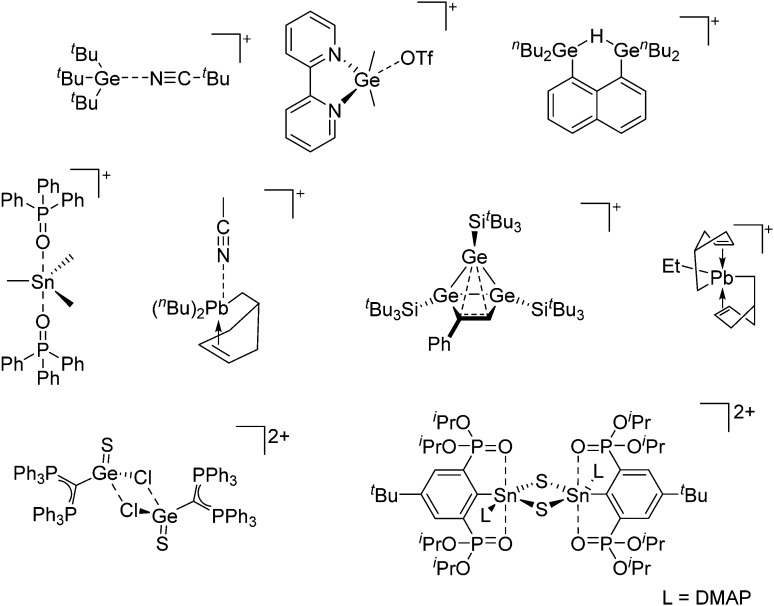
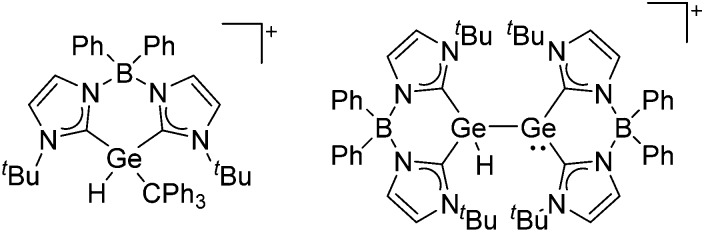
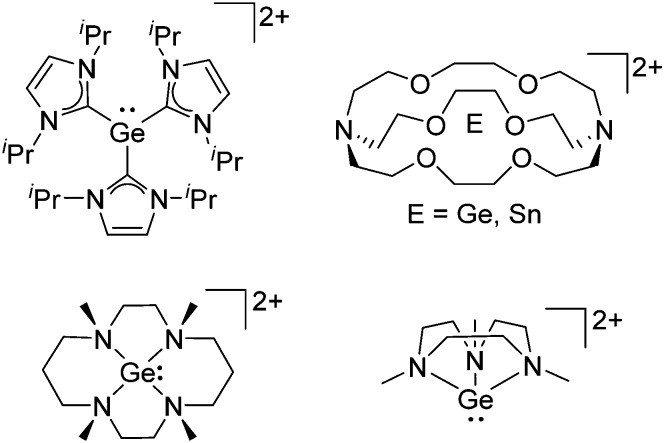
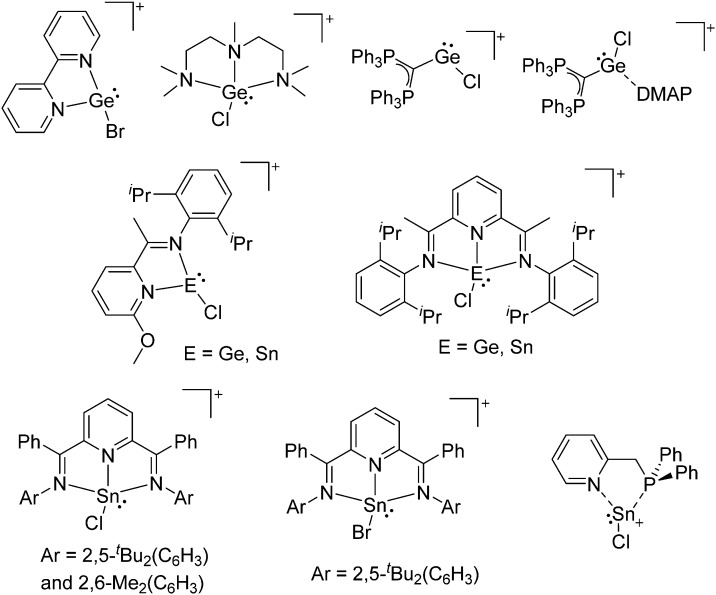
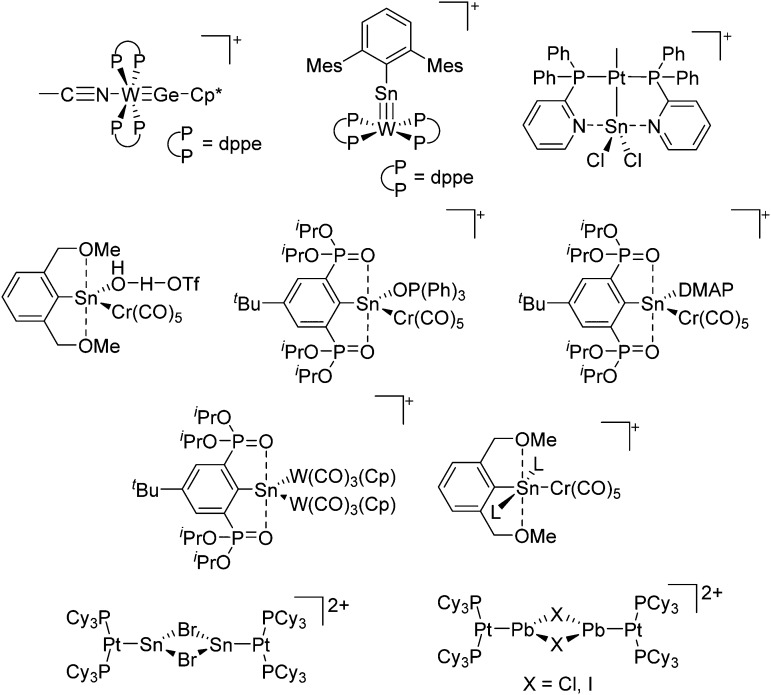
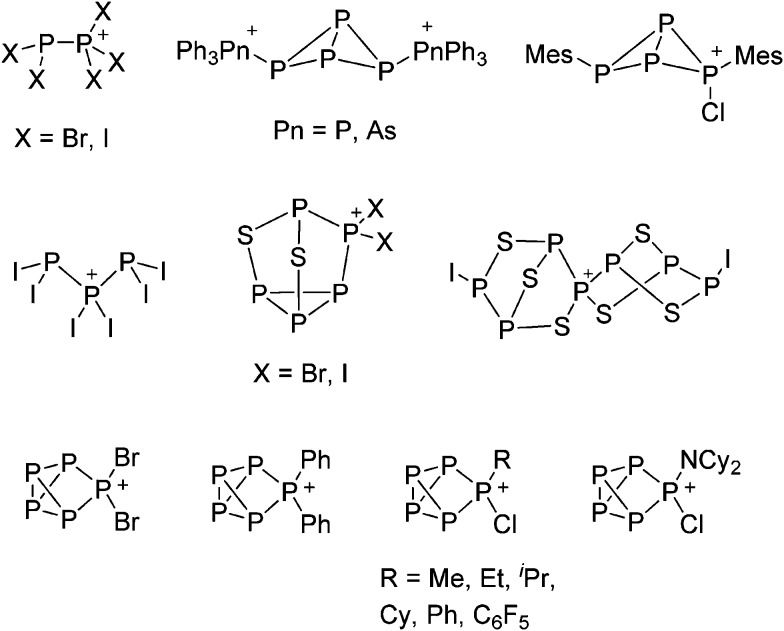
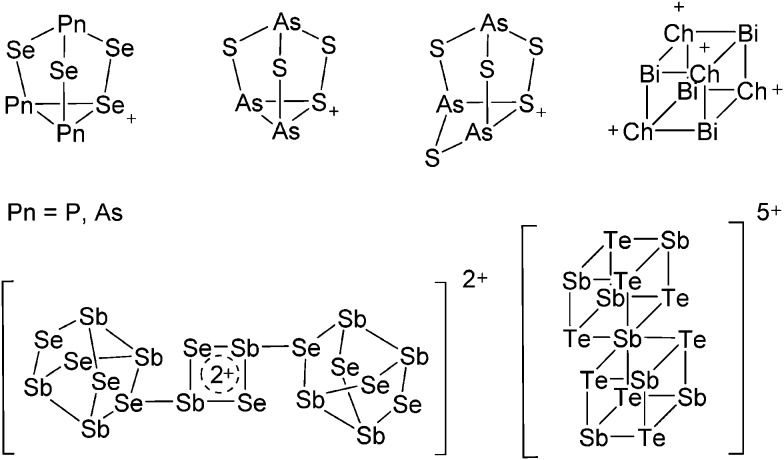
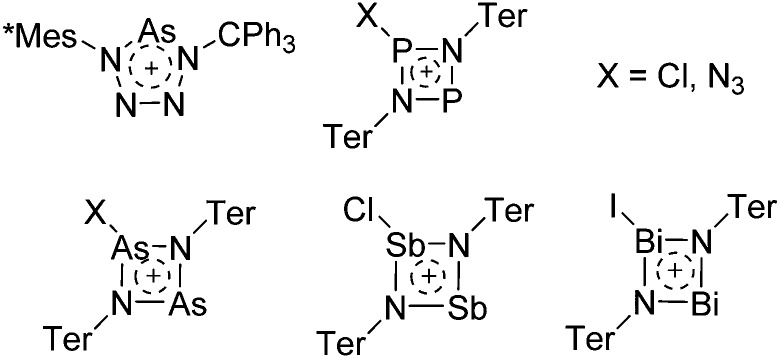
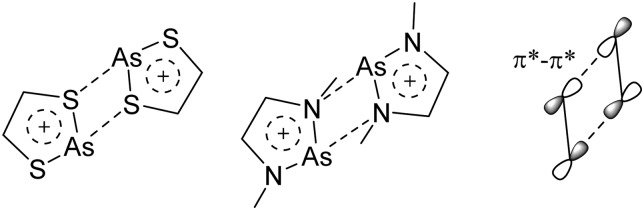
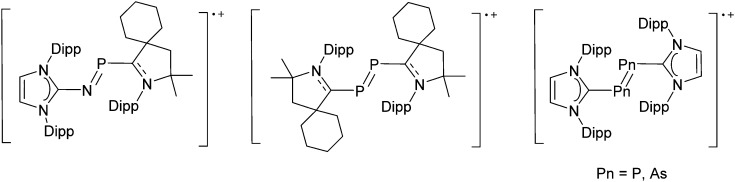
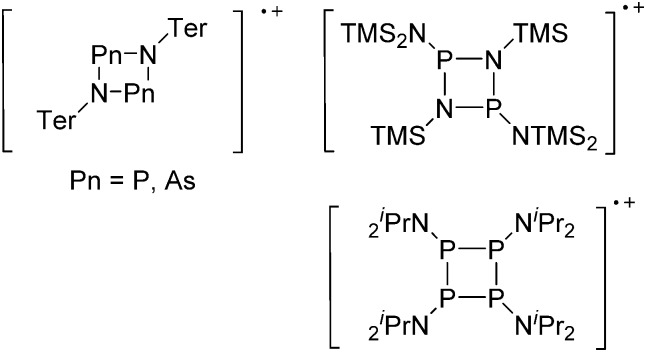
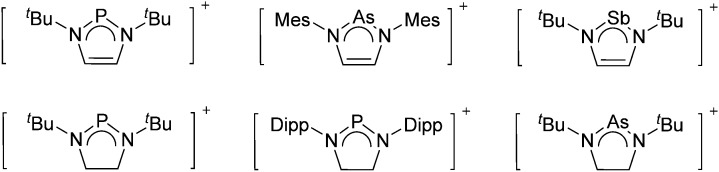
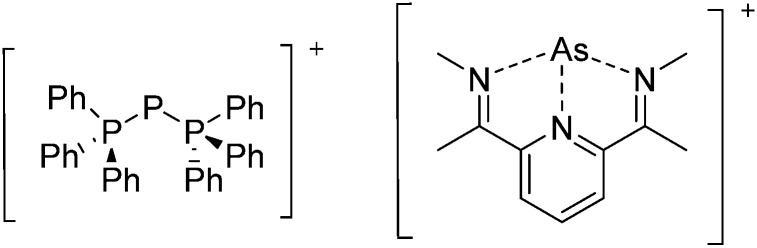
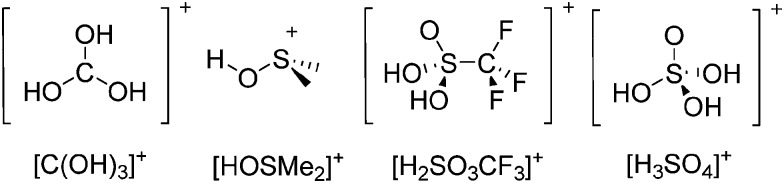
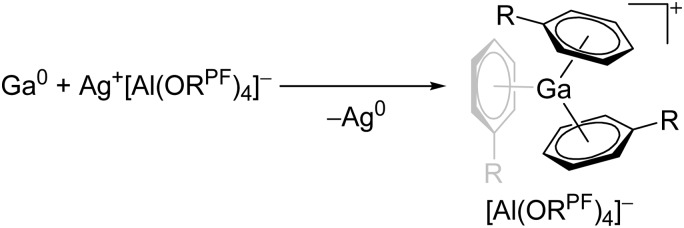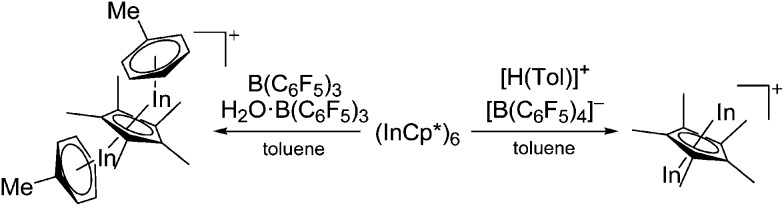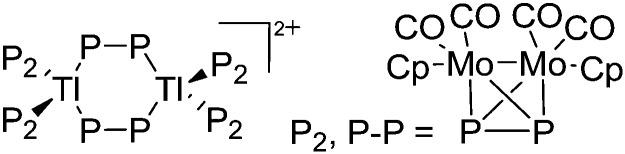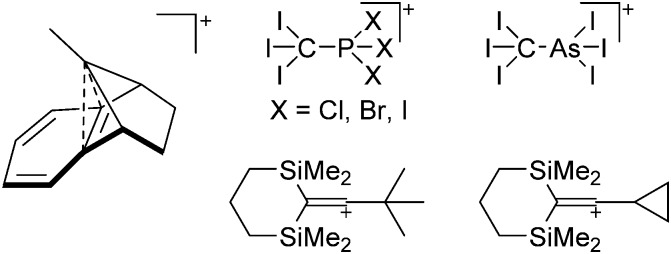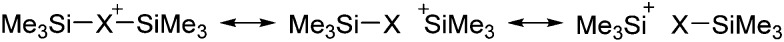 The chemistry of the p-block elements is a huge playground for fundamental and applied work.
The chemistry of the p-block elements is a huge playground for fundamental and applied work.
Abstract
The chemistry of the p-block elements is a huge playground for fundamental and applied work. With their bonding from electron deficient to hypercoordinate and formally hypervalent, the p-block elements represent an area to find terra incognita. Often, the formation of cations that contain p-block elements as central ingredient is desired, for example to make a compound more Lewis acidic for an application or simply to prove an idea. This review has collected the reactive p-block cations (rPBC) with a comprehensive focus on those that have been published since the year 2000, but including the milestones and key citations of earlier work. We include an overview on the weakly coordinating anions (WCAs) used to stabilize the rPBC and give an overview to WCA selection, ionization strategies for rPBC-formation and finally list the rPBC ordered in their respective group from 13 to 18. However, typical, often more organic ion classes that constitute for example ionic liquids (imidazolium, ammonium, etc.) were omitted, as were those that do not fulfill the – naturally subjective – “reactive”-criterion of the rPBC. As a rule, we only included rPBC with crystal structure and only rarely refer to important cations published without crystal structure. This collection is intended for those who are simply interested what has been done or what is possible, as well as those who seek advice on preparative issues, up to people having a certain application in mind, where the knowledge on the existence of a rPBC that might play a role as an intermediate or active center may be useful.
Introduction
Main group chemistry continues to reside at the heart of fundamental as well as applied chemistry. As such, recent years have seen an enormous growth of concepts that shed new light on hitherto undiscovered, or more correctly, underdeveloped areas of main group chemistry. Thus, the availability of stable singlet carbenes 1 as strong donors offered tremendous new perspectives as did the establishment of the frustrated Lewis pairs (FLP) concept 2 or the systematic investigation of (often low valent) cationic mixed main group-transition metal salts. 3–5 In the framework of those approaches, next to other fundamental 6 and applied questions, 7–10 also the stabilization or use of reactive p-block cations (rPBC) with weakly coordinating anions (WCAs) was one focus that led to fascinating new rPBC. This review gives a comprehensive overview on recent rPBC developments since about 2000, but also cites all-time classics in the field. It also includes the fascinating class of transition metal substituted rPBC for which the assignment of the positive charge to one specific moiety is often not clear.
Scope of this review
Many of the p-block elements have relatively high ionization potentials and electronegativities. Thus, most of the stable examples base on delocalization and other electronic or steric effects. In addition, rPBC are often very electrophilic and/or oxidizing. Therefore, chemically stable and inert weakly coordinating anions (WCAs) and solvents are needed to access their salts. These ingredients allowed the syntheses of a large number of fundamentally interesting rPBC of the groups 13–18 in the condensed phase. We discuss typical synthesis routes, give a brief overview of the WCAs, and describe the rPBC ordered according to their main group as well as cation class. However, typical, often more organic ion classes that constitute for example ionic liquids (imidazolium, ammonium, etc.) were omitted, as were those that do not fulfill the – naturally subjective – “reactive”-criterion of the rPBC. As a rule, we only included rPBC with crystal structure and only rarely refer to important cations published without crystal structure.
Handling of substance classes with recent reviews
Some of the substance classes, which fit into this review were just recently and sometimes very comprehensively reviewed (cf. our contribution describing the advances in the synthesis of homopolyatomic cations of the non-metals since 2000 11 ). To reduce the overlap, we decided to give an overview on general aspects such as WCAs in Table 1 and include a short table with relevant reviews for each main group at the beginning of each main group chapter and only list the compounds in these cases. Therefore, we mainly list, but do not describe the cations of this category in the chapters of their corresponding element. Nevertheless, the scope of this review is rather large, which in any case precludes extensive discussions and mainly serves as an overview on what is known.
Table 1. General reviews with focus on WCAs.
| Year | Topic | Title | Ref. |
| 1993 | WCAs | The search for larger and more weakly coordinating anions | 15 |
| 1998 | WCAs | Carboranes: a new class of weakly coordinating anions for strong electrophiles, oxidants, and superacids | 16 |
| 2004 | WCAs | Noncoordinating anions—fact or fiction? A survey of likely candidates | 13 and 12 |
| 2006 | WCAs | Chemistry with weakly-coordinating fluorinated alkoxyaluminate anions: gas phase cations in condensed phases? | 14, 15 and 17 |
| 2006 | WCAs | Chemistry of the carba-closo-dodecaborate(–) anion, [CB11H12]– | 18 |
| 2008 | π-Complexation of post-transition metals by neutral aromatic hydrocarbons: the road from observations in the 19th century to new aspects of supramolecular chemistry | 19 | |
| 2013 | WCAs | Weakly coordinating anions: halogenated borates and dodecaborates | 20 |
| 2013 | WCAs | Weakly coordinating anions: fluorinated alkoxyaluminates | 21 |
| 2013 | WCAs | Weakly coordinating anions: highly fluorinated borates | 22 |
| 2015 | WCAs | Taming the cationic beast: novel developments in the synthesis and application of weakly coordinating anions (Publication in progress by IK) | 23 |
Reactive p-block cations
The rPBC in this article need a WCA as counterion and, therefore, we first briefly describe typical WCAs and give some advice on their selection before turning to typical ionization and synthetic procedures for rPBC preparations. Thereafter, the ordering of the cation classes for the individual sections is described, and finally the rPBC are grouped according to the main group of the relevant cationic entry. In addition, first applications emerged for rPBC salts and will be highlighted in the respective cation sections.
WCA overview
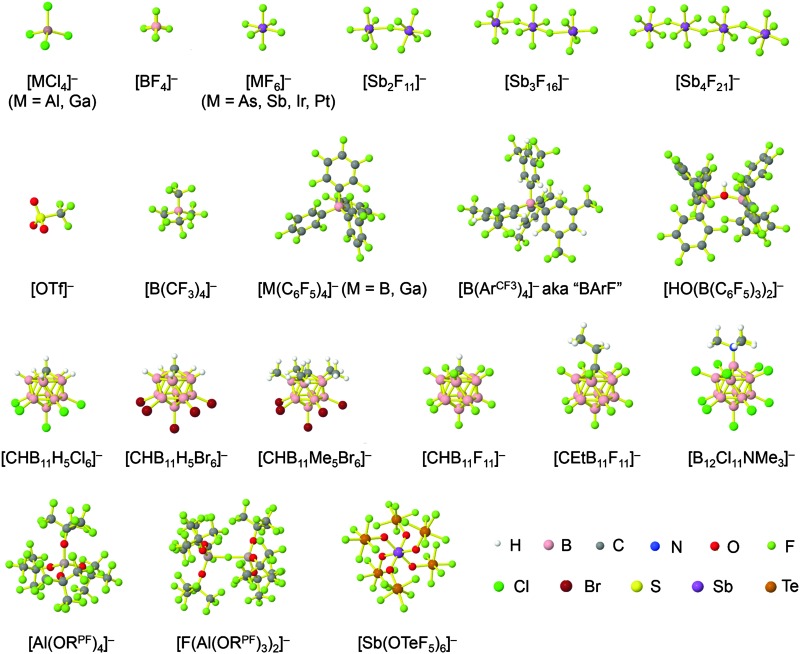
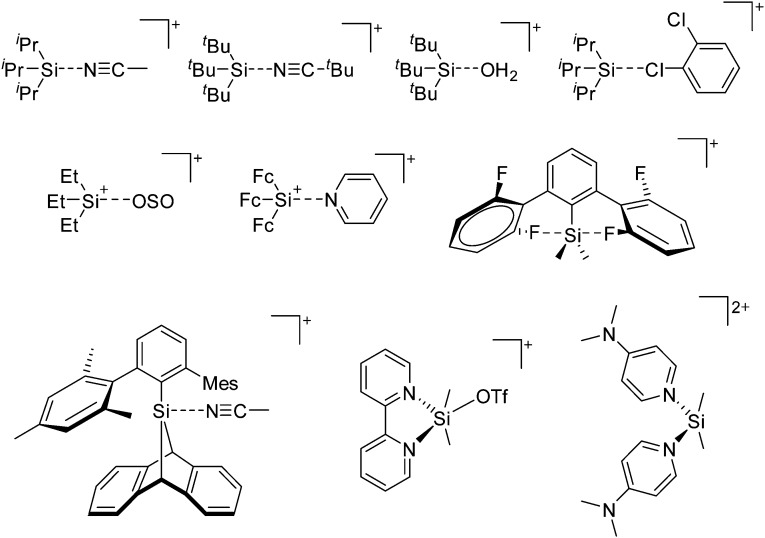
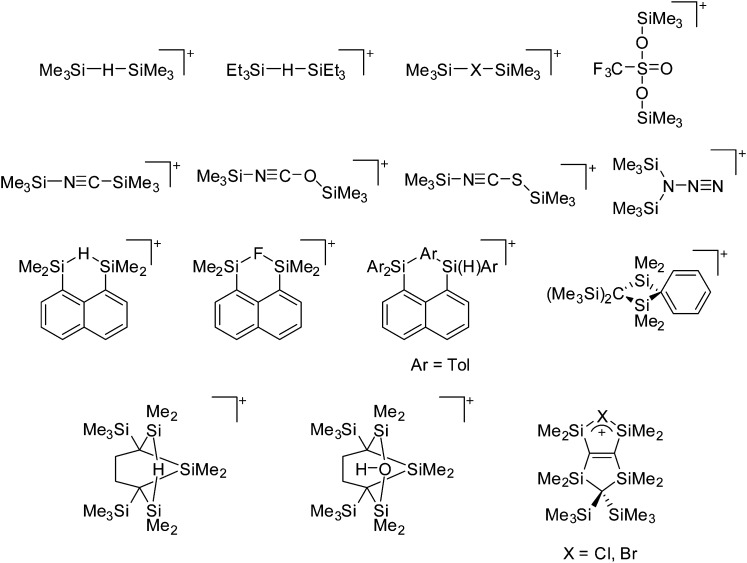
Because of their potential in fundamental and applied chemistry, 12–15 a great variety of different WCA types are currently known (Fig. 2) and was frequently reviewed (Table 1).
Fig. 2. Some of the weakly coordinating anions discussed in this review.
But which out of the multitude of published WCAs shown in Fig. 2 should be used for a given problem…? Is there one best WCA that fulfills all needs…?
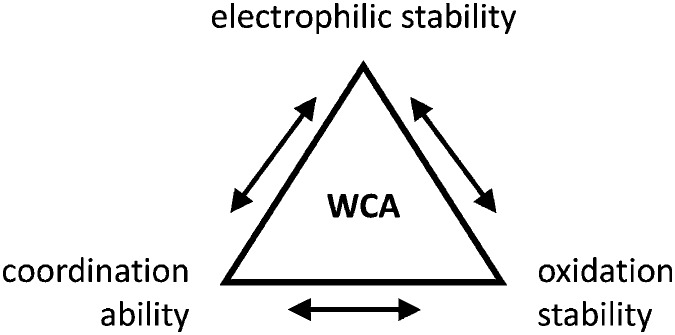
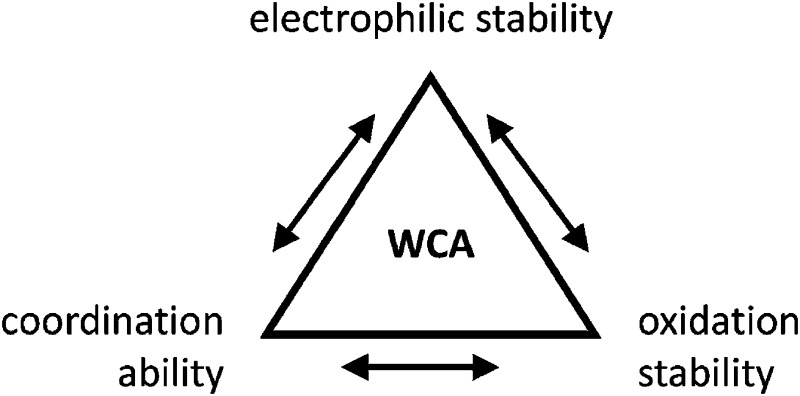
Clearly holds: the more reactive the rPBC are, the more demanding is the task for the anions, to meet the requirements for a successful stabilization in the condensed phase. Some of this reactivity may be dampened kinetically by the use of suitable bulky ligands, e.g. for the silylium ions. However, there is not one ultimate WCA that fulfills all requirements to allow for use with all in here described rPBC. Typically, rPBC follow at least one of the following classifications:
• Being a strong electrophile, thus having a strong tendency to coordinate an anion or solvent. Silylium ions SiR3 + are good examples for this. This coordination is often the entrance towards an anion degradation by heterolytic cleavage of a bond in the WCA.
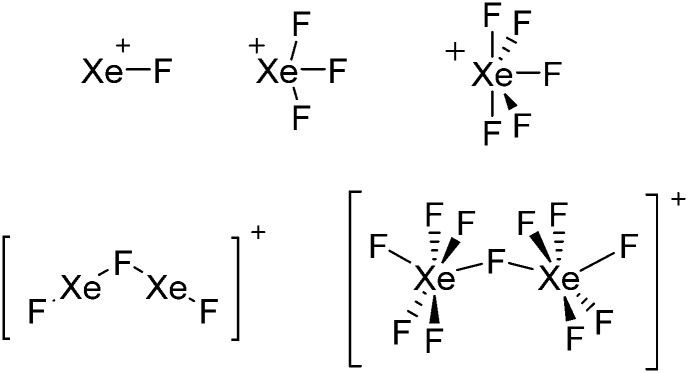
• Being a strong oxidant, thus needing anions and solvents compatible with this need. Halogen and noble gas cations are typical examples.
• Being a weakly bound complex, in which the interesting main group particle can easily be displaced by anion or solvent, just as in many metal–non-metal clusters. This includes protonated, weakly basic molecules that tend to pass the proton to more basic and more coordinating anions or solvents.
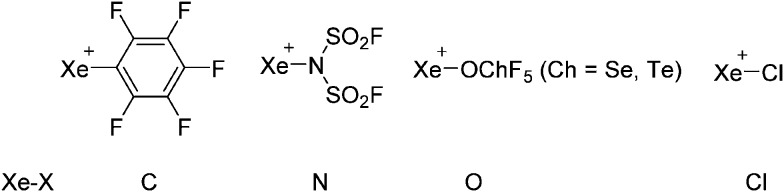
Thus, the demand for very weak coordination behaviour is only medium for several very oxidizing cations, but the necessity of the WCA being stable against oxidation is a prerequisite of highest importance. For example, the typical counterions of group 16 to 18 rPBC are fluorometallates like [MF6]– or [M2F11]– (M = As, Sb) compatible with (i) the oxidizing power of the cation and (ii) the typically used super acid solvents. However, despite the fact that fluoroantimonates allow for the synthesis of tremendously oxidizing cations like [Xe2]+, they fail to stabilize the extreme electrophiles [SiR3]+ and form F-SiR3 and antimony pentafluoride. On the other hand, with some steric protection at the silylium ion, already the [B(C6F5)4]– WCA suffices to stabilize for example the [Si(Mes)3]+ cation. By contrast, and due to the aromatic system, [B(C6F5)4]– is not compatible with the only mildly oxidizing [NO]+ or [NO2]+ cation. Some thoughts that allow for the selection of a suitable WCA for a given problem may be summarized by the triangle shown in Fig. 1.
Fig. 1. Triangle delineating the independent demands of a rPBC that lead to different mixtures of the WCA properties necessary for its successful stabilization.
With Fig. 1 in mind, a personal selection of the “best WCAs” includes [1-H-CB11Me5Br6]–, 24 [1-Et-CB11F11]–, 25 [CB11(CF3)12]–, 26 [Sb4F21]–, 27 [Sb(OTeF5)6]–, 28 [Al(ORPF)4]–, 29–31 [B(C6F5)4]– 32–34 and [B(CF3)4]–. 35 A recent noteworthy addition overcoming the frequent disorder of the also towards fluoride abstraction less stable [B(ArCF3 )4]– anion is the [B(ArCl)4]– WCA. 36
Other aspects that will influence the choice, are the synthetic availability of the entire WCA class, or the specific starting material necessary to ionize the system of interest. In this respect, most of the WCAs known so far also do have disadvantages: the carborates are hard to synthesize and have often low yields. [CB11(CF3)12]– is even explosive, as is the LiC6F5 intermediate needed for the [B(C6F5)4]– synthesis. In addition, starting materials such as solvent free Ag+ salts or [NO]+, [NO2]+ are not accessible as salts of [B(C6F5)4]–. Anions with multiple –CF3 groups often tend to disorder in the solid state, which sometimes makes it hard to solve or refine the crystal structure. The problems associated with the refinement of structures containing the [Al(ORPF)4]– WCA even led to the development of the software tool DSR. 37 It allows for the simplified refinement of such disordered structures and is now implemented with standard programs like OLEX2. 38
Therefore, the search for new useful anions is still in progress. With the amminated chloroborate cluster anion [1-Me3N-B12Cl11]– another promising candidate that refined earlier ideas by S. Strauss et al., 39 was just recently presented by Jenne et al. in 2014. 40 The positive charge of the ammonium function leads to an overall –1 charge and makes it possible to use the in 30 g scale accessible –B12Cl11 cluster residue. Important starting materials M+[1-Me3N-B12Cl11]– (M+ = Na+, [HNMe3]+, [HNOct3]+, [NO]+, [CPh3]+, [N n Bu4]+, [Et3Si]+) have been described facilitating the application. 40,41 More details on typical WCA starting materials to introduce a counterion into the given system can be found in the synthesis section below as well in the numerous WCA reviews cited in Table 1.
Synthesis routes to reactive main group cation salts
At the beginning, each proposal to prepare a target-rPBC needs to consider the choice of the WCA as delineated in the preceding section, as well as the available starting materials, ionization method and reaction medium.
WCA starting materials
A suitable starting material, should be accessible in good yields and contain a useful cation that typically acts as either a strong oxidant (e.g. [O2]+, 42 [NO]+, 29 [NO2]+, 43 N(arene)3 + 44 ) a halide (e.g. Li+, 45 Na+, Ag+ 46 ), hydride- or alkyl-abstractor ([CPh3]+ 47 ), a Brønsted acid ([H(OEt2)2]+, 48 [H(NMe2Ph)]+) or a metal cation, if a simple metal complex is desired as product (e.g. Cu+ 49,50 ) (Table 2). Neutral Lewis acids for bond heterolysis are available in great variety and include the classical simple halides MIIIX3 and MVX5 (MIII = B, Al, Ga; MV = P, As, Sb, Bi; X = F, Cl, Br, I; not all combinations useful), the rather fine tunable B(aryl)3 acids (aryl = fluorinated, 51 chlorinated 52 or fluoroalkylated 53 aromatic residue), or aluminum based systems like Al(C6F5)3 54 and Al(ORF)3. 55 Also the ion-like R3Si(WCA) compounds have frequently been used. 56,57 Recent systematic work analyzed the potency of a given Lewis acid versus fluoride, chloride, hydride and methanide as a base. It includes benchmark Lewis acidity values for a smaller set of simple MX n acids. 58 Neutral Brønsted acids like HF, HNTf2 and derivatives thereof, 59 or combinations of Brønsted and Lewis acids like HBr/nAlBr3 60,61 are suitable for protonations. Novel, and in large quantity available very strong acids like RHFOSO3H 62 should also be mentioned.
Table 2. Acronym (Acr.) and type of the classified synthesis routes leading to rPBC.
| Acr. | Type | Example | Ref. |
| Com | Complexation reaction |
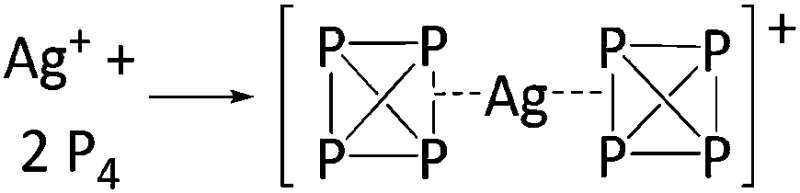
|
72 |
| Ox | Oxidation reaction; including 1e– and 2e– oxidations. |
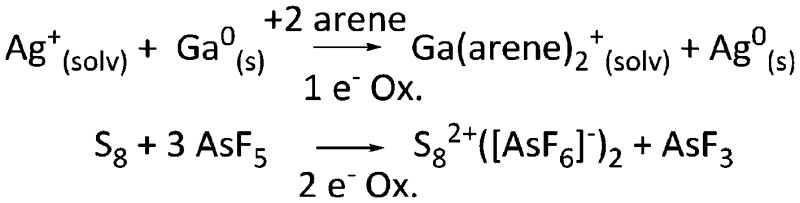
|
30 and 31 |
| Lewis | Lewis acid induced halogen bond heterolysis with neutral Lewis acids, including ion-like compounds. |
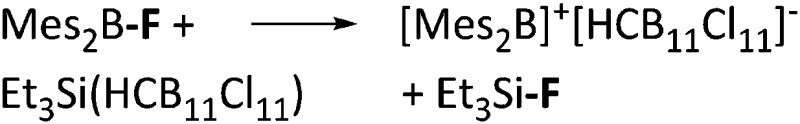
|
32, 33 and 73 |
| Salt | Salt elimination reaction |

|
74 |
| Hyd | Hydride metathesis reaction with neutral or ionic H–-acceptor |
 a
a
|
76 |
| Alk | Alkyl metathesis reaction with neutral or ionic R–-acceptor |
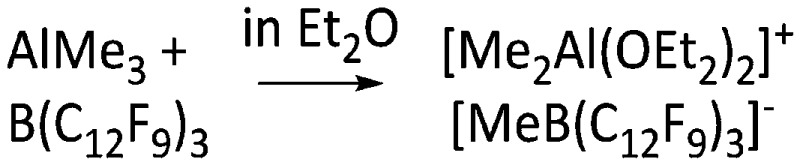
|
77 |
| Ins | Insertion reaction |
|
78 |
| Prot | Protonation reaction |
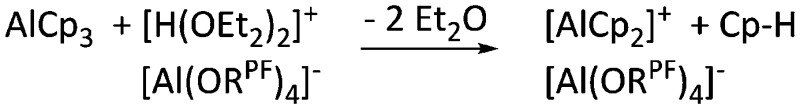
|
79 |
| Lig | Ligand exchange reaction |
|
80 |
| Ion | Ionization |
|
81 |
| Other | Other reaction not classified as one of the above | — | — |
a This type of reaction is sometimes referred to as Bartlett–Condon–Schneider (BCS) type hydride transfer reaction. 75
Suitable media/solvents
Since the synthesis of reactive ions is aspired, a suitable reaction medium should favorably be polar but not itself be a base or a nucleophile. This often rules out classical polar solvents that are itself good donors such as ethers or nitriles. Often chlorinated solvents CH2Cl2 (ε r = 8.9), 1,2-Cl2C2H4 (ε r = 10.4) or Cl–Ph (ε r = 5.7) tend to be good choices that nevertheless are incompatible with strong electrophiles like the silylium ions [SiR3]+. Fluorinated arenes like F–Ph (ε r = 5.5) and 1,2-F2C6H4 (ε r = 13.4) are good additions that became cheaper (but not cheap) over the last decade. However, they are incompatible with oxidants like [NO]+ or [NO2]+ due to nitration/nitrosation reactions. Especially for non-metal cations, often superacids or SO2 (ε r = 16.3), SO2ClF (ε r = n.a.) etc. are the solvents of choice. ILs 63 like acidic BMIM[AlCl4] 64,65 and others were shown in recent years to be very promising media for rPBC cation synthesis. 65–67 Especially for group 15 cations, solvent free reactions using Me3Si-OSO2CF3 or MX3 (M = Al, Ga; X = Cl, Br) were shown to provide quantitative yields of the desired salts. By contrast, several of such reactions do work only incomplete or not at all in solution. 68 Similarly, protonations with HBr/nAlBr3 turned out to be best done solvent free. 60,61
The recently established concepts of absolute acidity, 69 absolute reducity 70 and their two-dimensional combination as the protoelectric potential map 70 can be used to understand protonation and/or redox chemistry over medium/solvent and even phase boundaries. This also includes ILs and therefore a thermodynamically sound pH definition has been introduced for IL media. 61,71
Ionization protocols
Overall, we have categorized the rPBC included with the tables in the following sections by an acronym describing the synthetic approach used for their preparation. The synthesis routes are collected, explained and abbreviated in Table 2. Almost all the early approaches to reactive main group cations used halide abstractors as the Lewis acids AsF5 or SbF5, which form the conjugated [AsF6]– or [SbF6]– WCAs through the reaction. The trityl cation is a hydride abstractor, which is especially in case of silanes as starting materials very useful to produce silylium cations. Most of the metal–non-metal complexes were synthesized by complexation of a non-metal molecule (e.g. P4, S8, Cl2, Xe) with a metal salt of a WCA. The coinage metals CuI, AgI and AuI with their d10 electron configuration induce positive charge on the main group elements, stabilize the almost undistorted non-metal clusters, and provide insights in their bonding situation. If the cation is a strong oxidant, it is also possible to oxidize neutral substrates directly to give reactive cations, which are in turn stabilized by the corresponding WCA. An interesting recent addition are the transfer oxidation of e.g. the simple diorganodichalcogenides R2E2 (E = S, Se) with the combination of XeF2 (primary oxidant and source of fluoride) and a Lewis acid. 82
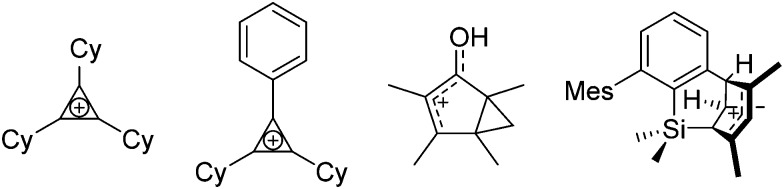
On the representations of the cation chemical structures
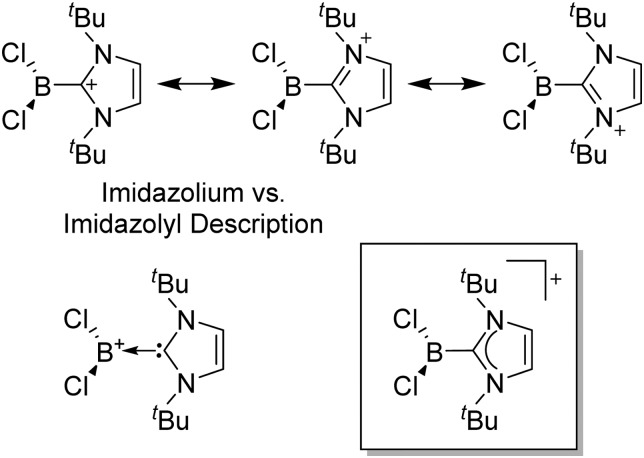
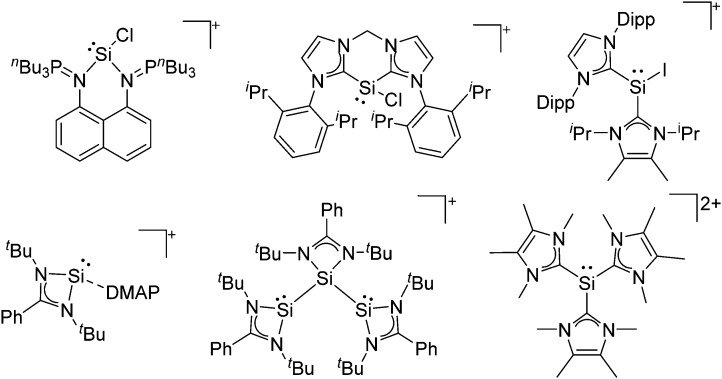
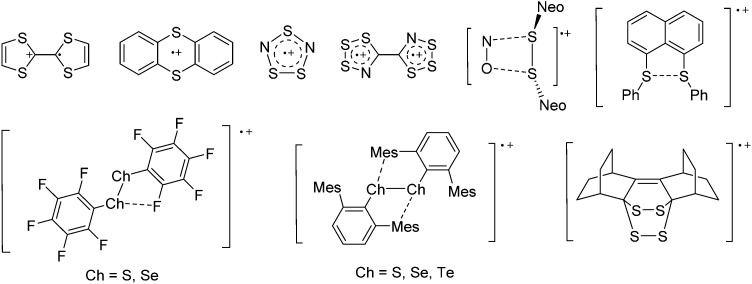
Note that the structural diagrams used throughout this review obey a distance criterion for interactions, but not necessarily a 2e2c-understanding of every interaction line. However, at least in the organic residue we attempted to follow an electron precise 2e2c picture. Necessarily, this gets difficult for structures with N-heterocyclic carbenes that formally allow for a neutral dative (imidazolyl) as well as ionic (imidazolium) description (Fig. 3).
Fig. 3. Possible descriptions of NHC-containing structures exemplified for the simple [Cl2B-I t Bu]+ cation.
For simplicity, we chose the representation shown in the box in Fig. 3 and adopted similar drawings for related cases throughout. Thus, we only use arrows for relatively weak interactions with the bonding situation in ammine-borane H3B←:NH3 being the prototype as suggested by A. Haaland, 83 and later contributions. 84 For thoughts on these ongoing discussions, see these recent publications. 85 Only if the positive charge can clearly (and not just formally) be attributed to one atom, we assigned the charge to this atom. More common is the case in the box in Fig. 3, in which the charge may be delocalized to quite a series of atoms and therefore we placed the charge at the upper right corner.
Ordering of the cation classes
The rPBC were as far as possible ordered according to accepted cation classes that may either refer to the number of valence electrons (i.e. the onium/enium/inium-series) or to the structure. In each subchapter, we intend to go from homoatomic, to binary and then to more complex cation compositions. The not always consistently used classification according to onium- (8 VE), enium- (6 VE) and inium-cations (4 VE) presents some problems. Note, that the coordination number of a group 14 onium ion may not always be four, as the σ-donation of π-density of a donating double bond may increase the coordination number to 5 as, for example, in the 2-norbornyl cation, a carbonium ion. 86 Similar considerations hold for other donor coordinated onium- and enium-ions. Thus, we typically include the coordination number in the cation classification, for example as ligand substituted, (CN = 2). By contrast to these cation assignments, the group 15 to 18 cations were in addition classified by the oxidation state of the central atoms. This is often used for such rPBC. In addition, we included ion-like compounds that were initially defined for silylium compounds with coordinated counterions that structurally have to be addressed as a tight ion-pair but from the reactivity still bear a considerable amount of reactivity related to the free cation, e.g. see the ion like silylium compounds R3Si(WCA) in Fig. 4. Related cases were published for coordinated aluminum cations, e.g. R2Al(WCA), and were used in a similar manner. 87 For ion-like compounds we keep the notation with the anion in parentheses and no charges written, as in R3Si(WCA) and R2Al(WCA). Heteropolyatomic clusters were discussed in the group of their most electropositive element (e.g. [P3Se4]+ in group 15, but [S4N4]2+ in group 16).
Fig. 4. Definition of ion-like compounds as exemplified for silylium ions.
In the following chapters we describe the rPBCs of the Group 13 to 18 elements and give selected representative examples for each cation type. However, for reasons of legibility, the full tables that comprehensively cover the rPBC entries of the groups, are collected in landscape format at the end of this document.
Group 13 cations
Traditionally, group 13 chemistry is dominated by compounds in the +III oxidation state. 88 Of those, the simple halides are commonly applied as Lewis acid catalysts and initiators (e.g. BF3) and usually associated with anion formation (e.g. [BF4]–). However, discrete trivalent group 13 cations have been found to be more reactive, owing to their greater electrophilicity if paired with coordinative unsaturation. 10,89 Except for boron, it has become increasingly possible to stabilize group 13 cations in their +I oxidation state, e.g. by employing bulky substituents and/or WCAs 31,90–92 (for thallium, this is the favored oxidation state due to the inert pair effect 93 ). Featuring a lone pair of electrons and empty p-orbitals, the +I cations are ambiphilic and can function both as Lewis base or acid, thus offering unique reactivities and selectivities in organometallic chemistry, 94–97 as well as organic 98 and polymer 7,99 syntheses. Overall, different aspects of the chemistry of cationic group 13 compounds were reviewed and these contributions are compiled in Table 3. In this context, this sections intends to give a comprehensive overview of reactive group 13 cations of the larger WCAs since about 2000. Due to the large scope of this chapter, we mainly omit rPBC with the simple halometallate based counterions and only include those in special cases of high relevance.
Table 3. Review articles including cationic group 13 compounds.
| Year | Group | Title | Ref. |
| 1985 | 13 | Arene complexes of univalent gallium, indium and thallium | 100 and 101 |
| 1998 | 13 | Cationic group 13 complexes | 102 |
| 2004 | 13 | From group 13–group 13 donor–acceptor bonds to triple-decker cations | 94 |
| 2005 | 13 | Borinium, borenium, and boronium ions: synthesis, reactivity, and applications | 89 |
| 2007 | 13 | Development of the chemistry of indium in formal oxidation states lower than +III | 103 |
| 2008 | 13 | Borylene transfer from transition metal borylene complexes | 13 and 12 |
| 2008 | 13 | Synthesis, characterization, and applications of group 13 cationic compounds | 95 |
| 2009 | 13 | Highly electrophilic main group compounds: ether and arene thallium and zinc complexes | 90 |
| 2009 | 13 | Transition metal borylene complexes: boron analogues of classical organometallic systems | 104 |
| 2010 | 13 | Electron-precise coordination modes of boron-centered ligands | 105 |
| 2011 | 13 | Coordination chemistry of group 13 monohalides | 96 |
| 2011 | 13 | New light on the chemistry of the group 13 metals | 88 |
| 2011 | 13 | The chemistry of the group 13 metals in the +I oxidation state | 106 |
| 2011 | 13 | Mixed or intermediate valence group 13 metal compounds | 107 |
| 2011 | 13 | Coordination and solution chemistry of the metals: biological, medical and environmental relevance | 108 |
| 2012 | 13 | Cationic tricoordinate boron intermediates: borenium chemistry from the organic perspective | 109 |
| 2012 | 13 | Cyclopentadiene based low-valent group 13 metal compounds: ligands in coordination chemistry and link between metal rich molecules and intermetallic materials | 110 |
| 2012 | 13 | Low-oxidation state indium-catalyzed C–C bond formation | 98 |
| 2013 | 13 | 1.17-low-coordinate main group compounds – group 13 | 97 |
| 2013 | 13 | Transition metal borylene complexes | 5 |
| 2013 | 13 | Boron, aluminum, gallium, indium and thallium | 111 |
| 2015 | 13 | Discrete cationic complexes for ring-opening polymerization catalysis of cyclic esters and epoxides | 10 |
Boron cations
For a long time, boron cations have remained a chemical curiosity due to their redox lability. However and partly owing to the developments in the field of WCAs, more and more boron-based cations are being reported. Overall, the cations can be classified according to the coordination number at boron: i.e., di-, tri-, and tetra-coordinated boron cations are referred to as borinium, borenium and boronium cations. To this day, the boron cations have been most notably reviewed by Nöth (1985; a milestone in cationic boron chemistry), 112 Piers (2005; structural and bonding aspects) 89 and Vedejs (2012; reactivties and applications). 109
Alkyl-/aryl substituted (CN = 2)
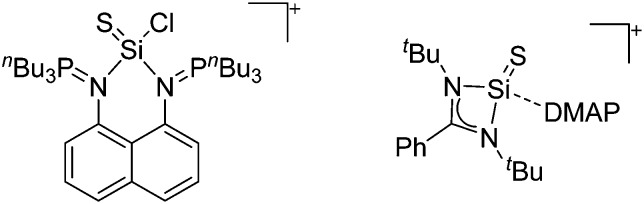
To our knowledge, there is only one contribution to this class of compounds: i.e., the recently reported [Mes2B]+ borinium cation with the very good [HCB11Cl11]–/[B(C6F5)4]– WCAs. 32,113 Herein, the boron atom adopts a linear di-coordinated structure and the Mes substituents are aligned orthogonal to each other, allowing for a perfect shielding as well as π-donation into the empty p-orbitals of the highly electrophilic borinium cation (cf. the modelled delocalized molecular orbitals). The [Mes2B]+ cation is likely to become a textbook compound as it is the first borinium cation that does not rely on strongly π-donating heteroatom substituents (cf. the earlier reported [( t Bu3PN)2B]+ cation 114 in the section ligand substituted (CN = 2) in Table 8).
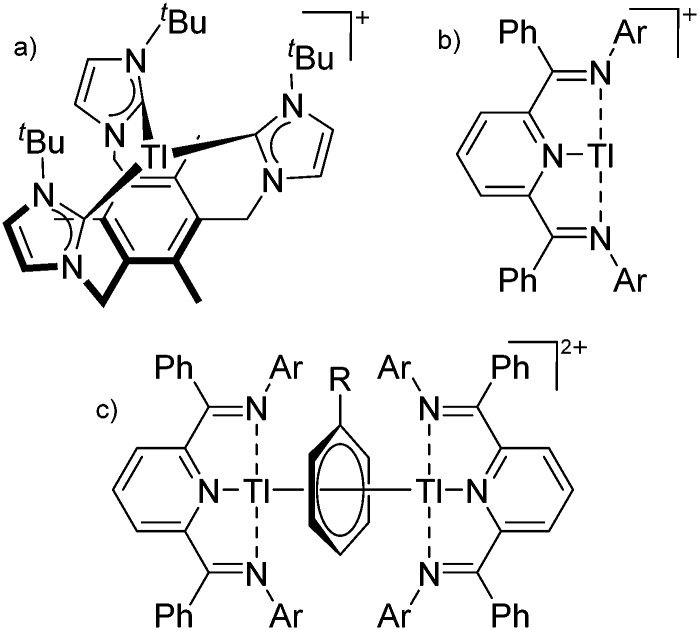
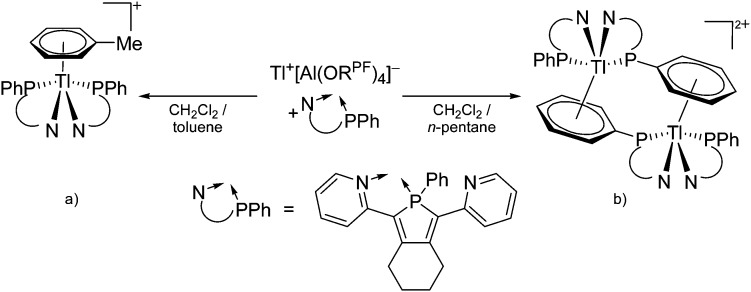
Table 8. Group 13 cations, their counterions (WCA) as well as the synthesis routes. The entries are ordered as follows: (i) from boron- to thallium-based cations, (ii) from unsubstituted, via alkyl/aryl, Cp, arene and ligand to transition-metal substituted cations, (ii) from low to high CNs, (iv) from mono- to multinuclear group 13 complexes. Note that the structural diagrams obey a distance criterion for interactions, but not necessarily a 2e2c-understanding of every interaction line. See comment in the Introduction.
| Cation | WCA | Class a | Synthesis | Comment/structure | Ref. |
| Unsubstituted | |||||
| In+ | [OTf]– | Prot | InCp* + H+[WCA]– | Soluble in organic solvents in contrast to the In(i) halides | 182 |
| Tl+ | [B(ArCF3 )4]–/[B(C6F5)4]– | Prot | TlOEt + [H(OEt2)2]+[WCA]– | — | 196 and 197 |
| Tl+ | [B(OTeF5)4]– | Lewis | Tl+[OTeF5]– + B(OTeF5)3 in CH2Cl2/1,2-C2Cl3F3 | — | 198 |
| Tl+ | [Al(ORPF)4]–/[Al(ORHF)4]–/[Al(ORMeF)4]– | Salt | TlF + Li+[WCA]– | — | 199 and 200 |
| Alkyl/aryl substituted | |||||
| [Mes2B]+ | [HCB11Cl11]–/[B(C6F5)4]– | Salt | Mes2BF + Et3Si(HCB11Cl11)/[Et3Si(Mes)]+[WCA]– |
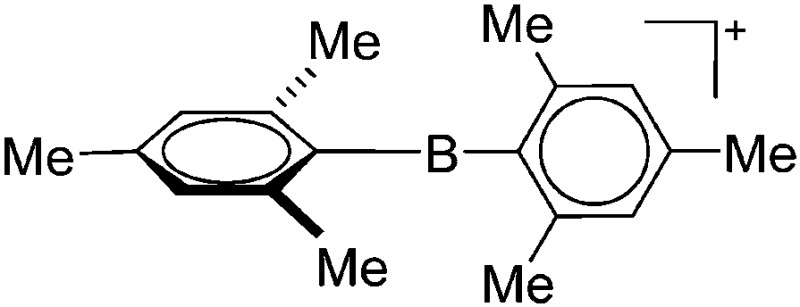
|
32 and 113 |
| (R2Al) (R = Me, Et) | [B12Cl12]2– | Alk | R3Al + {[CPh3]+}2[B12Cl12]2– | Ion-like compound | 87 |
| (Et2Al) | [CB11H6X6]– (X = Cl, Br) | Alk | Et3Al + [CPh3]+[CB11H6X6]– | Ion-like compound | 128 |
| [(2,6-Mes2C6H3)2Al]+ | [B(C6F5)4]– | Hyd | (2,6-Mes2C6H3)2AlH + [CPh3]+[WCA]– | Related structure to the [Mes2B]+ cation, though the Mes moieties of the 2,6-Mes2C6H3 substituent additionally shield the aluminum cation | 129 |
| [(2,6-Mes2C6H3)2Ga]+ | [Li{Al(ORHF)4}2]– | Salt | (2,6-Mes2C6H3)2GaCl + 2Li+[WCA]– | Similar structure as the [(2,6-Mes2C6H3)2Al]+ cation | 158 |
| Cyclopentadienyl complexed | |||||
| [(η5-Cp)2Al]+ | [Al(ORPF)4]– | Prot | AlCp3 + [H(OEt2)2]+[WCA]– | — | 79 |
| [(η5-Cp′)2Al]+ | [B(C6F5)4]– | Alk | Cp′3Al + [CPh3]+[WCA]– | — | 131 |
| [(η5-Cp*)2Al]+ | [{Ph(Me)B(η5-C5H4)2}ZrCl2]– | Alk | Cp*2AlMe + {Ph(SMe2)B-(η5-C5H4)2}ZrCl2 + [Ph3P N PPh3]+Cl– | — | 132 |
| [(η5-Cp*)2Al]+ | [MeB(C6F5)3]– | Alk | Cp*2AlMe + B(C6F5)3 | — | 133 |
| [(η5-Cp)2(Et2O)2Al]+ | [Al(ORPF)4]– | Prot | AlCp3 + [H(OEt2)2]+[WCA]– | Et2O can coordinate the [(η5-Cp)2Al]+ cation | 79 |
| [Ga2(η5-Cp*)]+ | [B(ArCF3 )4]– | Prot | [H(OEt2)2]+[WCA]– + GaCp* |
|
159 |
| [(η1-Cp*)(η3-Cp*)Ga]+ | [BF4]– | Prot | Cp*3Ga + HBF4 | cf. [B(η5/η1-Cp*)2]+ and [Al(η5/η5-Cp*)2]+ | 160 |
| [In2(η5-Cp)]+ | [Cp3In–Cp–InCp3]– | Com | In+[OTf]– + Cp2Mn in C6H5Me | Inverted sandwich structure (cf. the related [Ga2(η5-Cp*)]+ cation) | 183 |
| [In2(η5-Cp*)]+ | [B(C6F5)4]– | Prot | [(C6H5Me)H]+[WCA]– + InCp* | Similar structure to the [Ga2(η5-Cp*)]+ cation | 185 |
| [(μ-η5-C5Me5)In2(η6-Tol)2]+ | [(C6F5)3BO(H)B(C6F5)3]– | Prot, Com | (Cp*In)6 + B(C6F5)3 + H2O·B(C6F5)3 |
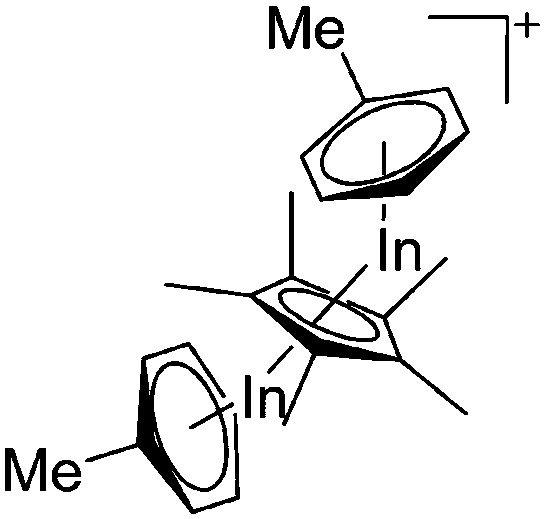
|
184 and 185 |
| Arene complexed | |||||
| [Ga(η6-C6H5R) n ]+ (R = F, Me; n = 2, 3) | [Al(ORPF)4]– | Ox | Ga0 + Ag+[WCA]– in arene |
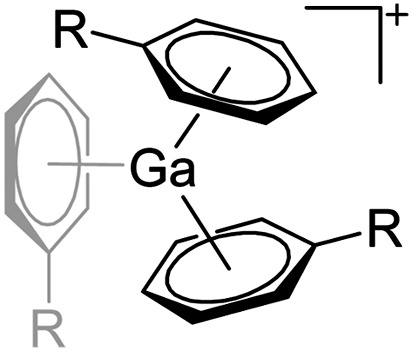
|
31, 91 and 92 |
| [Ga(η6-arene) n ]+ (n = 2, 3) | [Al(ORPF)4]– | Com | [Ga(C6H5F)2–3]+[WCA]– + arene (arene = Mes, p-Xyl, C6Me6) | Bent-sandwich (2 ligands) or tubby coordinated complex (3 ligands) | 7 |
| [Ga(η6-DPE)]+ | [Al(ORPF)4]– | Com | [Ga(C6H5F)2–3]+[WCA]– + DPE | First structurally characterized bent-sandwich ansa-arene complex | 8 and 99 |
| [In(η6-C6H5F) n ]+ (n = 2, 3) | [Al(ORPF)4]– | Ox | In0 + Ag+[WCA]– in C6H5F | Bent-sandwich complex (cf. gallium analogue) | 162 |
| [In(η6-o-C6H4F2)2]+ | [Al(ORPF)4]– | Salt | InCl + Li+[WCA]– in o-C6H4F2 | Bent-sandwich complex (cf. gallium analogue) | 186 |
| [Tl(η6-C6Me6)]+ | [H2N{B(C6F5)3}2]– | Other | [Tl(C6Me6)2]+ in Et2O + C6H5Me, vacuum | First example of a mono-η6-coordinated thallium complex | 204 |
| [Tl(η6-C6H5Me)2]+ | [HCB11H5Br6]– | Salt | Cs+[HCB11H5Br6]– + TlF | Bent-sandwich complex (cf. gallium analogue) | 201 |
| [Tl(η6-C6H5Me)3]+ | [H2N{B(C6F5)3}2]– | Com | [Tl(OEt2)2]+[WCA]– + C6H5Me | Tubby coordinated complex (cf. gallium analogue) | 203 |
| [Tl(η6-Mes)2]+ | [B(OTeF5)4]– | Lewis, Com | Tl+[OTeF5]– + B(OTeF5)3 in Mes | Tubby coordinated complex (cf. gallium analogue) | 202 |
| [Tl(η6-C6Me6)2]+ | [H2N{B(C6F5)3}2]– | Com | [Tl(OEt2)3]+[WCA]– + C6Me6 | Tubby coordinated complex (cf. gallium analogue) | 203 |
| Ligand substituted (CN = 2) | |||||
| [Cp*B(IMes)]2+ | [AlCl4]– | Lewis | Cp*BCl2(IMes) + 2AlCl3 |
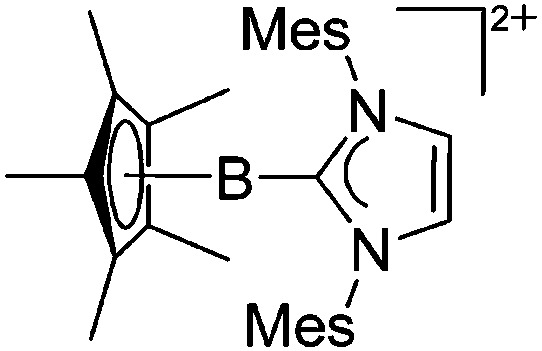
|
210 |
| [( t Bu3PN)2B]+ | [B(C6F5)4]– | Hyd | ( t Bu3PN)2BH + [Ph3C]+[WCA]– |
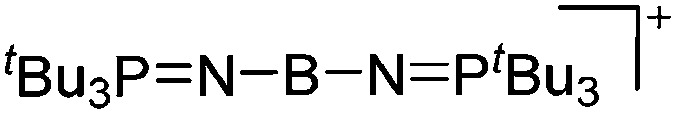
|
114 |
| [ t Bu2MeSi–Al–Si t Bu2–Si t Bu2Me]+ | [B(C6F5)4]– | Alk | Al(SiMe t Bu2)3 + [Et3Si]+[WCA]– | Hyperconjugation with a neighboring Si–Si bond | 134 |
| [Ga(IR)2]+ (R = Pr, Mes) | [Al(ORPF)4]– | Com | [Ga(C6H5F)2]+[WCA]– + IR |
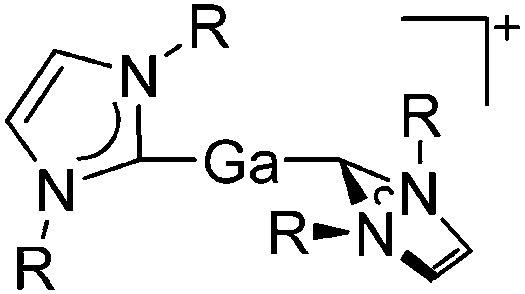
|
161 |
| [ t Bu2MeSi–Ga–Si t Bu2–Si–Me t Bu2]+ | [B(C6F5)4]– | Alk | Ga(SiMe t Bu2)3 + [Et3Si(C6H6)]+[WCA]– | Stabilized by hyperconjugation with a neighboring Si–Si bond | 134 |
| [ t Bu3Si–Ga–Si t Bu3]+ | [Al(ORPF)4]– | Salt | ( t Bu3Si)2GaCl + Ag+[WCA]– | Linear arrangement | 163 |
| [Ga(P t Bu3)2]+ | [Al(ORPF)4]– | Com | [Ga(C6H5F)2]+[WCA]– + P t Bu3 |
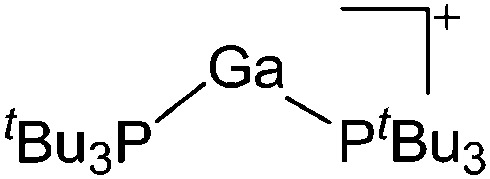
|
162 |
| [In(Mes2py)(η6-C6H5F)]+ | [B(ArCF3 )4]– | Salt, Com | InBr + Na+[WCA]– + Mes2py |
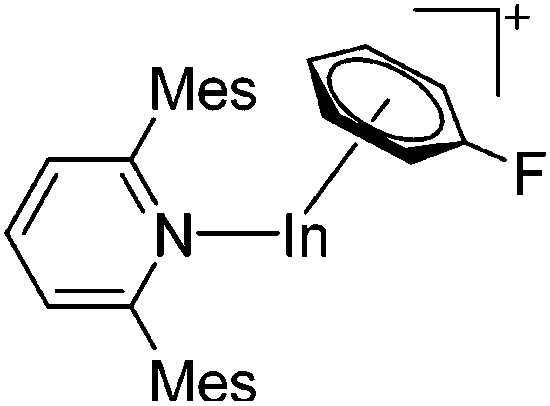
|
187 |
| [In(IPr)2]+ | [Al(ORPF)4]– | Com | [In(C6H5F)2]+ + IPr |
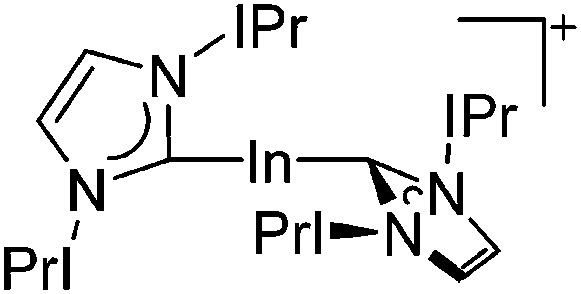
|
161 |
| [In(Mes2py)2]+ | [B(ArCF3 )4]– | Salt | In+Br– + Na+[WCA]– + 2Mes2py |
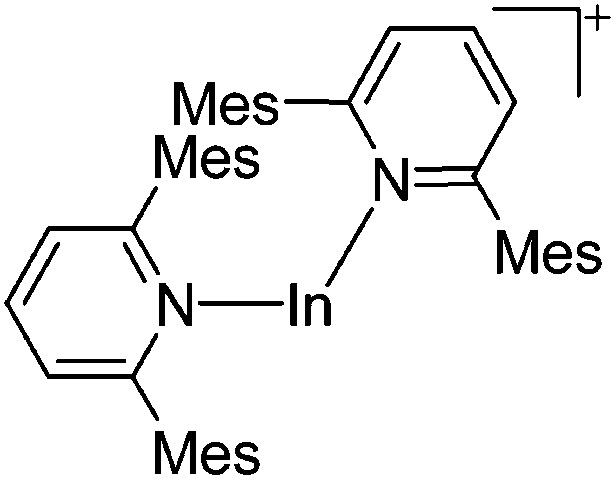
|
187 |
| [Tl(1,2-Cl2C2H4)]+ | [B(OTeF5)4]– | Lewis, Com | Tl+[OTeF5]– + B(OTeF5)3 in 1,2-C2H4Cl2 |
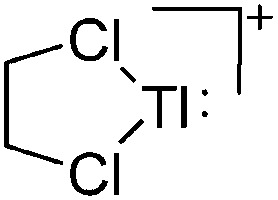
|
198 |
| Ligand substituted (CN = 3) | |||||
| [BMes2(IMe)]+ | [OTf]– | Salt | Mes2BF + [Me3Si]+[OTf]– + [Ag(IMe)2]+[Ag2I3]– |
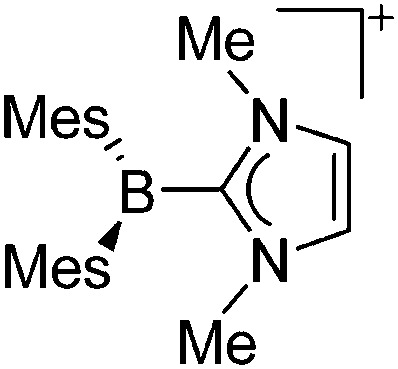
|
211 |
| [BCl2(I t Bu)]+ | [B(ArCl)4]– | Salt | BCl3(I t Bu) + Na+[WCA]– |
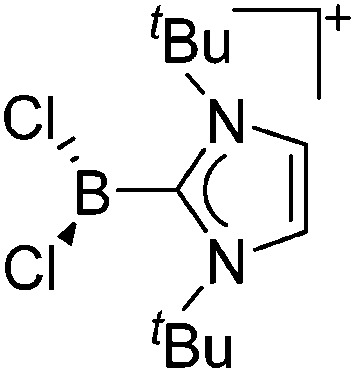
|
74 |
| [{(PPh3)2C}BH2]+ | [HB(C6F5)3]– | Hyd |
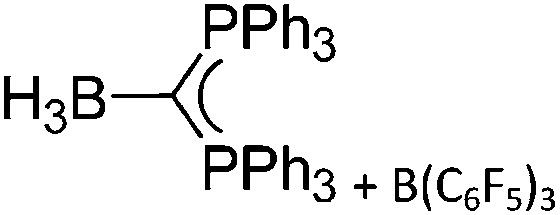
|

|
118 and 119 |
| [BMes2(DMAP)]+/[B(ArN)2(DMAP)]+ ArN = 4-(Me2N)-2,6-Me2-C6H2) | [OTf]– | Salt | Mes2BF + Me3Si-OTf + ArN 2BF + DMAP |
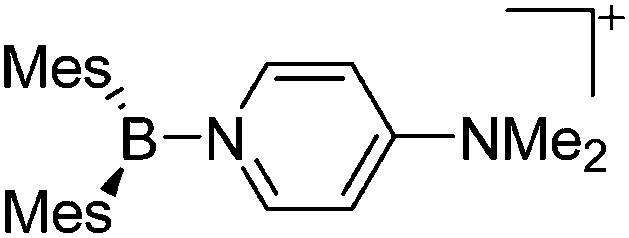
|
57 |
| [B(SubPc)]+(Sub = C24H12N6) | [HCB11Me5Br6]– | Salt | B(SubPc)Cl + Et3Si(HCB11Me5Br6) |
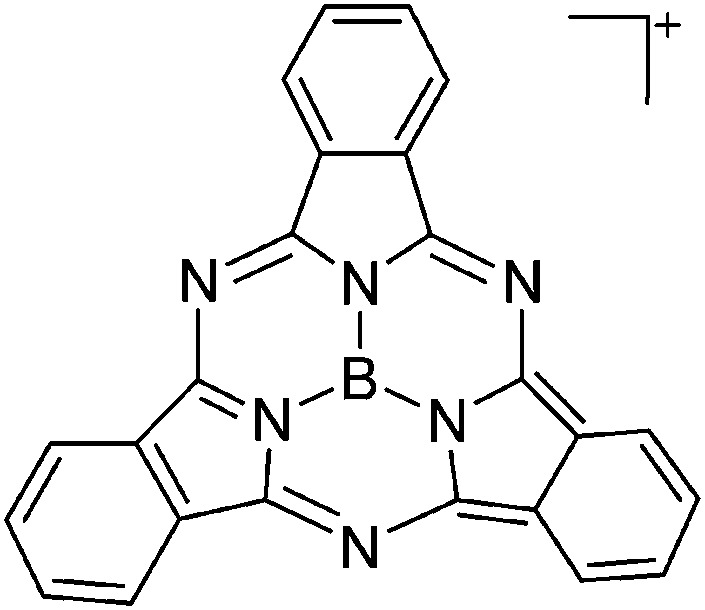
|
115 |
| [CatB(O PEt3)]+ | [HCB11H5Br6]– | Salt, Com | Ag+[WCA]– + CatBBr + OPEt3 |
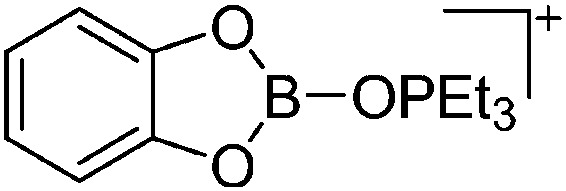
|
116 |
| [(CatB)(PNP)PdH]+ | [B(ArCF3 )4]–/[CB11H12]– | Other | [(PNP)Pd(THF)]+[WCA]– + CatBH |
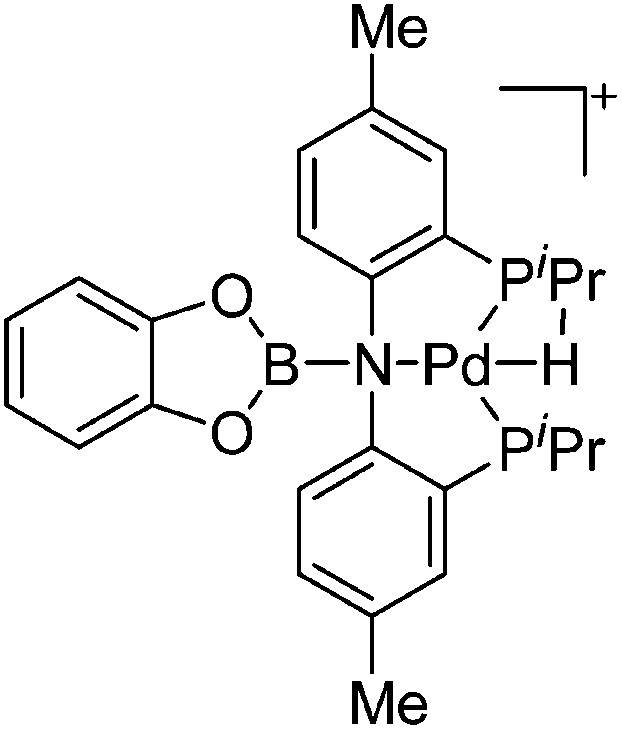
|
117 |
| [ArN(C( CH2)NAr)(C(Me)NAr)AlH]+ (Ar = DIPP) | [B(C6F5)4]– | Hyd | ArN(CMeNAr)2 + AlH3·NMe2Et + [Ph3C]+[WCA]– |
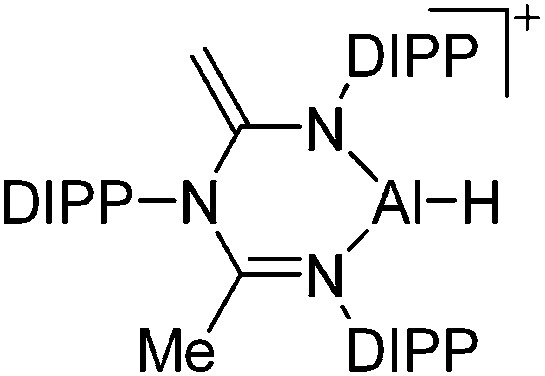
|
135 |
| [{HC(CMeNAr)2}AlMe]+ (Ar = DIPP) | [B(C6F5)4]–/[MeB(C6F5)3]– | Alk | {HC(CMeNAr)2}AlMe2 + [CPh3]+[WCA]–/B(C6F5)3 |
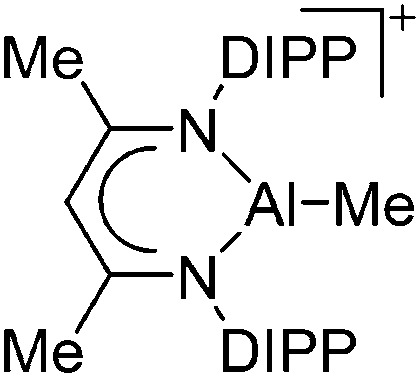
|
136 |
| [Ga(η6-C6H5F)2(DTBMP)]+ | [Al(ORPF)4]– | Com | [Ga(η6-μC6H5F) n ]+[WCA]– + DTBMP (n = 2, 3) |
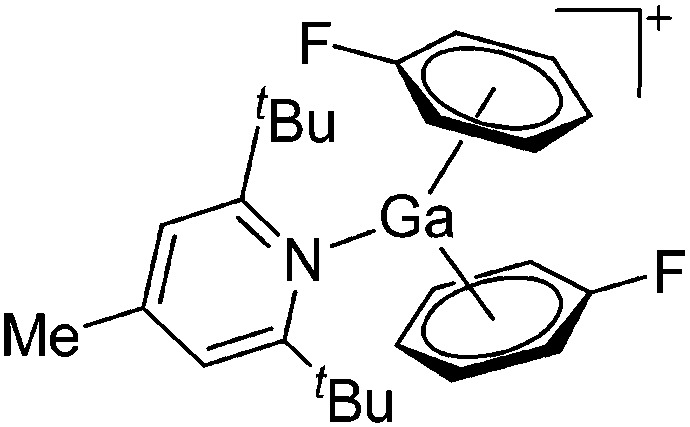
|
164 |
| [Ga(pyrazine)3]+/[{Ga(μ-pyrazine)2-(η1-pyrazine)}+]∞ | [Al(ORPF)4]– | Com | [Ga(C6H5F)]+[WCA]– + pyrazine (n = 2, 3) |
|
164 |
| [Ga(PPh3)3]+ | [Al(ORPF)4]– | Com | [Ga(C6H5Me)2]+[WCA]– + PPh3 |
|
31, 91 and 92 |
| [(iPr2-ATI)InMe]+ | [B(C6F5)4]– | Other | Thermolysis of [{iPr2-ATI(CPh3)}InMe2]+[WCA]– |
|
190 |
| [{ArN CPh}2(NC5H3)In]+ (Ar = 2,4- t Bu2C6H3, 2,5- t Bu2C6H3, 2,6-Et2C6H3, 2,6-iPr2C6H3,) | [OTf]– | Com | In+[WCA]– + bis(imino)pyridine ligand |
|
188 and 189 |
| [In(PPh3)3]+ | [Al(ORPF)4]– | Com | [In(C6H5F) n ]+[WCA]– + 3 PPh3 (n = 2, 3) | Trigonal pyramidal (cf. gallium analogue) | 162 |
| [Tl(Mes2py)(η6-C6H5R)2]+ (R = F, Me) | [B(ArCF3 )4]– | Salt, Com | TlCl + Na+[WCA]– + Mes2py in C6H5R |
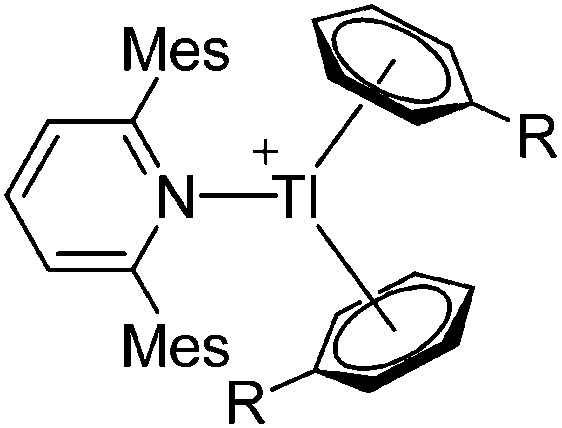
|
187 |
| [Tl(timtmb tBu)]+ | [OTf]– | Com | Tl+[WCA]– + timtmb tBu |
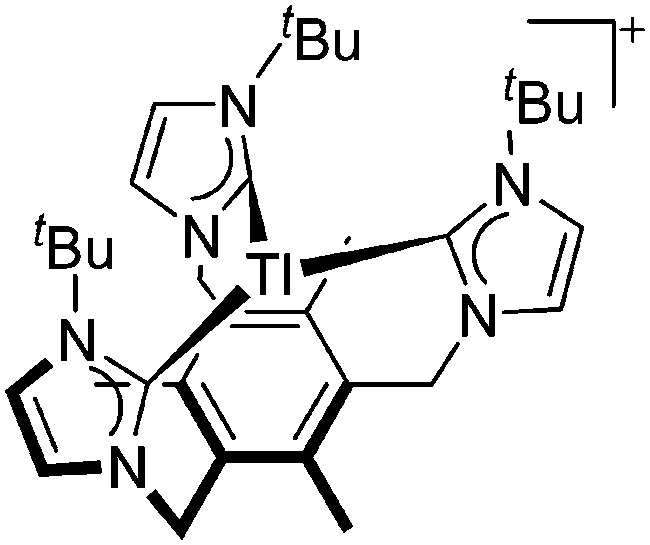
|
205 |
| [{ArN CPh}2(NC5H3)Tl]+ (Ar = 2,6-Et2C6H3, 2,5- t Bu2C6H3) | [OTf]– | Com | Tl+[WCA]– + bis(imino)pyridine ligand |
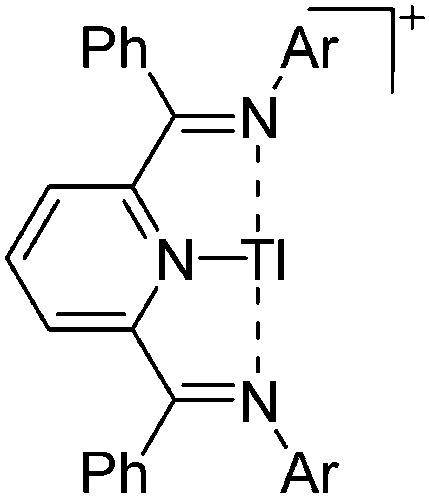
|
206 |
| Ligand substituted (CN = 4) | |||||
| [{(PPh3)2C}BH2(DMAP)]+ | [HB(C6F5)3]– | Com | [{(PPh3)2C}BH2]+[WCA]– + DMAP |
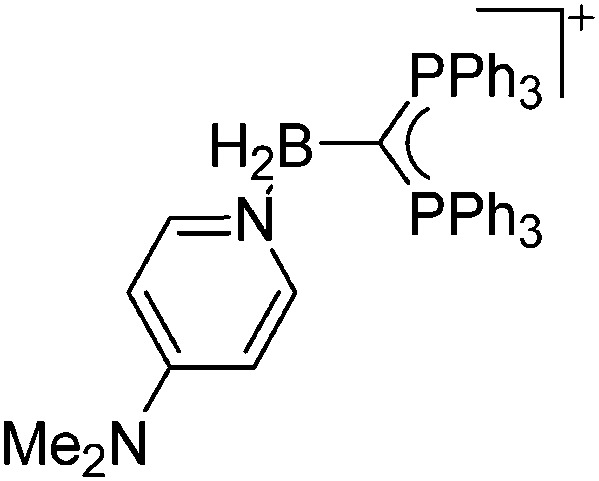
|
118 and 119 |
| [BH2(PR2H)2]+ (R = t Bu, Cy, Ph) | [B(ArCF3 )4]– | Salt | [BH2(PR2H)]+Br– + Na+[WCA]– |
|
120 |
| [(1-MIM)2(9BBN)]+ | [B(ArCF3 )4]– | Com | [PMAF–9BBN)]+[WCA]– + 1-MIM |
|
212 |
| [Me2Al(OEt2)2]+ | [MeB(C12F9)3]– | Alk | AlMe3 + B(12F9)3 in Et2O | — | 77 |
| [Me2Al(THF)2]+ | [{Me2Si(NDIPP)2}2Zr2Cl5]– | Alk | Al2Me6 + {Me2Si(NDIPP)2}ZrCl2(THF)2 | — | 143 |
| [Me2Al(NPhMe2)2]+ | [B(C6F5)4]– | Prot, Com | Al2Me6 + [HNMe2Ph]+[WCA]– | — | 144 |
| [H2Al(NMe3)2]+ | [(AlH)8(CCH2 t Bu)6]2– | Other | t Bu CLi + AlH3·NMe3 + ClAlH2·NMe3 + [ t BuCH2(Bzl)NMe2]+Cl– | — | 145 |
| [(Pytsi)AlMe]+ | [MeB(C6F5)3]– | Alk | (Pytsi)AlMe2 + B(C6F5)3 |
|
137 |
| [H2C{hpp}2AlMe2]+ | [BPh4]– | Prot | [{hpp}H2C{hpp}H]+[WCA]– + AlMe3 |
|
138 |
| [{H2C C(BOX-Me2)2}Al-(Me)2]+ | [B(C6F5)4]– | Hyd | {BOX-Me2}Al(Me)2 + [CPh3]+[WCA]– |
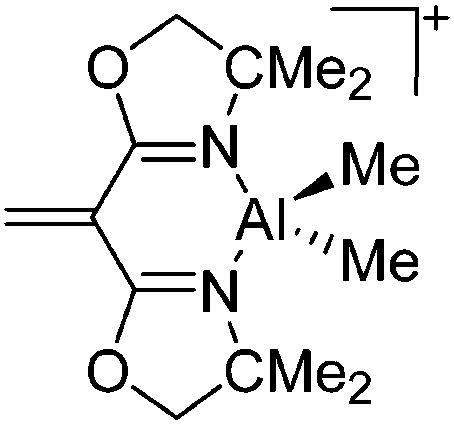
|
139 |
| [{BOX-Me2}Al(Me)(NMe2Ph)]+ | [MeB(C6F5)3]– | Alk | {BOX-Me2}Al(Me)2 + B(C6F5)3 in NMe2Ph |
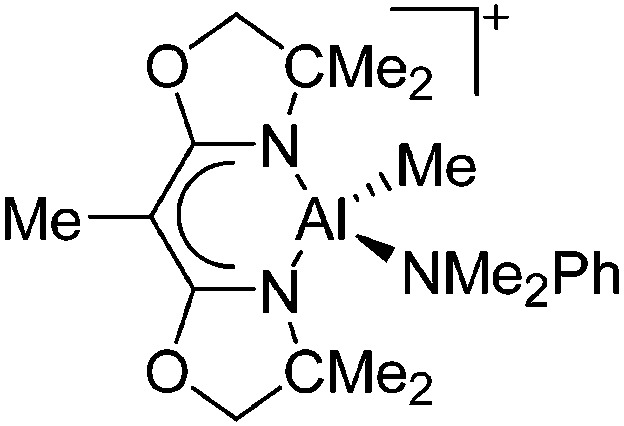
|
139 |
| [{6-(CH2NMe2)-2-CPh3-4-Me-C6H2O}Al(iBu)(NMe2Ph)]+ | [HB(C6F5)3]– | Hyd | {6-(CH2NMe2)-2-CPh3-4-Me-C6H2O}Al-(iBu)2 + B(C6F5)3 + NMe2Ph |
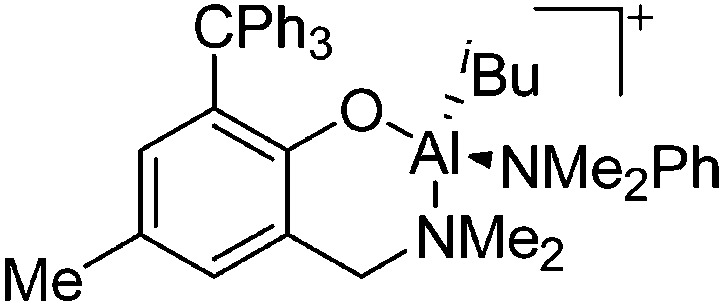
|
213 and 214 |
| [{HC(CPhNSiMe3)2}-Al(Do)Me]+ (Do = Et2O, THF) | [B(C6F5)4]–/[MeB(C6F5)3]– | Prot/Alk, Com | {HC(CPhNSiMe3)2}AlMe2 + [HNMe2Ph]+[WCA]– + Et2O/B(C6F5)3 + THF |
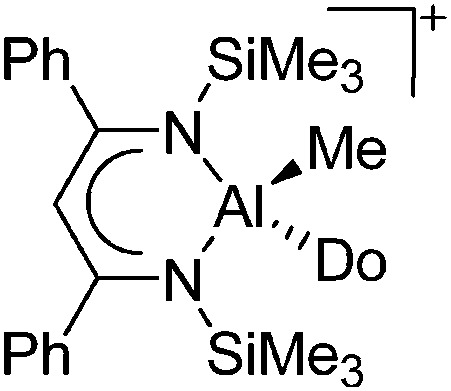
|
215 |
| [(ArN)C(Me)CHPPh2(NAr)AlMe(OEt2)]+ (Ar = DIPP) | [B(C6F5)4]– | Alk, Com | (ArN)C(Me)CHPPh2(NAr)MMe2 + [Ph3C]+[WCA]– in Et2O |
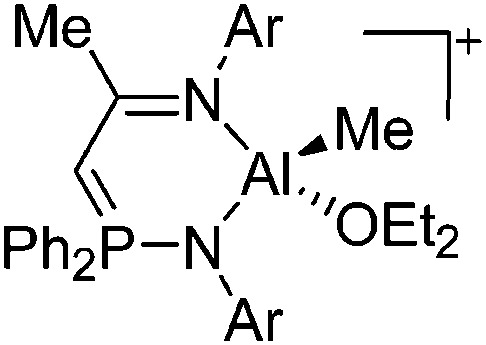
|
216 |
| [(iPr2-ATI)Al(Et)(Do)]+ (Do = ClPh, NCMe) | [B(C6F5)4]– | Alk, Com | (iPr2-ATI)AlEt2 + [CPh3]+[WCA]– in PhCl/ + MeCN |
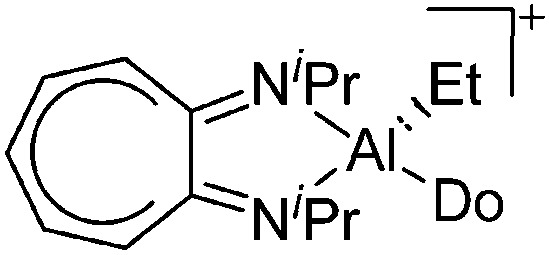
|
140 and 141 |
| [(SchNMe2)AlMe]+ | [BPh4]– | Salt | (SchNMe2)AlMeCl + Na+[WCA]– |
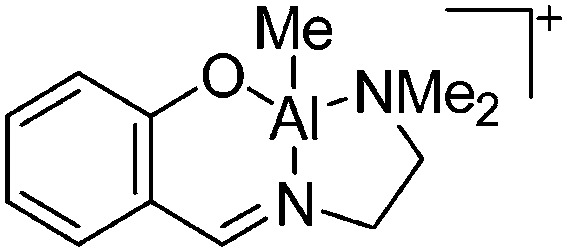
|
142 |
| [{η2-O,P-(2-PPh2-4-Me-6- t Bu-C6H2O)}2Al]+ | [MeB(C6F5)3]– | Alk | {η2-O,P-(2-PPh2-4-Me-6- t Bu-C6H2O)}2AlMe + B(C6F5)3 |
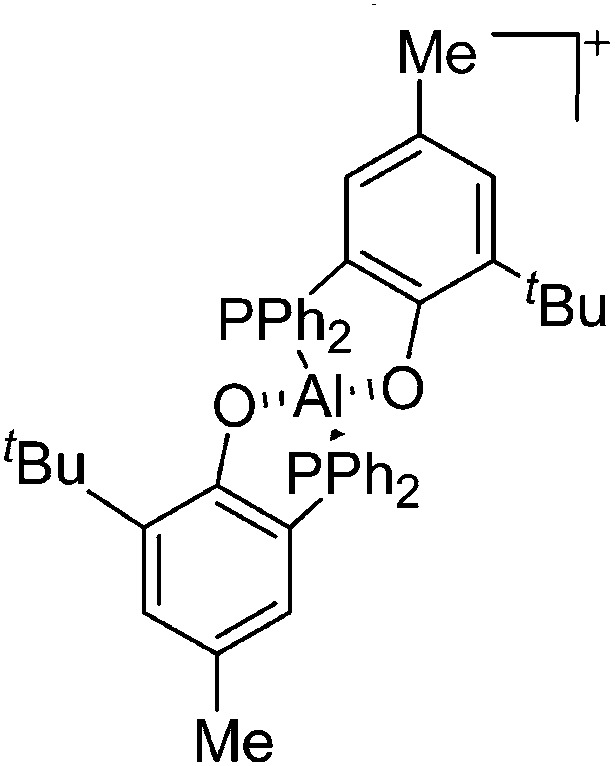
|
217 |
| [{H2C C(BOX-Me2)2}Ga-(Me)2]+ | [B(C6F5)4]– | Hyd | {BOX-Me2}Ga(Me)2 + [CPh3]+[WCA]– |
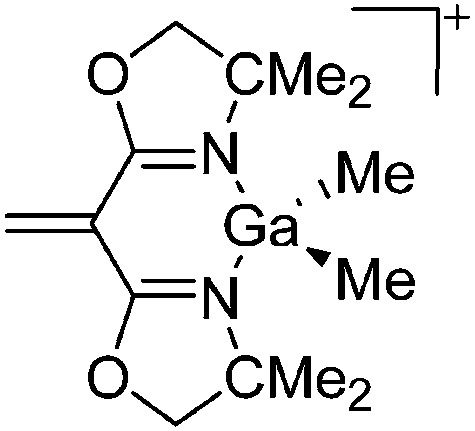
|
165 |
| [{BOX-Me2}Ga(Me)]+ | [MeB(C6F5)3]– | Alk | {BOX-Me2}Ga(Me)2 + B(C6F5)3 in NMe2Ph |
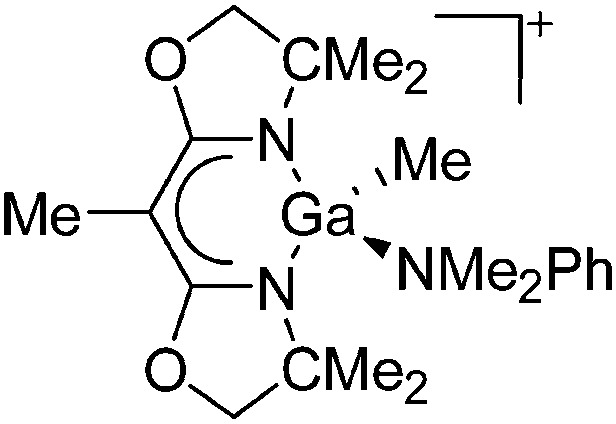
|
165 |
| [(iPr2-ATI)Ga(Me)(ClPh)]+ | [B(C6F5)4]– | Alk, Com | (iPr2-ATI)GaMe2 + [CPh3]+[WCA]– in PhCl |
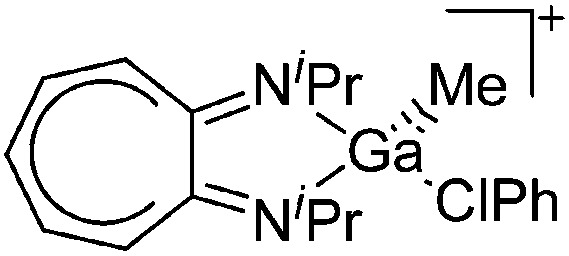
|
140 |
| [{1,2-(NiPr)2-5-CPh3-cyclohepta-3,6-diene}InMe2]+ | [B(C6F5)4]– | Other | (iPr2-ATI)InMe2 + [Ph3C]+[WCA]– |
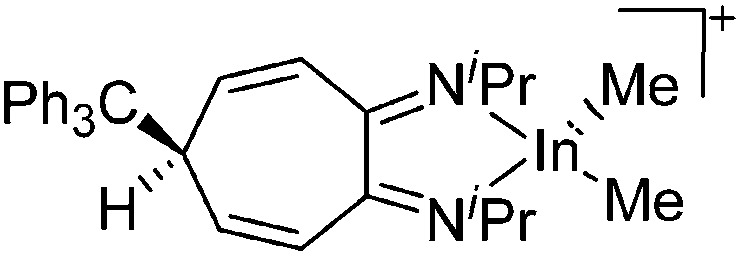
|
190 |
| [(iPr2-ATI)In(Me)(NMe2Ph)]+ | [B(C6F5)4]– | Prot | (iPr2-ATI)InMe2 + [HNMe2Ph]+[WCA]– |
|
190 |
| [Tl(OEt2)4]+ | [H2N{B(C6F5)3}2]– | Prot | TlOEt + [H(OEt2)2]+[WCA]– in Et2O |
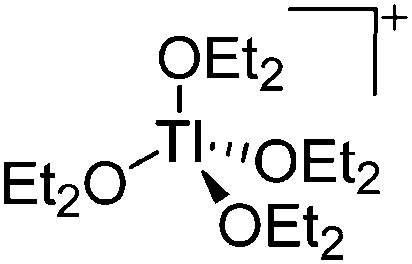
|
203 |
| Ligand substituted (CN = 5) | |||||
| [{SalenCF3 }Al(OEt2)]+ | [MeB(C6F5)3]– | Alk | {SalenCF3 }AlMe + B(C6F5)3 in Et2O |
|
147 |
| [Ga(η1-C3H5)2(THF) n ]+ (n = 2, 3) | [B(C6F5)4]–/[B(ArCl)4]– | Prot | Ga(η1-C3H5)3(THF) + [HNMe2Ph]+[WCA]– |
|
166 |
| [In(CH2SiMe3)2(THF)3]+ | [B(C6F5)4]– | Prot | In(CH2SiMe3)3 + [HNMe2Ph]+[WCA]– in THF |
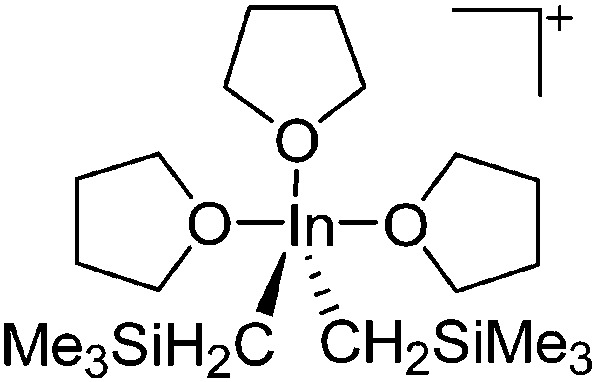
|
191 |
| [Tl(NPPh)2(η6-C6H5Me)]+ NPPh = 2,5-bis(2-pyridyl)-1-phenylphosphole | [Al(ORPF)4]– | Com |
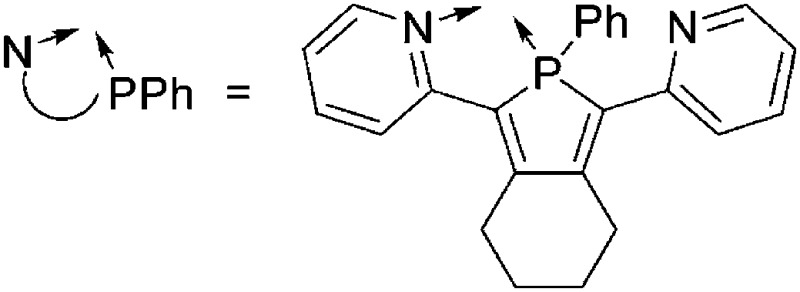
|
|
218 |
| Ligand substituted (CN ≥ 6) | |||||
| [DoAl(MeOH)2]+ (Do = Salen, Acen) | [BPh4]– | Salt, Com | DoAlCl + Na+[WCA]– + MeOH |
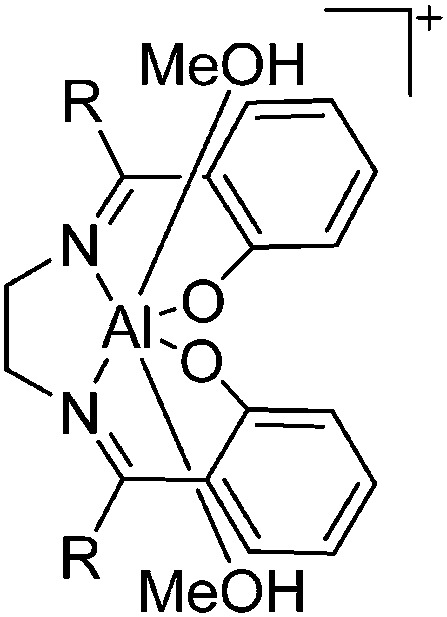
|
148 and 149 |
| [Salpen( t Bu)Al(THF)2]+ | [BPh4]– | Salt, Com | Salpen( t Bu)AlCl + Na+[WCA]– + THF |
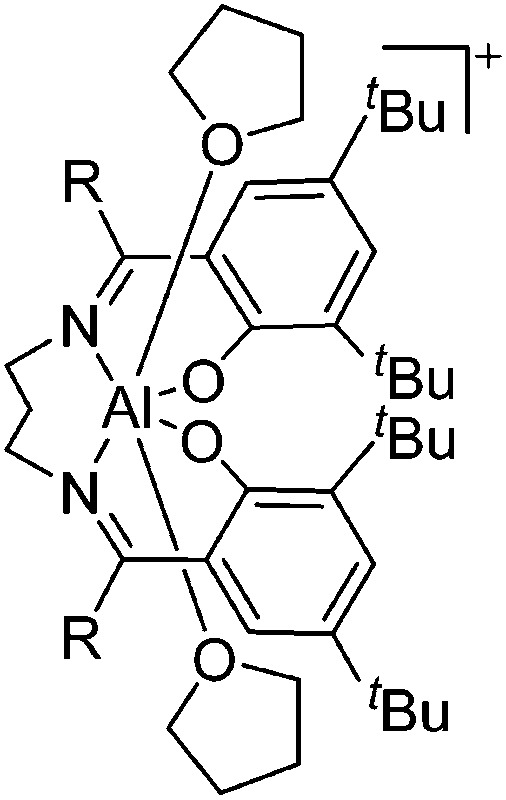
|
150 and 151 |
| [(SchNMe2)Al(OPh)-(THF)2]+ | [BPh4]– | Com | [(SchNMe2)AlPh]+[WCA]– + O2 in THF |
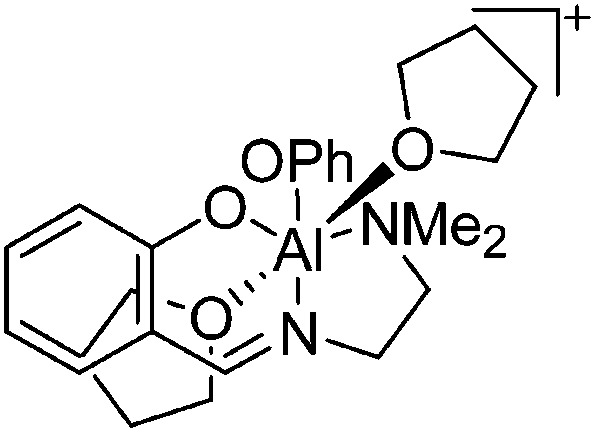
|
142 |
| [GaH(THF)4(OTf)]+ | [Ga(THF)4(OTf)2]– | Prot | GaCp* + HOSO2CF3 in THF |
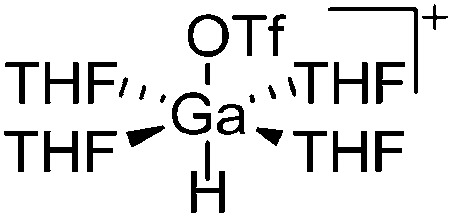
|
167 |
| [GaIII{(bipy)3}˙]2+ | [Al(ORPF)4]– | Com |
|

|
17 |
| [Ga([18]crown-6)(η6-/η1-C6H5F)2]+ | [Al(ORPF)4]– | Com | [Ga(η6-C6H5F)]+[WCA]– + [18]crown-6 (n = 2, 3) |
|
168 |
| [In([18]crown-6)]+ | [OTf]– | Com | In+[WCA]– + [18]crown-6 | No coordinated solvent, but a strong anion–cation interaction: cf. In–O = 227.2 pm and 278.5 pm (sum of the van der Waals radii 345 pm) | 192 and 193 |
| [In([18]crown-6)(η6-/η1-C6H5F)2]+ | [Al(ORPF)4]– | Com | [In(η6-C6H5F) n ]+[WCA]– + [18]crown-6 (n = 2, 3) | Similar structure to the gallium analogue (see above) | 168 |
| [In([15]crown-5)2]+ | [OTf]– | Com | In+[WCA]– + [15]crown-5 | Sandwich complex | 194 |
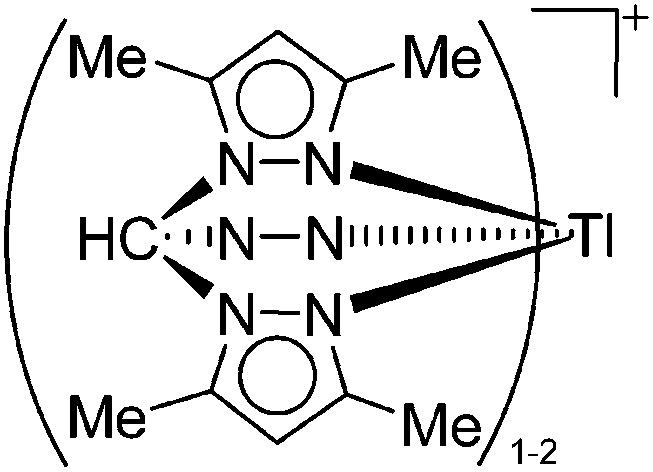
| [{HC(3,5-Me2pz)3} n Tl]+ (n = 1, 2) | [PF6]– | Com | Tl+[WCA]– + HC(3,5-Me2pz)3 |
|
219 |
| [Tl([18]crown-6)]+ | [H2N{B(C6F5)3}2]– | Com | [Tl(C6H5Me)2]+[WCA]– + [18]crown-6 | Similar to gallium analogue, yet featuring significant Tl–F interactions to two counteranions | 203 |
| Transition-metal substituted | |||||
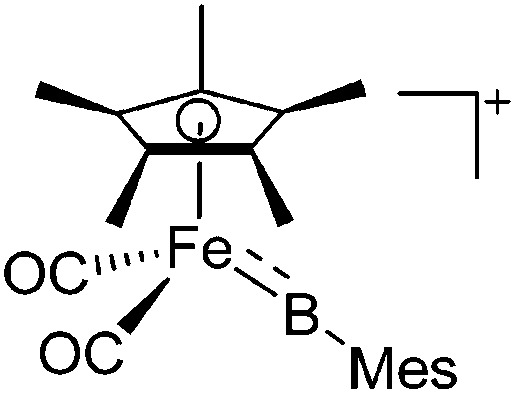
| [(FP*)(BMes)]+ | [B(ArCF3 )4]– | Salt | (FP*)(BMes)Br + Na+[WCA]– |
|
220 |
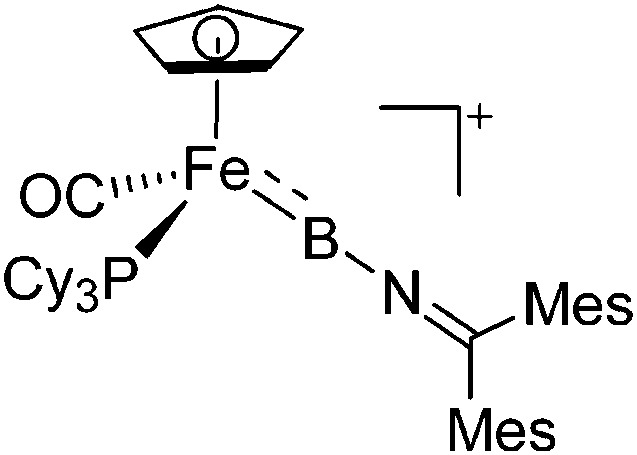
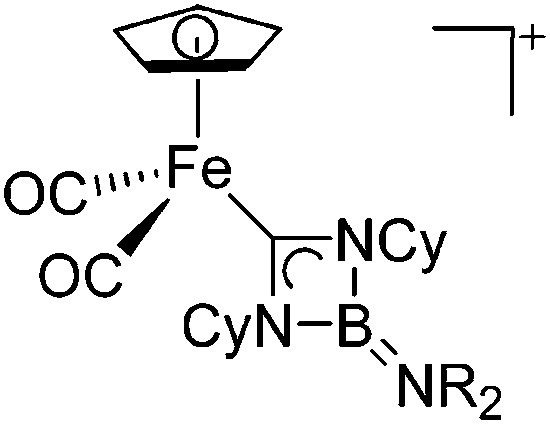
| [CpFe(CO)(PCy3)-(BNCMes2)]+ | [B(ArCl)4]– | Salt | CpFe(CO)(PCy3)(B(Cl)-NCMes2) + Na+[WCA]– |
|
221 |
| [CpM(CO)(R){B(NCy2)}]+ (M = Fe, Ru; Do = CO, PMe3, PPh3) | [B(ArCF3 )4]– | Salt | CpM(CO)(R){B(NCy2)Cl} + Na+[WCA]– |
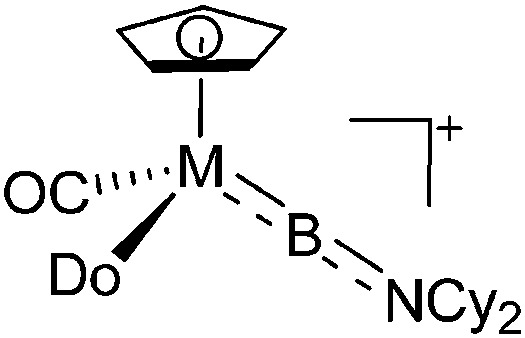
|
12 and 124 |
| [(Cy3P)2(MeCN)Pt(B O)]+ | [B(ArCF3 )4]– | Salt, Com | (Cy3P)2Pt(B O)(Br) + Ag+[WCA]– + MeCN |
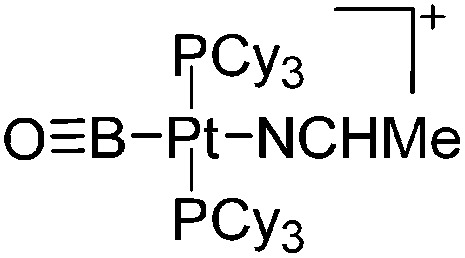
|
121 |
| [{(OC)5Mn}2(μ-B)]+ | [B(ArCF3 )4]– | Salt | {(OC)5Mn}2(μ-BBr) + Na+[WCA]– |
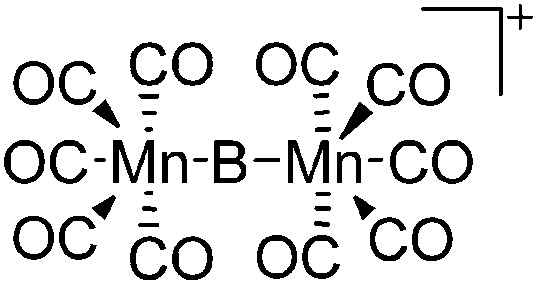
|
222 |
| [(FP’)2(μ-B)]+ | [B(ArCF3 )4]– | Salt | (FP′)2B(Cl) + Na+[WCA]– |
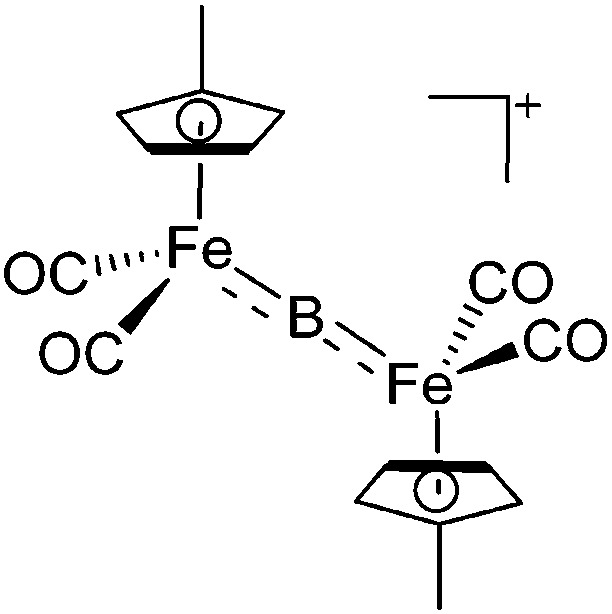
|
222 |
| [Fc(NC5H2Me2)BPh]+ | [Al(ORPF)4]– | Salt |
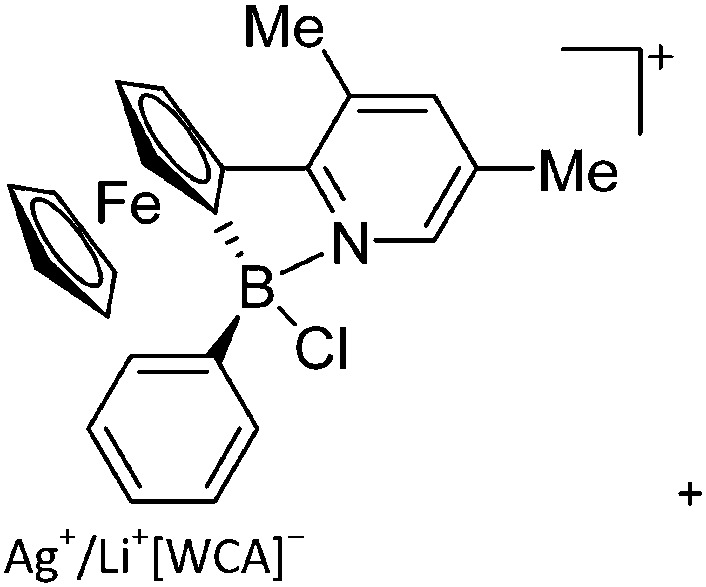
|
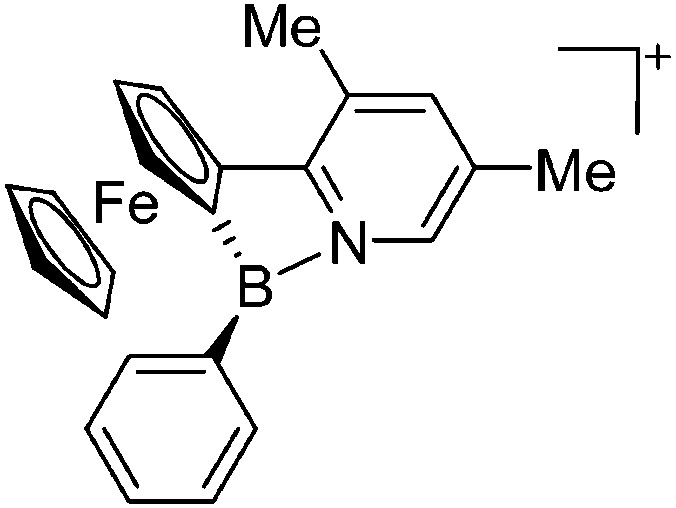
|
223 |
| [(FP){B(NiPr2)(OPPh3)}]+ | [B(ArCF3 )4]– | Com | [(FP)B(NiPr2)]+[WCA]– + Ph3PO |
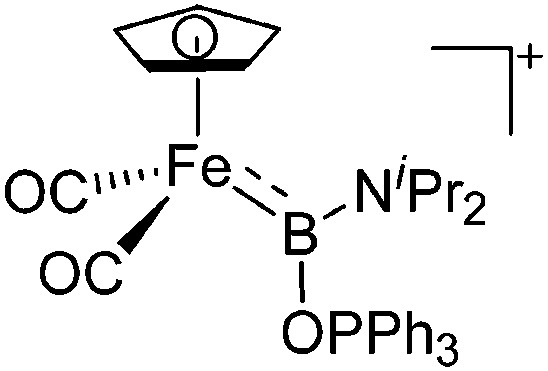
|
224 |
| [(FP)B{N(iPr)(CMe2)}(Do)]+ (Do = Ph2C O, Me2C NiPr) | [B(ArCF3 )4]– | Com, other | [(FP) B NiPr2]+[WCA]– + Do |
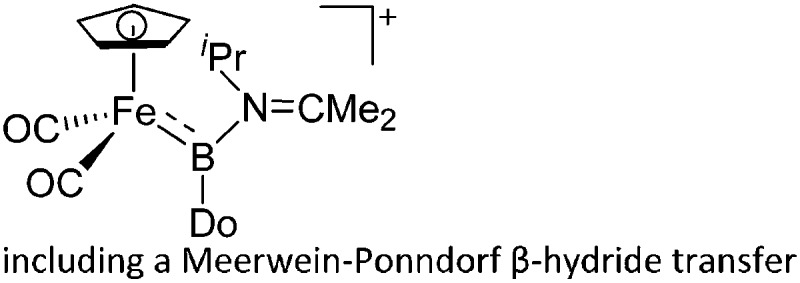
|
225 |
| [(FP)B(NCy2)(Do)]+ (Do = C5H4PPh3, 4-Pic) | [B(ArCF3 )4]– | Com | [(FP) B NCy2]+[WCA]– + Do |
|
12 |
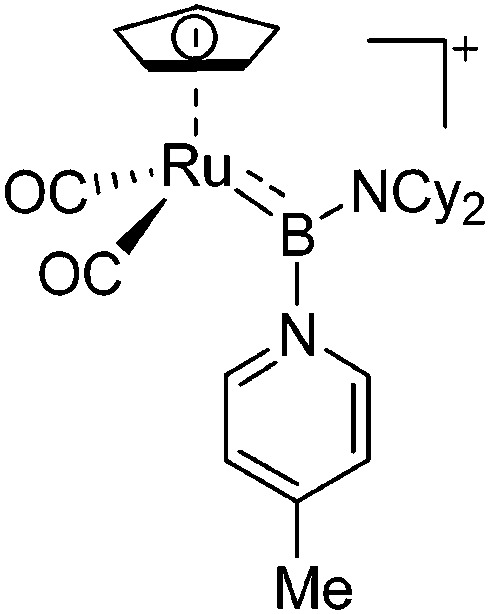
| [CpRu(CO)2{B(NCy2)-(4-Pic)}]+ | [B(ArCF3 )4]– | Salt, Com | CpRu(CO)2{B(NCy2)Cl} + Na+[WCA]– + 4-Pic |
|
124 |
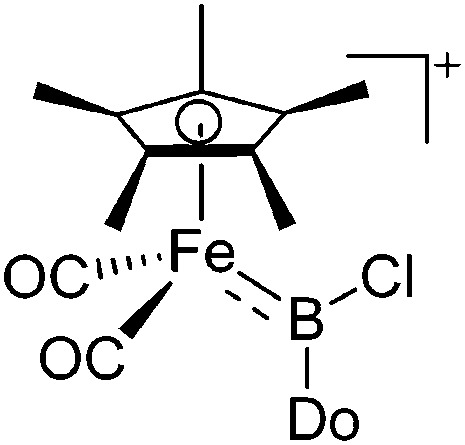
| [(FP*)B(Cl)(LB)]+ (Do = 3,5-lutidine, PMe3, IMe) | [B(ArCl)4]– | Salt | (FP*)B(Cl2)(Do) + Na+[WCA]– |
|
226 |
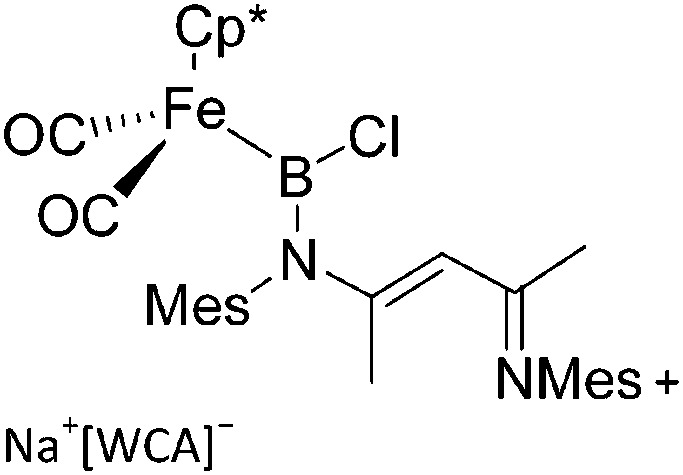
| [(FP*)B(nacnac)]+ | [B(ArCF3 )4]– | Salt, other |
|

|
227 |
| [(FP)C(NCy)2BNR2]+ (R = iPr, Cy) | [B(ArCF3 )4]– | Ins | [(FP)(BNR)2]+[WCA]– + RN C NR (substoichiometric) |
|
228 |
| [(H)(PNP)Pd(BCat)]+ | [B(ArCF3 )4]– | Other | [(BCat)(PNP)Pd(BCat)]+[WCA]– + H2O |
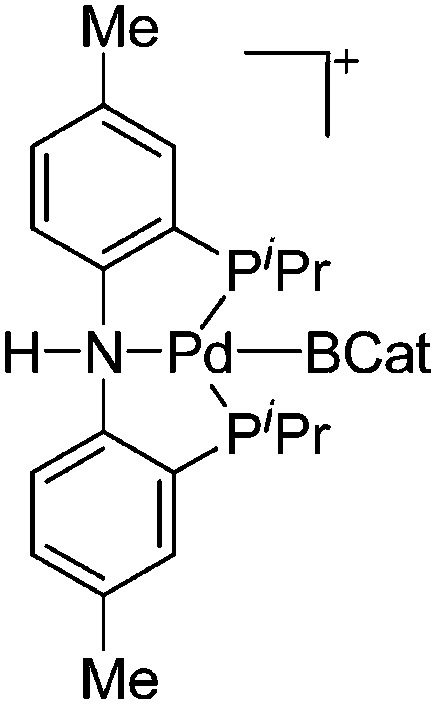
|
117 |
| [(R3P)2Pt{B(Fc)Br}]+ (R = iPr, Cy) | [B(ArCF3 )4]– | Salt | (R3P)2Pt(Br){B(Fc)Br} + Na+[WCA]– |
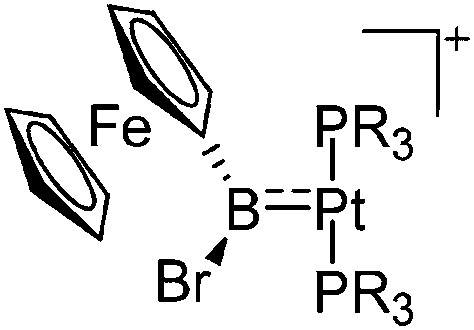
|
229 and 230 |
| [(Cy3P)2Pt{B(X)X′}]+ (X = Br; X′ = ortho-tolyl, t Bu, NMe2, Pip, Br; XX′ = (NMe2)2, CatB) | [B(ArCF3 )4]–/[B(C6F5)4]– | Salt | (Cy3P)2Pt(Br){B(X)X′} + Na+/K+[WCA]– |
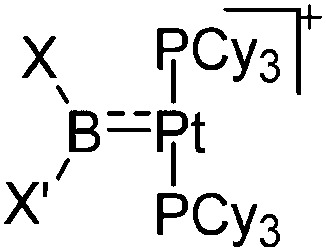
|
231 |
| [(Cy3P)2Pt(Br){B(NC5H4-4-R)X}]+ (R = Me, X = NMe2, Pip, Br; R = t Bu, X = Pip) | [B(ArCF3 )4]– | Salt | (Cy3P)2Pt(Br){B(Br)-(NC5H4-4-R)X} + Na+[WCA]– |
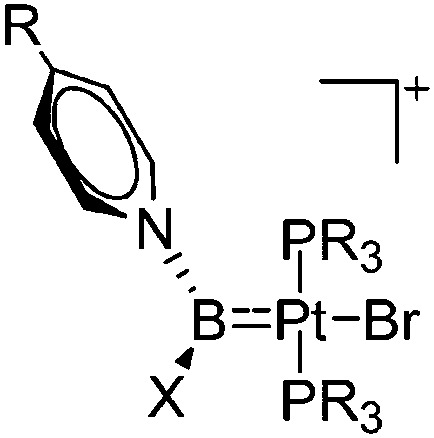
|
125 |
| [(Cy3P)2Pt{B(Br)(NMe2)}-(NCMe)]+ | [B(ArCF3 )4]–/[B12Cl12]2– | Com/salt, Com | (Cy3P)2Pt{B(Br)(NMe2)} + NCMe/(Cy3P)2Pt{B(Br)(NMe2)}Br + {Na+}2[WCA]2– + MeCN |
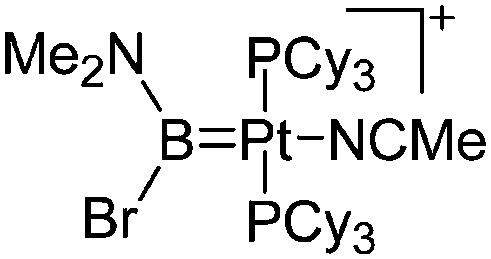
|
230 |
| [(Cy3P)2Pt(BCl2)]+ | [B(ArCF3 )4]– | Salt | (Cy3P)2Pt(BCl2)Cl + Na+[WCA]– |
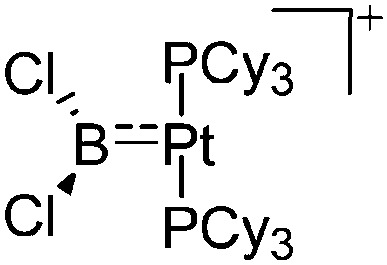
|
230 |
| [Cp*Ru(PiPr3)(BH2Mes)]+ | [B(C6F5)4]– | Salt | Cp*Ru(PiPr3)-(BH2MesCl) + Li+[WCA]–·2.5OEt2 |
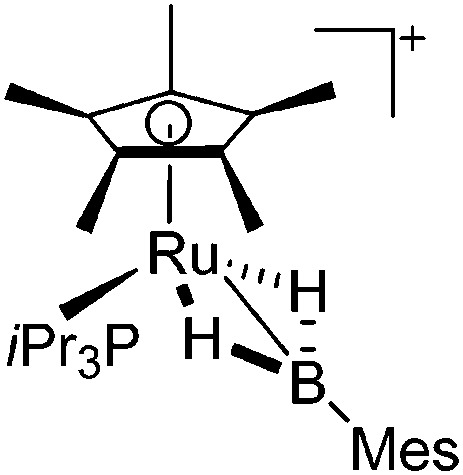
|
232 |
| [(PMAF)2BH2]+ | [B(C6F5)4]– | Hyd, Com | PMAF–BH3 + [CPh3]+[WCA]– + PMAF |
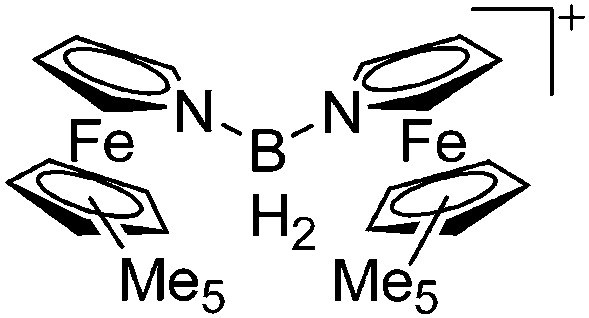
|
212 |
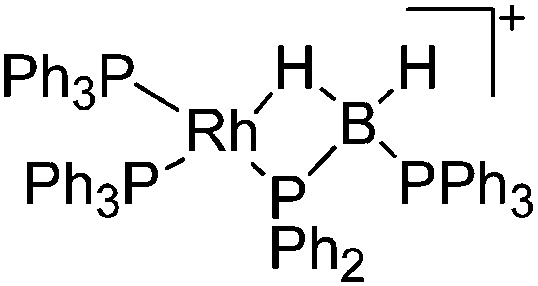
| [Rh(PPh3)2(κ1,η-PPh2BH2·PPh3)]+ | [B(ArCF3 )4]– | Salt, Com | ClRh(PPh3)3 + Na+[WCA]– + H3B·PPh2H |
|
120 |
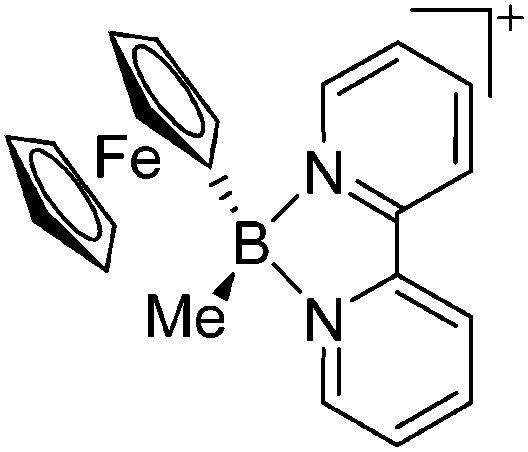
| [FcBMe(bipy)]+ | [PF6]– | Salt, Com | FcBBrMe + bipy + [NH4]+[WCA]– |
|
126 |
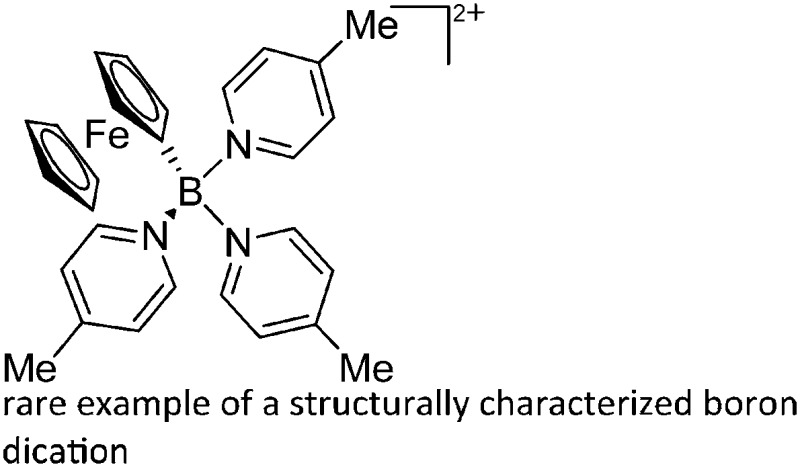
| [FcB(Pic)3]2+ | [B(ArCF3 )4]– | Salt, Com | Br2BFc + 2Na+[WCA]– + 3Pic |
|
122 |
| [(FP){C(NCy)2B-(NCy)2CNR2}]+ (R = iPr, Cy) | [B(ArCF3 )4]– | Ins | [(FP){B(NR2)}]+ + CyN C NCy |
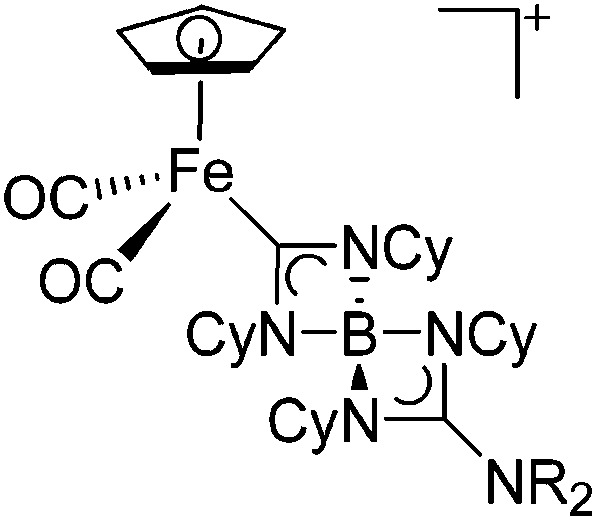
|
124 and 228 |
| [(dppe)Cp*FeGaI]+ | [B(ArCF3 )4]– | Salt | (dppe)Cp*FeGaI2 + Na+[WCA]– |
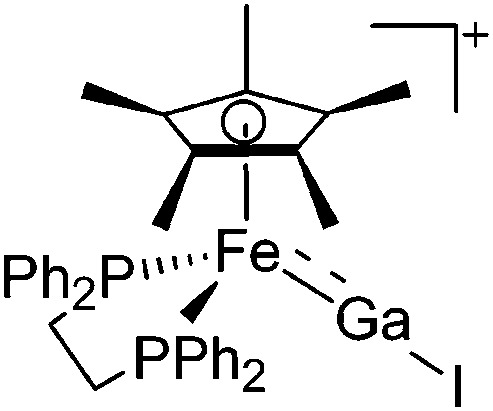
|
233 |
| [(FP*)2Ga]+ | [B(ArCF3 )4]– | Salt | (FP*)2GaCl + Na+[WCA]– |
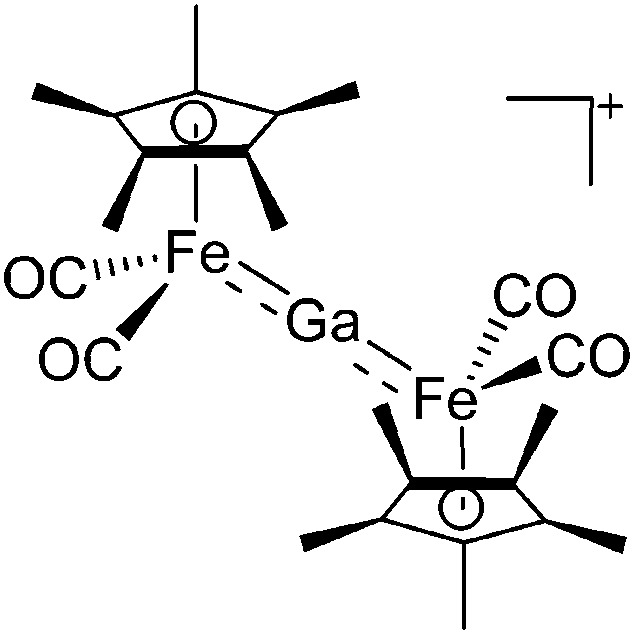
|
169 |
| [(FP*)2Ga(4-Pic)]+ | [B(ArCF3 )4]– | Salt, Com | (FP*)2GaCl + Na+[WCA]– + 4-Pic |
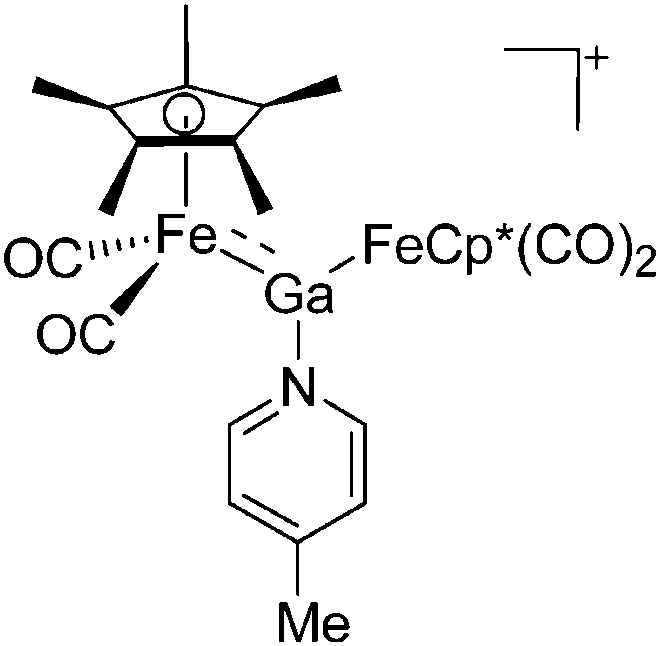
|
170 |
| [(FP*)Ga(Mes)(dtbpy)]+ | [B(ArCF3 )4]– | Salt, Com | (FP*)Ga(Mes)I + Na+[WCA]– + dtbpy |
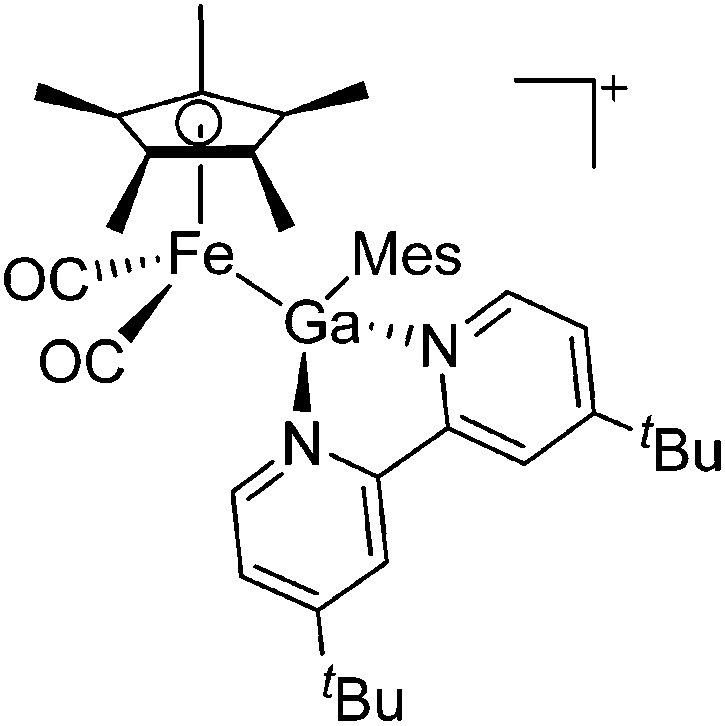
|
234 |
| [(FP*)Ga(phen)(Y)]+ (Y = Cl, S p Tol) | [BPh4]– | Salt, Com/Lewis | 2(FP*)GaCl2 + Na+[WCA]– + phen/[(FP*)Ga(phen)(Cl)]+ + Me3SiS p Tol |
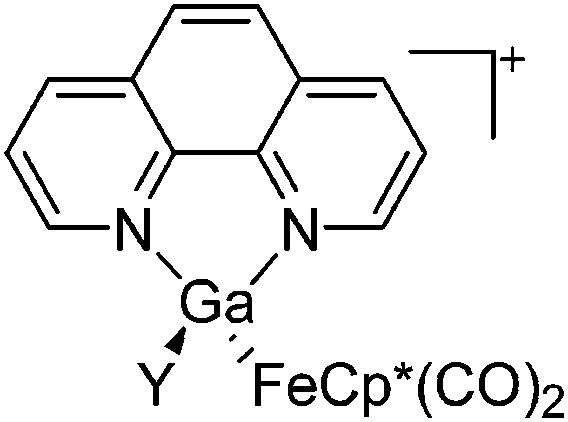
|
171 |
| [(FP)Ga(OEt2){(NCy)2-C t Bu}]+ | [B(ArCF3 )4]– | Salt, Com | (FP)Ga(Cl){(NCy)2C t Bu} + Na+[WCA]– in Et2O |
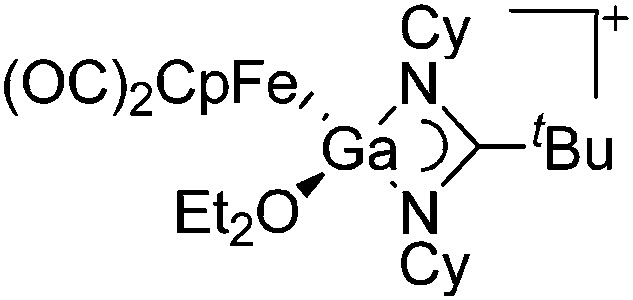
|
235 |
| [(FP)2Ga(bipy)]+ | [Cl2Ga(FP)2]– | Lewis, Com | 2ClGa(FP)2 + bipy |
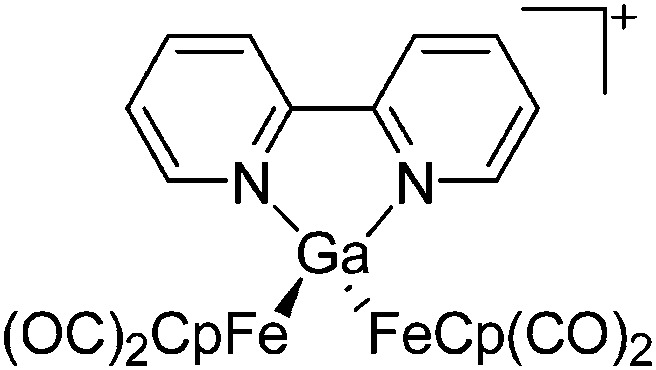
|
236 |
| [InPt(PPh3)3]+ | [B(ArCF3 )4]– | Com | In+[WCA]– + Pt(PPh3)4 |
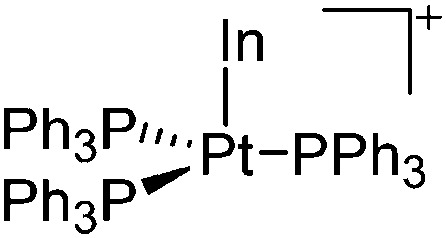
|
179 and 180 |
| [(phen)2In-Ag(η3-C6H5F)]2+ | [Al(ORPF)4]– | Com |
|
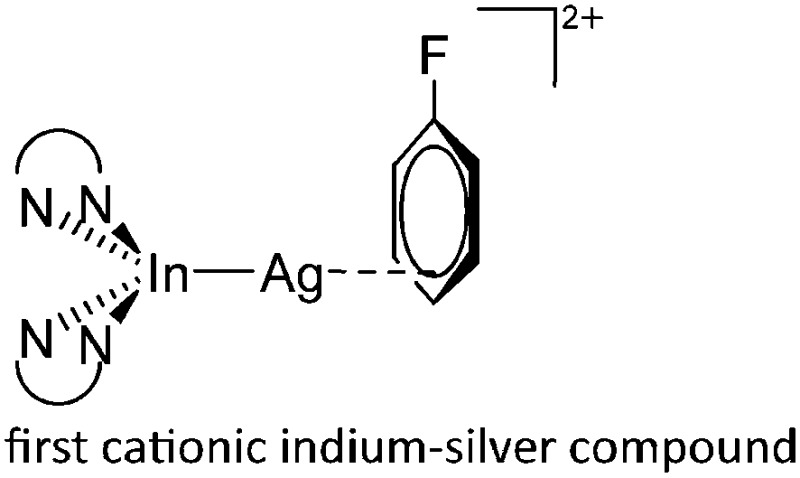
|
17 |
| [(FP*)2In]+ | [B(ArCF3 )4]– | Salt | (FP*)2InCl + Na+[WCA]– |
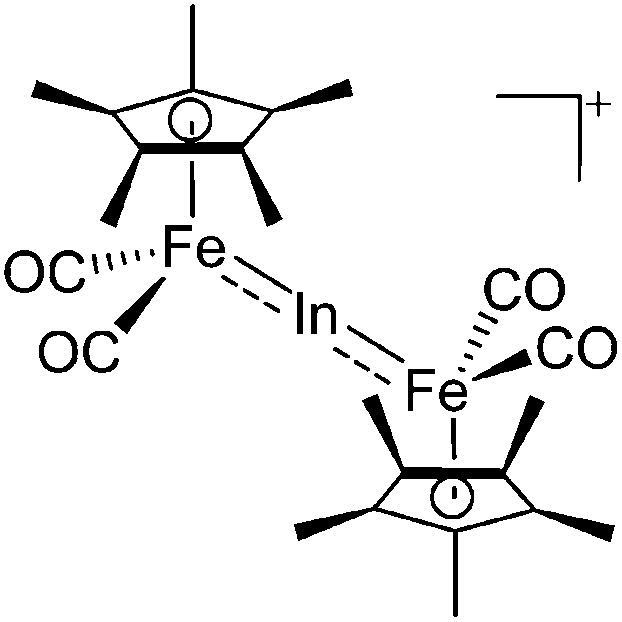
|
195 |
| [(FP*)2In(THF)]+ | [B(ArCF3 )4]– | Com | [(FP*)2In]+[WCA]– + THF |
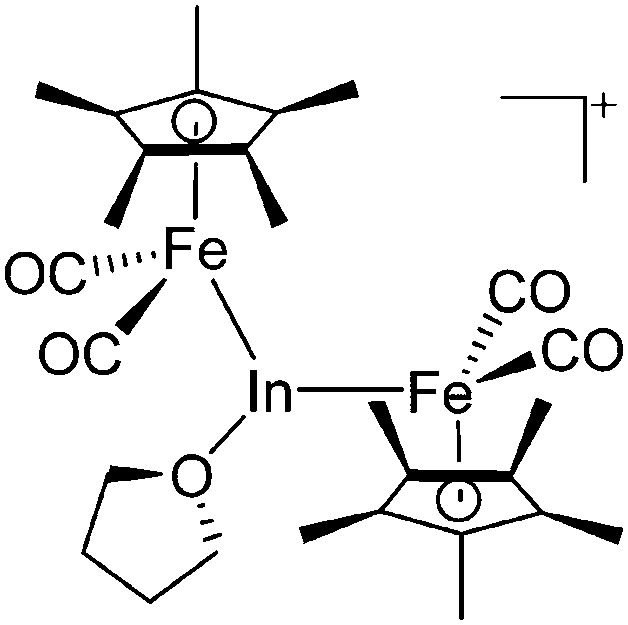
|
195 |
| [Tl(η5-FeCp2)]+ | [H2N{B(C6F5)3}2]– | Others | [Tl(η6-C6H5Me)3]+[WCA]– + 2.2FeCp2 |
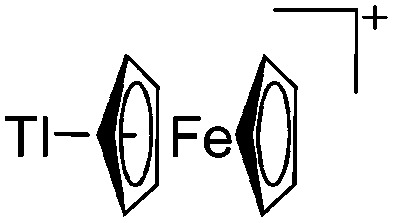
|
204 |
| [Tl2(η5-FeCp2)3]2+ | [H2N{B(C6F5)3}2]– | Com | [Tl(η6-C6H5Me)2]+[WCA]– + FeCp2 |
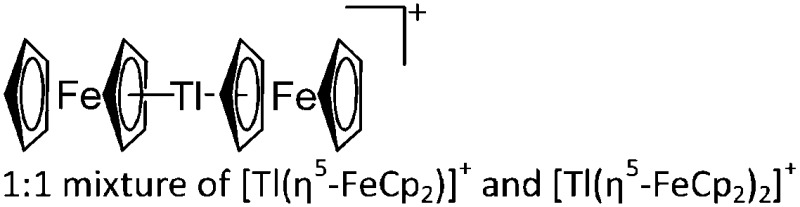
|
203 |
| [Tl{(η5-As5)FeCp*}3]+ | [FAl{OC6F10(C6F5)}3]– | Salt, Com |
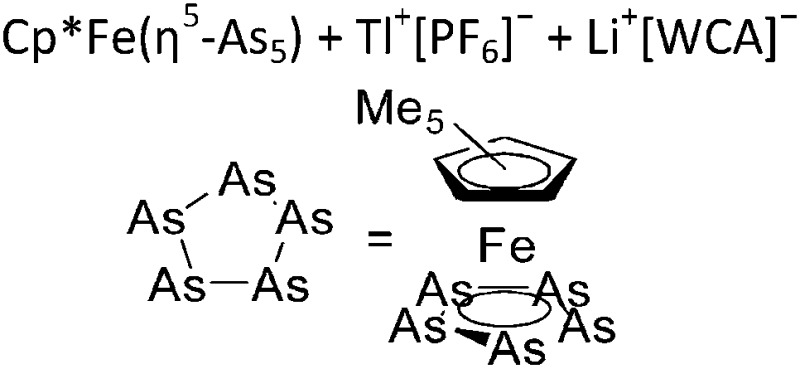
|

|
176 |
| [{P(Ph2)CH2ox}(Cl)(Tl)Pt-CH2Ph}]+ | [PF6]– | Other | Tl+[WCA]– + {P(Ph2)CH2ox}Pt(Cl)-CH2Ph} |
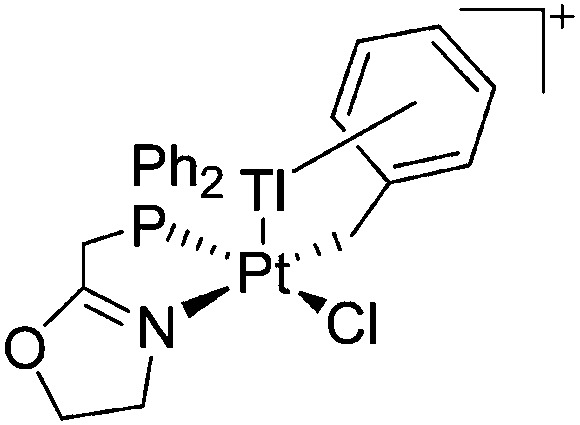
|
208 |
| Multinuclear | |||||
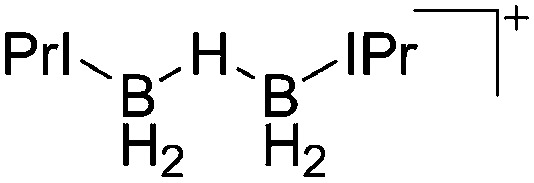
| [{IPr(H2B)}2(μ-H)]+ | [HB(C6F5)3]– | Hyd | IPr + B(C6F5)3 |
|
118 and 119 |
| [{Me3N(H2B)}2(μ-H)]+ | [B(C6F5)4]– | Hyd | Me3N–BH3 + [CPh3]+[WCA]– |
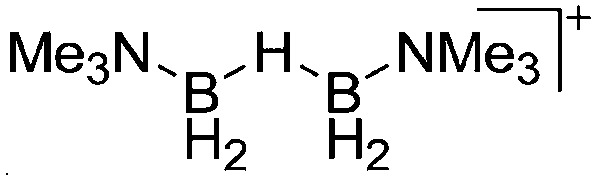
|
237 |
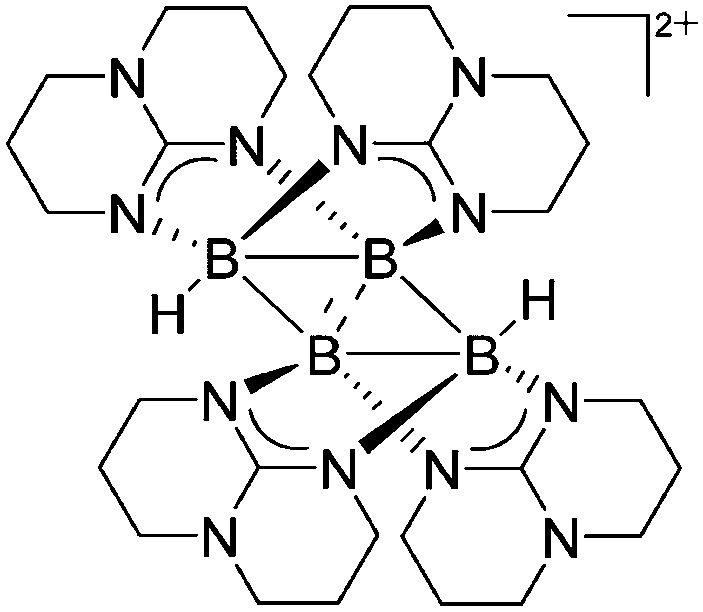
| [B4H2(μ-hpp)4]2+ | [HB(C6F5)3]– | Hyd, Com | [HB(μ-hpp)]2 + B(C6F5)3 |
|
123 |
| [[{6-(CH2NMe2)-2-CPh3-4-Me-C6H2O}Al(R)]2]2+ (R = C6H13) | [B(C6F5)4]– | Com | [{6-(CH2NMe2)-2-CPh3-4-Me-C6H2O}Al(iBu)(BrPh)]+[WCA]– + 1-hexene |
|
152 |
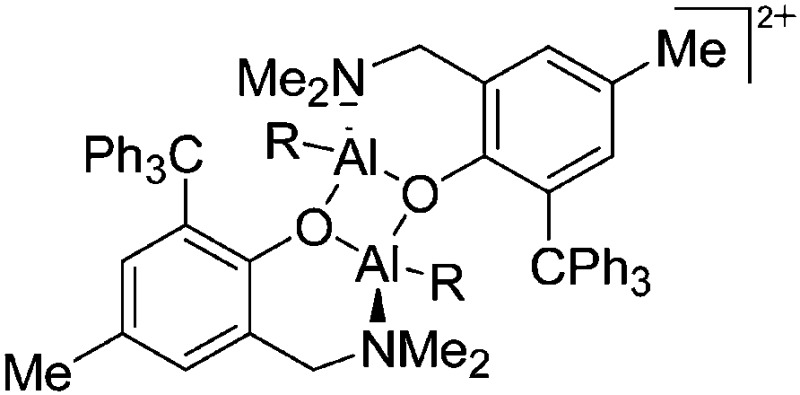
| [{2-(CH2Do)-6-R-C6H3O}AlMe({2-(CH2Do)-6-R-C6H3O}AlMe2)]+ (R = Ph, t Bu; Do = NMe2, NC4H8, NC5H10) | [MeB(C6F5)3]– | Alk | {2-(CH2Do)-6-R-C6H3O}AlMe2 + B(C6F5)3 |
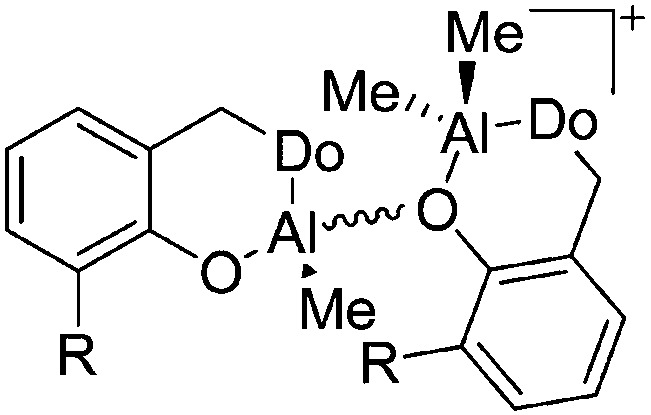
|
238 and 214 |
| [{MeC(NR)2}2Al2Me3]+ (R = iPr, Cy) | [B(C6F5)4]–/[MeB(C6F5)3]– | Alk, Com | {MeC(NR)2}AlMe2 + [CPh3]+[WCA]–/B(C6F5)3 |
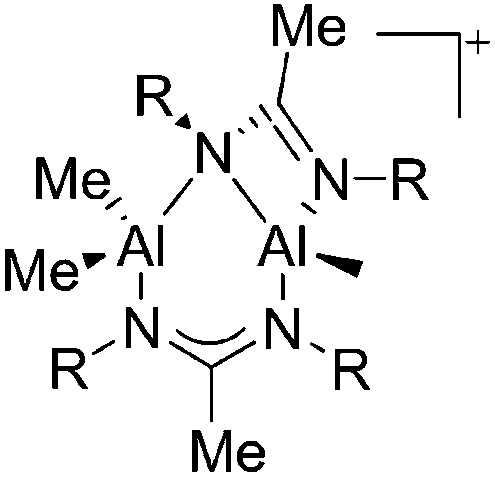
|
156 |
| [AlEt(μ-η2,η1-iPr2-ATI)-(μ-Et)AlEt2]+ | [B(C6F5]– | Com | [(iPr2-ATI)Al(Et)]+[WCA]–+AlEt3 |
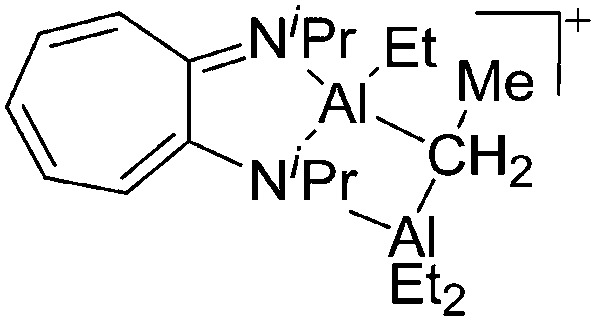
|
141 |
| [{(iPr2-ATI)AlMe}2(μ-Me)]+ | [B(C6F5)4]– | Alk, Com | (iPr2-ATI)AlMe2 + [CPh3]+[WCA]– |
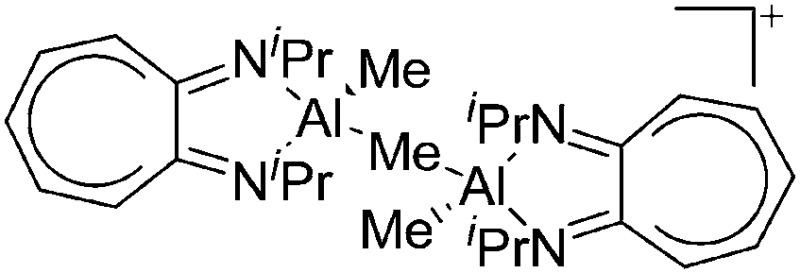
|
141 and 239 |
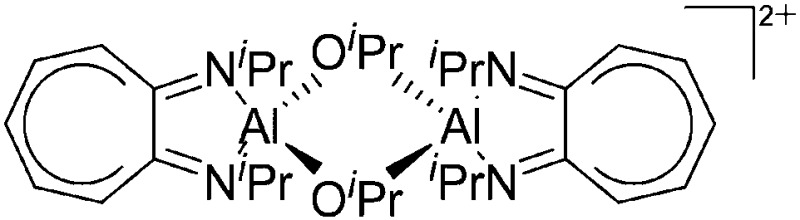
| [{(iPr2-ATI)Al(μ-OiPr)}2]2+ | [B(C6F5)4]– | Com | [(iPr2-ATI)Al(Et)]+[WCA]– + acetone |
|
141 and 153 |
| [Me2Al(μ-OSi(R123)3)2Al-Me(NMe2Ph)]+ (R1, R2 = Me; R3 = Me, t Bu) | [B(C6F5)4]– | Prot | Me2Al(μ-OSiR3)2AlMe2 + [HNMe2Ph]+[WCA]– |
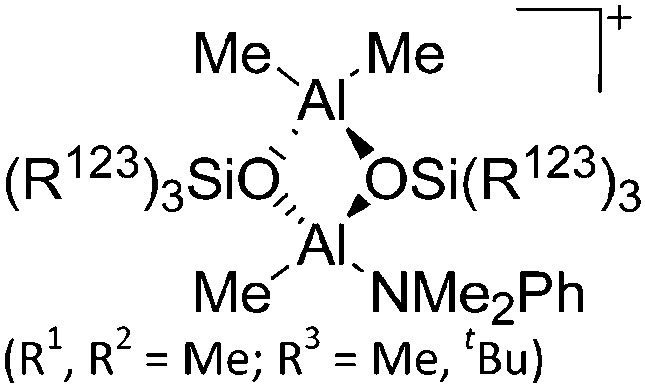
|
154 |
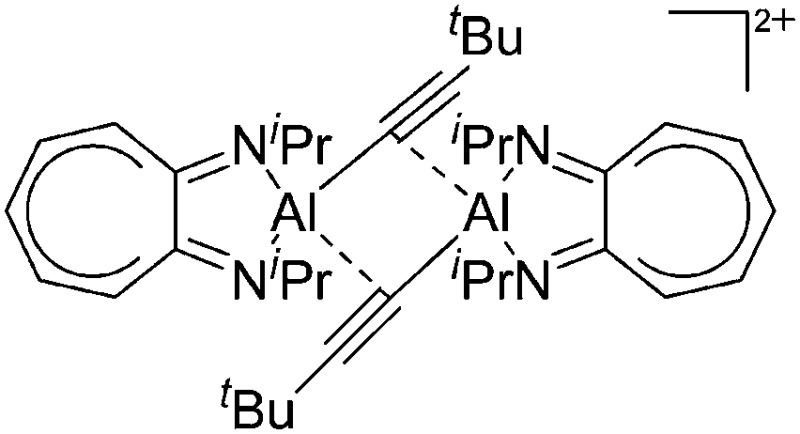
| [{(iPr2-ATI)Al-(μ-C C t Bu)}2]2+ | [B(C6F5)4]– | Com | [(iPr2-ATI)Al(Et)]+[WCA]– + tert-butyl acetylene |
|
141 and 153 |
| [{(tacn)AlMe}2]2+ | [MeB(C6F5)3]– | Alk | [(tacn)AlMe2]2 + B(C6F5)3 |
|
240 |
| [{(OSSO)Al}2]2+ | [MeB(C6F5)3]– | Alk | (OSSO)AlMe + B(C6F5)3 |
|
155 |
| [(η6-C6H5F)Ga-(μ-η6-m-TP)2-Ga(η6-C6H5F)]2+ | [Al(ORPF)4]– | Com | [Ga(η6-C6H5F) n ]+[WCA]– + m-TP (n = 2, 3) |
|
8 and 99 |
| [{ t BuC(NiPr)2}GaMe-{ t BuC(NiPr)2}GaMe2]+ | [B(C6F5)4]– | Alk, Com | { t BuC(NiPr)2}GaMe2 + [Ph3C]+[WCA]– |
|
156 |
| [{(iPr2-ATI)GaMe}2(μ-OH)]+ | [B(C6F5)4]– | Other | [(iPr2-ATI)Ga(Me)(NMe2Ph)]+[WCA]– + H2O |
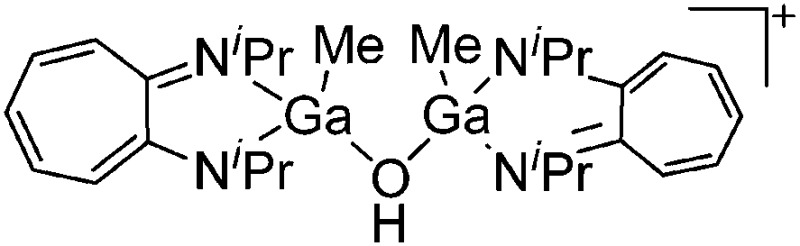
|
172 |
| [{(Salomphen)Ga}(μ-Cl)]+ | [BPh4]– | Salt | (Salomphen)GaCl + Na+[BPh4]– |
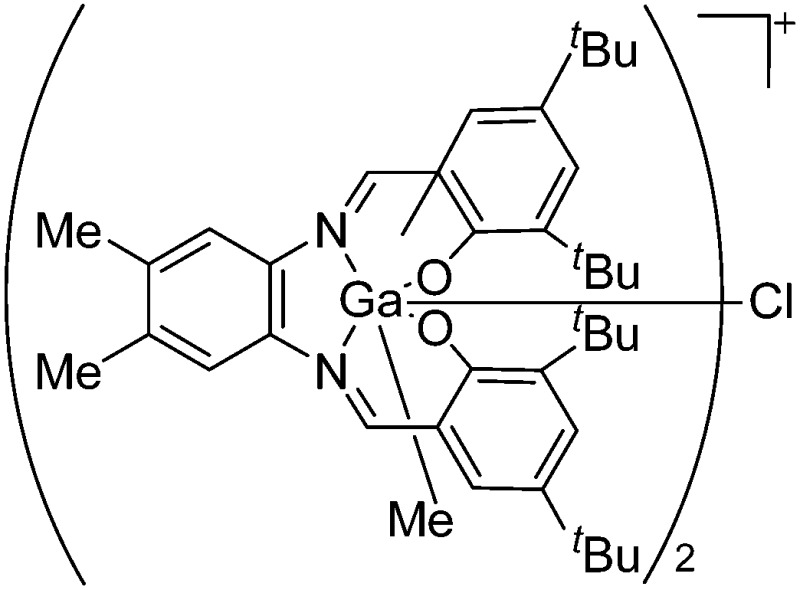
|
241 |
| [(BuGa)4(μ-OH)6]2+ | [HCB11Br6Me5]– | Other | [(2,6-Mes2C6H3)GaBu]+ [WCA]– + H2O |
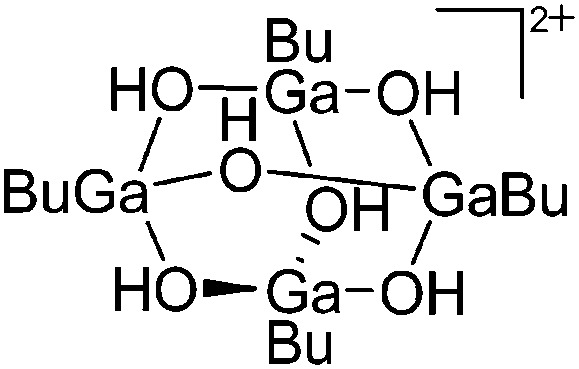
|
173 |
| [{(PPh3)3In}2(μ-PPh3)]2+ | [Al(ORPF)4]– | Com | [In(C6H5F) n ]+[WCA]– + PPh3 (n = 2, 3) | One PPh3 moiety functions as a bridge between both InI cations | 162 |
| [In3(bipy)5–6]3+ | [Al(ORPF)4]– | Com |
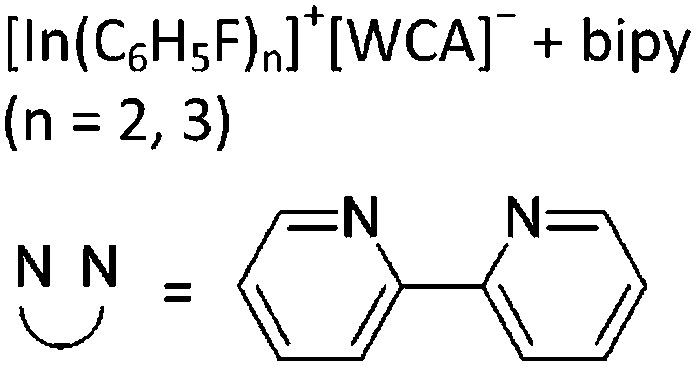
|
|
17 |
| [In4(Do)6]4+ (Do = bipy, phen) | [Al(ORPF)4]– | Com |
|
|
17 |
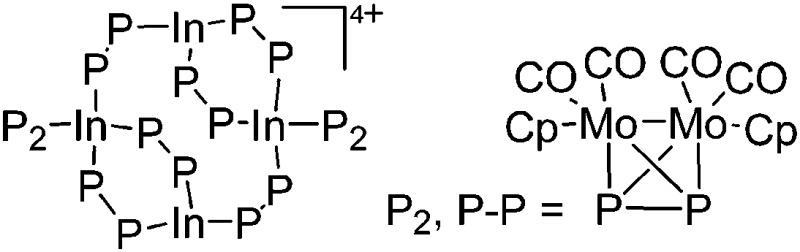
| [In4{(CpMo(CO)2)2P2}8]4+ | [Al(ORPF)4]– | Com | [In(o-C6H4F2)2]+[WCA]– + {CpMo(CO)2}2(P2) |
|
186 |
| [{{ArN CPh}2(NC5H3)Tl}2(μ-η6-C6H5R)]2+ (Ar = 2,6-Et2C6H3, 2,5- t Bu2C6H3; R = H, Me) | [OTf]– | Com | 2[(ArN CPh)2(C5H3N)Tl]+[WCA]– + C6H5R |
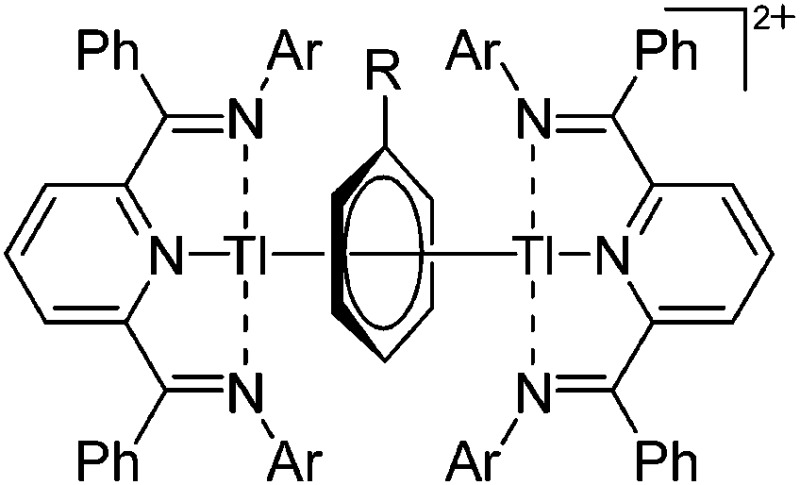
|
206 |
| [Tl2(NPPh)4]2+ NPPh = 2,5-bis(2-pyridyl)-1-phenylphosphole | [Al(ORPF)4]– | Com |
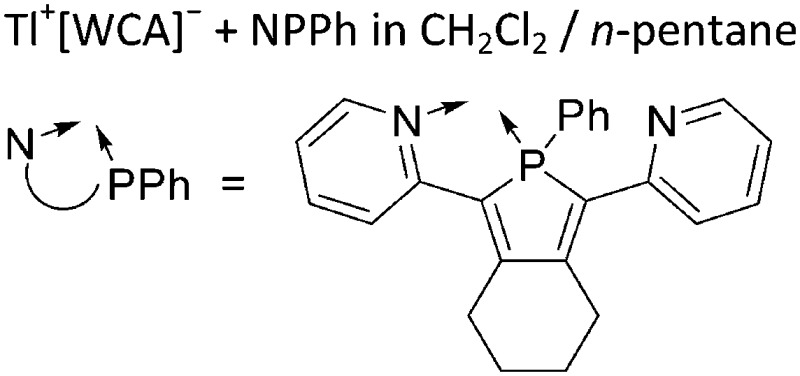
|
|
218 |
| [Tl(β-triketimine)2]2+(R = Me, t Bu) | [B(ArCF3 )4]– | Com | Tl+[WCA]– + β-triketimine |
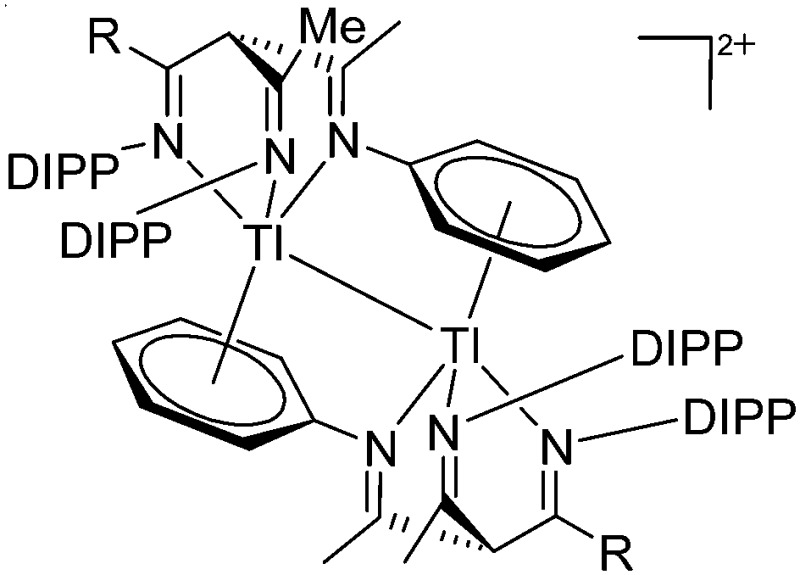
|
14 |
| [Tl2({CpMo(CO)2}2)6]2+ | [Al(ORPF)4]– | Com | {CpMo(CO)2}2(P2) + Tl+[WCA]– |
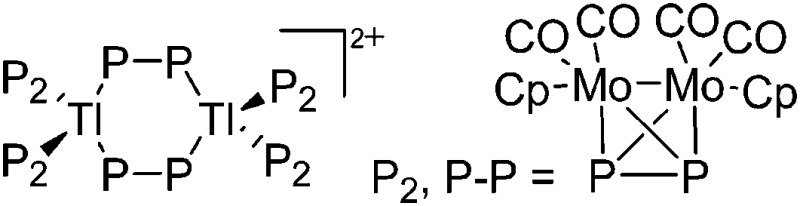
|
207 |
| [Tl3F2Al(ORHF)3]+ | [Al(ORHF)4]– | Salt, other | TlF + 2Li+[WCA]– |
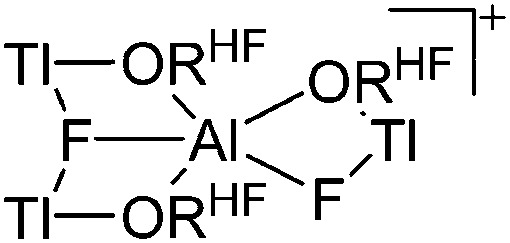
|
200 |
| [Tl4(μ-OH)2]2+ | [H2N{B(C6F5)3}2]– | Other | [Tl(OEt2)2]+[WCA]– + H2O |
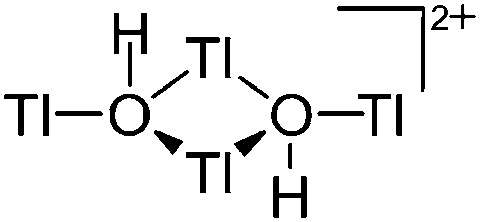
|
203 |
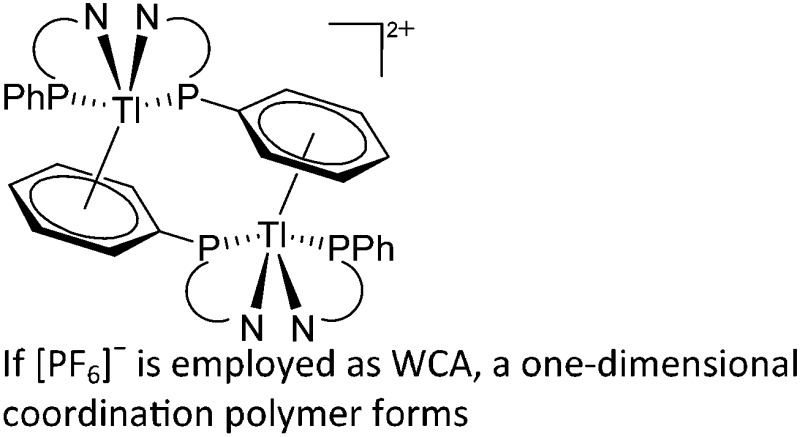
| [{Tl(OR)4(μ-Cl)2}+] n | [PF6]– | Other | RuCl2(DMeOPrPE)2 + Tl+[WCA]– | “Arrested” chloride abstraction yielding a one-dimensional coordination polymer | 209 |
| Multinuclear transition-metal substituted | |||||
| [(CpFe(CO){B(NCy2)})2-(μ-dmpe)]2+ | [B(ArCF3 )4]– | Com, salt | (FP){B(NCy2)}Cl + dmpe + Na+[WCA]– |
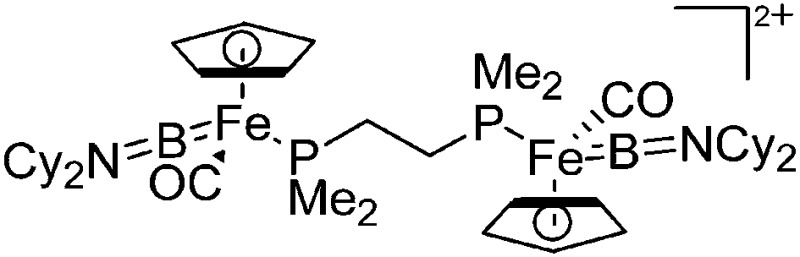
|
124 |
| [(BCat)(PNP)Pd(BCat)]+ | [B(ArCF3 )4]–/[CB11H12]– | Other | [(PNP)Pd(THF)]+[WCA]–/(PNP)Pd(CB11H12) + CatB–BCat |
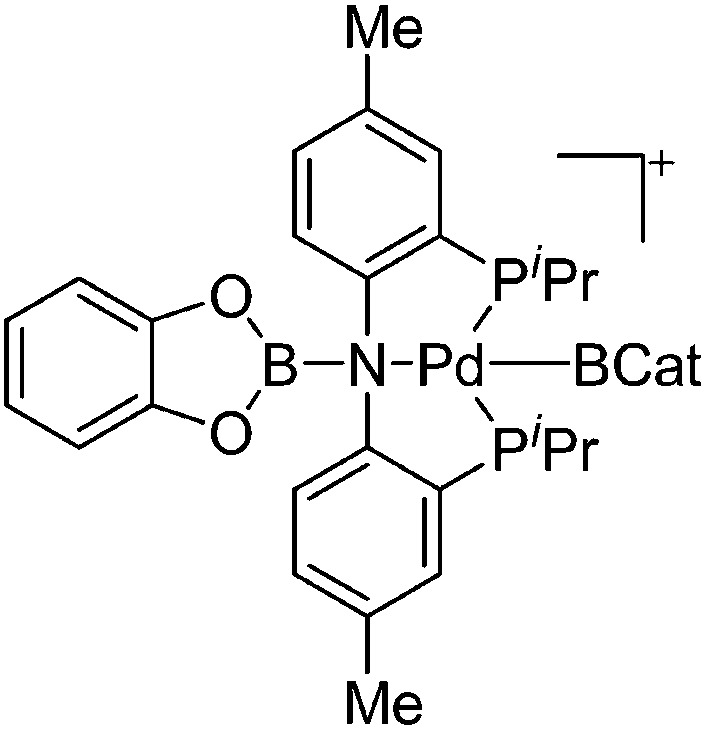
|
117 |
| [(Cy3P)2{Pt(BBr)}2-(μ-C6H4)]2+ | [B(C6F5)4]– | Salt | {(Cy3P)2Pt(Br)(BBr2)}2-(μ-C6H4) + K+[WCA]– |
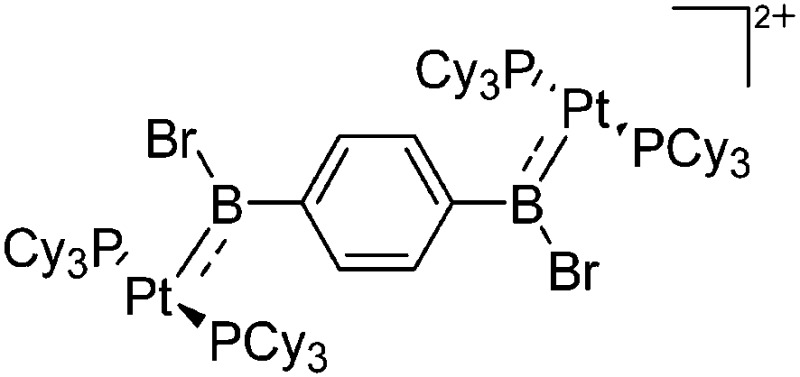
|
125 |
| [{(Cy3P)2PtB}2(μ-O)2]2+ | [Al(ORPF)4]– | Salt | (Cy3P)2BrPt(B O) + Ag+[WCA]– |
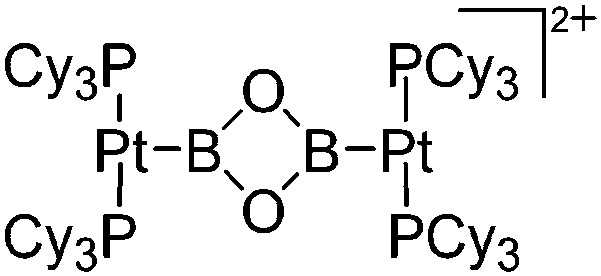
|
242 |
| [{(bipy)(Me)B}2(μ-Fc)]2+ | [PF6]– | Salt, Com | Fc(BBrMe)2 + 2bipy + [NH4]+[WCA]– |
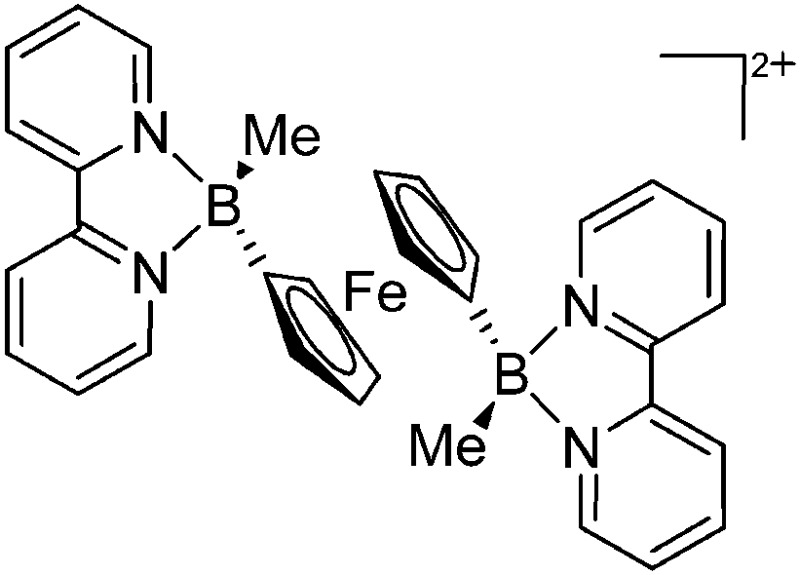
|
126 and 127 |
| [{(FP)Ga(Mes)}2(μ-Cl)]+ | [B(ArCF3 )4]– | Salt, Com | (FP)Ga(Mes)(Cl) + Na+[WCA]– |
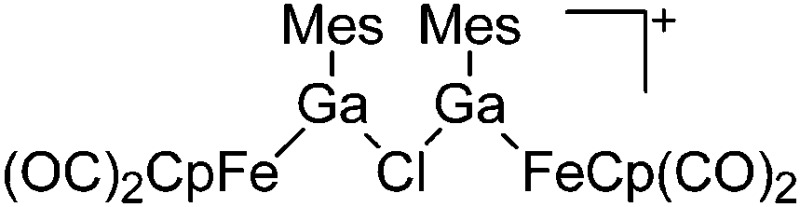
|
195 |
| [{{FeCp(CO)2}Ga{(NCy)2-C t Bu}}2(μ-OH)]+ | [B(ArCF3 )4]– | Other | [FpGa(OEt2)-{(NCy)2C t Bu}]+[WCA]– + H2O |
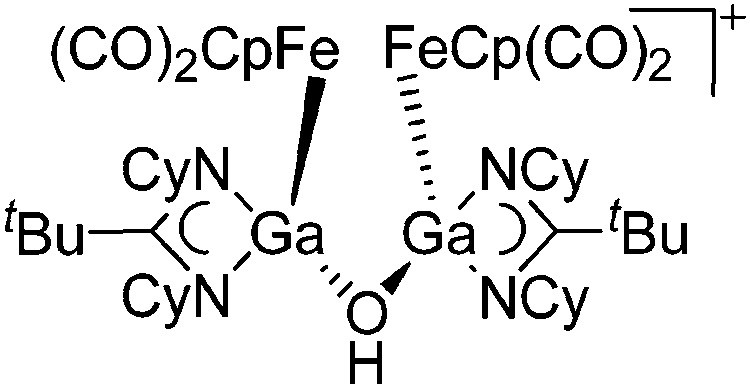
|
235 |
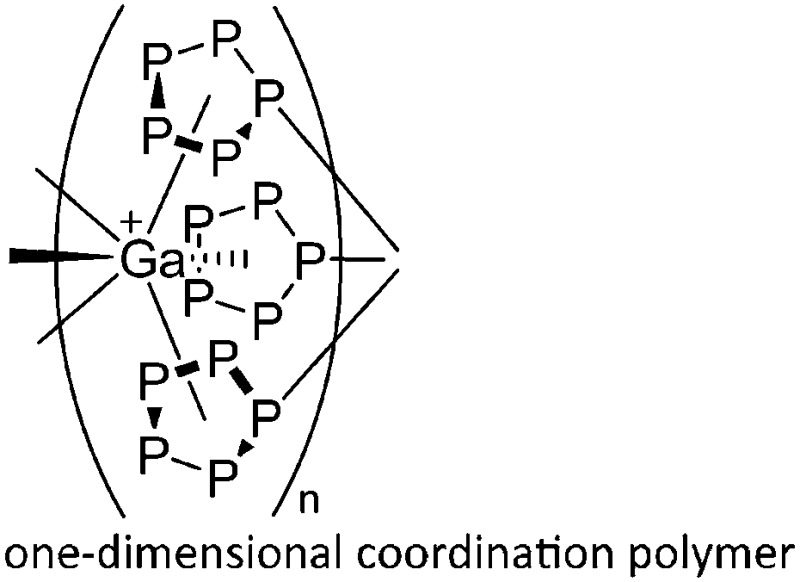
| [{Ga(P5FeCp*)3}+] n | [Al(ORPF)4]– | Com |
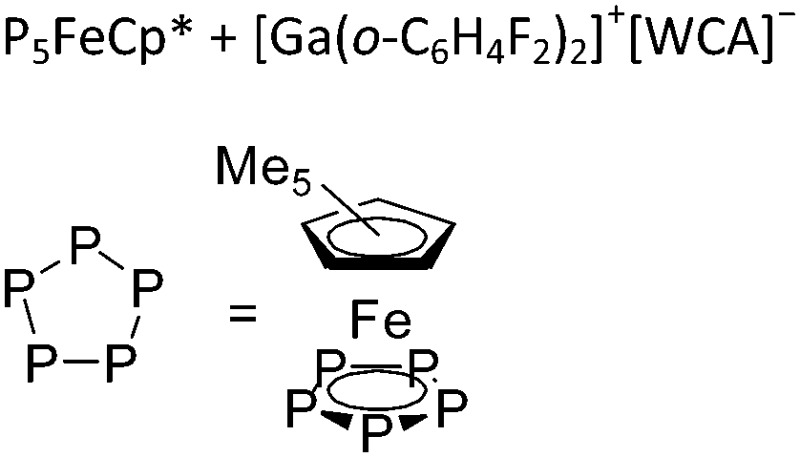
|
|
176 |
| [{(DDP)(THF)Ga}2Au]+ | [B(ArCF3 )4]– | Salt, Com | {(DDP)Ga}2AuCl + Na+[WCA]– in THF |
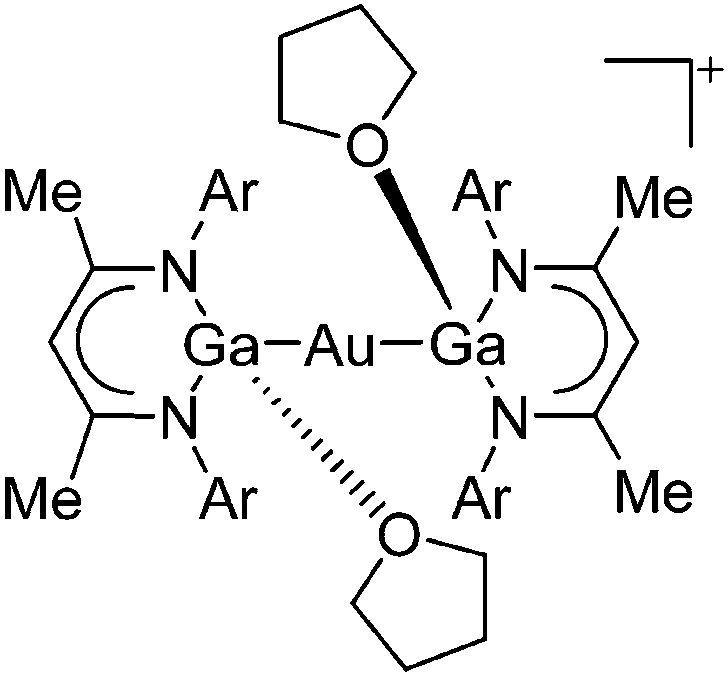
|
174 |
| [{(THF)(DDP)GaZn(THF)}2-(μ-Cl)2]2+ | [B(ArCF3 )4]– | Salt, Com | (DDP)(Cl)GaZn(Cl)(THF)2 + Na+[WCA]– in THF |
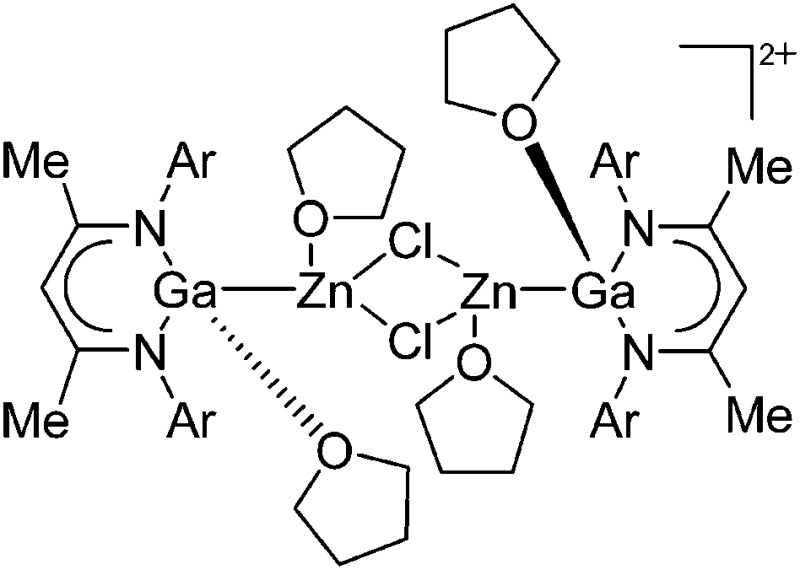
|
175 |
| [{In{η5-E5)FeCp*}3}+] n (E = P, As) | [Al(ORPF)4]– | Com |
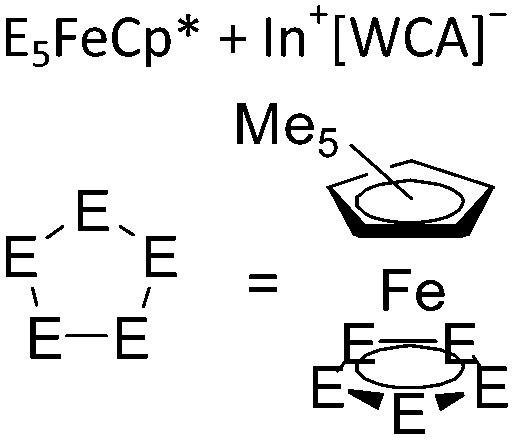
|
One-dimensional coordination polymer/similar structure to [{Ga(P5FeCp*)3}+] n | 176 |
| [{Tl{(η5-E5)FeCp*}3}+] n (E = P, As) | [Al(ORPF)4]– | Com |
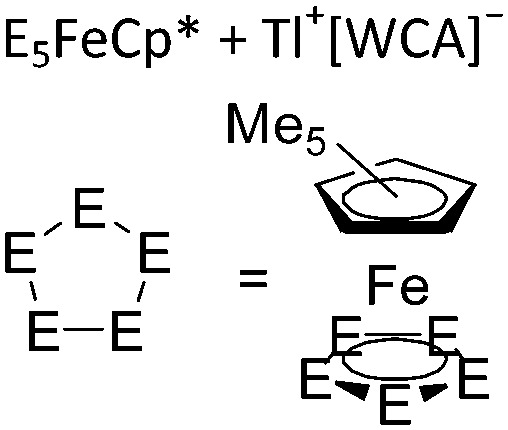
|
One-dimensional coordination polymer/similar structure to [{Ga(P5FeCp*)3}+] n | 207 and 176 |
| ECp* substituted (E = Al, Ga) | |||||
| [Rh(COD)(AlCp*)3]+ | [B(ArCF3 )4]– | Com | [Rh(COD)2]+[WCA]– + 3AlCp* |
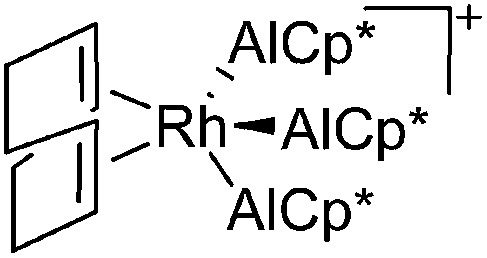
|
157 |
| [Cp*Fe(GaCp*)3]+ | [B(ArCF3 )4]– | Com | [Fe(MeCN)6]2+{[WCA]–}2 + 4GaCp* |
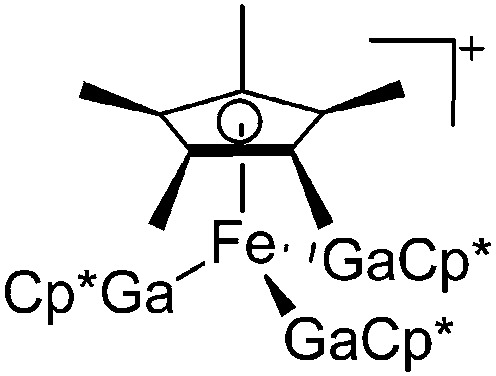
|
243 |
| [Cp*Co(GaCp*)3]2+ | [B(ArCF3 )4]– | Ox, Com | [Co(MeCN)6]2+{[WCA]–}2 + 4GaCp* |
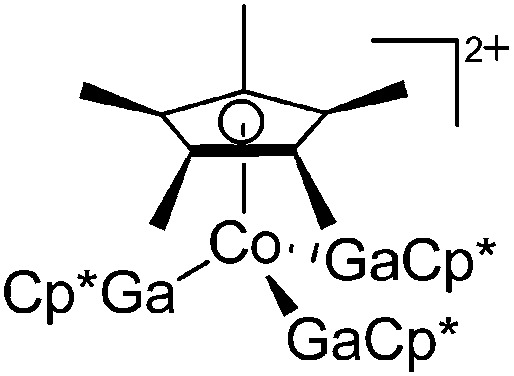
|
243 |
| [Cu(GaCp*)4]+ | [B(ArCF3 )4]– | Com | [Cu(MeCN)4]+[WCA]– + 4GaCp* |
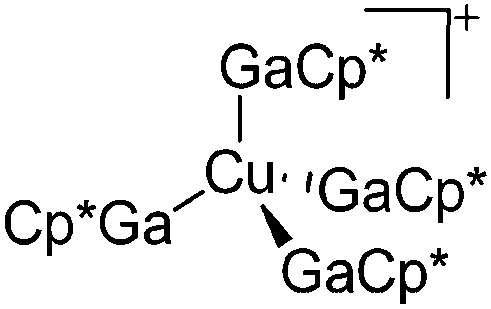
|
243 |
| [Zn(GaCp*)4]2+ | [B(ArCF3 )4]– | Prot, Com | ZnMe2 + [H(OEt2)2]+[WCA]– + 4GaCp* |
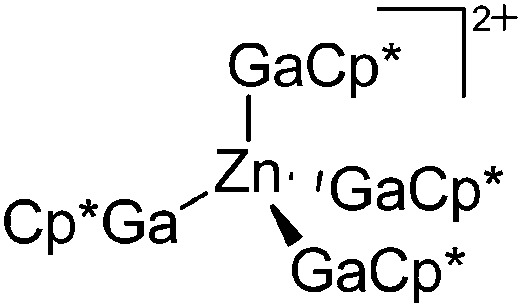
|
175 |
| [Zn2(GaCp*)6]2+ | [B(ArCF3 )4]– | Other | Zn2Cp*2 + [Ga2Cp*]+[WCA]– mechanism unclear |
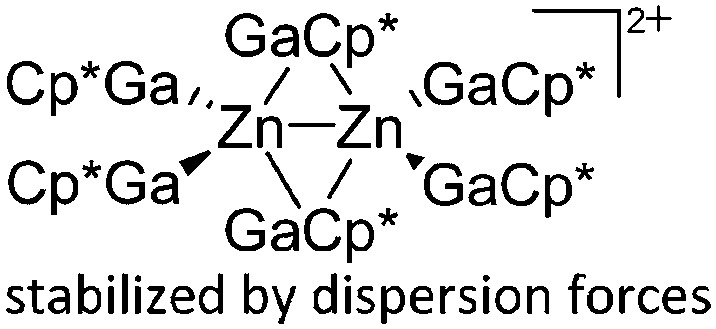
|
244 |
| [Rh(COD)(GaCp*)3]+ | [B(ArCF3 )4]– | Com | [Rh(COD)2]+[WCA]– + 3GaCp* |
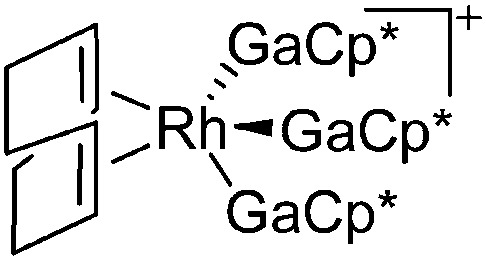
|
157 |
| [{Rh(NBD)(PCy3)-(GaCp*)2}]+ | [B(ArCF3 )4]– | Com | [Rh(NBD)(PCy3)2]+[WCA]– + 2GaCp* |
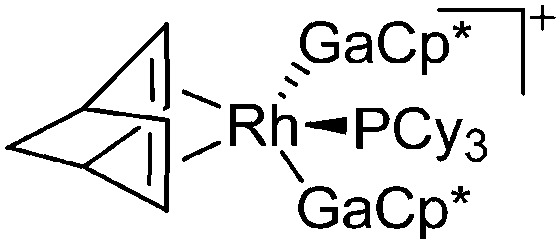
|
157 |
| [Pt(H)(GaCp*)4]+ | [B(ArCF3 )4]– | Prot | Pt(GaCp*)4 + [H(OEt2)2*]+[WCA]– |
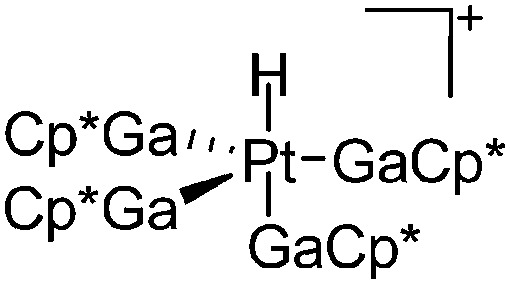
|
180 |
| [(Ga)Ru(PCy3)2(GaCp*)2]+ | [B(ArCF3 )4]– | Other | Ru(PCy3)2(GaCp*)2(H)2 + [Ga2Cp*]+[WCA]– |
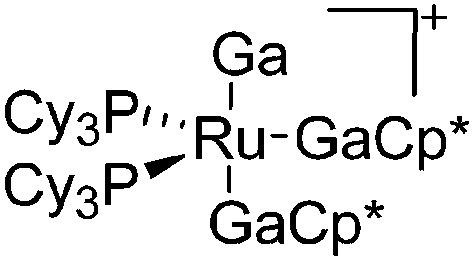
|
177 |
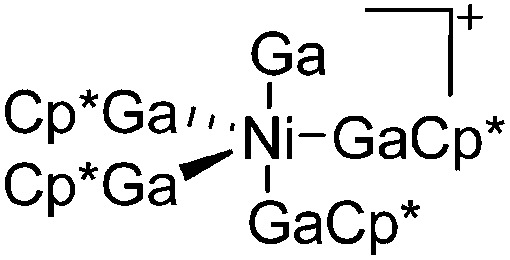
| [(Ga)Ni(GaCp*)4]+ | [B(ArCF3 )4]– | Other | Ni(GaCp*)4 + [FeCp2]+[WCA]– |
|
178 |
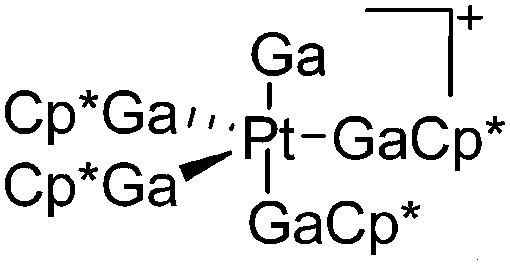
| [(Ga)Pt(GaCp*)4]+ | [B(ArCF3 )4]– | Com | Pt(GaCp*)4 + [Ga2Cp*]+[WCA]– |
|
179 and 180 |
| [(Cp*Ga)4Rh{Ga(Me)}]+ | [B(ArCF3 )4]– | Prot | (Cp*Ga)4Rh-(η1-Cp*GaMe) + [H(OEt2)2]+[WCA]– |
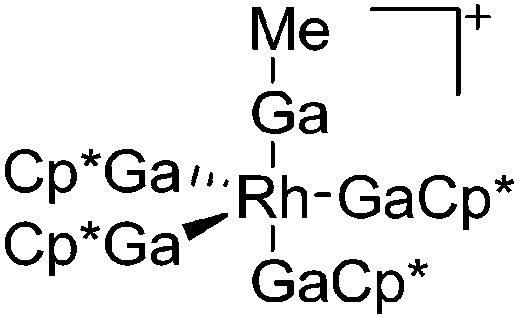
|
181 |
| [(Cp*Ga)4Rh{Ga(Me)-(py)}]+ | [B(ArCF3 )4]– | Com | [(Cp*Ga)4Rh(GaMe)]+[WCA]– + py |
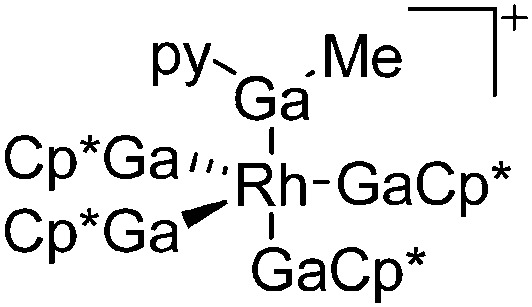
|
181 |
| [Ru(COD)(H)(GaCp*)3]+ | [B(ArCF3 )4]– | Com | [Ru(COD)(H)(DMH)3]+[WCA]– + 3GaCp* |
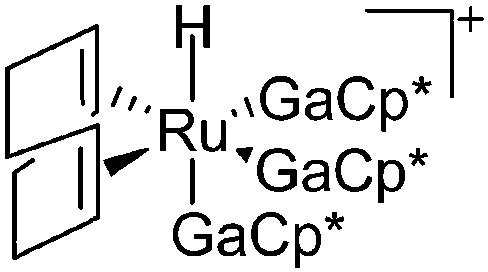
|
245 |
| [Ru(GaCp*)4-{η3-(CH2)2C(Me)}]2+ | [B(ArCF3 )4]– | Prot | Ru(GaCp*)3(TMM) + [H(OEt2)2]+[WCA]– TMM = η4-C(CH2)3 |
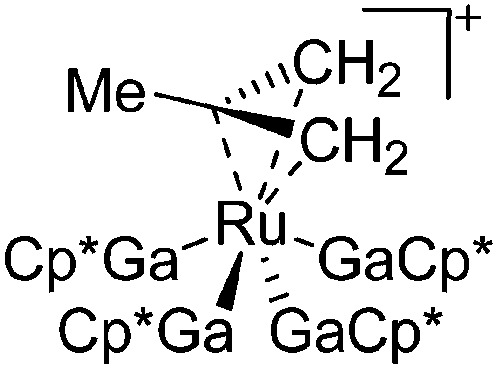
|
177 |
| [{Ru(GaCp*)3-[(CH2)2C{CH2(μ-Ga)}]}2]+ | [B(ArCF3 )4]– | Com | Ru(GaCp*)3(TMM) + [Ga2Cp*]+[WCA]– |
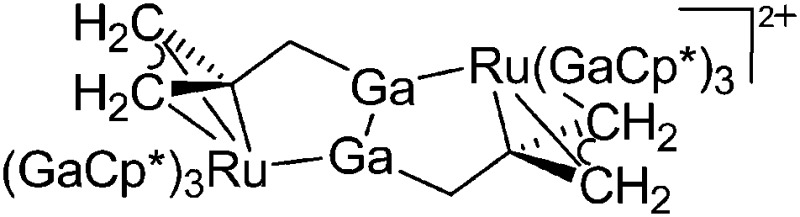
|
177 |
| [{(GaCp*)4Pt}{Pt(H)-(GaCp*)3}(μ-Ga)]2+ | B(ArCF3 )4]– | Prot, Com | Pt(GaCp*)4 + [H(OEt2)2*]+[WCA]– |
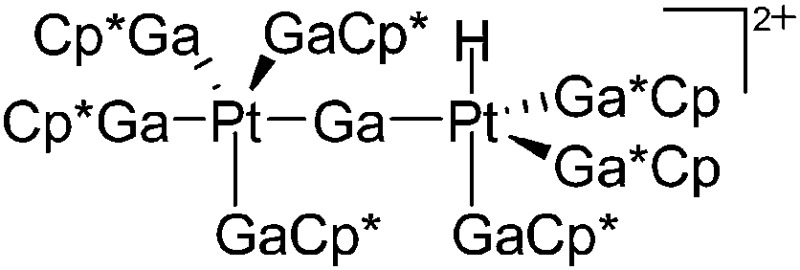
|
180 |
| [Pt3(GaCp*)6(μ-Ga)]+ | [B(ArCF3 )4]– | Other | Pt(GaCp*)4 + [FeCp2]+[WCA]– (substoichiometric) |
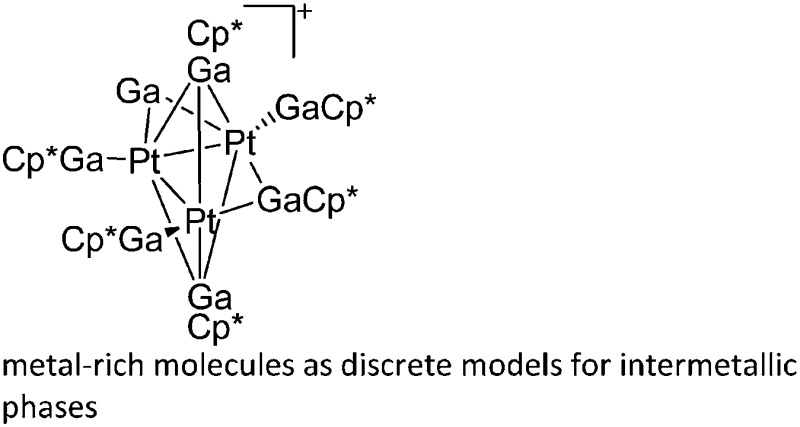
|
178 |
a Classification according to the introduction (Table 2).
Ligand substituted (CN = 3)
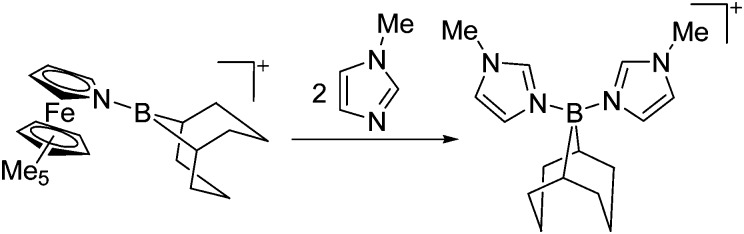
Tricoordinate borenium cations are not as electron deficient as the borinium cations and therefore more stable. Nonetheless, the cations can only be isolated in the solid state if chelating (e.g. phthalocyanine 115 and catecholborane 116,117 ) or strongly σ-donating ligands (e.g. N-heterocyclic carbene I t Bu 74 or hexaphenylcarbodiphosphorane 118,119 ) are applied. For the synthesis and reactivity of the [BCl2(I t Bu)]+ cation, the nature of the WCA is crucial. Hence and though the cation can be prepared in the presence of [AlCl4]–, [OTf]– or [B(ArCl)4]–, only the latter allows for a structure with no notable cation–anion contact. This leads to an increased reactivity of the [BCl2(I t Bu)]+[B(ArCl)4]– salt. 74
Ligand substituted (CN = 4)
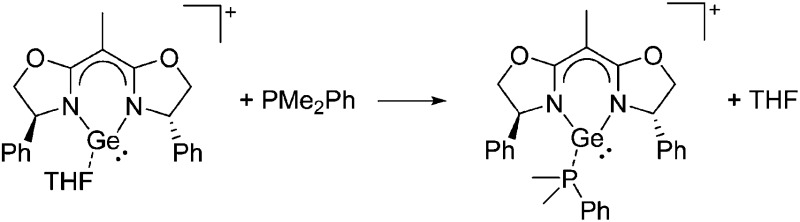
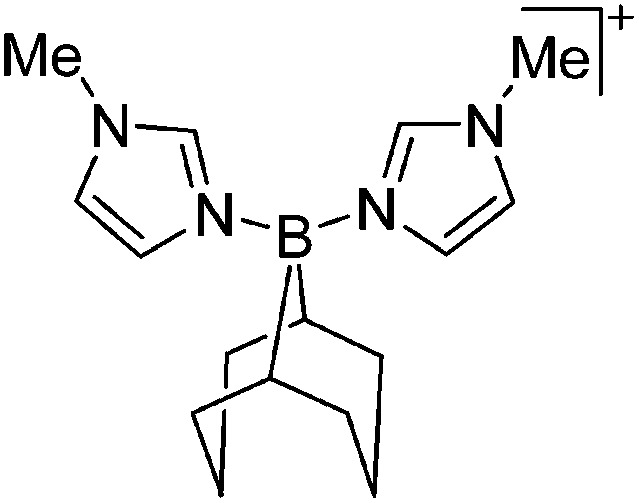
Some of the tetra-coordinated boronium cations directly derive from the corresponding borenium cations: i.e., the tricoordinate [PMAF–9BBN]+ cation (only stable in solution, as monitored by 11B NMR spectroscopy) reacts with 1-MIM in a ligand exchange and addition reaction to form the tetra-coordinated [(1-MIM)2(9BBN)]+ cation (Fig. 5). 212
Fig. 5. Synthesis of the [(1-MIM)2(9BBN)]+ boronium cation via a borenium cation precursor.
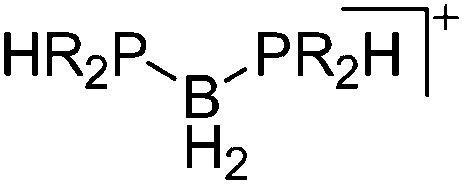
For the isolation of the discrete [BH2(PR2H)2]+ cation, the nature of the WCA is again essential: compared to [OTf]–, [B(ArCF3 )4]– features no hydrogen bond with the cation, thus allowing for increased reactivities. 120
Transition-metal substituted
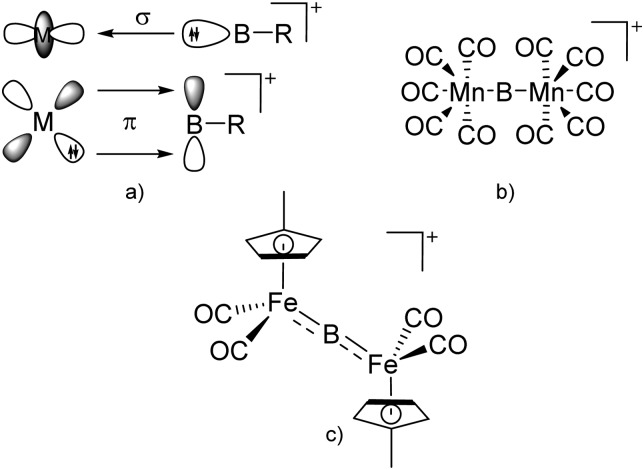
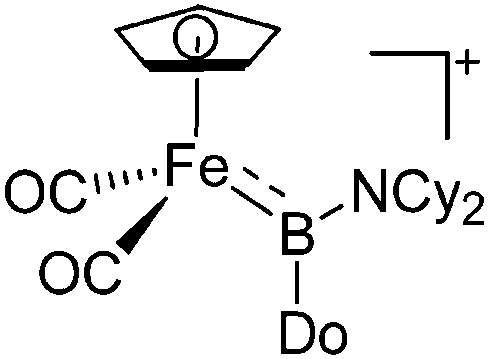
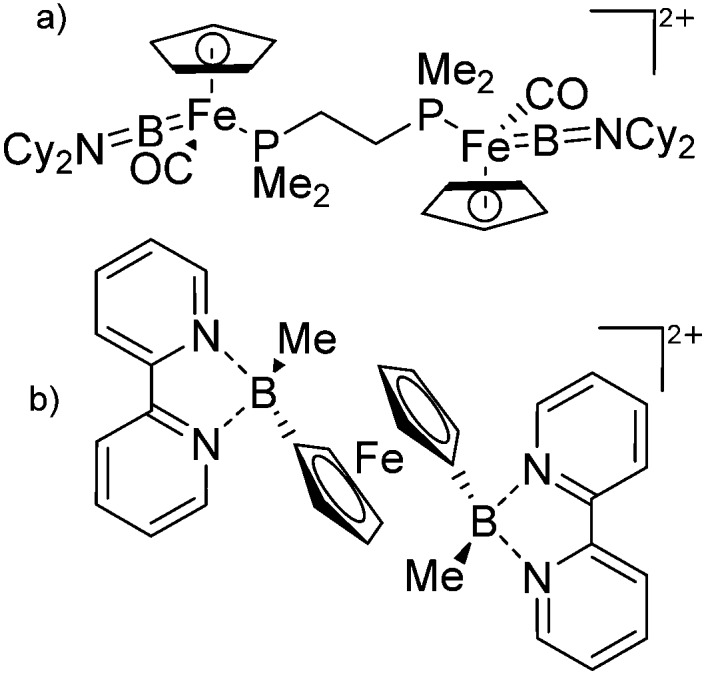
The number of transition-metal substituted boron cations is much higher than the one of related alkyl-/aryl- or heteroatom substituted compounds. Numerous contributions have been made by Braunschweig and Aldridge and both authors recently reviewed the chemistry of transition-metal borylene complexes. 3,5,104 The d-orbitals of the transition-metals allow for stabilizing σ- and π-interactions with the orbitals of boron (Fig. 6) and of all the ligands the FeCp(CO)2/FeCp′(CO)2/FeCp*(CO)2 substituents protrude: e.g., various linear borinium cations, such as [CpFe(CO)2B(NCy2)]+, and borenium cations derived thereof, such as [(CpFe(CO)2)B(NCy2)(4-Pic)]+, have been isolated. 12
Fig. 6. (a) Orbital interaction between borylenes and transition-metal fragments; (b) and (c) exemplarily selected transition-metal substituted borinium cations.
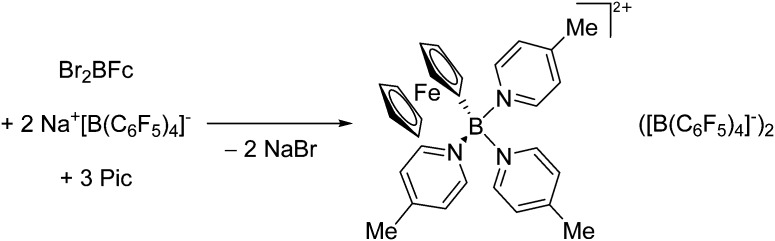
Another notable substance class are the cationic T-shaped platinum boryl complexes that are usually accessible via salt metathesis reactions: e.g., [(Cy3P)2(MeCN)Pt(B O)]+ can be synthesized by reacting (Cy3P)2Pt(B O)(Br) with the halide abstracting reagent Ag+[B(ArCF3 )4]–. 121 Employing a ferrocenyl ligand on the other hand, Braunschweig et al. were able to isolate a rare example of a structurally characterized boron dication: [FcB(Pic)3]2+ (Fig. 7). 122
Fig. 7. Synthesis of the boron dication [FcB(Pic)3]2+ via bromide abstraction and subsequent complexation.
Multinuclear
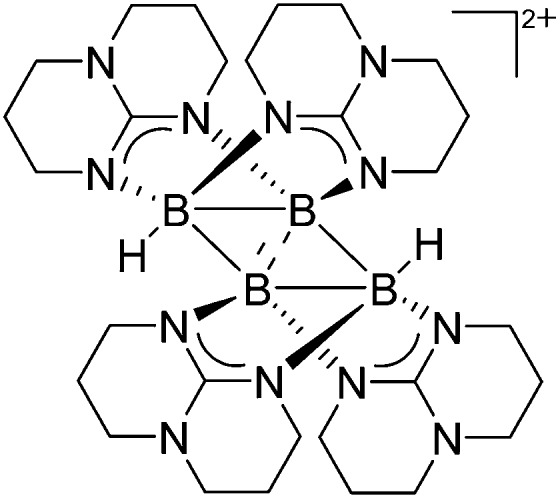
Due to the pronounced electron deficiency of boron there are not many contributions to the field of cationic multinuclear boron-based rPBC. The neutral diborane [HB(μ-hpp)]2 complex however, is an excellent precursor for hydride abstractions and via unexpected boron–boron coupling reactions the unprecedented tetraborane dication [B4H2(μ-hpp)4]2+ was isolated (Fig. 8). 123
Fig. 8. The tetraborane dication [B4H2(μ-hpp)4]2+. The bonding properties in the rhomboid B4 core of the product can be described as two B–B units connected by 3c-2e bonds, sharing a short diagonal.
Multinuclear transition-metal substituted
Compared to their mononuclear congeners, both the ligands and coordination modes in the multinuclear borinium, borenium and boronium cations are very similar: (i) linear in the [(CpFe(CO){B(NCy2)})2(μ-dmpe)]2+ complex, 124 (ii) trigonal-planar in the [(Cy3P)2{Pt(B(Br))}2(μ-Ph)]2+ dication 125 and (iii) tetrahedral in [{(bipy)(Me)B}2(μ-Fc)]2+. 126,127 The aggregation usually occurs via bi-functional ligands like dmpe or via the transition-metal ligand itself (Fig. 9).
Fig. 9. Dicationic (a) [(CpFe(CO){B(NCy2)})2(μ-dmpe)]2+ 124 and (b) [{(bipy)(Me)B}2(μ-Fc)]2+ 126,127 complexes.
Aluminum cations
Among the group 13 cations, the lower- and higher-coordinated derivatives of aluminum have been of significant interest as they feature increased Lewis acidities and ligand labilities, thus allowing for higher catalytic activities compared to their neutral analogs. 95 While Atwood (1998) 102 and Dagorne (2008) 95 have given a good overview on cationic aluminum species from a fundamental perspective, Sarazin and Carpentier (2015) 10 recently reviewed various discrete cationic aluminum complexes that are able to catalyze ring-opening polymerizations.
Alkyl or aryl substituted
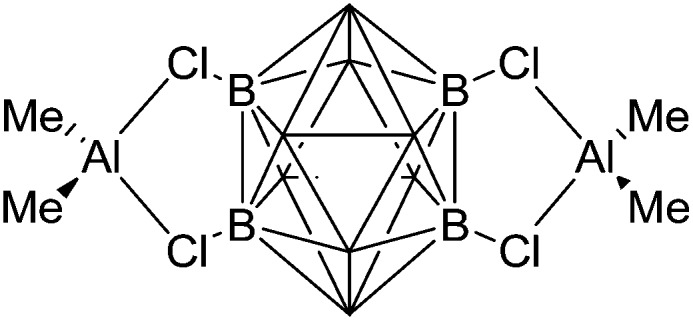
The synthesis of di-coordinated alkyl complexes of aluminum [R2Al]+ (R = Me, Et, 2,6-Mes2C6H3) is only viable, if extremely weakly coordinating anions (e.g. borate 87 and carboranes 128 ) and/or bulky substituents 129 are applied. In the case of “[Me2Al]+” and “[Et2Al]+”, the Lewis acidity of the aluminum cations is so significant that the latter feature distinct contacts to the corresponding WCAs and should therefore be described as ion-like compounds (Fig. 10). However, preliminary investigations showed that ion-like (Et2Al)2B12Cl12 is a very active initiator for the cationic polymerization of isobutylene. 130
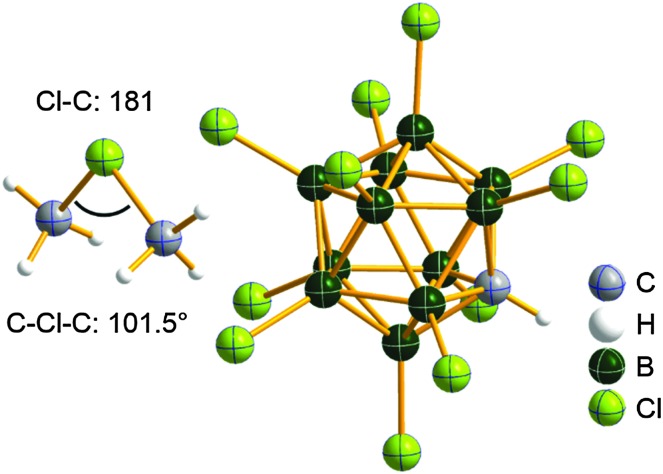
Fig. 10. The ion-like (Me2Al)2B12Cl12 salt. 87 For clarity, all BCl moieties of the perchlorinated closo-dodecaborate that feature no contact to the “[Me2Al]+” cation have been omitted.
The [(2,6-Mes2C6H3)2Al]+ cation on the other hand, is a discrete and therefore almost linear di-coordinate aluminum cation that features no contact to the WCA [B(C6F5)4]–. 129 The occurrence of the highly Lewis acidic aluminum cation is attributable to the intrinsic stabilization effect of the 2,6-Mes2C6H3 ligand: i.e., bending of the flanking Mes-moieties towards the aluminum center.
Cyclopentadienyl complexed
This class of compounds is to some extent related to the just mentioned alkyl substituted [(2,6-Mes2C6H3)2Al]+ complex. Hence, the Cp ligands are η5- but not σ-bonding, and allow for the synthesis of discrete aluminum cations with different WCAs as counterions: [(η5-Cp)2Al]+[Al(ORPF)4]–, 79 [(η5-Cp′)2Al]+[B(C6F5)4]– 131 and [(η5-Cp*)2Al]+ [MeB(C6F5)3]–. 132,133 Moreover, the salts offer insights into the relationship between the nucleophilicity of Cp, Cp′ and Cp*, the corresponding WCAs and the resultant Lewis acidities and reactivities of the aluminum cations: i.e., with increasing nucleophilicity of the Cp ligands (Cp < Cp′ < Cp*) the WCAs can be less coordinating ([MeB(C6F5)3]– > [B(C6F5)4]– > [Al(ORPF)4]–). The more interacting anions induce decreased Lewis acidities and lower reactivities of the aluminum cations for the initiation of olefin polymerizations: [(η5-Cp)2Al]+ > [(η5-Cp′)2Al]+ > [(η5-Cp*)2Al]+. 79
Ligand substituted (CN = 2)
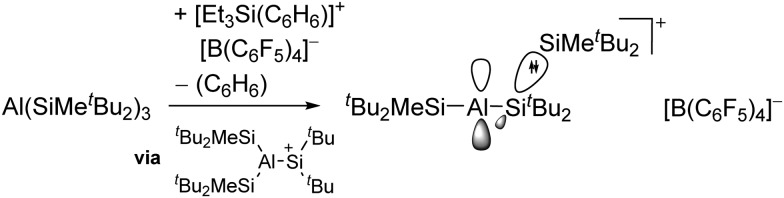
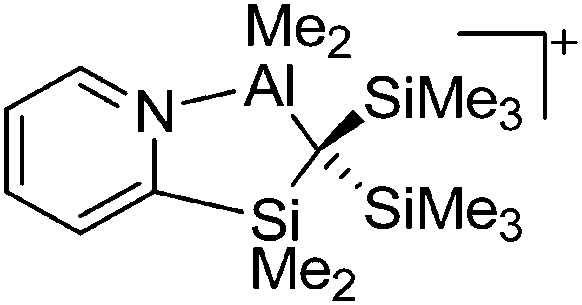
The above mentioned σ-coordinated [R2Al]+ complexes are either stabilized by intermolecular interactions with the corresponding WCAs or intramolecularly by two bulky terphenyl ligands. Within this context, Sekiguchi et al. were able to contribute another cationic di-coordinated, yet differently intramolecularly stabilized aluminum species: the [ t Bu2MeSi–Al–Si t Bu2–SiMe t Bu2]+ cation. 134 As supported by the solid-state structure and theoretical calculations, the stabilizing element is a σ–π hyperconjugation of the aluminum cation and the neighboring Si–Si σ bond (Fig. 11).
Fig. 11. Synthesis of the [ t Bu2MeSi–Al–Si t Bu2–MeSi t Bu2]+ cation via demethylation and subsequent migration of a t Bu2MeSi group.
Ligand substituted (CN = 3)
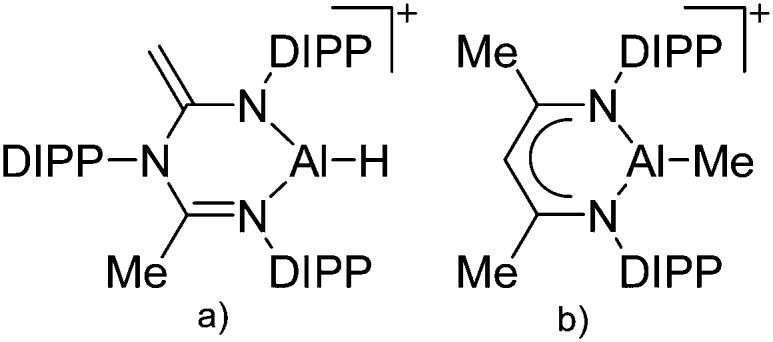
Tricoordinate aluminum cations are a bit less electrophilic than their di-coordinated congeners but nevertheless still very reactive. The few examples that have been reported, require chelating and sterically demanding β-diketiminate ligands, thus allowing for the successful synthesis of cationic [(β-diketiminate)Al–H]+ 135 and [(β-diketiminate)Al–Me]+ 136 complexes, respectively (Fig. 12).
Fig. 12. (a) The [(β-diketiminate)Al–H]+ cation derives from the reaction of a N-imidoylamidine ligand with AlH3·NMe2Et and [Ph3C]+[B(C6F5)4]–. 135 (b) The [(β-diketiminate)Al–Me]+ cation is formed by reacting the neutral precursor (β-diketiminate)Al(Me)2 with the demethylating reactants [CPh3]+[B(C6F5)4]– and B(C6F5)3, respectively. 136 .
Ligand substituted (CN = 4)
In their recent review on group 13 cations, Dagorne and Atwood state that “four-coordinate cations are most common … as they incorporate an electronically saturated metal center”. 95 In all compounds the aluminum cations are coordinated in a tetrahedral fashion with at least one coordination site being occupied by a heteroatom (N, O, P). Moreover, the vast majority of aluminum cations are incorporated into heterocycles, which derive from chelating ligands, such as Pytsi, 137 hpp, 138 BOX, 139 iPr2-ATI 140,141 and SchNMe2. 142 The usual synthesis routes are alkyl or hydride abstractions. On the other hand, there are a few examples where aluminum is coordinated by four discrete ligands: [Me2Al(OEt2)2]+, 77 [Me2Al(THF)2]+, 143 [Me2Al(NPhMe2)2]+ 144 and [H2Al(NMe3)2]+ 145 (cf. Fig. 13 for the complex synthesis of the [H2Al(NMe3)2]+ cation and the in situ generation of the corresponding WCA).
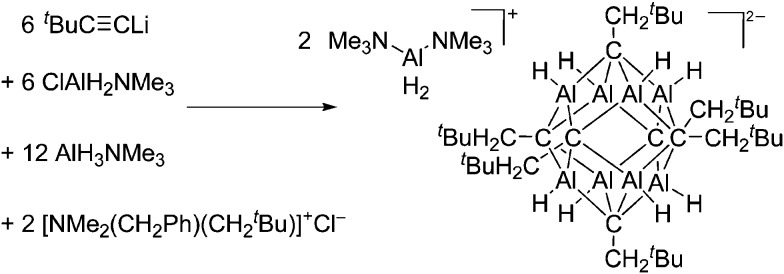
Fig. 13. Salt metathesis and hydroalumination reactions lead to the formation of the weakly coordinating carbaalanate cluster that allows for the synthesis of two equivalents of the [H2Al(NMe3)2]+ cation. 145 .
Ligand substituted (CN ≥ 5)
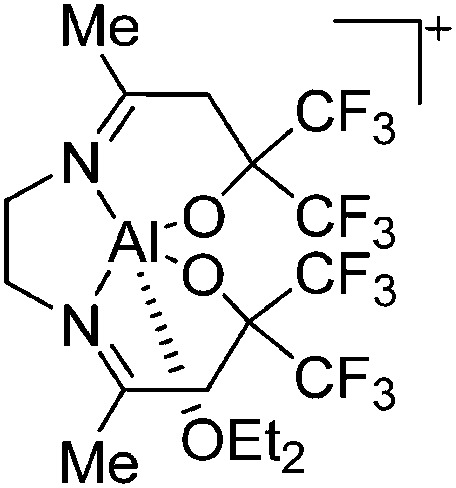
As mentioned in the previous sub-chapter, chelating ligands are of significant importance in terms of stabilizing cationic highly coordinated (CN ≥ 5) aluminum cations. Of all the different chelates, the Salen derivatives 146 protrude, allowing for the synthesis of distorted square pyramidal/octahedral aluminum cations that interact with one 147 or two 148–151 equivalents of Lewis base, such as Et2O and THF (Fig. 14).
Fig. 14. Octahedral or quadratic-pyramidal coordinated [SalenAl(Do) n ]+ cations (Do = Et2O, THF) with n = 1,2.
Multinuclear
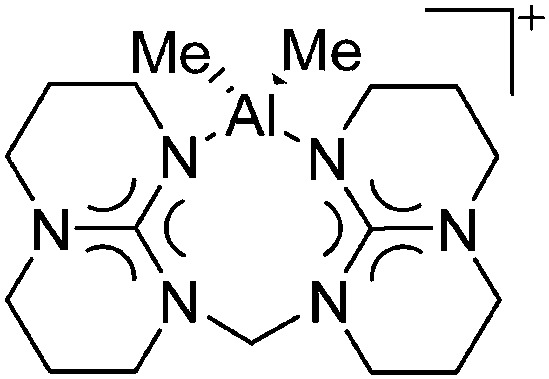
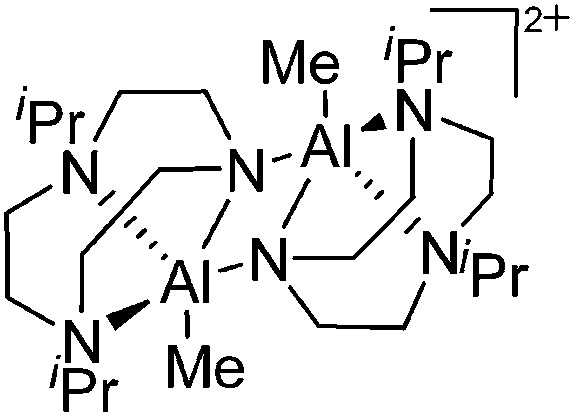
A common structural motif of dicationic and dinuclear aluminum cations are the often centrosymmetric [Al2O2]-rhomboids 141,152–154 as seen in the recently reported [{(OSSO)Al}2]2+ cation (Fig. 15). 155
Fig. 15. In the [{(OSSO)Al}2]2+ cation one aluminum atom is coordinated in a trigonal-bipyramidal and the other in a distorted-square-pyramidal fashion. The cationic species is a potential catalyst for the ring opening polymerization of propylene oxide. 155 .
On the other hand, there are various dinuclear, yet singly charged aluminum cations in which the latter usually feature different coordination modes. Notable contributions to this field of research have been made by Jordan et al., such as the cationic aluminum aminotroponiminate 141 and amidinate 156 complexes in Table 8.
AlCp* substituted
The coordination chemistry of low-valent group 13 organyls such as AlCp* to transition-metals is a growing field in inorganic chemistry, though more contributions were reported using the heavier homologue GaCp* (see below). Nonetheless, Fischer et al. were able to isolate the cationic [Rh(COD)(AlCp*)3]+ complex by reacting [Rh(COD)2]+ with three equivalents of AlCp*. 157
Gallium cations
As mentioned above, gallium in its +I oxidation state is thermodynamically unstable and usually disproportionates into the metal and the +III ions. Notable contributions to the field of reactive gallium cations therefore allow for the stabilization of the +I oxidation state of gallium. 31
Alkyl or aryl substituted
The isolation of the linear di-coordinated [(2,6-Mes2C6H3)2Ga]+ cation 158 was performed by Wehmschulte et al. as a test run for the above mentioned structurally related [(2,6-Mes2C6H3)2Al]+ cation. 129 Hence, the bowl-shaped terphenyl substituents are potential ligands in terms of shielding highly electrophilic cations. Moreover, both syntheses were only possible due to the presence of very good WCAs, such as the [Li{Al(ORHF)4}2]– and [B(C6F5)4]– anion.
Cyclopentadienyl complexed
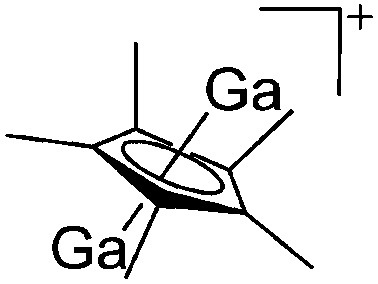
Partial protolysis of GaCp* with [H(OEt2)2]+[B(ArCF3 )4]– yields the bipyramidal double-cone structured [Ga2(η5-Cp*)]+ cation. 159 The latter can be seen as a GaCp*-substituted “naked” Ga+ cation, thus reacting as a gallium(i) source with ligands such as DDP (Fig. 16).
Fig. 16. The [Ga2(η5-Cp*)]+[B(ArCF3 )4]– salt cleanly reacts as a gallium(i) source with ligands such as DDP.
The coordination mode of the Cp* ligands in the [(η1-Cp*)(η3-Cp*)Ga]+ cation on the other hand differs. 160 Hence, the originally expected η5,η5-ferrocene-like structure that was also observed for the aluminum analogue is likely perturbed by the more interacting [BF4]– counterion.
Arene complexed
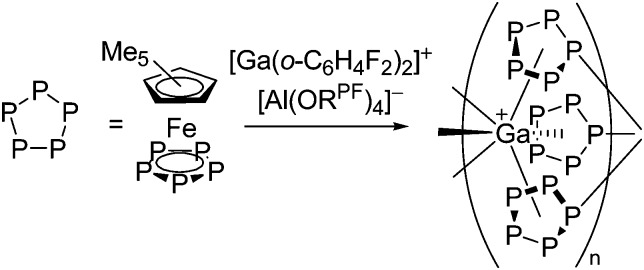
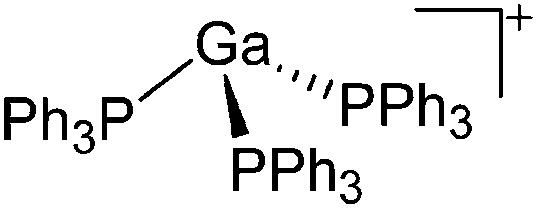
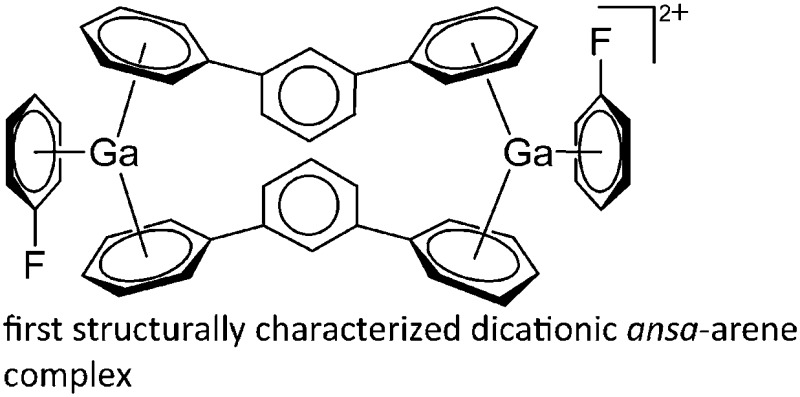
Cationic arene complexes of univalent gallium are known for more than 30 years and Schmidbaur et al. have made notable contributions to this field of research. 100,101 Yet, the reported compounds feature strong cation–anion interactions and are labile towards com- and disproportionations. More recently, Krossing et al. developed a simple oxidative route to [Ga(η6-arene) n ]+ complexes of the weakly coordinating [Al(ORPF)4]– anion with n = 2, 3 (Fig. 17). 31,91,92 The arene complexes have proven to be a powerful starting material for further gallium(i) chemistry (e.g. various ligand exchange reactions) but also highly efficient catalyst for the polymerization of isobutylene. 7,8,99
Fig. 17. Oxidative access to [Ga(η6-arene) n ]+ complexes with n = 2, 3(R = F, Me).
Ligand substituted (CN = 2)
The arene ligands in the [Ga(η6-arene) n ]+ cations with n = 2, 3 can be substituted by electron-richer analogues. In addition, σ-donating ligands such as carbenes IR (R = Pr, Mes) 161 or phosphines P t Bu3 162 can also be applied, yielding bent [Ga(IR)2]+ and [Ga(P t Bu3)2]+ complexes (cf. the stereoactive electron lone pair at the gallium(i) cation). Another notable di-coordinated gallium(iii) cation is the linear [ t Bu3Si–Ga–Si t Bu3]+ complex (Fig. 18), which could be isolated in the presence of the [Al(ORPF)4]– WCA, but not the simple [GaCl4]– anion. 163
Fig. 18. Molecular structure of the [ t Bu3Si–Ga–Si t Bu3]+ cation. A. Budanow, T. Sinke, J. Tilmann, M. Bolte and M. Wagner, Two-coordinate gallium ion [ t Bu3Si–Ga–Si t Bu3]+ and the halonium ions [ t Bu3Si–X–Si t Bu3]+ (X = Br, I): sources of the supersilyl cation [ t Bu3Si]+, Organometallics, 2012, 31, 7298–7301. Data from this reference were used to draw this figure and the hydrogen atoms were omitted for clarity. 163 .
Ligand substituted (CN = 3)
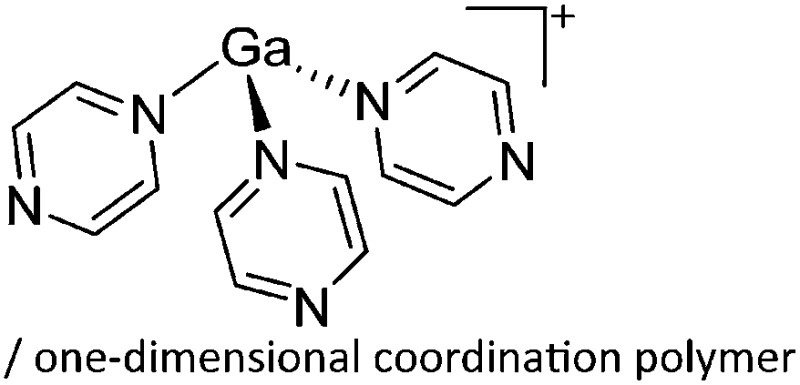
This class of tricoordinate gallium(i) cations again derives from the above mentioned [Ga(η6-arene) n ]+ cations with n = 2, 3. The coordination mode for the gallium(i) cations is trigonal-pyramidal due to the stereoactive lone pair electrons. Besides sterically less demanding phosphines, N-heterocylic arenes like pyrazine and DTBMP (a σ-, and not a π-donating ligand, proving its perception of being non-nucleophilic wrong) were also applied as potential ligands. 164 Due to the bifunctionality of pyrazine, both the monomeric [Ga(pyrazine)3]+ complex and the one-dimensional coordination polymer [{Ga(μ-pyrazine)2(η1-pyrazine)}+]∞ were isolated (Fig. 19).
Fig. 19. (a) Monomeric [Ga(pyrazine)3]+ complex and (b) one-dimensional coordination polymer [{Ga(μ-pyrazine)2(η1-pyrazine)}+]∞. The propagation of the polymer into the second dimension was not possible as each cationic strand is surrounded by strands of the corresponding [Al(ORPF)4]– anions. 164 .
Ligand substituted (CN = 4)
Using the BOX ligand, Dagorne et al. isolated tetra-coordinate neutral gallium complexes. 165 The latter were easily ionized by applying [CPh3]+[B(C6F5)4]– or B(C6F5)3 in NMe2Ph. Interestingly, the trityl cation functions as hydride and B(C6F5)3 as methyl abstracting reactant (Fig. 20).
Fig. 20. Hydride vs. methyl abstraction of neutral BOX ligated gallium complexes. 165 .
Ligand substituted (CN ≥ 5)
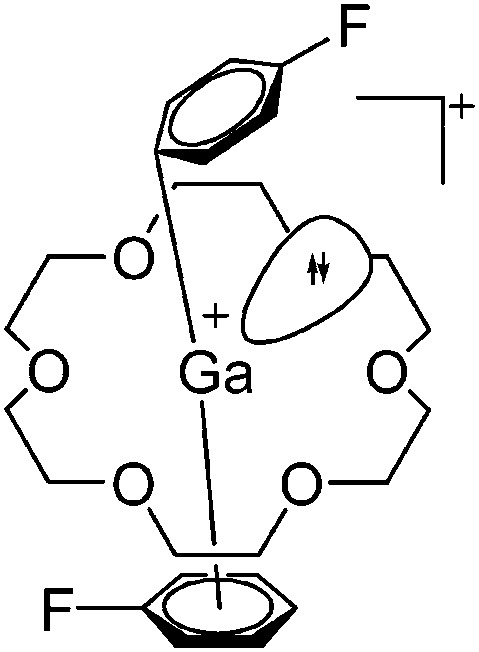
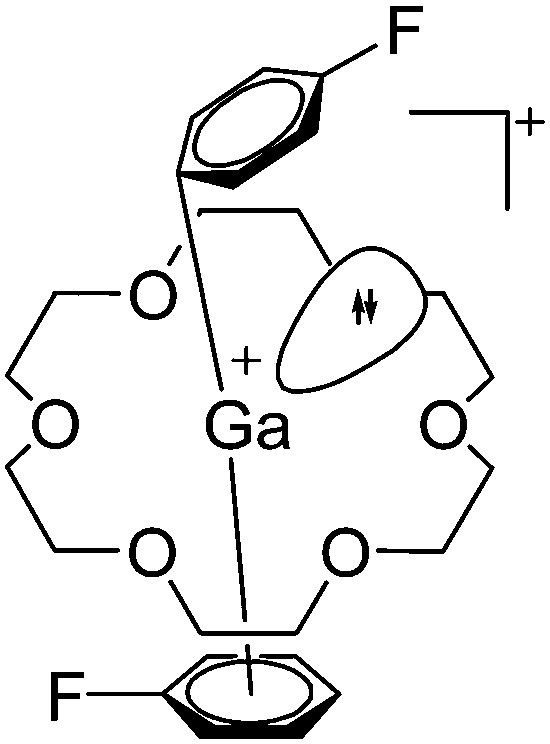
Cationic penta- and hexa-coordinated gallium complexes are synthesized via protonation 166,167 or complexation. 168 Within this context, the [Ga([18]crown-6)(C6H5F)2]+ complex is of special interest as the gallium(i) cation features no contact to the corresponding [Al(ORPF)4]– anion and the C6H5F ligands coordinate in different fashions (Fig. 21). 168
Fig. 21. The [Ga([18]crown-6)(η6-/η1-C6H5F)2]+ cation. The η6- and η1-coordination modes could be an indication for a stereoactive lone pair on the side of the weaker and only η1-coordinated C6H5F molecule. 168 .
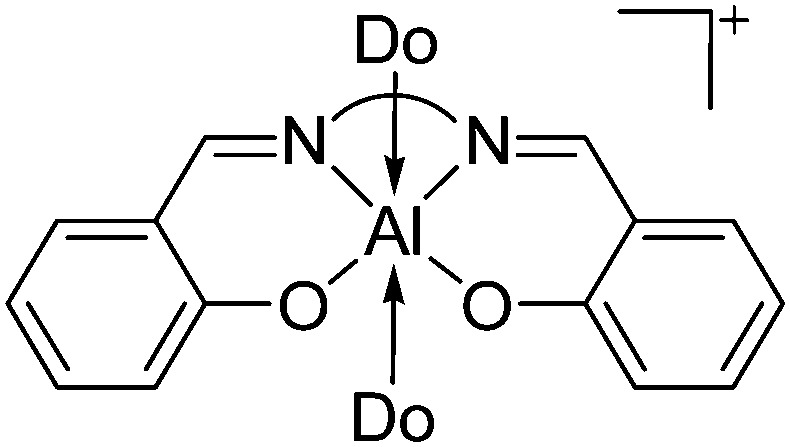
As the N-heterocyclic arenes are potential ligands for univalent gallium (see above), Krossing et al. additionally reacted the chelating bipy with the [Ga(η6-C6H5F)2]+ complex. Instead of witnessing a simple ligand exchange reaction, they isolated the paramagnetic and distorted octahedral [GaIII{(bipy)3}˙]2+ complex due to the non-innocence of the bipy ligand. 17 This is reminiscent to transition metal chemistry where for example the [RuIII{(bipy)3}˙]2+ complex features similar bonding.
Transition-metal substituted
Similar to the transition-metal substituted boron cations, CpFe(CO)2 (FP) and Cp*Fe(CO)2 (FP*) are the most important ligands in terms of stabilizing di-, tri- and tetra-coordinated gallium cations: cf. the [(FP*)2Ga]+, 169 [(FP*)2Ga(4-Pic)]+, 170 [(FP*)Ga(phen)(Y)]+ (Y = Cl, S p Tol) 171 cations (Fig. 22).
Fig. 22. (a) The linear di-coordinated cation [(FP*)2(μ-Ga)]+ derives from a salt metathesis of (FP*)2GaCl and Na+[B(ArCF3 )4]–. The Fe–Ga–Fe moiety features a significant π bonding component (population analysis). 169 (b) The [(FP*)2(μ-Ga)(4-Pic)]+ cation is an addition product of [(FP*)2Ga]+ and 4-Pic and the second structurally characterized complex containing a cationic tricoordinate gallium centre. 170 (c) Applying the chelating phen ligand, Ueno et al. isolated the tetra-coordinated [(FP*)Ga(phen)(Y)]+ (Y = Cl, S p Tol) cations, i.e. the first transition-metal complex with a thiolate group on the gallium atom. 171 .
Multinuclear
There are not many contributions to this field of research and some cationic multinuclear gallium complexes are a product of hydrolysis. 172,173 Two remarkable examples however are the dinuclear [(η6-C6H5F)Ga(μ-η6-m-TP)2-Ga(η6-C6H5F)]2+ complex 8,99 in which the gallium(i) cations are solely π-coordinated by arene ligands as well as the σ-coordinated amidinate-bridging [{ t BuC(NiPr)2}GaMe{ t BuC(NiPr)2}GaMe2]+ cation. 156
Multinuclear transition-metal substituted
A notable class of contributions are the β-diketiminate/THF coordinated gallium cations that can be bridged by a gold atom 174 or a {ZnClTHF}2-rhomboid. 175 Reaction of Cp*Fe(η5-P5) with the [Ga(o-C6H4F2)2]+ complex on the other hand resulted in aggregation and the formation of a cationic one-dimensional coordination polymer (Fig. 23). 176
Fig. 23. Reaction of [Ga(o-C6H4F2)2]+ and Cp*Fe(η5-P5) results in aggregation and formation of a cationic one-dimensional coordination polymer.
GaCp* substituted
As of today, GaCp* is a widely used ligand concerning cationic transition-metal complexes, thus leading to an enormous variety of cationic gallium species. This area of research has been intensively reviewed by Fischer et al. 110 and we would like to refer to the multiple entries in Table 8 of this review. Yet, some of the compounds also include “naked” and bridging gallium atoms: e.g. [(Ga)Ru(PCy3)2(GaCp*)2]+, 177 [(Ga)M(GaCp*)4]+ (M = Ni, 178 Pt 179,180 ), [(Cp*Ga)4Rh{Ga(Me)}]+, 181 [(Cp*Ga)4Rh{Ga(Me)(py)}]+, 181 [{Ru(GaCp*)3-[(CH2)2C{CH2(μ-Ga)}]}2]+, 177 [{(GaCp*)4Pt}{Pt(H)-(GaCp*)3}(μ-Ga)]2+. 180 Contrary to GaCp* (a strong σ-donor and weak π-acceptor, cf. similarity to the boron related compounds in Fig. 6), the “naked” gallium cations function as pure acceptor ligands, with significant components of σ- and π-symmetry contributing to the M–Ga linkages. 179,180
Indium cations
Compared to the lighter homologue gallium, well-defined indium(i) halides exist, though they are practically insoluble in organic solvents. The synthesis of In+[OTf]– by Macdonald et al. as a soluble alternative is therefore an important development concerning the indium(i) chemistry. 182
Cyclopentadienyl complexed
Using the just mentioned In+[OTf]– salt as starting material and reacting it with manganocene, the inverse sandwich complex [In2(η5-Cp)]+ was successfully synthesized. 183 Interestingly, the counterion is the complex [Cp3InIII–Cp–InIIICp3]– ion, deriving form a partial oxidation of the starting material. The formation of the mixed valence species seems to be preferred over an alternative indium(ii) species. Reacting InCp* (a hexamer in the solid state) with a mixture of B(C6F5)3 and H2O·B(C6F5)3, the first indium-based triple-decker cation [(η6-Tol)In(μ-η5-C5Me5)In(η6-Tol)]+ was formed. 184 Reducing the size of the counterion from [(C6F5)3BO(H)B(C6F5)3]– to [B(C6F5)4]– on the other hand, results in the formation of the inverse sandwich complex [In2(η5-Cp*)]+ in which the indium(i) cations are not capped by toluene molecules but rather interact with the [B(C6F5)4]– anions (Fig. 24). 185
Fig. 24. Reducing the size of the counterion from [(C6F5)3BO(H)B(C6F5)3]– to [B(C6F5)4]– “squeezes” the toluene molecules from the triple-decker cation, yielding the inverse sandwich complex [In2(η5-Cp*)]+.
Arene complexed
By reacting elemental indium with Ag+[Al(ORPF)4]–, Krossing et al. expanded the above mentioned oxidative route to gallium(i) salts towards the synthesis of [In(arene) n ]+ complexes with n = 2, 3. 162 Identical compounds can also be synthesized by using the salt metathesis reactions of Scheer et al., with insoluble InCl as starting material. 186
Ligand substituted (CN = 2)
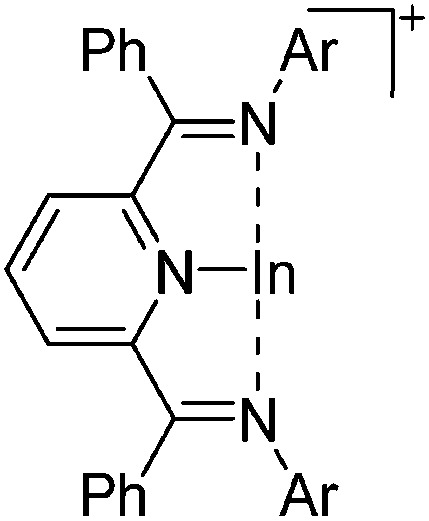
These [In(arene) n ]+ complexes with n = 2, 3 are an ideal starting material for further indium(i) chemistry: e.g. the arene ligands can be substituted for N-heterocyclic carbenes such as IPr. 161 Salt metathesis reactions on the other hand are still very important: i.e., using the isosteric and isoelectronic terphenyl Mes2py ligand, Aldridge et al. were able to isolate mixed-leptic [In(Mes2py)(η6-C6H5F)]+ (both σ- and π-coordinated) and homo-leptic [In(Mes2py)2]+ complexes (only σ-coordinated, though the flanking mesityl rings of the Mes2py ligands also partly π-coordinate). 187 The latter features an indium(i) cation wholly encapsulated by two Mes2py ligands and remarkably long In–N distances, which the authors explain with an energy mismatch between the (low lying) pyridine ligand donor and (high energy) metal acceptor orbitals.
Ligand substituted (CN = 3)
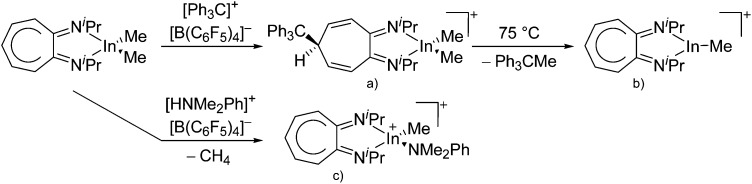
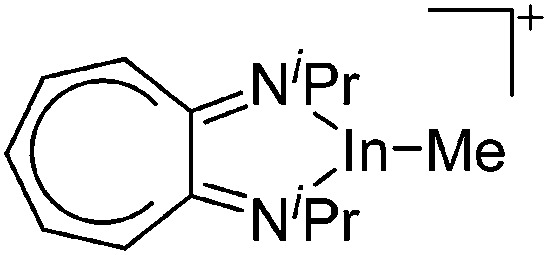
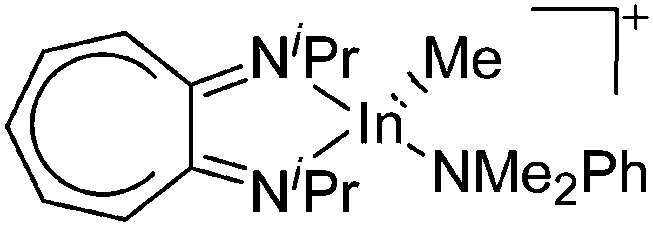
Besides complexation and ligand exchange reactions of In+[OTf]– and [In(arene) n ]+ (n = 2, 3) with ligands such as bis(imino)pyridines 188,189 and PPh3, 162 tricoordinate indium cations can also be isolated by thermolysis of [{iPr2-ATI(CPh3)}InMe2]+, a cationic tetra-coordinated indium precursor (Fig. 25, conversion of (a) to (b)). 190
Fig. 25. Formation of tetra- and tricoordinate cationic diimine substituted indium complexes: (a) [{iPr2-ATI(CPh3)}InMe2]+, (b) [(iPr2-ATI)InMe]+ and (c) [(iPr2-ATI)In(Me)(NMe2Ph)]+. For each complex, the counterion is [B(C6F5)4]–.
Ligand substituted (CN = 4)
The cationic diimine [{iPr2-ATI(CPh3)}InMe2]+ complex was synthesized by reacting the neutral (iPr2-ATI)InMe2 precursor with the ionizing [Ph3C]+[B(C6F5)4]– salt (Fig. 25). 190 Surprisingly, the latter does not function as methyl abstracting reactant but rather adds to the C5 carbon of (iPr2-ATI)InMe2. Reacting (iPr2-ATI)InMe2 with the protonating [HNMe2Ph]+[B(C6F5)4]– on the other hand, results in CH4 formation and the labile adduct [(iPr2-ATI)In(Me)(NMe2Ph)]+ (Fig. 25). 190
Ligand substituted (CN ≥ 5)
Compared with the lighter homologues aluminum and gallium, indium shows a tendency to expand its coordination sphere. 95,102 Protonolysis of the neutral In(CH2SiMe3)3 complex in THF therefore yields a penta-coordinated indium cation: [In(CH2SiMe3)2(THF)3]+. 191 Moreover, In+[OTf]– 192,193 and [In(arene) n ]+ 168 (n = 2, 3) can be reacted with the crown ether [18]crown-6, yielding cationic indium complexes with similar structures to the gallium congener (cf. Fig. 21) and strong anion–cation interactions in the case of the [OTf]– anion. Reacting In+[OTf]– with [15]crown-5 on the other hand, the sandwich complex [In([15]crown-5)2]+ was isolated. 194
Transition-metal substituted
The class of cationic transition-metal substituted indium compounds very much relates to the related gallium structures: i.e., the [InPt(PPh3)3]+ complex with a “naked” Pt-substituted indium cation 179,180 as well as the di- and tricoordinate [(FP*)2In]+ and [(FP*)2In(THF)]+ complexes. 195 Reacting the chelating phen ligand with the [In(C6H5F)2]+ complex in the presence of silver salt, Krossing et al. isolated the silver bound indium dication 17 [(phen)2In–Ag(η3-C6H5F)]2+ that is related to the [InPt(PPh3)3]+ complex. 179,180 In this complex the tetragonal-pyramidal [In(phen)2]+ cation reacts as a Lewis basic donor (cf. the stereoactive 5s lone pair at indium), while the [Ag(η3-C6H5F)]+ complex is the corresponding Lewis acidic acceptor.
Multinuclear
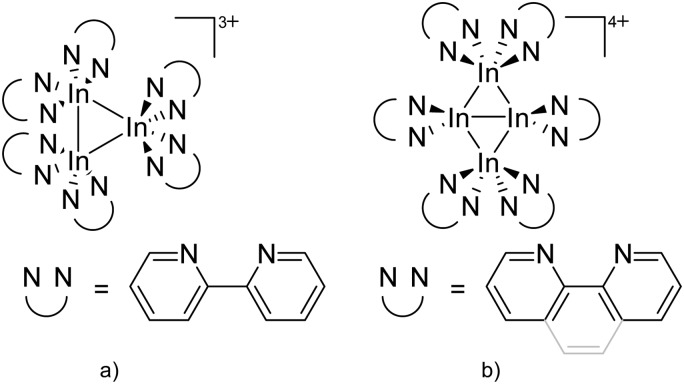
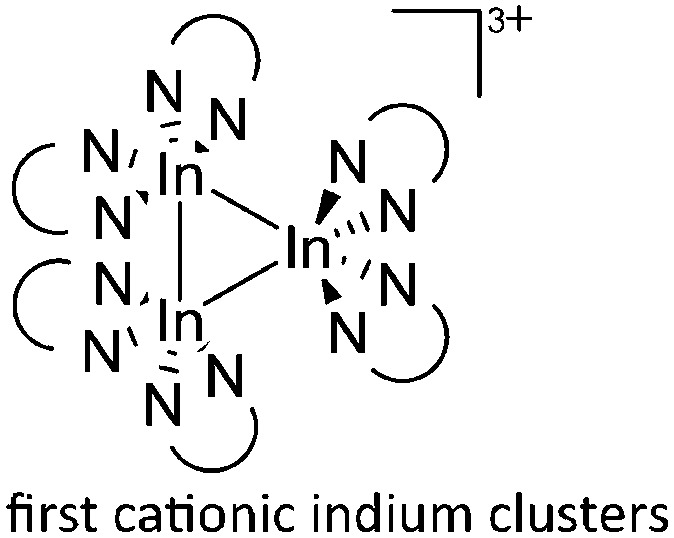
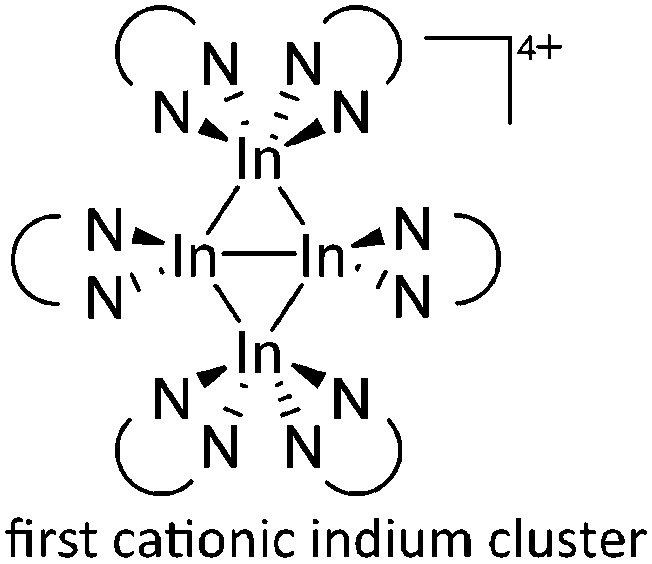
For the synthesis of multinuclear indium cations, the [In(arene) n ]+ 168 complexes with n = 2, 3 are a powerful starting material. Hence a dicationic [{(PPh3)3In}2(μ-PPh3)]2+ complex in which one PPh3 ligand bridges both indium(i) cations was isolated. 162 Applying the non-innocent and chelating bipy and phen ligands on the other hand, Krossing et al. surprisingly isolated the first cationic tri- and tetra-nuclear indium clusters: [In3(bipy)5–6]3+ and [In4(Do)6]4+ (Do = phen, bipy) (Fig. 26). 17 This result very much differs from the above mentioned synthesis of the paramagnetic [GaIII{(bipy)3}˙]2+ complex and can be attributed to the higher redox-stability of indium compared to gallium. In addition and to our knowledge, these are the first higher charged clusters that have been reported to this day: i.e., for cluster formations usually reductive syntheses are applied, yielding neutral and anionic clusters.
Fig. 26. Cationic (a) [In3(bipy)5–6]3+ and (b) [In4(Do)6]4+ (Do = bipy, phen) complexes synthesized via ligand exchange reactions and aggregations.
Thallium cations
In contrast to the lighter homologues, thallium's thermodynamic most stable oxidation state is +I. Hence, various syntheses of unsubstituted thallium(i) cations of different WCAs have been reported: i.e., the protonation of TlOEt using [H(OEt2)2]+[B(ArCF3 )4]–/[B(C6F5)4]–, 196,197 the Lewis acid base reaction of Tl+[OTeF5]– and B(OTeF5)3 198 and the salt metathesis of TlF and Li+[Al(ORHF/PF)4]–. 199,200 The thallium(i) salts are relatively stable (cf. the silver congeners decompose upon exposure to light) and mainly used as reactants to introduce WCAs (e.g. salt metathesis reactions). Tl+[Al(ORHF)4]– could only be isolated, if the precursors were applied in an exact 1 : 1 stoichiometry. An excess of TlF however, led to the formation of the cationic multinuclear [Tl3F2Al(ORHF)3]+ complex. 200
Arene complexed
Various cationic thallium(i) arene complexes have been reported. While the di- and tricoordinate [Tl(η6-arene) n ]+ complexes (arene = C6H5Me, n = 2, 3; 201 Mes, n = 2; 202 C6Me6, n = 2 203 ) are structurally related to the lighter homologues, C6Me6 additionally allows for the first mono-coordinated [Tl(η6-C6Me6)]+ complex (DFT calculations gave a remarkable Tl–C6Me6 π-bonding energy of 163 kJ mol–1). 204
Ligand substituted (CN = 2)
Reacting Tl+[OTeF5]– with B(OTeF5)3 in 1,2-dichloroethane, the solvent functions as chelating ligand, thus forming the five-membered TlCl2C2-ring in [Tl(1,2-Cl2C2H4)]+ (after silver and ruthenium, thallium was at that time the third reported metal atom coordinated by a simple chlorocarbon). 198 By contrast, from CH2Cl2 the “naked” Tl+[B(OTeF5)4]– salt was isolated.
Ligand substituted (CN = 3)
Similar to the lighter homologues gallium and indium, the [Tl(η6-C6H5R)2]+ (R = F, Me) bent-sandwich complexes can interact with N-heterocyclic ligands such as Mes2Py, thus forming tricoordinate [Tl(Mes2py)(η6-C6H5R)2]+ complexes. 187 On the other hand tri-dentate chelating ligands like timtmb tBu 205 and bis(imino)pyridines 206 can be applied to isolate tricoordinate thallium(i) cations (Fig. 27).
Fig. 27. Both the (a) [Tl(timtmb tBu)]+ and (b) [{ArN CPh}2(NC5H3)Tl]+ complexes derive from Tl+[OTf]– and are synthesized via complexation reactions of the corresponding ligands. In addition, the inverted sandwich structure (c) [{{ArN CPh}2(NC5H3)Tl}2(μ-η6-C6H5R)]2+ (Ar = 2,6-Et2C6H3, 2,5- t Bu2C6H3; R = H, Me) was isolated.
Ligand substituted (CN = 4)
The protonation of TlOEt with [H(OEt2)2]+[H2N{B(C6F5)3}2]– in Et2O yielded the tetrahedral coordinated cationic [Tl(OEt2)4]+ complex, which shows no contact to the corresponding WCA. 203
Ligand substituted (CN ≥ 5)
2,5-Bis(2-pyridyl)-1-phenylphosphole (NPPh) exhibits a rich coordination chemistry towards thallium(i) cations and dependent on the nature of the solvents and WCAs, different structures were obtained: i.e., reacting Tl+[Al(ORPF)4]– with NPPh in CH2Cl2/C6H5Me, the tetra-coordinated and C6H5Me-capped [Tl(NPPh)2(η6-C6H5Me)]+ complex formed, whereas in CH2Cl2/n-pentane the dinuclear and dicationic [Tl2(NPPh)4]2+ was isolated (Fig. 28). If Tl+[PF6]– was applied as starting material a coordination polymer with strong cation–anion interactions was formed.
Fig. 28. Solvent effects on the formation of cationic thallium(i) complexes of NPPh. (a) If toluene is applied, the solvent-stabilized penta-coordinated [Tl(NPPh)2(η6-C6H5Me)]+ complex forms. (b) If non-coordinating n-pentane is applied two [Tl2(NPPh)2]+ cations aggregate via their phenyl substituents, forming the dinuclear and dicationic [Tl2(NPPh)4]2+ complex.
An even higher coordinated thallium cation is the [Tl([18]crown-6)]+ complex, which features a similar structure as the [18]crown-6 complexes of the lighter homologues gallium and indium. 203
Transition-metal substituted
Reacting the above mentioned [Tl(η6-C6H5Me) n ]+ complexes (n = 2, 3) with FeCp2, Sarazin et al. were able to isolate the [Tl2(FeCp2)3]+{[H2N{B(C6F5)3}2]–}2 salt that contains the mono- and di-coordinated [Tl(FeCp2) n ]+ complexes with n = 1, 2 in a 1 : 1 ratio. 203 Increasing the amount of FeCp2 from 1 to 2.2 equivalents, only the [Tl(FeCp2)]+ complex was isolated. 204 In contrast to the lighter homologues, the reaction of Cp*Fe(η5-As5) with Tl+[PF6]– and Li+[FAl{OC6F10(C6F5)}3]– did not result in aggregation and formation of a cationic one-dimensional coordination polymer, but rather yielded the pseudo-trigonal-planar [Tl{(η5-As5)FeCp*}3]+ complex. 176 Performing a similar chemistry in the presence of the very good WCA [Al(ORPF)4]– however, one-dimensional polymers were isolated (cf. Fig. 23), proving the importance of the WCA. 207 Reacting the neutral Pt(CH2Ph)Cl(PCH2-ox) complex with Tl+[PF6]–, Braunstein et al. did not isolate any chloride abstraction product but a “trapped” thallium(i) cation: the cationic [{P(Ph2)CH2ox}(Cl)(Tl)Pt-CH2Ph}]+ complex. 208 Herein, the ligand functions as a chelate and interacts with thallium via a Pt–Tl bond and a η6-benzyl coordination (Fig. 29).
Fig. 29. The first fully characterized metal–metal bonded Tl–Pt–Cl complex. If Ag+[OTf]– and Ag+[BF4]– is applied, the expected chloride abstraction takes place. 208 .
Multinuclear
Some of the cationic multinuclear thallium complexes have already been mentioned in the text above. A further example is the [{Tl(β-triketimine)}2]2+ complex that features Tl–η6-aryl and weak thallophilic interactions, allowing to overcome the Coulomb repulsion of both cations (cf. Fig. 28). 14 The reaction of the P n -ligand {CpMo(CO)2}2(P2) with Tl+[Al(ORPF)4]–, yields the dicationic [Tl2({CpMo(CO)2}2)6]2+ complex that features a distorted Tl2P4 ring (Fig. 30). 207
Fig. 30. Formation of the [Tl2({CpMo(CO)2}2)6]2+ complex.
Reacting RuCl2(DMeOPrPE)2 with Tl+[PF6]– an “arrested” chloride abstraction occurs. 209 In the resultant one-dimensional coordination polymer, the thallium(i) cations are coordinated in an unusual octahedral fashion with a stereoactive 6s2 lone pair at thallium.
Group 14 cations
Already in 1887, Henderson described the synthesis of trityl malonate starting from triphenylmethyl bromide and ethylic sodiomalonate 246 and 15 years later, Bayer and Villiger realized that the yellow color of a solution of triphenylmethane in concentrated sulfuric acid is the result of the formation of a carbocation. 247 Despite these early discoveries, it took another 63 years until the structure of this cation could be determined. 248 While the first structure determination succeeded with [ClO4]–, the structure of the trityl cation is nowadays known with several different anions (e.g. ref. 249) and it has become a common reagent for the generation of various other cations. Only one earlier structure determination of a carbocation was published: the structure of triphenylcyclopropenium perchlorate in 1963. 250 Since then, many rPBCs of group 14 were synthesized and characterized. Carbocations have drawn a lot of interest, due to their role as intermediates in organic chemistry. Silylium ions are more electrophilic and more reactive than their carbon analogues, so that the structural characterization of a truly free silylium ion was only achieved in 2002 24 and is still in the focus of interest. But also the heavier elements of group 14 were subject to extensive research and today a multitude of interesting rPBCs are known, part of which have been reviewed in the articles included with Table 4.
Table 4. Review articles including cationic group 14 compounds.
| Year | Title | Ref. |
| 1995 | Modern approaches to silylium cations in condensed phase | 251 |
| 2005 | Cations of group 14 organometallics | 252 |
| 2005 | Carbon, silicon, germanium, tin and lead | 253 |
| 2010 | Silylium ions in catalysis | 254 |
| 2010 | H+, CH3 +, and R3Si+ carborane reagents: when triflates fail | 255 |
| 2011 | N-heterocyclic carbene analogues with low-valent group 13 and group 14 elements: syntheses, structures, and reactivities of a new generation of multitalented ligands | 256 |
| 2013 | Catenated compounds – group 14 (Ge, Sn, Pb) | 257 |
| 2015 | Cations and dications of heavier group 14 elements in low oxidation states | 258 |
Carbon
Homopolyatomic and cluster cations
In group 14, carbon is the only element for which homopolyatomic cations are known in condensed phase. While [C76]+, 44 and [C60]+, 259 are already known for more than ten years, there is only one more recently published compound of that class. In [C60]2+([AsF6]–)2, 260 the cations build a 1D polymeric structure, in which the [C60]2+ cations are connected alternatingly by single C–C bonds and four-membered carbon rings. Along with the before mentioned [C60]+, the protonated buckminsterfullerene [HC60]+ was published 259 and by oxidation of the [C59N]2 dimer, [C59N]+ was synthesized and structurally characterized. 261
Carbonium ions
As mentioned before, the classification according to onium-, enium and inium-cations is not always consistent and in literature, the term carbonium ion is often used to describe what is mostly a carbenium ion. A prototype for a carbonium ion is the 2-norbornyl cation, whose structure has been controversially discussed. In 2013, 49 years after its first preparation under stable ion conditions, 262 its structure could be determined by scXRD. 86 This finally provided a crystallographic proof that the 2-norbornyl cation adopts the non-classical structure ( Fig. 31). It remains the only structurally characterized non-classical carbonium ion. Substituted relatives exhibit distinctly distorted structures that are better classified as carbenium ions. 263
Fig. 31. Non-classical vs. classical structure of the 2-norbornyl cation.
Carbenium ions
The first simple structurally characterized alkyl cation, was the tert-butyl cation with [Sb2F11]– as the counterion, 264 and later also with [HCB11Me5Cl6]–. 265 In the same publication, two more carbocations with slight variations in the alkyl chains were presented (Fig. 32). 265 Recent additions include the super-acidic room temperature ionic liquid [(CH3)3C]+[Al2Br7]– 266 and an additional structure of the tert-butyl cation with the [HCB11Cl11]– anion. 267 In 2000, ion-like (CH3)2CF(AsF6) was the first structural characterized example of a fluorinated carbocation and was published together with a higher substituted variant. 268 In both compounds, each cation is stabilized by two stronger contacts to the anion. The higher substituted [(m-CF3C6H4)(C6H5)CF]+ derivative, contains the less coordinating [As2F11]– anion in the structure with only weak interaction between the ions. 268 With [HCB11I11]–, two more fluoro-substituted carbocations and one with fluorine substituted aryl residues could be isolated (see Fig. 32). 56 Apart from [CF3]+, all [CX3]+ cations are now synthesized and structurally characterized (see Fig. 32). First, [CI3]+[Al(ORPF)4]– was published in 2003 269 and shortly after [CCl3]+ and [CBr3]+ with [Sb(OTeF5)6]– as the counterion. 270 In addition, the latter was used to stabilize related [C(OTeF5)3]+. 270 Later, also [CCl3]+ and [CBr3]+ were synthesized with the [Al(ORPF)4]– and the [(RPFO)3Al–F–Al(ORPF)3]– counterions. 271 In all of those compounds containing [CX3]+ cations, still some weak interactions between cation (mainly halogen atoms) and anion exist. These interactions are weaker between [Br-C(SBr)2]+ and the mentioned alkoxyaluminate, due to delocalization of the charge. 117 Although comparable, far stronger interaction between cation and anion was found in [(MeO)(MeS)CSH]+[SbF6]–. 272 However, there is no close contact between the carbon atom and the fluorine atoms of the anion. Instead, the anion forms hydrogen bonds to the thiol group of the cation.
Fig. 32. Structurally characterized carbenium ion salts.
In 2004, the structure of the benzonorbornenyl cation was published, with an intramolecular stabilization of the cationic center by the aromatic ring. 273 Intermolecular stabilized carbenium cations are known of the [CI3]+ with the weak bases PX3 (X = Cl, Br, I) and AsI3 (Fig. 33). 274 Only two related vinyl cations are known (see Fig. 33). 275,276 Both are β-substituted by two silyl groups, which help to stabilize the positive charge.
Fig. 33. Structurally characterized ligand-stabilized carbenium ions and vinyl cation salts.
Delocalized (cyclic) carbocations
Only shortly after the first structural characterization of an alkyl cation, the first structure determination of an arenium ion – [C6Me7]+[AlCl4]– – was published. 277 To date, more structurally characterized arenium ions with several WCAs are known (Fig. 34).
Fig. 34. Protonated and methylated structurally characterized arenium ion salts.
An exception is the radical cation [C6F6]˙+ in the solid state structures with [Sb2F11]– and [Os2F11]–: it yields two different forms. 278 One cation can be described as a quinoidal cation and the other as a bisallyl cation (see Fig. 35) and both are separated by a barrier of around 13 kJ mol–1 according to calculations.
Fig. 35. Lewis structures of the canonical forms of the quinoid and the bisallyl cationic form of [C6F6]˙+.
Shortly after the publication of the radical cation of the hexafluorobenzene, some more related structures were presented. Among them, the other perhalogenated benzene radical cations 279,280 and some partially and mixed substituted analogs, including the [C6F5–C6F5]˙– 280,281 (Fig. 36). The only other example displaying both a quinoidal and a bisallyl cationic form is [2,4,6- t Bu3C6H2NH2]˙+. 282 At 123 K, this cation adopts the bisallylic structure but upon heating, a transition to the quinoidal form occurs.
Fig. 36. Structurally characterized substituted benzene radical cation salts.
A different type of delocalized cations are the allyl cations amongst which the cyclopropenyl cations take a special position. Already since 1986, two examples, [(Cy)3C3]+ and [(Cy)2(Ph)C3]+, are known 283 and in the same year, an allyl cation stabilized by an hydroxyl group has been published (Fig. 37). 284 In 2002, the structure of [C5Me5H2]+ was determined although it was by mistake addressed as an [C5Me5]+ cation, probably due to its unexpected formation during the reaction of C5Me5H with [Ph3C]+. 285 Finally a silyl stabilized allyl cation was characterized, which formed via an interesting mechanism that starts with the formation of a silylium cation (Fig. 37). 286
Fig. 37. Structurally characterized delocalized cation salts.
Ion-like carbon compounds
As mentioned before, in the analog structure of [(m-CF3-C6H4)(Ph)CF]+ with [AsF6]– instead of [AsF11]–, stronger interactions to the anions are present. 268 The same applies to the related Me2CF(AsF6). 268 Also known is the ion-like (Me2CH)(HCB11Me5Br6), which displays a covalent C–Br distance of about 210 pm. 287 Along with the latter, the preparation of H3C(HCB11Me5Br6) and H3C(HCB11Me5Cl6) was reported, but no structural data from XRD was presented. In 2010, the strongly methylating ion-like Me2B12Cl12 was structurally characterized with a C–Cl bond length of 182 pm. 288
Silicon
Silylium ions (CN = 3)
Silylium ions are certainly amongst the most electrophilic cations known and thus exhibit an enormous Lewis acidity. Most of them are either stabilized by bulky ligands, or display a strong interaction with the corresponding WCA and have therefore to be categorized as ion-like compounds. In addition, the first claimed “stable silyl cation” [Et3Si]+ in 1993 contained a coordinating toluene ligand – a feature typical for many silylium ions. 289 In order to obtain a truly tricoordinate silylium ion without stabilization through the anion or an additional ligand, bulkier substituents were needed. Hence, the first structurally characterized compound featuring a free silylium ion was [Mes3Si]+[HCB11Me5Br6]– 24 and in 2013, ([Pemp3Si]+)2[B12Cl12]2– was published (Fig. 38). 290 The latter was afterwards also synthesized and characterized with [Al(ORPF)4]–. 291 In all three structures, the cation has no closer contacts to the anion.
Fig. 38. Structurally characterized tricoordinate silylium ions.
Delocalized cyclic cations
Despite the early characterization of cyclopropenyl cations, the first example for a comparable silicon ion was published only in 2000 (Fig. 39, left). 292 In this compound, however, it is not the three-membered silicon-ring with a delocalized π-system but rather a silicon butterfly with one Si–Si–σ-bond stabilizing the positive charge. A direct equivalent of a cyclopropenyl cation was finally published in 2005 (Fig. 39). 293 One more example is known with the positive charge being partially delocalized over four silicon atoms. 294 An example for a Si(ii) cation with 6π-aromaticity provides the silyliumylidene-like species introduced by Driess et al. (Fig. 39, right). 295 This compound is stabilized by delocalization so that, although produced through protonation with [H(OEt2)2]+[B(C6F5)4]–, no ether molecule remains coordinated to the cation.
Fig. 39. Delocalized cyclic silicon centered cations.
Ligand-stabilized silicon cations
Already in 1983, pyridine stabilized [Me3Si]+ was reported. 296 Yet, this compound is stable to such an extent that Br– and I– are sufficient as anions and that it can be prepared just by reacting Me3SiX with pyridine. Many of these [R3Si-L]+ ions stabilized by different σ-donors are known: with nitriles, 297–299 pyridine, 296,300 water, 301 o-dichlorobenzene, 81 sulfur dioxide 81 and bipyridine. 302 Even though 2,6-bis(2,6-difluorophenyl)phenyldimethylsilylium ion has no additional ligand acting as a σ-donor, the cationic center is stabilized by one fluorine of each 2,6-difluorophenyl-substituent (Fig. 40). 303 With the stronger stabilizing DMAP, the dication [Me2Si(dmap)2]2+ has been synthesized. 302
Fig. 40. Structurally characterized silylium ions stabilized by σ-donors.
[R3Si-L]+ ions with π-donor ligands L = arenes like the before mentioned [Et3Si(C7H8)] are less stabilized than those with σ-donors (Fig. 42). 289 Several different arene adducts of [Me3Si]+ were reported by Schulz and Villinger et al. (Fig. 42). 304 As can be seen in Fig. 41, some of these compounds are coordinated by a second arene molecule binding in an η6-fashion to the proton ipso to the silylium center. This shows that these arena adducts are also very strong cationic Brønsted acids.
Fig. 42. Structurally characterized silylium ions stabilized by internal or external π-donors. Ar = benzene, toluene, ethylbenzene, n-propylbenzene, and iso-propylbenzene, o-xylene, m-xylene, p-xylene, 1,2,3-trimethylbenzene, 1,2,4-trimethylbenzene, mesitylene.
Fig. 41. Molecular structure of [Me3Si(C8H10)·(C8H10)]+. The weak interaction between the stabilized cation and the adjacent ethylbenzene is indicated by the dashed bonds. M. F. Ibad, P. Langer, A. Schulz and A. Villinger, J. Am. Chem. Soc., 2011, 133, 21016–21027. Data from this reference were used to draw this figure.
Comparable to the before mentioned 2,6-bis(2,6-difluorophenyl)phenyldimethylsilylium ion without any additional ligand, a 2,6-diarylphenyldimethylsilyl cation is existing, which is stabilized by intramolecular π-donation (Fig. 42). 76
Compounds of the type [R3Si–X–SiR3]+ have to be treated as a special case of ligand stabilization. The first example of this type is the initially as [Et3Si]+ misinterpreted [Et3Si–H–SiEt3]+, 305 whose structure determination has been published about two years ago. 306 [Me3Si–H–SiMe3]+ 81 is also known as well as the analogous [Me3Si–X–SiMe3]+ compounds with X = F, Cl, Br, I 307 and trifluoromethanesulfonate. 308 The X-bridged species are typically addressed as halonium ions, but it appears more reasonable to address them as ligand-stabilized silylium ions (see Fig. 43).
Fig. 43. Canonical structures of the halogen-bridged bis-silylium ions.
Calculations state that the positive charge is still located at the silicon atoms and F, Cl and Br are negatively charged. 307 Only in the case of iodine, a small positive charge is located at the bridging atom. 307 Additionally, bissilylated pseudohalonium cations [Me3Si–X–SiMe3]+ with X = CN, OCN, SCN, and NNN are known. 309 Of these, only in [(Me3Si)2NNN]+ both silyl groups are attached to the same atom, 309 so that the structure of the cation is analog to the protonated hydrogen azide 310 (see Fig. 44 and Table 5 for [H2N3]+[SbF6]–). Some more examples with bridged SiR3-groups, in which both groups are connected with each other, are known (Fig. 44). 311–315
Fig. 44. Structurally characterized bridged bissilylium ion salts.
Table 5. Review articles including cationic group 15 compounds.
| Year | Title | Ref. |
| 2004 | Homoatomic cages and clusters of the heavier group 15 elements. Neutral species and cations | 394 |
| 2008 | Catena–phosphorus cations | 392 |
| 2011 | Homo- and heteroatomic polycations of groups 15 and 16. recent advances in synthesis and isolation using room temperature ionic liquids | 66 |
| 2012 | Multiple-charged P1-centered cations: perspectives in synthesis | 395 |
| 2013 | Catenated phosphorus compounds | 391 |
| 2013 | Recent advances in the syntheses of homopolyatomic cations of the non-metallic elements C, N, P, S, Cl, Br, I and Xe | 11 |
| 2013 | Catenated compounds – group 15 (As, Sb, Bi) | 396 |
| 2014 | Interpnictogen cations: exploring new vistas in coordination chemistry | 393 |
| 2014 | The chemistry of cationic polyphosphorus cages – syntheses, structure and reactivity | 397 |
| 2015 | Coordination chemistry of homoatomic ligands of bismuth, selenium and tellurium | 398 and 399 |
A special case of intramolecular ligand stabilization can be observed in [FcSiMe t Bu]+. 316 Here the silicon is dipped towards the iron atom due to two 3c2e bonds between C ipso , Si and Fe and C′ ipso , Si and Fe, respectively (Fig. 45). 316
Fig. 45. Molecular structure of [FcSiMe t Bu]+. K. Müther, R. Fröhlich, C. Mück-Lichtenfeld, S. Grimme and M. Oestreich, J. Am. Chem. Soc., 2011, 133, 12442–12444. Data from this reference were used to draw this figure.
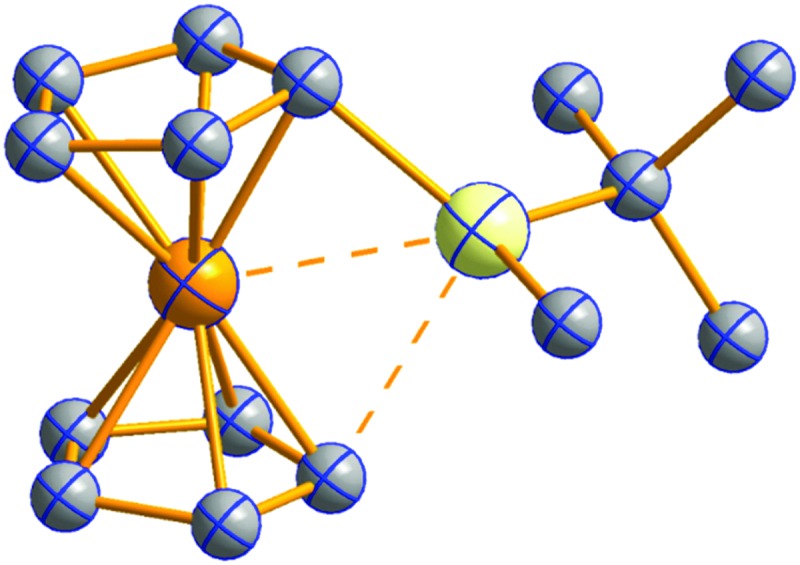
More intramolecular σ-donor stabilized silylium ions are known: [RSi(R′)2]+ or [RSi(R′)(R′′)]+ with R being a pincer ligand can be seen as an extra class of ligand-stabilized silicon cations. In 2009, several silylium ions with OCO and SCS pincer ligands were published by Jutzi et al. (Fig. 46). 317
Fig. 46. Structurally characterized silylium ion salts with intramolecular stabilization by pincer ligands.
All before mentioned ligand-stabilized silicon cations contain an inter- or intramolecularly by additional donor atoms stabilized [R3Si]+ cation. Two more different types of ligand-stabilized silicon cations were published with silicon in oxidation state +IV. Both were synthesized by oxidation of silicon(ii) cations through elemental sulfur (Fig. 47). 318,319 These cations containing subvalent silicon are very rare and most of the known examples are bearing a cyclopentadienyl substituent (see Cyclopentadienyl substituted cations). However, with well stabilizing ligands, two [LSiCl]+ cations were synthesized (Fig. 47). 318,320 Both are prepared just by adding the chelating ligand to NHC·SiCl2. The NHC ligand is being replaced by L and yields the [LSiCl]+ cation with chloride as the anion. This shows that the silicon cationic center is largely stabilized by coordination. By using well stabilizing NHCs, it was possible to generate an [(L)(L′)SiI]+[I]– and even the dication [L3Si]2+([I]–)2. 321 In addition, two related silicon(ii) monocations [RSi(L) n ]+ were structurally characterized in which the residue R is not a halogen atom (Fig. 48). 319
Fig. 47. Structurally characterized ligand-stabilized silathionium cations.
Fig. 48. Structurally characterized ligand-stabilized cations of subvalent silicon.
Cyclopentadienyl substituted cations
So far, two cyclopentadienyl substituted silicon cations without any further stabilization through additional ligands were structurally characterized. First [(C5Me5)Si]+ with [B(C6F5)4]– was published in 2004 322 and two years later, the synthesis and characterization of [(C5 iPr5)Si]+[Al(ORPF)4]– was presented. 323 However, the latter structure determination was of poor quality and did not allow to obtain any exact structural parameters. Additionally, two ether stabilized [(C5Me5)Si]+ cations are known (Fig. 49). 324
Fig. 49. Structurally characterized cyclopentadienyl substituted silicon cation salts with and without additional ligands.
Ion-like silylium compounds
In alkylsilylium ion salts without an additional stabilizing ligand, interactions between the cation and the corresponding WCA can be observed. The first structurally characterized R3Si(WCA) was the iso-propyl substituted compound in 1993. 325 Today, at least one example with the most common alkyl substituents t Bu, iPr, Et and Me is known. 81,297,326–330 Additionally, with t Bu2MeSi(CB11H6Br6) one mixed substituted ion-like silylium compound was published. 328 Another example might be Fc3Si(OTf). However, its Si–O interaction is with 175 pm in the range of a normal Si–O bond. 331
Germanium, tin and lead
Cluster cations
As for silicon, no homopolyatomic cations comparable to the fullerenium ions are known for germanium, tin and lead. Nevertheless, one example of a germanium cluster exists (Fig. 50). 332 The cluster is composed of ten germanium atoms, of which seven bear substituents. The remaining three unsubstituted germanium atoms carry the positive charge, which is evenly distributed.
Fig. 50. Lewis structure of the 5-iodo-2,4,6,8,9,10-hexakis(tri-tert-butylsilyl)heptacyclo[4.4.0.01,3.02,5.03,9.04,7.08,10]decagerman-7-ylium ion.
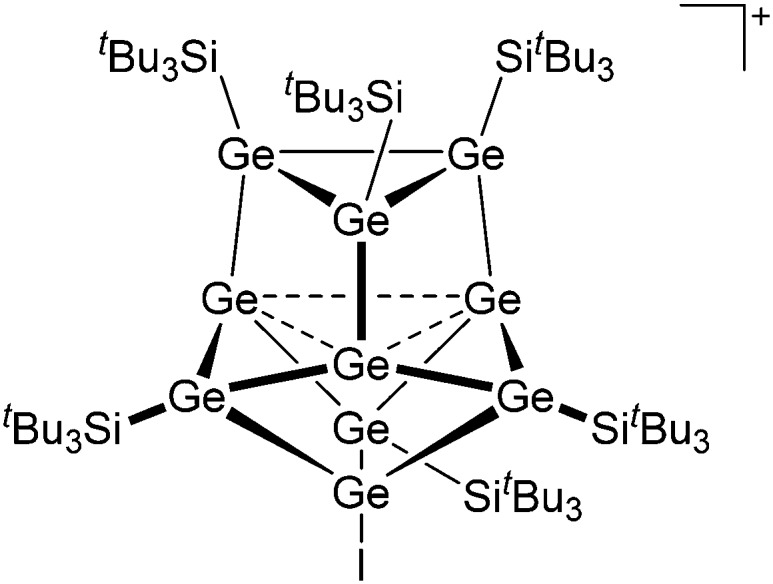
Enium ions
Just as for silicon, enium ions of Ge, Sn and Pb need substituents with a high steric demand to shield the cationic center. The first example of the heavier elements of group 14 – [ n Bu3Sn]+[CB11Me12]– – does have, as expected, interactions between cation and the [CB11Me12]– WCA. 333 At about the same time, Lambert et al. and Sekiguchi et al. published the first examples of free enium ions of germanium and tin. While Lambert relied on bulky aryl substituents to synthesize [(Tipp)3Sn]+, 34 Sekiguchi deployed silyl groups and managed to produce [( t Bu2MeSi)3Ge]+ 334 and [( t Bu2MeSi)3Sn]+, 335 all with [B(C6F5)4]– as their counterpart (Fig. 51). Although enium ions with aryl substituents have always been under the first examples for carbon, silicon and tin, it kept lacking an example for germanium until in 2009 [Ge({2,6-O t Bu}2C6H3)3]+[Al(ORPF)4]– was synthesized and characterized. 336 However, the cationic center is stabilized by contacts to the oxygen atoms of the tert-butoxy residues at 286 and 288 pm. 336 More recently, a mixed substituted enium ion of tin has been published (Fig. 51). 337 Examples for lead are still missing and the only formally [R3Pb]+ containing ion-like substance Et3Pb(HCB11H5Br6) has like its Si, Ge and Sn analogs stronger interactions between the ions. 338
Fig. 51. Structurally characterized enium ions of germanium and tin.
Delocalized cyclic cations
The germanium compound [Ge3(Si t Bu3)3]+, has been published long before the first silicon analog of a cyclopropenyl cation. 339 Although it is known with a few different anions, 340,341 it is still the only example of delocalized cyclic cations of the heavier elements of group 14 (similar to Fig. 39).
Ligand-stabilized
Far less ligand-stabilized cations of Ge, Sn and Pb in oxidation state +IV are known than of Si. [Me3Sn(OPPh3)2]+[(MeSO2)2N]– and [Ph3Sn(OPPh3)2]+[(MeSO2)2N]– were synthesized already in 1994 342 and six years later, the [ t Bu3E(NC- t Bu)]+ cations were synthesized with E = Ge and Sn, but only for the germanium compound the crystal structure is known. 298 In addition, together with the analogous silicon complex, [Me2Ge(bipy)(OTf)]+[OTf]– has been published. 302 Interesting is however, that the corresponding substances with DMAP coordinating to germanium and the ones with DMAP or bipyridine coordinating to tin have to be described as ion-like, since in all of them both [OTf]– anions do have close contacts to the cationic center. 302 As already stated for silicon, symmetrical compounds of the type [R3E–X–ER3]+ are somewhat special since the positive charge is evenly distributed and it is not possible to speak of an cation and a ligand anymore. Contrary to silicon, only one cation belonging to this type is known for the heavier homologues (Fig. 52). 343 Additionally, for germanium and tin ligand-stabilized dimeric cations are known, both synthesized by oxidation of their E(ii) precursors through elemental sulfur (Fig. 52). 344,345
Fig. 52. Ligand-stabilized cations of germanium, tin and lead in oxidation state IV.
Norbornyl cations with the heavier group 14 elements were classified in here as ligand-stabilized cations, although one may address them as onium ions. Although the heavier norbornyl cation analogues were all published – also with silicon – no crystal structure could be determined. 346 However, by addition of acetonitrile to the norbornyl cations, the stronger σ-donor replaces the weaker π-donating C C double bond. An exception is the plumbanorbornyl cation, which gets coordinated by acetonitrile additionally and remains coordinated by the alkene (scXRD). 346 A comparable π-stabilization as in the norbornyl cations can be found in the 1,4,5-trigermabicyclo[2.1.0]pent-2-en-5-ylium ion, in which the cationic center is coordinated intramolecularly by a C C double bond. 347 Another unique π-stabilization can be observed in bis(cyclopentenemethyl)plumbylium. 348 This cation is intramolecularly stabilized by the C–C double bonds of the two cyclopentene substituents (Fig. 52).
Hard to classify are two germanium cations stabilized by a monoanionic bidentate bis(NHC)borate ligand (Fig. 53). 349 Both originate from the attempt to synthesize a germanium dication stabilized by the before mentioned ligand through the reaction of LGeH with [Ph3C]+[B(C6F5)4]–. Instead of delivering the desired germanium dication, two different products were obtained. In one, instead of abstracting the hydride, the trityl cation attacks the lone pair of the Ge(ii) cation, forming the adduct. In the other, the hydride is indeed abstracted by the trityl cation, but the resulting germanium dication is coordinated by unreacted starting material.
Fig. 53. Germanium cations stabilized by a monoanionic bidentate bis(NHC)borate ligand.
Apart from those examples, the ligand-stabilized cations of the heavier group 14 elements are in oxidation state +II. Already as early as 1989, [Sn([15]crown-5)2]2+ has been published along with its crystal structure. 350 This cation is accessible directly through the reaction of SnCl2 with two equivalents of the crown ether, which is why [SnCl3]– serves as the counterion. In this or a similar fashion it has been possible to synthesize a portfolio of different crown ether complexes of tin(ii) and lead(ii). 351–353
To isolate the first related Ge(ii) compound, better stabilizing ligands were needed. By employing NHC ligands, a germanium dication was isolated (Fig. 54). 354 The germanium center is highly stabilized by its ligands, and – although iodides are the counterions – only weak interactions between the ions are present. Another germanium containing dication was synthesized with the encapsulating cryptand[2.2.2], 355 and a few years later the analogous tin complex. 356 Today, quite a few different crown ether complexes of germanium are known as well (Table 9). 357 By using other well stabilizing chelating N-donor ligands, it was also possible to isolate [(L)Ge]2+ cations. 358
Fig. 54. Structurally characterized dicationic compounds of germanium and tin.
Table 9. Overview on structurally characterized cations of group 14.
| Cation | Anion | Class. a | Synthesis | Comment | Ref. |
| Homopolyatomic and cage cations | |||||
| [C76]+ | [HCB11H5Br6]– | Ox | C76 + [Ar3N]+[WCA]– | 44 | |
| [C60]+ | [HCB11H5Cl6]– | Ox | C60 + [Ar3N]+[WCA]– | 259 | |
| [C60]2+ | [AsF6]– | Ox | C60 + 3 AsF5 | Polymeric | 260 |
| [HC60]+ | [HCB11H5Cl6]– | Prot | C60 + H(WCA) | 259 | |
| [C59N]+ | [Ag(HCB11H5Cl6)2]– | Ox | (C59N)2 + 2[HBPC]˙+[WCA]– | 261 | |
|
[B(C6F4H)4]– | Other |
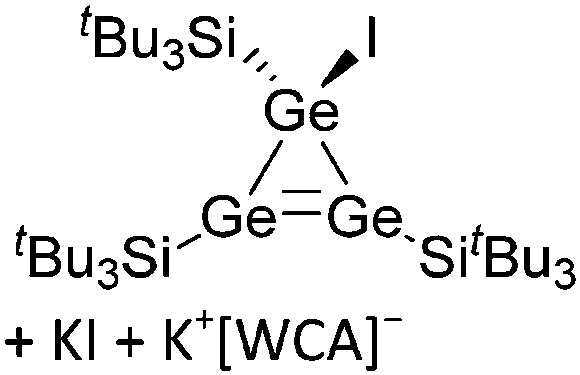
|
332 | |
| Onium ions | |||||
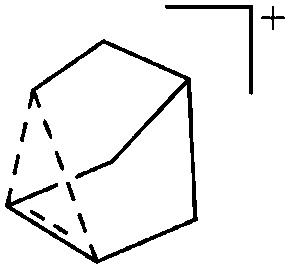
|
[Al2Br7]– | Lewis |
|
86 | |
| Enium ions | |||||
| [(CH3)3C]+ | [HCB11Me5Cl6]– | Hyd | n BuH + Me+[WCA]– or t BuH + Me(WCA) | 265 | |
| [(CH3)3C]+ | [Al2Br7]– | Lewis | t BuBr + 2 AlBr3 | 266 | |
| [(CH3)3C]+ | [HCB11Cl11]– | Other | Thermal decomposition of [Et2Cl][CHB11Cl11] | 267 | |
|
[HCB11Me5Br6]– | Hyd |
|
265 | |
|
[HCB11Me5Br6]– | Hyd |

|
265 | |
|
[HCB11I11]– | Lewis | p-CH3-C6F4-CF3 + Et3Si(WCA) + PhF | 56 | |
|
[HCB11Cl11]– | Lewis | p-CH3-C6F4-CF3 + Et3Si(WCA) + PhF | 56 | |
|
[As2F11]– | Lewis | C6H5CF3 + AsF5 | Excess AsF5 | 268 |
|
[HCB11I11]– | Lewis | p-F-C6H4CF3 + Et3Si(WCA) + PhF | 56 | |
|
[HCB11I11]– | Lewis | CH3CF3 + Et3Si(WCA) + PhF | 56 | |
| [CI3]+ | [Al(ORPF)4]– | Salt | CI4 + Ag+[WCA]– | 269 | |
| [CCl3]+ | [Sb(OTeF5)6]– | Ox | CCl4 + [XeOTeF5]+[WCA]– | 270 | |
| [CCl3]+ | [Al(ORPF)4]–, [(RPFO)3Al-F-Al(ORPF)3]– | Salt | CCl4 + Ag+[WCA]– | 271 | |
| [CBr3]+ | [Sb(OTeF5)6]– | Ox | CBr4 + [XeOTeF5]+[WCA]– | 270 | |
| [CBr3]+ | [Al(ORPF)4]–, [(RPFO)3Al-F-Al(ORPF)3]– | CBr4 + Ag+[WCA]– | 271 | ||
| [C(OTeF5)3]+ | [Sb(OTeF5)6]– | Ox | CBr4 + [XeOTeF5]+[WCA]– | 270 | |
|
[Al(ORPF)4]– | Ox | CS2 + [AsBr4]+[WCA]– and CS2 + Br2 + Ag+[WCA]– | 117 | |
|
[B(C6F5)4]– | Hyd |
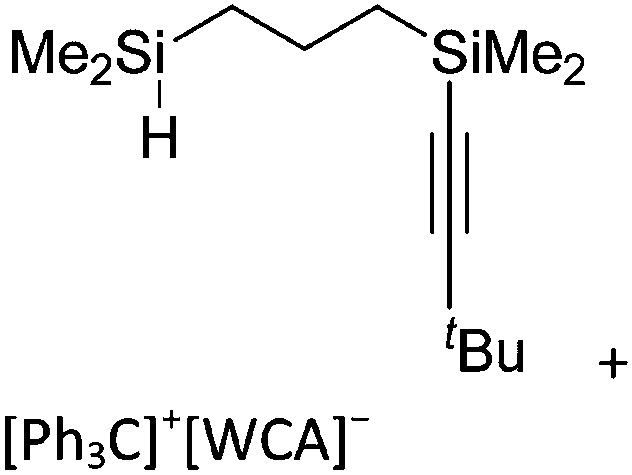
|
275 | |
|
[B(C6F5)4]– | Hyd |
|
276 | |
| [Mes3Si]+ | [HCB11Me5Br6]– | Other | Mes3Si(CH2CH CH2) + Et3Si(WCA) | 24 | |
| [Pemp3Si]+ | [B12Cl12]2– | Hyd | 2Pemp2MeSiH + [Ph3C]2 +[WCA]– | 290 | |
| [Pemp3Si]+ | [Al(ORPF)4]– | Hyd | 1.5Pemp2MeSiH + [Ph3C]+[WCA]– | 291 | |
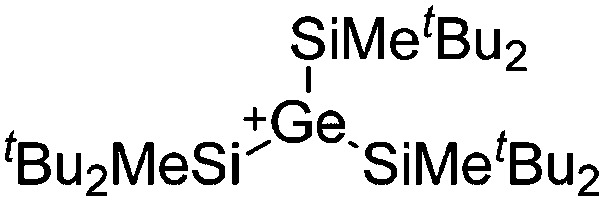
|
[B(C6F5)4]– | Ox |
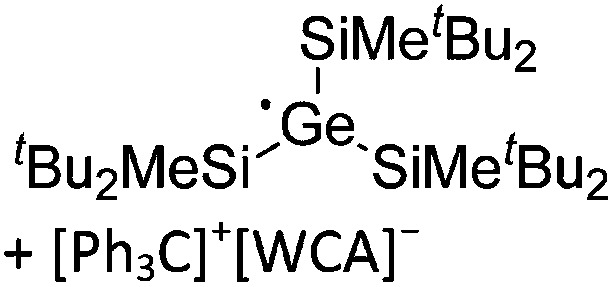
|
334 | |
|
[Al(ORPF)4]– | Salt | (Ar)3GeBr + Ag+[WCA]– | R = O t Bu | 336 |
|
[B(C6F5)4]– | Ox |
|
335 | |
| [(Tipp)3Sn]+ | [B(C6F5)4]– | Other | (allyl)(Tipp)3Sn + [E]+[WCA]– | [E]+ not exactly defined, likely [Et3Si(C6H6)]+ or comparable | 34 |
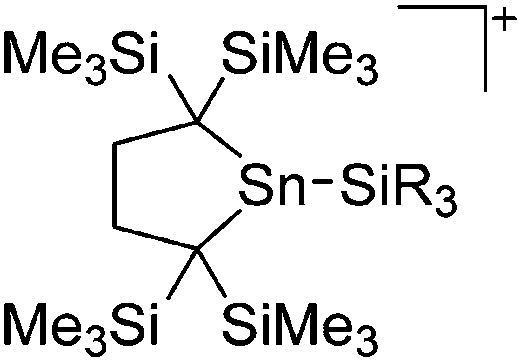
|
[B(C6F5)4]– | Com, Lig |
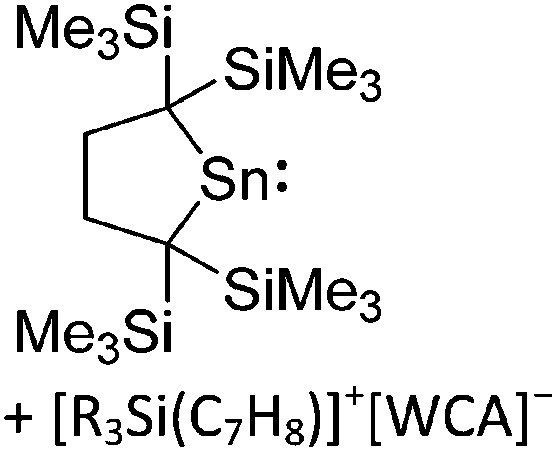
|
R = Et, iPr | 337 |
| Delocalized (cyclic) cations | |||||
| [C6H7]+ | [HCB11Me5Br6]– | Prot | C6H6 + H(WCA) | 386 | |
|
[HCB11H5Br6]– | Prot | C6H6 + H(WCA) | 387 | |
|
[HCB11H5Br6]– | Prot | C6Me2H4 + H(WCA) | 387 | |
|
[HCB11H5Br6]– | Prot | C6Me3H3 + H(WCA) | 387 | |
|
[B(C6F5)4]– | Prot | Et3Si(WCA) + HCl + C6(Me)5H | C–H···F–C interactions | 388 |
|
[HCB11H5Br6] | Prot | C6Me6 + H(WCA) | 387 | |
| [C6Me7]+ | [AlCl4]– | Other | C6Me6 + CH3Cl + AlCl3 | 277 | |
| [C6F6]˙+ | [Sb2F11]– | Ox | C6F6 + [O2]+[WCA]– | Crystallized out of HF | 278 |
| [C6F6]˙+ | [Os2F11]– | Ox | C6F6 + OsF6 + SbF5 | In HF | 278 |
| [C6Cl6]˙+ | [Sb2F11]– | Ox | C6F6 + SbF5 | 279 | |
| [C6Br6]˙+ | [As2F11]– | Ox | C6Br6 + [O2]+[AsF6]– + HSO3F | 280 | |
| [C6I6]˙+ | [AsF6]– | Ox | C6I6 + AsF5 | In HF | 279 |
| [C6I6]˙+ | [SbF6]– | Ox | C6I6 + SbF5 | In HF | 279 |
| [C6I6]˙+ | [OTf]– | Other | [C6I6][AsF6] + HOTf | 279 | |
| [C6HF5]˙+ | [AsF6]– | Ox | C6HF5 + [O2]+[WCA]– | 279 | |
|
[SbF6]– | Ox | C6H2F4 + [O2]+[WCA]– | 279 | |
|
[AsF6]– | Ox | C6H3F3 + [O2]+[WCA]– + AsF5 | 279 | |
|
[SbF6]– | Ox | C6H3F3 + [O2]+[Sb2F11]– | In HF | 279 |
|
[Sb2F11]– | Ox | C6F5(CF3) + [O2]+[WCA]– | In HF | 280 |
|
[Sb2F11]– | Ox | C6F4(CF3)2 + SbF5 | In HF | 280 |
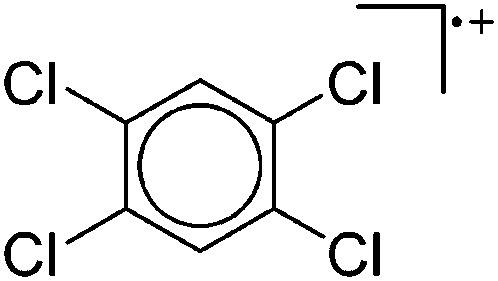
|
[Sb2F11]– | Ox | C6H2Cl4 + [O2]+[WCA]– | 280 | |
|
[Sb3F16]– | Ox | [C6F5–C6F5] + [O2]+[WCA]– + SbF5 | 280 | |
|
[Nb2F11]– | Ox |
|
281 | |
|
[SbF6]– | Ox |
|
282 | |
| [Cy3C3]+ | [SbF6]– | Salt | [Cy3C3]Cl + Ag+[WCA]– | 283 | |
| [Cy2PhC3]+ | [BF4]– | Lewis | [Cy2(Ph)C3]F + BF3 | 283 | |
|
[SbCl6]– | Prot |
|
284 | |
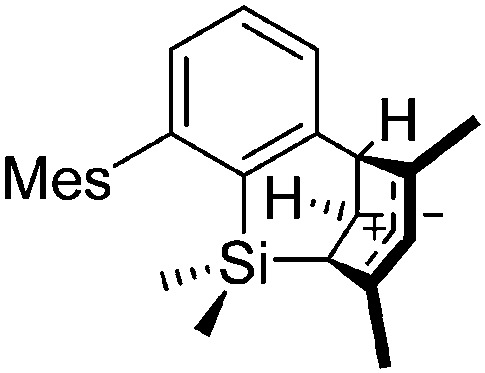
|
[HCB11Me5Br6]– | Hyd | ArMe2SiH + [Ph3C]+[WCA]– | 286 | |
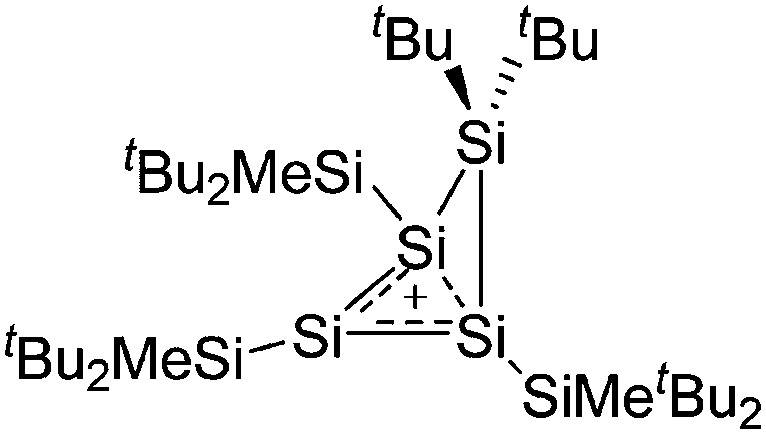
|
[B(C6F5)4]– | Other |
|
292 | |
|
[B(C6F4R)4]– | Other |
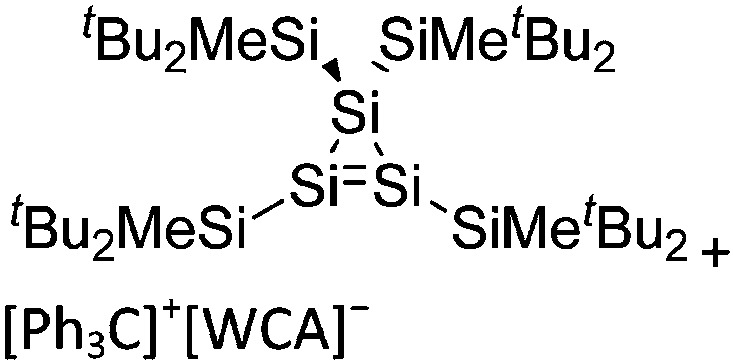
|
R = 4-SiMe2 t Bu | 293 |
|
[Zr2Cl7Cp*2]– | Lewis |
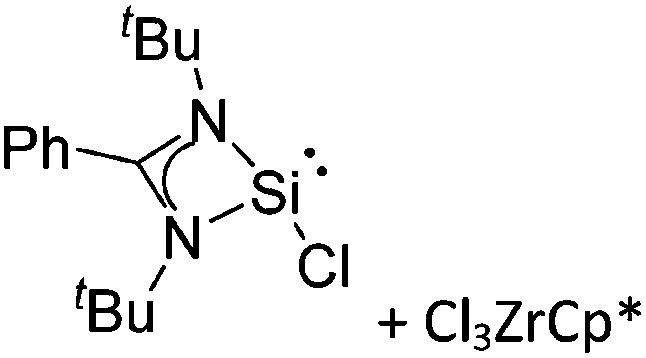
|
294 | |
|
[B(C6F5)4]– | Prot |
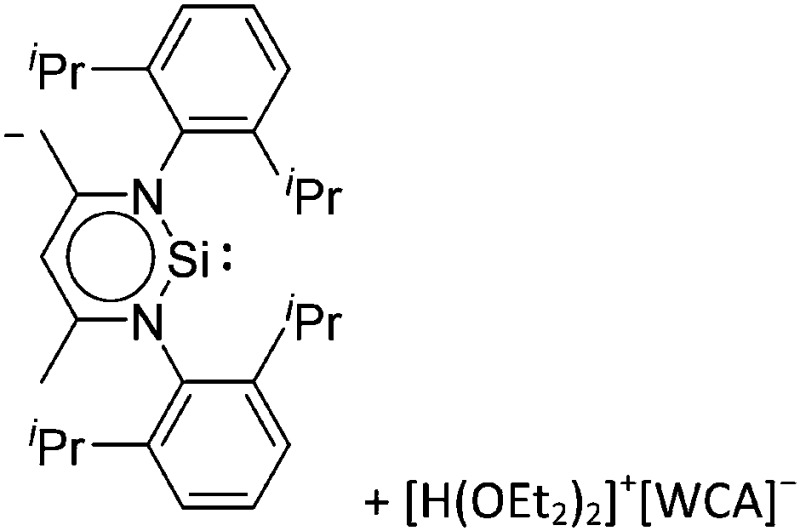
|
295 | |
|
[B(C6H5)]–, [B(ArCF3 )4]–, [B(C6F4R)4]– | Other |
|
R = 4-Si(Me)2( t Bu) | 339–341 |
| Ligand-stabilized cations | |||||
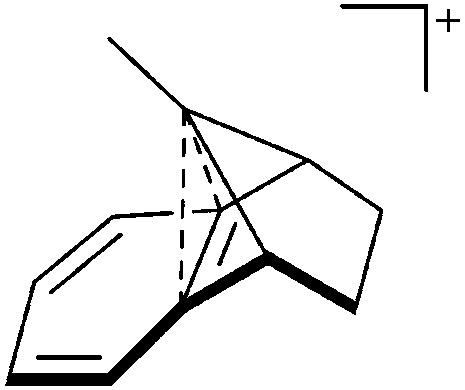
|
[Sb2F11]– | Lewis |
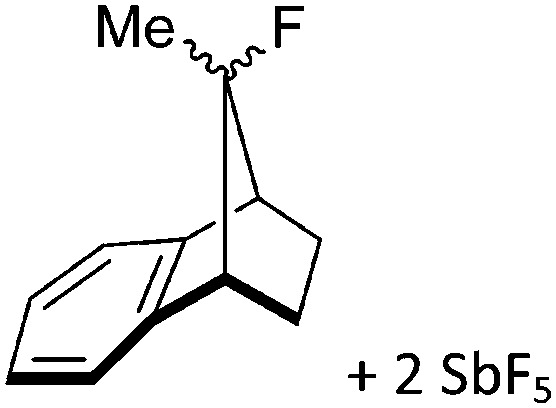
|
273 | |
| [I3C-PX3]+ | [Al(ORPF)4]– | Comp | [CI3]+[WCA]– + PX3 | X = Cl, Br, I | 274 |
| [I3C-PI3]+ | [(RPFO)3Al-F-Al(ORPF)3] | Comp | [CI3]+[WCA]– + PI3 | 274 | |
| [I3C-AsI3]+ | [Al(ORPF)4]– | Comp | [CI3]+[WCA]– + AsI3 | 274 | |
|
[HCB9H4Br5]– | Hyd | iPr3SiH + [Ph3C]+[WCA]– + MeCN | 297 | |
|
[B(C6F5)4]– | Other | t Bu3Si–Si t Bu3 + 2[Ph3C]+[WCA]– + t BuCN | 298 | |
|
[B(C6F5)4]– | Com |
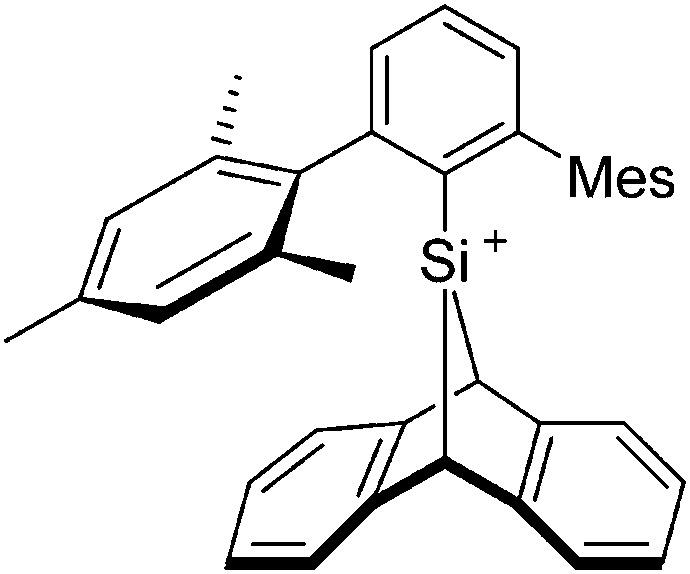
|
+ MeCN | 299 |
| [Fc3Sipy]+ | [B(ArCF3 )4]– | Lig | [Fc3Si(THF)]+ + py | 300 | |
|
[HCB11H5Br6]– | Ion | t Bu3Si(WCA) + H2O | 301 | |
|
[HCB11Cl11]– | Ion | iPr3Si(WCA) + C6H4Cl2 | 81 | |
|
[HCB11Me5Br6]– | Ion | Et3Si(WCA) + SO2 | 81 | |
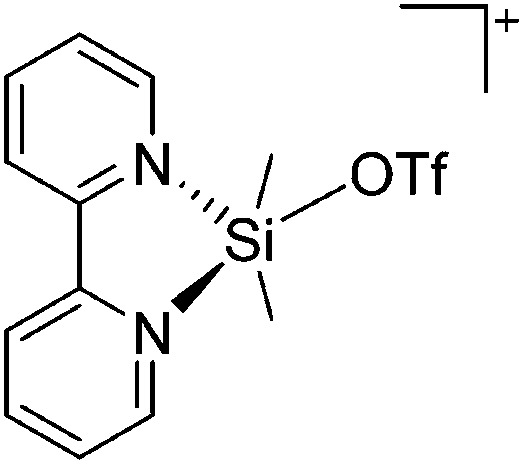
|
[OTf]– | Ion | Me2Si(OTf)2 + bipy | 302 | |
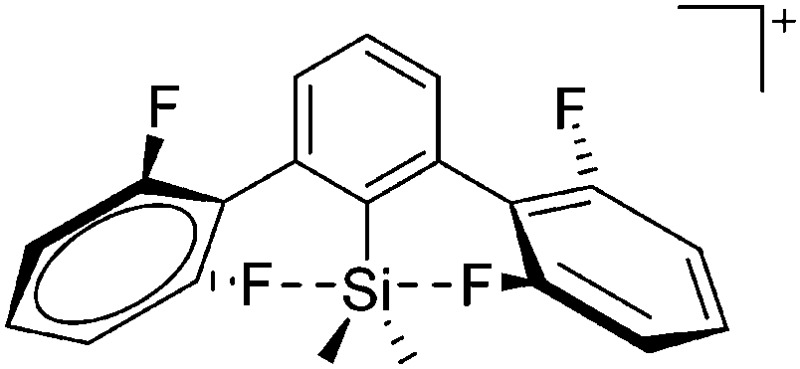
|
[B(C6F5)4]– | Hyd | Me2ArSiH + [Ph3C]+[WCA]– | 303 | |
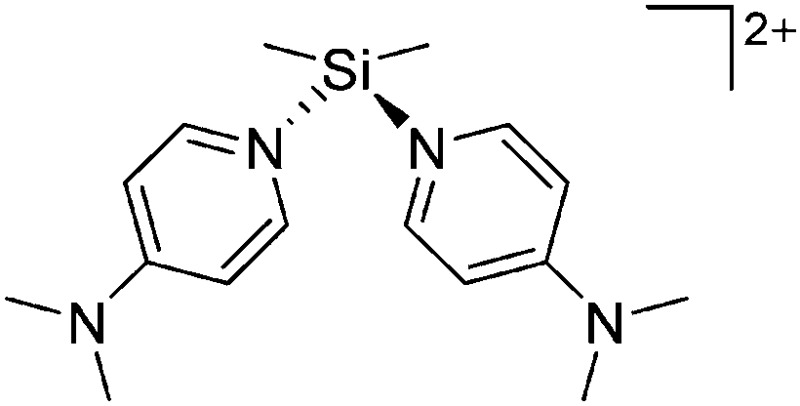
|
[OTf]– | Ion | Me2Si(OTf)2 + 2DMAP | 302 | |
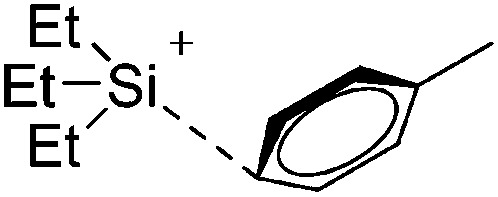
|
[B(C6F5)4]– | Hyd | Et3SiH + [Ph3C]+[WCA]– | 289 | |
| [Me3Si(Ar)]+ | [B(C6F5)4]– | Lig | [Me3SiHSiMe3]+[WCA]– + arene | Ar = benzene, toluene, ethylbenzene, n-propylbenzene, and iso-propylbenzene, o-xylene, m-xylene, p-xylene, 1,2,3-trimethylbenzene, 1,2,4-trimethylbenzene, mesitylene | 304 |
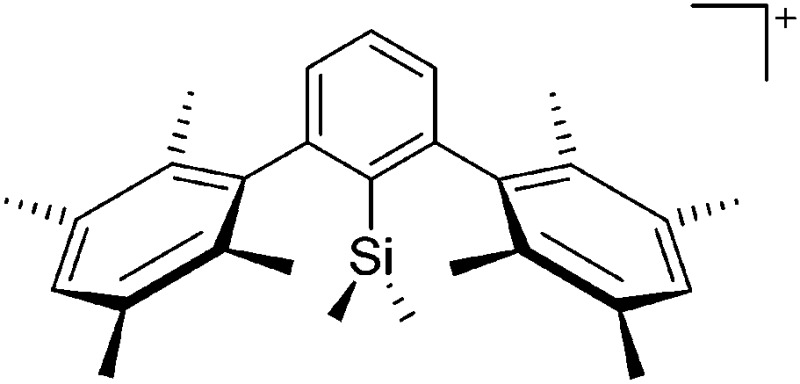
|
[B(C6F5)4]– | Hyd |
|
76 | |
| [Me3SiHSiMe3]+ | [HCB11HCl11]– | Hyd | 2Me3SiH + [Ph3C]+[WCA]– | 81 | |
| [Et3SiHSiEt3]+ | [B(C6F5)4]– | Hyd | 2Et3SiH + [Ph3C]+[WCA]– | 306 | |
| [Me3SiXSiMe3]+ | [B(C6F5)4]– | Ion | Me3SiX + Me3Si(WCA) | X = F, Cl, Br, I | 307 |
| [Me3Si-CN-SiMe3]+ | [B(C6F5)4]– | Ion | Me3SiCN + Me3Si(WCA) | 309 | |
| [Me3Si-OCN-SiMe3]+ | [B(C6F5)4]– | Ion | Me3SiOCN + Me3Si(WCA) | 309 | |
| [Me3Si-SCN-SiMe3]+ | [B(C6F5)4]– | Ion | Me3SiSCN + Me3Si(WCA) | 309 | |
| [(Me3Si)2NNN]+ | [B(C6F5)4]– | Ion | Me3SiNNN + Me3Si(WCA) | 309 | |
|
[B(C6F5)4]– | Ion | Me3Si(OTf) + Me3Si(WCA) | 308 | |
|
[B(C6F5)4]– | Hyd |
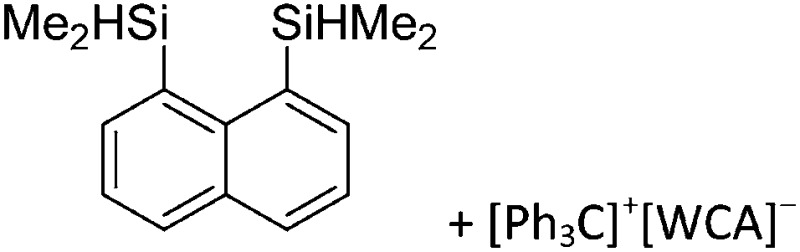
|
311 | |
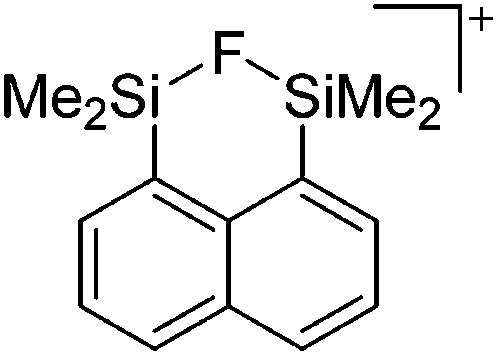
|
[B(C6F5)4]– | Other |
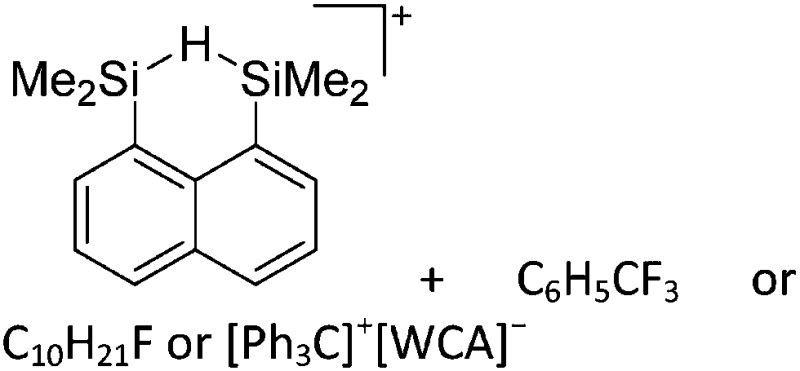
|
311 | |
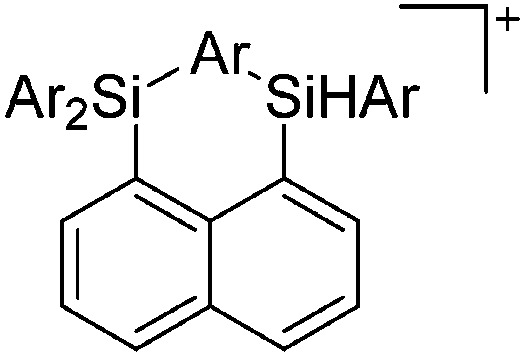
|
[B(C6F5)4]– | Hyd |
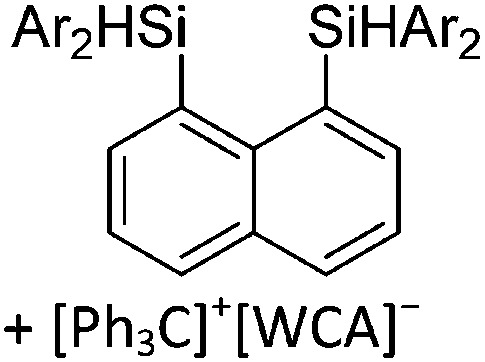
|
Ar = Tol | 312 |
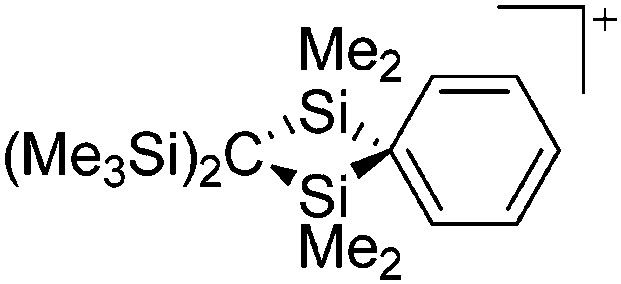
|
[B(C6F5)4]– | Other | (Me3Si)3CSiMePhH + [Ph3C]+[WCA]– | 313 | |
|
[B(C6F5)4]– | Other |
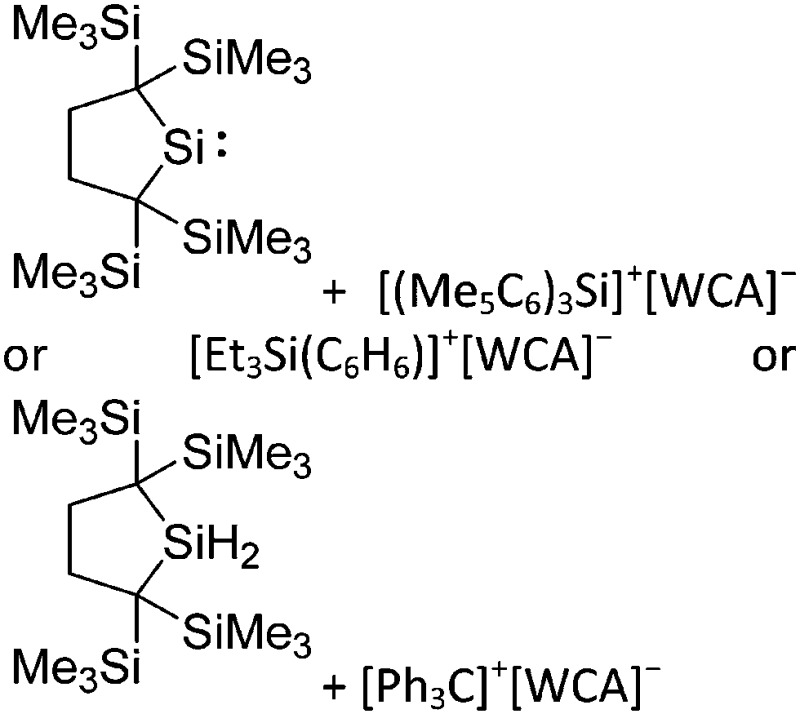
|
314 | |
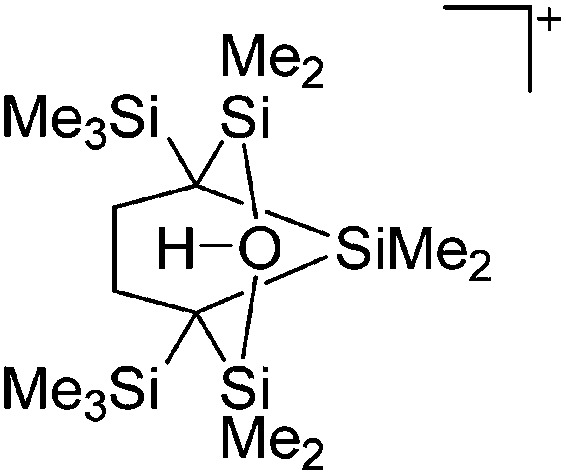
|
[B(C6F5)4]– | Other |
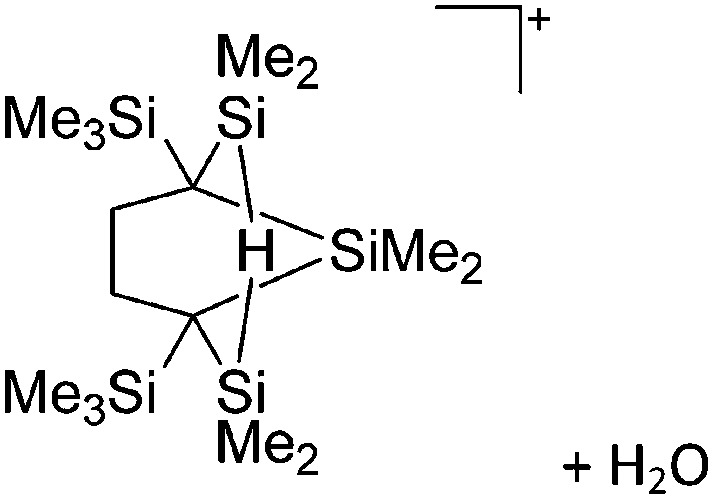
|
314 | |
|
[B(C6F5)4]– | Other |
|
X = Cl, Br | 315 |
|
[B12Cl12]2– | Hyd | 2FcMe t BuSiH + [Ph3C]2 +[WCA]– | 316 | |
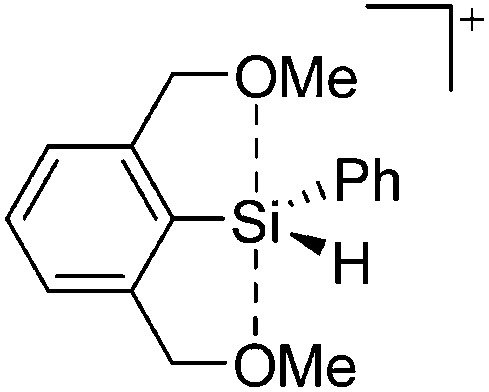
|
[OTf]– | Lewis |
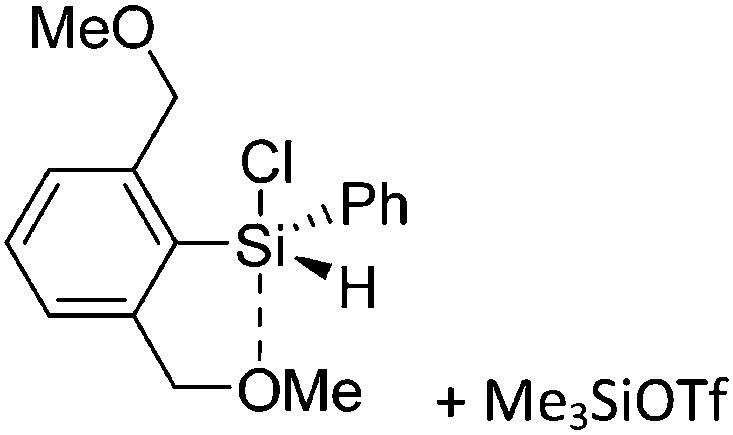
|
317 | |
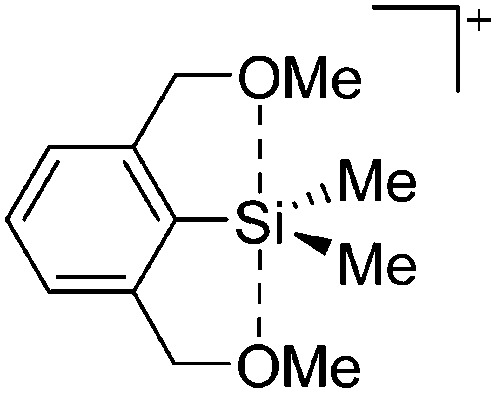
|
[OTf]– | Lewis |
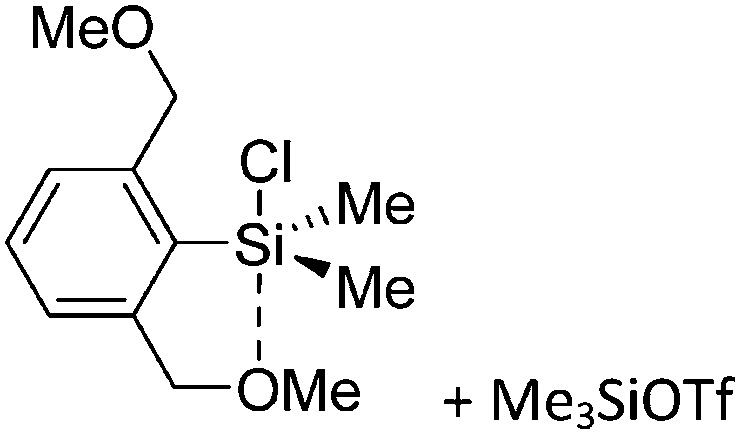
|
317 | |
|
[OTf]– | Lewis |
|
317 | |
|
[OTf]– | Lewis |
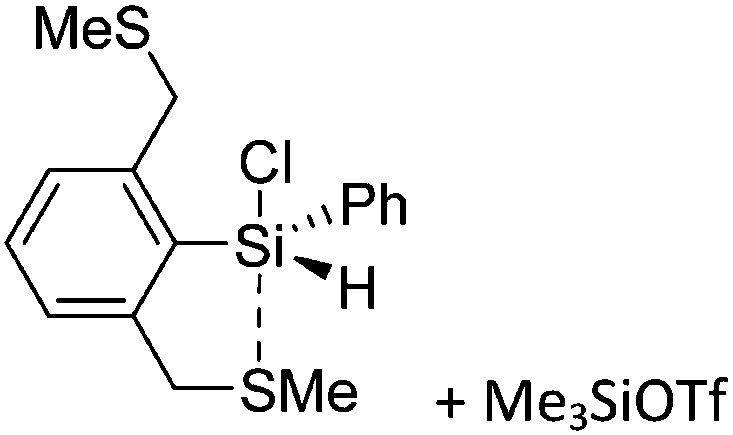
|
317 | |
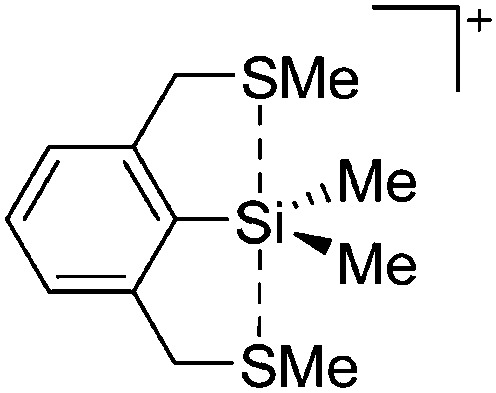
|
[OTf]– | Lewis |
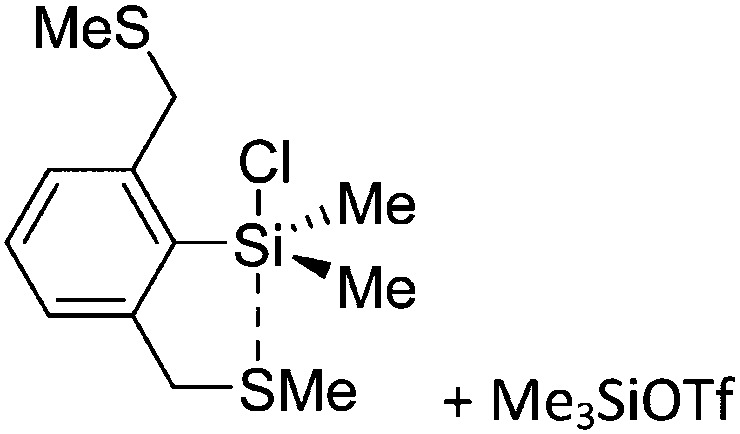
|
317 | |
|
Cl– | Ion |
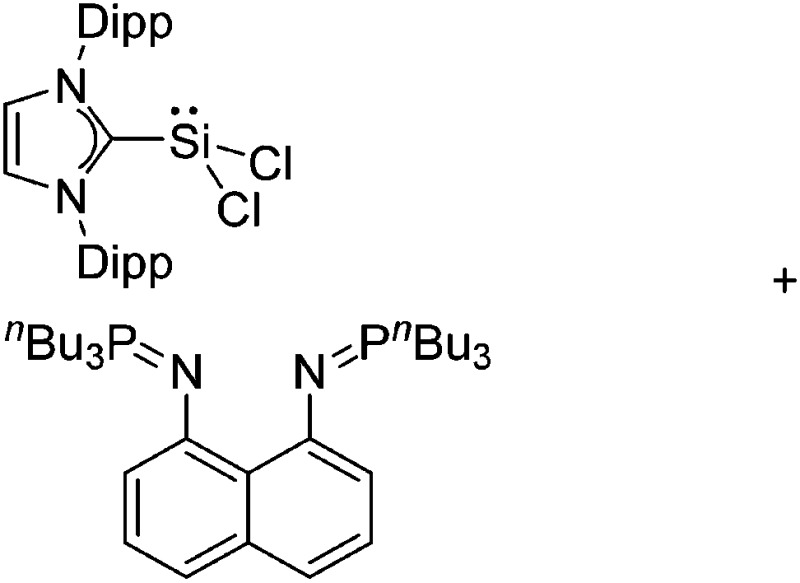
|
318 | |
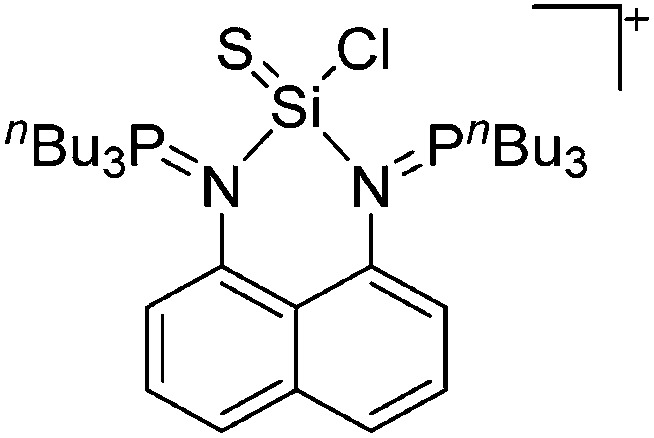
|
Cl– | Ox |
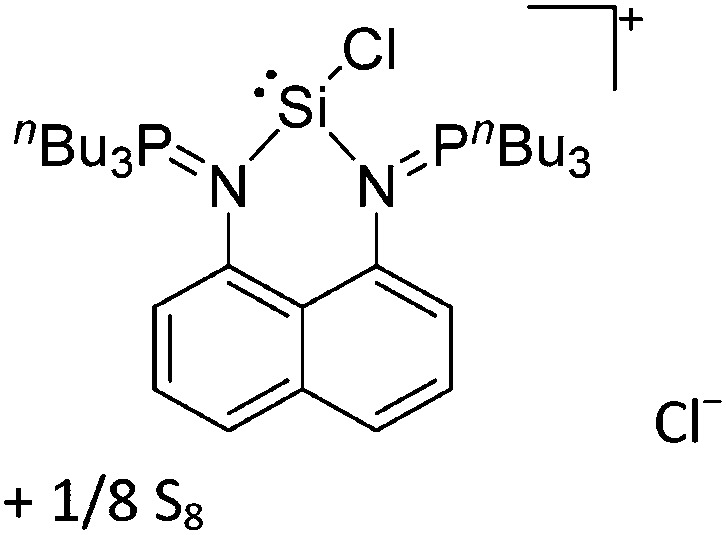
|
318 | |
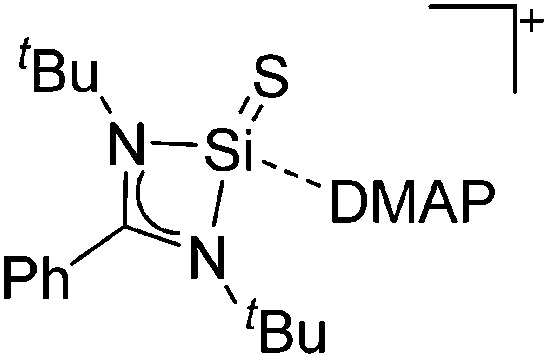
|
[OTf]– | Ox |
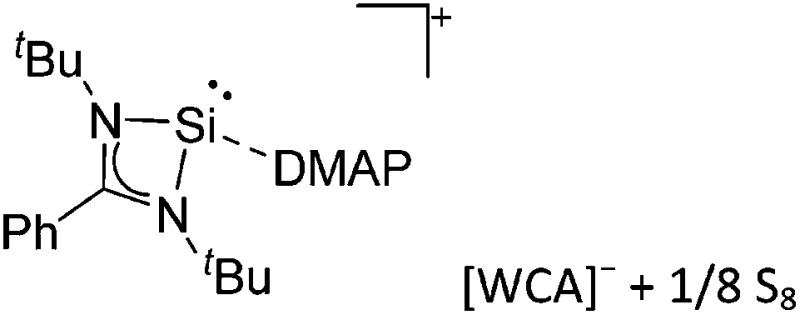
|
319 | |
|
Cl– | Ion |
|
320 | |
|
I– | Ion |
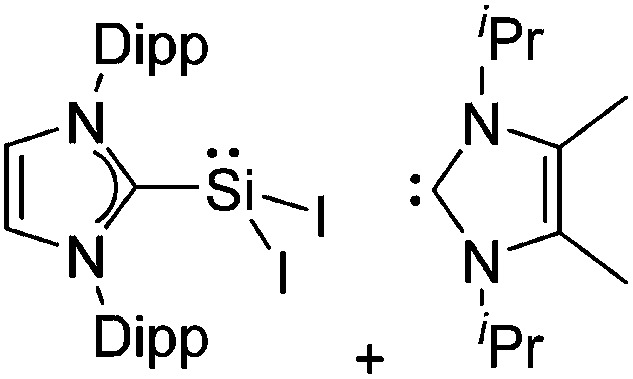
|
321 | |
|
[OTf]– | Ox |
|
319 | |
|
Other |
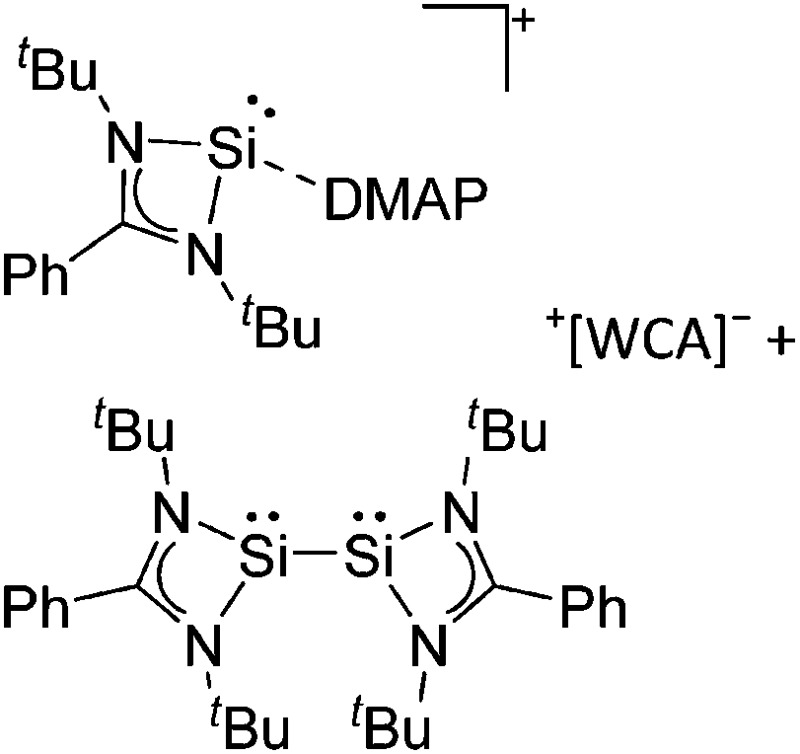
|
319 | ||
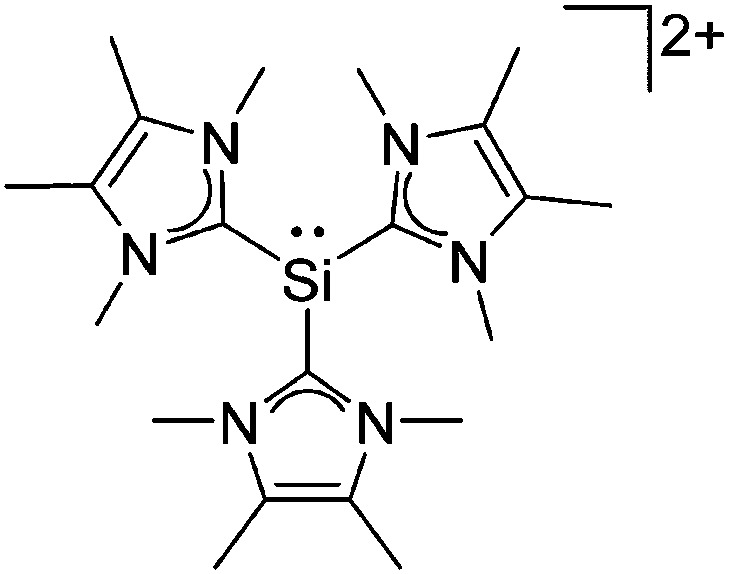
|
I– | Ion |
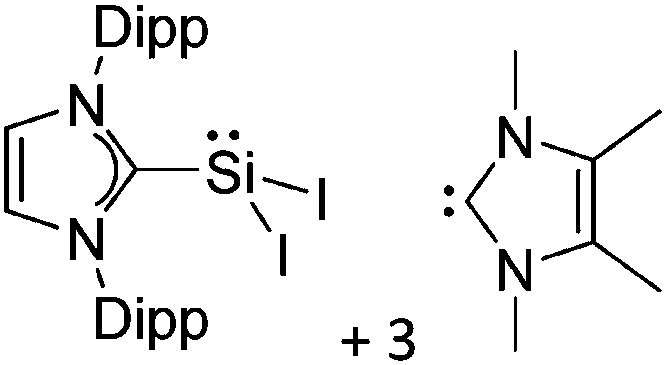
|
321 | |
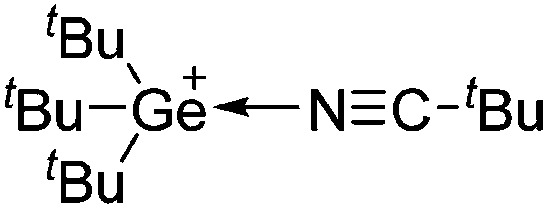
|
[B(C6F5)4]– | Ox | t Bu3Ge–Ge t Bu3 + 2[Ph3C]+[WCA]– + t BuCN | 298 | |
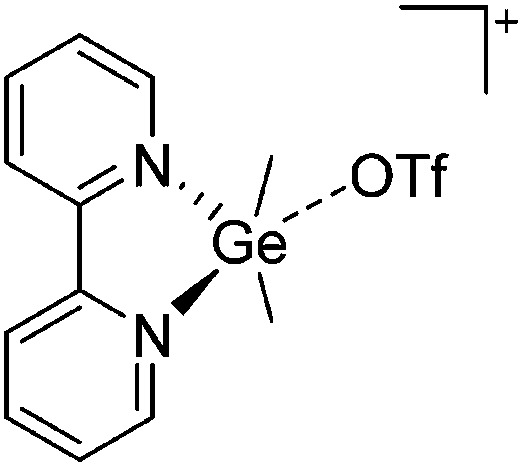
|
[OTf]– | Ion | Me2Ge(OTf)2 + bipy | 302 | |
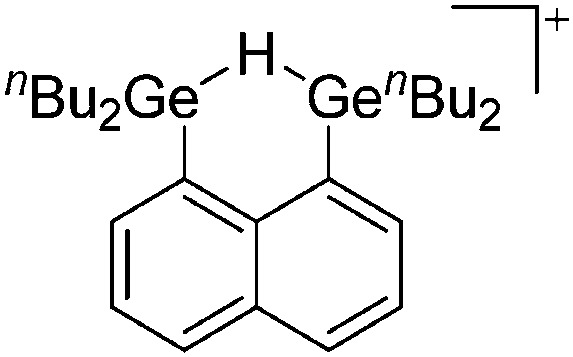
|
[B(C6F5)4]– | Hyd |
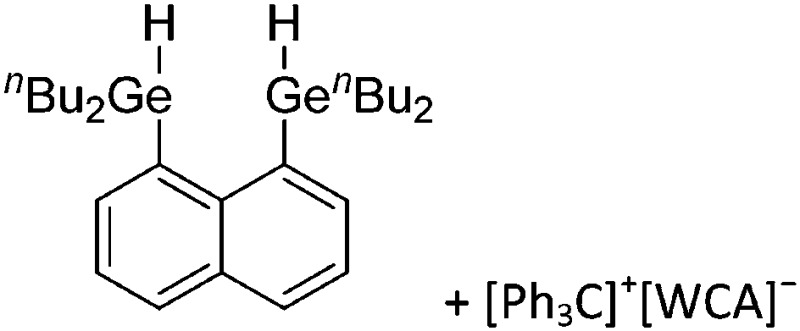
|
343 | |
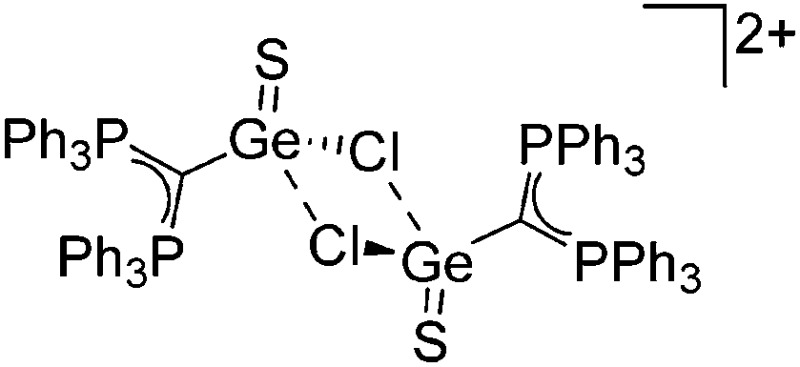
|
[AlCl4]– | Ox |
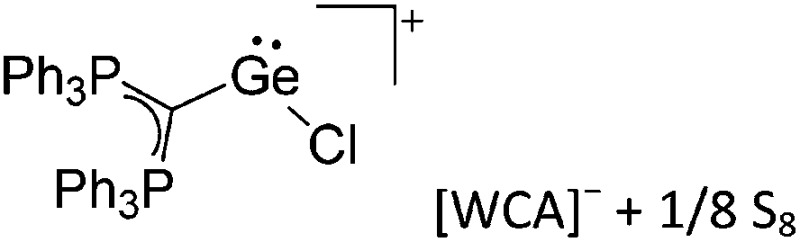
|
344 | |
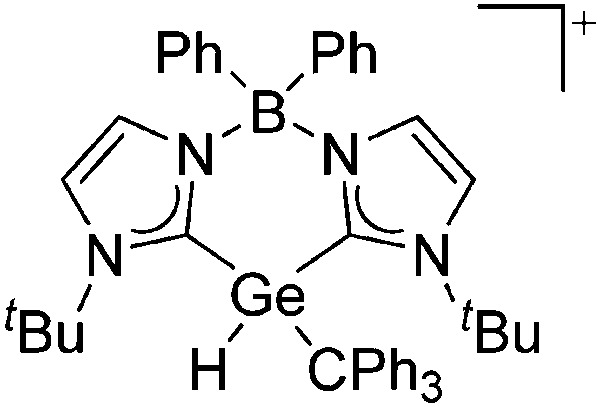
|
[B(C6F5)4]– | Ox or Com |
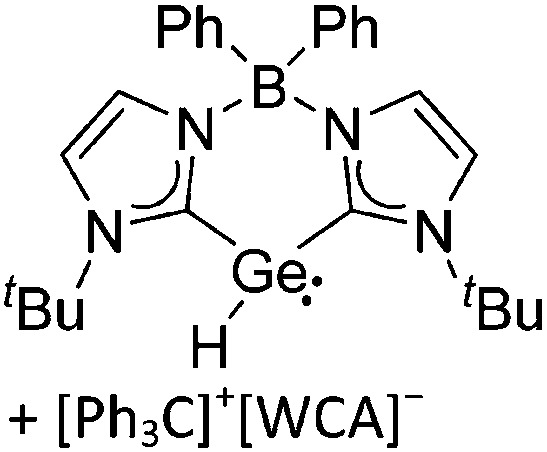
|
349 | |
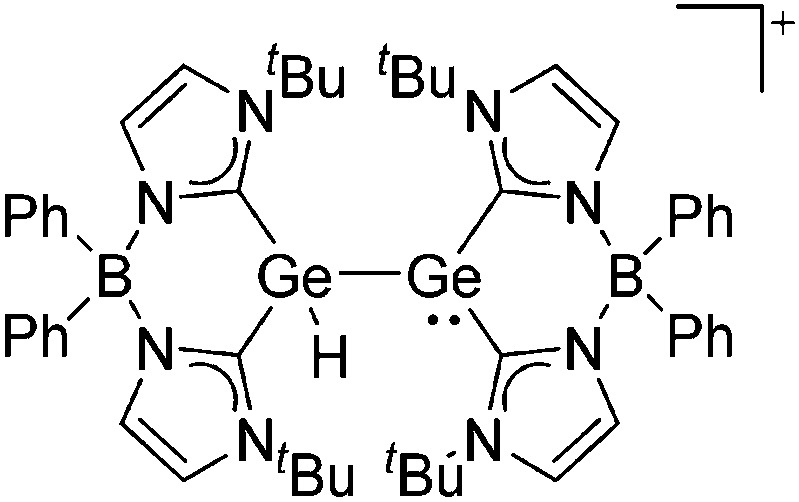
|
[B(C6F5)4]– | Hyd |
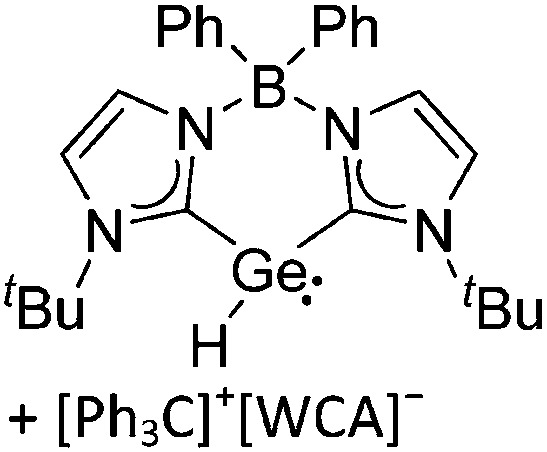
|
349 | |
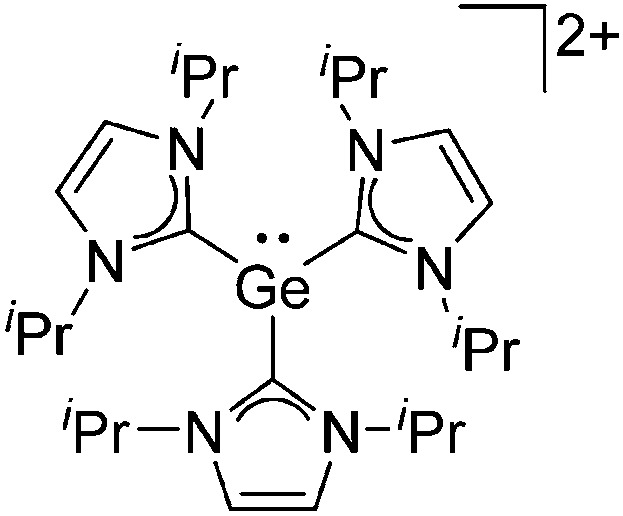
|
I– | Ion |
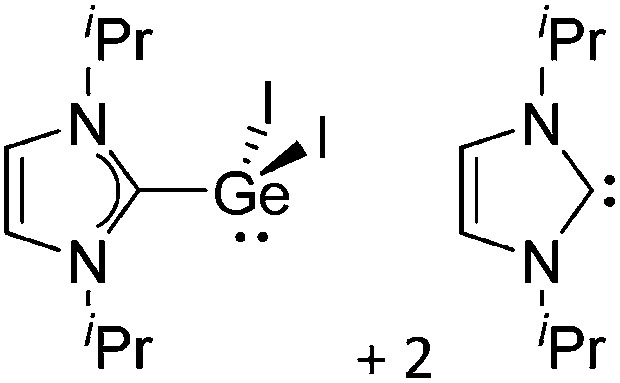
|
354 | |
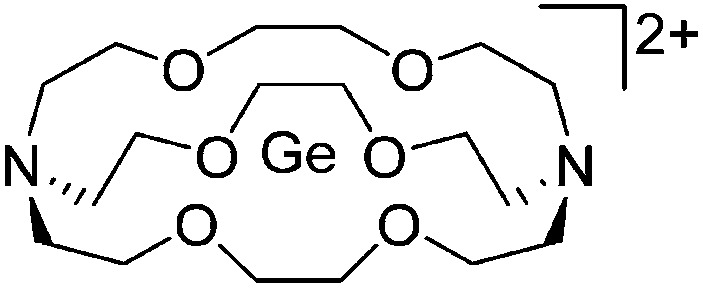
|
[OTf]– | Ion, Lig |
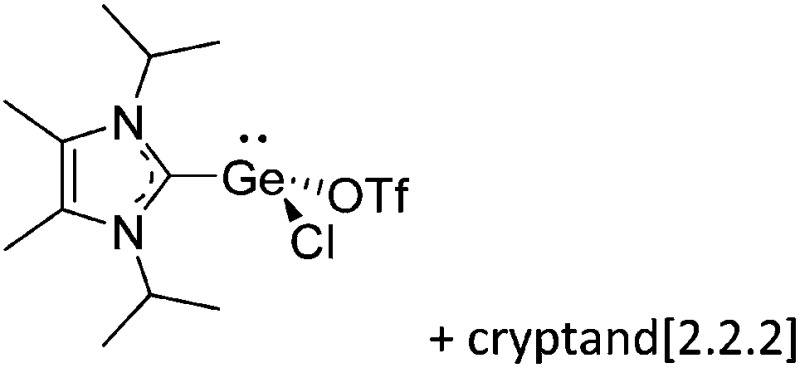
|
355 | |
| [Ge([12]-crown-4)2]2+ | [GeCl3]– | Ion | GeCl2·dioxane + [12]crown-4 | 357 | |
| [Ge([12]-crown-4)2]2+ | [OTf]– | Ion | GeCl2·dioxane + [12]crown-4 + 2Me3Si(OTf) | 357 | |
| [GeCl([15]-crown-5)]+ | [GeCl3]– | Ion | 2GeCl2·dioxane + [15]crown-5 | 357 | |
| [Ge(OTf)([15]-crown-5)]+ | [OTf]– | Ion | GeCl2·dioxane + [15]crown-5 + 2Me3Si(OTf) | 357 | |
| [GeCl([18]-crown-6)]+ | [GeCl3]– | Ion | 2GeCl2·dioxane + 1.5[18]crown-6 | 357 | |
|
[GeCl3]– | Ion |
|
360 | |
|
[GeCl3]– | Ion |
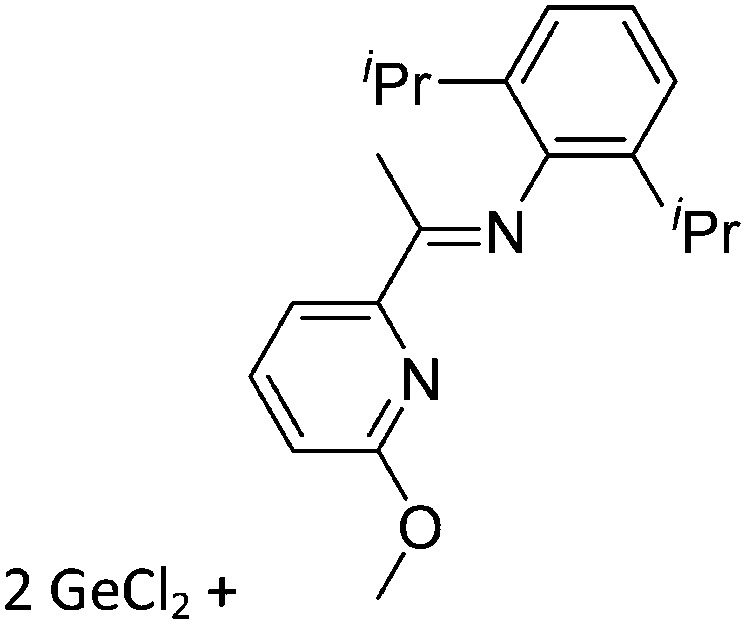
|
361 | |
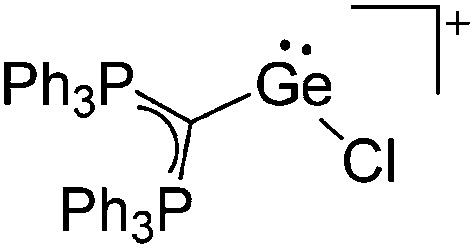
|
[AlCl4]– | Lewis |
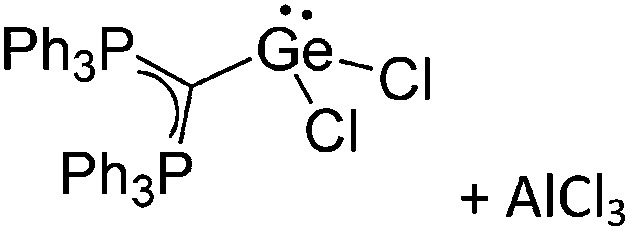
|
344 | |
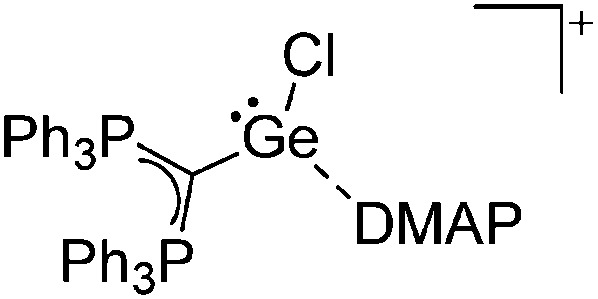
|
[B12Cl12]2– | Com |
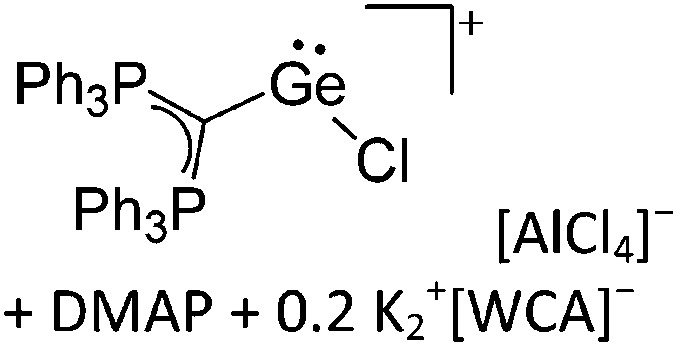
|
344 | |
|
[HO{B(C6F5)3}2]– | Lewis |
|
369 | |
|
[B(C6F5)4]– | Prot |
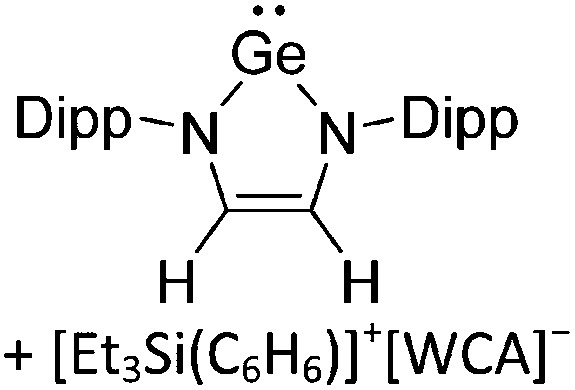
|
Protonated by acidified benzene | 365 |

|
[B(C6F5)4]– | Prot |
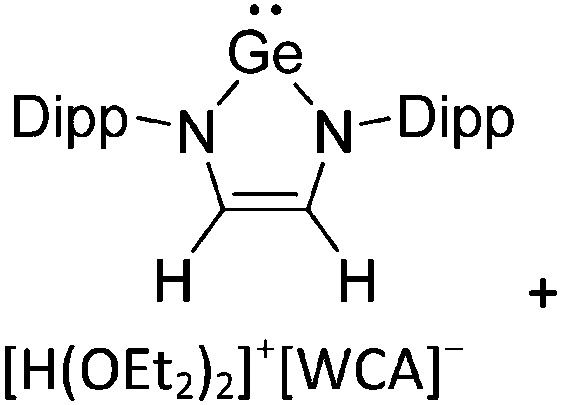
|
365 | |
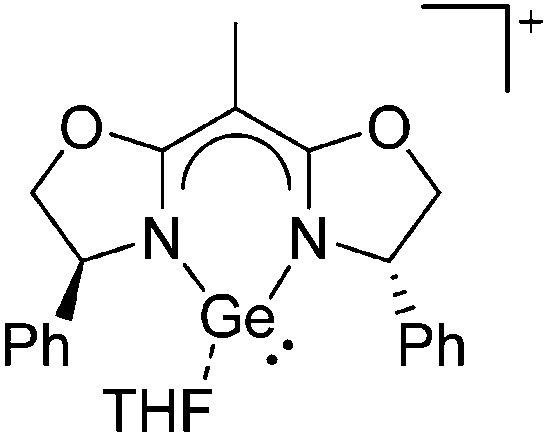
|
[SbF6]– | Salt |
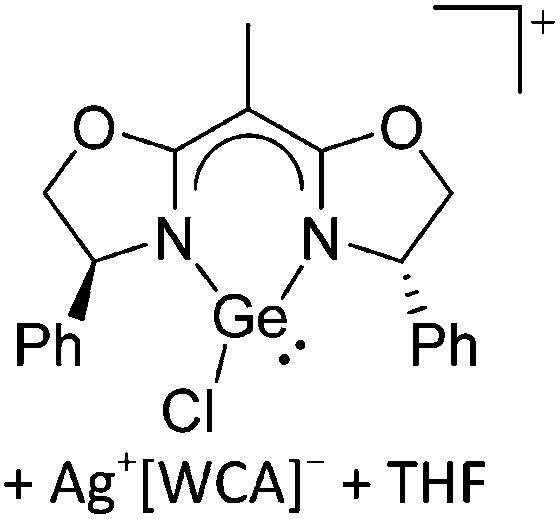
|
80 | |
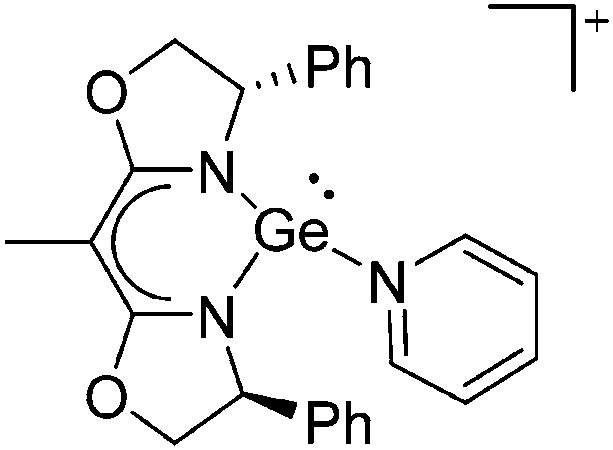
|
[SbF6]– | Lig |
|
80 | |
|
[SbF6]– | Lig |
|
80 | |
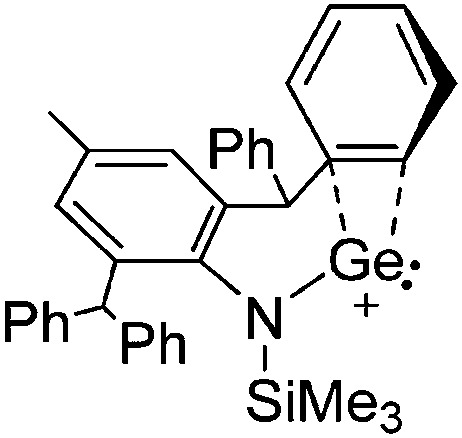
|
[Al(ORPF)4]– | Salt |
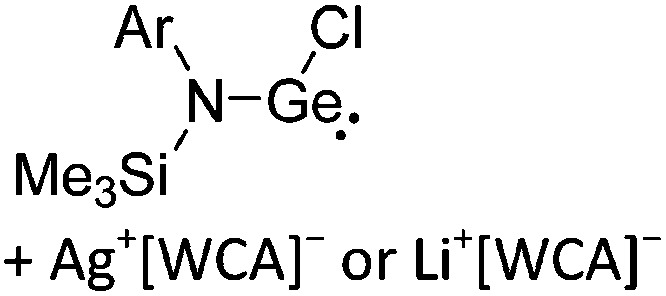
|
366 | |
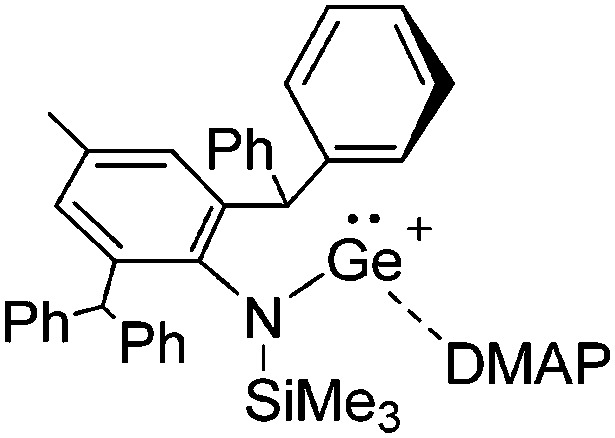
|
[Al(ORPF)4]– | Lig |
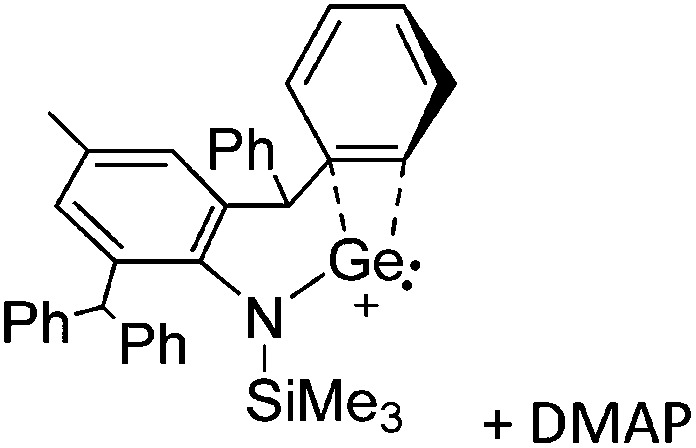
|
366 | |
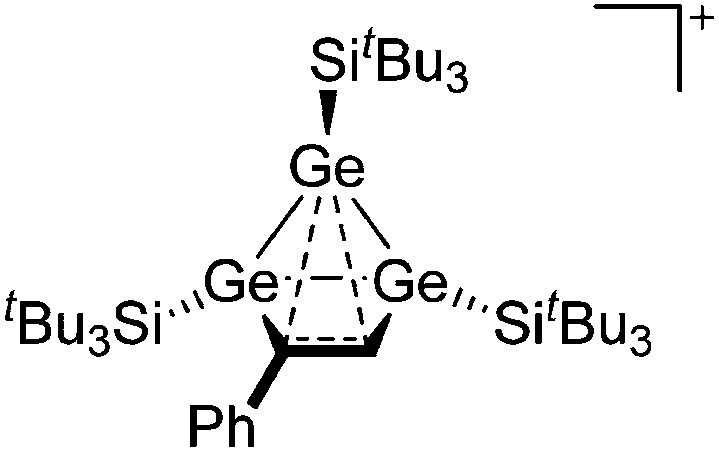
|
[B(C6F5)4]– | Lewis |
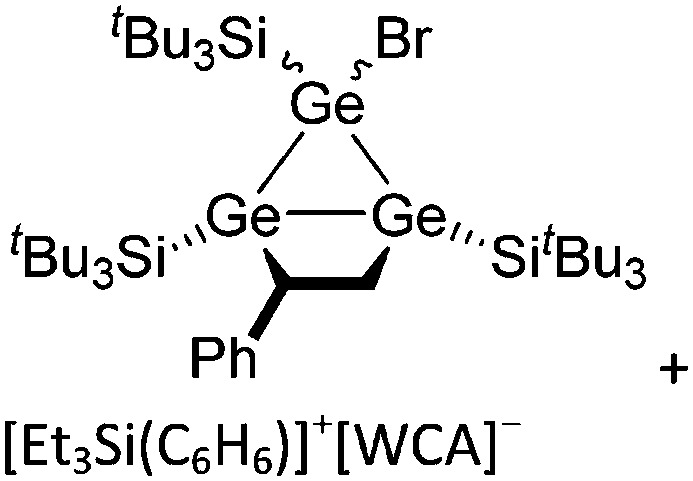
|
347 | |
| [Me3Sn(OPPh3)2]+ | [(MeSO2)2N]– | Ion | Me3SnN(SO2Me)2 + 2OPPh3 | 342 | |
|
[B(Ph)4]– | Ox |
|
L = DMAP | 345 |
| [Sn([15]crown-5)2]2+ | [SnCl3]– | Ion | 2SnCl2 + 2[15]crown-5 | 350 | |
| [Sn([12]crown-4)2]2+ | [OTf]– | Ion | Sn(OTf)2 + 2[12]crown-4 | 352 | |
| [Sn([15]crown-5)2]2+ | [OTf]– | Ion | Sn(OTf)2 + 2[15]crown-5 | 352 | |
| [Sn([18]crown-6)(OTf)]+ | [OTf]– | Ion | Sn(OTf)2 + [18]crown-6 | 352 | |
| [Sn([18]crown-6)(F)]+ | [PF6]– | Other | [Sn([18]crown-6)]2+[WCA]2 – + KF | 353 | |
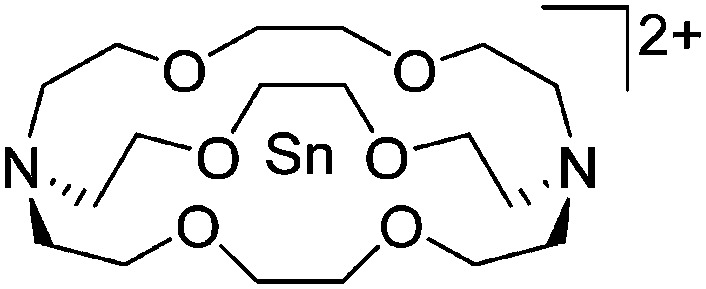
|
[OTf]– | Ion | Sn(OTf)2 + cryptand[2.2.2] or 2SnCl2 + cryptand[2.2.2] + 4Me3Si(OTf) | 356 | |
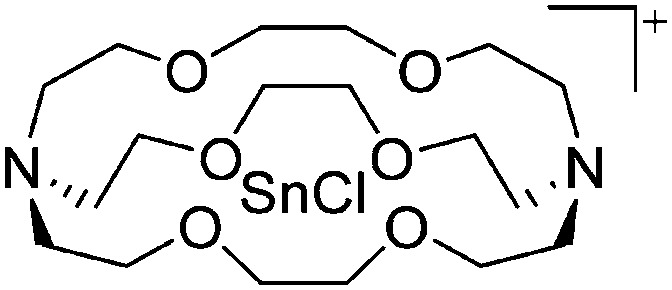
|
[SnCl3]– | Ion | 2SnCl2 + cryptand[2.2.2] | 356 | |
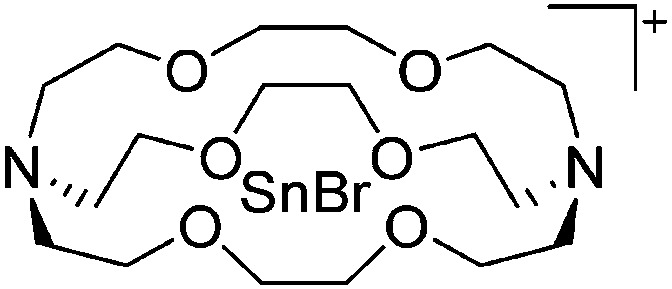
|
[SnBr3]– | Ion | 2SnCl2 + cryptand[2.2.2] + 4Me3SiBr | 356 | |
|
[B(C6F5)4]– | Com |
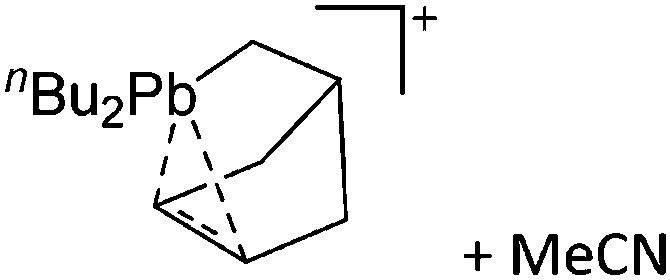
|
346 | |
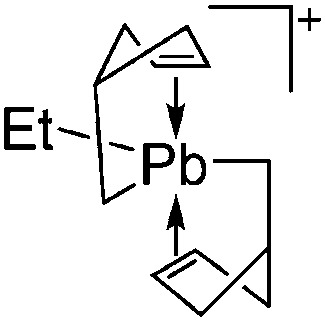
|
[B(C6F5)4]– | Other |
|
348 | |
|
[SnCl3]– | Salt |
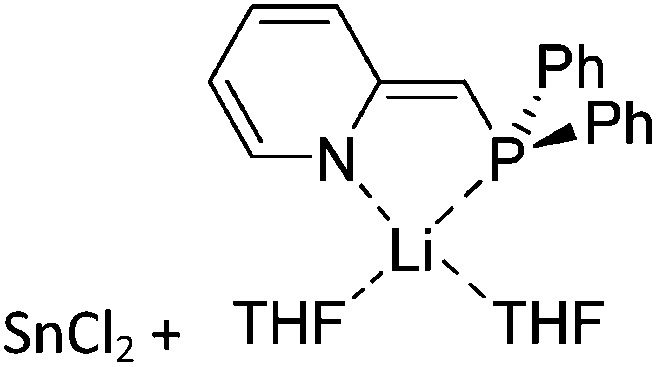
|
389 | |
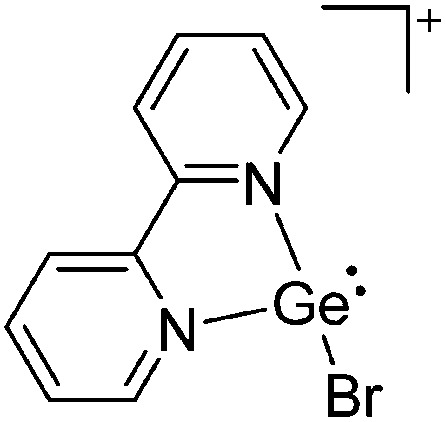
|
[GeBr3]– | Ion | GeBr2 + bipy | Bulk product is GeBr2(bipy) | 359 |
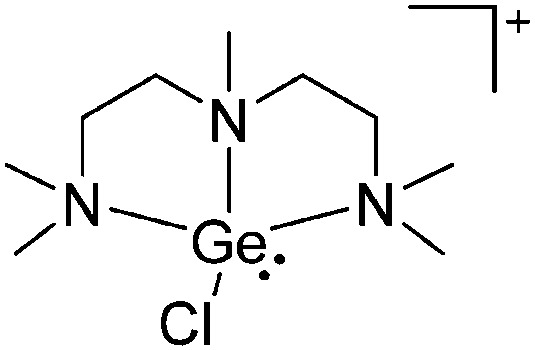
|
[GeCl3]– | Ion | GeCl2·dioxane + pmdta | 359 | |
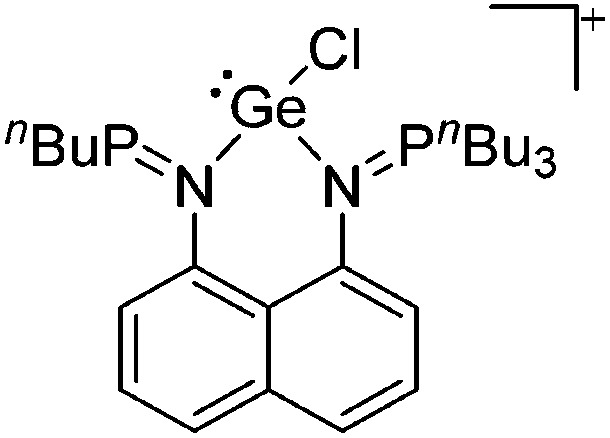
|
Cl– | Ion |
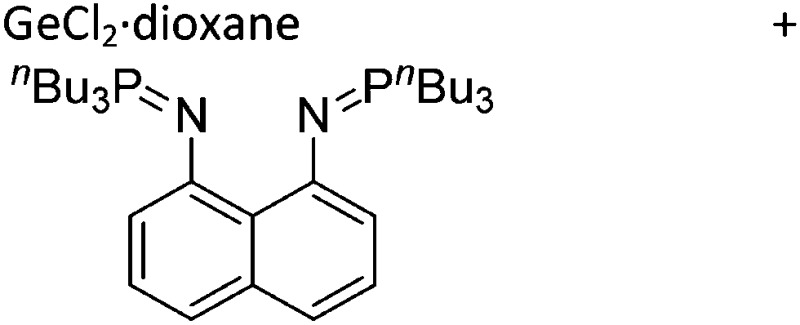
|
363 | |
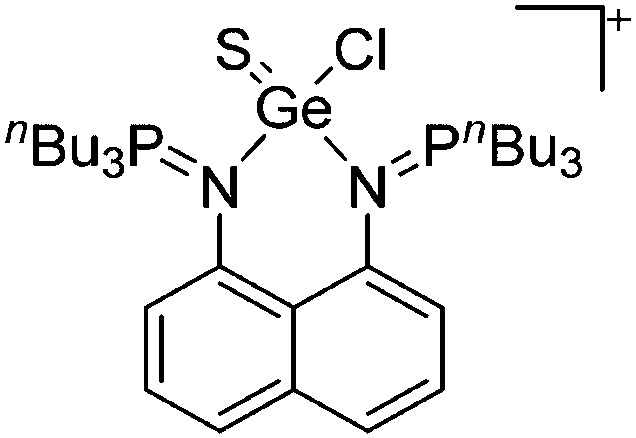
|
Cl– | Ox |
|
363 | |
|
Cl– | Ion |
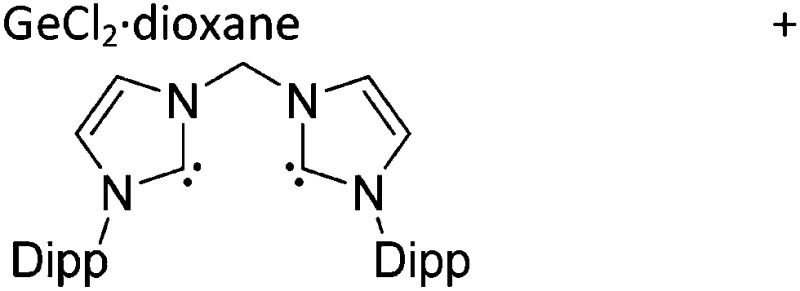
|
364 | |
|
[GeCl3]– | Ion | GeCl2·dioxane + Me4-cyclam | 358 | |
|
[GeBr3]– + Br– | Ion | GeBr2 + Me3-tacn | 358 | |
|
[SnCl3]– | Ion |
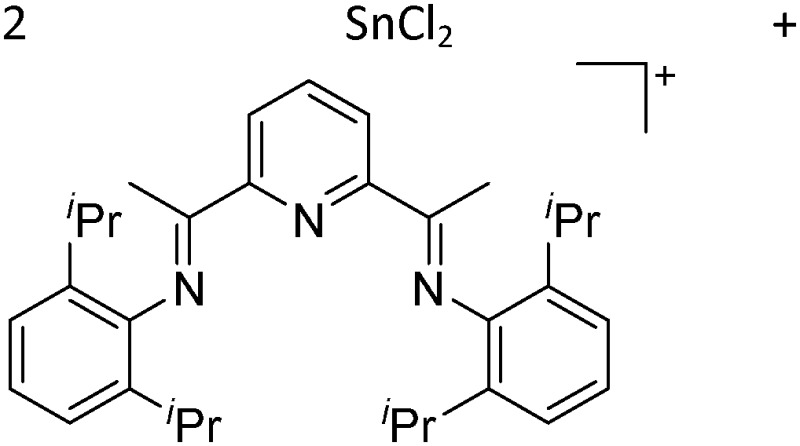
|
360 | |
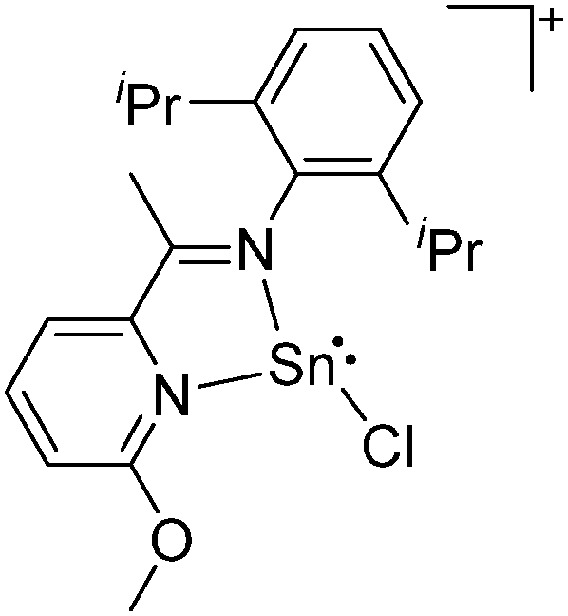
|
[SnCl3]– | Ion |
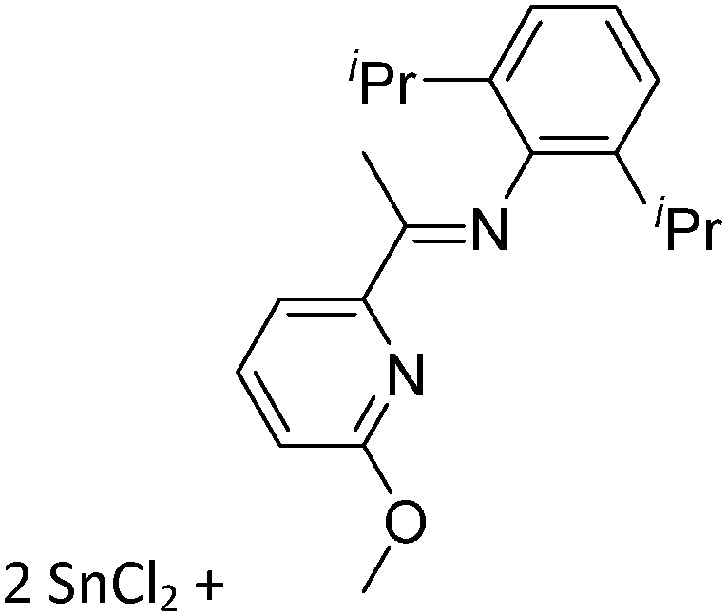
|
361 | |
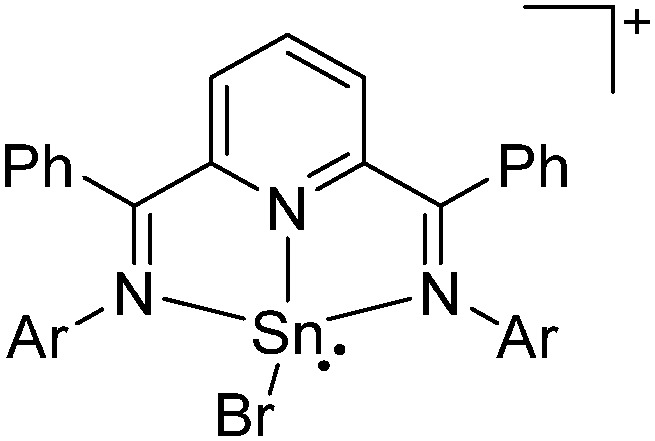
|
[SnBr3]– | Ion |
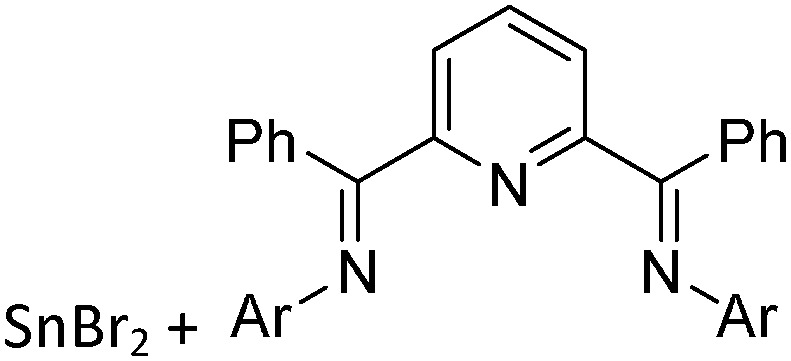
|
Ar = 2,5- t Bu2(C6H3) | 362 |
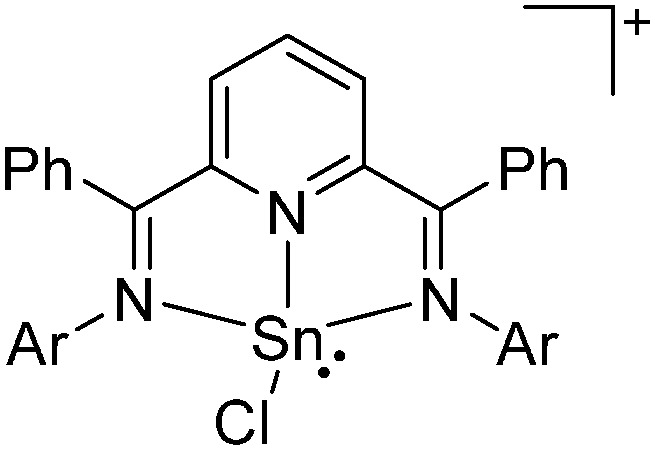
|
[SnCl3]– | Ion |
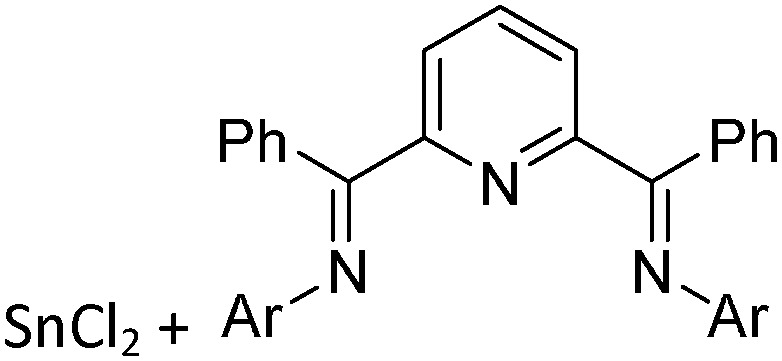
|
Ar = 2,5- t Bu2(C6H3) and 2,6-Me2(C6H3) | 362 |
|
[Al(ORPF)4]– | Salt |
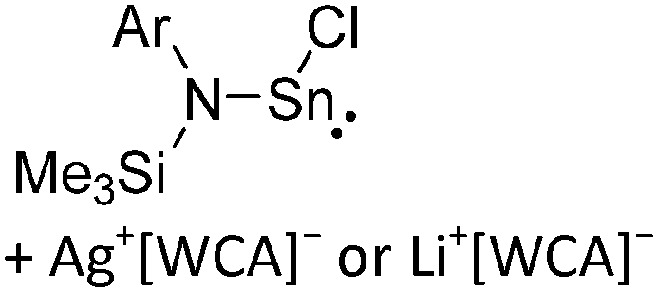
|
366 | |
|
[Al(ORPF)4]– | Lig |
|
366 | |
|
[MeB(C6F5)3]– | Lewis |
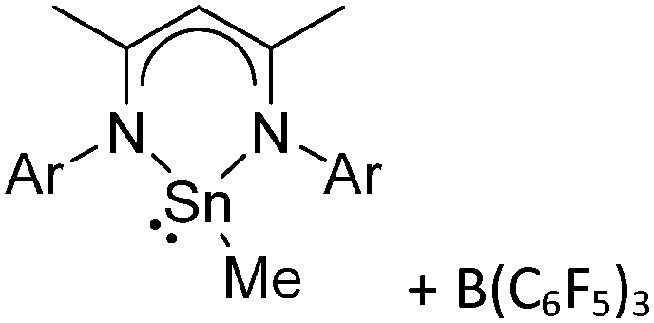
|
367 | |
|
[SbF6]– | Salt |
|
368 | |
|
[B(Ph)4]– | Salt |
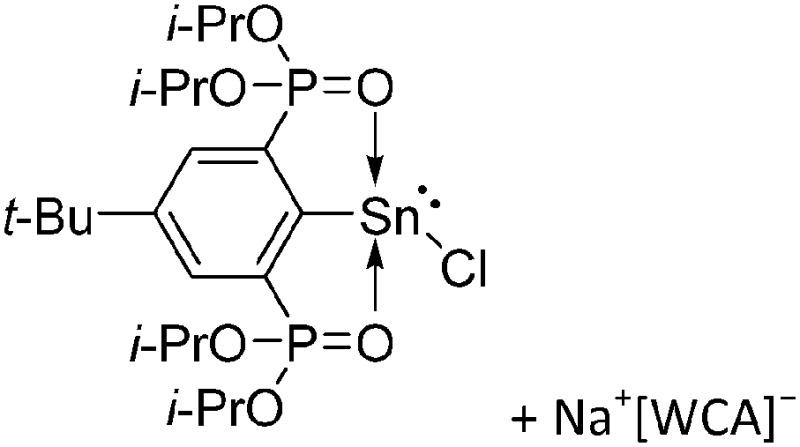
|
345 | |
|
[B(ArCF3 )4]– | Salt |
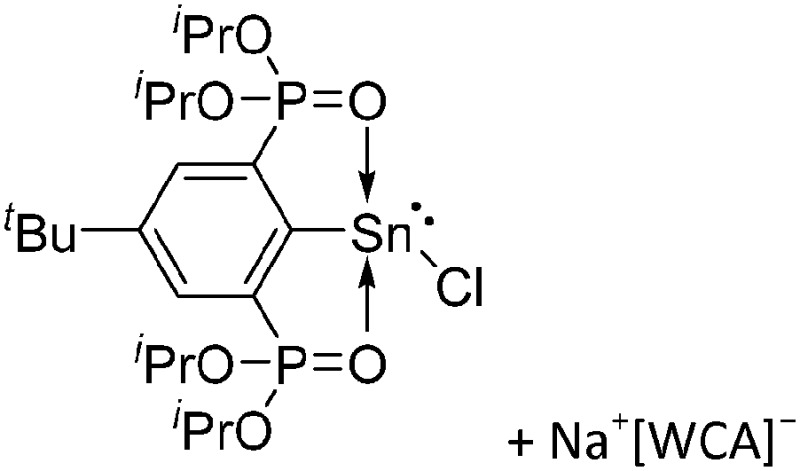
|
345 | |
|
[B(C6F5)4]– | Other | (Tipp)2Sn + [Et3Si(C7H8)]+[WCA]– | 371 | |
| [Pb(NO3)([12]-crown-4)2]+ | [Pb(NO3)3([12]crown-4)]– | Ion | Pb(NO3)2 + [12]crown-4 | 351 | |
| [Pb([15]-crown-5)2]2+ | [Pb(NO3)3([15]crown-5)]– | Ion | Pb(NO3)2 + 2[15]crown-5 | 351 | |
| [Pb(benzo-[15]-crown-5)2]2+ | [Pb(NO3)3(benzo-[15]crown-5)]– | Ion | Pb(NO3)2 + benzo-[15]crown-5 | 351 | |
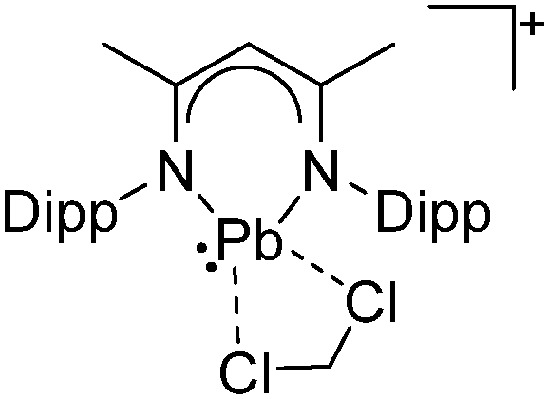
|
[B(C6F5)4]– | Salt |
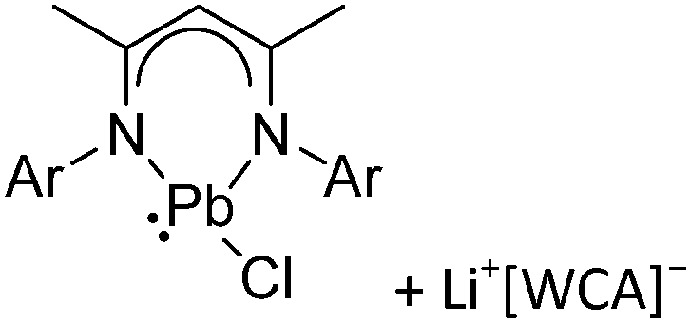
|
367 | |
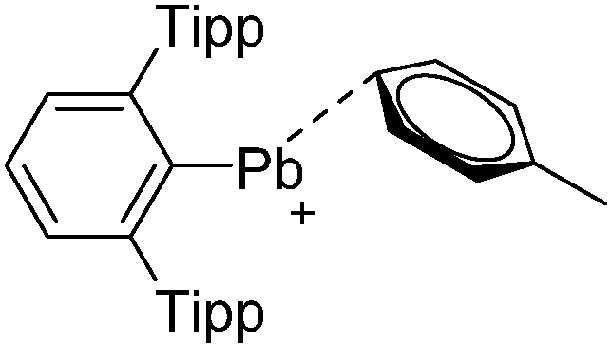
|
[B(C6F5)3(CH3)]– | Lewis |
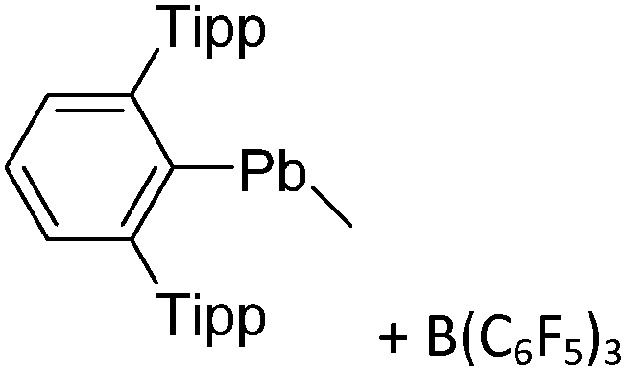
|
370 | |
| Cyclopentadienyl substituted cations | |||||
|
[B(C6F5)4]– | Prot | (Me5C5)2Si + [Me5C5H2]+[WCA]– | 322 | |
|
[Al(ORPF)4]– | Prot | (Me5C5)(iPr5C5)Si + [H(OEt2)2]+[WCA]– | 323 | |
|
[B(C6F5)4]– | Com | [(Me5C5)Si]+[WCA]– + dme | 324 | |
|
[B(C6F5)4]– | Com | [(Me5C5)Si]+[WCA]– + [12]crown-4 | 324 | |
|
[BF4]– | Prot | (C5Me5)2Ge + H(WCA) | 376 | |
|
[SnCl3]– | Lewis | (C5Me5)GeCl + SnCl2 | 377 | |
|
[BF4]– | Prot | (C5Me5)2Ge + H(WCA) | 373 | |
|
[B(C6F5)4]– | Salt | (C5Me5)SnCl + Li+[WCA]– | 375 | |
|
[B(C6F5)4]– | Salt | (C5Me5)PbCl + Li+[WCA]– | 375 | |
|
[Ga(C6F5)4]– | Other | (C5Me5)2Sn + Ga(C6F5)3 | 378 | |
|
[B(C6F5)4]– | Com | [(C5Me5)Sn]+[WCA]– + (C5Me5)2Sn | 375 | |
|
[B(C6F5)4]– | Com | [(C5Me5)Pb]+[WCA]– + (C5Me5)2Pb | 375 | |
| Ion-like compounds | |||||
| Me2(B12Cl12) | Salt | [Li]2 +[WCA]– + 2.2MeF + 2.6AsF5 | 1 : 1 mixture with [Li]2 +[WCA]– | 288 | |
| (Me2CH)(HCB11Me5Br6) | Lewis | (H3C)2CHCl + (H3C)(HCB11H5Br6) | 287 | ||
| Me2CF(AsF6) | Lewis | (H3C)2CF2 + AsF5 | 268 | ||
| (m-CF3-C6H4)(Ph)CF(AsF6) | Lewis | C6H5CF3 + AsF5 | 268 | ||
| Me3Si(HCB11F11) | Hyd | Me3SiH + [Ph3C]+[WCA]– | 326 | ||
| Me3Si(C2H5CB11F11) | Hyd | Me3SiH + [Ph3C]+[WCA]– | 326 | ||
| Me3Si(FAl(ORPF)3) | Other | Ag+[Al(ORPF)4]– + Me3SiCl or AlEt3 + 3HORPF + Me3SiF | 327 | ||
| Et3Si(HCB11H5Br6) | Hyd | Et3SiH + [Ph3C]+[WCA]– | 328 | ||
| Et3Si(HCB11Cl11) | Hyd | Et3SiH + [Ph3C]+[WCA]– | 81 | ||
| iPr3Si(HCB11H5Br6) | Hyd | iPr3SiH + [Ph3C]+[WCA]– | 325 | ||
| iPr3Si(HCB11H5Cl6) | Hyd | iPr3SiH + [Ph3C]+[WCA]– | 329 | ||
| iPr3Si(HCB11H5I6) | Hyd | iPr3SiH + [Ph3C]+[WCA]– | 329 | ||
| iPr3Si(HCB9H4Br5) | Hyd | iPr3SiH + [Ph3C]+[WCA]– | 297 | ||
| t Bu3Si(HCB11H5Br6) | Hyd | t Bu3SiH + [Ph3C]+[WCA]– | 328 | ||
| t Bu3Si(FAl(ORPF)3) | Other | t Bu3SiI + 15 ( t Bu)3SiF + Ag+[Al(ORPF)4]– | 330 | ||
| t Bu2MeSi(HCB11H5Br6) | Hyd | t Bu2MeSiH + [Ph3C]+[WCA]– | 328 | ||
| Fc3Si(OTf) | Prot |
|
331 | ||
| Et3Ge(HCB11H5Br6) | Hyd | Et3GeH + [Ph3C]+[WCA]– | 338 | ||
| Et3Sn(HCB11H5Br6) | Lewis | Et3SnCl + (Et)3Si(WCA) | 338 | ||
| n Bu3Sn(CB11Me12) | Ox | n Bu6Sn2 + 2 CB11Me12 | 333 | ||
| Et3Pb(HCB11H5Br6) | Lewis | Et3PbCl + (Et)3Si(WCA) | 338 | ||
| Transition-metal substituted cations | |||||
|
[B(C6F5)4]– | Salt |
|
380 | |
|
[PF6]– | Salt |
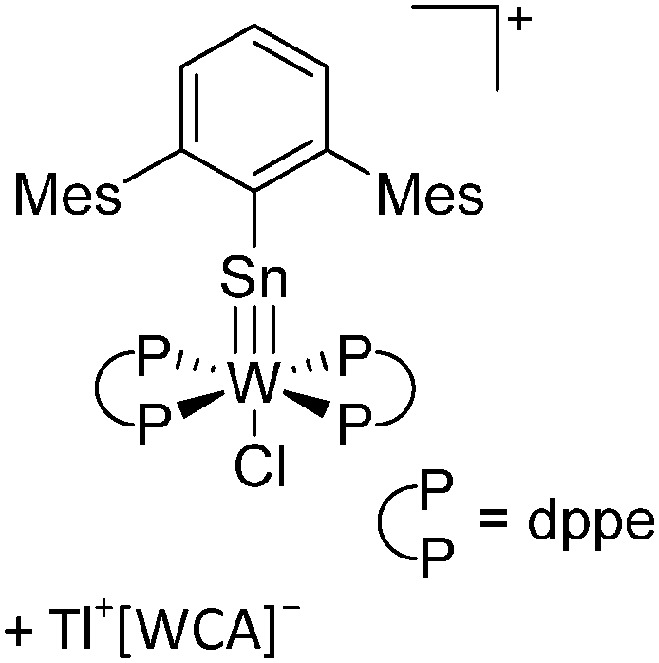
|
379 | |
|
[BF4]– | Com |
|
381 | |
|
Salt |
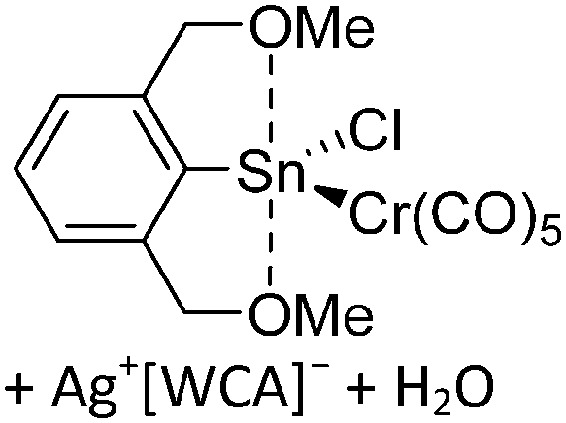
|
382 | ||
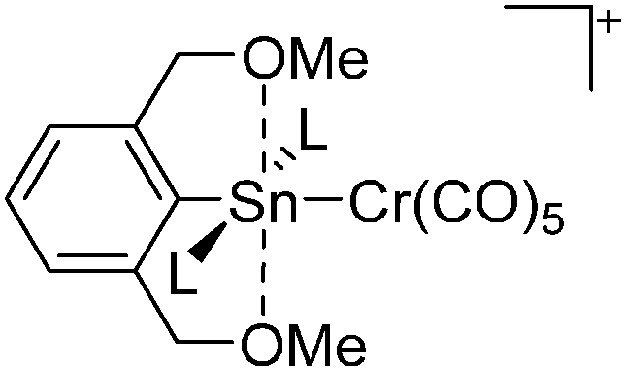
|
[CB11H12]– | Salt |
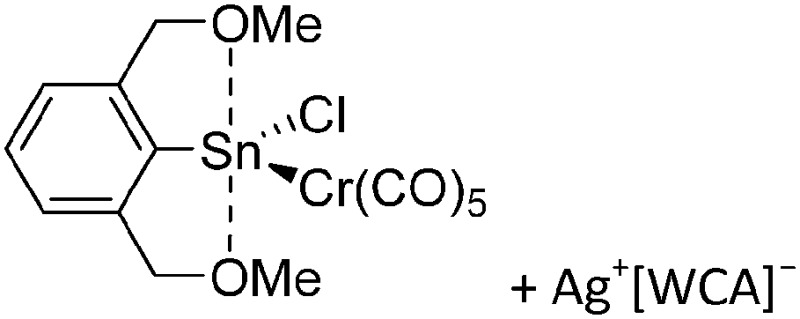
|
L = THF | 382 |

|
[ClO4]– | Ion |
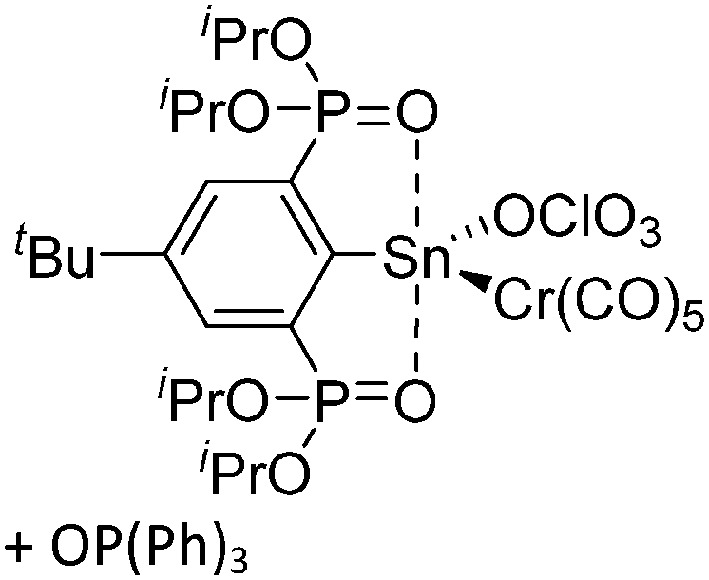
|
383 | |
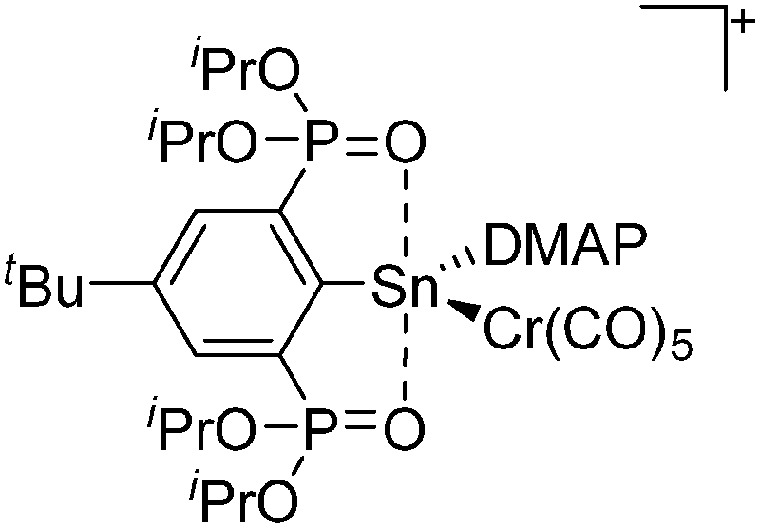
|
[ClO4]– | Ion |
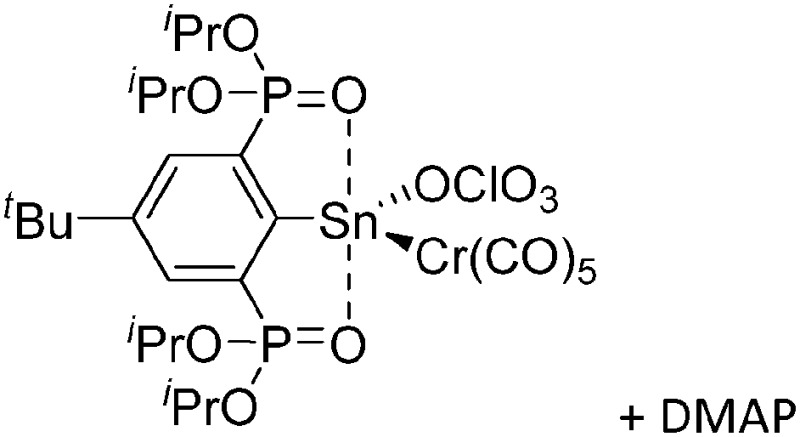
|
383 | |
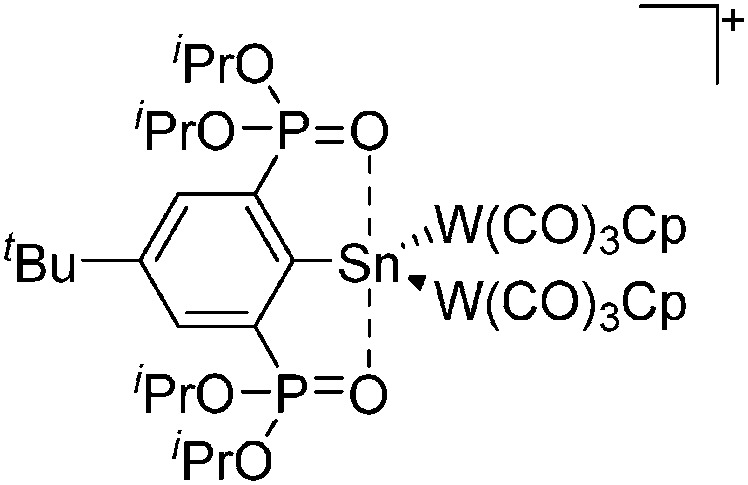
|
[W(CO)3Cp]– | Prot |
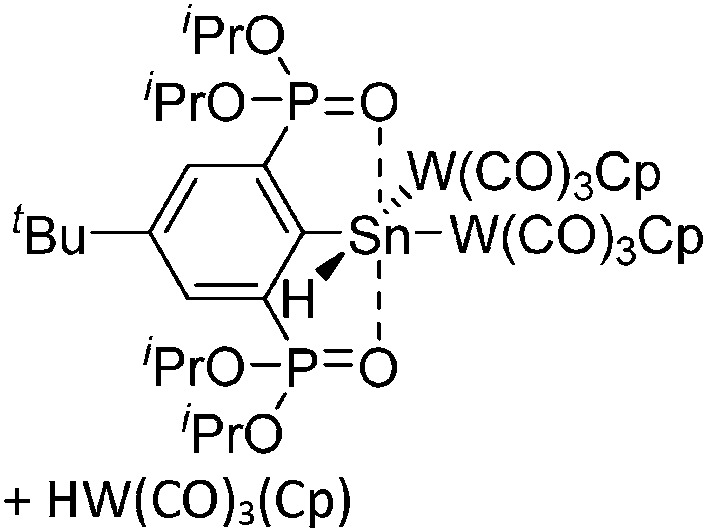
|
384 | |
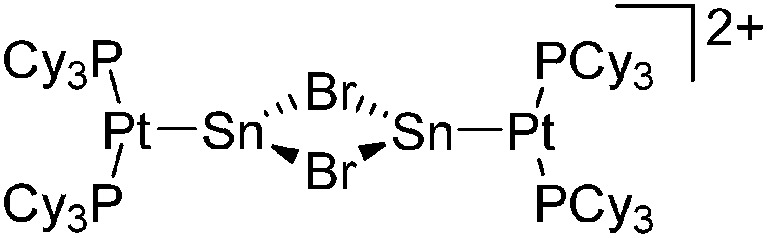
|
[AlBr4]– | Lewis |
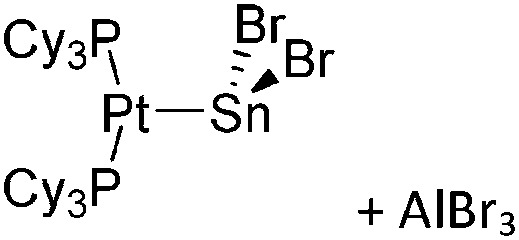
|
385 | |
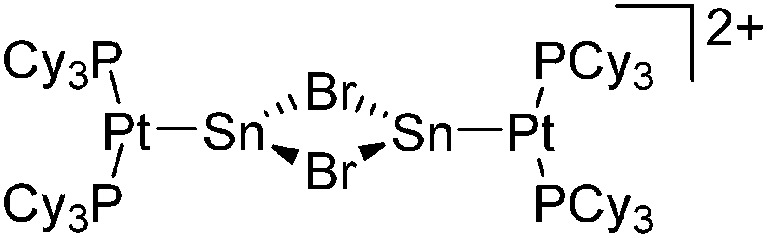
|
[B(ArCl)4]– | Salt |
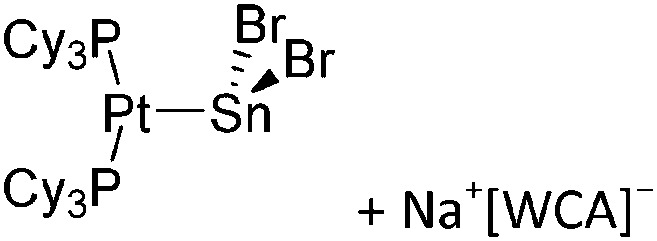
|
385 | |
| {(Cy3P)2Pt}2Sn(AlBr4)2 | Lewis |
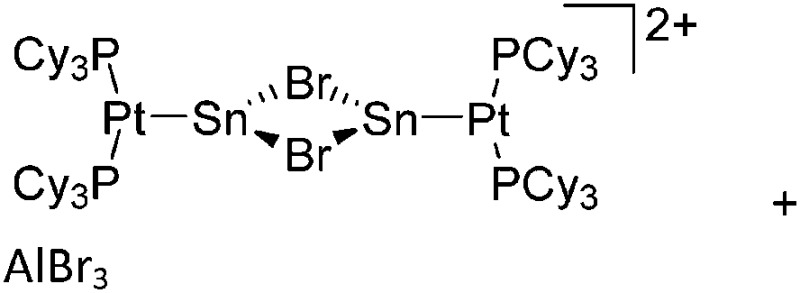
|
385 | ||
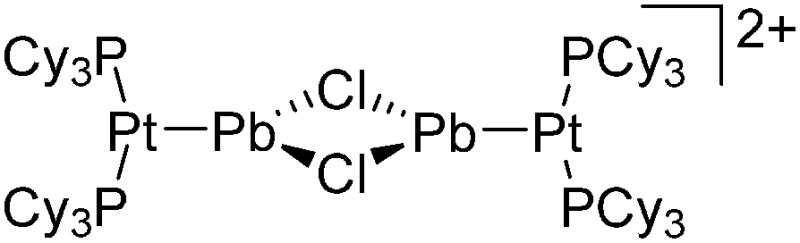
|
[AlCl4]– | Lewis |
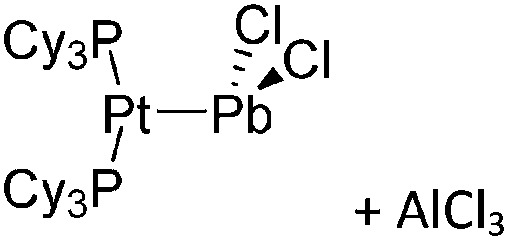
|
385 | |
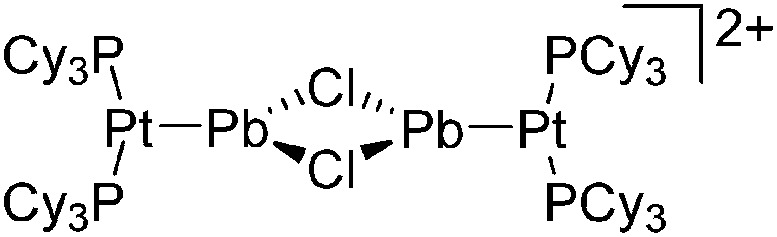
|
[B(ArCl)4]– | Salt |
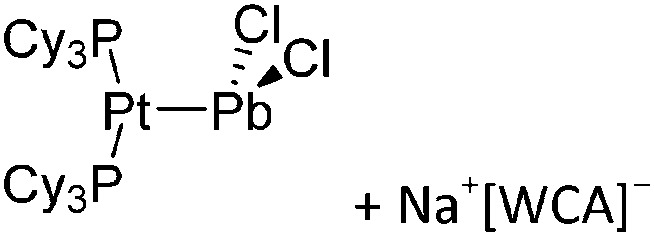
|
385 | |
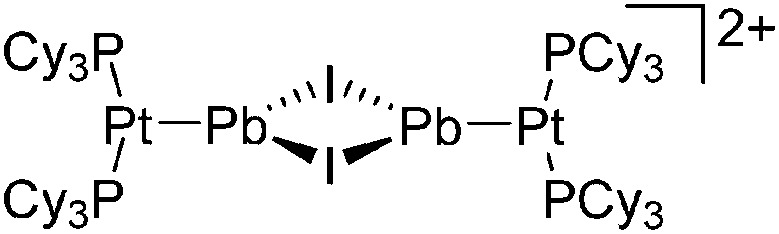
|
[AlCl4]– | Salt |
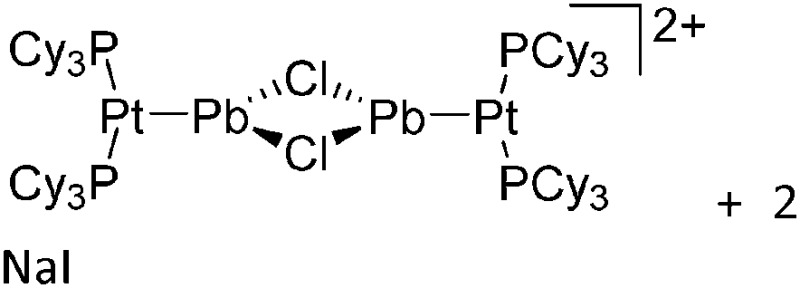
|
385 | |
| {(Cy3P)2Pt}2Pb(AlCl4)2 | Lewis |
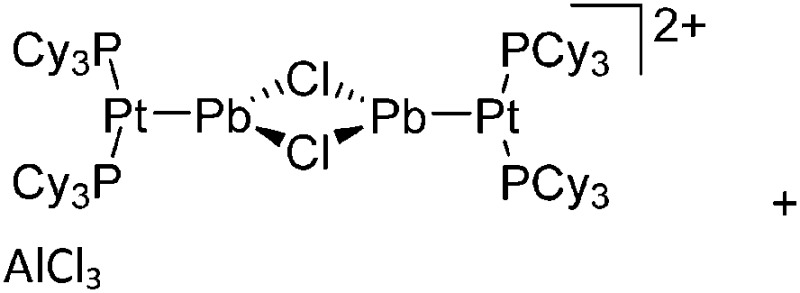
|
385 | ||
The autoionization reaction used for the preparation of many of the crown ether complexes has also been applied to synthesize most of the structurally characterized [(L)EX]+[WCA]– compounds of germanium and tin (Fig. 55). 344,359–362,389 With even stronger donating ligands, comparable salts [(L)GeCl]+Cl– were prepared. 363,364 These compounds are strongly stabilized so that even halides are sufficient as anions (Fig. 56). Related [RE(L)]+ cations with the residue R not being an halogen atom are also known. In these cations, the residue is capable to stabilize the cationic center by an additional σ- or π-donation (Fig. 57). 80,345,365–368 In case of bulky residues it was possible to work without an additional ligand and to obtain the free [RE]+ cations (Fig. 57). 365,366,369 For lead, one additional [RE(L)]+ cation is known with R being a bulky aryl ligand and with a toluene molecule coordinating to the lead atom. 370
Fig. 55. Ligand-stabilized cations [(L)EX]+ of germanium and tin.
Fig. 56. Ligand-stabilized cations [(L)GeCl]+ with chloride as their counterion.
Fig. 57. Structurally characterized [RE(L)]+ and [RE]+ cations of germanium, tin and lead.
A rather special case is [Sn(C7H8)3]2+, in which a tin(ii) cation is coordinated by three toluene molecules. 371 Although lots of arene complexes of tin(ii) are known, almost all of them do still have strong interactions to the anions, mostly halides and/or [AlCl4]– (see for some examples ref. 19). An exception is the Sn(ii) complex with [2.2.2]paracyclophane. 372 Only one of the two [AlCl4]– ions is coordinated to the tin atom, the other one does not have interactions with the cation. However, [Sn(C7H8)3]2+([B(C6F5)4]–)2 is the first example of a tin(ii) complex with independent arenes and without additional stabilization by the anion (Fig. 58).
Fig. 58. Molecular structure of [Sn(C7H8)3]2+. A. Schäfer, F. Winter, W. Saak, D. Haase, R. Pöttgen and T. Müller, Chem. – Eur. J, 2011, 17, 10979–10984. Data from this reference were used to draw this figure.
Cyclopentadienyl substituted cations
The tin analog of the [(C5Me5)Si]+ cation was already published in 1979, 373 about 25 years before the silicon compound was characterized by XRD. This is due to the fact, that [(C5Me5)Sn]+ could be synthesized as its [BF4]– salt, which is not possible in case of [(C5Me5)Si]+ because of its instantaneous decomposition. 374 In [(C5Me5)Sn][BF4] are still some stronger interactions present between the fluorine atoms and tin. In 2005, the structure of the [(C5Me5)Sn][B(C6F5)4] was determined in which these interactions are a lot weaker 375 and with the same anion, [(C5Me5)Pb]+ was synthesized and structurally characterized. 375 The sole exception is germanium, whose [(C5Me5)Ge]+ was only characterized by XRD with [BF4]– 376 and [SnCl3]– 377 as its counterion and not with any larger WCA. In addition, interesting triple-decker cations are known for tin and lead: [({Me5C5}Sn)2(μ-Me5C5)]+ was first synthesized and structurally characterized with the [Ga(C6F5)4]– anion, 378 its structure was subsequently published with [B(C6F5)4]– together with the analogous lead compound (Fig. 59). 375
Fig. 59. Structurally characterized cyclopentadienyl substituted cations of germanium, tin and lead.
Ion-like compounds of germanium, tin and lead
As for silicon, alkyl substituted enium ions of the heavier group 14 elements without any additional ligand need stabilizing interactions with the WCA. However, far less examples are known for E = Ge, Sn and Pb, although already in 2000, the first example was published with n Bu3Sn(CB11Me12). 333 The other known examples are the Et3E(HCB11H5Br6) compounds already mentioned before. 338
Transition-metal substituted cations of germanium, tin and lead
Other than for silicon, more transition metal coordinated cations are known for the heavier elements of group 14, especially for tin. Via a salt elimination reaction, the complex cation [(dppe)2W Sn-C6H3-2,6-Mes2]+ was synthesized with [PF6]– as its counterion in which the W–Sn–C angle is close to 180°. 379 A similar germanium compound was published one year after, in 2004. In [(MeCN)(dppe)2W Ge-(η1-Cp*)]+, the germanium is substituted by a Cp* and the tungsten atom is coordinated additionally by an acetonitrile molecule. 380 As WCA serves [B(C6F5)4]– in this case. A new complex cation featuring a Sn–Pt bond was published in 2010. In trans-[Pt(Me)(SnCl2)(2-PyPPh2)2][BF4], the tin atom is pentacoordinated and adopts a trigonal-bipyramidal geometry. 381 Along with that, comparable compounds were synthesized with the remaining group 10 metals, but no crystal structure determination was performed. By using an OCO-pincer ligand, a chromiumpentacorbonyl coordinated tin(ii) cation was synthesized. Two variants were published, {2,6-(MeOCH2)2C6H3}(H2O)SnCr(CO)5(OTf), in which the tin is coordinated additionally by a water molecule and [{2,6-(MeOCH2)2C6H3}(THF2)SnCr(CO)5]+[CB11H12]–, in which the tin is hexacoordinated with two THF molecules complementing the coordination sphere. 382 The former has indeed no contact between the triflate and the tin atom, but a strong hydrogen bond between the coordinated water molecule and the anion is existing, with an O–O distance of about 261 pm. Two more chromiumpentacorbonyl coordinated tin(ii) cations were published in 2013, both also with a pincer-type ligand (Fig. 60). 383 The same ligand was used to prepare the [RSn{W(CO)3Cp}2]+, with R = R = 4- t Bu-2,6-{P(O)(OiPr)2}2C6H2) and [W(CO)3Cp]– as its counterion. 384 Recently, new platinum-coordinated cations of tin and lead were published. Starting from (Cy3P)2Pt(SnBr2), [{(Cy3P)2Pt-SnBr}2]+ was synthesized with two different anions. 385 The analogous dimeric lead cation [{(Cy3P)2Pt-PbCl}2]+ was accessible by using (Cy3P)2Pt(PbCl2) as a starting material. 385 Through further reaction with AlX3, {(Cy3P)2Pt}2Sn(AlBr4)2 respectively {(Cy3P)2Pt}2Pb(AlCl4)2 were synthesized. 385 However, in both dications some interactions between the ions are present. Additionally, the dimeric lead cation was also synthesized with iodine instead of chlorine. 385
Fig. 60. Structurally characterized transition metal substituted cations of germanium, tin and lead.
Group 15 cations
Of all the pnictogen elements, especially phosphorus has a rich cation chemistry. The analogy between CR4 and [PR4]+ displays the possibility of creating a large variety of cationic phosphorus frameworks (Fig. 61).
Fig. 61. Analogy between cationic phosphorus atom and carbon.
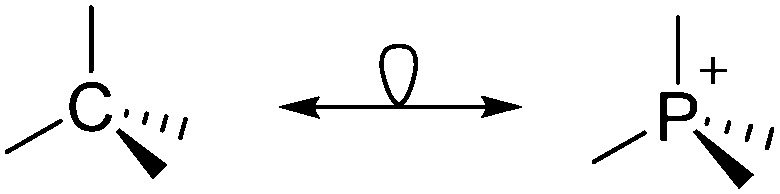
Over the last decades a multitude of catenated phosphorus cations were synthesized. The classical phosphino-phosphonium cation (Fig. 62, left), which can be synthesized through halide abstraction from PR2Cl and formal insertion/coordination of the resulting [PR2]+ (see section “Oxidation state +III” below) into a R2P–R bond/to PR3 stands for an entire substance class of compounds typically containing organic residues R. 390 However, we refer the interested reader to the multitude of recent reviews especially on these cations, 391,392 the analogous interpnictogen cations (Fig. 63) 393 and other types of cationic pnictogen compounds (Table 5).
Fig. 62. Examples for catenated phosphorus cations in the formal phosphino-phosphonium or diphosphonium form. Also cyclic versions are available.
Fig. 63. Example for a catenated interpnictogen cation.
Homopolyatomic cations
Except for the long-known bismuth cations, all homopolyatomic pnictogen cations were synthesized in the last 16 years. In late 1999 the third all-nitrogen molecule [N5]+ – besides N2 and N3 – – was prepared through a reaction of [N2F]+[AsF6]– and HN3. 400 The obtained compound [N5]+[AsF6]– is explosive but an anion exchange led to the more stable [N5]+[SbF6]– and to the crystal structure of [N5]+[Sb2F11]–. 401 In 2004 the reduction of SbCl3 with [Ga]+[GaCl4]– in a GaCl3–benzene solution led to the square-antiprismatic arachno-[Sb8]2+ Wade cluster cation. 402 Recently also the first – formally electron precise and Zintl type – phosphorus cation [P9]+ was synthesized through the reaction of P4 and the nitrosyl salt of the [Al(ORPF)4]– WCA. 29 By contrast, bismuth has a rich cation chemistry. The first structure of a bismuth cation was measured already in 1962. Most of them were synthesized through high temperature solid state reactions. The newer room temperature approaches are based on ionic liquids. 403 Normally the clusters formed are badly soluble, but there is evidence that the use of very weakly coordinating anions like [Al(ORPF)4]– can lead to [Bi n ]+ clusters, which are soluble in solvents like CH2Cl2 or SO2. 404 Besides the lighter noble gases and fluorine, only arsenic has still no homopolyatomic cation. Yellow arsenic (As4), which has now a relatively stable storage form (see section “Metal–pnictogen complexes”) might be a good starting point for a future synthesis (Fig. 64).
Fig. 64. Examples for homopolyatomic pnictogen cations (only for bismuth, several entries are known).
Metal–pnictogen complexes
There are still only a few complexes with pnictogen modifications as ligands in the literature. Early examples of tetrahedro-P4 complexes like (PPh3)2RhICl(η2-P4) are better viewed as phosphide complex (PPh3)2RhIII(P4 2–). By contrast, the d10-metal cation Ag+ is ideal for the stabilization of the non-metallic clusters and the electronic structure of the ligand stays relatively unaffected (see also chapter chalcogen cations). In 2001, the WCA [Al(ORPF)4]– made it possible to crystallize the [Ag(P4)2]+ complex and later through salt metathesis with CuI also the copper complex [Cu(P4)2]+ was accessible. In 2012 the gold complex was obtained as [GaCl4]– salt and completed the whole series [M(P4)2]+ (M = Cu, Ag, Au). Recently light-stable (!) [Ag(As4)2]+[Al(ORPF)4]– was synthesized, which finally serves as a good storage form of yellow arsenic (As4). As such, As4 is both thermally and photochemically unstable. The salt made it possible to transfer the As4 tetrahedron to gold in [Ph3PAu(As4)]+ and opens new possibilities in the synthesis of arsenic complexes (Fig. 65). 405
Fig. 65. Molecular structure of [Ag(As4)2]+[Al(ORPF)4]–. C. Schwarzmaier, M. Sierka, M. Scheer, Angew. Chem., Int. Ed., 2013, 52, 858–861. C. Schwarzmaier, M. Sierka and M. Scheer, Angew. Chem., 2013, 125, 891–894. Data from this reference were used to draw this figure. The disorder of the anion was omitted for clarity.
Diazonium cations and heavier homologues
There is a multitude of crystal structures of different cluster or cluster-like cations that contain pnictogen atoms. 391–393,397 We decided to give an overview and to list parent (model)-compounds like [N2Ph]+ and [N2Mes]+ in case of diazonium cations 406 as examples for the entire diazonium substance classes. The heavier homologues of the diazonium cations [RNPn]+ (Pn = P, As) need sterically demanding groups like Mes* (2,4,6- t Bu3C6H2) to protect the highly reactive triple bonds (Fig. 66). 407,408
Fig. 66. The diazonium cation and its heavier homologues. R e.g. Mes*.
Cluster and cage cations
The reaction of phosphorus halides PX3 with halide abstractors led to very reactive carbene-analogous [PX2]+ cations (see chapter “Phosphenium ions”), which are able to formally insert in the X2P–X bonds of a second equivalent to form [P2X5]+ clusters or in the P–P bond of white phosphorus to produce [P5X2]+ for instance. Insertion in P4S3 or halide abstraction from P4S3I2 followed by rearrangements led to the phosphorus–sulphur cluster cations [P5S2X2]+ 134 and [P7S6I2]+. 234 Ref. 134 contains investigations on the nature of this formal insertion reaction, which is not as simple as thought and rather follows a concerted, orbital controlled mechanism (Fig. 67).
Fig. 67. Typical examples for phosphorus cations.
The binary group 15 and 16 cations have also a strong tendency to form clusters. The newer examples like the antimony-chalcogen cations [Sb10Se10]2+ and [Sb7Te8]5+ were synthesized in ionic liquids or GaCl3 melts. 409,410 Very recently [P3Se4]+, the first binary P–Se-cation was characterized by six different groups with three different approaches. 411 It is accessible from solution, but also through solid state syntheses. 412 In 2004 the synthesis of the sulphur- and selenium-bismuth cations from a chloroaluminate melt completed the series of the heterocubane cluster cations [Bi4Ch4]4+ (Ch = S, Se, Te) (Fig. 68). 413,414
Fig. 68. Examples for binary pnictogen containing cations.
(4n + 2)π-cations
The pnictogen cations with planar delocalized π-systems can be described as (pseudo-)aromatic systems. The four-membered rings were all synthesized through halide abstraction from the neutral rings with two halogen atoms. The five-membered As–N ring was prepared through cycloaddition of the highly reactive [AsNMes*]+, which reacts as dienophile with the 1,3 dipole tritylazide N3CPh3 (Fig. 69).
Fig. 69. Heteroatomic, cationic aromatic 6π-systems containing pnictogen atoms.
π*–π*-complexes
Like the chalcogen compounds, the pnictogen cations containing π*–π*-interactions can be described as dimers of chalcogen radicals, whose half-occupied interacting orbitals have π*-character. The interannular π*–π*-bonds between the “monomers” are relatively weak. They were synthesized through halide abstraction from the chlorides of the monomers (Fig. 70). 415
Fig. 70. Arsenic cations containing π*–π*-interactions.
Radical cations
Radical cations of pnictogens can be obtained through direct oxidation of Pn2 fragments with stabilizing ligands like N-heterocyclic carbenes (NHC) or cyclic alkylaminocarbenes (CAAC). As one-electron-oxidants the trityl salt of [B(C6F5)4]– was used (Fig. 71). 208,416,417
Fig. 71. Cationic phosphorus radicals stabilized by NHCs or CAACs.
Bulky arylphosphines and -diphosphines (Fig. 72) can also be oxidized to their radical cations, if the cation is stabilized by a WCA. 418,419
Fig. 72. Phosphorus radical cations.
The delocalization of the single electron over a ring system also leads to stabilization. The four-membered radical cation ring systems with different pnictogen atoms (Fig. 73) were obtained through direct oxidation with silver and nitrosyl salts of WCAs. 420
Fig. 73. Cyclic pnictogen radical cations.
Formal oxidation state +I
In some compounds like [P3Ph6]+ 421 the oxidation state of the central pnictogen “P+” can be described as +I, which is for example supported by the unusual high field shift in 31P-NMR of the central phosphorus atom in these cations (–210 to –270 ppm). 422 In case of the ligand-stabilized arsenic cation [AsDppDIMPY]+ this is also supported by the synthesis: (DppDIMPY = [α,α′-{2,6-iPr2PhNC(Me)}2(C5H3N)]). The reduction of AsCl3 with SnCl2 led to a cation with a planar carbenoid-like structure. 423 Under the same conditions with a different ligand an arsa-carbenoid of type [As(NR)2C2H2]+ was obtained (Fig. 78). This displays the difficulty of a clear assignment of oxidation states in such systems (Fig. 74).
Fig. 78. Examples for pnictogen carbenoids.
Fig. 74. Phosphorus and arsenic cations in formal oxidation state +I.
Phosphenium ions (oxidation state +III)
The chemistry of the highly reactive phosphenium ions [P(R/Y)2]+ (Fig. 75) was part of many studies in the past. The stability increases with the π-donor-ability of the substituent and the Lewis acidity with a stronger negative inductive effect (FIA: [P(NH2)2]+ < [PCl(NH2)]+ < [PCl2]+). 424
Fig. 75. Structurally characterized simple phosphenium ions.
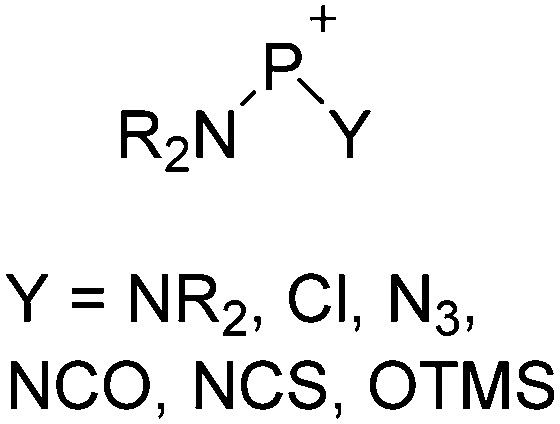
For most of the reactive phosphorus cations, the decomposition is normally accompanied by the formation of strong P–X bonds (X = F, Cl…). This makes it necessary to use weakly coordinating anions stable against electrophilic cations. For the homoleptic halogen substituted cations, extremely weak anions are needed. The first examples of the less, but still highly reactive mixed amino-halogen substituted phosphenium cations were published already in 1976. Through the use of a halide-abstractor (MCl3, M = Al, Ga, Fe) is was possible to prepare [P(NR2)Cl]+ (R = Me, Et, iPr) (X = [AlCl4]–) but no crystallographic data was obtained. It was not until 2012 that the first crystal structure of a halogen and a pseudohalogen mono-substituted phosphenium cation was determined. The structures of [P(NR2)X]+ (R = TMS; X = Cl, N3, NCO, NCS) and (R = iPr; X = Cl, N3) were determined by scXRD. All cations were stabilized with the [GaCl4]– anion. Especially the azidophosphenium compound turned out to be a versatile starting material for further chemistry, and made it possible to derive more complex phosphor-centered cations like iminophosphorane-substituted-phosphonium salts [iPr2NPNP(Cl)2NR2]+[GaCl4]– [R = iPr, SiMe3] (Fig. 76) – for instance through the reaction with the corresponding chlorophosphane R2NPCl2.
Fig. 76. Iminophosphorane-substituted phosphorus cation.
Miscellaneous cations in oxidation state +III
There are also some examples of the heavier homologues in oxidation state +III (Fig. 77). They were typically synthesized through halide abstraction with Lewis acids.
Fig. 77. Pnictogen cations with pnictogen atoms in formal oxidation state +III.
Another example of ligand-stabilized pnictogen cations are the N-heterocyclic carbenoid rings [Pn(NR)2C2H2]2+ (Pn = P, As, Sb), which are formally 1,4-diaza-1,3-butadiene complexes of a pnictogen cation in oxidation state +III, but the delocalization of the positive charge supports also a description as a neutral pnictogen atom. In case of the 1,3,2-diazaphospholidinium rings [Pn(NR)2C2H4]+ (Pn = P, As) the double bond between C4 and C5 is missing.
Reactive pnictonium cations (oxidation state +V)
The halo-pnictonium cations [PnX4]+ with pnictogen atoms in oxidation state +V (Fig. 79), have a very different presence in the literature. For phosphorus, all four cations (X = F, Cl, Br, I) have been synthesized but for [PF4]+ no crystal structure was determined. A multitude of structures of [PX4]+ with different anions was characterized, but only a few of [AsX4]+ and [SbX4]+ are known. The cations are normally prepared from PnX3, X2 and a Lewis acid.
Fig. 79. Classical halo-pnictonium cations in oxidation state +V.
But there are also some newer, highly oxidized cations in the literature: the formal [PnPh3]2+ cations (Fig. 80), which have a strong contact to the anion, serve as useful starting materials for further coordination chemistry of PnV compounds. 425 The ligand stabilized formal “PO+” cations were prepared through the oxidation of a phosphorus carbenoid (Fig. 80, see also the phosphorus carbenoids above) with the amine-N-oxides Me3NO and pyO. 426 The charge of the carbene-stabilized formal “[PFPh2]2+”, which was prepared from the carbene-stabilized “[PF2Ph2]+” through fluoride abstraction is likely partially localised on the strongly bound ligand (Fig. 80). 427
Fig. 80. Examples for recent pnictogen cations in oxidation state +V.
Protonated cations
With the super acidic system HF/MF5 (M = As, Sb) it is possible to protonate hydrazoic or phosphoric acid for instance and obtain the aminodiazonium [H2N3]+ and phosphatacidium (tetrahydroxyphosphonium) cation [P(OH)4]+ (Table 10). The structure determinations of the [SbF6]– salts revealed the structures of the cations (Fig. 81). 428
Table 10. Overview on selected and structurally characterized pnictogen cations.
| Cation | Anion | Class. a | Synthesis | Comment b | Ref. |
| Homopolyatomic cations | |||||
| [N5]+ | [Sb2F11]– | Other | [N2F]+[SbF6]– + HN3 in aHF | 401 | |
| [P9]+ | [Al(ORPF)4]– | Ox | P4 + [NO]+[Al(OC(CF3)3)4]– | 2.5 equiv. of P4, no X-ray | 29 |
| [Sb8]2+ | [GaCl4]– | Lewis | SbCl3 + Ga+[GaCl4]– in GaCl3/C6H6 | 402 | |
| [Bi2]4+·[Bi9]5+ | [Ag3Bi3Br15]3–·6[Br]– | Lewis, Ox | Bi + BiBr3 + Ag | HTS (350 °C) | 429 |
| 2[Bi5]+·[Bi6]2+ | 2[IrBi6Br12]–·[IrBi6Br13]2– | Lewis, Ox | Bi + Ir + BiBr3 | HTS (1000 °C) | 430 |
| [Bi5]3+ | [AlCl4]– | Lewis, Ox | Bi + BiCl3 + AlCl3 | HTS | 431 |
| [Bi5]3+ | [AlCl4]– | Lewis, Ox | Bi + BiCl3 + [BMIM]Cl/AlCl3 | Ionic liquid based synthesis | 403 |
| [Bi5]3+ | [AlX4]– (X = Br, I) | Lewis, Ox | Bi + BiX3 + AlX3 | HTS (490 °C (I), 520 °C (Br)) | 432 |
| [Bi5]3+·2SO2 | [AsF6]– | Lewis, Ox | Bi + AsF5 in SO2 | No X-ray | 196 |
| [Bi8]2+ | [AlCl4]– | Lewis, Ox | Bi + BiCl3 + AlCl3 | HTS | 433 |
| [Bi8]2+ | [Ta2O2Br7]– | Lewis, Ox | Bi + BiBr3 + TaBr5 | HTS (570 °C), traces of H2O | 434 |
| [Bi9]5+ | 4[BiCl5]2–·[Bi2Cl8]2– | Lewis, Ox | Bi + BiCl3 | HTS (325 °C), Bi6Cl7 | 435 and 236 |
| [Bi9]5+ | [Bi]+·3[HfCl6]2– | Lewis, Ox | Bi + BiCl3 + HfCl4 | HTS | 436 |
| [Bi9]5+ | [Bi]+·3[NbCl6]2– | Lewis, Ox | Bi + BiCl3 + NbCl5 | HTS (550 °C), Nb(v) to Nb(iv) reduction | 434 |
| [Bi9]5+ | [Sn7Br24]10– | Lewis, Ox | Bi + BiBr3 + Sn | HTS (250 °C), C 4v symmetric | 437 |
| Metal–nonmetal-cluster complexes | |||||
| [Cu(P4)2]+ | [Al(ORPF)4]– | Com | CuI + [Ag]+[Al(ORPF)4]– + P4 | 438 | |
| [Cu(P4)]+ | [GaCl4]– | Com | CuCl + GaCl3 + P4 | 439 | |
| [Ag(P4)2]+ | [Al(ORPF)4]– | Com | Ag+[Al(ORPF)4]– + P4 | 72 | |
| [Ag(P4)]+ | [GaCl4]– | Com | AgCl + GaCl3 + P4 | 439 | |
| [Au(P4)2]+ | [GaCl4]– | Com | AuCl + GaCl3 + P4 | 439 | |
| [Cp*M(dppe)(P4)]+ (M = Fe, Ru) | [BPh4]– | Salt | [Cp*M(dppe)Cl] + P4 + Na+[BPh4]– | 440 | |
| [CpOs(PPh3)2(P4)]+ | [OTf]– | Salt | [CpOs(PPh3)2Cl] + Ag+[OTf]– | 441 | |
| [{CpRu(PPh3)2}2(P4)]2+ | [OTf]– | Com, salt | [CpRu(PPh3)2Cl] + P4 + Ag+[OTf]– | 442 | |
| [{CpRu(PPh3)2}-{CpOs(PPh3)2}(P4)]2+ | [OTf]– | Com | [CpOs(PPh3)2(P4)]+ + [{CpRu(PPh3)2}]+ | Bridging end-on/end-on | 441 |
| [Ag(As4)2]+ | [Al(ORPF)4]– | Com | Ag+[Al(ORPF)4]– + As4 | 405 | |
| [AuPPh3(As4)]+ | [Al(ORPF)4]– | Com, salt | [Ag(As4)2]+[Al(ORPF)4]– + AuPPh3Cl | 405 | |
| [Cp*Ru-(dppe)(As4)]+ | [Al(ORPF)4]– | Com, salt | [Ag(As4)2]+[Al(ORPF)4]– + Cp*Ru-(dppe)Cl | 443 | |
| [Ag(P4S3) n ]+ (n = 1, 2) | [Al(ORPF)4]– | Com | Ag+[Al(ORPF)4]– + P4S3 | 444 | |
| [Ag2(P4S3)6]+ | [Al(ORPF)4]– | Com | Ag+[Al(ORPF)4]– + P4S3 | 170 | |
| [{CpRu(PPh3)2}2(P4S3)]2+ | [OTf]– | Com, salt | [CpRu(PPh3)2Cl] + P4S3 + Ag+[OTf]– | 442 | |
| Clusters, cluster-like and catenated cations | |||||
| [N2Ph]+ | [BF4]– | Other | PhNH2 + NaNO2 in HCl(aq) and Na+[BF4]– | N–N triple bond | 445 |
|
[BF4]– | Other | H2NC6(CF3)5 + [NO]+[BF4]– | 446 | |
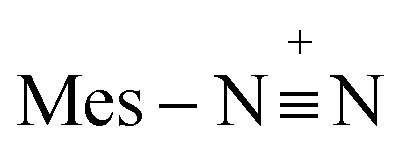
|
[OsO2(NO3)2(Mes)]– | Other | [N2Mes]+[NO3]– + [OsO2(NO3)(Mes)]+ | 447 | |
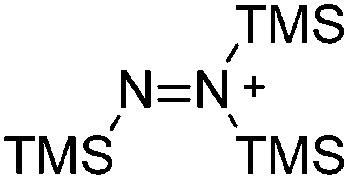
|
[GaCl4]– | Other | Hg(N2(TMS)3)2 + Ag+[GaCl4]– or Bi(N2(TMS)3)2 + GaCl3 + Cl2 | LTS (–80 °C) | 448 |
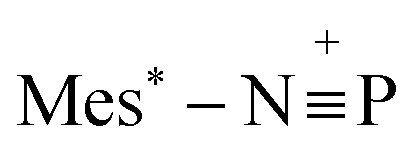
|
[AlCl4]– | Lewis | Mes*-NPCl + AlCl3 | N–P triple bond | 407 |
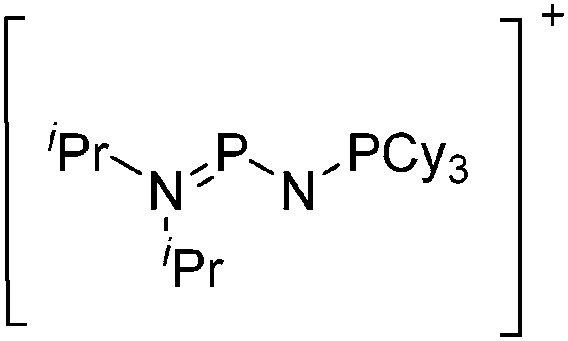
|
[GaCl4]– | Other | [P(NiPr2)N3]+[GaCl4]– + PCy3 | 449 | |
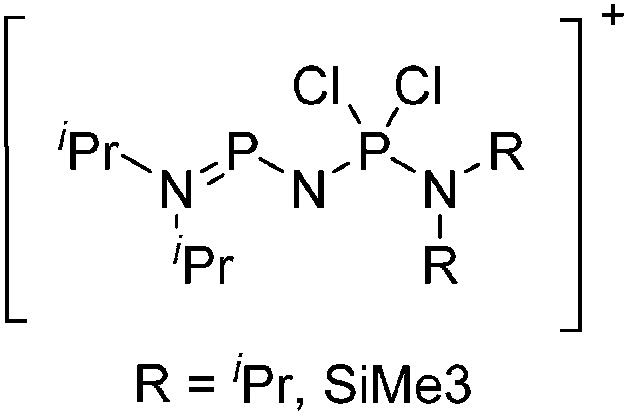
|
[GaCl4]– | Other | [P(NiPr2)N3]+[GaCl4]– + P(NR2)Cl2 | N2 as leaving group | 449 |
|
[SbF6]– | Salt | [( t BuIM)NPCl(C(PPh3)2)]+[Cl]– + Ag+[SbF6]– | Dicationic iminophosphorane | 450 |
| [P2Ph5]+ | [OTf]– | Lewis | Ph2PCl + TMSOTf + PPh3 | First homoleptic phosphine-phosphonium | 451 |
| [P2Me6]2+ | [OTf]– | Lewis | P2Me4 + MeOTf | 452 | |
| [P2Br5]+ | [Al(ORPF)4]– | Salt | PBr3 + Ag+[Al(ORPF)4]– | 453 | |
| [P2I5]+ | AlI4 – | Lewis | PI3 + AlI3 | 454 | |
| [P2I5]+ | [Al(ORPF)4]– | Salt | PI3 + Ag+[Al(ORPF)4]– | 453 | |
|
[AlCl4]– | Lewis | PPh3 + PCl3 + AlCl3 | [P(PPh3)2]+ | 421 |
|
[OTf]– | Lewis | P2Me4 + PMe2Cl + TMSOTf | [(PMe2)3]+ | 455 |
|
[AlCl4]– | Prot | [P(PPh3)2]+[AlCl4]– + AlCl3 + HCl | 456 | |
| [P3I6]+ | [F(Al(ORPF)3)2]– | Salt | P4 + I2 + Ag+[Al(ORPF)4]– | [(PI2)3]+ | 457 |
| [PMe(AsMe3)2]+ | [OTf]– | Other | MePCl2 + AsMe3 + TMSOTf | [PRL2]+ | 458 |
| [P4Ph8]2+ | [OTf]– | Lewis | PPhCl2 + PPh3 + TMSOTf | 459 | |
| [P4NO]+ | [Al(ORPF)4]– | Other | P4 + [NO]+[Al(ORPF)4]– | Insertion in P4, no X-ray | 78 |
|
[GaCl4]– | Lewis | ClP(PMes*)2PCl + GaCl3 | Bicyclic phosphine-phosphonium | 460 |
| [(PPh)2(PnPh3)2]2+ (Pn = As, Sb) | [AlCl4]– | Lewis | PPhCl2 + PnPh3 + AlCl3 | 461 | |
| [P5Br2]+ | [Al(ORPF)4]– | Lewis, other | P4 + PBr3 + Ag+[Al(ORPF)4]– | Insertion in P4 | 453 and 462 |
| [P5Ph2]+, [P7Ph6]3+ | [GaCl4]– | Lewis, other | P4 + Ph2PCl + GaCl3 | Different stoichiometries, insertion in P4, HTS (60–70 °C) | 68 |
| [P5RCl]+ (R = Me, Et, iPr, Cy, Ph, C6F5) | [GaCl4]– | Lewis, other | P4 + RPCl2 + GaCl3 | Insertion in P4 | 463 |
| [P5R2]+ (R = Me, Et, iPr, Cy, Mes, Dipp) | [GaCl4]– | Lewis, other | P4 + R2PCl + GaCl3 | Insertion in P4 | 464 |
| [P5(NCy2)Cl]+ | [GaCl4]– | Lewis, other | P4 + P(NCy2)Cl2 + GaCl3 | Insertion in P4 | 465 |
|
[Al(ORPF)4]– | Salt, other | P4S3 + PX3 + Ag+[Al(ORPF)4]– | Initial insertion in P4S3 | 134 |
|
[AlCl4]– | Other | [P4(AsPh3)2]2+([AlCl4]–)2 + PPh3 | Ligand exchange | 466 |
|
[AlCl4]– | Other | AsPh3 + PCl3 + AlCl3 | 466 | |
|
[Al(ORPF)4]– | Salt | P4S3I2 + Ag+[Al(ORPF)4]– | 234 | |
|
[OTf]– | Other | (AsPh3)(OTf)2 + PCl3 + Ph3As | 467 | |
|
[AlCl4]– | Lewis, other | P(red) + Se + SeCl4 + BMImCl/AlCl3 | Ionic liquid based synthesis, rhombohedral and orthorhombic modification | 412 |
| [P3Se4]+ | [AlCl4]– | Lewis, other | P3Se4 + Me5C6Br + AlCl3 | In solution, orthorhombic modification | 412 |
| [P3Se4]+ | [Ga2Cl7]– | Lewis, other | PCl3 + SeTMS2 + GaCl3 | In solution | 412 |
| [P5Se2Ph2]+ | [GaCl4]– | Ox | [P5Ph2]+[GaCl4]– + Se | HTS (160 °C) | 464 |
| [P5Se2Cy2]+ | [GaCl4]– | Ox | [P5Cy2]+ + GaCl3 + Se | HTS (140 °C) | 464 |
| Mes*–N As | [GaCl4]– | Lewis | Mes*NAsCl + GaCl3 | N–As triple bond | 408 |
| [As3N3Ph3Cl2]+ | [GaCl4]– | Lewis | N2As2Ph2Cl2 + GaCl3 | As3N3 ring | 468 |
| [As2Me6]2+ | [OTf]– | Other | Me3As + PCl3 + TMSOTf | 458 | |
| [As2Ph6]2+ | [AlCl4]– | Ox | AsPh3 + PCl3 + AlCl3 | 466 | |
|
[AsF6]– | Ox | As4S4 + AsF5 in SO2 | 469 | |
| [As3S4]+ | [SbF6]– | Ox | As4S4 + SbF5 in SO2 | 469 | |
|
[AlCl4]– | Ox, Lewis | As + AsCl3 + S + AlCl3 | HTS (80 °C) | 470 |
|
[SbF6]– | Ox | As + Se + SbF5 in SO2 | 469 | |
| [As3Se4]+ | [AlCl4]– | Ox, Lewis | As + AsCl3 + Se + AlCl3 | HTS (80 °C) | 470 |
| ([Sb2Se2]+) n | [AlCl4]– | Ox | Sb + Se + BMImCl/AlCl3 | Ionothermal, HTS (160 °C) | 67 |
|
[AlCl4]– | Ox, others | Sb + Se + SeCl4 + BMImCl/AlCl3 | Ionic liquid based synthesis | 409 |
| ([Sb2Te2]+) n | [AlCl4]– | Ox, Lewis | Sb + Te + SbCl3 + NaCl + AlCl3 | HTS (130 °C), polymeric | 15 |
| [Sb7Te8]5+ | 3[Ga2Cl7]–·2[GaCl4]–/2[Ga2Cl7]–·3[GaCl4]– | Ox, Lewis | Sb + Te + SbCl3 + GaCl3 | AlCl3/GaCl3 melt | 410 |
|
[MCl4]– (M = Al, Ga) | Ox, Lewis | Sb + Te + SbCl3 + MCl3 + NaCl | AlCl3/GaCl3 melt | 410 |
| [Bi2X4]2+ (X = Cl, Br) | [AlX4]– | Lewis | BiX3 + AlX3 | 471 | |
| [Bi4OF2Cl6(C6Me6)4]2+ | [Al(ORPF)4]– | Other | [Bi5]+[AsF6]– + Li+[Al(ORPF)4]– + C6Me6 | Evidence for soluble [Bi n ] x+ salts, partial decomposition of anion and solvent | 404 |
|
[AlCl4]– | Ox, Lewis | Bi + BiCl3 + Ch in AlCl3/NaCl | HTS (130 °C), heterocubane, series Bi4Ch4 4+ (Ch = S, Se, Te) complete | 413 |
|
[AlCl4]– | Ox, Lewis | Bi + BiCl3 + Te in AlCl3/NaCl | HTS (130 °C), heterocubane | 414 |
| [PdBi10]4+ | ([BiBr4]–)∞ | Ox | Bi2Pd + Bi + Br2 | HTS (1000 °C) Pd@[Bi10]4+ | 472 |
| (4n + 2)π-cations | |||||
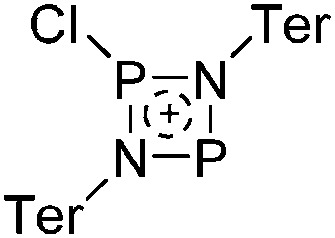
|
[GaCl4]– | Lewis | ClP(μ-NTer)2PCl + GaCl3 | P2N2 ring | 473 |
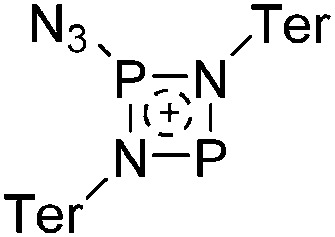
|
[N3(GaCl3)2]– | Other | [ClP(μ-NTer)2P]+[GaCl4]– + TMSN3 + GaCl3 | P2N2 ring | 473 |
|
[GaCl4]– | Other | [Mes*-NAs]+[GaCl4]– + Ph3CN3 | Cycloaddition | 408 |
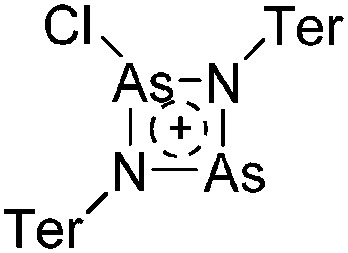
|
[GaCl4]– | Lewis | ClAs(μ-NTer)2AsCl + GaCl3 | As2N2 ring | 474 |
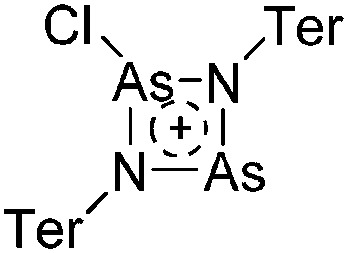
|
[OTf]– | Salt | ClAs(μ-NTer)2AsCl + Ag+[OTf]– | As2N2 ring | 474 |
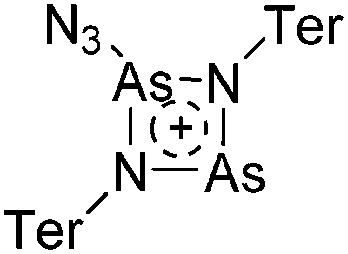
|
[N3(GaCl3)2]– | Lewis | [ClAs(μ-NTer)2As]+[GaCl4]– + TMSN3 + GaCl3 | As2N2 ring | 474 |
| [As(μ-NTer)2As]2+ | [OTf]– | Salt | ClAs(μ-NTer)2AsCl + Ag+[OTf]– | As2N2 ring, 4π system, 2 equiv. of Ag+[OTf]– | 474 |
| [ClSb(μ-NTer)2Sb]+ | [GaCl4]– | Lewis | ClSb(μ-NTer)2SbCl + GaCl3 | Sb2N2 ring | 475 |
| [Sb(μ-NTer)2Sb]2+ | [OTf]– | Salt | ClSb(μ-NTer)2SbCl + Ag+[OTf]– | Sb2N2 ring, 4π system, 2 equiv. of Ag+[OTf]– | 475 |
| [IBi(μ-NTer)2Bi]+ | [B(C6F5)4]– | Salt | IBi(μ-NTer)2BiI + [Ag(Tol)3]+[B(C6F5)4]– | Bi2N2 ring | 475 |
| [Bi(μ-NTer)2Bi]2+ | [OTf]– | Salt | ClBi(μ-NTer)2BiCl + Ag+[OTf]– | Bi2N2 ring, 4π system, 2 equiv. of Ag+[OTf]– | 475 |
| π*–π*-complexes | |||||
|
[GaCl4]– | Lewis | AsS2(CH)2Cl + GaCl3 | 415 | |
|
[MCl4]– (M = Al, Ga) | Lewis | As(NMe)2(CH)2Cl + MCl3 | 415 | |
| Radicals | |||||
|
[B(C6F5)4]– | Ox | [P(CN(Dipp)C10H18)N(C(N(Dipp))2C2H2)] + [CPh3]+[B(C6F5)4]– | CN(Dipp)C10H18 CAAC, cyclic alkylaminocarbene | 416 |
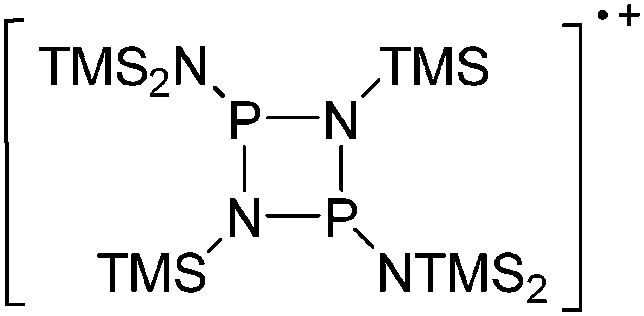
|
[SbF6]– | Ox | [(NTMS)2(PNTMS2)2] + [NO]+[SbF6]– | 420 | |
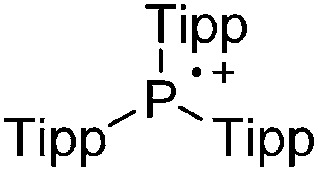
|
[X]– = [SbF6]–, [Al(ORPF)4]– | Ox | PTipp3 + Ag+[X]– | 418 | |
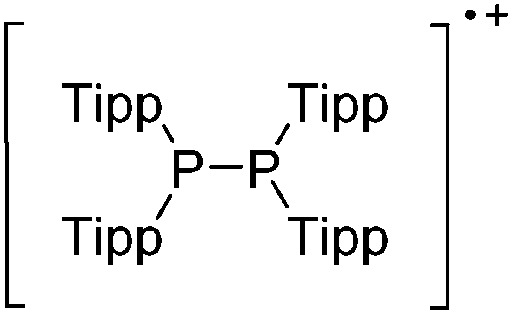
|
[Al(ORHT)4]– | Ox | P2Tipp4 + Ag+[Al(ORHT)4]– | 419 | |
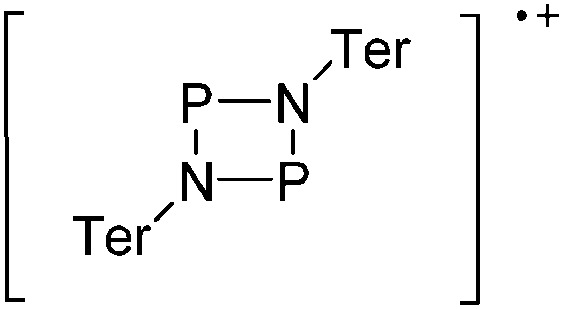
|
[B(C6F5)4]– | Ox | [P2(NTer)2] + [Ag(Tol)3]+[B(C6F5)4]– | 476 | |
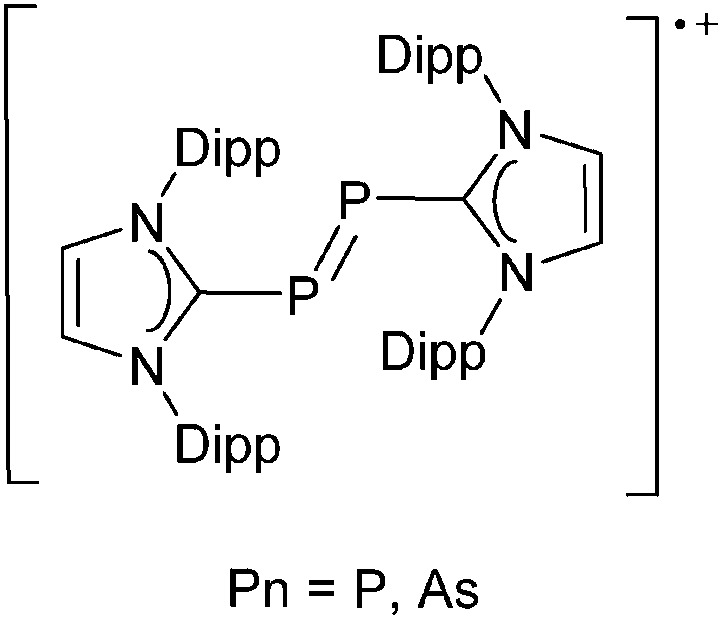
|
[B(C6F5)4]– | Ox | [P2(C(N(Dipp))2C2H2)2] + [CPh3]+[B(C6F5)4]– | C(N(Dipp))2C2H2 NHC, N-heterocyclic carbene | 208 |
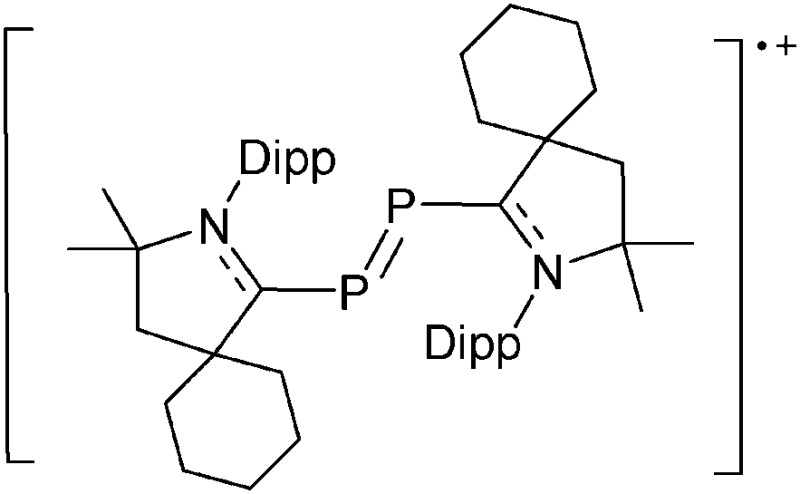
|
[B(C6F5)4]– | Ox | [P2(CN(Dipp)C10H18)2] + [CPh3]+[B(C6F5)4]– | NC10H18 = tetramethylamide | 477 |
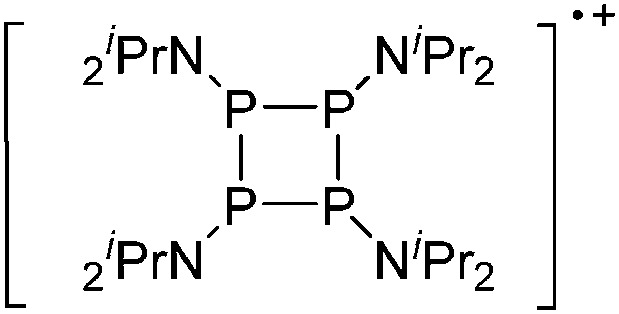
|
[Al(ORPF)4]– | Ox | [P4(NiPr2)4] + [NO]+[BF4]– + Li+[Al(ORPF)4]– | 420 | |
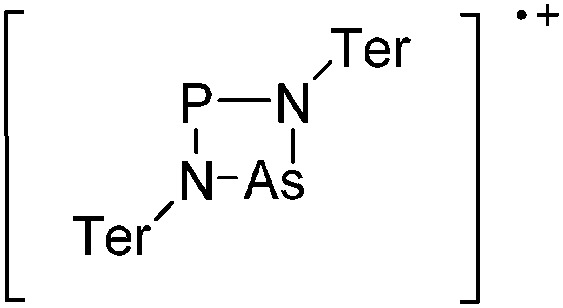
|
[B(C6F5)4]– | Ox | [AsP(NTer)2] + [Ag(Tol)3]+[B(C6F5)4]– | 476 | |
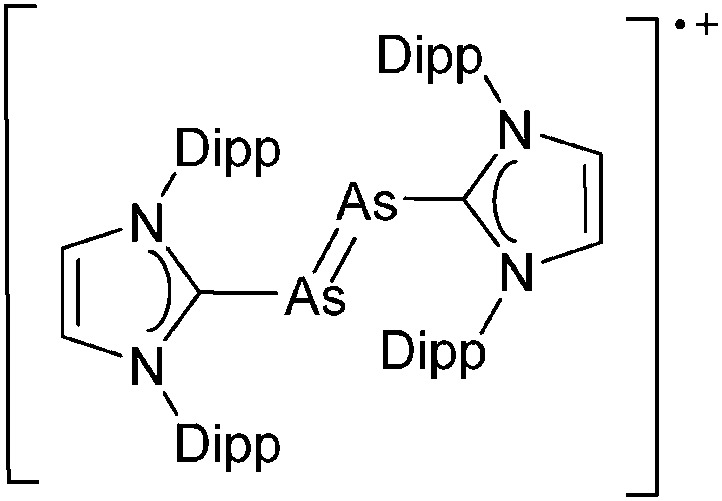
|
[GaCl4]– | Ox | [As2(C(N(Dipp))2C2H2)2] + GaCl3 | 417 | |
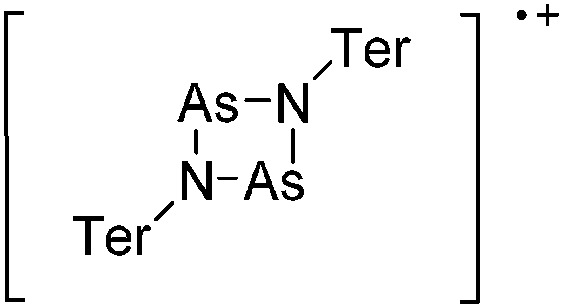
|
[B(C6F5)4]– | Ox | [As2(NTer)2] + [Ag(Tol)3]+[B(C6F5)4]– | 476 | |
| Oxidation state +I | |||||
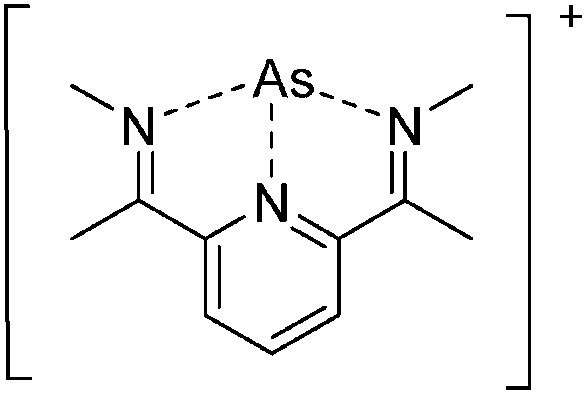
|
[SbCl5·THF]– | Lewis | AsCl3 + SnCl2 + DppDIMPY | [AsL3]+, DppDIMPY = [α,α′-{2,6-iPr2PhN-C(Me)}2(C5H3N)] | 423 |
| Oxidation state +III | |||||
| [P(NiPr2)2]+ | [GaCl4]– | Lewis | P(NiPr2)2Cl + GaCl3 | [PX2]+ | 478 and 479 |
| [P(NCy2)Cl]+ | [GaCl4]– | Lewis | P(NCy2)Cl2 + GaCl3 | [PX2]+ | 465 |
| [P(NiPr2)Cl]+ | [GaCl4]– | Lewis | P(NiPr2)Cl2 + GaCl3 | [PX2]+ | 449 |
| [P(NTMS 2)Cl]+ | [GaCl4]– | Lewis | P(NTMS2)Cl2 + GaCl3 | [PX2]+ | 480 |
| [P(NiPr2)N3]+ | [GaCl4]– | Other | [P(NiPr2)Cl]+[GaCl4]– + TMSN3 | [PX2]+ | 449 |
| [P(NTMS2)X]+ (X = N3, NCO, NCS) | [GaCl4]– | Other | [P(NTMS2)Cl]+[GaCl4]– + TMSX | [PX2]+ | 480 and 481 |
| [P(NTMS2)OTMS]+ | [GaCl4]– | Other | [P(NTMS2)Cl]+[GaCl4]– + TMSCNO | [PX2]+ | 481 |
| [PCp*Cl]+ | [Cl(Al(ORPF)3)2]– | Lewis | PCp*Cl2 + PhF·Al(ORPF)3 | [PR2]+ | 482 |
| [PCp*2]+ | [Cl(Al(ORPF)3)2]– | Lewis | PCp*2Cl + PhF·Al(ORPF)3 | [PR2]+, phosphocenium ion | 482 |
| [AsCp*Cl]+ | [Cl(Al(ORPF)3]– | Lewis | AsCp*Cl2 + PhF·Al(ORPF)3 | [AsRX]+ | 482 |
| [SbCl2(AsMe3)]+ | [OTf]– | Lewis | SbCl3 + AsMe3 + TMSOTf | [SbX2L]+ | 483 |
| [SbPhCl(AsPh3)]+ | [AlCl4]– | Lewis | SbPhCl2 + AsPh3 + AlCl3 | [SbRXL]+ | 483 |
| [BiPh(AsPh3)]2+ | [OTf]– | Lewis | BiCl2Ph + AsPh3 + TMSOTf | [BiRL]2+ | 484 |
| [BiCl(SbPh3)]2+·C6H6 | [AlCl4]– | Lewis | BiCl3 + AsPh3 + AlCl3 | [BiXL]2+ | 484 |
| [BiCl2(AsPh3)2]+ | [OTf]– | Lewis | BiCl3 + AsPh3 + TMSOTf | [BiX2L2]+ | 483 |
| [BiCl2(SbPh3)2]+·C7H8 | [AlCl4]– | Lewis | BiCl3 + SbPh3 + AlCl3 | [BiX2L]+ | 484 |
| [Bi(N2TMS3)2]+ | [GaCl4]– | Lewis | Bi(N2TMS3)2Cl + GaCl3 | [BiX2]+ | 485 |
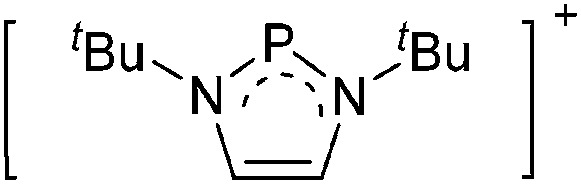
|
[BF4]– | Salt | PCl(N t Bu)2C2H2 + Ag+[BF4]– | P carbenoid | 486 |
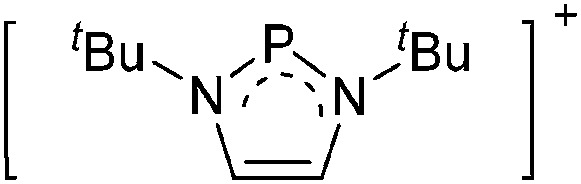
|
[PF6]– | Salt | PCl(N t Bu)2C2H2 + Ag+[PF6]– | P carbenoid | 487 |
|
[GaCl4]– | Lewis | PCl(NMe)2C2H4 + GaCl3 | P carbenoid | 479 |
|
[GeCl5]–·[Cl]– | Other | Ge(N t Bu)2C2H4 + PCl3 | P carbenoid | 488 |
|
[PF6]– | Salt | PCl(N t Bu)2C2H4 + Ag+[PF6]– | P carbenoid | 487 |
|
[GaCl4]– | Lewis | PCl(NDipp)2C2H4 + GaCl3 | P carbenoid | 426 |
|
[SbCl5·THF]– | Lewis | AsCl3 + SnCl2 + (MesN)2C2H2 | As carbenoid | 423 |
|
[GeCl5]–·[Cl]– | Other | Ge(N t Bu)2C2H4 + AsCl3 | As carbenoid | 488 |
|
[GaCl4]– | Lewis | As(iPrN)2C10H6Cl + GaCl3 | As carbenoid | 489 |
|
[AlCl4]– | Lewis | AsCl(HN)SC6H4 + AlCl3 | As carbenoid | 490 |
|
[AlCl4]– | Lewis | AsClS2C6H3CH3 + AlCl3 | As carbenoid | 490 |
|
[GaCl4]– | Lewis | AsCl(NMe)2C3H6 + GaCl3 | As carbenoid | 491 |
|
[OTf]– | Other | Sb(iPrN)2C10H6(NMe2) + HOTf | Not planar through the ligand NHMe2 | 489 |
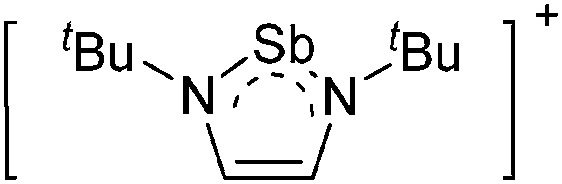
|
[Sb2Cl8]2– | Lewis | [Sb( t BuN)2C2H2]+ + SbCl3 | Sb carbenoid | 492 |
| Oxidation state +V | |||||
| [NF4]+ | [BF4]–, [SbF6]–, [Sb2F11]– | Lewis | NF3 + F2 + BF3 or SbF5 | 493 | |
| [PF4]+ | [Sb3F16]– | Lewis | PF5 + SbF5 | No X-ray | 494 |
| [PCl4]+ | [SnCl6]2– | Lewis | PCl5 + SnCl4 | For more structures see ref. 495 | 496 |
| [PBr4]+ | [Al(ORPF)4]– | Salt | PBr3 + Br2 + Ag+[Al(ORPF)4]– | 453 | |
| [PI4]+ | [AlCl4]– | Lewis | PI3 + ICl + AlCl3 in CS2 | 495 | |
| [PI4]+ | [AlBr4]– | Lewis | PI3 + IBr + AlBr3 in CS2 | 495 | |
| [PI4]+ | [AlI4]– | Lewis | PI3 + I2 + AlI3 in CS2 | 497 | |
| [PI4]+ | [GaI4]– | Lewis | PI3 + I2 + GaI3 in CS2 | 495 | |
| [PI4]+ | [Al(ORPF)4]– | Salt | PI3 + I2 + Ag+[Al(ORPF)4]– | 453 | |
| [AsCl4]+ | [AsF6]– | Lewis, Ox | AsCl3 + Cl2 + AsF5 | 498 | |
| [AsCl4]+ | [As(OTeF5)6]– | Lewis, Ox | AsCl3 + ClOTeF5 + As(OTeF5)5 | 499 | |
| [AsBr4]+ | [Al(ORPF)4]– | Salt | AsBr3 + Br2 + Ag+[Al(ORPF)4]– | 500 | |
| [AsBr4]+ | [FAs(OTeF5)5]– | Lewis, Ox | AsBr3 + BrOTeF5 + AsF(OTeF5)4 | 499 | |
| [SbCl4]+ | [Sb2F11]– | Lewis | SbCl5 + SbF5 | 501 | |
| [SbCl4]+ | [Sb(OTeF5)6]– | Ox | Sb(OTeF5)3 + Cl2 | 502 | |
| [SbBr4]+ | [Sb(OTeF5)6]– | Ox | Sb(OTeF5)3 + Br2 | 502 | |
|
[GaCl4]– | Ox | [(CH2)2(NDipp)2P]+ + OL | “PO+” cation | 426 |
|
[B(C6F5)4]– | Lewis | [(SIMes)PF2Ph2]+ + [Et3Si(Tol)]+[B(C6F5)4]– | “PFPh2 2+” cation | 427 |
| [Ph3Pn]2+ (Pn = Sb, Bi) | [OTf]– | Salt | Ph3PnCl2 + Ag+[OTf]– | Strong contact to the anion | 425 |
| Protonated cations | |||||
| [H2N3]+ | [SbF6]– | Prot | HN3 + HF/SbF5 | 310 | |
| [P(OH)4]+ | [SbF6]– | Prot | H3PO4 + HF/SbF5 | 428 | |
a Classification according to the introduction: Lewis = Lewis acid halogen bond heterolysis, Ox = oxidation, Com = complexation reaction, Prot = protonation, other = all other reactions not classified.
b HTS = high temperature synthesis.
Fig. 81. Structures of [H2N3]+ and [P(OH)4]+ in their [SbF6]– salts.
Group 16 cations
Hundreds of chalcogen cations are known to the literature (see Table 6 for reviews). The relatively strong Ch–Ch– and Ch–X-single bonds (Ch = S, Se, Te; X = F, Cl, Br, I) led to a great diversity of reactive compounds, which include homo- and heteropolyatomic clusters, radical cations and a large number of different [ChX3]+ structures for instance. To avoid the formation of the more stable neutral compounds, weakly coordinating anions are needed to stabilize the reactive chalcogen cations.
Table 6. Review articles including cationic group 16 compounds.
| Year | Title | Ref. |
| 2000 | Recent advances in the understanding of the syntheses, structures, bonding and energetics of the homopolyatomic cations of groups 16 and 17 | 503 |
| 2003 | Homoatomic sulfur cations | 504 |
| 2004 | Cages and clusters of the chalcogens | 505 |
| 2006 | Synthesis, reactions and structures of telluronium salts | 506 |
| 2011 | Homo- and heteroatomic polycations of groups 15 and 16. Recent advances in synthesis and isolation using room temperature ionic liquids | 66 |
| 2013 | Catenated sulfur compounds | 507 |
| 2013 | Catenated compounds – group 16 (Se, Te) | 508 |
| 2013 | Recent advances in the syntheses of homopolyatomic cations of the non-metallic elements C, N, P, S, Cl, Br, I and Xe | 11 |
| 2013 | RCNSSS+: a novel class of stable sulfur rich radical cations | 509 |
| 2015 | Coordination chemistry of homoatomic ligands of bismuth, selenium and tellurium | 398 and 399 |
Homopolyatomic cations
The first observation of homopolyatomic cations were the colored solutions of elemental sulfur, selenium and tellurium in sulfuric acid in the 18th and 19th century. Over the next centuries, the nature of these solutions stayed unclear and it was not before the middle of the 20th century that the use of superacidic media and better analytical methods made it possible to characterize the responsible species. Since then, starting with [O2]+[PtF6]– in 1962, 510 the crystal structures of a multitude of different homopolyatomic chalcogen cations were measured in the last 50 years (Fig. 82). All of them have more or less weakly coordinating anions as counter ions. In some cases, cationic clusters with unusual bonding situations including trans-annular interactions and negative hyperconjugation were found that presented quite a challenge for theory (e.g. the [S8]2+ dication). 11,503,511 The cations were mostly synthesized under superacidic conditions or through solid state or solvothermal reactions at higher temperature. Either the elemental chalcogen is directly oxidized with strong oxidants like MF5 (M = As, Sb) or WCl6 or a combination of the elemental chalcogen, chalcogen halides ChX4 (e.g. SeCl4, TeBr4) and a strong Lewis acid undergo a synproportionation.
Fig. 82. Selected examples for homopolyatomic chalcogen cations.
Metal–chalcogen complexes
The use of very weakly coordinated metal salts M[WCA] (e.g. M+ = Cu+, Ag+) made it possible to obtain complexes with very weak ligands like the elemental modifications of the chalcogen elements. Stable, metastable and hitherto unknown modifications were prepared, for example [Cu(S12)(S8)]+, 512 [Cu2Se19]2+ (Fig. 83) 50 and [Ag2Se6]2+ (Fig. 84). 513 In all such complexes, extensive charge delocalization from the metal cation to the chalcogen ring took place as evidenced by cation–anion contacts as well as accompanying quantum chemical calculations.
Fig. 83. Molecular structure of [Cu2Se19]2+([Al(OC(CF3)3)4]–)2·C6F4H2. (a) J. Schaefer, A. Steffani, D. A. Plattner and I. Krossing, Angew. Chem., Int. Ed., 2012, 51, 6009–6012; Angew. Chem., 2012, 124, 6112–6115. Data from this reference were used to draw this figure.
Fig. 84. Molecular structure of [(SO2)2Ag2(Se6)]2+([Sb(OTeF5)6]–)2. D. Aris, J. Beck, A. Decken, I. Dionne, I. Krossing, J. Passmore, E. Rivard, F. Steden and X. Wang, Phosphorus, Sulfur Silicon Relat. Elem., 2004, 179, 859–863. Data from this reference were used to draw this figure. One anion of the formula unit and the disorder of the anion and SO2 molecules were omitted for clarity.
Clusters/cluster-like cations
Chalcogens have a strong tendency to form clusters. There are examples for chains, rings and cages with almost every combination of the groups 15 and 16 (Fig. 68 and 85). The clusters often have delocalized charges, positive and negative hyperconjugation, or show pseudo-aromaticity (Fig. 86) or π*–π*-interactions (Fig. 87). The clusters were often synthesized through direct oxidation of neutral clusters like S4N4 or mixtures of the elements (e.g. Se and Te) with strong oxidants like MF5 in SO2. [NS]+, a useful starting material for the syntheses of further rPBC, can be obtained by halide abstraction from trichlorocyclotrithiazene (NSCl)3 with [Ag]+[WCA]–. 514
Fig. 85. Selected examples for cationic chalcogen clusters or cluster-like structures.
Fig. 86. Heteroatomic cationic aromatic (6π) systems containing chalcogen atoms. (S4N3 + is a 10π system).
Fig. 87. Selected chalcogen cations containing different π*–π*-interactions.
(4n + 2)π-cations
Some planar cationic conjugated π-systems containing chalcogen atoms can be described as (pseudo-)aromatic systems. The four-membered rings are related to the homopolyatomic cations Ch4 2+ and were synthesized through direct oxidation of mixtures of the elemental chalcogens instead of the pure elements. The five-membered rings were obtained by cycloadditions of [NS]+ and [NS2]+ 515 or in case of the selenium containing rings with Se, [Se8]2+ or EtSeCl and [NS]+ as starting materials. 516–518 [S4N3]+, a 10π-system, was synthesized through the reaction of S4N4 with Se2Cl2 and is stabilized by the polymeric ([SeCl5]–)∞ anion. 519
π*–π*-complexes
The chalcogen cations containing π*–π*-interactions can be described as dimers of chalcogen radicals, whose half-occupied interacting orbitals have π*-character. The two [Ch2I4]2+ cations (Ch = S, Se) have a very similar structure, but include different orbital interactions. In case of [S2I4]2+ two 2e4c-bonds were formed through the π* of the diatomic molecules ([I2···S2···I2]2+). 520 In [Se2I4]2+ the two delocalized π* orbitals of the “monomer” [SeI2]˙+ are overlapping. 521 In the last 10 years, chalcogen systems, which are analogous to the [I4]2+ cation were characterized by scXRD. The isolobality of [Ch2R2]+˙ and [X2]+˙ leads to the same rectangular structural motif with two long π*–π*- and two short σ-interactions. Overall those cations are typically diamagnetic in the solid state (Fig. 88).
Fig. 88. Selected chalcogen radical cations, which were stabilized by WCAs.
Radical cations
The chalcogen elements have a rich radical cation chemistry. Most of the cations contain (pseudo-)aromatic systems or Ch–Ch fragments, over which the unpaired electron is delocalized. Strong one-electron oxidants like [NO]+ or XeF2 combined with a Lewis acid were frequently used to synthesize the cations. There are also examples of diradicals like (CNS3˙+)2, obtained by the reaction of homopolyatomic sulfur cations (a formal [S3]+ equivalent) and dicyanogen. 522,523 We also refer the reader to a recently published comprehensive review about [RCNSSS]˙+ radical cations and their properties. 509
Oxidation state +II
Chalcogen cations [RCh]+ in the formal oxidation state +II are only known in combination with stabilizing donor ligands. It was for example possible to stabilize the formal selenium cation [RSe]+ with the two amine-arms of a pincer ligand 524 (Fig. 89). Two different Te(ii) cations with the strong donors DMAP and the carbene iPrIM as ligands (Fig. 89) were synthesized with the useful starting material [(Dipp2BIAN)Te]2+([OTf]–)2, a base stabilized “Te(OTf)2”, which was presented in 2009 and can also be understood as a tellurium analogue of a carbene. 525 The thi-, selen- and tellur-irenium cations (in the figure drawn as coordination complexes of [RCh]+ ions) were all synthesized with starting materials that contain Ch–Ch bonds like Me2S2 82 or [Se3Me3]+ 526 or already oxidized chalcogens like [PhTe]+[SbF6]– 526 and alkynes.
Fig. 89. Selected chalcogen cations with chalcogen atoms in the formal oxidation state of +II.
Another example of ligand-stabilized chalcogen cations are the N-heterocyclic carbenoidic rings [R2C2N2Ch]2+ (Ch = S, Se, Te), which are formally 1,4-diaza-1,3-butadiene complexes of a chalcogen cation in oxidation state +II, but the delocalization of the positive charge supports a description as a chalcogen in oxidation state +IV (Fig. 90). 527 The carbene-analogues were prepared through complexation of an in situ generated Ch2+ dication, which can be obtained through halide abstraction from SCl2, 528 SeCl4 529 or (Dipp2BIAN)TeI2 525 (Dipp2BIAN = 1,4-(2,6-diisopropyl)phenyl-bis(arylimino)-acenaphthene).
Fig. 90. Carbene-analogous chalcogen dications.
Oxidation state +IV
The [ChX3]+ cations were one of first structurally characterized reactive chalcogen cations (Fig. 91). The first experiments mainly on [TeCl3]+ were published already in the 1950s. 530 Now, a multitude of crystal structures can be found in the literature. Due to the relatively simple vibrational spectra of the four-atomic molecules, they can serve as a probe for the coordination power of the anion. The stronger the secondary interaction is, the weaker are the intramolecular Ch–X bonds and thus they get red-shifted. 531 There are also over 60 crystal structures of triorganyltelluronium [TeR3]+ cations with different anions. We decided only to list the “classics” [TePh3]+ 532 and [Te(C6F5)3]+ 533 and to refer to a recently published review about the chemistry and structures of these cations. 506 Some newer examples of compounds with chalcogen atoms in oxidation state +IV are the triazidetelluronium cation [Te(N3)3]+ 534 and a fluoride bridged version of [TeCl3]+. 28
Fig. 91. Chalcogen cations with chalcogen atoms in oxidation state +IV. * Every combination but [SI3]+ is known.
Oxidation state +VI
To our knowledge, the only chalcogen cation with a chalcogen atom in oxidation state +VI is [TePh5]+. It was obtained through halide abstraction from TePh5Cl with silver triflate and crystallized with the classical WCAs [B(C6F5)4]– 532 and [B(ArCF3 )4]– (Fig. 92). 535
Fig. 92. [TePh5]+, a cation with a chalcogen atom in oxidation state +VI.
Protonated chalcogen cations
The use of super-acidic conditions, makes it possible to obtain the protonated forms of very weakly basic molecules like trifluorosulfonic acid (Fig. 93). 536 In some cases, it is not possible to isolate the neutral form of a molecule, but the conjugated positively charged acid (e.g. carbonic acid 537 ). We decided to list the protonated carbonic acid together with the sulphur species like protonated sulphuric acid in this chapter (Table 11). 538
Fig. 93. Examples for protonated molecules obtained under superacidic conditions.
Table 11. Overview on structurally characterized chalcogen cations.
| Cation | Anion | Class a | Synthesis | Comment b | Ref. |
| Homopolyatomic cations | |||||
| [O2]+ | [PtF6]– | Ox | O2 + PtF6 | 510 | |
| [O2]+ | [PtF6]– | Ox | O2 + PtF6 | Neutron diffraction | 539 |
| [S4]2+ | [AsF6]– | Ox | S + AsF5 in SO2 | 540 | |
| [S4]2+·4[S7I]+ | [AsF6]– | Ox, Lewis | S + I2 + AsF5 in SO2 | 540 and 541 | |
| [S4]2+ | [Sb9F39]2–(= [(SbF6)5(Sb2F4)(Sb2F5)]2–) | Ox | S + SbF5 in SO2 | Traces of Br2 were added | 542 |
| [S4]2+·AsF3 | [AsF6]– | Ox | S + AsF5 + AsF3 in HF | Traces of Br2 were added | 543 |
| [S8]2+ | [AsF6]– | Ox | S + HF/AsF5 | 544 | |
| [S8]2+ | [SbF6]–·[Sb3F14]–(=[(SbF6)2(SbF2)]–) | Ox | S + SbF5 in SO2 | 542 | |
| [S8]2+ | [AsF6]– | Ox | [S8]2+([AsF6]–)2 in SO2/SO2ClF | 73 | |
| [S19]2+ | [AsF6]– | Ox | S + AsF5 in SO2/SO2ClF | 545 | |
| [S19]2+ | [SbF6]– | Ox | S + SbF5 in SO2 | 542 | |
| [Se4]2+ | [AlCl4]– | Lewis, Ox | Se + SeCl4 + AlCl3 | 546 | |
| [Se4]2+ | [MCl6]2– (M = Zr, Hf) | Lewis, Ox | Se + SeCl4 + MCl4 | HTS (130 °C) | 235 |
| [Se4]2+ | [SbF6]–·[Sb4F11]–(=[(SbF6)2(Sb2F5)2]–) | Ox | Se + SbF5 in SO2 | 547 | |
| [Se4]2+ | [Sb9F39]2–(=[(SbF6)5(Sb2F4)(Sb2F5)]2–) | Ox | S + Se + SbF5 in SO2 | 546 | |
| [Se4]2+ | [MoOCl4]– | Ox | Se + MoOCl4 | HTS (190 °C) | 548 |
| [Se4]2+ | [Mo2O2Cl8]–·[MCl6]– (M = Zr, Hf) | Lewis, Ox | [Se4]2+[Mo2O2Cl8]2– + [Se4]2+[MCl6]2– or Se, SeCl4 + MoOCl4 + MCl4 | HTS (120 °C) | 549 |
| [Se8]2+ | [AlCl4]– | Lewis, Ox | Se + SeCl4 + AlCl3 | Crystals from vapour-phase transport | 550 |
| [Se8]2+·[Te6]4+·SO2 | [AsF6]– | Ox | Se + Te + AsF5 in SO2 | 551 | |
| [Se10]2+ | [SbF6]– | Ox | Se + SbF5 in SO2 | 552 | |
| [Se10]2+ | [SO3F]– | Ox | Se + AsF5 in SO2 | 553 | |
| [Se10]2+ | [Bi4Cl14]– | Lewis, Ox | Se + SeCl4 + BiCl3 | HTS (90 °C) | 554 |
| [Se17]2+ | [WCl6]– | Ox | Se + WCl6 | 555 | |
| [Se17]2+ | [NbCl6]– | Lewis, Ox | Se + SeCl4 + NbCl5 in SnCl4 | Solvothermal (150 °C) | 556 |
| [Se17]2+ | [TaBr6]– | Lewis, Ox | Se + SeBr4 + TaBr5 in SiBr4 | Solvothermal (150 °C) | 556 |
| [Te4]2+ | [AlCl4]–[Al2Cl7]– | Lewis, Ox | Te + TeCl4 + AlCl3 | HTS (250 °C) | 557 |
| [Te4]2+ | [SbF6]– | Ox | Te + SbF5 in SO2 | Germanium was added to obtain mixed cations | 546 |
| [Te4]2+ | [WCl6]– | Ox | Te + WCl6 | HTS (190 °C) | 558 |
| [Te4]2+ | [WCl6]– | Ox | Te + WCl6 | Traces of Br2 were added, β-mod. | 559 |
| [Te4]2+ | [Zr2Br10]2– | Lewis, Ox | Te2Br + ZrBr4 | HTS (210 °C) | 560 |
| [Te4]2+ | [HfCl6]– | Lewis, Ox | Te + TeCl4 + HfCl4 | HTS (200 °C) | 235 |
| [Te4]2+ | [MCl6]– (M = Nb, Ta), [TaBr6]–, [Ta2Cl10O]2– | Lewis, Ox | Te + TeCl4 + MCl5 | HTS (170 °C) | 561 |
| [Te4]2+ | [Bi6Cl10]2–[Bi2Br8]2– | Lewis, Ox | Te + TeX4 + BiX3 (X = Cl, Br) | HTS (170 °C) | 562 |
| [Te4]2+ | [Nb2Cl10O]2– | Lewis, Ox | Te + TeCl4 + NbCl5 + NbOCl3 | HTS (200 °C) | 563 |
| [Te4]2+ | [MoCl4O]– | Ox | Te + MoOCl4 | HTS (250 °C) | 564 |
| (Te10 2+) n ·Te4 2+ | [Bi4Cl16]2– | Lewis, Ox | Te + TeCl4 + BiCl3 | HTS (150 °C) | 565 |
| [Te6]2+ | [MCl6]2– (M = Zr, Hf) | Lewis, Ox | Te + TeCl4 + MCl4 | HTS (220 °C) | 103 |
| [Te6]2+ | [WCl4O]– | Ox | Te + WOCl4 | HTS (150 °C) | 566 |
| [Te6]2+ | [NbCl4O]– | Lewis, Ox | Te + TeCl4 + NbOCl3 | HTS (200 °C) | 567 |
| [Te6]4+·2AsF3 | [AsF6]– | Ox | Te + AsF5 in SO2 | 568 | |
| (Te7 2+) n | [AsF6]– | Other | [Te4]2+([AsF6]–)2 + Fe(CO)5 in SO2 | Reduction of Te4 2+ | 569 |
| (Te7 2+) n | [Be2Cl6]2– | Lewis, Ox | Te + TeCl4 + BeCl2 | HTS (250 °C) | 565 |
| (Te7 2+) n | [WBr4O]–·[Br]– | Ox | Te + WOBr4/WBr5 | HTS (230 °C) | 570 |
| (Te7 2+) n | [WCl4O]–·[Cl]– | Ox | Te + WOCl4/WCl5 | HTS (150 °C) | 571 |
| (Te7 2+) n | [NbCl4O]–·[Cl]– | Ox | Te + TeCl4 + NbOCl3 | HTS (225 °C) | 101 |
| (Te7 2+) n | [NbBr4O]–·[Br]– | Ox | Te2Br + NbOBr3 | HTS (220 °C) | 101 |
| [Te8]2+ | [WCl6]– | Ox | Te + WCl6 | HTS (200 °C) | 572 |
| [Te8]2+ | [ReCl6]2– | Lewis, Ox | Te + TeCl4 + ReCl4 | HTS (230 °C) | 573 |
| [Te8]2+ | [Bi4Cl14]2– | Lewis, Ox | Te + TeCl4 + BiCl3 | HTS (160 °C) | 574 |
| [Te8]2+ | [U2Br10]2– | Lewis, Ox | Te + TeBr4 + UBr5 in SiBr4 | Solvothermal 200 °C | 575 |
| [Te8]2+ | [Ta4O4Cl16]4– | Lewis, Ox | Te + TeCl4 + TaCl5 + TaOCl3 + [BMIM]+Cl– | Ionic liquid based synthesis | 576 |
| [Te8]4+ | ([VCl4O]2–) n | Ox | Te + VOCl3 | HTS (270 °C), cubic | 577 |
| Metal–nonmetal-cluster complexes | |||||
| [Cu(S12)(S8)]+ | [Al(ORPF)4]– | Com | Cu+ + S | 512 and 578 | |
| [Cu(S12)(CH2Cl2)]+ | [Al(ORPF)4]– | Com | Cu+ + S | 512 and 578 | |
| [Ag(S8)2]+ | [Al(ORPF)4]– | Com | Ag+ + S | 579 | |
| [Cu2Se19]2+ | [Al(ORPF)4]– | Com | Cu+ + Se(red) | 50 | |
| ([Ag2(Se6)]2+)∞ | [AsF6]– | Com | Ag+ + Se | 513 | |
| [Ag(Se6)]+ | [Ag2(SbF6)3]– | Com | Ag+ + Se | 513 | |
| [Ag2(Se6)(SO2)2]2+ | [Sb(OTeF5)6]– | Com | Ag+ + Se(grey) | 513 | |
| [Ag2(Se6)(SO2)4]2+ | [Al(ORPF)4]– | Com | Ag+ + Se(grey) | 580 | |
| [Ag2Se12]2+ | [FAl(OC(C5F10)(C6F5))3]–, [Al(ORPF)4]–) | Com | Ag+ + Se(red) | 176 and 581 | |
| Clusters/cluster-like | |||||
| NS+ | [AlCl4]+ | Lewis | (NSCl)3 + AlCl3 | 517 | |
| [NS2]+ | [AlCl4]+ | Other | S4N4 + AlCl3 | 582 | |
| [S4N4]2+ | [SbCl6]– | Ox | S4N4/S3N3Cl3 + SbCl5 in SO2 | 583 | |
| [S4N4]2+ | [Sb3F14]–·[SbF6]– | Ox | S4N4 + SbF5 in SO2 | 583 | |
| [S5N5]+ | [SbCl6]– | Lewis | S3N3Cl3 + SbCl5 in SOCl2 | 584 | |
| [S3Cl3]+ | [AsF6]– | Other | [SCl3]+[AsF6]– + S8 in SO2 | 585 | |
| [(S2N2C)2]2+ | [SbF6]–, [Sb2F11]– | Other | [NS2]+[AsF6]– + (CN)2 in SO2 | 586 | |
| [S4Te4]2+·SO2 | [AsF6]– | Ox | Te + Sn + AsF5 in SO2 | 587 | |
| [S3Te3]2+ | [AsF6]– | Ox | S + Te + AsF5 in SO2 | 588 | |
| [Se3Cl3]+ | [AsF6]– | Other | [SeCl3]+[AsF6]– + Se in SO2 | 585 | |
| [Se6Ph2]2+·2SO2 | [AsF6]– | Other | [Se4]2+([AsF6]–)2 + Ph2Se2 | 589 | |
| [Se6I2]2+·2SO2 | [AsF6]– | Ox | Te + I2 + AsF5 in SO2 or [SeI3]+[AsF6]– 2 + [Se8]2+([AsF6]–)2 | 590 | |
| [Te6I2]2+ | [WCl6]– | Ox | Te + I2 + WCl6 | HTS (150 °C) | 566 |
| ([Te15X4]2+) n | ([MOX4]–) n (M = Mo, X = Cl, Br; M = W, X = Br) | Other | Te2Br + MoOBr3, TeCl4 + MoNCl2/MoOCl3, Te + WBr5/WOBr3 | 591 | |
| [Se4Te2]2+ | [MF6]– (M = As, Sb) | Ox | Se + Te + MF5 in SO2 | 588 | |
| [Se4Te3]2+ | [MOCl4]– | Ox | Se + [Te6]2+([MOCl4]–)2 | HTS (190 °C) | 592 |
| [Se6Te2]2+·[Se8Te2]2+·2SO2 | [AsF6]– | Ox | S + Se + Te + AsF5 in SO2 | Heterocubane | 593 |
| [Se8Te2]2+ | [MF6]– (M = As, Sb) | Ox | Se + Te + MF5 in SO2 | Isostructural to Se10 2+ | 593 |
| [Se8Te2]2+·SO2 | [AsF6]– | Ox | Te + [Se8]2+([AsF6]–)2 in SO2 | 594 | |
| (4n + 2)π-cations | |||||
| [S2N3]+ | [Hg2Cl6]2– | Lewis | NSCl + HgCl2 | 595 | |
| [S3N2]2+ | [MF6]– (M = As, Sb) | Other | [SN]+[MF6]– + [S2N]+[MF6]– in SO2 | Cycloaddition | 515 |
| [S4N3]+ | ([SeCl5]–)∞ | Ox | S4N4 + Se2Cl2 in SOCl2 | 10π-aromatic | 519 |
| [S3Se]2+ | [Sb3F16]–·3[SbF6]– | Ox | S + Se + SbF5 in SO2 | Traces of Br2 were added, contains disordered mixture of [S x Se x–4]2+ | 596 |
| [SSe2N2]2+ | [AsF6]– | Ox | [(SSe2N2)2]2+([AsF6]–)2 + AsF5 in SO2 | 597 | |
| [S2SeN2]2+ | [AsF6]– | Other | [NS]+[AsF6]– + [Se8]2+[AsF6]– | 516 | |
| [S2SeN2Cl]+ | [AlCl4]– | Other | [NS]+[AlCl4]– + Se/EtSeCl | 517 and 518 | |
| [Se2Te2]2+ | [Sb3F14]3–·[SbF6]– | Ox | Se + Te + SbF5 in SO2 | 598 | |
| [SeTe3]2+ | [Sb3F14]3–·[SbF6]– | Ox | Se + Te + SbF5 in SO2 | Contains disordered mixture of [SeTe3]2+, [Te4]2+ and [Se2Te2]2+ | 598 |
| π*–π*-complexes | |||||
|
[AsF6]– | Ox | S4N4 + AsF5 in SO2 | 599 | |
| [S6N4]2+ | [S2O2F]–, [SO3F]– | Ox | S4N4 + HSO3F in SO2 | 600 | |
|
[AsF6]– | Ox | S + I2 + AsF5 in SO2 | 520 | |
|
[Sb2F11]– | Ox | Se + [I2]+[Sb2F11]– in SO2 | 521 | |
|
[MF6]– (M = As, Sb) | Other | S4N4 + [Se4]2+([MF6]–)2 in SO2 | 601 | |
|
[BF4]– | Ox | Me2Se2 + XeF2 + BF3·OEt2 | 82 | |
| [(MeSe)4]2+ | [OTf]– | Ox | Et2Te2 + [NO]+[OTf]– | 602 | |
|
[OTf]– | Ox | Et2Te2 + [NO]+[OTf]– | 82 and 602 | |
| Radicals | |||||
|
[AsF6]– | Ox | S4N4 + [Te4]2+([AsF6]–)2 in SO2 | 600 | |
|
[NTf2]– | Ox | TTF + 0.5XeF2 + TMSNTf2 | TTF = Tetrathiafulvalene | 82 |
|
[SbCl6]– | Ox | (C8H10)2S2 + SbCl5 | Strong transannular interactions | 603 |
|
[OTf]– | Ox | (NeoS)2 + [NO]+ | Diorgano disulfide–nitrosonium adduct | 604 |
|
[FAl(ORPF)3]– | Ox | C12H8S2 + XeF2 + Al(OC(CF3)3)3 | C12H8S2 = thianthrene | 82 |
|
[Al(ORPF)4]– | Ox | 1,8-(SPh)2Nap + [NO]+ | S–S-3e–σ-bond Nap = naphthalene | 605 |
|
[AsF6]– | Ox | S + AsF5 + (CN)2 in SO2 | Traces of Br2 were added | 522 |
| (CNS3˙+)2 | [SbF6]–, [Sb2F11]– | Ox | S + SbF5 + (CN)2 in SO2 | Traces of Br2 were added | 523 |
|
[Sb2F11]– | Ox | (C6F5S)2 + SbF5 | 606 | |
|
[As2F11]– | Ox | (C6F5Se)2 + AsF5 | 606 | |
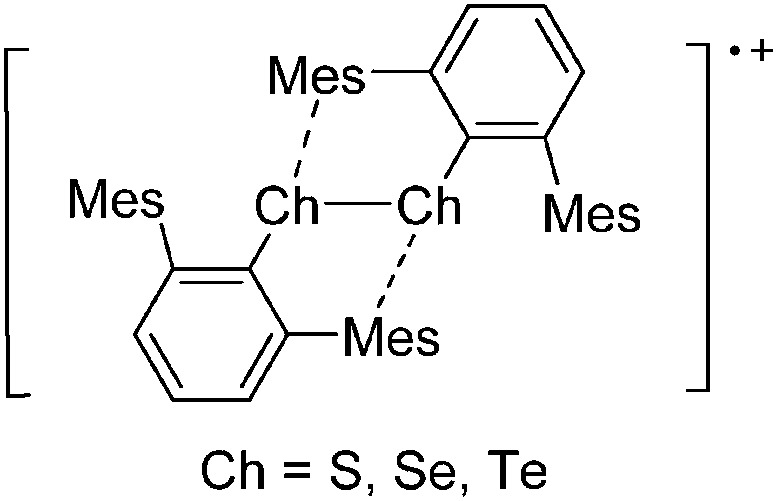
|
[SbF6]– | Ox | (2,6-Mes2C6H3Ch)2 + [NO]+ | 606 | |
| Oxidation state +II | |||||
| [Me2S-SMe]+ | [SbCl6]– | — | — | 607 | |
| [MeS-S(Me)-SMe]+ | [SbCl6]– | Ox, Lewis | S2Me2 + SbCl5 | =[S3Me3]+ | 607 |
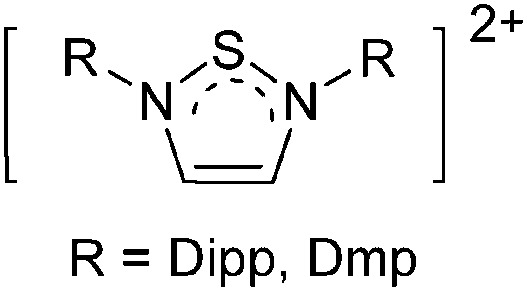
|
[OTf]– | Com | S(OTf)2 + (NR)2C2H2 | Sulfur carbenoid, oxidation state unclear | 528 |
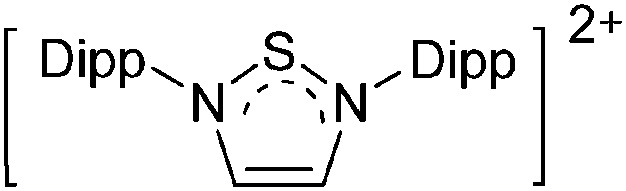
|
[B(C6F5)4]– | Other | [S(NDipp)2C2H2]2+ ([OTf]–)2 + K+[B(C6F5)4]– | Sulfur carbenoid, salt metathesis, oxidation state unclear | 528 |
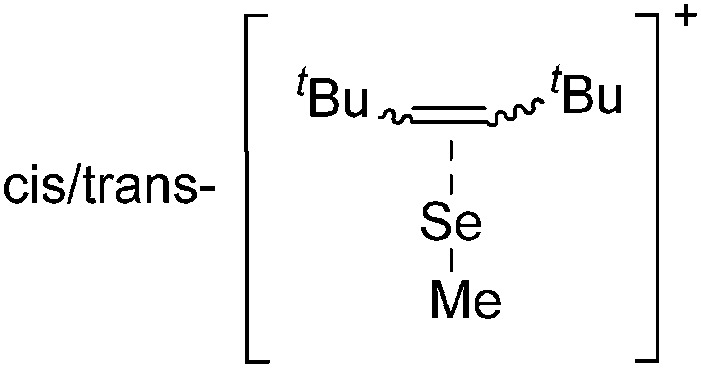
|
[X]– = [SbCl6]–, [BF4]– | Other | [S3Me3]+[X]– + C2H2 t Bu2 | Thiiranium ion | 608 |
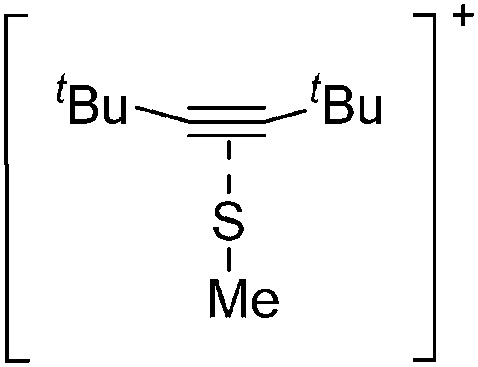
|
[X]– = [BF4]– [PF6]– | Other | [S3Me3]+[X]– + C2 t Bu2 | Thiirenium ion | 608 |
| [MeSC2 t Bu2]+·CH2Cl2 | [CHB11Cl11]– | Ox | Me2S2 + C2 t Bu2 + XeF2 + Me3Si(CHB11Cl11) | 82 | |
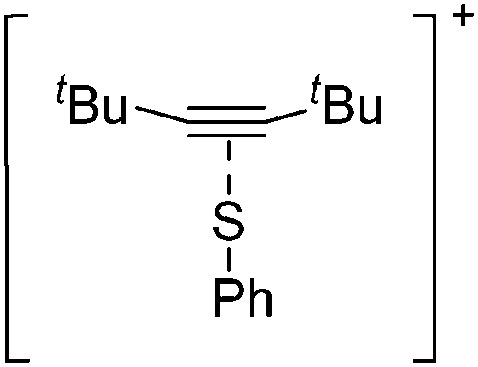
|
[B(C6F5)4]– | Ox | Ph2S2 + C2 t Bu2 + XeF2 + [Me3Si(Tol)]+[B(C6F5)4]– | Thiirenium ion | 82 |
| [MeSe-Se(Me)-SeMe]+ | [SbCl6]– | Ox, Lewis | Se2Me2 + SbCl5 | =[Se3Me3]+ | 607 |
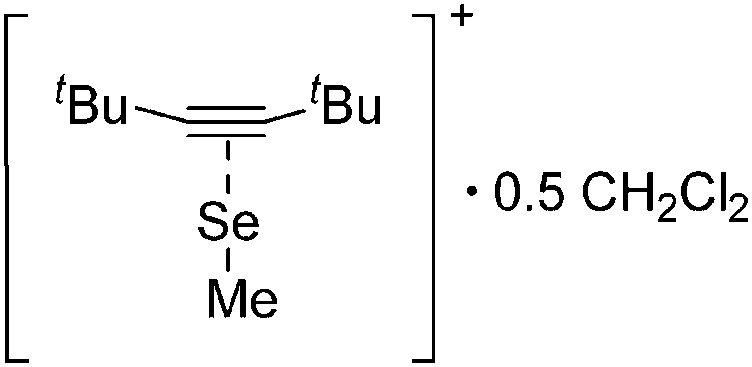
|
[SbCl6]– | Other | [Se3Me3]+[SbCl6]– + C2 t Bu2 | Selenirenium ion | 526 |
| [PhSeC2Ad2]+·CH2Cl2 | [SbCl6]– | Other | [PhSe]+[SbCl6]– + C2Ad2 | 526 | |
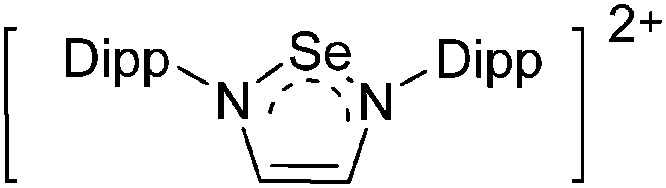
|
[SnCl6]2– | Ox, Lewis | (NDipp)2C2H2 + SnCl2 + SeCl4 | Selenium carbenoid | 529 |
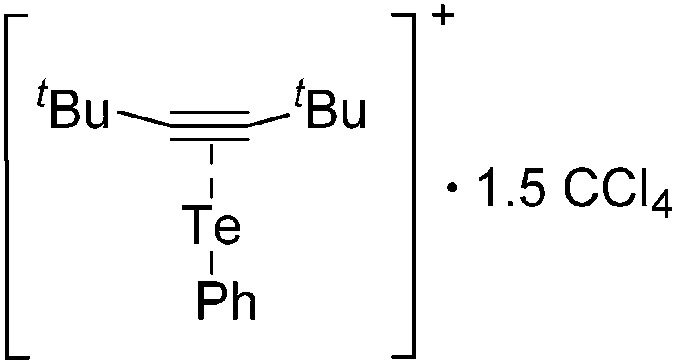
|
[SbF6]– | Other | [PhTe]+[SbF6]– + C2 t Bu2 | Tellurirenium ion | 526 |
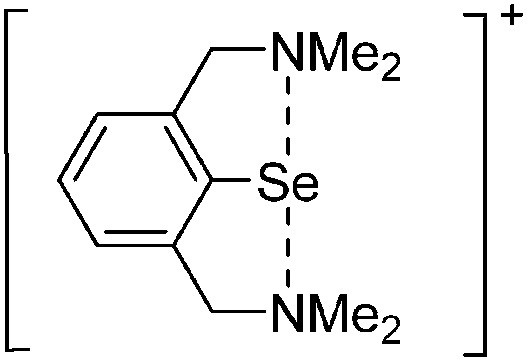
|
[PF6]– | Other | C6H3(CH2NMe2)2SeMe + t BuOCl + K+[PF6]– | 524 | |
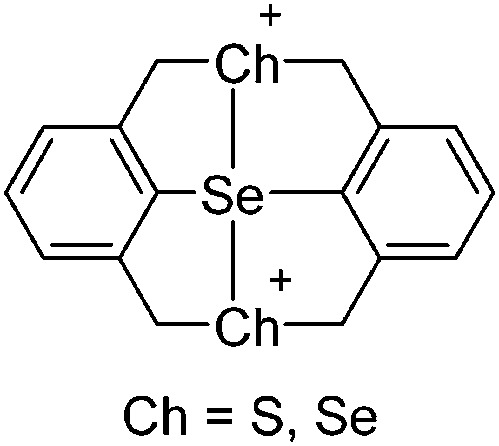
|
[PF6]– | Ox | (C6H3(CH2)2Ch)2Se + [NO]+[PF6]– | 609 | |
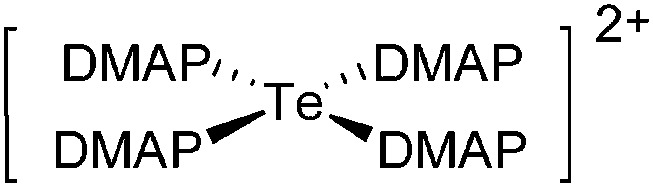
|
[OTf]– | Other | [(Dipp2BIAN)Te]2+ ([OTf]–)2 + DMAP | Ligand exchange | 525 |
|
[OTf]– | Other | [(Dipp2BIAN)Te]2+ ([OTf]–)2 + 2 iPrIM | Ligand exchange, anions coordinating | 525 |
|
[OTf]– | Other | [(Dipp2BIAN)Te]2+ ([OTf]–)2 + iPrIM | Ligand exchange, 4 equivalents of iPrIM | 525 |
|
[OTf]– | Hal | (Dipp2BIAN)TeI2 + Ag+[OTf]– | Tellurium carbenoid, Dipp2BIAN = 1,4-(2,6-diisopropyl)phenyl-bis(arylimino)acenaphthene | 525 |
| Oxidation state +IV | |||||
| [SF3]+ | [BF4]– | Lewis | SF4 + BF3 | Crystals through sublimation | 610 |
| [SF3]+ | [GeF6]2– | Lewis | SF4 + GeF4 | 611 | |
| [SCl3]+ | [ICl4]– | Ox, Lewis | S + Cl2 + I2 | 612 | |
| [SCl3]+ | [ICl4]– | Ox, Lewis | S + Cl2 + I2 | Second modification | 613 |
| [SCl3]+ | [UCl6]– | Lewis | SOCl2 + UCl5 | 614 | |
| [SCl3]+ | [AlCl4]– | Lewis | SCl4 + AlCl3 | 615 | |
| [SCl3]+ | [SbCl6]– | Ox, Lewis | As4S4 + SbCl5 in SO2 | 531 | |
| [SCl3]+ | [MoOCl4] | Ox | S + MOCl4 | HTS (100 °C), large excess of ICl3 was added | 616 |
| [SBr1.2Cl1.8]+ | [SbCl6]– | Ox, Lewis | S + Br2 + SbCl5 in SO2 | Attempt to prepare [SBr3]+[SbCl6]– | 531 |
| [SBr3]+ | [AsF6]– | Lewis | S + Br2 + AsF5 in SO2 | 617 and 618 | |
| [(SX2)2N]+ (X = F, Cl) | [AsF6]– | Ox | [S2N]+[AsF6]– + X2 in SO2 | 619 | |
| [SeF3]+ | [NbF6]–, [Nb2F11]–, [TaF6]– | Lewis | SeF4 + NbF5 | First structure with a [ChX3]+ cation (Ch = S, Se, Te; X = F, Cl, Br, I) | 620 |
| [SeCl3]+ | [AlCl4]– | Lewis | SeCl4 + AlCl3 in SO2Cl2 | 621 | |
| [SeCl3]+ | [SbCl6]– | Ox, Lewis | As + Se + SbCl5, or SeCl4 + SbCl5 in SO2 | Melt | 531 |
| [SeCl3]+ | [MoOCl4]– | Others | [Se4]2+[MoOCl4]2– in SOCl2 | Decomposition at 150 °C, β-modification | 622 |
| [SeCl3]+ | [AuCl4]– | Lewis | SeCl4 + AuCl3 | 623 | |
| [SeBr3]+ | [AsF6]– | Ox, Lewis | Se + Br2 + AsF5 in SO2 | Small amount of AsF3 was added | 624 |
| [SeBr3]+ | [SbF6]– | Ox, Lewis | [Se4]2+([SbF6]–)2 + Br2 + AsF5 in SO2 | 624 | |
| [SeBr3]+ | [AlBr4]– | Lewis | SeBr4 + AlBr3 | HTS (150 °C) | 625 |
| [SeI3]+ | [AsF6]– | Ox, Lewis | [Se4]2+([SbF6]–)2 + I2 in AsF3 | 617 and 618 | |
| [SeI3]+ | [SbF6]– | Ox, Lewis | Se + I2 + SbF5 in SO2 | ||
| [TeF3]+ | [Sb2F11]– | Lewis | TeF4 + SbF5 | 626 | |
| [TeF3]+ | [SO4]2– | Ox, Lewis | Te + Br2 + AsF5 in SO2 | 531 | |
| [TeCl3]+ | [AlCl4]– | Lewis | TeCl4 + AlCl3 | Monoclinic | 627 |
| [TeCl3]+ | [AlCl4]– | Lewis | S7TeCl2 + AlCl3 | TeCl3[AlCl4] (triclinic) | 531 |
| [TeCl3]+ | [AsF6]– | Lewis | TeCl4 + AsF5 | 531 | |
| [TeCl3]+ | [SbF6]– | Lewis | TeF4 + SbF5 in CH2Cl2 | Solvent decomposition | 531 |
| [TeCl3]+ | [AuCl4]– | Lewis | TeCl4 + AuCl3 | 628 | |
| [TeCl3]+ | [MoCl4O]– | Lewis | TeCl4 + MoOCl3 | HTS (180 °C) | 629 |
| [TeCl3]+ | [MoCl6]2– | Lewis | TeCl4 + MoCl4 | HTS (195 °C) | 630 |
| [TeCl3]+ | [Re2Cl6]– | Lewis, Ox | Te + TeCl4 + ReCl5 | HTS (150 °C), β-modification | 630 |
| [TeCl3]+ | [MoCl6]2–·[Cl]– | Lewis | TeCl4 + MoCl4 | HTS (300 °C) | 631 |
| [TeCl3]+ | [MCl6]– (M = Nb, Ta) | Lewis | TeCl4 + MCl5 | α- and β-modification | 632 |
| [TeCl3]+ | [WCl6]– | Lewis | TeCl4 + WCl6 | 632 | |
| [TeBr3]+ | [AsF6]– | Ox, Lewis | Te + Br2 + AsF5 in SO2 | 624 | |
| [TeBr3]+·1/2Br2 | [AuBr4]– | Ox, Lewis | Te + Au + Br2 | HTS (160 °C) | 633 |
| [TeBr3]+ | [Zr2Br9]– | Other | [Te4]2+[Zr2Br10]2– | Decomposition above 250 °C | 560 |
| [TeBr3]+ | [MBr6]– (M = Ta, W) | Lewis | TeBr4 + MBr5 | 632 | |
| [TeI3]+ | [AsF6]– | Ox, Lewis | Te + I2 + AsF5 in SO2 | 634 | |
| [TeI3]+ | [SbF6]– | Ox, Lewis | Te + I2 + SbF5 in SO2 | 617 and 618 | |
| [TeI3]+ | [AlI4]– | Lewis, Ox | Te + I2 + AlI3 | HTS (150 °C) | 625 |
| [TeI3]+ | [MI4]–, (M = Ga, In) | Lewis, Ox | Te + M + I2 | HTS (350 °C) | 635 |
| [TeI3]+·1/2SO2 | [AsF6]– | Ox, Lewis | Te + I2 + AsF5 in SO2 | Hemisolvate of [TeI3]+[AsF6]– | 636 |
| [TeI3]+ | [Al(ORPF)4]– | Salt | TeX4 + Ag+[Al(ORPF)4]– | Good solubility | 637 |
| [TePh3]+ | [B(C6F5)4]– | Other | [TePh5]+[B(C6F5)4]– | Thermal decomposition at 150 °C | 532 |
| [Te(C6F5)3]+ | [OTf]– | Other | Te(C6F5)4 + TMSOTf | 533 | |
| [Te(N3)3]+ | [SbF6]– | Ox | [Te4]2+([SbF6]–)2 + KN3 in SO2 | Side products may be explosive! | 534 |
| [F(TeCl3)2]+ | [Sb(OTeF5)6]– | Ox, Lewis | TeBr4 + Ag+[Sb(OTeF5)6]– in SO2ClF | 28 | |
|
[BF4]– | Ox | (C6H3(CH2ChPh)2)TePh + [NO]+[BF4]– | 638 | |
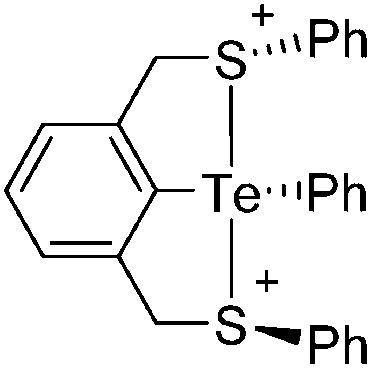
|
[OTf]– | Ox | (C6H3(CH2SPh)2)TePh + t BuOCl + O(Tf)2 | 638 | |
| Oxidation state +VI | |||||
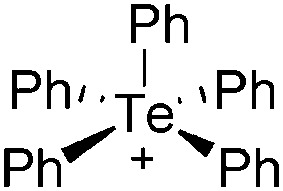
|
[B(C6F5)4]– | Hal | TePh5Cl + Ag+[OTf]– + Li+[B(C6F5)4]– | 532 | |
| [TePh5]+ | [ClO4]– | Hal | TePh5Cl + Ag+[ClO4]– | 535 | |
| [TePh5]+···MeCN | [B(ArCF3 )4]– | Hal | TePh5Cl + Ag+[OTf]– + [B(ArCF3 )4]– | 535 | |
| Protonated cations | |||||
|
[AsF6]– | Prot | OC(OTMS)2 + HF/AsF5 | 537 | |
|
[Ge3F16]4– | Prot | Me2SO + HF/GeF4 | 639 | |
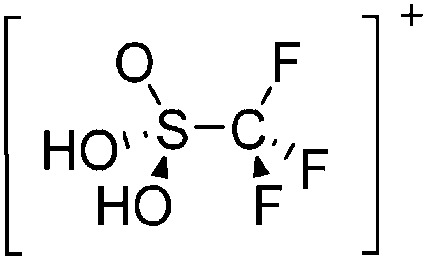
|
[SbF6]– | Prot | OTf2 + HF/SbF5 | 536 | |
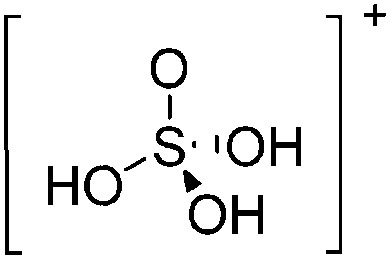
|
[SbF6]– | Prot | SO2(OTMS)2 + HF/SbF5 | 538 | |
a Classification according to the introduction (Table 2): Com = complexation reaction, Lewis = Lewis acid halogen bond heterolysis, Prot = protonation, Ox = oxidation, other = all other reactions not classified.
b HTS = high temperature synthesis.
Group 17 cations
The electronegative halogen atoms are strongly oxidizing and have high ionization energies as well as electron affinities. This makes halogen cations good electrophiles, which need very oxidation-resistant WCAs to be stabilized in condensed phases. Concerning the synthesis of these highly reactive compounds, there are many similarities to the noble gas cations. Almost every halogen cation, for which a crystal structure is known, was synthesized through a halide abstraction by a strong Lewis acid like MF5 (M = As, Sb). The starting materials are normally neutral interhalogen compounds like ClF3, BrF5, IF7, I2Cl6, or IBr, and the majority of the obtained cations contains halogen atoms in oxidation state +III, +V and +VII (Table 7).
Table 7. Review articles including cationic group 17 compounds.
| Year | Title | Ref. |
| 2000 | Recent advances in the understanding of the syntheses, structures, bonding and energetics of the homopolyatomic cations of groups 16 and 17 | 503 |
| 2008 | Polyvalent perfluoroorgano- and selected polyfluoroorgano-halogen(iii and v) compounds | 640 |
| 2013 | Recent advances in the syntheses of homopolyatomic cations of the non-metallic elements C, N, P, S, Cl, Br, I and Xe | 11 |
Fluorine cations
Because of the high electronegativity and ionization potential of fluorine, it would be very difficult to oxidize it and obtain actual fluorine cations in the condensed phase. By contrast, in the gas phase this is possible. 641 Some bulk cationic compounds contain fluorine, but it is very unlikely that the positive charge is actually localized on the fluorine atom. Such compounds like for example the in Fig. 94 shown compound that was published as a formal disilylfluoronium ion are discussed in the chapters of the element, which has a larger positive charge density (here silicon). 642
Fig. 94. Left: Formal disilylfluoronium ion that bears a larger positive charge density at the silicon atoms. Therefore, it is discussed with the silylium cations in the section on group 14 rPBC. Right: The Selectfluor reagent.
It should be mentioned that also electrophilic “F+-” or “N–F-” reagents like “Selectfluor” belong to this class of compounds that are very useful for organic transformations and compatible with solvents like CH2Cl2. 643
Chlorine, bromine and iodine cations
Homopolyatomic cations
Two homopolyatomic chlorine cations are known in the solid state and its crystal structures of [Cl3]+ and [Cl4]+ were published in 1999 42 and 2000. 644 [Cl2]+, which would be the lighter homologue to the known [Br2]+ and [I2]+, was only detected in the gas phase. [Cl3]+ can be synthesized through a reaction of ClF with AsF5, with the adduct δ+Cl–F δ– → AsF5 as intermediate. This leads to a more activated ClF with a more positively charged chlorine atom and results in the formation of [Cl2F]+[AsF6]–, which can be described as a formal “Cl+” stabilized by a second equivalent of ClF. When elemental chlorine is used instead of a second equivalent of ClF, [Cl3]+ is formed. [Cl4]+, which is a homopolyatomic cation but also a π*–π*-complex (see below: π*–π*-complexes of group 17) can be obtained by direct oxidation of chlorine with the strong one-electron oxidant IrF6. For bromine, three cations are known. Of those, [Br2]+ was one of the first homopolyatomic cations of the non-metals for which the crystal structure was determined. It was already in 1968 that Edwards et al. stabilized it with the very good [Sb3F16]– WCA. 663 The structures of the other two known cations [Br3]+ and [Br5]+ were measured relatively late in 1991. [Br5]+ can be synthesized by oxidation of elemental bromine with the strong oxidant [XeF]+ 211 and the only measurable crystals of a [Br3]+ salt were obtained from a 20 year old [BrF2]+[AsF6]– solution. 645 Iodine has five known cations. [I2]+, [I3]+ and [I5]+ are isostructural to the lighter homologues and can all be obtained by oxidation from I2 with the strong Lewis acids MF5 (M = As, Sb). [I4]2+ was synthesized through the entropically unfavorable dimerization of the paramagnetic [I2]+˙ radical cation at low temperature and can be described as an rectangular planar diamagnetic π*–π*-complex (see below: π*–π*-complexes of group 17) (Fig. 95).
Fig. 95. Structurally characterized homopolyatomic cation of chlorine, bromine and iodine.
Metal–halogen complexes
The very weak coordination power of the neutral dihalogen molecules X2 (F2, Cl2, Br2, I2) made it very difficult to obtain metal–halogen-complexes. Only for diiodine, which is the strongest donor, it was possible to get polymeric [{Ag(I2)} n ] n+ cations through the reaction of [Ag]+[MF6]– (M = As, Sb) and I2 in liquid SO2. 646 Also one example of a neutral complex with diiodine as ligand is known: [Rh2(O2CCF3)4(I2)]·I2. 647 Dibromine and dichlorine complexes remained unknown. However, very recently, 648 the use of the very weakly coordinating solvent perfluorohexane and one of the weakest anions [Al(ORPF)4]– led to the isolation of the first dichlorine and dibromine complexes [Ag(X2)]+ (X = Cl, Br, also I) (Fig. 96). Moreover, diiodine turned out to have a rich coordination chemistry and formed three further structures with Ag2I2-moiety as well as isolated [Ag2(I2)4]2+ as well as [Ag2(I2)6]2+ dications well separated from the counterion (Fig. 96). It should be noted that the [Ag(X2)]+ cations are structurally related to the [X3]+ cations and the polymeric [{Ag(I2)} n ] n+ cations bear some similarity to [I5]+.
Fig. 96. Structurally characterized metal complexes with dihalogen molecules as ligands.
π*–π*-complexes
The three halogen containing cations that can be described as π*–π* complexes, can be understood as adducts of two homonuclear diatomic (radical) cations. According to its synthesis from chlorine and [O2]+[SbF6]–, [Cl2O2]+˙ can be described as complex of the paramagnetic [O2]+˙ cation with Cl2. For [Cl4]+˙, which is made from Cl2 and the strong oxidant IrF6, an initially formed paramagnetic [Cl2]+˙ could react with another equivalent of Cl2. In the case of [I4]2+, which was obtained from an [I2]+˙ solution at low temperature, a dimerization of the two paramagnetic [I2]+˙ units to give the [I4]2+ dimer is obvious. All three syntheses have in common that at least one molecule with a half-filled π* orbital as HOMO is involved, for [I4]2+ even both “starting materials”. The formed π*–π* interaction includes two quarter bonds for [Cl2O2]+˙ (243/241 pm, Σ vdW radii: 327 pm) and [Cl4]+˙ (293 pm, Σ vdW radii: 350 pm) and two half bonds for [I4]2+, where the interaction leads to 2e-4c π*–π* bond (328 pm, Σ vdW radii: 396 pm, Fig. 97). 11,503
Fig. 97. Structurally characterized halogen cations, which include π*–π*-interactions. Bond lengths: [Cl2O2]+: O–O 121, Cl···O 241/243, Cl–Cl 191; [Cl4]+: Cl–Cl 194, Cl···Cl 293; [I4]2+: I–I 258, I···I 328 [pm].
Oxidation state +I
The isolated halogens X in this oxidation state would either correspond to a triplet state X+ or the triplet dimer [X = X]2+ – isoelectronic to the dichalcogens O2 to Te2. Neither the monomer, nor the dimer cation are hitherto known in condensed phase. Only one cation type with a formal oxidation state of +I is known: [I(Donor)2]+, in which the electrophilicity of I+ is dampened by coordination of neutral donor molecules. Thus, [I3]+[AsF6]– reacted with acetonitrile to form the structurally characterized salt [I(NCMe)2]+[AsF6]– (Fig. 98). 649 The closely related [I(py)2]+[BF4]– is a useful reagent in organic chemistry. 650 For the latter it is disputable, if this is better assigned as being a pyridinium cation.
Fig. 98. [I(NCMe)2]+ cation in [I(NCMe)2][AsF6] with iodine in the formal oxidation state +I. 649 .
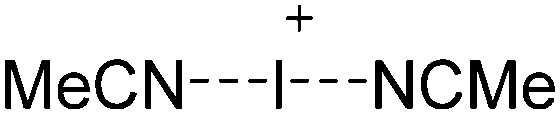
Oxidation state +III
The known interhalonium cations with the central halogen atoms in oxidation state +III are [ClF2]+, [BrF2]+, [ICl2]+, [IBrCl]+ and [IBr2]+ (Fig. 99). All were synthesized by halide abstraction from a neutral interhalogen. Others, like [Cl2F]+ and [IF2]+ are also accessible, but no crystal structures are known. 651 The cations [I3Cl2]+ and [I3Br2]+ can also be understood as interhalonium compounds with two iodine atoms in oxidation state +III and one in –I and are in some way the lighter homologues of the homopolyatomic cation I5 +. 652–654 Two recently published examples are the dialkyl chloronium cations [ClR2]+ (R = Me, Et), which were synthesized through alkylation of chloromethane and –ethane, both stabilized by the very good carborate WCA [CHB11Cl11]– (Fig. 100). The superacid H(CHB11Cl11) was used to generate ion-like methyl and ethyl cations “R+” by protonation of the chloroalkanes RCl, which than react with a second equivalent of RCl to the chloronium cations. 655
Fig. 99. Structurally characterized halonium cations.
Fig. 100. Molecular structure of [ClMe2]+[CHB11Cl11]–. E. S. Stoyanov, I. V. Stoyanova, F. S. Tham and C. A. Reed, J. Am. Chem. Soc., 2010, 132, 4062–4063. Data from this reference were used to draw this figure. Bond lengths in [pm].
In case of bromine and iodine, also some new examples were synthesized during the last 20 years. The stable cyclic bromonium and iodonium ions of sterically hindered olefins (Fig. 101) were stabilized by the [OTf]– anion and can be seen as stable intermediates of the halogenation of olefins. 656
Fig. 101. Stable [OTf]– salts of cyclic bromonium and iodonium ions. 656 .
Oxidation state +V
There are also some examples of cations in oxidation state +V. Besides the classical [XF4]+ cations (X = Cl, Br, I), the two oxocations [ClO2]+ and [BrO2]+ as well as since 2008 also one example of a cation with a XV–carbon bond were structurally characterized. This electrophilic [IF2(C6F5)2]+ cation was synthesized as [BF4]– salt through halide abstraction and ligand exchange from C6F5IF4 and C6F5BF2. 657 Examples without crystal structures include [OClF2]+ 658 and [OBrF2]+ (Fig. 102). 659
Fig. 102. Structurally characterized halogen cations with halogen atoms in oxidation state +V. 657,660–662 .
Oxidation state +VII
In 2004, with the octahedral complexes of the series [XF6]+ (X = Cl, Br, I) the first structures of cations with oxidation state +VII were published (Fig. 103). All three cations were crystallized with the anion [Sb2F11]– and have very weak contact to the anion (Fig. 104). It is also possible to obtain the [AsF6]– and [SbF6]– salts, but the differentiation between the octahedral [XF6]+ and [MF6]– would not be easily done by X-ray crystallography (e.g., for [BrF6]+[AsF6]– or [IF6]+[SbF6]–). The use of [Sb2F11]– allowed for a clear differentiation between cations and anions (Table 12). 660
Fig. 103. Structurally characterized halogen cations with halogen atoms in oxidation state +VII. 660 .
Fig. 104. Molecular structure of [BrF6]+[Sb2F11]–. J. F. Lehmann, G. J. Schrobilgen, K. O. Christe, A. Kornath and R. J. Suontamo, Inorg. Chem., 2004, 43, 6905–6921. Data from this reference were used to draw this figure. Bond lengths in [pm].
Table 12. Overview on all structurally characterized halogen cations.
| Cation | Anion | Class a | Synthesis | Comment | Ref. |
| Homopolyatomic cations | |||||
| [Cl3]+ | [AsF6]– | Lewis | [Cl2F]+[AsF6]– + Cl2 + AsF5 | 42 | |
| [Cl3]+ | [X]– = [SbF6]–, [Sb2F11]–, [Sb3F16]– | Other | [Cl2O2]+[X]– + Cl2 in HF at RT | 42 | |
| [Br2]+ | [Sb3F16]– | Ox | Br2 + HSO3F/SbF5/3SO3 | 663 | |
| [Br3]+ | [AsF6]– | Other | [BrF2]+[AsF6]– decomposition | Store [BrF2]+[AsF6]– for 20 years (!) | 645 |
| [Br5]+ | [MF6]– (M = As, Sb) | Ox | [XeF]+[MF6]– + Br2 | 211 | |
| [I2]+ | [Sb2F11]– | Ox | I2 + SbF5 | 15 | |
| [I3]+ | [AsF6]– | Ox | I2 + AsF5 | 15 | |
| [I5]+ | [AsF6]– | Ox | I2 + AsF5 | 664 | |
| [I15]3+ | [SbF6]– | Ox | I2 + SbF5 | 15 | |
| Metal–nonmetal-cluster complexes | |||||
| [Ag(Cl2)]+ | [Al(ORPF)4]– | Com | Ag+[Al(ORPF)4]– + Cl2 | Only stable at low temperature | |
| [Ag(Br2)]+ | [Al(ORPF)4]– | Com | Ag+[Al(ORPF)4]– + Br2 | ||
| [Ag2(I2) x ]2+ (x = 1, 4, 6) | [Al(ORPF)4]– | Com | Ag+[Al(ORPF)4]– + I2 | ||
| [Ag(I2)] n n+ | [MF6]– (M = Sb, As) | Com | Ag+[MF6]– + I2 | First complex with halogen as donor | 646 |
| π*–π*-complexes | |||||
| [Cl4]+ | [IrF6]– | Ox | Cl2 + IrF6 | Radical, homo-polyatomic cation | 644 |
| [Cl2O2]+ | [X]– = [SbF6]–, [Sb2F11]– | Com | [O2]+ + Cl2 | 42 | |
| [I4]2+ | [MF6]– (M = As, Sb), and [Sb3F14]–/[SbF6]– | Com | 2[I2]+ | Also homopoly-atomic cation | 665 |
| Oxidation state +I | |||||
| [I(NCMe)2]+ | [AsF6]– | Other | [I3]+ + MeCN | [I3]+ as I+ donor | 649 |
| Oxidation state +III | |||||
| [ClF2]+ | [AsF6]– | Lewis | ClF3 + AsF5 | 666 | |
| [ClF2]+ | [SbF6]– | Lewis | ClF3 + SbF5 | 667 | |
| [ClF2]+ | [RuF6]– | Lewis | ClF3 + RuF5 | 668 | |
| [ClMe2]+[ClEt2]+ | [CHB11Cl11]– | Prot | H(CHB11Cl11) + RCl (R = Me, Et) | Protonation of RCl | 655 |
| [BrF2]+ | [SbF6]– | Lewis | BrF3 + SbF5 | 669 | |
| [Br(C6F5)2]+ | [BF4]– | Lewis | BrF3 + (C6F5)2BF | 670 | |
|
[OTf]– | Lewis | C20H28 + Br2 + MeOTf | Stable bromonium ion C20H28 = adamantyli-deneadamantane | 656 |
| [ICl2]+ | [SbF6]– | Lewis | I2Cl6 + SbF5 | 671 | |
| [IBr2]+ | [Sb2F11]– | Lewis | IBr + SbF5 | 672 | |
| [IBr0.75Cl1.25]+ | [SbCl6]– | Lewis | IBr + Cl2 + SbF5 | 672 | |
| [I3Cl2]+ | [X]– = [SbCl6]–, [AlCl4]– | Lewis, other | I2 + SbCl5 | 652–654 | |
| [I3Br2]+ | [SbCl6]– | Lewis, other | I2 + SbCl5 | 653 | |
| [I(C6F5)R]+ | [BF4]– | Lewis | I(C6F5)F2 + RBF2 R = C6H5, o-C6H4F, m-C6H4F, p-C6H4F, 2,4,6-C6H2F3, C6F5 | Strong interactions with the anions | 673 |
|
[OTf]– | Lewis | C20H28 + I2 + Ag+[OTf]– | Stable iodonium ion C20H28 = adamantyli-deneadamantane | 656 |
| Oxidation state +V | |||||
| [ClF4]+ | [SbF6]– | Lewis | ClF5 + SbF5 | 661 | |
| [ClO2]+ | [SbF6]– | Lewis | ClO2F + HF/SbF5 | 674 | |
| [ClO2]+ | [Sb2F11]– | Lewis | ClO2F + 2SbF5 | Also an oxidation product of [ClF2]+ | 675 |
| [ClO2]+ | [X]– = [BF4]–, [GeF5]– | Lewis | ClO2F + BF3, GeF4 | 611 and 676 | |
| [ClO2]+ | [ClO4]– | Ox | ClO2 + O3 | Cl2O6 | 677 |
| [ClO2]+ | [RuF6]– | Lewis Ox | ClO2F + 2RuF5 ClF3 + HF/RuO4 | 668 | |
| [BrF4]+ | [Sb2F11]– | Lewis | BrF5 + 2SbF5 | 662 and 678 | |
| [BrO2]+ | [SbF6]– | Lewis, other | BrO3F + SbF5 | Reduction of BrVII | 674 |
| [IF4]+ | [SbF6]– | Lewis | IF5 in HF/SbF5 | 662 and 679 | |
| [IF4]+ | [Sb2F11]– | Lewis | IF5 + 2HF/SbF5 | 662 and 680 | |
| [I(C6F5)2F2]+ | [BF4]– | Lewis | C6F5IF4 + C6F5BF2 | 657 | |
| Oxidation state +VII | |||||
| [ClF6]+ | [Sb2F11]– | Ox, Lewis | ClF5 + F2 + SbF5 | 660 | |
| [BrF6]+ | [Sb2F11]– | Ox, Lewis | BrF5 + F2 + SbF5 | 660 | |
| [IF6]+ | [Sb2F11]– | Lewis | IF7 + SbF5 | 660 | |
a Classification according to the introduction (Table 2): Lewis = Lewis acid halogen bond heterolysis, Ox = oxidation, Com = complexation reaction, Other = all other reactions not classified.
Group 18 cations
Due to the high ionization potential of the noble gases, their cations all need weakly coordinating and very oxidation resistant anions to be stabilized. The history of noble gas compounds started in the early 1960s, when Bartlett obtained “[Xe]+[PtF6]–” from a reaction of [O2]+[PtF6]– and xenon – the noble gas with the lowest ionization potential. Over the next decades the composition of this product stayed unclear. In 2000 a very comprehensive review about the nature of the product was published with the result that it is very likely a [XeF]+ salt of a polymeric (weakly coordinating) [PtF5] n anion. 681 All of the hitherto known and structurally characterized noble gas cations are included with Table 13.
Table 13. Cationic noble gas compounds with weakly coordinating anions.
| Cation | Anion | Class. a | Synthesis | Comment | Ref. |
| Homopolyatomic cations | |||||
| [Xe2]+ | [Sb4F21]– | Lewis | [XeF]+/Xe + SbF5 | First homopoly-atomic cation | 27 |
| [Xe4]+ | [Sb x F5x+1]– | Lewis | [XeF]+[Sb2F11]–, SbF5 + Xe | No X-ray | 696 |
| Metal–nonmetal-cluster complexes | |||||
| [AuXe4]2+ | [Sb2F11]– | Lewis, Com | AuF3, Xe in HF/SbF5 | First xenon metal complex, gold(ii) 2 modifications: triclinic and tetragonal | 695 and 697 |
| [trans-AuXe2]2+ | [SbF6]– | Com | HAuCl4, XeF2, Xe in HF/SbF5 | Gold(ii) | 695 |
| [cis-AuXe2]2+ | [Sb2F11]– | Com | [AuXe4]2+([Sb2F11]–)2–Xe at RT | Gold(ii) | 695 |
| [trans-AuXe2F]2+ | [SbF6]– + [Sb2F11]– | Com | Au, XeF2, Xe in HF/SbF5 | Gold(iii) | 695 |
| [Au2Xe2F]3+ | [SbF6]– | Com | Au, XeF2, Xe in HF/SbF5 | Gold(ii) | 695 |
| [(F3As)AuXe]+ | [Sb2F11]– | Com | AuF3, Xe, AsF3 in HF/SbF5 | 698 | |
| [HgXe]2+ | [SbF6]– + [Sb2F11]– | Com | HgF2, Xe in HF/SbF5 | 698 | |
| Oxidation state +II | |||||
| [KrF]+ | [MF6]– (M = As, Sb, Bi) | Lewis | KrF2 + MF5 | First krypton cation | 699 |
| [KrF]+ | [AuF6]– | Lewis | KrF2 + Au | 682 | |
| [Kr2F3]+·KrF2, [Kr2F3]+·1/2KrF2, [Kr2F3]+·[KrF]+ | [SbF6]–[SbF6]–[AsF6]– | Lewis | KrF2 + MF5 | 699 | |
| [XeF]+ | [Sb2F11]– | Lewis | XeF2 + 2SbF5 | 687 | |
| [XeF]+ | [RuF6]– | Lewis | XeF2 + RuF5 | 700 | |
| [XeF]+ | [AsF6]– | Lewis | XeF2 + AsF5 | 701 | |
| [XeF]+ | [N(SO2F)2]– | Lewis | XeF2 + HN(SO2F)2 | First Xe–N bond, strong interaction with the anion | 702 |
| [XeF]+·HF | [Sb2F11]– | Lewis | XeF2 + 2SbF5 | 27 | |
| [XeF]+ | [X]– = [AsF6]–, [SbF6]–, [Sb2F11]–, [BiF6]–, [Bi2F11]– | Lewis | XeF2 + AsF5, SbF5, BiF5 | Better structures for [XeF]+[X]–: [X]– = [MF6]–, [SbF6]–, [Sb2F11]– | 691 |
| [Xe2F3]+ | [AsF6]– | Lewis | 2XeF2 + AsF5 | Monoclinic structure | 703 and 704 |
| [Xe2F3]+ | [AuF6]– | Lewis | XeF2 + AuF5 | 686 | |
| [Xe2F3]+ | [MF6]– (M = As, Sb) | Com | [XeF]+ as starting material | [Xe2F3]+[AsF6]– trigonal structure | 705 |
| [Xe(N(SO2F)2)]+ | [Sb3F16]– | Lewis | 3 step synthesis with AsF5 and SbF5 | 706 | |
| [XeC6F5]+ | [(F5C6)2BF2]– | Lewis | XeF2 + B(C6F5)3 | First Xe–C bond | 693 |
| [(MeCN)XeC6F5]+[XeC6F5]+ | [BX]– (X = CF3, C6F5) [BX]– (X = CF3, CN) | Lewis, Com | XeF2 + C6F5BF2 | Salt metatheses with [XeC6F5]+[BF4]– | 707 |
| [(C6F5Xe)2Cl]+ | [AsF6]– | Other | [XeC6F5]+ + TMSCl | First Xe–Cl bond | 708 |
| [XeOChF5]+ (Ch = Se, Te) | [AsF6]– | Lewis | FXeOChF5 + AsF5 | First Xe–O bond | 709 |
| [XeOTeF5]+·SO2ClF | [Sb(OTeF5)6]– | Other | Xe(OTeF5)2 + Sb(OTeF3)3 | OTeF5 – abstraction | 710 |
| [XeCl]+ | [Sb2F11]– | Other | [XeF]+ + [Cl]– | Ligand exchange | 711 |
| [XeN(H)TeF5]+ | [AsF6]– | Lewis | [F5TeNH3]+[AsF6]– + XeF2 | First Xe–N(sp3) bond | 712 |
| [(F3SN)XeF]+ | [AsF6]– | Com | F3SN + [XeF]+ | First Xe–N(sp) bond | 713 |
| [XeNSF4]+ | [AsF6]– | Other | In solid state and HF/BrF5 solution | Rearrangement of [F3SNXeF]+[AsF6]– | 714 |
| [(F3SN)XeNSF4]+ | [AsF6]– | Com | [F3SNXeF]+ + F3SN | 715 | |
| [Xe3OF3]+ | [MF6]– (M = As, Sb) | Other | H2O + XeF2/[XeF]+ | Hydrolysis to XeFOH followed by a reaction with [Xe2F3]+ | 716 |
| Oxidation state +IV | |||||
| [XeF3]+ | [Sb2F11]– | Lewis | XeF4 + 2SbF5 | 717 | |
| [XeF3]+ | [BiF6]– | Lewis | XeF4 + BiF5 | 718 | |
| [XeF3]+ | [Sb2F11]– | Lewis | XeF4 + SbF5 | Better structure | 691 |
| [XeF3]+·HF [H5F4]+·2([XeF3]+·HF) [XeF3]+ | [Sb2F11]–[SbF6]·2[Sb2F11]–[SbF6]– | Lewis | XeOF2·xHF + HF/SbF5 | 719 | |
| Oxidation state +VI | |||||
| [XeF5]+ | [PtF6]– | Lewis | XeF6 + PtF5 | First xenon cation structure | 688 and 689 |
| [XeF5]+ | [AsF6]– | Lewis | XeF6 + AsF5 | 704 | |
| [XeF5]+ | [SbF6]– | Ox | [XeF]+[SbF6]– + F2 in aHF | 692 | |
| [XeF5]+ | [Sb2F11]– | Other | [XeF5]+[SbF6]– | Crystals from a O2SbF6/XeF5SbF6 mixture | 692 |
| [XeF5]+ | [RuF6]– | Lewis | XeF6 + RuF5 | 700 | |
| [XeF5]+ | [PdF6]2– | Lewis | 2XeF6 + PdF4 | 720 | |
| [XeF5]+ | [Ti4F19]3– | Lewis | XeF6 + TiF4 | XeF6 from XeF2, F2 and UV radiation | 721 |
| [XeF5]+ | [m-F(OsO3F2)2]–[OsO3F2]– | Lewis | XeF6 + (OsO3F2)∞ | 690 | |
| [XeF5]+ | [Cu(SbF6)3]– | Other | [XeF5]+[SbF6]– + Cu+[SbF6]– in aHF | Anion exchange | 43 |
| [XeF5]+ | [AgF4]– | Lewis | AgF2 + KrF2 + XeF6 in aHF | 722 | |
| [XeF5]+ | [AuF4]– | Lewis | XeF6 + BrF3 AuF3 | 722 | |
| [XeF5]+·XeF2·[XeF5]+·2XeF2 [XeF5]+·1/2XeF2 | [AsF6]–[AsF6]–[AsF6]– | Com | XeF2 + [XeF5]+[AsF6]– | 723 | |
| [XeF5]+·XeOF4 | [SbF6]– | Lewis | XeF6 + [H3O]+[SbF6]– | 694 | |
| [XeF5]+·NO2 + | [SbF6]– | Other | [XeF5]+[SbF6]– + [NO2]+[SbF6]– | 43 | |
| [Xe2F11]+ | [AuF6]– | Lewis | 2XeF6 + AuF5 | 724 | |
| [Xe2F11]+ | [OsO3F2]– | Lewis | XeF6 + (OsO3F2)∞ | 690 | |
| [XeO2F]+[F(XeO2F)2]+ | [MF6]– (M = As, Sb) [AsF6]– | Lewis | XeO2F2 + MF5 (M = As, Sb) | α- and β-modification of [XeO2F]+[SbF6]– | 694 |
a Classification according to the introduction (Table 2): Lewis = Lewis acid halogen bond heterolysis, Com = complexation reaction, other = all other reactions not classified.
Krypton cations
The only other noble gas besides xenon, for which cations are known, is krypton. The oxidation state +II is the only one known yet. The diversity of the amount of compounds and the number of hitherto realized Kr–X bonds is much smaller. Only two different fluorine-containing cations are known ([KrF]+ and [Kr2F3]+) and some nitrile complexes, which can be described as Kr–N compounds. [KrF]+ is a very strong oxidative fluorinating reagent 682 and made is possible to synthesize [XeF5]+ from Xe, 683 [ClF6]+ from ClF5, 684 [BrF6]+ from BrF5 685 and [O2]+ from O2. 686 There are only seven crystal structures of krypton cations in the literature and five of them with the classical [AsF6]– and [SbF6]– anions.
Xenon cations
The lower ionization potential calls for a richer chemistry of xenon. Thus, since the birth of noble gas chemistry, a multitude of different xenon cations containing the element in the oxidation state II, IV and VI were synthesized.
Homopolyatomic cations
The two known homopolyatomic cations are [Xe2]+ and [Xe4]+. It was only possible to obtain a crystal structure from [Xe2]+, but because of its importance [Xe4]+, which was assigned based on spectroscopic and computational evidence as being stable at higher Xe pressure, is also mentioned.
Fluoroxenon cations and related
The majority of such cations include Xe–F bonds ([XeF m ]+, m = 1, 3, 5; [Xe2F n ]+, n = 3, 11, Fig. 105), but over the decades a lot of different cations with Xe–X (X = F, Cl, O, N, C) bonds were obtained and are included with Table 13 and in part also with Fig. 106. Some of them were already published in the late sixties, 687–689 but also recently with [XeF]+[SbF6]– and [XeF3]+[SbF6]– some new structures were presented 690,691 and in 2015 the crystal structures of [XeF5]+ with the classical WCAs [SbF6]– and [Sb2F11]– were measured for the first time, 692 which shows that the investigation of xenon cations is still in progress.
Fig. 105. Fluoroxenon cations.
Fig. 106. Examples of Xe–X bonds (X = C, Cl, N, O) in cations.
Most of the syntheses use Lewis acids to abstract fluoride from the neutral xenon fluoride (XeF2, XeF4, XeF6). Most structures contain the conjugated weakly coordinating anions of these Lewis acids ([MF6]–, M = As, Sb, Au, Ru). Another mentionable approach is the reaction of XeF2 with the Lewis acid B(C6F5)3, which led to the [F5C6Xe]+ cation containing the first Xe–C bond and the unsymmetric anion [(F5C6)2BF2]–. 693 Through the use of the dioxydifluoride XeO2F2 as starting material, it was also possible to obtain the mixed cation [XeO2F]+ or the fluoride bridged [F(XeO2F)2]+ (Fig. 107). 694
Fig. 107. Examples of Xe–O–F cations.
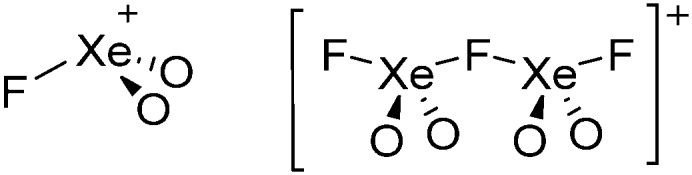
Metal–xenon complexes
There are also some examples of metal–xenon complexes (M = AuI, AuII, AuIII and HgII), which can be described as part of the stabilization of modifications of main group elements as metal complexes ([M m (E n )]+). Formally, Xe is isoelectronic to iodide I–, and thus complex formation appeared to be difficult, but feasible. The syntheses proceed under the superacidic conditions of the systems HF/MF5 (M = As, Sb). In case of these compounds, the role of the anions can be described as unreactive, but not really weakly coordinating. Most of them have relatively short contacts to the anions (shorter than the sum of the van der Waals-radii) and show typical coordination spheres with the anions included (e.g. square planar trans-AuIIXe2(SbF6)2) (Fig. 108). 695
Fig. 108. Molecular structure of trans-AuIIXe2(SbF6)2. T. Drews, S. Seidel and K. Seppelt, Angew. Chem., 2002, 114, 470–473; Angew. Chem., Int. Ed., 2002, 41, 454–456. Data from this reference were used to draw this figure.
Only the [AuXe4]2+ dication exists in a truly ionic structure with two [Sb2F11]– counterions in the lattice (Fig. 109).
Fig. 109. Molecular structure of tetragonal [AuIIXe4]2+([Sb2F11]–)2. T. Drews, S. Seidel and K. Seppelt, Angew. Chem., 2002, 114, 470–473; Angew. Chem., Int. Ed., 2002, 41, 454–456. Data from this reference were used to draw this figure.
Conclusion
Sparked by the availability of new WCAs and new WCA starting materials in combination with novel concepts like FLP and others, the number of rPBC exploded over the last one to two decades. Noteworthy additions were found for each p-block element and, despite their quite high moisture and air sensitivity, true applications of rPBC salts emerged.
Where will this lead to over the next one or two decades…? To our understanding the blue sky synthesis of rPBC salts barely accessible with good/novel WCAs in combination with suitable media will continue to function as an “eye-opener” of what is possible. Many surprising discoveries will force us to sharpen our use of bonding concepts or lead to novel applications. It is often the combination of structural knowledge (“Wow, this crazy cation is stable…? I would have never thought so.”) that leads to the right moment of wonder and then inspiration (“Hm, if this cation is really straight forward accessible, one could use its electrophilic/acidic/oxidizing/activating properties in application XY”). In the 21st century, it is our duty as creative scientists to use this potential from fundamentals to the first application. Do not hesitate to really seek for application of your rPBC salt, as rarely others will pick up on these ideas, since the activation barrier for synthesizing a to this application group unknown rPBC is simply too high. So do not give up until you have demonstrated a possible application – yourself or through collaborations – to a level that others will continue. And on the other hand this compilation of rPBC should encourage application based groups to identify interesting cations that may have an application. Contact the people, the chances are very good that through an informal collaboration showing a proof-of-principle new and relevant application areas may be developed.
In this respect, we are looking forward to all the scientific creativity that is breaking loose, and to realize what potentially could be done with the rPBC. This is an integral element of innovation and the justification for preparing blue sky or simply beautiful and esoteric compounds.
Abbreviations
- CN
Coordination number
- DFT
Density functional theory
- ε r
Relative permittivity of a solvent (static dielectric constant)
- FLP
Frustrated Lewis pairs
- IL
Ionic liquid
- n.a.
Not available
- rPBC
Reactive p-block cations
- WCA
Weakly coordinating anion
- ArCF3
3,5-(CF3)2C6H3
- ArCl
3,5-Cl2-C6H3
- 9BBN
9-Bora[3.3.1]bicyclononane
- bipy
1,2-Bipyridine
- BOX
Bis(oxazoline)
- CatBH
Catecholborane/1,3,2-benzodioxaborole
- COD
1,5-Cyclooctadiene
- Cp
C5H5
- Cp′
C5Me4H
- Cp*
C5Me5
- Cy
Cyclohexyl
- DDP
2-(DIPP)amino-4-(Dipp)imino-2-pentene
- Dipp
2,6-iPr2-C6H3
- DMAP
4-Dimethylaminopyridine
- DMeOPrPE
1,2-(Bis(dimethoxypropyl)-phosphino)ethane
- DMH
1,1-Me2N2H4
- Dmp
2,6-Dimethyl-phenyl
- dmpe
1,2-Bis(dimethylphosphino)ethane
- Do
Donor
- DPE
1,2-Diphenylethane
- dppe
1,2-Bis(diphenylphosphino)ethane
- DTBMP
2,6-Di-tert-butyl-4-methylpyridine
- dtbpy
4,4′-Di-tert-butyl-2,2′-bipyridyl
- Et
Ethyl
- Fc
Ferrocenyl
- FP
CpFe(CO)2
- FP′
Cp′Fe(CO)2
- FP*
Cp*Fe(CO)2
- hppH
1,3,4,6,7,8-Hexahydro-2H-pyrimido-[1,2-a]pyrimidine
- IMe
1,3-Bis(methyl)imidazol-2-ylidene
- IMes
1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene
- iPr
iso-Propyl
- iPr2-ATI
N,N′-Diisopropylaminotroponiminate
- IPr
1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene
- I t Bu
1,3-Bis(tert-butyl)imidazol-2-ylidene 3,5-lutidine 3,5-dimethylpyridine
- Me
Methyl
- Me4-cyclam
N,N′,N′′,N′′′-Tetramethyl-1,4,8,11-tetraazacyclotetradecane
- Mes
2,4,6-Me3C6H2
- Me3SiS p Tol
1-SSiMe3-4-Me-C6H4
- Me3-tacn
N,N′,N′′-Trimethyl-1,4,7-triaza-cyclononane
- nacnac
(NMesCMe)2CH
- NBD
2,5-Norbornadiene
- NPPh
2,5-Bis(2-pyridyl)-1-phenylphosphole
- 1-MIM
N-Methylimidazole
- m-TP
meta-Terphenyl
- OSSO
trans-1,2-Cyclooctanediyl-bridged[OSSO]-type bis(phenolate)
- ORPF
–OC(CF3)3
- ORHT
–OC(CH3)(CF3)2
- ORHF
–OC(H)(CF3)2
- ORMeF
–OC(CH3)(CF3)2
- Ph
–C6H5
- Phen
1,10-Phenanthroline
- 4-Pic
4-Methylpyridine
- Pip
Piperidyl
- PMAF
Pentamethylazaferrocene
- pmdta
N,N,N′,N′,N′′-Pentamethyldiethylenetriamine
- PNP
Bis(2-iPr2 P4-Me-phenyl)amido
- Py
Pyridine
- Pytsi
C(SiMe3)2SiMe2(2-C5H4N)
- p-Xyl
para-Xylene
- R
Typical univalent organic residue
- Salen
N,N′-Ethylenebis(2-hydroxyphenyl)imine
- SalenCF3
N,N′-Ethylenebis(2-hydroxy-2-(CF3)2-ethyl)imine
- Salomphen
N,N′-(4,5-Dimethyl)phenylene-bis(2-hydroxyphenyl)imine
- Salpen
N,N′-Propylenebis(2-hydroxyphenyl)imine
- Sch
Tridentate Schiff base
- SubPc
Subphthalocyanine
- tacn
1,4-iPr2-1,4,7-Triaza-cyclononane
- t Bu
tert-Butyl
- Tf
–SO2CF3
- THF
Tetrahydrofuran
- timtmb tBu
1,3,5-{Tris(3-tert-butylimidazol-2-ylideno)methyl}-2,4,6-trimethylbenzene
- TMM
η4-C(CH2)3
- Tol
Toluene
- Tipp
2,4,6-iPr3-C6H2
- X
Halogen
Acknowledgments
This work was supported by the Albert-Ludwigs-Universität Freiburg, the ERC in the grant UniChem, and by the DFG in the Normalverfahren.
Biographies

Tobias A. Engesser
Dipl.-Chem. Tobias Engesser obtained his intermediate diploma at the Technische Universität Karlsruhe (now KIT) and continued his studies at the Albert-Ludwigs-Universität Freiburg, where he also wrote his diploma thesis about reactive tellurium cations and started his PhD in 2011 at the Institute of Inorganic Chemistry under the supervision of Prof. Ingo Krossing. His research interests are reactive phosphorus cations and gold(i) starting materials, both in combination with weakly coordinating anions. On the basis of his interest for phosphorus compounds he stayed for three months in the group of Prof. Christopher “Kit” Cummins at the Massachusetts Institute of Technology in 2015.

Martin R. Lichtenthaler
Martin R. Lichtenthaler (1986) received his Diploma (2010) and PhD (2015) from the Albert-Ludwigs-Universität Freiburg, Germany. As a member of the Krossing group, he has developed a novel class of highly efficient, main group metal-based olefin polymerization catalysts and discovered unprecedented cationic clusters of univalent indium. After a research stay with the New Technology Office of Merck Ltd. Japan, he is likely to join the University of California, Berkeley as a postdoc in 2016. His research interests are organometallic and polymer chemistry, catalysis, molecular modelling and materials science.

Mario Schleep
Dipl.-Chem. Mario Schleep is a PhD student in the group of Prof. Ingo Krossing at the University of Freiburg, where he received his Diploma in 2012. During his studies, he has undertaken a research stay dedicated to poly-metallic chromium and vanadium clusters in the group of Prof. Eric McInnes at the University of Manchester (England) for two semesters. While dealing with electrolytes for lithium ion batteries during his thesis, his current research focuses on the synthesis of reactive tin(ii) cations stabilized by weakly coordinating anions.

Ingo Krossing
Ingo Krossing studied chemistry in Munich (LMU) and finished his PhD thesis 1997 (with Prof. H. Nöth). From 1997 to 1999, he worked as Feodor Lynen postdoc with Prof. J. Passmore at UNB, Canada. In 1999, he started his independent career as a Liebig- and DFG-Heisenberg-Fellow at the Universität Karlsruhe (TH) (mentor: Prof. H. Schnöckel). 2004 he changed as assistant professor to the Ecole Polytechnic Federale de Lausanne (EPFL), before being appointed Chair of Inorganic Chemistry at the Albert-Ludwigs-Universität Freiburg in 2006. His research interests cover ionic systems from reactive cations to ionic liquids, as well as electrochemical energy storage. With an ongoing ERC Advanced Grant he develops absolute acidity and reducity scales.
References
- Bourissou D., Guerret O., Gabbaï F. P., Bertrand G. Chem. Rev. 2000;100:39. doi: 10.1021/cr940472u. [DOI] [PubMed] [Google Scholar]
- Welch G. C., San Juan R. R., Masuda J. D., Stephan D. W., Stephan D. W. Science. Org. Biomol. Chem. 2006;2008;3146:1124. 1535. doi: 10.1126/science.1134230. [DOI] [PubMed] [Google Scholar]
- Anderson C. E., Braunschweig H., Dewhurst R. D. Organometallics. 2008;27:6381. [Google Scholar]
- Vidovic D., Pierce G. A., Aldridge S. Chem. Commun. 2009:1157. doi: 10.1039/b816042b. [DOI] [PubMed] [Google Scholar]
- Braunschweig H., Dewhurst R. D., Gessner V. H. Chem. Soc. Rev. 2013;42:3197. doi: 10.1039/c3cs35510a. [DOI] [PubMed] [Google Scholar]
- Reed C. A. Acc. Chem. Res. 1998;31:325. [Google Scholar]
- Lichtenthaler M. R., Higelin A., Kraft A., Hughes S., Steffani A., Plattner D. A., Slattery J. M., Krossing I. Organometallics. 2013;32:6725. [Google Scholar]
- Lichtenthaler M. R., Maurer S., Mangan R. J., Stahl F., Mönkemeyer F., Hamann J., Krossing I. Chem. – Eur. J. 2015;21:471. doi: 10.1002/chem.201404833. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler M. R., Maurer S., Mangan R. J., Stahl F., Mönkemeyer F., Hamann J., Krossing I. Chem. – Eur. J. 2015;21:157. doi: 10.1002/chem.201404833. [DOI] [PubMed] [Google Scholar]
- Sarazin Y., Carpentier J.-F. Chem. Rev. 2015;115:3564. doi: 10.1021/acs.chemrev.5b00033. [DOI] [PubMed] [Google Scholar]
- Engesser T. A., Krossing I. Coord. Chem. Rev. 2013;257:946. [Google Scholar]
- Aldridge S., Jones C., Gans-Eichler T., Stasch A., Kays D. L., Coombs N. D., Willock D. J. Angew. Chem., Int. Ed. 2006;45:6118. doi: 10.1002/anie.200602162. [DOI] [PubMed] [Google Scholar]
- Krossing I., Raabe I. Angew. Chem., Int. Ed. 2004;43:2066. doi: 10.1002/anie.200300620. [DOI] [PubMed] [Google Scholar]
- Cullinane J., Jolleys A., Mair F. S. Dalton Trans. 2013;42:11971. doi: 10.1039/c3dt51233a. [DOI] [PubMed] [Google Scholar]
- Strauss S. H. Chem. Rev. 1993;93:927. [Google Scholar]
- Reed C. A. Acc. Chem. Res. 1998;31:133. [Google Scholar]
- Lichtenthaler M. R., Stahl F., Kratzert D., Heidinger L., Schleicher E., Hamann J., Himmel D., Weber S., Krossing I. Nat. Commun. 2015;6:8288. doi: 10.1038/ncomms9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körbe S., Schreiber P. J., Michl J. Chem. Rev. 2006;106:5208. doi: 10.1021/cr050548u. [DOI] [PubMed] [Google Scholar]
- Schmidbaur H., Schier A. Organometallics. 2008;27:2361. [Google Scholar]
- Knapp C., in Comprehensive Inorganic Chemistry II, ed. J. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 651–679. [Google Scholar]
- Krossing I., in Comprehensive Inorganic Chemistry II, ed. J. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 681–705. [Google Scholar]
- Chen E. Y.-X. and Lancaster S. J., in Comprehensive Inorganic Chemistry II, ed. J. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 707–754. [Google Scholar]
- Malinowski P., Kraft A., Schaefer J. and Krossing I., 2015, invited review by Angew. Chem., to be submitted within 2015. [Google Scholar]
- Kim K.-C., Reed C. A., Elliott D. W., Mueller L. J., Tham F., Lin L., Lambert J. B. Science. 2002;297:825. doi: 10.1126/science.1073540. [DOI] [PubMed] [Google Scholar]
- Ivanova S. M., Ivanov S. V., Miller S. M., Anderson O. P., Solntsev K. A., Strauss S. H., Lupinetti A. J., Havighurst M. D., Miller S. M., Anderson O. P., Strauss S. H. Inorg. Chem. J. Am. Chem. Soc. 1999;1999;38121:3756. 11920. [Google Scholar]
- King B. T., Michl J. J. Am. Chem. Soc. 2000;122:10255. [Google Scholar]
- Drews T., Seppelt K. Angew. Chem., Int. Ed. 1997;36:273. [Google Scholar]
- Cameron T. S., Dionne I., Krossing I., Passmore J. Solid State Sci. 2002;4:1435. [Google Scholar]
- Köchner T., Engesser T. A., Scherer H., Plattner D. A., Steffani A., Krossing I. Angew. Chem., Int. Ed. 2012;51:6529. doi: 10.1002/anie.201201262. [DOI] [PubMed] [Google Scholar]
- Slattery J. M., Higelin A., Bayer T., Krossing I. Angew. Chem., Int. Ed. 2010;49:3228. doi: 10.1002/anie.201000156. [DOI] [PubMed] [Google Scholar]
- Wehmschulte R. J. Angew. Chem., Int. Ed. 2010;49:4708. doi: 10.1002/anie.201001102. [DOI] [PubMed] [Google Scholar]
- Reus C., Wagner M. Nat. Chem. 2014;6:466. doi: 10.1038/nchem.1953. [DOI] [PubMed] [Google Scholar]
- Shoji Y., Tanaka N., Mikami K., Uchiyama M., Fukushima T. Nat. Chem. 2014;6:498. doi: 10.1038/nchem.1948. [DOI] [PubMed] [Google Scholar]
- Lambert J. B., Lin L., Keinan S., Müller T. J. Am. Chem. Soc. 2003;125:6022. doi: 10.1021/ja034362t. [DOI] [PubMed] [Google Scholar]
- Wilson W. W., Vij A., Vij V., Bernhardt E., Christe K. O. Chem. – Eur. J. 2003;9:2840. doi: 10.1002/chem.200304973. [DOI] [PubMed] [Google Scholar]
- Anulewicz-Ostrowska R., Kliś T., Krajewski D., Lewandowski B., Serwatowski J. Tetrahedron Lett. 2003;44:7329. [Google Scholar]
- Kratzert D., Holstein J. J., Krossing I. J. Appl. Crystallogr. 2015;48:933. doi: 10.1107/S1600576715005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H. J. Appl. Crystallogr. 2009;42:339. doi: 10.1107/S0021889811041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov S. V., Davis J. A., Miller S. M., Anderson O. P., Strauss S. H. Inorg. Chem. 2003;42:4489. doi: 10.1021/ic0344160. [DOI] [PubMed] [Google Scholar]
- Bolli C., Derendorf J., Jenne C., Scherer H., Sindlinger C. P., Wegener B. Chem. – Eur. J. 2014;20:13783. doi: 10.1002/chem.201403625. [DOI] [PubMed] [Google Scholar]
- Wegener M., Huber F., Bolli C., Jenne C., Kirsch S. F. Chem. – Eur. J. 2015;21:1328. doi: 10.1002/chem.201404487. [DOI] [PubMed] [Google Scholar]
- Drews T., Koch W., Seppelt K. J. Am. Chem. Soc. 1999;121:4379. [Google Scholar]
- Mazej Z., Goreshnik E. Eur. J. Inorg. Chem. 2015:1453. [Google Scholar]
- Bolskar R. D., Mathur R. S., Reed C. A. J. Am. Chem. Soc. 1996;118:13093. [Google Scholar]
- Ivanova S. M., Nolan B. G., Kobayashi Y., Miller S. M., Anderson O. P., Strauss S. H. Chem. – Eur. J. 2001;7:503. doi: 10.1002/1521-3765(20010119)7:2<503::aid-chem503>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Krossing I. Chem. – Eur. J. 2001;7:490. doi: 10.1002/1521-3765(20010119)7:2<490::aid-chem490>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Xie Z., Jelinek T., Bau R., Reed C. A. J. Am. Chem. Soc. 1994;116:1907. [Google Scholar]
- Jutzi P., Müller C., Stammler A., Stammler H.-G. Organometallics. 2000;19:1442. [Google Scholar]
- Santiso-Quiñones G., Higelin A., Schaefer J., Brückner R., Knapp C., Krossing I. Chem. – Eur. J. 2009;15:6663. doi: 10.1002/chem.200900244. [DOI] [PubMed] [Google Scholar]
- Schaefer J., Steffani A., Plattner D. A., Krossing I. Angew. Chem., Int. Ed. 2012;51:6009. doi: 10.1002/anie.201201642. [DOI] [PubMed] [Google Scholar]
- Massey A. G., Park A. J. J. Organomet. Chem. 1964;2:245. [Google Scholar]
- Ashley A. E., Herrington T. J., Wildgoose G. G., Zaher H., Thompson A. L., Rees N. H., Krämer T., O’Hare D. J. Am. Chem. Soc. 2011;133:14727. doi: 10.1021/ja205037t. [DOI] [PubMed] [Google Scholar]
- Kolychev E. L., Bannenberg T., Freytag M., Daniliuc C. G., Jones P. G., Tamm M. Chem. – Eur. J. 2012;18:16938. doi: 10.1002/chem.201202840. [DOI] [PubMed] [Google Scholar]
- Belgardt T., Storre J., Roesky H. W., Noltemeyer M., Schmidt H.-G. Inorg. Chem. 1995;34:3821. [Google Scholar]
- Müller L. O., Himmel D., Stauffer J., Steinfeld G., Slattery J., Santiso-Quiñones G., Brecht V., Krossing I., Müller L. O., Himmel D., Stauffer J., Steinfeld G., Slattery J., Santiso-Quiñones G., Brecht V., Krossing I., Kraft A., Trapp N., Himmel D., Böhrer H., Schlüter P., Scherer H., Krossing I. Angew. Chem., Int. Ed. Angew. Chem. Chem. – Eur. J. 2008;2008;2012;4712018:7659. 7772, 9371. doi: 10.1002/anie.200800783. [DOI] [PubMed] [Google Scholar]
- Douvris C., Stoyanov E. S., Tham F. S., Reed C. A. Chem. Commun. 2007:1145. doi: 10.1039/b617606b. [DOI] [PubMed] [Google Scholar]
- Chiu C.-W., Gabbaï F. P. Organometallics. 2008;27:1657. [Google Scholar]
- Bohrer H., Trapp N., Himmel D., Schleep M., Krossing I. Dalton Trans. 2015;44:7489. doi: 10.1039/c4dt02822h. [DOI] [PubMed] [Google Scholar]
- Kögel J. F., Linder T., Schröder F. G., Sundermeyer J., Goll S. K., Himmel D., Krossing I., Kütt K., Saame J., Leito I. Chem. – Eur. J. 2015;21:5769. doi: 10.1002/chem.201405391. [DOI] [PubMed] [Google Scholar]
- Scholz F., Himmel D., Eisele L., Unkrig W., Krossing I. Angew. Chem., Int. Ed. 2014;53:1689. doi: 10.1002/anie.201308120. [DOI] [PubMed] [Google Scholar]
- Scholz F., Himmel D., Eisele L., Unkrig W., Martens A., Schlüter P., Krossing I. Chem. – Eur. J. 2015;21:7489. doi: 10.1002/chem.201405952. [DOI] [PubMed] [Google Scholar]
- Beichel W., Panzer J. M. U., Hätty J., Ye X., Himmel D., Krossing I., Beichel W., Panzer J. M. U., Hätty J., Ye X., Himmel D., Krossing I. Angew. Chem., Int. Ed. Angew. Chem. 2014;2014;53126:6637. 6755. doi: 10.1002/anie.201402577. [DOI] [PubMed] [Google Scholar]
- Welton T., Weingärtner H. Chem. Rev. Angew. Chem., Int. Ed. 1999;2008;9947:2071. 654. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- Müller U., Isaeva A., Ruck M. Z. Anorg. Allg. Chem. 2014;640:1564. [Google Scholar]
- Ahmed E., Breternitz J., Groh M. F., Isaeva A., Ruck M. Eur. J. Inorg. Chem. 2014:3037. [Google Scholar]
- Ahmed E., Ruck M. Coord. Chem. Rev. 2011;255:2892. [Google Scholar]
- Groh M. F., Breternitz J., Ahmed E., Isaeva A., Efimova A., Schmidt P., Ruck M. Z. Anorg. Allg. Chem. 2015;641:388. [Google Scholar]
- Weigand J. J., Holthausen M., Fröhlich R. Angew. Chem., Int. Ed. 2009;48:295. doi: 10.1002/anie.200804903. [DOI] [PubMed] [Google Scholar]
- Himmel D., Goll S. K., Leito I., Krossing I., Himmel D., Goll S. K., Leito I., Krossing I., Himmel D., Goll S. K., Leito I., Krossing I., Himmel D., Goll S. K., Leito I., Krossing I. Angew. Chem., Int. Ed. Angew. Chem. Chem. – Eur. J. Chem. – Eur. J. 2010;2010;2011;2012;491221718:6885. 7037, 5808, 9333. doi: 10.1002/anie.201000252. [DOI] [PubMed] [Google Scholar]
- Radtke V., Himmel D., Pütz K., Goll S. K., Krossing I. Chem. – Eur. J. 2014;20:4194. doi: 10.1002/chem.201302473. [DOI] [PubMed] [Google Scholar]
- Himmel D., Goll S. K., Scholz F., Radtke V., Leito I., Krossing I. ChemPhysChem. 2015;16:1428. doi: 10.1002/cphc.201402906. [DOI] [PubMed] [Google Scholar]
- Krossing I. J. Am. Chem. Soc. 2001;123:4603. doi: 10.1021/ja002007m. [DOI] [PubMed] [Google Scholar]
- Cameron T. S., Deeth R. J., Dionne I., Du H., Jenkins H. D. B., Krossing I., Passmore J., Roobottom H. K. Inorg. Chem. 2000;39:5614. doi: 10.1021/ic990760e. [DOI] [PubMed] [Google Scholar]
- Muthaiah S., Do D. C. H., Ganguly R., Vidovic D. Organometallics. 2013;32:6718. [Google Scholar]
- Bartlett P. D., Condon F. E., Schneider A. J. Am. Chem. Soc. 1944;66:1531. [Google Scholar]
- Duttwyler S., Do Q.-Q., Linden A., Baldridge K. K., Siegel J. S. Angew. Chem., Int. Ed. 2008;47:1719. doi: 10.1002/anie.200705291. [DOI] [PubMed] [Google Scholar]
- Klosin J., Roof G. R., Chen E. Y. X., Abboud K. A. Organometallics. 2000;19:4684. [Google Scholar]
- Köchner T., Riedel S., Lehner A. J., Scherer H., Raabe I., Engesser T. A., Scholz F. W., Gellrich U., Eiden P., Paz-Schmidt R. A., Plattner D. A., Krossing I. Angew. Chem., Int. Ed. 2010;49:8139. doi: 10.1002/anie.201003031. [DOI] [PubMed] [Google Scholar]
- Huber M., Kurek A., Krossing I., Mülhaupt R., Schnöckel H. Z. Anorg. Allg. Chem. 2009;635:1787. [Google Scholar]
- Arii H., Nakadate F., Mochida K., Kawashima T. Organometallics. 2011;30:4471. [Google Scholar]
- Hoffmann S. P., Kato T., Tham F. S., Reed C. A. Chem. Commun. 2006:767. doi: 10.1039/b511344j. [DOI] [PubMed] [Google Scholar]
- Poleschner H., Seppelt K. Angew. Chem., Int. Ed. 2013;52:12838. doi: 10.1002/anie.201307161. [DOI] [PubMed] [Google Scholar]
- Haaland A., Haaland A. Angew. Chem., Int. Ed. Angew. Chem. 1989;1989;28101:992. 1017. [Google Scholar]
- Himmel D., Krossing I., Schnepf A., Schmidbaur H., Schier A., Schmidbaur H., Schier A. Angew. Chem., Int. Ed. Angew. Chem., Int. Ed. Angew. Chem. 2014;2013;2013;5352125:370. 176, 187. [Google Scholar]
- Koppe R., Schnoeckel H., Holzmann N., Hermann M., Frenking G., Frenking G., Frenking G., Himmel D., Krossing I., Schnepf A., Himmel D., Krossing I., Schnepf A. Chem. Sci. Chem. Sci. Angew. Chem., Int. Ed. Angew. Chem. Angew. Chem. Angew. Chem., Int. Ed. 2015;2015;2014;2014;2014;2014;665312612653:1199. 4089, 6040, 6152, 6159, 6047. [Google Scholar]
- Scholz F., Himmel D., Heinemann F. W., von Ragué Schleyer P., Meyer K., Krossing I. Science. 2013;341:62. doi: 10.1126/science.1238849. [DOI] [PubMed] [Google Scholar]
- Kessler M., Knapp C., Zogaj A. Organometallics. 2011;30:3786. [Google Scholar]
- Downs A. J. and Himmel H.-J., The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, John Wiley & Sons, Ltd, 2011, pp. 1–74. [Google Scholar]
- Piers W. E., Bourke S. C., Conroy K. D. Angew. Chem., Int. Ed. 2005;44:5016. doi: 10.1002/anie.200500402. [DOI] [PubMed] [Google Scholar]
- Bochmann M. Coord. Chem. Rev. 2009;253:2000. [Google Scholar]
- Corbett J. D., Prince D. J., Garbisch B. Inorg. Chem. 1970;9:2731. [Google Scholar]
- Gillespie R. J., Pez G. P. Inorg. Chem. 1969;8:1233. [Google Scholar]
- Akhbari K., Morsali A. Coord. Chem. Rev. 2010;254:1977. [Google Scholar]
- Cowley A. H. Chem. Commun. 2004:2369. doi: 10.1039/b409497m. [DOI] [PubMed] [Google Scholar]
- Dagorne S., Atwood D. A. Chem. Rev. 2008;108:4037. doi: 10.1021/cr078351k. [DOI] [PubMed] [Google Scholar]
- Vidovic D., Aldridge S. Chem. Sci. 2011;2:601. [Google Scholar]
- Allan C. J. and Macdonald C. L. B., in Comprehensive Inorganic Chemistry II, ed. J. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 485–566. [Google Scholar]
- Schneider U., Kobayashi S. Acc. Chem. Res. 2012;45:1331. doi: 10.1021/ar300008t. [DOI] [PubMed] [Google Scholar]
- Barr J., Gillespie R. J., Kapoor R., Malhotra K. C. Can. J. Chem. 1968;46:149. [Google Scholar]
- Schmidbaur H. Angew. Chem., Int. Ed. Engl. 1985;24:893. [Google Scholar]
- Beck J., Bock G. Z. Anorg. Allg. Chem. 1994;620:1971. [Google Scholar]
- Atwood D. A. Coord. Chem. Rev. 1998;176:407. [Google Scholar]
- Baumann A., Beck J. Z. Anorg. Allg. Chem. 2004;630:2078. [Google Scholar]
- Vidovic D., Pierce G. A., Aldridge S. Chem. Commun. 2009:1157. doi: 10.1039/b816042b. [DOI] [PubMed] [Google Scholar]
- Braunschweig H., Dewhurst R. D., Schneider A. Chem. Rev. 2010;110:3924. doi: 10.1021/cr900333n. [DOI] [PubMed] [Google Scholar]
- Jones C. and Stasch A., The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, John Wiley & Sons, Ltd, 2011, pp. 285–341. [Google Scholar]
- Cooper B. F. T. and Macdonald C. L. B., The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, John Wiley & Sons, Ltd, 2011, pp. 342–401. [Google Scholar]
- Brothers P. J. and Ruggiero C. E., The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, John Wiley & Sons, Ltd, 2011, pp. 519–611. [Google Scholar]
- De Vries T. S., Prokofjevs A., Vedejs E. Chem. Rev. 2012;112:4246. doi: 10.1021/cr200133c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gallardo S., Bollermann T., Fischer R. A., Murugavel R. Chem. Rev. 2012;112:3136. doi: 10.1021/cr2001146. [DOI] [PubMed] [Google Scholar]
- Ingleson M. J. Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem. 2013;109:28. [Google Scholar]
- Koelle P., Noeth H. Chem. Rev. 1985;85:399. [Google Scholar]
- Shoji Y., Tanaka N., Mikami K., Uchiyama M., Fukushima T. Nat. Chem. 2014;6:498. doi: 10.1038/nchem.1948. [DOI] [PubMed] [Google Scholar]
- Courtenay S., Mutus J. Y., Schurko R. W., Stephan D. W. Angew. Chem., Int. Ed. 2002;41:498. doi: 10.1002/1521-3773(20020201)41:3<498::aid-anie498>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kato T., Tham F. S., Boyd P. D. W., Reed C. A. Heteroat. Chem. 2006;17:209. [Google Scholar]
- Del Grosso A., Pritchard R. G., Muryn C. A., Ingleson M. J. Organometallics. 2010;29:241. [Google Scholar]
- Gonsior M., Krossing I. Chem. – Eur. J. 2004;10:5730. doi: 10.1002/chem.200400046. [DOI] [PubMed] [Google Scholar]
- Gillespie R. J., Milne J. B. Inorg. Chem. 1966;5:1577. [Google Scholar]
- Inés B., Patil M., Carreras J., Goddard R., Thiel W., Alcarazo M. Angew. Chem., Int. Ed. 2011;50:8400. doi: 10.1002/anie.201103197. [DOI] [PubMed] [Google Scholar]
- Shuttleworth T. A., Huertos M. A., Pernik I., Young R. D., Weller A. S. Dalton Trans. 2013;42:12917. doi: 10.1039/c3dt50817j. [DOI] [PubMed] [Google Scholar]
- Braunschweig H., Radacki K., Schneider A. Chem. Commun. 2010;46:6473. doi: 10.1039/c0cc01802c. [DOI] [PubMed] [Google Scholar]
- Braunschweig H., Kaupp M., Lambert C., Nowak D., Radacki K., Schinzel S., Uttinger K. Inorg. Chem. 2008;47:7456. doi: 10.1021/ic800876p. [DOI] [PubMed] [Google Scholar]
- Litters S., Kaifer E., Enders M., Himmel H.-J. Nat. Chem. 2013;5:1029. doi: 10.1038/nchem.1776. [DOI] [PubMed] [Google Scholar]
- Pierce G. A., Vidovic D., Kays D. L., Coombs N. D., Thompson A. L., Jemmis E. D., De S., Aldridge S. Organometallics. 2009;28:2947. [Google Scholar]
- Braunschweig H., Radacki K., Uttinger K. Organometallics. 2008;27:6005. [Google Scholar]
- Fabrizi de Biani F., Gmeinwieser T., Herdtweck E., Jäkle F., Laschi F., Wagner M., Zanello P. Organometallics. 1997;16:4776. [Google Scholar]
- Ding L., Ma K., Durner G., Bolte M., Fabrizi de Biani F., Zanello P., Wagner M. J. Chem. Soc., Dalton Trans. 2002:1566. [Google Scholar]
- Kim K.-C., Reed C. A., Long G. S., Sen A. J. Am. Chem. Soc. 2002;124:7662. doi: 10.1021/ja0259990. [DOI] [PubMed] [Google Scholar]
- Young J. D., Khan M. A., Wehmschulte R. J. Organometallics. 2004;23:1965. [Google Scholar]
- Kessler M., Knapp C., Krossing I., Lichtenthaler M. R., 2011, unpublished results.
- Lee S.-J., Shapiro P. J., Twamley B. Organometallics. 2006;25:5582. [Google Scholar]
- Burns C. T., Stelck D. S., Shapiro P. J., Vij A., Kunz K., Kehr G., Concolino T., Rheingold A. L. Organometallics. 1999;18:5432. [Google Scholar]
- Burns C. T., Shapiro P. J., Budzelaar P. H. M., Willett R., Vij A. Organometallics. 2000;19:3361. [Google Scholar]
- Gonsior M., Krossing I., Matern E. Chem. – Eur. J. 2006;12:1703. doi: 10.1002/chem.200500138. [DOI] [PubMed] [Google Scholar]
- Masuda J. D., Stephan D. W. Dalton Trans. 2006:2089. doi: 10.1039/b513531a. [DOI] [PubMed] [Google Scholar]
- Radzewich C. E., Guzei I. A., Jordan R. F. J. Am. Chem. Soc. 1999;121:8673. [Google Scholar]
- Stanga O., Lund C. L., Liang H., Quail J. W., Müller J. Organometallics. 2005;24:6120. [Google Scholar]
- Saez P. J. A., Oakley S. H., Coles M. P., Hitchcock P. B. Chem. Commun. 2007:816. doi: 10.1039/b611343e. [DOI] [PubMed] [Google Scholar]
- Dagorne S., Bellemin-Laponnaz S., Welter R. Organometallics. 2004;23:3053. [Google Scholar]
- Korolev A. V., Delpech F., Dagorne S., Guzei I. A., Jordan R. F. Organometallics. 2001;20:3367. [Google Scholar]
- Korolev A. V., Ihara E., Guzei I. A., Young V. G., Jordan R. F. J. Am. Chem. Soc. 2001;123:8291. doi: 10.1021/ja010242e. [DOI] [PubMed] [Google Scholar]
- Lewiński J., Horeglad P., Dranka M., Justyniak I. Inorg. Chem. 2004;43:5789. doi: 10.1021/ic049337i. [DOI] [PubMed] [Google Scholar]
- Hill M. S., Hitchcock P. B. Organometallics. 2002;21:3258. [Google Scholar]
- Wrobel O., Schaper F., Brintzinger H. H. Organometallics. 2004;23:900. [Google Scholar]
- Stasch A., Roesky H. W., Noltemeyer M., Schmidt H.-G. Inorg. Chem. 2005;44:5854. doi: 10.1021/ic048594k. [DOI] [PubMed] [Google Scholar]
- Atwood D. A., Harvey M. J. Chem. Rev. 2001;101:37. doi: 10.1021/cr990008v. [DOI] [PubMed] [Google Scholar]
- Dagorne S., Bouyahyi M., Vergnaud J., Carpentier J.-F. Organometallics. 2010;29:1865. [Google Scholar]
- Atwood D. A., Jegier J. A., Rutherford D. J. Am. Chem. Soc. 1995;117:6779. [Google Scholar]
- Atwood D. A., Jegier J. A., Rutherford D. Inorg. Chem. 1996;35:63. doi: 10.1021/ic950574i. [DOI] [PubMed] [Google Scholar]
- Muñoz-Hernandez M.-A., Parkin S., Yearwood B., Wei P., Atwood D. J. Chem. Crystallogr. 2000;30:215–218. [Google Scholar]
- Munoz-Hernandez M.-A., McKee M. L., Keizer T. S., Yearwood B. C., Atwood D. A. J. Chem. Soc., Dalton Trans. 2002:410. [Google Scholar]
- Dagorne S., Le Bideau F., Welter R., Bellemin-Laponnaz S., Maisse-Franois A. Chem. – Eur. J. 2007;13:3202. doi: 10.1002/chem.200601112. [DOI] [PubMed] [Google Scholar]
- Korolev A. V., Guzei I. A., Jordan R. F. J. Am. Chem. Soc. 1999;121:11605. [Google Scholar]
- Wrobel O., Schaper F., Wieser U., Gregorius H., Brintzinger H. H. Organometallics. 2003;22:1320. [Google Scholar]
- Nakata N., Saito Y., Ishii A. Organometallics. 2014;33:1840. [Google Scholar]
- Dagorne S., Guzei I. A., Coles M. P., Jordan R. F. J. Am. Chem. Soc. 2000;122:274. [Google Scholar]
- Cadenbach T., Gemel C., Bollermann T., Fischer R. A. Inorg. Chem. 2009;48:5021. doi: 10.1021/ic802283r. [DOI] [PubMed] [Google Scholar]
- Wehmschulte R. J., Steele J. M., Young J. D., Khan M. A. J. Am. Chem. Soc. 2003;125:1470. doi: 10.1021/ja029142e. [DOI] [PubMed] [Google Scholar]
- Buchin B., Gemel C., Cadenbach T., Schmid R., Fischer R. A. Angew. Chem., Int. Ed. 2006;45:1074. doi: 10.1002/anie.200503028. [DOI] [PubMed] [Google Scholar]
- Macdonald C. L. B., Gorden J. D., Voigt A., Cowley A. H. J. Am. Chem. Soc. 2000;122:11725. [Google Scholar]
- Higelin A., Keller S., Göhringer C., Jones C., Krossing I. Angew. Chem., Int. Ed. 2013;52:4941. doi: 10.1002/anie.201209757. [DOI] [PubMed] [Google Scholar]
- Higelin A., Sachs U., Keller S., Krossing I. Chem. – Eur. J. 2012;18:10029. doi: 10.1002/chem.201104040. [DOI] [PubMed] [Google Scholar]
- Budanow A., Sinke T., Tillmann J., Bolte M., Wagner M., Lerner H.-W. Organometallics. 2012;31:7298. [Google Scholar]
- Lichtenthaler M. R., Stahl F., Kratzert D., Benkmil B., Wegner H. A., Krossing I. Eur. J. Inorg. Chem. 2014:4335. [Google Scholar]
- Dagorne S., Bellemin-Laponnaz S., Maisse-Francois A., Rager M.-N., Juge L., Welter R. Eur. J. Inorg. Chem. 2005:4206. [Google Scholar]
- Lichtenberg C., Spaniol T. P., Okuda J. Inorg. Chem. 2012;51:2254. doi: 10.1021/ic202293t. [DOI] [PubMed] [Google Scholar]
- Linti G., Seifert A. Z. Anorg. Allg. Chem. 2008;634:1312. [Google Scholar]
- Higelin A., Haber C., Meier S., Krossing I. Dalton Trans. 2012;41:12011. doi: 10.1039/c2dt30379e. [DOI] [PubMed] [Google Scholar]
- Bunn N. R., Aldridge S., Coombs D. L., Rossin A., Willock D. J., Jones C., Ooi L.-L. Chem. Commun. 2004:1732. doi: 10.1039/b405943c. [DOI] [PubMed] [Google Scholar]
- Coombs N. D., Bunn N. R., Kays D. L., Day J. K., Ooi L.-L., Aldridge S. Inorg. Chim. Acta. 2006;359:3693. [Google Scholar]
- Ueno K., Watanabe T., Ogino H. Appl. Organomet. Chem. 2003;17:403. [Google Scholar]
- Bartlett N., Lohmann D. H. J. Chem. Soc. 1962:5253. [Google Scholar]
- Gillespie R. J., Milne J. B. Inorg. Chem. 1966;5:1236. [Google Scholar]
- Kempter A., Gemel C., Hardman N. J., Fischer R. A. Inorg. Chem. 2006;45:3133. doi: 10.1021/ic0521731. [DOI] [PubMed] [Google Scholar]
- Kempter A., Gemel C., Cadenbach T., Fischer R. A. Inorg. Chem. 2007;46:9481. doi: 10.1021/ic7013804. [DOI] [PubMed] [Google Scholar]
- Fleischmann M., Welsch S., Krauss H., Schmidt M., Bodensteiner M., Peresypkina E. V., Sierka M., Groeger C., Scheer M. Chem. – Eur. J. 2014;20:3759. doi: 10.1002/chem.201304466. [DOI] [PubMed] [Google Scholar]
- Cadenbach T., Gemel C., Bollermann T., Fernandez I., Frenking G., Fischer R. A. Chem. – Eur. J. 2008;14:10789. doi: 10.1002/chem.200801328. [DOI] [PubMed] [Google Scholar]
- Halbherr M., Bollermann T., Gemel C., Fischer R. A. Angew. Chem., Int. Ed. 2010;49:1878. doi: 10.1002/anie.200905920. [DOI] [PubMed] [Google Scholar]
- Aldridge S. Angew. Chem., Int. Ed. 2006;45:8097. doi: 10.1002/anie.200603643. [DOI] [PubMed] [Google Scholar]
- Buchin B., Gemel C., Cadenbach T., Fernández I., Frenking G., Fischer R. A. Angew. Chem., Int. Ed. 2006;45:5207. doi: 10.1002/anie.200601007. [DOI] [PubMed] [Google Scholar]
- Cadenbach T., Gemel C., Zacher D., Fischer R. A. Angew. Chem., Int. Ed. 2008;47:3438. doi: 10.1002/anie.200705031. [DOI] [PubMed] [Google Scholar]
- Macdonald C. L. B., Corrente A. M., Andrews C. G., Taylor A., Ellis B. D. Chem. Commun. 2004:250. doi: 10.1039/b312983g. [DOI] [PubMed] [Google Scholar]
- Andrews C. G., Macdonald C. L. B. J. Organomet. Chem. 2005;690:5090. [Google Scholar]
- Cowley A. H., Macdonald C. L. B., Silverman J. S., Gorden J. D., Voigt A. Chem. Commun. 2001:175. [Google Scholar]
- Jones J. N., Macdonald C. L. B., Gorden J. D., Cowley A. H. J. Organomet. Chem. 2003;666:3. [Google Scholar]
- Welsch S., Bodensteiner M., Dušek M., Sierka M., Scheer M. Chem. – Eur. J. 2010;16:13041. doi: 10.1002/chem.201002484. [DOI] [PubMed] [Google Scholar]
- Mansaray H. B., Tang C. Y., Vidovic D., Thompson A. L., Aldridge S. Inorg. Chem. 2012;51:13017. doi: 10.1021/ic3021386. [DOI] [PubMed] [Google Scholar]
- Jurca T., Lummiss J., Burchell T. J., Gorelsky S. I., Richeson D. S. J. Am. Chem. Soc. 2009;131:4608. doi: 10.1021/ja901128q. [DOI] [PubMed] [Google Scholar]
- Beck J., Brendel C. J., Bengtsson-Kloo L., Krebs B., Mummert M., Stankowski A., Ulvenlund S. Chem. Ber. 1996;129:1219. [Google Scholar]
- Delpech F., Guzei I. A., Jordan R. F. Organometallics. 2002;21:1167. [Google Scholar]
- Peckermann I., Robert D., Englert U., Spaniol T. P., Okuda J. Organometallics. 2008;27:4817. [Google Scholar]
- Andrews C. G., Macdonald C. L. B. Angew. Chem., Int. Ed. 2005;44:7453. doi: 10.1002/anie.200502901. [DOI] [PubMed] [Google Scholar]
- Cooper B. F. T., Macdonald C. L. B. New J. Chem. 2010;34:1551. [Google Scholar]
- Cooper B. F. T., Macdonald C. L. B. J. Organomet. Chem. 2008;693:1707. [Google Scholar]
- Bunn N. R., Aldridge S., Kays D. L., Coombs N. D., Rossin A., Willock D. J., Day J. K., Jones C., Ooi L.-L. Organometallics. 2005;24:5891. [Google Scholar]
- Burns R. C., Gillespie R. J., Luk W.-C. Inorg. Chem. 1978;17:3596. [Google Scholar]
- Alberti D., Pörschke K.-R. Organometallics. 2004;23:1459. [Google Scholar]
- Hurlburt P. K., Anderson O. P., Strauss S. H. Can. J. Chem. 1992;70:726. [Google Scholar]
- Barbarich T. J., Miller S. M., Anderson O. P., Strauss S. H. J. Mol. Catal. A: Chem. 1998;128:289. [Google Scholar]
- Gonsior M., Krossing I., Mitzel N. Z. Anorg. Allg. Chem. 2002;628:1821. [Google Scholar]
- Mathur R. S., Drovetskaya T., Reed C. A. Acta Crystallogr. 1997;C53:881. [Google Scholar]
- Noirot M. D., Anderson O. P., Strauss S. H. Inorg. Chem. 1987;26:2216. [Google Scholar]
- Sarazin Y., Hughes D. L., Kaltsoyannis N., Wright J. A., Bochmann M. J. Am. Chem. Soc. 2007;129:881. doi: 10.1021/ja0657105. [DOI] [PubMed] [Google Scholar]
- Sarazin Y., Kaltsoyannis N., Wright J. A., Bochmann M. Organometallics. 2007;26:1811. [Google Scholar]
- Nakai H., Tang Y., Gantzel P., Meyer K. Chem. Commun. 2003:24. doi: 10.1039/b209071f. [DOI] [PubMed] [Google Scholar]
- Jurca T., Korobkov I., Gorelsky S. I., Richeson D. S. Inorg. Chem. 2013;52:5749. doi: 10.1021/ic302552v. [DOI] [PubMed] [Google Scholar]
- Welsch S., Gregoriades L. J., Sierka M., Zabel M., Virovets A. V., Scheer M. Angew. Chem., Int. Ed. 2007;46:9323. doi: 10.1002/anie.200704015. [DOI] [PubMed] [Google Scholar]
- Back O., Donnadieu B., Parameswaran P., Frenking G., Bertrand G. Nat. Chem. 2010;2:369. doi: 10.1038/nchem.617. [DOI] [PubMed] [Google Scholar]
- Szymczak N. K., Han F., Tyler D. R. Dalton Trans. 2004:3941. doi: 10.1039/b415259j. [DOI] [PubMed] [Google Scholar]
- Shen C.-T., Liu Y.-H., Peng S.-M., Chiu C.-W., Shen C.-T., Liu Y.-H., Peng S.-M., Chiu C.-W. Angew. Chem., Int. Ed. Angew. Chem. 2013;2013;52125:13293. 13535. [Google Scholar]
- Hartl H., Nowicki J., Minkwitz R. Angew. Chem., Int. Ed. 1991;30:328. [Google Scholar]
- Bentivegna B., Mariani C. I., Smith J. R., Ma S., Rheingold A. L., Brunker T. J. Organometallics. 2014;33:2820. [Google Scholar]
- Dagorne S., Janowska I., Welter R., Zakrzewski J., Jaouen G. Organometallics. 2004;23:4706. [Google Scholar]
- Dagorne S. C. R. Chim. 2006;9:1143. [Google Scholar]
- Cosledan F., Hitchcock P. B., Lappert M. F. Chem. Commun. 1999:705. [Google Scholar]
- Masuda J. D., Walsh D. M., Wei P., Stephan D. W. Organometallics. 2004;23:1819. [Google Scholar]
- Haddad M., Laghzaoui M., Welter R., Dagorne S. Organometallics. 2009;28:4584. [Google Scholar]
- Welsch S., Lescop C., Reau R., Scheer M. Dalton Trans. 2009:2683. doi: 10.1039/b822721g. [DOI] [PubMed] [Google Scholar]
- Reger D. L., Collins J. E., Layland R., Adams R. D. Inorg. Chem. 1996;35:1372. doi: 10.1021/ic951167+. [DOI] [PubMed] [Google Scholar]
- Coombs D. L., Aldridge S., Jones C., Willock D. J., Braunschweig H., Rais D. J. Am. Chem. Soc. Heteroat. Chem. 2003;2005;12516:6356. 566. doi: 10.1021/ja0346936. [DOI] [PubMed] [Google Scholar]
- Niemeyer J., Addy D. A., Riddlestone I., Kelly M., Thompson A. L., Vidovic D., Aldridge S., Niemeyer J., Addy D. A., Riddlestone I., Kelly M., Thompson A. L., Vidovic D., Aldridge S. Angew. Chem., Int. Ed. Angew. Chem. 2011;2011;50123:8908. 9070. doi: 10.1002/anie.201103757. [DOI] [PubMed] [Google Scholar]
- Braunschweig H., Kraft K., Kupfer T., Radacki K., Seeler F. Angew. Chem., Int. Ed. 2008;47:4931. doi: 10.1002/anie.200800533. [DOI] [PubMed] [Google Scholar]
- Chen J., Lalancette R. A., Jakle F. Chem. Commun. 2013;49:4893. doi: 10.1039/c3cc41556b. [DOI] [PubMed] [Google Scholar]
- Kays D. L., Day J. K., Ooi L.-L., Aldridge S., Kays D. L., Rossin A., Day J. K., Ooi L.-L., Aldridge S. Angew. Chem., Int. Ed. Dalton Trans. 2005;2006;44:7457. 399. [Google Scholar]
- Kays D. L., Day J. K., Aldridge S., Harrington R. W., Clegg W. Angew. Chem., Int. Ed. 2006;45:3513. doi: 10.1002/anie.200600714. [DOI] [PubMed] [Google Scholar]
- Bissinger P., Braunschweig H., Damme A., Dewhurst R. D., Kraft K., Kramer T., Radacki K. Chem. – Eur. J. 2013;19:13402. doi: 10.1002/chem.201302263. [DOI] [PubMed] [Google Scholar]
- Firinci E., Bates J. I., Riddlestone I. M., Phillips N., Aldridge S. Chem. Commun. 2013;49:1509. doi: 10.1039/c3cc38073d. [DOI] [PubMed] [Google Scholar]
- Pierce G. A., Aldridge S., Jones C., Gans-Eichler T., Stasch A., Coombs N. D., Willock D. J. Angew. Chem., Int. Ed. 2007;46:2043. doi: 10.1002/anie.200604838. [DOI] [PubMed] [Google Scholar]
- Braunschweig H., Radacki K., Rais D., Scheschkewitz D., Braunschweig H., Radacki K., Rais D., Scheschkewitz D. Angew. Chem., Int. Ed. Angew. Chem. 2005;2005;44117:5651. 5796. doi: 10.1002/anie.200501588. [DOI] [PubMed] [Google Scholar]
- Arnold N., Braunschweig H., Brenner P., Jimenez-Halla J. O. C., Kupfer T., Radacki K. Organometallics. 2012;31:1897. [Google Scholar]
- Braunschweig H., Radacki K., Uttinger K. Chem. – Eur. J. 2008;14:7858. doi: 10.1002/chem.200800879. [DOI] [PubMed] [Google Scholar]
- Hesp K. D., Kannemann F. O., Rankin M. A., McDonald R., Ferguson M. J., Stradiotto M. Inorg. Chem. 2011;50:2431. doi: 10.1021/ic1022328. [DOI] [PubMed] [Google Scholar]
- Coombs N. D., Clegg W., Thompson A. L., Willock D. J., Aldridge S. J. Am. Chem. Soc. 2008;130:5449. doi: 10.1021/ja800876k. [DOI] [PubMed] [Google Scholar]
- Coombs N. D., Vidovic D., Day J. K., Thompson A. L., Le Pevelen D. D., Stasch A., Clegg W., Russo L., Male L., Hursthouse M. B., Willock D. J., Aldridge S. J. Am. Chem. Soc. 2008;130:16111. doi: 10.1021/ja806655f. [DOI] [PubMed] [Google Scholar]
- Beck J., Schlitt K.-J. Chem. Ber. 1995;128:763. [Google Scholar]
- Hershaft A., Corbett J. D. Inorg. Chem. 1963;2:979. [Google Scholar]
- Prokofjevs A., Vedejs E. J. Am. Chem. Soc. 2011;133:20056. doi: 10.1021/ja208093c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagorne S., Lavanant L., Welter R., Chassenieux C., Haquette P., Jaouen G. Organometallics. 2003;22:3732. [Google Scholar]
- Ihara E., Young V. G., Jordan R. F. J. Am. Chem. Soc. 1998;120:8277. doi: 10.1021/ja010242e. [DOI] [PubMed] [Google Scholar]
- Cui C., Giesbrecht G. R., Schmidt J. A. R., Arnold J. Inorg. Chim. Acta. 2003;351:404. [Google Scholar]
- Hill M. S., Atwood D. A. Eur. J. Inorg. Chem. 1998:67. [Google Scholar]
- Braunschweig H., Radacki K., Schneider A. Angew. Chem., Int. Ed. 2010;49:5993. doi: 10.1002/anie.201002300. [DOI] [PubMed] [Google Scholar]
- Bollermann T., Puls A., Gemel C., Cadenbach T., Fischer R. A. Dalton Trans. 2009:1372. doi: 10.1039/b815319a. [DOI] [PubMed] [Google Scholar]
- Freitag K., Banh H., Gemel C., Jerabek P., Seidel R. W., Frenking G., Fischer R. A. Inorg. Chem. 2015;54:352. doi: 10.1021/ic502532g. [DOI] [PubMed] [Google Scholar]
- Cadenbach T., Bollermann T., Gemel C., Fischer R. A. Dalton Trans. 2009:322. doi: 10.1039/b807917j. [DOI] [PubMed] [Google Scholar]
- Henderson G. G. J. Chem. Soc., Trans. 1887;51:224. [Google Scholar]
- Baeyer A., Villiger V. Ber. Dtsch. Chem. Ges. 1902;35:1189. [Google Scholar]
- Gomes de Mesquita A. H., MacGillavry C. H., Eriks K. Acta Crystallogr. 1965;18:437. [Google Scholar]
- Krauße J., Heublein G., Rudakoff G., Leibnitz P., Reck G., Krossing I., Brands H., Feuerhake R., Koenig S., Zhou J., Lancaster S. J., Walker D. A., Beck S., Thornton-Pett M., Bochmann M. J. Crystallogr. Spectrosc. Res. J. Fluorine Chem. J. Am. Chem. Soc. 1991;2001;2001;21112123:45. 83, 223. [Google Scholar]
- Sundaralingam M., Jensen L. H. J. Am. Chem. Soc. 1963;85:3302. [Google Scholar]
- Lambert J. B., Kania L., Zhang S. Chem. Rev. 1995;95:1191. [Google Scholar]
- Müller T., Cations of Group 14 Organometallics, Elsevier, 2005, vol. 53, pp. 155–215. [Google Scholar]
- Parr J. Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem. 2005;101:74. [Google Scholar]
- Klare H. F. T., Oestreich M. Dalton Trans. 2010;39:9176. doi: 10.1039/c003097j. [DOI] [PubMed] [Google Scholar]
- Reed C. A. Acc. Chem. Res. 2010;43:121. doi: 10.1021/ar900159e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asay M., Jones C., Driess M. Chem. Rev. 2011;111:354. doi: 10.1021/cr100216y. [DOI] [PubMed] [Google Scholar]
- Marschner C. and Hlina J., Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 83–117. [Google Scholar]
- Swamy V. S. V. S. N., Pal S., Khan S., Sen S. S. Dalton Trans. 2015;44:12903. doi: 10.1039/c5dt01912e. [DOI] [PubMed] [Google Scholar]
- Reed C. A. Science. 2000;289:101. doi: 10.1126/science.289.5476.101. [DOI] [PubMed] [Google Scholar]
- Riccò M., Pontiroli D., Mazzani M., Gianferrari F., Pagliari M., Goffredi A., Brunelli M., Zandomeneghi G., Meier B. H., Shiroka T. J. Am. Chem. Soc. 2010;132:2064. doi: 10.1021/ja909614x. [DOI] [PubMed] [Google Scholar]
- Kim K.-C., Hauke F., Hirsch A., Boyd P. D. W., Carter E., Armstrong R. S., Lay P. A., Reed C. A. J. Am. Chem. Soc. 2003;125:4024. doi: 10.1021/ja034014r. [DOI] [PubMed] [Google Scholar]
- von Ragué Schleyer P., Watts W. E., Fort R. C., Comisarow M. B., Olah G. A., Saunders M., von Ragué Schleyer P., Olah G. A. J. Am. Chem. Soc. J. Am. Chem. Soc. 1964;1964;8686:5679. 5680. [Google Scholar]
- Montgomery L. K., Grendze M. P., Huffman J. C., Laube T., Laube T., Laube T. J. Am. Chem. Soc. J. Am. Chem. Soc. Angew. Chem., Int. Ed. Engl. Angew. Chem., Int. Ed. Engl. 1987;1989;1987;1986;1091112625:4749. 9224, 560, 349. [Google Scholar]
- Hollenstein S., Laube T. J. Am. Chem. Soc. 1993;115:7240. [Google Scholar]
- Kato T., Reed C. A. Angew. Chem., Int. Ed. 2004;43:2908. doi: 10.1002/anie.200453931. [DOI] [PubMed] [Google Scholar]
- Scholz F., Himmel D., Scherer H., Krossing I. Chem. – Eur. J. 2013;19:109. doi: 10.1002/chem.201203260. [DOI] [PubMed] [Google Scholar]
- Stoyanov E. S., Stoyanova I. V., Tham F. S., Reed C. A. Angew. Chem., Int. Ed. 2012;51:9149. doi: 10.1002/anie.201203958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christe K. O., Zhang X., Bau R., Hegge J., Olah G. A., Prakash G. K. S., Sheehy J. A. J. Am. Chem. Soc. 2000;122:481. [Google Scholar]
- Krossing I., Bihlmeier A., Raabe I., Trapp N. Angew. Chem., Int. Ed. 2003;42:1531. doi: 10.1002/anie.200250172. [DOI] [PubMed] [Google Scholar]
- Mercier H. P. A., Moran M. D., Schrobilgen G. J., Steinberg C., Suontamo R. J. J. Am. Chem. Soc. 2004;126:5533. doi: 10.1021/ja030649e. [DOI] [PubMed] [Google Scholar]
- Lehner A. J., Trapp N., Scherer H., Krossing I. Dalton Trans. 2011;40:1448. doi: 10.1039/c0dt01076f. [DOI] [PubMed] [Google Scholar]
- Minkwitz R., Neikes F. Eur. J. Inorg. Chem. 2000:2283. doi: 10.1021/ic9902673. [DOI] [PubMed] [Google Scholar]
- Laube T. J. Am. Chem. Soc. 2004;126:10904. doi: 10.1021/ja040115t. [DOI] [PubMed] [Google Scholar]
- Raabe I., Röhr C., Krossing I. Dalton Trans. 2007:5376. doi: 10.1039/b711639j. [DOI] [PubMed] [Google Scholar]
- Müller T., Juhasz M., Reed C. A. Angew. Chem., Int. Ed. 2004;43:1543. doi: 10.1002/anie.200352986. [DOI] [PubMed] [Google Scholar]
- Klaer A., Saak W., Haase D., Müller T. J. Am. Chem. Soc. 2008;130:14956. doi: 10.1021/ja8069055. [DOI] [PubMed] [Google Scholar]
- Baenziger N. C., Nelson A. D. J. Am. Chem. Soc. 1968;90:6602. [Google Scholar]
- Shorafa H., Mollenhauer D., Paulus B., Seppelt K. Angew. Chem., Int. Ed. 2009;48:5845. doi: 10.1002/anie.200900666. [DOI] [PubMed] [Google Scholar]
- Molski M. J., Mollenhauer D., Gohr S., Paulus B., Khanfar M. A., Shorafa H., Strauss S. H., Seppelt K. Chem. – Eur. J. 2012;18:6644. doi: 10.1002/chem.201102960. [DOI] [PubMed] [Google Scholar]
- Molski M. J., Khanfar M. A., Shorafa H., Seppelt K. Eur. J. Org. Chem. 2013:3131. [Google Scholar]
- Marchetti F., Pinzino C., Zacchini S., Pampaloni G. Angew. Chem., Int. Ed. 2010;49:5268. doi: 10.1002/anie.201001572. [DOI] [PubMed] [Google Scholar]
- Chen X., Wang X., Sui Y., Li Y., Ma J., Zuo J., Wang X. Angew. Chem., Int. Ed. 2012;51:11878. doi: 10.1002/anie.201205478. [DOI] [PubMed] [Google Scholar]
- Moss R. A., Shen S., Krogh-Jespersen K., Potenza J. A., Schugar H. J., Munjal R. C. J. Am. Chem. Soc. 1986;108:134. [Google Scholar]
- Chadda S. K., Childs R. F., Faggiani R., Lock C. J. L. J. Am. Chem. Soc. 1986;108:1694. [Google Scholar]
- Lambert J. B., Lin L., Rassolov V., Otto M., Scheschkewitz D., Kato T., Midland M. M., Lambert J. B., Bertrand G. Angew. Chem., Int. Ed. Angew. Chem., Int. Ed. 2002;2002;4141:1429. 2275. [Google Scholar]
- Duttwyler S., Zhang Y., Linden A., Reed C. A., Baldridge K. K., Siegel J. S. Angew. Chem., Int. Ed. 2009;48:3787. doi: 10.1002/anie.200900098. [DOI] [PubMed] [Google Scholar]
- Kato T., Stoyanov E., Geier J., Grützmacher H., Reed C. A. J. Am. Chem. Soc. 2004;126:12451. doi: 10.1021/ja047357d. [DOI] [PubMed] [Google Scholar]
- Bolli C., Derendorf J., Keßler M., Knapp C., Scherer H., Schulz C., Warneke J. Angew. Chem., Int. Ed. 2010;49:3536. doi: 10.1002/anie.200906627. [DOI] [PubMed] [Google Scholar]
- Lambert J. B., Zhang S., Stern C. L., Huffman J. C. Science. 1993;260:1917. doi: 10.1126/science.260.5116.1917. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Reißmann M., Jung S., Schäfer A., Saak W., Brendler E., Müller T. Organometallics. 2013;32:4713. [Google Scholar]
- Reißmann M., PhD dissertation, University of Oldenburg, 2014. [Google Scholar]
- Sekiguchi A., Matsuno T., Ichinohe M. J. Am. Chem. Soc. 2000;122:11250. doi: 10.1021/ja011617z. [DOI] [PubMed] [Google Scholar]
- Ichinohe M., Igarashi M., Sanuki K., Sekiguchi A. J. Am. Chem. Soc. 2005;127:9978. doi: 10.1021/ja053202+. [DOI] [PubMed] [Google Scholar]
- Inoue S., Epping J. D., Irran E., Driess M. J. Am. Chem. Soc. 2011;133:8514. doi: 10.1021/ja2033475. [DOI] [PubMed] [Google Scholar]
- Driess M., Yao S., Brym M., van Wüllen C. Angew. Chem., Int. Ed. 2006;45:6730. doi: 10.1002/anie.200602327. [DOI] [PubMed] [Google Scholar]
- Hensen K., Zengerly T., Pickel P., Klebe G. Angew. Chem., Int. Ed. 1983;22:725. [Google Scholar]
- Xie Z., Liston D. J., Jelínek T., Mitro V., Bau R., Reed C. A. J. Chem. Soc., Chem. Commun. 1993:384. [Google Scholar]
- Ichinohe M., Fukui H., Sekiguchi A. Chem. Lett. 2000:600. [Google Scholar]
- Gerdes C., Saak W., Haase D., Müller T. J. Am. Chem. Soc. 2013;135:10353. doi: 10.1021/ja400306h. [DOI] [PubMed] [Google Scholar]
- MacLachlan M. J., Bourke S. C., Lough A. J., Manners I. J. Am. Chem. Soc. 2000;122:2126. [Google Scholar]
- Xie Z., Bau R., Reed C. A. J. Chem. Soc., Chem. Commun. 1994:2519. [Google Scholar]
- Robertson A. P. M., Friedmann J. N., Jenkins H. A., Burford N. Chem. Commun. 2014;50:7979. doi: 10.1039/c4cc01109k. [DOI] [PubMed] [Google Scholar]
- Romanato P., Duttwyler S., Linden A., Baldridge K. K., Siegel J. S. J. Am. Chem. Soc. 2010;132:7828. doi: 10.1021/ja9109665. [DOI] [PubMed] [Google Scholar]
- Ibad M. F., Langer P., Schulz A., Villinger A. J. Am. Chem. Soc. 2011;133:21016. doi: 10.1021/ja209693a. [DOI] [PubMed] [Google Scholar]
- Lambert J. B., Zhang S., Nava M., Reed C. A. J. Chem. Soc., Chem. Commun. Organometallics. 1993;2011;30:383. 4798. [Google Scholar]
- Connelly S. J., Kaminsky W., Heinekey D. M. Organometallics. 2013;32:7478. [Google Scholar]
- Lehmann M., Schulz A., Villinger A. Angew. Chem., Int. Ed. 2009;48:7444. doi: 10.1002/anie.200902992. [DOI] [PubMed] [Google Scholar]
- Schulz A., Thomas J., Villinger A. Chem. Commun. 2010;46:3696. doi: 10.1039/c0cc00013b. [DOI] [PubMed] [Google Scholar]
- Schulz A., Villinger A. Chem. – Eur. J. 2010;16:7276. doi: 10.1002/chem.201000289. [DOI] [PubMed] [Google Scholar]
- Christe K. O., Wilson W. W., Dixon D. A., Khan S. I., Bau R., Metzenthin T., Lu R. J. Am. Chem. Soc. 1993;115:1836. [Google Scholar]
- Panisch R., Bolte M., Müller T. J. Am. Chem. Soc. 2006;128:9676. doi: 10.1021/ja061800y. [DOI] [PubMed] [Google Scholar]
- Panisch R., Bolte M., Müller T. Organometallics. 2007;26:3524. [Google Scholar]
- Choi N., Lickiss P. D., McPartlin M., Masangane P. C., Veneziani G. L. Chem. Commun. 2005:6023. doi: 10.1039/b513203g. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Reißmann M., Schäfer A., Schmidtmann M., Müller T. Chem. – Eur. J. 2014;20:9381. doi: 10.1002/chem.201402874. [DOI] [PubMed] [Google Scholar]
- Sekiguchi A., Murakami Y., Fukaya N., Kabe Y. Chem. Lett. 2004;33:530. [Google Scholar]
- Müther K., Fröhlich R., Mück-Lichtenfeld C., Grimme S., Oestreich M. J. Am. Chem. Soc. 2011;133:12442. doi: 10.1021/ja205538t. [DOI] [PubMed] [Google Scholar]
- Bockholt A., Jutzi P., Mix A., Neumann B., Stammler A., Stammler H.-G. Z. Anorg. Allg. Chem. 2009;635:1326. [Google Scholar]
- Xiong Y., Yao S., Inoue S., Irran E., Driess M. Angew. Chem., Int. Ed. 2012;51:10074. doi: 10.1002/anie.201205840. [DOI] [PubMed] [Google Scholar]
- Yeong H.-X., Xi H.-W., Li Y., Lim K. H., So C.-W. Chem. – Eur. J. 2013;19:11786. doi: 10.1002/chem.201300255. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Yao S., Inoue S., Epping J. D., Driess M. Angew. Chem., Int. Ed. 2013;52:7147. doi: 10.1002/anie.201302537. [DOI] [PubMed] [Google Scholar]
- Filippou A. C., Lebedev Y. N., Chernov O., Straßmann M., Schnakenburg G. Angew. Chem., Int. Ed. 2013;52:6974. doi: 10.1002/anie.201301363. [DOI] [PubMed] [Google Scholar]
- Jutzi P. Science. 2004;305:849. doi: 10.1126/science.1099879. [DOI] [PubMed] [Google Scholar]
- Jutzi P., Mix A., Neumann B., Rummel B., Stammler H.-G. Chem. Commun. 2006:3519. doi: 10.1039/b607236d. [DOI] [PubMed] [Google Scholar]
- Leszczynska K., Mix A., Berger R. J. F., Rummel B., Neumann B., Stammler H.-G., Jutzi P. Angew. Chem., Int. Ed. 2011;50:6843. doi: 10.1002/anie.201101139. [DOI] [PubMed] [Google Scholar]
- Reed C. A., Xie Z., Bau R., Benesi A. Science. 1993;262:402. doi: 10.1126/science.262.5132.402. [DOI] [PubMed] [Google Scholar]
- Küppers T., Bernhardt E., Eujen R., Willner H., Lehmann C. W. Angew. Chem., Int. Ed. 2007;46:6346. doi: 10.1002/anie.200701136. [DOI] [PubMed] [Google Scholar]
- Rohde M., Müller L. O., Himmel D., Scherer H., Krossing I. Chem. – Eur. J. 2014;20:1218. doi: 10.1002/chem.201303671. [DOI] [PubMed] [Google Scholar]
- Xie Z., Bau R., Benesi A., Reed C. A. Organometallics. 1995;14:3933. [Google Scholar]
- Xie Z., Manning J., Reed R. W., Mathur R., Boyd P. D. W., Benesi A., Reed C. A. J. Am. Chem. Soc. 1996;118:2922. [Google Scholar]
- Budanow A., Bolte M., Wagner M., Lerner H.-W. Eur. J. Inorg. Chem. 2015:2524. [Google Scholar]
- Bourke S. C., MacLachlan M. J., Lough A. J., Manners I. Chem. – Eur. J. 2005;11:1989. doi: 10.1002/chem.200400859. [DOI] [PubMed] [Google Scholar]
- Sekiguchi A., Ishida Y., Kabe Y., Ichinohe M. J. Am. Chem. Soc. 2002;124:8776. doi: 10.1021/ja020395h. [DOI] [PubMed] [Google Scholar]
- Zharov I., King B. T., Havlas Z., Pardi A., Michl J. J. Am. Chem. Soc. 2000;122:10253. [Google Scholar]
- Sekiguchi A., Fukawa T., Lee V. Y., Nakamoto M., Ichinohe M. Angew. Chem., Int. Ed. 2003;42:1143. doi: 10.1002/anie.200390300. [DOI] [PubMed] [Google Scholar]
- Sekiguchi A., Fukawa T., Lee V. Y., Nakamoto M. J. Am. Chem. Soc. 2003;125:9250. doi: 10.1021/ja030156+. [DOI] [PubMed] [Google Scholar]
- Schenk C., Drost C., Schnepf A. Dalton Trans. 2009:773. doi: 10.1039/b818931e. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Saak W., Haase D., Müller T. J. Am. Chem. Soc. 2011;133:14562. doi: 10.1021/ja206338u. [DOI] [PubMed] [Google Scholar]
- Wright J. H., Mueck G. W., Tham F. S., Reed C. A. Organometallics. 2010;29:4066. [Google Scholar]
- Sekiguchi A. Science. 1997;275:60. doi: 10.1126/science.275.5296.60. [DOI] [PubMed] [Google Scholar]
- Ichinohe M., Fukaya N., Sekiguchi A. Chem. Lett. 1998:1045. [Google Scholar]
- Sekiguchi A., Fukaya N., Ichinohe M., Ishida Y. Eur. J. Inorg. Chem. 2000:1155. [Google Scholar]
- Lange I., Krahl J., Jones P. G., Blaschette A. J. Organomet. Chem. 1994;474:97. [Google Scholar]
- Kordts N., Borner C., Panisch R., Saak W., Müller T. Organometallics. 2014;33:1492. [Google Scholar]
- Khan S., Gopakumar G., Thiel W., Alcarazo M. Angew. Chem., Int. Ed. 2013;52:5644. doi: 10.1002/anie.201300677. [DOI] [PubMed] [Google Scholar]
- Wagner M., Zöller T., Hiller W., Prosenc M. H., Jurkschat K. Chem. – Eur. J. 2013;19:9463. doi: 10.1002/chem.201301477. [DOI] [PubMed] [Google Scholar]
- Müller T., Bauch C., Ostermeier M., Bolte M., Auner N. J. Am. Chem. Soc. 2003;125:2158. doi: 10.1021/ja021234g. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Sekiguchi A., Kabe Y. J. Am. Chem. Soc. 2003;125:11468. doi: 10.1021/ja030327a. [DOI] [PubMed] [Google Scholar]
- Müller T., Bauch C., Bolte M., Auner N. Chem. – Eur. J. 2003;9:1746. doi: 10.1002/chem.200390200. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Szilvási T., Yao S., Tan G., Driess M. J. Am. Chem. Soc. 2014;136:11300. doi: 10.1021/ja506824s. [DOI] [PubMed] [Google Scholar]
- Hough E., Nicholson D. G., Vasudevan A. K. J. Chem. Soc., Dalton Trans. 1989:2155. [Google Scholar]
- Rogers R. D., Bond A. H. Inorg. Chim. Acta. 1992;192:163. [Google Scholar]
- Bandyopadhyay R., Cooper B. F., Rossini A. J., Schurko R. W., Macdonald C. L. J. Organomet. Chem. 2010;695:1012. [Google Scholar]
- Beattie C., Farina P., Levason W., Reid G. Dalton Trans. 2013;42:15183. doi: 10.1039/c3dt51889b. [DOI] [PubMed] [Google Scholar]
- Rupar P. A., Staroverov V. N., Ragogna P. J., Baines K. M. J. Am. Chem. Soc. 2007;129:15138. doi: 10.1021/ja0775725. [DOI] [PubMed] [Google Scholar]
- Rupar P. A., Staroverov V. N., Baines K. M. Science. 2008;322:1360. doi: 10.1126/science.1163033. [DOI] [PubMed] [Google Scholar]
- Avery J. C., Hanson M. A., Herber R. H., Bladek K. J., Rupar P. A., Nowik I., Huang Y., Baines K. M. Inorg. Chem. 2012;51:7306. doi: 10.1021/ic3006713. [DOI] [PubMed] [Google Scholar]
- Rupar P. A., Bandyopadhyay R., Cooper B., Stinchcombe M. R., Ragogna P. J., Macdonald C., Baines K. M. Angew. Chem., Int. Ed. 2009;48:5155. doi: 10.1002/anie.200901351. [DOI] [PubMed] [Google Scholar]
- Cheng F., Hector A. L., Levason W., Reid G., Webster M., Zhang W. Angew. Chem., Int. Ed. 2009;48:5152. doi: 10.1002/anie.200901247. [DOI] [PubMed] [Google Scholar]
- Cheng F., Dyke J. M., Ferrante F., Hector A. L., Levason W., Reid G., Webster M., Zhang W. Dalton Trans. 2010;39:847. doi: 10.1039/b911016j. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Roesky H. W., Carl E., Stalke D., Demers J.-P., Lange A. J. Am. Chem. Soc. 2012;134:4998. doi: 10.1021/ja300563g. [DOI] [PubMed] [Google Scholar]
- Bouška M., Dostál L., Růžička A., Jambor R. Organometallics. 2013;32:1995. [Google Scholar]
- Jurca T., Hiscock L. K., Korobkov I., Rowley C. N., Richeson D. S. Dalton Trans. 2014;43:690. doi: 10.1039/c3dt52227j. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Yao S., Inoue S., Berkefeld A., Driess M. Chem. Commun. 2012;48:12198. doi: 10.1039/c2cc36926e. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Yao S., Tan G., Inoue S., Driess M. J. Am. Chem. Soc. 2013;135:5004. doi: 10.1021/ja402477w. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Saak W., Haase D., Müller T. Chem. – Eur. J. 2009;15:3945. doi: 10.1002/chem.200900222. [DOI] [PubMed] [Google Scholar]
- Li J., Schenk C., Winter F., Scherer H., Trapp N., Higelin A., Keller S., Pöttgen R., Krossing I., Jones C. Angew. Chem., Int. Ed. 2012;51:9557. doi: 10.1002/anie.201204601. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Saunders A. J., Coles M. P., Fulton J. R. Organometallics. 2011;30:1334. [Google Scholar]
- Arii H., Matsuo M., Nakadate F., Mochida K., Kawashima T. Dalton Trans. 2012;41:11195. doi: 10.1039/c2dt31187a. [DOI] [PubMed] [Google Scholar]
- Stender M., Phillips A. D., Power P. P. Inorg. Chem. 2001;40:5314. doi: 10.1021/ic0155582. [DOI] [PubMed] [Google Scholar]
- Hino S., Brynda M., Phillips A. D., Power P. P. Angew. Chem., Int. Ed. 2004;43:2655. doi: 10.1002/anie.200353365. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Winter F., Saak W., Haase D., Pöttgen R., Müller T. Chem. – Eur. J. 2011;17:10979. doi: 10.1002/chem.201101938. [DOI] [PubMed] [Google Scholar]
- Probst T., Steigelmann O., Riede J., Schmidbaur H. Angew. Chem., Int. Ed. 1990;29:1397. [Google Scholar]
- Jutzi P., Kohl F., Krüger C. Angew. Chem., Int. Ed. Engl. 1979;18:59. [Google Scholar]
- Jutzi P., Holtmann U., Bögge H., Müller A. J. Chem. Soc., Chem. Commun. 1988:305. [Google Scholar]
- Jones J. N., Moore J. A., Cowley A. H., Macdonald C. L. B. Dalton Trans. 2005:3846. doi: 10.1039/b510337a. [DOI] [PubMed] [Google Scholar]
- Winter J. G., Portius P., Kociok-Köhn G., Steck R., Filippou A. C. Organometallics. 1998;17:4176. [Google Scholar]
- Rouzaud J., Joudat M., Castel A., Delpech F., Rivière P., Gornitzka H., Manriquez J., Chavez I. J. Organomet. Chem. 2002;651:44. [Google Scholar]
- Cowley A. H., Macdonald C. L. B., Silverman J. S., Gorden J. D., Voigt A. Chem. Commun. 2001:175. [Google Scholar]
- Filippou A. C., Philippopoulos A. I., Schnakenburg G. Organometallics. 2003;22:3339. [Google Scholar]
- Filippou A. C., Philippopoulos A. I., Portius P., Schnakenburg G. Organometallics. 2004;23:4503. [Google Scholar]
- Cabon Y., Kleijn H., Siegler M. A., Spek A. L., Klein Gebbink R. J. M., Deelman B.-J. Dalton Trans. 2010;39:2423. doi: 10.1039/b924783c. [DOI] [PubMed] [Google Scholar]
- Dostálová R., Dostál L., Růžička A., Jambor R. Organometallics. 2011;30:2405. [Google Scholar]
- Wagner M., Henn M., Dietz C., Schürmann M., Prosenc M. H., Jurkschat K. Organometallics. 2013;32:2406. [Google Scholar]
- Krabbe S., Wagner M., Löw C., Dietz C., Schürmann M., Hoffmann A., Herres-Pawlis S., Lutter M., Jurkschat K. Organometallics. 2014;33:4433. [Google Scholar]
- Braunschweig H., Celik M. A., Dewhurst R. D., Heid M., Hupp F., Sen S. S. Chem. Sci. 2015;6:425. doi: 10.1039/c4sc02948h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasko D., Reed C. A. J. Am. Chem. Soc. 2002;124:1148. doi: 10.1021/ja0118800. [DOI] [PubMed] [Google Scholar]
- Reed C. A., Kim K.-C., Stoyanov E. S., Stasko D., Tham F. S., Mueller L. J., Boyd P. D. W. J. Am. Chem. Soc. 2003;125:1796. doi: 10.1021/ja027336o. [DOI] [PubMed] [Google Scholar]
- Reed C. A., Fackler N. L. P., Kim K.-C., Stasko D., Evans D. R., Boyd P. D. W., Rickard C. E. F. J. Am. Chem. Soc. 1999;121:6314. [Google Scholar]
- Objartel I., Ott H., Stalke D. Z. Anorg. Allg. Chem. 2008;634:2373. [Google Scholar]
- Burford N., Ragogna P. J., McDonald R., Ferguson M. J. J. Am. Chem. Soc. 2003;125:14404. doi: 10.1021/ja036649w. [DOI] [PubMed] [Google Scholar]
- Weigand J. J. and Burford N., Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 119–149. [Google Scholar]
- Dyker C. A., Burford N. Chem. – Asian J. 2008;3:28. doi: 10.1002/asia.200700229. [DOI] [PubMed] [Google Scholar]
- Robertson A. P. M., Gray P. A., Burford N. Angew. Chem., Int. Ed. 2014;53:6050. doi: 10.1002/anie.201307658. [DOI] [PubMed] [Google Scholar]
- Krossing I., Driess M. and Nöth H., Molecular Clusters of the Main Group Elements, Wiley-VCH, 2004, p. 223. [Google Scholar]
- Feldmann K.-O., Weigand J. J. Angew. Chem., Int. Ed. 2012;51:6566. doi: 10.1002/anie.201201600. [DOI] [PubMed] [Google Scholar]
- Breunig H. J. and Raţ C. I., Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 151–178. [Google Scholar]
- Holthausen M. H., Weigand J. J. Chem. Soc. Rev. 2014;43:6639. doi: 10.1039/c4cs00019f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruck M., Locherer F. Coord. Chem. Rev. 2015;297–298:208. [Google Scholar]
- Ruck M., Locherer F. Coord. Chem. Rev. 2015;285:1. [Google Scholar]
- Christe K. O., Wilson W. W., Sheehy J. A., Boatz J. A. Angew. Chem., Int. Ed. 1999;38:2004. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<2004::AID-ANIE2004>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vij A., Wilson W. W., Vij V., Tham F. S., Sheehy J. A., Christe K. O. J. Am. Chem. Soc. 2001;123:6308. doi: 10.1021/ja010141g. [DOI] [PubMed] [Google Scholar]
- Lindsjö M., Fischer A., Kloo L. Angew. Chem., Int. Ed. 2004;43:2540. doi: 10.1002/anie.200353578. [DOI] [PubMed] [Google Scholar]
- Ahmed E., Köhler D., Ruck M. Z. Anorg. Allg. Chem. 2009;635:297. [Google Scholar]
- Decken A., Nikiforov G. B., Passmore J. Polyhedron. 2005;24:2994. [Google Scholar]
- Schwarzmaier C., Sierka M., Scheer M. Angew. Chem., Int. Ed. 2013;52:858. doi: 10.1002/anie.201208226. [DOI] [PubMed] [Google Scholar]
- Glaser R., Horan C. J. Can. J. Chem. 1996;74:1200. [Google Scholar]
- Niecke E., Nieger M., Reichert F. Angew. Chem., Int. Ed. 1988;27:1715. [Google Scholar]
- Kuprat M., Schulz A., Villinger A. Angew. Chem., Int. Ed. 2013;52:7126. doi: 10.1002/anie.201302725. [DOI] [PubMed] [Google Scholar]
- Ahmed E., Isaeva A., Fiedler A., Haft M., Ruck M. Chem. – Eur. J. 2011;17:6847. doi: 10.1002/chem.201100398. [DOI] [PubMed] [Google Scholar]
- Eich A., Schlüter S., Schnakenburg G., Beck J. Z. Anorg. Allg. Chem. 2013;639:375. [Google Scholar]
- Feldmann K.-O., Wiegand T., Ren J., Eckert H., Breternitz J., Groh M. F., Müller U., Ruck M., Maryasin B., Ochsenfeld C., Schön O., Karaghiosoff K., Weigand J. J. Chem. – Eur. J. 2015;21:9577. doi: 10.1002/chem.201406476. [DOI] [PubMed] [Google Scholar]
- Feldmann K.-O., Wiegand T., Ren J., Eckert H., Breternitz J., Groh M. F., Müller U., Ruck M., Maryasin B., Ochsenfeld C., Schön O., Karaghiosoff K., Weigand J. J. Chem. – Eur. J. 2015;21:9697. doi: 10.1002/chem.201406476. [DOI] [PubMed] [Google Scholar]
- Beck J., Schlüter S., Zotov N. Z. Anorg. Allg. Chem. 2004;630:2512. [Google Scholar]
- Beck J., Dolg M., Schlüter S. Angew. Chem., Int. Ed. 2001;40:2287. doi: 10.1002/1521-3773(20010618)40:12<2287::AID-ANIE2287>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Burford N., Parks T. M., Royan B. W., Borecka B., Cameron T. S., Richardson J. F., Gabe E. J., Hynes R. J. Am. Chem. Soc. 1992;114:8147. [Google Scholar]
- Kinjo R., Donnadieu B., Bertrand G. Angew. Chem., Int. Ed. 2010;49:5930. doi: 10.1002/anie.201002889. [DOI] [PubMed] [Google Scholar]
- Abraham M. Y., Wang Y., Xie Y., Gilliard R. J., Wei P., Vaccaro B. J., Johnson M. K., Schaefer H. F., von Ragué Schleyer P., Robinson G. H. J. Am. Chem. Soc. 2013;135:2486. doi: 10.1021/ja400219d. [DOI] [PubMed] [Google Scholar]
- Pan X., Chen X., Li T., Li Y., Wang X. J. Am. Chem. Soc. 2013;135:3414. doi: 10.1021/ja4012113. [DOI] [PubMed] [Google Scholar]
- Pan X., Su Y., Chen X., Zhao Y., Li Y., Zuo J., Wang X. J. Am. Chem. Soc. 2013;135:5561. doi: 10.1021/ja402492u. [DOI] [PubMed] [Google Scholar]
- Su Y., Zheng X., Wang X., Zhang X., Sui Y., Wang X. J. Am. Chem. Soc. 2014;136:6251. doi: 10.1021/ja502675d. [DOI] [PubMed] [Google Scholar]
- Schmidpeter A., Lochschmidt S., Sheldrick W. S. Angew. Chem., Int. Ed. 1985;24:226. [Google Scholar]
- Barnham R. J., Deng R. M. K., Dillon K. B., Goeta A. E., Howard J. A. K., Puschmann H. Heteroat. Chem. 2001;12:501. [Google Scholar]
- Reeske G., Cowley A. H. Chem. Commun. 2006:1784. doi: 10.1039/b602017h. [DOI] [PubMed] [Google Scholar]
- Slattery J. M., Hussein S. Dalton Trans. 2012;41:1808. doi: 10.1039/c1dt11636c. [DOI] [PubMed] [Google Scholar]
- Robertson A. P. M., Burford N., McDonald R., Ferguson M. J. Angew. Chem., Int. Ed. 2014;53:3480. doi: 10.1002/anie.201310613. [DOI] [PubMed] [Google Scholar]
- Hendsbee A. D., Giffin N. A., Zhang Y., Pye C. C., Masuda J. D. Angew. Chem., Int. Ed. 2012;51:10836. doi: 10.1002/anie.201206112. [DOI] [PubMed] [Google Scholar]
- Holthausen M. H., Mehta M., Stephan D. W. Angew. Chem., Int. Ed. Engl. 2014;53:6538. doi: 10.1002/anie.201403693. [DOI] [PubMed] [Google Scholar]
- Minkwitz R., Schneider S. Angew. Chem., Int. Ed. 1999;38:210. doi: 10.1002/(SICI)1521-3773(19990301)38:5<714::AID-ANIE714>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wahl B., Ruck M. Z. Anorg. Allg. Chem. 2008;634:2873. [Google Scholar]
- Ruck M. Z. Anorg. Allg. Chem. 1998;624:521. [Google Scholar]
- Krebs B., Mummert M., Brendel C. J. Less-Common Met. 1986;116:159. [Google Scholar]
- Ruck M., Steden F. Z. Anorg. Allg. Chem. 2007;633:1556. [Google Scholar]
- Krebs B., Hucke M., Brendel C. J. Angew. Chem., Int. Ed. Engl. 1982;21:1108. [Google Scholar]
- Beck J., Hilbert T. Eur. J. Inorg. Chem. 2004:2019. [Google Scholar]
- Hershaft A., Corbett J. D. J. Chem. Phys. 1962;36:551. [Google Scholar]
- Friedman R. M., Corbett J. D. Inorg. Chem. 1973;12:1134. [Google Scholar]
- Wahl B., Ruck M. Z. Anorg. Allg. Chem. 2010;636:337. [Google Scholar]
- Santiso-Quinones G., Reisinger A., Slattery J., Krossing I. Chem. Commun. 2007:5046. doi: 10.1039/b710899k. [DOI] [PubMed] [Google Scholar]
- Forfar L. C., Clark T. J., Green M., Mansell S. M., Russell C. A., Sanguramath R. A., Slattery J. M. Chem. Commun. 2012;48:1970. doi: 10.1039/c2cc15291f. [DOI] [PubMed] [Google Scholar]
- de los Rios I., Hamon J.-R., Hamon P., Lapinte C., Toupet L., Romerosa A., Peruzzini M. Angew. Chem., Int. Ed. 2001;40:3910. doi: 10.1002/1521-3773(20011015)40:20<3910::AID-ANIE3910>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Caporali M., Di Vaira M., Peruzzini M., Seniori Costantini S., Stoppioni P., Zanobini F. Eur. J. Inorg. Chem. 2010:152. [Google Scholar]
- Barbaro P., Di Vaira M., Peruzzini M., Seniori Costantini S., Stoppioni P. Chem. – Eur. J. 2007;13:6682. doi: 10.1002/chem.200601846. [DOI] [PubMed] [Google Scholar]
- Schwarzmaier C., Timoshkin A. Y., Scheer M. Angew. Chem., Int. Ed. 2013;52:7600. doi: 10.1002/anie.201302882. [DOI] [PubMed] [Google Scholar]
- Adolf A., Gonsior M., Krossing I. J. Am. Chem. Soc. 2002;124:7111. doi: 10.1021/ja012760v. [DOI] [PubMed] [Google Scholar]
- Cygler M., Przybylska M., Elofson R. M. Can. J. Chem. 1982;60:2852. [Google Scholar]
- Kütt A., Werner F., Kaljurand I., Leito I., Koppel I. A. ChemPlusChem. 2013;78:932. doi: 10.1002/cplu.201300160. [DOI] [PubMed] [Google Scholar]
- McGilligan B. S., Arnold J., Wilkinson G., Hussain-Bates B., Hursthouse M. B. J. Chem. Soc., Dalton Trans. 1990:2465. [Google Scholar]
- Baumann W., Michalik D., Reiß F., Schulz A., Villinger A. Angew. Chem., Int. Ed. 2014;53:3250. doi: 10.1002/anie.201310186. [DOI] [PubMed] [Google Scholar]
- Hering C., Hertrich M., Schulz A., Villinger A. Inorg. Chem. 2014;53:3880. doi: 10.1021/ic500332s. [DOI] [PubMed] [Google Scholar]
- Loh Y. K., Gurnani C., Ganguly R., Vidović D. Inorg. Chem. 2015;54:3087. doi: 10.1021/acs.inorgchem.5b00003. [DOI] [PubMed] [Google Scholar]
- Burford N., Cameron T. S., Ragogna P. J., Ocando-Mavarez E., Gee M., McDonald R., Wasylishen R. E. J. Am. Chem. Soc. 2001;123:7947. doi: 10.1021/ja011075l. [DOI] [PubMed] [Google Scholar]
- Weigand J. J., Riegel S. D., Burford N., Decken A. J. Am. Chem. Soc. 2007;129:7969. doi: 10.1021/ja071306+. [DOI] [PubMed] [Google Scholar]
- Gonsior M., Krossing I., Müller L., Raabe I., Jansen M., van Wüllen L. Chem. – Eur. J. 2002;8:4475. doi: 10.1002/1521-3765(20021004)8:19<4475::AID-CHEM4475>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pohl S. Z. Anorg. Allg. Chem. 1983;498:20. [Google Scholar]
- Burford N., Dyker C. A., Decken A. Angew. Chem., Int. Ed. 2005;44:2364. doi: 10.1002/anie.200462997. [DOI] [PubMed] [Google Scholar]
- Schmidpeter A., Lochschmidt S., Karaghiosoff K., Sheldrick W. S. J. Chem. Soc., Chem. Commun. 1985:1447. [Google Scholar]
- Krossing I. J. Chem. Soc., Chem. Commun. 2002:500. [Google Scholar]
- Conrad E., Burford N., McDonald R., Ferguson M. J. Inorg. Chem. 2008;47:2952. doi: 10.1021/ic800287e. [DOI] [PubMed] [Google Scholar]
- Dyker C. A., Burford N., Lumsden M. D., Decken A. J. Am. Chem. Soc. 2006;128:9632. doi: 10.1021/ja062972y. [DOI] [PubMed] [Google Scholar]
- Bresien J., Faust K., Schulz A., Villinger A. Angew. Chem., Int. Ed. 2015;54:6926. doi: 10.1002/anie.201500892. [DOI] [PubMed] [Google Scholar]
- Conrad E., Burford N., McDonald R., Ferguson M. J. J. Am. Chem. Soc. 2009;131:17000. doi: 10.1021/ja907613c. [DOI] [PubMed] [Google Scholar]
- Krossing I., Raabe I. Angew. Chem., Int. Ed. 2001;40:4406. doi: 10.1002/1521-3773(20011203)40:23<4406::aid-anie4406>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Holthausen M. H., Feldmann K.-O., Schulz S., Hepp A., Weigand J. J. Inorg. Chem. 2012;51:3374. doi: 10.1021/ic2013304. [DOI] [PubMed] [Google Scholar]
- Holthausen M. H., Hepp A., Weigand J. J. Chem. – Eur. J. 2013;19:9895. doi: 10.1002/chem.201204337. [DOI] [PubMed] [Google Scholar]
- Holthausen M. H., Weigand J. J. Z. Anorg. Allg. Chem. 2012;638:1103. [Google Scholar]
- Donath M., Conrad E., Jerabek P., Frenking G., Fröhlich R., Burford N., Weigand J. J. Angew. Chem., Int. Ed. 2012;51:2964. doi: 10.1002/anie.201109010. [DOI] [PubMed] [Google Scholar]
- Donath M., Bodensteiner M., Weigand J. J. Chem. – Eur. J. 2014;20:17306. doi: 10.1002/chem.201405196. [DOI] [PubMed] [Google Scholar]
- Burford N., Landry J. C., Ferguson M. J., McDonald R. Inorg. Chem. 2005;44:5897. doi: 10.1021/ic050532m. [DOI] [PubMed] [Google Scholar]
- Christian B. H., Gillespie R. J., Sawyer J. F. Inorg. Chem. 1981;20:3410. [Google Scholar]
- Beck J., Schlüter S., Zotov N. Z. Anorg. Allg. Chem. 2005;631:2450. [Google Scholar]
- Beck J., Hengstmann M., Schlüter S. Z. Kristallogr. 2005;220:147. [Google Scholar]
- Ruck M., Dubenskyy V., Söhnel T. Angew. Chem., Int. Ed. 2003;42:2978. doi: 10.1002/anie.200250801. [DOI] [PubMed] [Google Scholar]
- Michalik D., Schulz A., Villinger A., Weding N. Angew. Chem., Int. Ed. 2008;47:6465. doi: 10.1002/anie.200801674. [DOI] [PubMed] [Google Scholar]
- Schulz A., Villinger A. Inorg. Chem. 2009;48:7359. doi: 10.1021/ic900821h. [DOI] [PubMed] [Google Scholar]
- Lehmann M., Schulz A., Villinger A. Angew. Chem., Int. Ed. 2012;51:8087. doi: 10.1002/anie.201203267. [DOI] [PubMed] [Google Scholar]
- Brückner A., Hinz A., Priebe J. B., Schulz A., Villinger A. Angew. Chem., Int. Ed. 2015;54:7426. doi: 10.1002/anie.201502054. [DOI] [PubMed] [Google Scholar]
- Back O., Celik M. A., Frenking G., Melaimi M., Donnadieu B., Bertrand G. J. Am. Chem. Soc. 2010;132:10262. doi: 10.1021/ja1046846. [DOI] [PubMed] [Google Scholar]
- Burford N., Losier P., Bakshi P. K., Cameron T. S. J. Chem. Soc., Dalton Trans. 1993:201. [Google Scholar]
- Burford N., Losier P., Macdonald C., Kyrimis V., Bakshi P. K., Cameron T. S. Inorg. Chem. 1994;33:1434. [Google Scholar]
- Hering C., Schulz A., Villinger A. Angew. Chem., Int. Ed. 2012;51:6241. doi: 10.1002/anie.201201851. [DOI] [PubMed] [Google Scholar]
- Hering C., Schulz A., Villinger A. Inorg. Chem. 2013;52:5214. doi: 10.1021/ic4001285. [DOI] [PubMed] [Google Scholar]
- Kraft A., Beck J., Krossing I. Chem. – Eur. J. 2011;17:12975. doi: 10.1002/chem.201102077. [DOI] [PubMed] [Google Scholar]
- Conrad E., Burford N., McDonald R., Ferguson M. J. J. Am. Chem. Soc. 2009;131:5066. doi: 10.1021/ja900968j. [DOI] [PubMed] [Google Scholar]
- Conrad E., Burford N., McDonald R., Ferguson M. J. Chem. Commun. 2010;46:4598. doi: 10.1039/c0cc00111b. [DOI] [PubMed] [Google Scholar]
- Baumann W., Schulz A., Villinger A. Angew. Chem., Int. Ed. 2008;47:9530. doi: 10.1002/anie.200803987. [DOI] [PubMed] [Google Scholar]
- Gudat D., Haghverdi A., Hupfer H., Nieger M. Chem. – Eur. J. 2000;6:3414. doi: 10.1002/1521-3765(20000915)6:18<3414::aid-chem3414>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Denk M. K., Gupta S., Lough A. J. Eur. J. Inorg. Chem. 1999:41. [Google Scholar]
- Carmalt C. J., Lomeli V. Chem. Commun. 1997:2095. [Google Scholar]
- Spinney H. A., Korobkov I., Richeson D. S. Chem. Commun. 2007:1647. doi: 10.1039/b617434e. [DOI] [PubMed] [Google Scholar]
- Burford N., Parks T. M., Royan B. W., Richardson J. F., White P. S. Can. J. Chem. 1992;70:703. [Google Scholar]
- Burford N., Macdonald C. L., Parks T. M., Wu G., Borecka B., Kwiatkowski W., Cameron T. S. Can. J. Chem. 1996;74:2209. [Google Scholar]
- Gudat D., Gans-Eichler T., Nieger M. Chem. Commun. 2004:2434. doi: 10.1039/b409657f. [DOI] [PubMed] [Google Scholar]
- Christe K. O., Lind M. D., Thorup N., Russell D. R., Fawcett J., Bau R. Inorg. Chem. 1988;27:2450. [Google Scholar]
- Chen G. S. H., Passmore J. J. Chem. Soc., Dalton Trans. 1979:1251. [Google Scholar]
- Aubauer C., Kaupp M., Klapotke T. M., Noth H., Piotrowski H., Schnick W., Senker J., Suter M. J. Chem. Soc., Dalton Trans. 2001:1880. [Google Scholar]
- Shamir J., Luski S., Bino A., Cohen S., Gibson D. Inorg. Chem. 1985;24:2301. [Google Scholar]
- Pohl S. Z. Anorg. Allg. Chem. 1983;498:15. [Google Scholar]
- Minkwitz R., Nowicki J., Borrmann H. Z. Anorg. Allg. Chem. 1991;596:93. [Google Scholar]
- Gerken M., Kolb P., Wegner A., Mercier H. P. A., Borrmann H., Dixon D. A., Schrobilgen G. J. Inorg. Chem. 2000;39:2813. doi: 10.1021/ic000118g. [DOI] [PubMed] [Google Scholar]
- Gonsior M., Krossing I. Dalton Trans. 2005:1203. doi: 10.1039/b417629d. [DOI] [PubMed] [Google Scholar]
- Miller H. B., Baird H. W., Bramlett C. L., Templeton W. K. J. Chem. Soc., Chem. Commun. 1972:262. [Google Scholar]
- Casteel W. J., Kolb P., LeBlond N., Mercier H. P. A., Schrobilgen G. J. Inorg. Chem. 1996;35:929. doi: 10.1021/ic950818z. [DOI] [PubMed] [Google Scholar]
- Brownridge S., Krossing I., Passmore J., Jenkins H., Roobottom H. K. Coord. Chem. Rev. 2000;197:397. [Google Scholar]
- Krossing I. Top. Curr. Chem. 2003;230:135. [Google Scholar]
- Sheldrick W. S., Molecular Clusters of the Main Group Elements, Wiley-VCH, 2004, p. 230. [Google Scholar]
- Zakharov A. V., Sadekov I. D., Minkin V. I. Russ. Chem. Rev. 2006;75:207. [Google Scholar]
- Kelly P. F. and King R., Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 179–196. [Google Scholar]
- Laitinen R. S. and Oilunkaniemi R., Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 197–231. [Google Scholar]
- Shuvaev K. V., Passmore J., Recent Developments in Main Group Chemistry, 2013, vol. 257, p. 1067. [Google Scholar]
- Bartlett N., Lohmann D. H. Proc. Chem. Soc. 1962:115. [Google Scholar]
- Hujo W., Grimme S. J. Chem. Theory Comput. 2013;9:308. doi: 10.1021/ct300813c. [DOI] [PubMed] [Google Scholar]
- Santiso-Quiñones G., Brückner R., Knapp C., Dionne I., Passmore J., Krossing I. Angew. Chem., Int. Ed. 2009;48:1133. doi: 10.1002/anie.200804021. [DOI] [PubMed] [Google Scholar]
- Aris D., Beck J., Decken A., Dionne I., Krossing I., Passmore J., Rivard E., Steden F., Wang X. Phosphorus, Sulfur Silicon Relat. Elem. 2004;179:859. [Google Scholar]
- Apblett A., Banister A. J., Biron D., Kendrick A. G., Passmore J., Schriver M., Stojanac M. Inorg. Chem. 1986;25:4451. [Google Scholar]
- Brooks W. V. F., Cameron T. S., Parsons S., Passmore J., Schriver M. J. Inorg. Chem. 1994;33:6230. [Google Scholar]
- Awere E. G., Passmore J., White P. S. J. Chem. Soc., Dalton Trans. 1993:299. [Google Scholar]
- Apblett A., Chivers T., Fait J. F. J. Chem. Soc., Chem. Commun. 1989:1596. [Google Scholar]
- Apblett A., Chivers T., Fait J. F. Inorg. Chem. 1990;29:1643. [Google Scholar]
- Stammler H.-G., Weiß J. Z. Naturforsch. 1989;44b:1483. [Google Scholar]
- Passmore J., Sutherland G., Whidden T., White P. S. J. Chem. Soc., Chem. Commun. 1980:289. [Google Scholar]
- Shantha Nandana W. A., Passmore J., White P. S., Wong C.-M. J. Chem. Soc., Chem. Commun. 1982:1098. [Google Scholar]
- Boyle P. D., Parsons S., Passmore J., Wood D. J. J. Chem. Soc., Chem. Commun. 1993:199. [Google Scholar]
- Cameron T. S., Decken A., Grein F., Knapp C., Passmore J., Rautiainen J. M., Shuvaev K. V., Thompson R. C., Wood D. J. Inorg. Chem. 2010;49:7861. doi: 10.1021/ic100760t. [DOI] [PubMed] [Google Scholar]
- Fujihara H., Mima H., Furukawa N. J. Am. Chem. Soc. 1995;117:10153. [Google Scholar]
- Dutton J. L., Tuononen H. M., Ragogna P. J. Angew. Chem., Int. Ed. 2009;48:4409. doi: 10.1002/anie.200901495. [DOI] [PubMed] [Google Scholar]
- Poleschner H., Seppelt K. Angew. Chem., Int. Ed. 2008;47:6461. doi: 10.1002/anie.200801691. [DOI] [PubMed] [Google Scholar]
- Chivers T., Konu J. Angew. Chem., Int. Ed. 2009;48:3025. doi: 10.1002/anie.200806010. [DOI] [PubMed] [Google Scholar]
- Martin C. D., Jennings M. C., Ferguson M. J., Ragogna P. J. Angew. Chem., Int. Ed. 2009;48:2210. doi: 10.1002/anie.200805198. [DOI] [PubMed] [Google Scholar]
- Dutton J. L., Tuononen H. M., Jennings M. C., Ragogna P. J. J. Am. Chem. Soc. 2006;128:12624. doi: 10.1021/ja064381d. [DOI] [PubMed] [Google Scholar]
- Gerding H., Houtgraaf H. Recl. Trav. Chim. Pays-Bas. 1954;73:759. [Google Scholar]
- Christian B. H., Collins M. J., Gillespie R. J., Sawyer J. F. Inorg. Chem. 1986;25:777. [Google Scholar]
- Minoura M., Mukuda T., Sagami T., Akiba K.-y. J. Am. Chem. Soc. 1999;121:10852. [Google Scholar]
- Naumann D., Tyrra W., Herrmann R., Pantenburg I., Wickleder M. S. Z. Anorg. Allg. Chem. 2002;628:833. [Google Scholar]
- Johnson J. P., MacLean G. K., Passmore J., White P. S. Can. J. Chem. 1989;67:1687. [Google Scholar]
- Minoura M., Mukuda T., Sagami T., Akiba K.-Y. Heteroat. Chem. 2001;12:380. [Google Scholar]
- Soltner T., Goetz N. R., Kornath A. Eur. J. Inorg. Chem. 2011:3076. [Google Scholar]
- Minkwitz R., Schneider S. Angew. Chem., Int. Ed. 1999;38:714. doi: 10.1002/(SICI)1521-3773(19990301)38:5<714::AID-ANIE714>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Minkwitz R., Seelbinder R., Schöbel R. Angew. Chem., Int. Ed. 2002;41:111. [PubMed] [Google Scholar]
- Ibers J. A. J. Chem. Phys. 1966;44:1748. [Google Scholar]
- Passmore J., Sutherland G., White P. S. J. Chem. Soc., Chem. Commun. 1980:330. [Google Scholar]
- Passmore J., Sutherland G., White P. S. Inorg. Chem. 1982;21:2717. [Google Scholar]
- Faggiani R., Gillespie R. J., Sawyer J. F., Vekris J. E. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1989;45:1847. [Google Scholar]
- Cameron T. S., Dionne I., Jenkins H. D. B., Parsons S., Passmore J., Roobottom H. K. Inorg. Chem. 2000;39:2042. doi: 10.1021/ic990850j. [DOI] [PubMed] [Google Scholar]
- Davies C. G., Gillespie R. J., Park J. J., Passmore J. Inorg. Chem. 1971;10:2781. [Google Scholar]
- Burns R. C., Gillespie R. J., Sawyer J. F. Inorg. Chem. 1980;19:1423. [Google Scholar]
- Cardinal G., Gillespie R. J., Sawyer J. F., Vekris J. E. J. Chem. Soc., Dalton Trans. 1982:765. [Google Scholar]
- Minkwitz R., Borrmann H., Nowicki J. Z. Naturforsch. 1991;46b:629. [Google Scholar]
- Beck J. Z. Anorg. Allg. Chem. 1995;621:131. [Google Scholar]
- Beck J., Kellner M., Kreuzinger M. Z. Anorg. Allg. Chem. 2002;628:2656. [Google Scholar]
- Corbett J. D., McMullan R. K., Prince D. J., Buchin B., Gemel C., Cadenbach T., Schmid R., Fischer R. A. Inorg. Chem. Angew. Chem. 1971;2006;10118:1749. 1091. [Google Scholar]
- Collins M. J., Gillespie R. J., Sawyer J. F. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1988;44:405. [Google Scholar]
- Burns R. C., Chan W.-L., Gillespie R. J., Luk W.-C., Sawyer J. F., Slim D. R. Inorg. Chem. 1980;19:1432. [Google Scholar]
- Collins M. J., Gillespie R. J., Sawyer J. F., Schrobilgen G. J. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1986;42:13. [Google Scholar]
- Beck J., Eck S. J. Z. Anorg. Allg. Chem. 2010;636:1910. [Google Scholar]
- Beck J., Wetterau J. Inorg. Chem. 1995;34:6202. [Google Scholar]
- Beck J., Fischer A. Z. Anorg. Allg. Chem. 1997;623:780. [Google Scholar]
- Couch T. W., Lokken D. A., Corbett J. D. Inorg. Chem. 1972;11:357. [Google Scholar]
- Beck J. Z. Naturforsch. 1990;45b:413. [Google Scholar]
- Beck J. Z. Naturforsch. 1994;49b:1159. [Google Scholar]
- Beck J. Chem. Ber. 1991;124:677. [Google Scholar]
- Beck J., Bock G. Z. Naturforsch. 1996;51b:119. [Google Scholar]
- Beck J., Kasper M., Stankowski A. Chem. Ber. 1997;130:1189. [Google Scholar]
- Collins M. J., Gillespie R. J., Kolis J. W., Sawyer J. F. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1987;43:2033. [Google Scholar]
- Beck J. Z. Naturforsch. 1990;45b:1610. [Google Scholar]
- Beck J., Fischer A., Stankowski A. Z. Anorg. Allg. Chem. 2002;628:2542. [Google Scholar]
- Beck J. Chem. Ber. 1995;128:23. [Google Scholar]
- Beck J., Bock G. Z. Anorg. Allg. Chem. 1996;622:823. [Google Scholar]
- Gillespie R. J., Luk W., Slim D. R., Burns R. C., Gillespie R. J., Luk W.-C., Slim D. R. J. Chem. Soc., Chem. Commun. Inorg. Chem. 1976;1979;18:791. 3086. [Google Scholar]
- Drake G. W., Schimek G. L., Kolis J. W. Inorg. Chem. 1996;35:1740. doi: 10.1021/ic951021a. [DOI] [PubMed] [Google Scholar]
- Beck J. Angew. Chem., Int. Ed. 1991;30:1128. [Google Scholar]
- Beck J. Z. Anorg. Allg. Chem. 1993;619:237. [Google Scholar]
- Beck J. Angew. Chem., Int. Ed. 1990;29:293. [Google Scholar]
- Beck J., Müller-Buschbaum K. Z. Anorg. Allg. Chem. 1997;623:409. [Google Scholar]
- Beck J., Stankowski A. Z. Naturforsch. 2001;56b:453. [Google Scholar]
- Beck J., Fischer A. Z. Anorg. Allg. Chem. 2002;628:369. [Google Scholar]
- Freudenmann D., Feldmann C. Z. Anorg. Allg. Chem. 2011;637:1481. [Google Scholar]
- Beck J., Bock G. Angew. Chem., Int. Ed. 1995;34:2559. [Google Scholar]
- Santiso-Quiñones G., Higelin A., Schaefer J., Brückner R., Knapp C., Krossing I. Chem. – Eur. J. 2009;15:6663. doi: 10.1002/chem.200900244. [DOI] [PubMed] [Google Scholar]
- Cameron T. S., Decken A., Dionne I., Fang M., Krossing I., Passmore J. Chem. – Eur. J. 2002;8:3386. doi: 10.1002/1521-3765(20020802)8:15<3386::AID-CHEM3386>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Aris D., Beck J., Decken A., Dionne I., Schmedt auf der Günne J., Hoffbauer W., Köchner T., Krossing I., Passmore J., Rivard E., Steden F., Wang X. Dalton Trans. 2011;40:5865. doi: 10.1039/c0dt01251c. [DOI] [PubMed] [Google Scholar]
- Köchner T., Trapp N., Engesser T. A., Lehner A. J., Röhr C., Riedel S., Knapp C., Scherer H., Krossing I. Angew. Chem., Int. Ed. 2011;50:11253. doi: 10.1002/anie.201104666. [DOI] [PubMed] [Google Scholar]
- Thewalt U., Berhalter K., Müller P. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1982;38:1280. [Google Scholar]
- Gillespie R. J., Slim D. R., Tyrer J. D. J. Chem. Soc., Chem. Commun. 1977:253. [Google Scholar]
- Gillespie R. J., Sawyer J. F., Slim D. R., Tyrer J. D. Inorg. Chem. 1982;21:1296. [Google Scholar]
- Bakshi P., Boyle P. D., Cameron T. S., Passmore J., Schatte G., Sutherland G. W. Inorg. Chem. 1994;33:3849. [Google Scholar]
- Parsons S., Passmore J., White P. S. J. Chem. Soc., Dalton Trans. 1993:1499. [Google Scholar]
- Faggiani R., Gillespie R. J., Vekris J. E. J. Chem. Soc., Chem. Commun. 1988:902. [Google Scholar]
- Gillespie R. J., Luk W., Maharajh E., Slim D. R. Inorg. Chem. 1977;16:892. [Google Scholar]
- Faggiani R., Gillespie R. J., Kolis J. W. J. Chem. Soc., Chem. Commun. 1987:592. [Google Scholar]
- Shantha Nandana W. A., Passmore J., White P. S., Wong C. M., Passmore J., White P. S., Wong C.-M. Inorg. Chem. J. Chem. Soc., Chem. Commun. 1989;1985;28:3320. 1178. [Google Scholar]
- Beck J., Richter J., Pell M. A., Ibers J. A. Z. Anorg. Allg. Chem. 1996;622:473. [Google Scholar]
- Beck J., Schlörb T. Phosphorus, Sulfur Silicon Relat. Elem. 1997;124:305. [Google Scholar]
- Collins M. J., Gillespie R. J., Sawyer J. F. Inorg. Chem. 1987;26:1476. [Google Scholar]
- Boldrini P., Brown I. D., Gillespie R. J., Ireland P. R., Luk W., Slim D. R., Vekris J. E. Inorg. Chem. 1976;15:765. [Google Scholar]
- Herler S., Mayer P., Nöth H., Schulz A., Suter M., Vogt M. Angew. Chem., Int. Ed. 2001;40:3173. doi: 10.1002/1521-3773(20010903)40:17<3173::AID-ANIE3173>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Collins M. J., Gillespie R. J., Sawyer J. F., Schrobilgen G. J. Inorg. Chem. 1986;25:2053. [Google Scholar]
- Awere E. G., Passmore J., White P. S., Awere E. G., Passmore J., White P. S., Klapotke T. J. Chem. Soc., Dalton Trans. J. Chem. Soc., Chem. Commun. 19921989:1267. 1415. [Google Scholar]
- Boldrini P., Brown I. D., Collins M. J., Gillespie R. J., Maharajh E., Slim D. R., Sawyer J. F. Inorg. Chem. 1985;24:4302. [Google Scholar]
- Gillespie R. J., Ireland P. R., Vekris J. E. Can. J. Chem. 1975;53:3147. [Google Scholar]
- Gillespie R. J., Kent J. P., Sawyer J. F. Inorg. Chem. 1981;20:3784. [Google Scholar]
- Gillespie R. J., Kent J. P., Sawyer J. F. Inorg. Chem. 1981;20:4053. [Google Scholar]
- Mueller B., Poleschner H., Seppelt K. Dalton Trans. 2008:4424. doi: 10.1039/b802259n. [DOI] [PubMed] [Google Scholar]
- Wakamiya A., Nishinaga T., Komatsu K. J. Am. Chem. Soc. 2002;124:15038. doi: 10.1021/ja028297j. [DOI] [PubMed] [Google Scholar]
- Mueller B., Takaluoma T. T., Laitinen R. S., Seppelt K. Eur. J. Inorg. Chem. 2011:4970. [Google Scholar]
- Zhang S., Wang X., Sui Y., Wang X. J. Am. Chem. Soc. 2014;136:14666. doi: 10.1021/ja507918c. [DOI] [PubMed] [Google Scholar]
- Mallow O., Khanfar M. A., Malischewski M., Finke P., Hesse M., Lork E., Augenstein T., Breher F., Harmer J. R., Vasilieva N. V., Zibarev A., Bogomyakov A. S., Seppelt K., Beckmann J. Chem. Sci. 2015;6:497. doi: 10.1039/c4sc02964j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen R., Steudel R., Weiss R. J. Chem. Soc., Dalton Trans. 1986:1095. [Google Scholar]
- Destro R., Lucchini V., Modena G., Pasquato L. J. Org. Chem. 2000;65:3367. doi: 10.1021/jo991731o. [DOI] [PubMed] [Google Scholar]
- Fujihara H., Mima H., Erata T., Furukawa N. J. Am. Chem. Soc. 1992;114:3117. [Google Scholar]
- Gibler D. D., Adams C. J., Fischer M., Zalkin A., Bartlett N. Inorg. Chem. 1972;11:2325. [Google Scholar]
- Mallouk T. E., Rosenthal G. L., Mueller G., Brusasco R., Bartlett N. Inorg. Chem. 1984;23:3167. [Google Scholar]
- Edwards A. J. J. Chem. Soc., Dalton Trans. 1978:1723. [Google Scholar]
- Finch A., Gates P. N., Page T. H., Dillon K. B., Waddington T. C. J. Chem. Soc., Dalton Trans. 1980:2401. [Google Scholar]
- Sawodny W., Rediess K., Thewalt U. Z. Anorg. Allg. Chem. 1983;499:81. [Google Scholar]
- Trojanow S. I., Kolditz L., Radde A. Z. Chem. 1983;23:136. [Google Scholar]
- Beck J., Hengstmann M. Z. Naturforsch. 1996;51b:1415. [Google Scholar]
- Johnson J. P., Murchie M., Passmore J., Tajik M., White P. S., Wong C.-M. Can. J. Chem. 1988;66:2671. [Google Scholar]
- Johnson J. P., Murchie M., Passmore J., Tajik M., White P. S., Wong C.-M. Can. J. Chem. 1987;65:2744. [Google Scholar]
- Brooks W. V. F., MacLean G. K., Passmore J., White P. S., Wong C.-M. J. Chem. Soc., Dalton Trans. 1983:1961. [Google Scholar]
- Edwards A. J., Jones G. R., Edwards A. J., Jones G. R., Edwards A. J., Jones G. R. J. Chem. Soc. A. J. Chem. Soc. A. Chem. Commun. 197019701968:1491. 1891, 346. [Google Scholar]
- Stork-Blaisse B. A., Romers C. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1971;27:386. [Google Scholar]
- Baumann A., Beck J. Z. Anorg. Allg. Chem. 1998;624:1725. [Google Scholar]
- Jones P. G., Schelbach R., Schwarzmann E. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1987;43:607. [Google Scholar]
- Passmore J., Richardson E. K., Whidden T. K., White P. S. Can. J. Chem. 1980;58:851. [Google Scholar]
- Beck J., Fischer A. Z. Anorg. Allg. Chem. 1995;621:1042. [Google Scholar]
- Edwards A. J., Taylor P. J. Chem. Soc., Dalton Trans. 1973:2150. [Google Scholar]
- Krebs B., Buss B., Altena D. Z. Anorg. Allg. Chem. 1971;386:257. [Google Scholar]
- Jones P. G., Jentsch D., Schwarzmann E. Z. Naturforsch. 1986;41b:1483. [Google Scholar]
- Beck J. Z. Naturforsch. 1991;46b:183. [Google Scholar]
- Beck J., Biedenkopf P., Müller-Buschbaum K. Z. Naturforsch. 1996;51b:727. [Google Scholar]
- Beck J. Z. Naturforsch. 1996;51b:1127. [Google Scholar]
- Beck J., Schlörb T. Z. Kristallogr. 1999;214:780. [Google Scholar]
- Freire-Erdbrügger C., Jentsch D., Jones P. G., Schwarzmann E. Z. Naturforsch. 1987;42b:1553. [Google Scholar]
- Passmore J., Sutherland G., White P. S. Can. J. Chem. 1981;59:2876. [Google Scholar]
- Schulz Lang E., Abram U., Strähle J., Vazquez Lopez E. M. Z. Anorg. Allg. Chem. 1998;624:999. [Google Scholar]
- Beck J., Steden F. Z. Naturforsch. 2003;58b:711. [Google Scholar]
- Engesser T. A., Hrobárik P., Trapp N., Eiden P., Scherer H., Kaupp M., Krossing I. ChemPlusChem. 2012;77:643. [Google Scholar]
- Bergholdt A. B., Kobayashi K., Horn E., Takahashi O., Sato S., Furukawa N., Yokoyama M., Yamaguchi K. J. Am. Chem. Soc. 1998;120:1230. [Google Scholar]
- Hopfinger M., Lux K., Kornath A. ChemPlusChem. 2012;77:476. [Google Scholar]
- Frohn H.-J., Hirschberg M. E., Wenda A., Bardin V. V. J. Fluorine Chem. 2008;129:459. [Google Scholar]
- Tuckett R. P., Dale A. R., Jaffey D. M., Jarrett P. S., Kelly T., Porter T. L. Mol. Phys. J. Chem. Phys. 1983;1968;4948:475. 2071. [Google Scholar]
- Schlöder T., Riedel S. RSC Adv. 2012;2:876. [Google Scholar]
- Singh R. P., Shreeve J. M. Acc. Chem. Res. 2004;37:31. doi: 10.1021/ar030043v. [DOI] [PubMed] [Google Scholar]
- Seidel S., Seppelt K. Angew. Chem., Int. Ed. 2000;39:3923. doi: 10.1002/1521-3773(20001103)39:21<3923::AID-ANIE3923>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Christe K. O., Bau R., Zhao D. Z. Anorg. Allg. Chem. 1991;593:46. [Google Scholar]
- Cameron T. S., Passmore J., Wang X. Angew. Chem., Int. Ed. 2004;43:1995. doi: 10.1002/anie.200352969. [DOI] [PubMed] [Google Scholar]
- Rogachev A. Y., Hoffmann R., Cotton F. A., Dikarev E. V., Petrukhina M. A. J. Am. Chem. Soc. Angew. Chem., Int. Ed. 2013;2000;13539:3262. 2362. [Google Scholar]
- Malinowski P., Himmel D. and Krossing I., 2015, to be submitted. [Google Scholar]
- Crawford M.-J., Göbel M., Karaghiosoff K., Klapötke T. M., Welch J. M. Inorg. Chem. 2009;48:9983. doi: 10.1021/ic9015492. [DOI] [PubMed] [Google Scholar]
- Fañanás F. J., Alvarez-Pérez M., Rodríguez F. Chem. – Eur. J. 2005;11:5938. doi: 10.1002/chem.200500070. [DOI] [PubMed] [Google Scholar]
- Gillespie R. J., Morton M. J., Schmeißer M., Ludovici W., Naumann D., Sartori P., Scharf E. Inorg. Chem. Chem. Ber. 1970;1968;9101:811. 4214. [Google Scholar]
- Thorup N., Shamir J. Inorg. Nucl. Chem. Lett. 1981;17:193. [Google Scholar]
- Pohl S., Saak W. Z. Naturforsch. 1981;36b:83. [Google Scholar]
- Birchall T., Myers R. D. Inorg. Chem. 1982;21:213. [Google Scholar]
- Stoyanov E. S., Stoyanova I. V., Tham F. S., Reed C. A. J. Am. Chem. Soc. 2010;132:4062. doi: 10.1021/ja100297b. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Nagorski R. W., Bennet A. J., McClung R. E. D., Aarts G. H. M., Klobukowski M., McDonald R., Santarsiero B. D. J. Am. Chem. Soc. 1994;116:2448. [Google Scholar]
- Frohn H.-J., Wenda A., Flörke U. Z. Anorg. Allg. Chem. 2008;634:764. [Google Scholar]
- Christe K. O., Curtis E. C., Schack C. J., Schack C. J., Lindahl C. B., Pilipovich D., Christe K. O. Inorg. Chem. Inorg. Chem. 1972;1972;1111:2212. 2201. [Google Scholar]
- Adelhelm M., Jacob E. Angew. Chem., Int. Ed. 1977;16:461. [Google Scholar]
- Lehmann J. F., Schrobilgen G. J., Christe K. O., Kornath A., Suontamo R. J. Inorg. Chem. 2004;43:6905. doi: 10.1021/ic040015o. [DOI] [PubMed] [Google Scholar]
- Christe K. O., Zhang X., Sheehy J. A., Bau R. J. Am. Chem. Soc. 2001;123:6338. doi: 10.1021/ja003347a. [DOI] [PubMed] [Google Scholar]
- Vij A., Tham F. S., Vij V., Wilson W. W., Christe K. O. Inorg. Chem. 2002;41:6397. doi: 10.1021/ic025900q. [DOI] [PubMed] [Google Scholar]
- Edwards A. J., Jones G. R., Sills R. J. C., Edwards A. J., Jones G. R. Chem. Commun. J. Chem. Soc. A. 19681971:1527. 2318. [Google Scholar]
- Apblett A., Grein F., Johnson J. P., Passmore J., White P. S. Inorg. Chem. 1986;25:422. [Google Scholar]
- Gillespie R. J., Kapoor R., Faggiani R., Lock C. J. L., Murchie M., Passmore J., Faggiani R., Gillespie R. J., Kapoor R., Lock C. J. L., Vekris J. E. J. Chem. Soc., Chem. Commun. Inorg. Chem. 1983;1988;27:8. 4350. [Google Scholar]
- Lynton H., Passmore J. Can. J. Chem. 1971;49:2539. [Google Scholar]
- Edwards A. J., Sills R. J. C. J. Chem. Soc. A. 1970:2697. [Google Scholar]
- Bougon R., Cicha W. V., Lance M., Meublat L., Nierlich M., Vigner J. Inorg. Chem. 1991;30:102. [Google Scholar]
- Edwards A. J., Jones G. R., Edwards A. J., Jones G. R. Chem. Commun. J. Chem. Soc. A. 19671969:1304. 1467. [Google Scholar]
- Frohn H.-J., Giesen M., Welting D. Eur. J. Solid State Inorg. Chem. 1996;33:841. [Google Scholar]
- Birchall T., Myers R. D. Inorg. Chem. 1981;20:2207. [Google Scholar]
- Birchall T., Myers R. D. Inorg. Chem. 1983;22:1751. [Google Scholar]
- Bailly F., Barthen P., Frohn H.-J., Köckerling M. Z. Anorg. Allg. Chem. 2000;626:2419. [Google Scholar]
- Lehmann J. F., Riedel S., Schrobilgen G. J. Inorg. Chem. 2008;47:8343. doi: 10.1021/ic800929h. [DOI] [PubMed] [Google Scholar]
- Edwards A. J., Sills R. J. C. J. Chem. Soc., Dalton Trans. 1974:1726. [Google Scholar]
- Mallouk T. E., Desbat B., Bartlett N. Inorg. Chem. 1984;23:3160. [Google Scholar]
- Tobias K. M., Jansen M. Angew. Chem., Int. Ed. 1986;25:993. [Google Scholar]
- Lind M. D., Christe K. O. Inorg. Chem. 1972;11:608. [Google Scholar]
- Baird H. W., Giles H. F. Acta Crystallogr. 1969;A25:115. [Google Scholar]
- Edwards A. J., Taylor P. J. Chem. Soc., Dalton Trans. 1975:2174. [Google Scholar]
- Graham L., Graudejus O., Jha N. K., Bartlett N. Coord. Chem. Rev. 2000;197:321. [Google Scholar]
- Lehmann J. F., Schrobilgen G. J. J. Fluorine Chem. 2003;119:109. [Google Scholar]
- Gillespie R. J., Schrobilgen G. J. Inorg. Chem. 1974;13:765. [Google Scholar]
- Christe K. O., Wilson W. W., Curtis E. C. Inorg. Chem. 1983;22:3056. [Google Scholar]
- Gillespie R. J., Schrobilgen G. J., Gillespie R. J., Schrobilgen G. J. J. Chem. Soc., Chem. Commun. Inorg. Chem. 1974;1974;13:90. 1230. [Google Scholar]
- Holloway J. H., Schrobilgen G. J. J. Chem. Soc., Chem. Commun. 1975:623. [Google Scholar]
- McRae V. M., Peacock R. D., Russell D. R. J. Chem. Soc. D. 1969:62. [Google Scholar]
- Bartlett N., Einstein F., Stewart D. F., Trotter J. J. Chem. Soc. A. 1967:1190. [Google Scholar]
- Bartlett N., Einstein F., Stewart D. F., Trotter J. Chem. Commun. 1966:550. [Google Scholar]
- Hughes M. J., Mercier H. P. A., Schrobilgen G. J. Inorg. Chem. 2010;49:3501. doi: 10.1021/ic100062x. [DOI] [PubMed] [Google Scholar]
- Elliott H. S. A., Lehmann J. F., Mercier H. P. A., Jenkins H. D. B., Schrobilgen G. J. Inorg. Chem. 2010;49:8504. doi: 10.1021/ic101152x. [DOI] [PubMed] [Google Scholar]
- Mazej Z., Goreshnik E. A. J. Fluorine Chem. 2015;175:47. [Google Scholar]
- Frohn H. J., Jakobs S., Henkel G. Angew. Chem., Int. Ed. 1989;28:1506. [Google Scholar]
- Pointner B. E., Suontamo R. J., Schrobilgen G. J. Inorg. Chem. 2006;45:1517. doi: 10.1021/ic0510260. [DOI] [PubMed] [Google Scholar]
- Drews T., Seidel S., Seppelt K. Angew. Chem., Int. Ed. 2002;41:454. doi: 10.1002/1521-3773(20020201)41:3<454::aid-anie454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Seidel S., Seppelt K., van Wüllen C., Sun X. Y. Angew. Chem., Int. Ed. 2007;46:6717. doi: 10.1002/anie.200701688. [DOI] [PubMed] [Google Scholar]
- Seidel S., Seppelt K. Science. 2000;290:117. doi: 10.1126/science.290.5489.117. [DOI] [PubMed] [Google Scholar]
- Hwang I.-C., Seidel S., Seppelt K. Angew. Chem., Int. Ed. 2003;42:4392. doi: 10.1002/anie.200351208. [DOI] [PubMed] [Google Scholar]
- Lehmann J. F., Dixon D. A., Schrobilgen G. J. Inorg. Chem. 2001;40:3002. doi: 10.1021/ic001167w. [DOI] [PubMed] [Google Scholar]
- Bartlett N., Gennis M., Gibler D. D., Morrell B. K., Zalkin A. Inorg. Chem. 1973;12:1717. [Google Scholar]
- Zalkin A., Ward D. L., Biagioni R. N., Templeton D. H., Bartlett N. Inorg. Chem. 1978;17:1318. [Google Scholar]
- Sawyer J. F., Schrobilgen G. J., Sutherland S. J. Inorg. Chem. 1982;21:4064. [Google Scholar]
- Sladky F. O., Bulliner P. A., Bartlett N., DeBoer B. G., Zalkin A. Chem. Commun. 1968:1048. [Google Scholar]
- Bartlett N., DeBoer B. G., Hollander F. J., Sladky F. O., Templeton D. H., Zalkin A. Inorg. Chem. 1974;13:780. [Google Scholar]
- Fir B. A., Gerken M., Pointner B. E., Mercier H. P. A., Dixon D. A., Schrobilgen G. J. J. Fluorine Chem. 2000;105:159. [Google Scholar]
- Faggiani R., Kennepohl D. K., Lock C. J. L., Schrobilgen G. J. Inorg. Chem. 1986;25:563. [Google Scholar]
- Koppe K., Frohn H.-J., Mercier H. P. A., Schrobilgen G. J. Inorg. Chem. 2008;47:3205. doi: 10.1021/ic702259c. [DOI] [PubMed] [Google Scholar]
- Frohn H.-J., Schroer T., Henkel G. Angew. Chem., Int. Ed. 1999;38:2554. [PubMed] [Google Scholar]
- Fir B. A., Mercier H. P. A., Sanders J. C. P., Dixon D. A., Schrobilgen G. J. J. Fluorine Chem. 2001;110:89. [Google Scholar]
- Mercier H. P. A., Moran M. D., Sanders J. C. P., Schrobilgen G. J., Suontamo R. J. Inorg. Chem. 2005;44:49. doi: 10.1021/ic0400890. [DOI] [PubMed] [Google Scholar]
- Seidel S., Seppelt K. Angew. Chem., Int. Ed. 2001;40:4225. doi: 10.1002/1521-3773(20011119)40:22<4225::AID-ANIE4225>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Fir B., Whalen J. M., Mercier H. P. A., Dixon D. A., Schrobilgen G. J. Inorg. Chem. 2006;45:1978. doi: 10.1021/ic051451t. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Mercier H. P. A., Schrobilgen G. J. Inorg. Chem. 2007;46:1369. doi: 10.1021/ic061899+. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Mercier H. P. A., Schrobilgen G. J. J. Am. Chem. Soc. 2009;131:7272. doi: 10.1021/ja901187n. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Schrobilgen G. J. Inorg. Chem. 2009;48:7714. doi: 10.1021/ic900651n. [DOI] [PubMed] [Google Scholar]
- Gerken M., Moran M. D., Mercier H. P. A., Pointner B. E., Schrobilgen G. J., Hoge B., Christe K. O., Boatz J. A. J. Am. Chem. Soc. 2009;131:13474. doi: 10.1021/ja905060v. [DOI] [PubMed] [Google Scholar]
- McKee D. E., Zalkin A., Bartlett N. Inorg. Chem. 1973;12:1713. [Google Scholar]
- Gillespie R. J., Martin D., Schrobilgen G. J., Slim D. R. J. Chem. Soc., Dalton Trans. 1977:2234. [Google Scholar]
- Brock D. S., Mercier H. P. A., Schrobilgen G. J. J. Am. Chem. Soc. 2013;135:5089. doi: 10.1021/ja312493j. [DOI] [PubMed] [Google Scholar]
- Leary K., Templeton D. H., Zalkin A., Bartlett N. Inorg. Chem. 1973;12:1726. [Google Scholar]
- Mazej Z., Goreshnik E. Eur. J. Inorg. Chem. 2009:4503. doi: 10.1021/ic9009338. [DOI] [PubMed] [Google Scholar]
- Lutar K., Jesih A., Leban I., Zemva B., Bartlett N. Inorg. Chem. 1989;28:3467. [Google Scholar]
- Zemva B., Jesih A., Templeton D. H., Zalkin A., Cheetham A. K., Bartlett N. J. Am. Chem. Soc. 1987;109:7420. [Google Scholar]
- Leary K., Zalkin A., Bartlett N. Inorg. Chem. 1974;13:775. [Google Scholar]