Abstract
The immune pathogenesis of dengue involves antibody production, B cell and T cell response and various pro-inflammatory and anti-inflammatory cytokines. VEGF, a potent permeability enhancing cytokine, is thought to play a pivotal role in mediating plasma leakage in DHF. It is a member of growing family of related proteins that includes VEGF B, VEGF C, VEGF D and placental growth factor. It promotes angiogenesis and vascular integrity. In addition to its role in promoting endothelial permeability & proliferation, it may contribute to inflammation and coagulation. This study was undertaken to investigate the role of VEGF in the patients with dengue infection. Sera were collected from 106 patients with various grades of dengue illness and 40 healthy controls and tested for VEGF levels using commercial ELISA kits. Viral serotypes were detected using specific primers. The results showed very low levels of VEGF (3.493 ± 1.982 pg/ml) in healthy controls. Levels of VEGF were higher in patients with severe dengue (428.170 ± 224.61 pg/ml) as compared to patients with non severe dengue with and without warning signs (290.407 ± 167.17 pg/ml). Significant correlation (p < 0.001) was found between raised VEGF levels and thrombocytopenia and raised haematocrit levels. The VEGF profile patterns discovered between the different phases of illness indicate an essential role in dengue pathogenesis and with further studies may serve as predictive markers for progression of dengue fever to severe dengue infection.
Keywords: Dengue fever, Severe dengue, Plasma leakage, Cytokines, Immunopathogenesis, VEGF
Introduction
Dengue is most emerging arthropod borne disease and is a major public health problem worldwide. It is an important cause of morbidity and mortality in the tropical and subtropical areas with around 2.5 billion people living in areas at risk [8]. At least 50–100 million infections are known to occur worldwide each year [5]. Frequency of dengue infection has increased in India and it has now become a major public health problem [2]. Illness caused by one of the four dengue virus serotypes can range from nonspecific febrile illness to classic dengue fever, which may then progress to severe disease.
Dengue virus (DENV) belongs to the family Flaviviridae, genus Flavivirus. It is a spherical, lipid-enveloped virus that contains a positive single stranded RNA genome, approximately 11 kb in length. The virion consists of three structural proteins (capsid, membrane, envelope) and seven non structural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, NS5) [9]. Virus is transmitted by two mosquito vectors; Aedes aegypti which thrives mainly in urban areas in tropical region and breeds in artificial collection of water and Aedes albopictus which resides in temperate regions where it may give rise to occasional outbreaks of dengue [1, 6, 7, 10].
Four immunologically distinct dengue serotypes (DENV 1, DENV 2, DENV 3 and DENV 4) coexist in many endemic areas. Infection with one serotype has been shown to provide lifelong immunity to that serotype, but no or only short term immunity to other serotypes [19]. Cross-strain infections are common and can have severe consequences, with extreme cases leading to death.
Dengue has a broad spectrum of clinical presentations, ranging from uncomplicated dengue fever to severe dengue. Classical dengue fever is a disease of older children and adults, characterized by biphasic fever of 102–105 °F and variety of non-specific signs & symptoms including frontal headache, retro-orbital pain, body aches, nausea, vomiting, joint pains & weakness. Rash is common, flushing or erythematous mottling occurs slightly before onset of fever & disappears 1–2 days after the onset of symptoms. Severe dengue includes patients with hemorrhagic manifestations, fluid accumulation with respiratory distress, shock and severe organ involvement [9].
The pathogenesis of dengue is not very clear, a secondary infection with a different serotype has been suspected to be a risk factor [14, 18]. This is explained by the theory of antibody dependent enhancement [12, 13]. The immune pathogenesis of dengue involves antibody production, B cell and T cell response and various pro-inflammatory and anti-inflammatory cytokines [3].
Vascular endothelial growth factor (VEGF), a potent permeability enhancing cytokine, is thought to play a pivotal role in mediating plasma leakage in DHF [24]. It is a member of growing family of related proteins that includes VEGF B, VEGF C, VEGF D and placental growth factor. At least two types of VEGF receptors are expressed on endothelial cells. Both the receptors are transmembrane receptors tyrosine kinases. VEGF promotes angiogenesis and vascular integrity [21]. In addition to its role in promoting endothelial permeability & proliferation, it may contribute to inflammation and coagulation. Few studies have demonstrated elevated circulating VEGF levels in adult severe dengue patients who were admitted during the early phases of dengue infection compared to patients with DF and study controls [4, 23]. A rise in circulating VEGF in DHF in the early febrile and defervescent stages of dengue infection but not during the convalescent stage has been observed. In view of the above, this study was under taken as among the few published reports investigating the role of VEGF in dengue patients, there have been inconstant findings. These conflicting findings emphasize the need for a thorough investigation to gain more knowledge regarding immune pathogenesis of dengue so that more could be done to control the severity and progression of disease.
Materials and methods
The present study was a cross sectional study conducted at the Dengue Sero-surveillance Laboratory of a 2000-bedded government hospital in Delhi. Ethical approval certificate for the study was obtained from the institutional ethical committee. Informed written consent was obtained from all the study subjects.
Cases
The study group included 106 patients with laboratory-confirmed diagnosis of dengue virus infection either by IgM antibody ELISA Pan-bio Dengue early ELISA kit or NS1 antigen ELISA Pan-bio Dengue Early ELISA kit (Inverness Medical Innovations Australia).
Cases were classified as primary and secondary dengue infection by using anti IgG ELISA (Pan-bio Dengue early ELISA) kit. Depending upon the severity of the illness, they were classified as non severe dengue fever and severe dengue fever according to the criteria of the World Health Organisation (2009). As controls, 40 normal age-matched healthy individuals without history of any febrile or other illnesses were included. A case was excluded, if routine laboratory testing suggested malaria or typhoid or any other bacterial and viral infection.
Specimen collection and transport
5 ml of venous blood was collected aseptically in a plain vial. Serum was separated from venous blood samples aseptically, aliquoted in eppendorf vials and immediately transferred to −70 °C until processed further. Samples positive for either NS1 antigen or IgM antibody or both and samples from healthy controls were tested for levels of VEGF by commercially available ELISA kits as per manufacturers instructions.
Measurement of VEGF
VEGF was measured in serum using Diaclone human VEGF ELISA kit. An anti-human VEGF-A coating antibody is adsorbed onto microwells. Human VEGF present in the sample or standard binds to antibodies adsorbed to the microwells. Following incubation unbound biological components are removed during a wash step. A biotin-conjugated antihuman VEGF-A antibody is added and binds to human VEGF-A captured by the first antibody. Following incubation unbound biotin-conjugated anti-human VEGF-A antibody is removed during a wash step. Streptavidin HRP is added and binds to the biotin-conjugated anti-human VEGF-A antibody. Following incubation unbound streptavidin—HRP is removed during a wash step and substrate solution reactive with HRP is added to the wells. A colored product is formed in proportion to the amount of human VEGF present in the sample. Data analysis was carried out by creating a standard curve by plotting the mean absorbance for each standard concentration on the ordinate against the VEGF concentration on the abscissa. Concentration of circulating VEGF for each sample was determined from the standard curve.
Detection of viral serotype in serum
Viral serotype was detected by using reverse-transcriptase polymerase chain reaction. RNA was extracted from acute phase serum (<5 days) using commercial kit (Hi pure RNA extraction kit). Specific primers designed by Lanciotti et al. [15] were used for the isolation of virus, while serotype specific primers were used to detect viral serotype.
Statistical analysis
Statistical analysis was done by using SPSS version 17. The comparison between the differences in means of data in dengue and severe dengue patients was done by Kruskal–Wallis test and Mann–Whitney test. Association between mean cytokines levels and primary and secondary infection and viral serotype was done by Mann–Whitney test. A p < 0.05 was considered significant. Pearson/spearman rank correlation was used to study the correlation between cytokines levels and clinical findings.
Results
Clinical features in the population
Out of the 106 cases studied, 22 cases were positive for anti dengue IgM antibody, 76 were positive for dengue NS1 antigen and 8 were positive for both. Among the patients, 58 were classified as suffering from non severe dengue fever and 48 as suffering from severe dengue fever. Out of total 106 cases, 68 were primary infection where as 38 were secondary infection. Total of 18 samples were tested positive for dengue virus RNA. Of the four serotypes DENV-2 was found to be predominant serotype (Table 1).
Table 1.
Laboratory diagnostic assay results in study group (N = 106)
| Non severe dengue | Severe dengue | |
|---|---|---|
| DENV RNA detection | 10 | 8 |
| DENV NS1 detection | 46 | 30 |
| DENV IgM detection | 10 | 12 |
| Both NS1 and IgM detection | 2 | 6 |
| Primary infection | 40 | 28 |
| Secondary infection | 18 | 20 |
Fever or history of fever, the commonest symptom encountered in the present study, was reported in all of the 106 study subjects. Other common manifestations were headache (88, 83.01 %), arthalgia (104, 98.11 %) myalgia (97, 91.5 %) and retro orbital pain (90, 84.9 %) The mean duration of fever was 2.99 days with median of 2 days (Table 2).
Table 2.
Distribution of clinical symptoms in study group (N = 106)
| Clinical symptoms | Total | Percentage |
|---|---|---|
| Fever | 106 | 100 |
| Headache | 88 | 83.01 |
| Retroorbital pain | 90 | 84.90 |
| Arthalgia | 104 | 98.11 |
| Myalgia | 97 | 91.50 |
Clinical signs included; rash (96, 90.56 %), gum bleeding (20, 18.86 %), melena (32, 30.18 %), epistaxis (12, 11.32 %), conjunctival bleeding (10, 9.4 %), haematuria (2, 1.8 %), hepatomegaly (17, 16.03) and splenomegaly (5, 4.71 %) (Table 3).
Table 3.
Distribution of clinical signs in the study group (N = 106)
| Clinical sign | Total | Percentage |
|---|---|---|
| Rash | 96 | 90.56 |
| Petechiae | 41 | 38.67 |
| Gum bleeding | 20 | 18.86 |
| Melena | 32 | 30.18 |
| Epistaxis | 12 | 11.32 |
| Conjunctival bleeding | 10 | 9.4 |
| Haematuria | 2 | 1.8 |
| Hepatomegaly | 17 | 16.03 |
| Splenomegaly | 5 | 4.71 |
Fever was present in every case of non severe dengue and severe dengue. No significant association was observed between rashes, arthralgia or splenomegaly and severity of dengue (p > 0.05). However, myalgia was found to be significantly associated with severe dengue (p < 0.05). The association of severe dengue with bleeding manifestations and hepatomegaly were also found to be highly significant (p < 0.001, p = 0.001 respectively) (Table 4).
Table 4.
Distribution of clinical features among group with different severity (N = 106)
| Clinical features | Dengue fever | Severe dengue | p value |
|---|---|---|---|
| Fever | 58 (100) | 48 (100) | 0.300 |
| Rash | 47 (81) | 41 (85.4) | 0.550 |
| Arthalgia | 56 (96.6) | 48 (100) | 0.194 |
| Myalgia | 49 (85.4) | 48 (100) | 0.004 |
| Melena | 0 (0) | 31 (64.6) | 0.000 |
| Gumbleed | 0 (0) | 20 (41.7) | 0.000 |
| Petechiae | 0 (0) | 41 (85.41) | 0.000 |
| Epistaxis | 0 (0) | 12 (25) | 0.000 |
| Conjunctival bleed | 0 (0) | 9 (18.8) | 0.000 |
| Hepatomegaly | 3 (5.2) | 14 (29.2) | 0.001 |
| Splenomegaly | 1 (1.7) | 4 (8.3) | 0.110 |
p value was calculated by Kruskal–Wallis test
Haematological profile revealed thrombocytopenia in 79 (74.52 %) patients, haemoconcentration in 47 patients (44.33 %), and leucopenia in 55 (51.88 %) patients (Table 5). The mean levels of platelet count were significantly lower in severe dengue patients as compared to non severe dengue patients (p < 0.05). Haematocrit was significantly raised in severe dengue patients as compared to non severe dengue patients (p < 0.05). Serum mean levels ALT, AST, and ALP levels were raised in severe dengue patients as compared to non severe dengue patients but it was not found to be significant (p < 0.001) (Table 6).
Table 5.
Association of haematological parameters with severity of disease
| Haematological parameters | Dengue fever mean ± SD | Severe dengue mean ± SD | p value |
|---|---|---|---|
| Haemoglobin (g/dl) | 14.457 ± 1.73 | 13.77 ± 2.44 | 0.426 |
| Platelet count (cells/mm3) | 94,231.03 ± 27,506.702 | 33,687.50 ± 21,797.08 | 0.000 |
| Total leucocyte count (cells/mm3) | 3955.17 ± 1336.948 | 3533.33 ± 1260.924 | 0.115 |
| Polymorphs (cells/mm3) | 30.36 ± 7.962 | 34.15 ± 13.29 | 0.359 |
| Lymphocytes (cells/mm3) | 25.83 ± 4.76 | 23.29 ± 4.005 | 0.009 |
| Monocytes (cells/mm3) | 25.10 ± 4.8 | 25.81 ± 4.66 | 0.392 |
| Eosinophils (cells/mm3) | 0.248 ± 0.197 | 0.256 ± 0.2153 | 0.640 |
| Haematocrit | 24.22 ± 5.419 | 50.31 ± 40.331 | 0.000 |
p value was calculated by Mann–Whitney test
Table 6.
Association of biochemical parameters with severity of disease
| Biochemical parameters | Dengue fever mean ± SD | Severe dengue mean ± SD | p value |
|---|---|---|---|
| ALT (IU) | 31.71 ± 20.985 | 79.62 ± 20.547 | 0.528 |
| AST (IU) | 33.62 ± 37.68 | 67.50 ± 50.449 | 0.116 |
| ALP (IU) | 42.95 ± 29.986 | 88.23 ± 71.088 | 0.176 |
| Serum bilirubin (mg/dl) | 0.753 ± 0.1203 | 0.719 ± 0.1394 | 0.219 |
| Serum albumin (g/dl) | 5.316 ± 0.9065 | 4.908 ± 1.289 | 0.115 |
| Total protein (g/dl) | 6.98 ± 0.802 | 6.56 ± 1.370 | 0.564 |
| Serum urea (mg/dl) | 27.33 ± 5.775 | 26.02 ± 4.255 | 0.513 |
| Sodium (mol/ml) | 141.45 ± 4.325 | 140.40 ± 4.37 | 0.207 |
| Potassium (mol/ml) | 4.54 ± 0.805 | 4.637 ± 0.8002 | 0.403 |
p value was calculated by Mann–Whitney test
Serum levels of VEGF
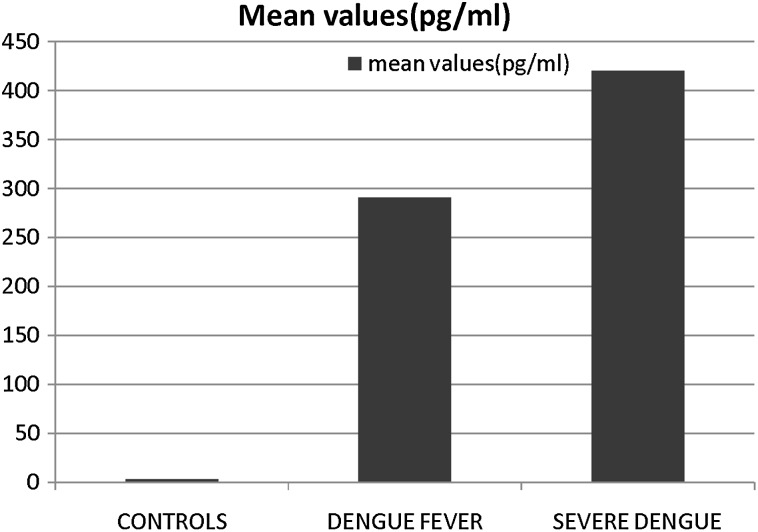
The mean levels of VEGF in control sera was 3.493 ± 1.982 pg/ml. The findings presented in Fig. 1 show that as compared to patients with DF (mean value of 290.407 ± 167.17 pg/ml), significantly higher levels of VEGF (p < 0.001) were detected in the sera of patients with severe dengue (428.170 ± 224.61 pg/ml). VEGF was present in 42 (72.41 %) patients with dengue fever while it was present in 44 (91.66 %) patients with severe dengue patients.
Fig. 1.
Level of VEGF in patients with dengue. Sera collected from the patients with various grades of illness were screened for VEGF concentration by ELISA using commercial kits. The mean value of data (pg/ml) have been presented
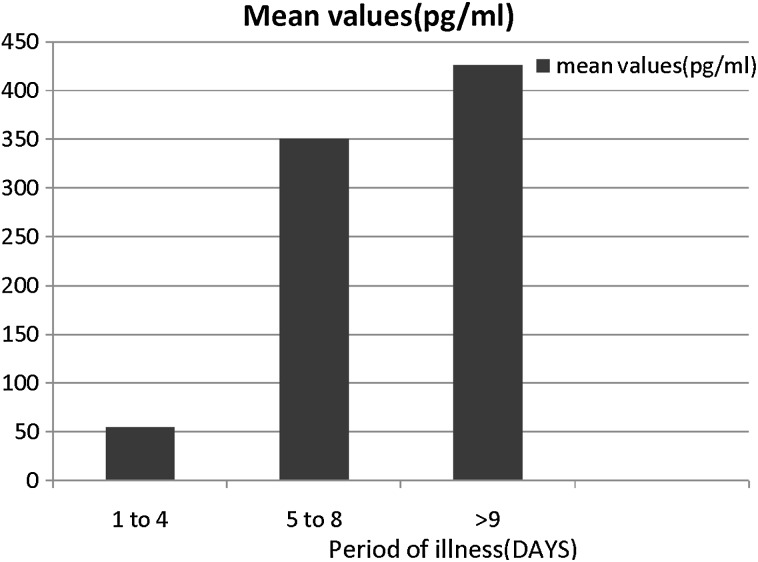
The patients were grouped according to the day of illness at the time of collection of the sera, as between days 1 and 4, between days 5 and 8 and day 9 onwards. When the data were analyzed with respect to the days of illness, it was observed that VEGF levels were lowest (55 ± 21.5 pg/ml) during the first 4 days of illness, the VEGF levels increased to 350.5 ± 125.17 pg/ml on days 5–8 and peaked to 426.70 ± 223.45 pg/ml on day 9 onwards (Fig. 2). The difference from the initial period was significant (p < 0.001).
Fig. 2.
Amount of VEGF in patient sera (pg/ml) as a function of stage of dengue illness
Relationship of VEGF levels with primary and secondary infections and viral serotypes
No significant association (p < 0.846) was found between difference in means of VEGF in primary (357.052 ± 81.27 pg/ml) and secondary infection (368.057 ± 26.40 pg/ml).
Dengue Virus was detected in 18 samples, of which DEN 2 was the commonest being reported from 16 cases. Both DENV 1 and DENV 3 could be detected from only 1 sample each. The significant difference in mean level of VEGF DEN 3 (640 pg/ml) as compared to DEN 2 (568.375 ± 135.99) and DENV1 (400 pg/ml) could not be established due to lesser number of samples of DENV 1 and DENV 3.
Association of clinical features with VEGF levels
Significant association of VEGF levels were found with clinical features of rashes, arthalgia and myalgia. However no significant association could be found between elevated VEGF levels and bleeding manifestations like melena, epistaxis and conjunctival bleed.
Correlation between mean VEGF levels and clinical, biochemical and haematological profile of the study group
Significant negative correlation was found between VEGF levels and total leucocyte count and platelet count. Significant positive correlation was found between VEGF levels and haematocrit. Significant correlation was found between VEGF levels and serum ALT, AST and ALP levels (Table 7).
Table 7.
Spearman rank correlation between VEGF levels and haematological and biochemical profile
| Variables | Correlation coefficient | p value |
|---|---|---|
| Haemoglobin | −0.016 | 0.878 |
| Total leucocyte count | −0.435 | 0.000 |
| Platelet count | −0.316 | 0.001 |
| Haematocrit | 0.215 | 0.032 |
| Serum ALT | 0.398 | 0.000 |
| Serum AST | 0.462 | 0.000 |
| Serum ALP | −0.238 | 0.017 |
| Total proteins | −0.236 | 0.018 |
p value <0.05
Discussion
Significantly higher levels of VEGF levels were found in severe dengue patients as compared to dengue patients and healthy controls. In addition to its role in promoting endothelial permeability and proliferation, VEGF may contribute to inflammation and coagulation. Therefore it may have a pivotal role in the pathogenesis of dengue. Tseng et al. [24] and Srikiatkhachorn et al. [23] demonstrated elevated levels of VEGF levels in dengue patients and its possible association with increase in vascular permeability in severe dengue patients. A subsequent study, however, had contradictory findings. Sathupan et al. [20] did not observe any increase in the concentration of circulating VEGF during the early febrile and toxic stages in severe dengue, but found instead lower VEGF concentrations in patients with more severe dengue infection. Several reasons may explain these differences, such as poor study design, small sample size, and the lack of a standardized collection methodology and storage of blood samples used for the measurement of VEGF.
VEGF levels were found to be significantly associated with clinical manifestations like rashes and myalgia. No significant association could be established between bleeding manifestations and VEGF levels. This is in contrast to previous studies that established the significant association between VEGF levels and bleeding manifestations in dengue patients. Significant correlation was found between VEGF levels and thrombocytopenia, leucopenia and raised haematocrit levels. This was in accordance with the study done by Seet et al. [22]. This was probably due to role of VEGF in causing vascular permeability and plasma leakage.
Also significant correlation was found between VEGF levels and liver enzymes. It might be due to increased angiogenesis and fibrogenesis by VEGF in liver leading to abnormal hepatic collateral formation and liver damage.
Earlier studies have demonstrated higher levels of VEGF in secondary dengue infection, hence establishing its role in the pathogenesis of dengue. Our study demonstrated its rise in secondary infection as compared to primary but this difference was not found to be significant. This discrepancy in results could be due to various reasons. The number of primary infection included in our study were relatively higher as compared to secondary infection. Comparatively higher number of primary severe dengue patients were found in our study as compared to secondary severe dengue cases. Also, these pro-inflammatory cytokines get raised in early phase of disease but most of our secondary severe dengue cases presented in later phase of disease when the levels of these cytokines already starts declining. Furthermore, T cell response of cytokines in secondary dengue infection is multifactorial and may vary according to viral serotypes (DEN2 > DEN1), host immune response and genetic factors such as TNF-α gene polymorphism and HLA gene association [16, 17].
Dengue viruses are flaviviruses, of which there are four different serotypes. It has been observed in several studies that sequential or secondary dengue virus infections are more likely to produce severe disease [11]. Since both pro-inflammatory cytokines and viral serotype plays role in mounting more severe response in dengue infection, we tried to establish the association between the two and to know whether release of these cytokines is affected by dengue viral serotype that is causing infection In our study, virus was isolated from 18 samples, out of which 16 belonged to DENV2 and one each to DENV1 and DENV3. Virus was isolated in lesser number of samples and hence the association could not be established. As dengue virus can be detected in acute phase serum (<5 days) only and is a RNA virus highly susceptible to external environmental condition, it is difficult to detect virus in all the samples. Moreover lack of a standardized collection methodology and proper storage of blood samples may contribute to this discrepancy. Thus in our study it has been shown that VEGF may contribute in the pathogenesis of dengue infection and can be associated with the severity of disease. However the immunopathogenesis of dengue infection is multifactorial and other aspects of the pathology such as gene polymorphism, HLA gene association etc. need to be studied to prevent the complications and outcome of disease. Over the past several years, greater awareness of the public health impact of DENV has led to increased focus on development of vaccines and antiviral therapies. The challenge now is to apply the knowledge gained regarding these growth factors and their role in the pathogenesis in order to prevent and control dengue and its severe manifestations.
Plasma leakage is the important feature of severe dengue infection. The pathogenic mechanisms of severe dengue involved cytokine activation, activation of coagulation and fibrinolysis. We demonstrated that plasma VEGF levels in patients with severe dengue were significantly higher than in dengue fever patients. These findings suggest that VEGF, as a factor of increasing vascular permeability, may contribute to the pathogenesis of severe dengue as well as predictive marker for the progression of disease and its severity.
Footnotes
Preeti Thakur, Anita Chakravarti, Sunita Aggarwal, Beena Uppal and Preena Bhalla have contributed equally to this work.
References
- 1.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2002;7(1):76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarti A, Kumaria R. Circulating levels of tumour necrosis factor-α & interferon-γ in patients with dengue & dengue haemorrhagic fever during an outbreak. Indian J Med Res. 2006;123:25–30. [PubMed] [Google Scholar]
- 3.Clyde K, Jennifer LK, Eva H. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80(3):11418–11431. doi: 10.1128/JVI.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuta T, Murao LA, Lan M, Hug NT, Huong VT. Association of mast cell-derived VEGF and proteases in Dengue shock syndrome. PloS Negl Trop Dis. 2012;6(2):e1505. doi: 10.1371/journal.pntd.0001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ. 2002;324(7353):1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grist NR. Aedes albopictus: the tyre—travelling tiger. J Infect. 1993;27:1–4. doi: 10.1016/0163-4453(93)93418-4. [DOI] [PubMed] [Google Scholar]
- 7.Grist NR, Burgess NRH. Aedes and dengue. Lancet. 1994;343(8895):477. doi: 10.1016/S0140-6736(94)92717-0. [DOI] [PubMed] [Google Scholar]
- 8.Gubler DJ. The global pandemic of dengue/dengue haemorrhagic fever: current status & prospects for the future. Ann Acad Med. 1998;27(2):227–234. [PubMed] [Google Scholar]
- 9.Gubler DJ. Dengue and dengue haemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gubler DJ, Clark GG. Community involvement in the control of Aedes aegypti. Acta Trop. 1996;61(2):169–179. doi: 10.1016/0001-706X(95)00103-L. [DOI] [PubMed] [Google Scholar]
- 11.Guzmán MG, Kourí G, Valdés L, Bravo J, Vázquez S, Halstead SB. Enhanced severity of secondary dengue-2 infections: death rates in 1981 and 1997 Cuban outbreaks. Rev Panam Salud Publica. 2002;11(4):223–227. doi: 10.1590/S1020-49892002000400003. [DOI] [PubMed] [Google Scholar]
- 12.Halstead SB. Observations related to pathogenesis of dengue haemorrhagic fever VI. Hypothesis and discussion. Yale J Biol Med. 1970;42:350–362. [PMC free article] [PubMed] [Google Scholar]
- 13.Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 14.Halstead SB. Antibody, macrophages, dengue virus infection, shock and haemorrhage: a pathogenic cascade. Clin Infect Dis. 1989;11(4):s830–s839. doi: 10.1093/clinids/11.Supplement_4.S830. [DOI] [PubMed] [Google Scholar]
- 15.Lanciotti R, Calisher C, Gubler DJ, Chang G, Vorndam V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase–polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loke H, Bethell DB, Phuong CXT, Dung M, Schneider J, White NJ, Hill AV. Strong HLA class I–restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J Infect Dis. 2001;184(11):1369–1373. doi: 10.1086/324320. [DOI] [PubMed] [Google Scholar]
- 17.Perez AB, Sierra B, Garcia G, Aguirre E, Babel N, Alvarez M, Guzman MG. Tumor necrosis factor-alpha, transforming growth factor-β1, and interleukin-10 gene polymorphisms: implication in protection or susceptibility to dengue hemorrhagic fever. Hum Immunol. 2010;71(11):1135–1140. doi: 10.1016/j.humimm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Rico-Hesse R. Molecular evolution and distribution of dengue viruses type 1 & 2 in nature. Virology. 1990;174(2):479–493. doi: 10.1016/0042-6822(90)90102-W. [DOI] [PubMed] [Google Scholar]
- 19.Sabin AB. Research on dengue during world war II. Am J Trop Med Hyg. 1952;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 20.Sathupan P, Khongphattanayothin A, Srisai J, Srikaen K, Poovorawan Y. The role of vascular endothelial growth factor leading to vascular leakage in children with dengue virus infection. Ann Trop Paediatr. 2007;27(3):179–184. doi: 10.1179/146532807X220280. [DOI] [PubMed] [Google Scholar]
- 21.Sawano A, Iwai S, Sakurai Y, Ito M, Shitare K et al. VEGFR1 is a novel cell surface marker for the lineage of monocyte-macrophage in humans. Blood 2001;97:785–91. [DOI] [PubMed]
- 22.Seet R, Chow A, Quekamy ML, Chan YH. Relationship between circulating vascular endothelial growth factor and its soluble receptors in adults with dengue virus infection: a case control study. Int J Infect Dis. 2009;13(5):248–253. doi: 10.1016/j.ijid.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Srikiatkhachorn C, Kharon A, Endy TB. Virus-induced decline in soluble vascular endothelium growth factor receptor 2 is associated with plasma leakage in DHF. J Virol. 2007;81(4):1592–1600. doi: 10.1128/JVI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng C, Lohw S, Teng H. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2005;43:99–102. doi: 10.1016/j.femsim.2004.10.004. [DOI] [PubMed] [Google Scholar]