for daunorubicin using oxidized porous silicon. J Control Release. 2014; 178: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sailor MJ. Porous Silicon in Practice: Preparation Characterization, and Applications. Hoboken, NJ: Wiley-VCH; 2012. [Google Scholar]
- 27. Wang C,, Hou H,, Nan K,, Sailor MJ,, Freeman WR,, Cheng L. Intravitreal controlled release of dexamethasone from engineered microparticles of porous silicon dioxide. Exp Eye Res. 2014; 129: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peyman GA,, Schulman J. Proliferative vitreoretinopathy and chemotherapeutic agents. Surv Ophthalmol. 1985; 29: 434–442. [DOI] [PubMed] [Google Scholar]
- 29. Davson H,, Luck CP. Chemistry and rate of turnover of the ocular fluids of the bush baby (Galago-Crassicaudatus-Agisymbanus). J Physiol-London. 1959; 145: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edelman JL,, Lutz D,, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005; 80: 249–258. [DOI] [PubMed] [Google Scholar]
- 31. Jaffuel D,, Roumestan C,, Balaguer P,, et al. Correlation between different gene expression assays designed to measure trans-activation potencies of systemic glucocorticoids. Steroids. 2001; 66: 597–604. [DOI] [PubMed] [Google Scholar]
- 32. Cheng L,, Anglin E,, Cunin F,, et al. Intravitreal properties of porous silicon photonic crystals: a potential self-reporting intraocular drug-delivery vehicle. Br J Ophthalmol. 2008; 92: 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kashanian S,, Harding F,, Irani Y,, et al. Evaluation of mesoporous silicon/polycaprolactone composites as ophthalmic implants. Acta Biomater. 2010; 6: 3566–3572. [DOI] [PubMed] [Google Scholar]
- 34. Nieto A,, Hou H,, Sailor MJ,, Freeman WR,, Cheng L. Ocular silicon distribution and clearance following intravitreal injection of porous silicon microparticles. Exp Eye Res. 2013; 116: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rong XF,, Yuan WE,, Lu Y,, Mo XF. Safety evaluation of poly(lactic-co-glycolic acid)/poly(lactic-acid) microspheres through intravitreal injection in rabbits. Int J Nanomed. 2014; 9: 3057–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varshochian R,, Riazi-Esfahani M,, Jeddi-Tehrani M,, et al. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J Biomed Mater Res A. 2015; 103: 3148–3156. [DOI] [PubMed] [Google Scholar]
- 37. Shmueli RB,, Ohnaka M,, Miki A,, et al. Long-term suppression of ocular neovascularization by intraocular injection of biodegradable polymeric particles containing a serpin-derived peptide. Biomaterials. 2013; 34: 7544–7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coscas G,, Augustin A,, Bandello F,, et al. Retreatment with Ozurdex for macular edema secondary to retinal vein occlusion. Eur J Ophthalmol. 2014; 24: 1–9. [DOI] [PubMed] [Google Scholar]
- 39. Zarranz-Ventura J,, Carreno E,, Johnston RL,, et al. Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, and reinjection frequency. Am J Ophthalmol. 2014; 158: 1136–1145. [DOI] [PubMed] [Google Scholar]
- 40. Chang-Lin JE,, Attar M,, Acheampong AA,, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011; 52: 80–86. [DOI] [PubMed] [Google Scholar]
- 41. Inoue M,, Takeda K,, Morita K,, Yamada M,, Tanigawara Y,, Oguchi Y. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol. 2004; 138: 1046–1048. [DOI] [PubMed] [Google Scholar]
- 42. Chew EY,, Glassman AR,, Beck RW,, et al. Ocular side effects associated with peribulbar injections of triamcinolone acetonide for diabetic macular edema. Retina. 2011; 31: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haller JA,, Bandello F,, Belfort R,, Jr,, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118: 2453–2460. [DOI] [PubMed] [Google Scholar]
- 44. Gerometta R,, Podos SM,, Danias J,, Candia OA. Steroid-Induced Ocular Hypertension in Normal Sheep. Invest Ophthalmol Vis Sci. 2009; 50: 669–673. [DOI] [PubMed] [Google Scholar]
- 45. Gerometta R,, Podos SM,, Candia OA,, et al. Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol-Chic. 2004; 122: 1492–1497. [DOI] [PubMed] [Google Scholar]