Abstract
Diarrheal diseases are responsible for a significant proportion of mortality and morbidity all around the globe. The contribution of viruses to gastroenteritis incidences in humans is well established. In the present study, we have studied the prevalence of rotavirus, norovirus and enterovirus in Himachal Pradesh, a north Indian state. A total of 287 (111 children and 176 adults) stool samples of gastroenteritis patients were screened for the viruses using RT-PCR method. 34.5 % samples were positive for the viral pathogens of gastroenteritis. Rotavirus was the predominant virus detected in the study with 49.5 and 14.8 % positivity in children and adults, respectively. Enterovirus was present in 5.6 % cases whereas norovirus had least prevalence (1.4 %). Co infection (rotavirus and enterovirus) was witnessed at the prevalence rate of 0.6 %. Among different age groups, the prevalence of studied viruses was highest in the children belonging to the age groups of <5 years. Rotavirus infections were found to be significantly associated with vomiting and trend of higher rates of fever and dehydration was seen in children along with diarrhea. Seasonal distribution shows circulation of diarrheagenic viruses throughout the year. This is the first report of prevalence of various diarrheagenic viruses circulating in this region. The outcome of the study from this cohort provides a baseline data which can be used to design the preventive strategies in the otherwise unexplored state of Himachal Pradesh.
Keywords: Viral Gastroenteritis, Diarrhea, RT-PCR, Rotavirus, Norovirus, Enterovirus
Introduction
Diarrhea is a major healthcare concern as it is responsible for a significant proportion of morbidity and mortality with 0.8 million annual fatalities to its account all around the globe [13]. The role of viral pathogens in diarrhea is well established and several diarrheagenic viruses have been reported till date [7, 38]. The disease burden associated with the viral gastroenteritis has been well documented in the studies from all over the world [30, 35]. Rotavirus (RV), the RNA (segmented double stranded) virus belonging to the family of Reoviridae is the major viral agent which is responsible for diarrhea basically in children below the age group of 5 years. This deadly virus is responsible for around 0.5 million deaths in this age group [29]. However, it has been found to cause diarrhea in adults and elderly also [2]. The underdeveloped and developing nations of the world bear the highest rotavirus associated disease burden [10, 37]. Noroviruses (NoVs) are also major viral pathogen associated with diarrhea among all age groups in humans. These single stranded positive sense RNA viruses are the leading cause of gastroenteritis outbreaks and are important agents involved in sporadic diarrheal cases [15, 23]. Out of the six (GI–GVI) genogroups of NoVs, GII is accountable for majority of the NoV associated gastroenteritis cases, followed by [14, 40]. Enteroviruses (EVs) are single stranded RNA viruses, belonging to the Picornaviridae family and they are responsible for a number of chronic and acute diseases in humans [26]. EVs are in fact a neglected pathogen for diarrhea but recent reports have showed its significance as a diarrheagenic virus particularly in children [9, 24, 27, 34]. Keeping in view the mortality, morbidity and financial burden attributed to these viruses, it is imperative to study their epidemiology. This will be crucial in formulating the preventive strategies accordingly.
A number of studies have been conducted to study the prevalence of various diarrheagenic viruses in various parts of India [4, 5, 8, 17, 27], but Himachal Pradesh, a north Indian state, is still unexplored in this context. Therefore, this study was done to evaluate the disease burden of viral gastroenteritis in the region. This is the first study which reports the prevalence of diarrheagenic viruses from this part of the country. In this study, we have studied the prevalence of Group A Rotavirus, Norovirus GI and GII, and Enterovirus. The outcomes of this study could be used to take necessary and appropriate measures to control and better management of diarrhea in the region.
Materials and methods
Samples
The stool samples were collected from the patients admitted to Indira Gandhi Medical College, Shimla and District Hospital, Solan, Himachal Pradesh, India from July 2013 through February 2015. The patients included in the study had a combination of symptoms including vomiting, abdominal pain, fever and dehydration along with diarrhea in common. The demographic details and medical/clinical features (vomiting, fever, dehydration, diarrheal episodes etc.) of the patients were documented and the samples were collected with the consent of the patients/their representative or the parents (in case of children). All the experiments were carried out according to Institutional Ethical Committee guidelines. The samples were transported to the laboratory within 3–4 h of collection.
RNA extraction, Reverse transcription and PCR amplification
Viral RNA was extracted from 10 % fecal suspensions using TRIzol (Invitrogen, Carlsbad, CA, USA) reagent following manufacturer’s instructions. Reverse transcription of the extracted RNA was performed using Verso cDNA synthesis kit (ThermoFisher scientific, Waltham, Massachusetts, USA). The detection of the viruses was done by subjecting cDNA to PCR amplification. The primers used for detection of various viruses were VP6(F) and VP6(R) for RV; Mon432 and Mon434 for NoV GI; Mon431 and Mon433 for NoV GII; and F1 and R1 for EV (Table 1) [19, 28, 41].
Table 1.
| Virus and primer | Sequence | Sense | Amplicon size (bp) | References |
|---|---|---|---|---|
| Group A rotavirus | ||||
| VP6 (F) | TTTGATCACTAAYTATTCACC | + | 227 | Mondal et al. [19] |
| VP6 (R) | GGTCACATCCTCTCACTA | − | ||
| Norovirus GI | ||||
| Mon432 | TGGACICGYGGICCYAAYCA | + | 213 | Richards et al. [28] |
| Mon434 | GAASCGCATCCARCGGAACAT | − | ||
| Norovirus GII | ||||
| Mon431 | TGGACIAGRGGICCYAAYCA | + | 213 | Richards et al. [28] |
| Mon433 | GAAYCTCATCCAYCTGAACAT | − | ||
| Enterovirus | ||||
| F1 | CAAGCACTTCTGTTTCCCCGG | + | 440 | Zoll et al. [41] |
| R1 | ATTGTCACCATAAGCAGCCA | − | ||
The PCR components contained 2.5 µl of 10X Standard Taq Reaction Buffer, 0.5 µl of 10 mM dNTPs, 0.5 µl of 10 µM forward and reverse primers each, 0.6U of Taq DNA Polymerase (New England Biolabs, Massachusetts, USA) and 3 µl of cDNA template. The volume of the mixture was made to 25 µl using nuclease free water (ThermoFisher Scientific, Waltham, Massachusetts, USA). The PCR conditions for the detection of RV were 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 48 °C for 30 s, 68 °C for 30 s and a final extension at 68 °C for 7 min. The PCR amplification conditions for the detection of NoV (GI and GII) and EV were used as described previously [28, 41]. The PCR products were subjected to electrophoresis on 1.5 % agarose gel and the bands were visualized in a UV transilluminator (Fig. 1).
Fig. 1.
Representative agarose gel electrophoresis of the PCR amplified products for the viruses included in the study. Lane 1–4 respectively represents rotavirus, enterovirus, norovirus GI and norovirus GII. Lane M is 100 bp DNA ladder and Lane 5 represents negative control with primers for RV and without cDNA
Statistical analysis
The prevalence of different viruses was estimated by the proportion of positive samples. Bivariate analysis of differences in clinical characteristics of RV positive and RV negative patients was done using Chi square test. Difference in RV infection in male and female patients was analyzed by Fisher’s exact test. P values <0.05 were considered statistically significant.
Results
Incidences of diarrheagenic viruses in the sample population
A total of 287 stool samples were collected and screened during this study for the presence of aforementioned diarrheagenic viruses. Ninety nine (34.5 %) out of 287 patients were positive for the viral pathogens (Table 2). Out of these, 97 (33.8 %) patients were positive for single virus whereas mixed infection was found in 2 (0.6 %) cases. Among single infections, RV was the most prevalent virus with 27.5 % (79/287) positivity among all cases. EV was the second most predominant virus (4.9 %, 14/287) followed by NoV GII (1.0 %). NoV GI was the least detected virus with presence in only one (0.3 %) sample. Two patients showing mixed infection had a coinfection of RV and EV in their stool samples. Out of the 81 RV positive cases (including single and mixed infection), 39 were male and among 206 RV negative cases, the number of male patients was 90. However, this correlation of RV infection with gender showed no statistical significance (P = 0.512).
Table 2.
Frequency of diarrheagenic viruses in the sample population
| Age Group | Rotavirus | Enterovirus | Norovirus GI | Norovirus GII | Coinfection (RV + EV) |
|---|---|---|---|---|---|
| <5 years (N = 111) |
55 (49.5 %) | 6 (5.4 %) | 1 (0.9 %) | 3 (2.7 %) | – |
| >5 years (N = 176) |
24 (13.6 %) | 8 (4.5 %) | 0 (0.0 %) | 0 (0.0 %) | 2 (1.1 %) |
| Total (N = 287) |
79 (27.5 %) | 14 (4.9 %) | 1 (0.3 %) | 3 (1.0 %) | 2 (0.7 %) |
Distribution of diarrheagenic viruses in different age groups
RV was the most commonly identified virus in all age groups (Fig. 2). A major fraction of RV infections were observed in the children up to 2 years of age. In this group, 50 % (49/98) of the diarrheic patients were positive for RV. The children belonging to the age group of 3–5 years showed 46.2 % (6/13) positivity for RV. Among the age groups of 6–15 years, 21.1 % samples were positive for RV. RV infections were lowest in adults and elderly belonging to the age groups of 16–65 and >65 years (15.3 and 5.0 %, respectively). The distribution of EV in different age groups was 5.1 % in <2 years, 7.7 % in 3–5 years, 15.8 % in 6–15 years and 5.1 % in 16–65 years of age. The elderly patients >65 years of age revealed no EV infection. It is to be noted that among 6–15 and 16–65 age groups each had one case of RV and EV mixed infection. As far as NoV infection is concerned, all the four cases were reported in children under the age of 2 years.
Fig. 2.
Distribution of diarrheagenic viruses in the patients of different age groups
Association of clinical features with RV infection
As RV was the predominant virus detected in both the children and adult patients, and it is extensively reported that RV infections are commonly and significantly associated with the additional clinical symptoms along with diarrhea [16, 31, 33], clinical features of the RV positive and RV negative patients were compared to ascertain the association of RV infection with the clinical features of vomiting, fever and dehydration (Table 3). In the children <5 years of age, 34.5 % of RV positive patients suffered from vomiting in contrast to the 10.7 % of the non-RV positive patients. This showed statistically significant association of vomiting with the RV infection (P = 0.0027). Similarly, the rates of fever and dehydration in the RV positive cases were higher compared to RV negative cases, but these differences showed no statistical significance. On the contrary, in the age group >5 years, the rates of vomiting, fever and dehydration in RV infected individuals were lower in comparison with the patients having non-RV diarrhea. However, these results were not statistically significant.
Table 3.
Comparison of clinical characteristics of RV positive and RV negative patients
| Age group | Clinical characteristics | Rotavirus positive (%) | Rotavirus negative (%) | P value |
|---|---|---|---|---|
| N = 55 | N = 56 | |||
| <5 years | Vomiting | 19 (34.5) | 6 (10.7) | 0.003 |
| Fever | 11 (20.0) | 9 (16.1) | 0.590 | |
| Dehydration | 18 (32.7) | 13 (23.2) | 0.264 |
| Age group | Clinical characteristics | Rotavirus positive (%) | Rotavirus negative (%) | P value |
|---|---|---|---|---|
| N = 26 | N = 150 | |||
| >5 years | Vomiting | 4 (15.4) | 33 (22.0) | 0.444 |
| Fever | 1 (3.8) | 14 (9.3) | 0.354 | |
| Dehydration | 4 (15.4) | 24 (16.0) | 0.936 |
The values in boldface indicate the statistical significance
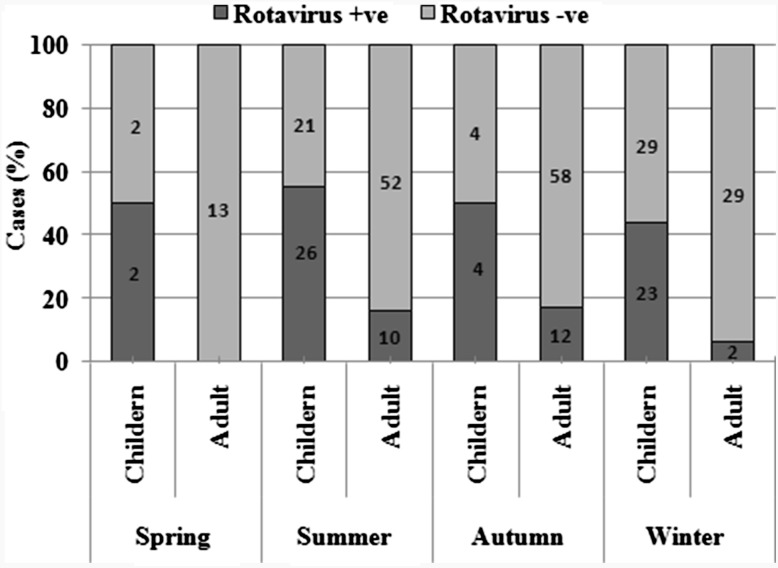
Seasonal distribution of diarrheagenic viruses
The infection rate of the gastroenteritis viruses in different seasons was studied. Among all the viruses under investigation, RV infections were observed throughout the year with no particular seasonal peaks (Fig. 3). Interestingly, the highest prevalence of RV was observed in the summer season where overall (both children and adults) infection rate was 33.0 %. Considerably high infection rate (30.1 %) was observed in the months of winter (November–February). The RV infectivity in children (<5 years) was around 50 % (±5 %) throughout the year. EV and NoV infections were found prominently in the winter and autumn seasons.
Fig. 3.
Seasonal distribution of rotavirus infections. Data labels represent the number of cases
Discussion
The present study was attempted to evaluate the prevalence of viral diarrhea in the region. In this study, RV was found to be the predominant diarrheagenic virus. Studies suggest that in India, the RV disease burden is quite high and this deadly virus causes 79,000 child deaths every year [11]. In this study also, RV was responsible for diarrhea in around 50 % children. However, this incidence rate is comparatively higher than the other parts of the country [4, 17, 22, 25]. Though RV associated diarrhea in adults is not extensively reported, but available reports have shown that it is responsible for as high as 63 % incidences in some cases [6]. In our study, RV, as a single pathogen or in mixed infection, was responsible for significant proportion (14.8 %) of adult diarrhea. These results signify that the RV associated morbidity in adults cannot be ignored and warrants the need to extensively and compulsorily monitor RV infection in adults. The role of EVs as a diarrheagenic pathogen is not very well recognized. However, their diarrheagenic potential is gaining more recognition after the emergence of reports claiming it as a pathogen identified in a number of diarrhea surveillance studies [9, 24, 27, 34]. It is interesting to note that, in our study, out of 16 EV positive cases, 10 were adults. This shows the competence of the enteroviruses to cause diarrhea in grown-up humans also. NoV is the well recognized viral pathogen which causes epidemic and sporadic diarrhea in both adults and children. Some studies have even reported it as the leading cause of diarrhea, especially in children where rotavirus is believed to be the foremost viral pathogen [35]. Among various NoV genogroups, the outcome of majority of the studies shows that GII is the foremost cause of diarrhea in humans. In this study also, NoV GII was detected in 1.0 % cases in contrast to the 0.3 % prevalence of NoV GI. However, it is very interesting to note that the prevalence of NoV in the region is exceptionally low unlike majority of studies from India and other counties which reports NoV as a very prominent diarrheagenic pathogen in both epidemic and sporadic cases [3, 18, 39]. This indicates the low circulation of NoV in the region. In the study, 188 (65.5 %) samples did not have any of the studied viruses. This signifies the prevalence of other enteric viruses such as adenovirus, astrovirus etc. or other etiological agents including bacteria and parasites in remaining cases which further needs to be investigated.
Altogether, among the three viruses, RV was the only pathogen which was detected in all the age groups. The most vulnerable of all the age groups were the children who are below the age of five years and it has been observed that the children >5 years of age have considerably increased RV infection rate in comparison with the adults (P < 0.0001). Studies have reported that the most RV infections in children occurs before the age of 2 years [1, 10, 32, 36] and in this study also the children <2 years of age demonstrated highest rate (50 %) of RV infections among all the patients belonging to different stages of life. EV infection was evenly distributed between the children (<5 years old) and adult (>5 years old) patient groups. This points out the diarrheagenic potential of the EVs in all stages of life and propels us to consider it as one of the major virus involved in both infant and adult diarrhea. All the NoV infections in the study emerged from the infants and thus strengthen the belief of considering NoV as important diarrheagenic pathogens in childhood diarrhea. To summarize, the affect of the diarrheagenic viruses on the subjects of different age groups, it can be observed that the most susceptible are the infants (<2 years) who suffer the major disease burden of the viruses included in the study. This could be because of the incompetence of the developing immune system of the children to set off the sufficient immune response which can combat the infecting pathogens.
As RV was the major pathogen detected in the study, the association of clinical features (vomiting fever and dehydration) with RV infection was studied. It was interesting to note that the children with RV infection have higher rates of vomiting, fever and dehydration in comparison with their RV negative counterparts. This shows that RV infection has a substantial association with the accompanying symptoms (along with diarrhea) and hence severity of illness is higher. On the contrary, in the adult subjects, the rates of vomiting, fever and dehydration was considerably lower in RV positive cases. This leads to the conviction that RV infection results in more severe illness in children than adults and this might be an explanation for the higher RV associated mortality in children than adults.
The seasonal distribution of the viruses revealed some interesting findings. It is reported that rotavirus infections are at peak in the season of winter [12, 21]. But in our study the rotavirus infections were evenly distributed among all the seasons of the year. In fact, the overall infection rate was highest in the months of summer followed by winters. This may be due to the geographical and climatic conditions of the region under study where the summers are not very hot because of the high altitude and hilly terrain. However, there are studies which observed relatively low rotavirus prevalence in winter season [20].
To conclude, this study indicates the predominance of RV in the region and a significant disease burden can be attributed to this virus among all age groups. EV is also detected in considerable fraction and thus imposing a need to consider it as a potential pathogen accountable for human diarrhea. However, a very important diarrheagenic virus, NoV was detected in very low frequency. This study not only throws light on the relative prevalence of the important diarrheagenic viruses but also furnish preliminary data which would be helpful in formulating the preventive strategies against them. In particular, the high infection rate of RV in children warrants immediate attention in order to reduce the morbidity and suspected mortality due to this pathogen. Nevertheless, this study suffers from some limitations including limited sample size and non-inclusion of other viral pathogens such as adenovirus, sapovirus and astrovirus in the study. However, we are in the process of characterization of various bacterial pathogens prevalent in these patients. Nevertheless a more extensive surveillance in the region is required to get the reliable and complete prevalence data of the viruses causing diarrhea in the population.
Acknowledgments
HC is thankful to Department of Science and Technology, Government of India and Department of Biotechnology, Government of India, respectively, for the grants SB/FT/LS-440/2012 and BT/PR6784/GBD/27/466/2012. JV is thankful to Indian Council of Medical Research for grant 5/9-1(26) 2011-12 ECD-II. SJ is thankful to Jaypee University of Information Technology, Solan, Himachal Pradesh, India and Indian Council of Medical Research, New Delhi, India for Junior and Senior Research Fellowships, respectively.
References
- 1.Albano F, Bruzzese E, Bella A, Cascio A, Titone L, Arista S, Izzi G, Virdis R, Pecco P, Principi N, Fontana M, Guarino A. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr. 2007;166:241–247. doi: 10.1007/s00431-006-0237-6. [DOI] [PubMed] [Google Scholar]
- 2.Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infect Dis. 2004;4:91–99. doi: 10.1016/S1473-3099(04)00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard SB, Reaves EJ, Luna CG, Silva ME, Rocha C, Heitzinger K, Saito M, Apaza S, Espetia S, Blazes DL, Tilley DH, Guzman Aguilar RC, Gilman RH, Bausch DG. Epidemiology and genetic characterization of noroviruses among adults in an endemic setting, Peruvian Amazon Basin, 2004–2011. PLoS One. 2015;10:e0131646. doi: 10.1371/journal.pone.0131646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broor S, Ghosh D, Mathur P. Molecular epidemiology of rotaviruses in India. Indian J Med Res. 2003;118:59–67. [PubMed] [Google Scholar]
- 5.Chhabra P, Dhongade RK, Kalrao VR, Bavdekar AR, Chitambar SD. Epidemiological, clinical, and molecular features of norovirus infections in western India. J Med Virol. 2009;81:922–932. doi: 10.1002/jmv.21458. [DOI] [PubMed] [Google Scholar]
- 6.del Refugio Gonzalez-Losa M, Polanco-Marin GG, Manzano-Cabrera L, Puerto-Solis M. Acute gastroenteritis associated with rotavirus in adults. Arch Med Res. 2001;32:164–167. doi: 10.1016/S0188-4409(00)00270-8. [DOI] [PubMed] [Google Scholar]
- 7.Eckardt AJ, Baumgart DC. Viral gastroenteritis in adults. Recent Pat Antiinfect Drug Discov. 2011;6:54–63. doi: 10.2174/157489111794407877. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Singh KP, Jain A, Srivastava S, Kumar V, Singh M. Aetiology of childhood viral gastroenteritis in Lucknow, north India. Indian J Med Res. 2015;141:469–472. doi: 10.4103/0971-5916.159298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada S, Okada M, Yahiro S, Nishimura K, Matsuo S, Miyasaka J, Nakashima R, Shimada Y, Ueno T, Ikezawa S, Shinozaki K, Katayama K, Wakita T, Takeda N, Oka T. Surveillance of pathogens in outpatients with gastroenteritis and characterization of sapovirus strains between 2002 and 2007 in Kumamoto Prefecture, Japan. J Med Virol. 2009;81:1117–1127. doi: 10.1002/jmv.21454. [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Vashistt J, Changotra H. Rotaviruses: is their surveillance needed? Vaccine. 2014;32:3367–3378. doi: 10.1016/j.vaccine.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 11.John J, Sarkar R, Muliyil J, Bhandari N, Bhan MK, Kang G. Rotavirus gastroenteritis in India, 2011–2013: revised estimates of disease burden and potential impact of vaccines. Vaccine. 2014;11:004. doi: 10.1016/j.vaccine.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Kawai K, O’Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine. 2012;30:1244–1254. doi: 10.1016/j.vaccine.2011.12.092. [DOI] [PubMed] [Google Scholar]
- 13.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 14.Kroneman A, Harris J, Vennema H, Duizer E, van Duynhoven Y, Gray J, Iturriza M, Bottiger B, Falkenhorst G, Johnsen C, von Bonsdorff CH, Maunula L, Kuusi M, Pothier P, Gallay A, Schreier E, Koch J, Szucs G, Reuter G, Krisztalovics K, Lynch M, McKeown P, Foley B, Coughlan S, Ruggeri FM, Di Bartolo I, Vainio K, Isakbaeva E, Poljsak-Prijatelj M, Grom AH, Bosch A, Buesa J, Fauquier AS, Hernandez-Pezzi G, Hedlund KO, Koopmans M. Data quality of 5 years of central norovirus outbreak reporting in the European Network for food-borne viruses. J Public Health. 2008;30:82–90. doi: 10.1093/pubmed/fdm080. [DOI] [PubMed] [Google Scholar]
- 15.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieberman JM. Rotavirus and other viral causes of gastroenteritis. Pediatr Ann. 1994;23:529–532. doi: 10.3928/0090-4481-19941001-06. [DOI] [PubMed] [Google Scholar]
- 17.Mathew A, Rao PS, Sowmyanarayanan TV, Kang G. Severity of rotavirus gastroenteritis in an Indian population: report from a 3 year surveillance study. Vaccine. 2014;11:038. doi: 10.1016/j.vaccine.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Menon VK, George S, Ramani S, Illiayaraja J, Sarkar R, Jana AK, Kuruvilla KA, Kang G. Genogroup IIb norovirus infections and association with enteric symptoms in a neonatal nursery in southern India. J Clin Microbiol. 2010;48:3212–3215. doi: 10.1128/JCM.02510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondal A, Sharma K, Malik YS, Joardar SN. Detection of group a rotavirus in faeces of diarrhoeic bovine porcine and human population from eastern India by reverse transcriptase–polymerase chain reaction. Adv Anim Vet Sci. 2013;1(1S):18–19. [Google Scholar]
- 20.Nafi O. Rotavirus gastroenteritis among children aged under 5 years in Al Karak, Jordan. East Mediterr Health J. 2010;16:1064–1069. [PubMed] [Google Scholar]
- 21.Namjoshi GS, Mitra M, Lalwani SK, Sachdeva A, Balasubramanian S, Babji S, Ghosh A, Pandey S, Kulkarni S, Goyal VK. Rotavirus gastroenteritis among children less than 5 years of age in private outpatient setting in urban India. Vaccine. 2014;11:070. doi: 10.1016/j.vaccine.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 22.Panda S, Deb AK, Chawla-Sarkar M, Ramamurthy T, Ganguly S, Pradhan P, Chakraborty A, Desai S, Gupte MD, Dhere R. Factors associated with diarrhoea in young children and incidence of symptomatic rotavirus infection in rural West Bengal, India. Epidemiol Infect. 2014;142:1848–1858. doi: 10.1017/S0950268814000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil PR, Chitambar SD, Gopalkrishna V. Molecular surveillance of non-polio enterovirus infections in patients with acute gastroenteritis in Western India: 2004–2009. J Med Virol. 2015;87:154–161. doi: 10.1002/jmv.23992. [DOI] [PubMed] [Google Scholar]
- 25.Paul A, Gladstone BP, Mukhopadhya I, Kang G. Rotavirus infections in a community based cohort in Vellore, India. Vaccine. 2014;11:039. doi: 10.1016/j.vaccine.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 26.Pons-Salort M, Parker EP, Grassly NC. The epidemiology of non-polio enteroviruses: recent advances and outstanding questions. Curr Opin Infect Dis. 2015;22:22. doi: 10.1097/QCO.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao DC, Ananda Babu M, Raghavendra A, Dhananjaya D, Kumar S, Maiya PP. Non-polio enteroviruses and their association with acute diarrhea in children in India. Infect Genet Evol. 2013;17:153–161. doi: 10.1016/j.meegid.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Richards GP, Watson MA, Fankhauser RL, Monroe SS. Genogroup I and II noroviruses detected in stool samples by real-time reverse transcription-PCR using highly degenerate universal primers. Appl Environ Microbiol. 2004;70:7179–7184. doi: 10.1128/AEM.70.12.7179-7184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotavirus vaccines WHO position paper January 2013—recommendations. Vaccine. 2013;31:6170–6171. doi: 10.1016/j.vaccine.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 30.Rovida F, Campanini G, Piralla A, Adzasehoun KM, Sarasini A, Baldanti F. Molecular detection of gastrointestinal viral infections in hospitalized patients. Diagn Microbiol Infect Dis. 2013;77:231–235. doi: 10.1016/j.diagmicrobio.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sai L, Sun J, Shao L, Chen S, Liu H, Ma L. Epidemiology and clinical features of rotavirus and norovirus infection among children in Ji’nan, China. Virol J. 2013;10:10–302. doi: 10.1186/1743-422X-10-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salinas B, Gonzalez G, Gonzalez R, Escalona M, Materan M, Schael IP. Epidemiologic and clinical characteristics of rotavirus disease during five years of surveillance in Venezuela. Pediatr Infect Dis J. 2004;23:S161–S167. doi: 10.1097/01.inf.0000142465.25992.c3. [DOI] [PubMed] [Google Scholar]
- 33.Saravanan P, Ananthan S, Ananthasubramanian M. Rotavirus infection among infants and young children in Chennai. South India. Indian J Med Microbiol. 2004;22:212–221. [PubMed] [Google Scholar]
- 34.Scarcella C, Carasi S, Cadoria F, Macchi L, Pavan A, Salamana M, Alborali GL, Losio MM, Boni P, Lavazza A, Seyler T. An outbreak of viral gastroenteritis linked to municipal water supply, Lombardy, Italy, June 2009. Euro Surveill. 2009;14:19274. doi: 10.2807/ese.14.29.19274-en. [DOI] [PubMed] [Google Scholar]
- 35.Thongprachum A, Takanashi S, Kalesaran AF, Okitsu S, Mizuguchi M, Hayakawa S, Ushijima H. Four-year study of viruses that cause diarrhea in Japanese pediatric outpatients. J Med Virol. 2015;87:1141–1148. doi: 10.1002/jmv.24155. [DOI] [PubMed] [Google Scholar]
- 36.Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 37.Vuletic B, Obradovic S, Stojkovic-Andjelkovic A, Igrutinovic Z, Radlovic P. Rotavirus gastroenteritis. Srp Arh Celok Lek. 2006;134:166–169. [PubMed] [Google Scholar]
- 38.Wilhelmi I, Roman E, Sanchez-Fauquier A. Viruses causing gastroenteritis. Clin Microbiol Infect. 2003;9:247–262. doi: 10.1046/j.1469-0691.2003.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Han J, Chen L, Xu D, Shen Y, Zha Y, Zhu X, Ji L. Prevalence and genetic diversity of noroviruses in adults with acute gastroenteritis in Huzhou, China, 2013–2014. Arch Virol. 2015;160:1705–1713. doi: 10.1007/s00705-015-2440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng DP, Widdowson MA, Glass RI, Vinje J. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J Clin Microbiol. 2010;48:168–177. doi: 10.1128/JCM.01622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zoll GJ, Melchers WJ, Kopecka H, Jambroes G, van der Poel HJ, Galama JM. General primer-mediated polymerase chain reaction for detection of enteroviruses: application for diagnostic routine and persistent infections. J Clin Microbiol. 1992;30:160–165. doi: 10.1128/jcm.30.1.160-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]