Abstract
Purpose
Antiangiogenic agents show significant antitumor activity against various tumor types. In a study evaluating the combination of sorafenib, bevacizumab, and low-dose cyclophosphamide in children with solid tumors, an unexpectedly high incidence of pneumothorax was observed. We evaluated patient characteristics and risk factors for the development of pneumothorax in patients receiving this therapy.
Patients and Methods
Demographics, clinical course, and radiographic data of 44 patients treated with sorafenib, bevacizumab and cyclophosphamide were reviewed. Risk factors associated with the development of pneumothorax were analyzed.
Results
Pneumothorax likely related to study therapy developed in 11 of 44 (25%) patients of whom 33 had pulmonary abnormalities. Median age of patients was 14.7 years (range, 1.08–24.5). Histologies associated with pneumothorax included rhabdoid tumor, synovial sarcoma, osteosarcoma, Ewing sarcoma, Wilms tumor, and renal cell carcinoma. Cavitation of pulmonary nodules in response to therapy was associated with pneumothorax development (P<0.001). Median time from start of therapy to development of pneumothorax was 5.7 weeks (range, 2.4–31).
Conclusion
The development of cavitary pulmonary nodules in response to therapy is a risk factor for pneumothorax. As pneumothorax is a potentially life-threatening complication of antiangiogenic therapy in children with solid tumors, its risk needs to be evaluated when considering this therapy.
Keywords: pneumothorax, antiangiogenic, solid tumors, cavitary pulmonary lesions
Antiangiogenic strategies are well characterized in clinical oncology and may involve the use of either single-agent, multitargeted tyrosine kinase inhibitors with activity against different proangiogenic factors [e.g., vascular endothelial growth factor (VEGF), VEGF receptors (VEGFRs) and platelet derived growth factor receptors (PDGFRs), fibroblast growth factor receptors (FGFRs)] or an antiangiogenic agent combined with cytotoxic chemotherapy. [1–6] The benefit of these approaches has thus far been seen only in adults, and therapeutic outcomes in children are not fully known. Further, the efficacy of commercially available drugs targeting the tumor vasculature, such as bevacizumab, sorafenib, pazopanib, and sunitinib, remains limited in the clinical setting because of the development of adaptive drug resistance.[7] One potential approach to overcome resistance is to combine drugs that work within the same vertical or horizontal angiogenic axis (e.g., VEGF and VEGFRs), and preclinical models support this strategy.[8, 9]
Considering the limited data available for antiangiogenic therapy in children and the concerns with resistance, we conducted a phase I trial evaluating a combination antiangiogenic strategy (sorafenib, bevacizumab, and low-dose cyclophosphamide) in children and young adults with refractory solid tumors.[10] Surprisingly, in this dose-finding safety study on heavily pretreated patients who had rapidly progressive disease, objective responses and disease stabilization were observed in more than 50% of patients. Dose-limiting toxicities included rash, increased lipase, anorexia, and thrombus, but we also observed a high incidence of spontaneous pneumothorax in patients on this therapy. In the current study, our objective was to identify patient characteristics and the risk factors associated with the development of pneumothorax.
1. Patients and Methods
1.1. Patient Population and Study Design
The medical records of the 44 patients with refractory or recurrent solid tumors enrolled on a single-institution phase I trial of bevacizumab and sorafenib combined with low-dose cyclophosphamide (NCT00665990) between November 2008 and May 2013 were reviewed. The dose-escalation phase of this study has been previously reported and included 19 of these patients.[10] The remaining 25 patients were treated in the extension phase of the study to better define the toxicity and anti-tumor activity at the recommended dose of sorafenib 90 mg/m2/dose by mouth twice daily, bevacizumab 15 mg/kg intravenously every 3 weeks, and cyclophosphamide 50 mg/m2 by mouth once daily. The trial was approved by the St. Jude Children’s Research Hospital Institutional Review Board. All patients, parents, or legal guardians provided written informed consent. The inclusion and exclusion criteria for the clinical trial have been previously reported.[10] Briefly, inclusion criteria were presence of a recurrent solid tumor or a solid tumor that was refractory to standard therapy; age ≤21 years at initial diagnosis, life expectancy ≥8 weeks, Karnofsky/Lansky performance score ≥50, body surface area ≥0.3 m2, and adequate bone marrow and organ function as specified in the protocol. Disease assessments which included a CT of the chest were required at baseline, at the end of course 1 and 2, then after every other course.
Patient demographics (age, gender, race) and cancer-related history (tumor type, sites of disease at the time of study enrollment, prior treatments) were noted. For patients who developed a pneumothorax, the timing of the pneumothorax, number and location of pulmonary nodules, history of prior lung intervention (surgery or radiation), treatment for the pneumothorax, and outcomes were reviewed.
The study radiologist (M.B.M) retrospectively reviewed the baseline and all follow-up chest computed tomography (CT) scans of patients with lung pathology and documented the number and laterality of pulmonary nodules, whether nodules were subpleural in location (within 3 mm of the pleural surface), and whether they were cavitary or solid. For participants who developed pneumothorax, the laterality and size of the pneumothorax were recorded. The radiologist estimated size categories on the basis of the following criteria: small, causing <10% lung compression; medium, causing up to 50% lung compression; and large, causing >50% lung compression. The presence of a tension component (mass effect or shift of midline structures) and outcome of pneumothorax were also recorded.
1.2. Statistical Analysis
The Chi-square test and logistic regression were used to study the association between potential risk factors and the development of pneumothorax. P-values less than 0.05 were considered statistically significant.
2. Results
2.1. Patient Characteristics
Table 1 summarizes the patient characteristics. Of the 44 patients (27 male; 8 white), 11 patients developed a pneumothorax that was thought to be related to the therapy. One patient developed a small pneumothorax as a consequence of central venous catheter placement, which resolved spontaneously. For this analysis, this patient was considered as having no pneumothorax. The median length of time from the start of therapy to the development of pneumothorax was 5.7 weeks (range 2.4–31 weeks). There were no significant differences in age, gender, race, tumor type, history of prior chest surgery or radiation therapy, number of prior chemotherapy regimens received, or timing of study entry from the last chest surgery between patients who did and did not develop a pneumothorax (Table 1). However, patients with pulmonary nodules were significantly more likely to develop a pneumothorax than those without pulmonary nodules (P = 0.041); no patients without pulmonary nodules subsequently developed pneumothorax.
Table 1.
Patient characteristics
| All Patients | Patients with Pneumothorax |
Patients without Pneumothorax |
P-value | ||
|---|---|---|---|---|---|
| No. of Patients | 44 | 11 | 33 | ||
| Age (yearsc | |||||
| Median (range) | 12.5 (1.08–24.5) | 14.7 (1.08–24.5) | 11.6 (1.19–22.4) | 0.126 | |
| Genderc | |||||
| Male | 27 | 6 | 21 | 0.593 | |
| Female | 17 | 5 | 12 | ||
| Racec | |||||
| White | 34 | 8 | 26 | 0.974 | |
| Non-white | 10 | 3 | 7 | ||
| Histologic Diagnosis | |||||
| NRSTSa | 9 | 2 | 7 | 0.135 | |
| Rhabdoid tumor | 8 | 1 | 7 | ||
| Osteosarcoma | 5 | 2 | 3 | ||
| Neuroblastoma | 5 | 0 | 5 | ||
| Wilms tumor | 4 | 2 | 2 | ||
| Rhabdomyosarcoma | 3 | 0 | 3 | ||
| Ewing sarcoma | 3 | 2 | 1 | ||
| Renal cell carcinoma | 3 | 2 | 1 | ||
| Otherb | 4 | 0 | 4 | ||
| Prior lung radiation therapyc | |||||
| Yes | 4 | 2 | 2 | 0.247 | |
| No | 40 | 31 | 9 | ||
| No. of prior chemotherapy courses receivedc | |||||
| Median (range) | 4 (1–23) | 6 (1–18) | 4 (1–23) | 0.417 | |
| Prior chest surgery | |||||
| Yes | 25 | 7 | 18 | 0.599 | |
| No | 19 | 4 | 15 | ||
| Time from prior-chest surgery to first day of drug (N = 25) (weeks)c | 20.9 (0.29–401) | 28.4 (7.1–401) | 16.4 (0.29–150.1) | 0.319 | |
| Pulmonary lesions at study entry | |||||
| Yes | 33 | 11 | 22 | 0.041 | |
| No | 11 | 0 | 11 | ||
Abbreviations: NRSTS, non-rhabdomyosarcoma soft tissue sarcoma.
Synovial sarcoma (N = 5), epithelioid sarcoma (N = 1), malignant peripheral nerve sheath tumor (N = 1), high-grade sarcoma, not specified (N = 1), clear cell sarcoma (N = 1).
Medulloblastoma (N = 1), atypical clear cell meningioma (N = 1), hepatocellular carcinoma (N = 1), adrenocortical carcinoma (N = 1).
P-values were calculated from a logistic regression model.
Table 2 compares the characteristics of pulmonary nodules in patients with and without pneumothorax. Eight of the 11 patients who developed pneumothorax had pulmonary metastases at study entry that were confirmed by biopsy or clinical judgment (nodules were multiple, bilateral or round and sharply defined). The benign versus malignant nature of pulmonary nodules in the remaining 3 patients was not apparent at study entry. The first patient had questionable nodules in the right lung that were initially thought to be infectious in etiology but later proved to be tumor. The second patient was thought to have postoperative changes at the site of an eventual pneumothorax associated with a small cavitary nodule. The third patient with synovial sarcoma developed a left pneumothorax in association with a posterior mediastinal mass and a cavitary nodule in the left lung (later biopsy proven to be tumor).
Table 2.
Characteristics of pulmonary nodules in patients with and without pneumothorax
| All Patients |
Patients with Pneumothorax |
Patients without Pneumothorax |
P-value | ||
|---|---|---|---|---|---|
| No. of Patients with Pulmonary Nodules | 33 | 11 | 22 | 0.080 | |
| Histologic Diagnosis | |||||
| NRSTSa | 6 | 2 | 4 | 0.174 | |
| Rhabdoid tumor | 6 | 1 | 5 | ||
| Osteosarcoma | 5 | 2 | 3 | ||
| Neuroblastoma | 1 | 0 | 1 | ||
| Wilms tumor | 3 | 2 | 1 | ||
| Rhabdomyosarcoma | 3 | 0 | 3 | ||
| Ewing sarcoma | 3 | 2 | 1 | ||
| Renal cell carcinoma | 3 | 2 | 1 | ||
| Otherb | 3 | 0 | 3 | ||
| Cavitary Nodule in Response to Therapyd | |||||
| Yes | 12 | 9 | 3 | 0.0008 | |
| No | 21 | 2 | 19 | ||
| No. of Pulmonary Nodules [median (range)]c | |||||
| Left | 5 (0–20+) | 5 (0–20+) | 4 (0–20+) | 0.839 | |
| Right | 6 (0–20+) | 5 (0–20+) | 7.5 (0–20+) | 0.234 | |
| No. of Pleural-Based Nodules | |||||
| Left | 2 (0–20+) | 3 (0–20+) | 2 (0–10) | 0.204 | |
| Right | 4 (0– 20+) | 4 (0–20+) | 4 (0–10) | 0.261 | |
Synovial sarcoma (N = 3), epithelioid sarcoma (N = 1), malignant peripheral nerve sheath tumor (N = 1), clear cell sarcoma (N = 1).
Atypical clear cell meningioma (N = 1), hepatocellular carcinoma (N = 1), adrenocortical carcinoma (N = 1).
Seven patients had too numerous to count (TNTC) pulmonary nodules, and were designated a value of 20+. Six patients had TNTC on the left and 5 patients had TNTC on the right. For this analysis, patients were assigned a value of 20 and P-values were calculated from a logistic regression model.
P-values were calculated from a logistic regression model.
Patients were more likely to develop a pneumothorax if the pulmonary nodule(s) became cavitary in response to therapy (P = 0.0008). Of the 12 patients with cavitary nodules in response to therapy, 9 (75%) developed a pneumothorax. However, of the 21 patients without cavitary nodules in response to therapy, only 2 (9.5%) developed a pneumothorax. Five patients had cavitation of existing nodules before the development of pneumothorax, and 4 of these patients presented asymptomatically with the pneumothorax. The number of pulmonary nodules and subpleural location were not associated with an increased risk of pneumothorax (P > 0.2). A histologic diagnosis of sarcoma was also not associated with the development of pneumothorax in the entire group of patients as well as the group with pulmonary nodules.
Table 3 summarizes the characteristics and treatment of the 11 patients with a pneumothorax. Seven patients had unilateral pneumothorax. Ten pneumothoraces were small or medium, with 2 causing a mediastinal shift. The pneumothorax was incidentally noted on routine CT imaging in 7 of the 11 patients; 4 patients were symptomatic (chest pain or shortness of breath) and further underwent a plain chest x-ray. One patient who had bilateral pneumothoraces presented initially with chest pain and then re-presented with a recurrence of a unilateral pneumothorax with shortness of breath.
Table 3.
Characteristics and treatment of pneumothorax (N = 11)
| Characteristic | Patients (No.) | |
|---|---|---|
| Bilateral | 4 | |
| Unilateral | 7 | |
| Left | 5 | |
| Right | 2 | |
| Size of Pneumothorax | ||
| Small | 6 | |
| Medium | 4 | |
| Large | 1 | |
| Presence of Tension Component by CT | 2 | |
| Symptomatica | 4 | |
| Chest pain | 3 | |
| Shortness of breath | 2 | |
| Therapy for Pneumothorax | 5 | |
| Pigtail catheter | 5 | |
| Chest tube | 2 | |
| Chemical pleurodesis with doxocycline | 2 | |
| Mechanical pleurodesis | 1 | |
| Video-assisted thoracoscopic surgery and chemical pleurodesis | 1 | |
| Thoracotomy | 1 | |
| Length of Therapy (days) [median (range)] | 12 (3–101) | |
| Outcome of Pneumothorax | ||
| Resolved | 5 | |
| Incomplete resolution | 4 | |
| Unknownb | 1 | |
| Mortality | 1 | |
Abbreviation: CT, computed tomography.
The same patient presented with shortness of breath and chest pain on separate admissions.
Patient lost to follow-up, and outcome of pneumothorax unknown.
2.2. Treatment and Outcome of Patients with Pneumothorax
Of the 11 patients with pneumothorax, 3 had resolution of pneumothorax while continuing study therapy. One patient required a pigtail catheter to evacuate the pneumothorax, whereas the other 2 patients required no intervention. The time to resolution of pneumothorax in these three patients was 27, 41, and 126 days from onset. The remaining 8 patients had persistent pneumothorax at the time they met off-study criteria. Reason for off study included progressive disease (N = 4), persistent pneumothorax requiring surgical intervention (N = 2), death due to pneumothorax (N = 1), and family preference (N = 1). The patient who was off study due to family preference was lost to follow up. Of the 4 patients with progressive disease, 1 patient had spontaneous resolution (i.e., no intervention) of pneumothorax whereas the other 3 patients had persistent pneumothorax at the time of last imaging. One of these 3 patients required bilateral chest tubes and chemical pleurodesis. The remaining 2 patients required no intervention before death due to their disease. Of the 2 patients removed from study because of persistent pneumothorax, one underwent a bilateral video-assisted thoracoscopy with chemical pleurodesis, followed by a thoracotomy with mechanical pleurodesis due to continued air leak. The other patient required a thoracotomy and wedge resection of what was suspected to be the source of an air leak approximately 3 weeks after his last dose of the study drug. Pathology from the wedge resection at the site of air leak revealed metastatic renal cell carcinoma with angiolymphatic spread. Of the 11 patients with pneumothoraces, 7 patients were identified incidentally on follow-up imaging. Of these 7, one patient required interventions on both thoracic cavities beginning with pigtail catheter placement and ultimately requiring chemical pleurodesis with doxycycline.
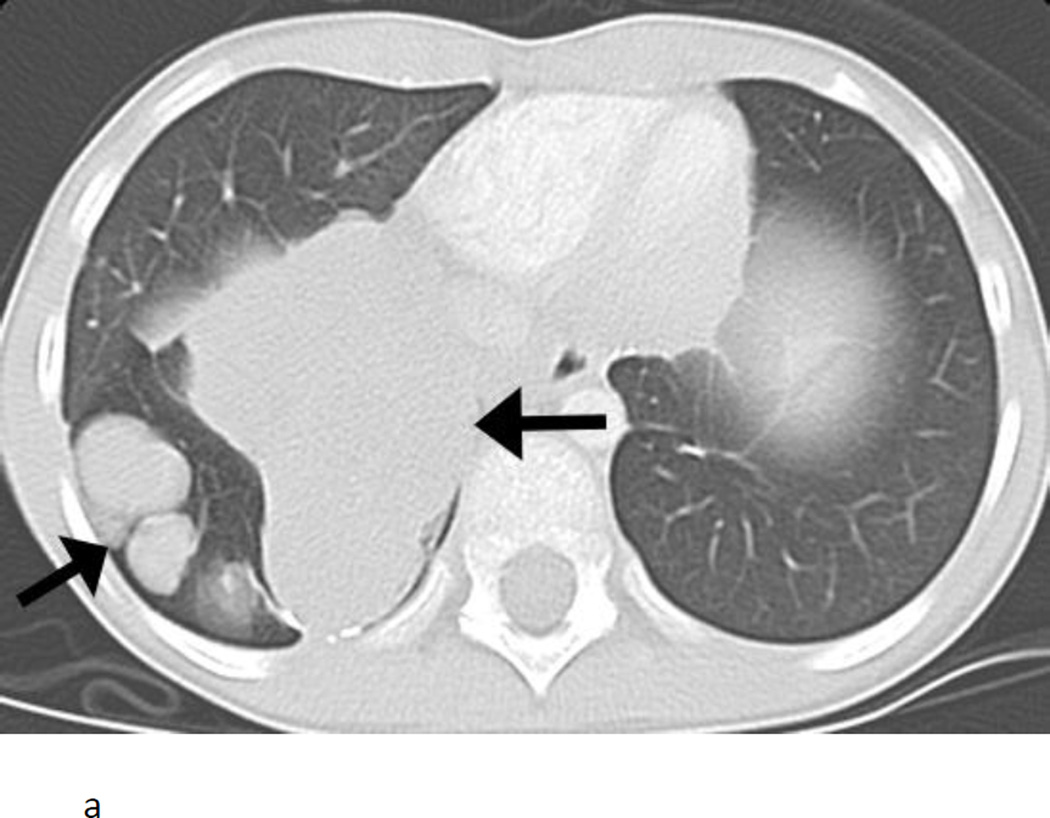
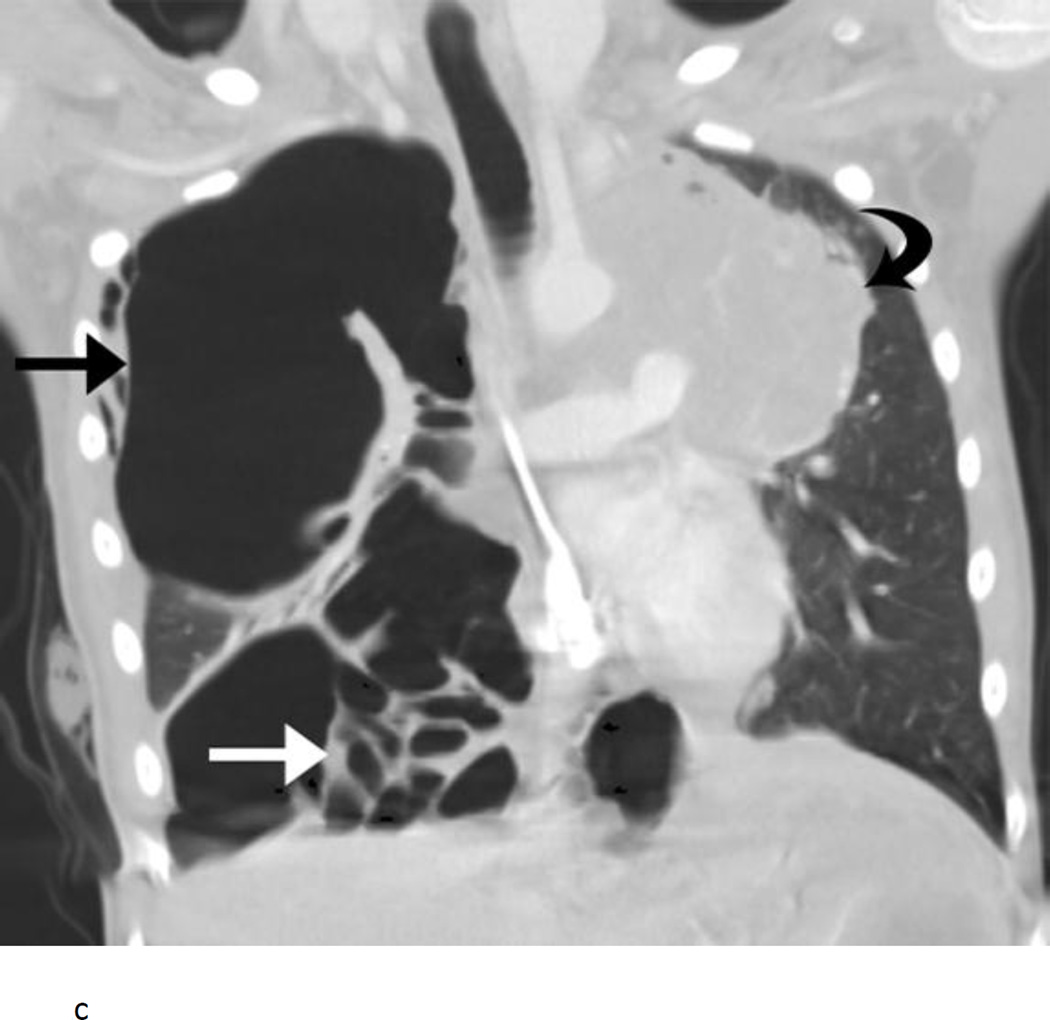
The patient who died from the complication of pneumothorax had rapidly progressive Wilms tumor with bilateral lung masses at the time of study entry (Fig. 1A). After 1 course of therapy, the tumors in the lungs had become smaller and largely cavitated with internal cyst formation (Fig. 1B). Imaging at the end of course 2 of therapy showed a large right pneumothorax with mediastinal shift. The patient was asymptomatic. A pigtail catheter was placed, the pneumothorax evacuated, and the catheter removed without incident. During course 3 of therapy, the patient developed acute onset of right-sided chest pain and shortness of breath. A chest radiograph revealed a right pneumothorax with mediastinal shift. A chest tube was placed, which led to improvement in mediastinal shift. The patient underwent a pleurodesis. Within 48 h, the patient developed acute respiratory distress following a coughing episode secondary to choking on a piece of food. A chest CT scan revealed multiple cystic changes in the right lung with right upper lobe atelectasis; pneumothorax could not be excluded (Fig. 1C). The patient’s respiratory status deteriorated. A catheter was placed in one of the cystic lesions, leading to some improvement, but the overall condition of the patient remained critical. The patient was sent home on hospice care and died the following day.
Fig. 1.
Nine year old female with pulmonary recurrence of Wilms tumor. A) Axial computed tomography (CT) image obtained just before initiation of therapy shows large solid pulmonary nodules and mass in the right lower lobe (arrows). B) At the end of course 1, the nodules and mass became cavitary with numerous cysts scattered throughout them. A small right pneumothorax had developed (arrow). C) Coronal CT image obtained approximately during course 3 of therapy shows right tension pneumothorax (straight black arrow) causing shift of mediastinal structures into the left chest. The right lower lobe is now largely replaced by cysts (straight white arrow). A large solid mass is present in the left upper lobe (curved arrow).
3. Discussion
Pneumothorax as a complication before treatment or after initiating cytotoxic chemotherapy, radiation, or targeted biologic agents, including antiangiogenic agents (e.g., pazopanib, cediranib, sunitinib, or bevacizumab) in patients with pulmonary malignancy has been reported for several tumor types.[11–26] In most series reported in the literature, the incidence of pneumothorax is less than 5%.[17, 21, 23, 27] However, we found a higher incidence (33%) of pneumothorax in our patients: 11 of 33 patients with pulmonary nodules developed pneumothorax in various tumor types while receiving sorafenib, bevacizumab, and low-dose cyclophosphamide, while no pneumothoraces were noted in the 11 patients without pulmonary nodules. To our knowledge, this is one of the largest and most comprehensive analyses of the incidence of pneumothorax in patients receiving antiangiogenic therapy or chemotherapy. Given the potential life-threatening nature of this complication, the risk of pneumothorax should be assessed and evaluated in children considered for this therapy that was primarily developed to target the tumor vasculature.
The pathophysiologic mechanisms of pneumothorax in the setting of treating a pulmonary malignancy are not known, but may include fistula formation between the lung parenchyma and pleural space due to necrosis of a subpleural tumor nodule, infarction and necrosis of tumor emboli, over distension and subsequent rupture of alveoli due to tumor progression, and increased intrathoracic pressure following emetogenic chemotherapy.[19–21, 24, 28, 29] In the case of antiangiogenesis treatment, sorafenib and bevacizumab both work by blocking VEGF signaling either by VEGFR tyrosine kinase inhibitor (sorafenib) or recombinant humanized anti-VEGF monoclonal antibody (bevacizumab), thereby disrupting the vascular architecture of the tumor-burdened tissue. Within this context, one can hypothesize that the use of VEGF blockers in combination with antiproliferative chemotherapy produces pulmonary nodule necrosis secondary to vascular perfusion compromise, subsequent rupture and pathologic pneumothorax formation.
The risk factors for spontaneous pneumothorax related to pulmonary metastasis and cytotoxic/biologic agents are poorly understood. Our results showed that the incidence of spontaneous pneumothorax events in patients receiving low-dose cyclophosphamide, bevacizumab, and sorafenib did not vary significantly among the selected variables (i.e., patient demographics, tumor type, prior lung radiation, chemotherapy, chest surgery, or the characteristics of lung nodules). However, we found a significant association between cavitation of pulmonary metastatic lesions in response to therapy and pneumothorax. Tumor cavitation after chemotherapy within both primary lung cancers and metastatic pulmonary nodules is well recognized; in particular, cavitation during antiangiogenic therapy in adult patients with lung cancer has been reviewed and may represent a class effect of angiogenesis inhibitors. Marom et al.[30] reported that 17 of 124 (14%) patients who received antiangiogenic therapy developed tumor cavitation. Cavitary pulmonary change was also reported in up to 24% of 33 patients with non-small cell lung cancer in a trial of angiogenesis inhibitors.[31] Interestingly, while a potential link with clinically relevant pulmonary hemorrhage has been suggested, neither of these studies reported pneumothorax as a complication in this patient population. Studies supporting the association of pneumothorax and cavitary pulmonary tumors after chemotherapy are limited and are restricted to case reports.[32] We postulate that some patients who develop pneumothorax during treatment are asymptomatic, and therefore pneumothorax goes unrecognized. In our cohort, 7 of 11 (64%) of patients were asymptomatic and only 1 asymptomatic patient required treatment.
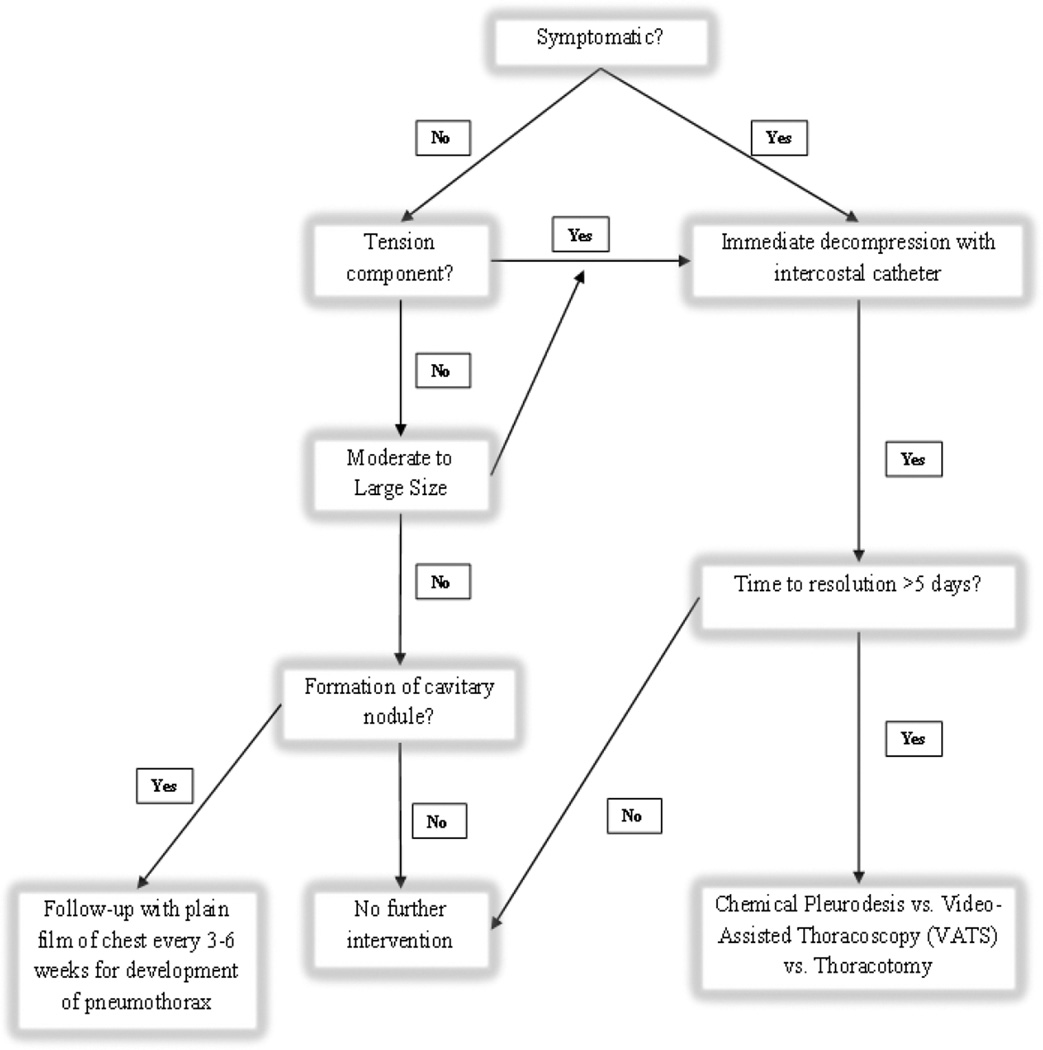
Appropriate management of a pneumothorax includes observation alone, observation with supplemental oxygen, needle aspiration, insertion of an intercostal catheter or thoracostomy tube, or invasive thoracoscopy or thoracotomy. Although treatment options are dictated by clinical symptoms and size of the pneumothorax, many aspects about the management of pneumothorax in children, especially those with cancer, are challenging and currently there are no established guidelines for children. Furthermore, establishing a standardized approach is difficult as the data in children are based on small-scale studies and mainly address primary spontaneous pneumothorax. Because of the link between the development of a pneumothorax and combination antiangiogenic chemotherapy-induced tumor cavitation in our patient population at a median of 5.7 weeks from the start of therapy, we recommend imaging with a 2-view chest x-ray at least 3–4 weeks from the initiation of therapy to evaluate for the presence of cavitary change in the lung nodules and an asymptomatic pneumothorax. Patients with these findings warrant close observation with serial imaging. The caregiver and patient should also be educated about potential symptoms necessitating further evaluation. Given the need for knowledge concerning appropriate management guidelines in children, we propose an algorithm for the management of spontaneous pneumothorax as a complication of treatment for advanced cancer (Fig. 2). Our algorithm was devised based on the American College of Chest Physicians (ACCP) consensus statement regarding treatment of secondary spontaneous pneumothorax in adults. We added our experience to formulate a pediatric-specific pneumothorax management protocol [33].
Fig. 2.
Treatment algorithm for the management of spontaneous pneumothorax in the setting of pediatric patients with pulmonary lesions receiving cancer-directed therapy. Chest X-ray every 3–6 weeks is recommended if formation of cavitary nodules is noted on follow-up CT. We define a moderate pneumothorax as one causing >10% and <50% lung compression.
This report is an in-depth analysis of pneumothorax in children with refractory or recurrent solid tumors receiving combination antiangiogenic therapy. The report is important for several reasons. First, it highlights the important complication of pneumothorax in patients receiving combinatorial antiangiogenic agents. Second, we identify risk factors for the development of pneumothorax that can be detected by radiographic imaging. Third, this study highlights the ethical challenges associated with phase I trials that recruit children with refractory cancer. Given that these trials are unlikely to cure a child [34], the implications of the treatments raise ethical concerns about high-risk pediatric research. The identification of an increased chance of pneumothorax and possible death due to this complication undoubtedly increases the complexity of an already difficult medical situation and places greater importance of parental understanding of informed consent. Cousino et al. [35] recently investigated physician–parent communication during informed consent conferences and parental understanding of the purpose of pediatric phase I studies. They found that of the 60 parents who were interviewed, 32% had a substantial understanding of the scientific purpose of phase I cancer trials, but 35% had little or no understanding of them. Although physician–parent communication and high-quality informed consent represent areas that are critically important to pediatric phase I research, the balance between risks and benefits also underscores the likelihood that the research will produce knowledge that is relevant to treating the particular disease. In our patients, we learned that although development of pneumothorax was associated with tumor cavitation/response, it represents an important adverse event in patients with evidence of pulmonary metastatic disease who receive the novel combination antiangiogenic therapy. Despite this complication, the results of the trial have allowed us to better select the patients who will receive this therapy. Although it may be challenged that this is a recognizable and treatable complication, in future trials we will offer this treatment as a maintenance therapy in children with advanced solid tumors without imaging evidence of pulmonary disease.
Acknowledgments
Supported in part by Cancer Center Grant CA23099 and Cancer Center Support CORE Grant P30CA21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Annual Meeting of the Tennessee Chapter of the American College of Surgeons (ACS), Nashville, TN, August 2013 and at the Annual Meeting of the American Pediatric Surgical Association (APSA), Phoenix, Arizona, May 2014.
References
- 1.El-Kenawi AE, El-Remessy AB. Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. Br J Pharmacol. 2013;170:712–729. doi: 10.1111/bph.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moschetta M, Cesca M, Pretto F, Giavazzi R. Angiogenesis inhibitors: implications for combination with conventional therapies. Curr Pharm Des. 2010;16:3921–3931. doi: 10.2174/138161210794455021. [DOI] [PubMed] [Google Scholar]
- 4.Nathan P, Zweifel M, Padhani AR, et al. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2012;18:3428–3439. doi: 10.1158/1078-0432.CCR-11-3376. [DOI] [PubMed] [Google Scholar]
- 5.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 7.Chen CT, Hung MC. Beyond anti-VEGF: dual-targeting antiangiogenic and antiproliferative therapy. Am J Transl Res. 2013;5:393–403. [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Mokhtari RB, Sheikh R, et al. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res. 2011;17:5656–5667. doi: 10.1158/1078-0432.CCR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose "chemo-switch" regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 10.Navid F, Baker SD, McCarville MB, et al. Phase I and clinical pharmacology study of bevacizumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin Cancer Res. 2013;19:236–246. doi: 10.1158/1078-0432.CCR-12-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias de la Vega F, Illarramendi JJ, Martinez E, et al. Spontaneous pneumothorax as the first manifestation of Ewing's sarcoma. Pediatr Pulmonol. 1995;19:182–184. doi: 10.1002/ppul.1950190307. [DOI] [PubMed] [Google Scholar]
- 12.Biran H, Dgani R, Wasserman JP, et al. Pneumothorax following induction chemotherapy in patients with lung metastases: a case report and literature review. Ann Oncol. 1992;3:297–300. doi: 10.1093/oxfordjournals.annonc.a058183. [DOI] [PubMed] [Google Scholar]
- 13.Escuissato DL, Adam GP, Urban LA, et al. Spontaneous bilateral pneumothoraces associated with Wilms tumor metastases. Pediatr Radiol. 2003;33:588–589. doi: 10.1007/s00247-003-0936-3. [DOI] [PubMed] [Google Scholar]
- 14.Fayda M, Kebudi R, Dizdar Y, et al. Spontaneous pneumothorax in children with osteosarcoma: report of three cases and review of the literature. Acta Chir Belg. 2012;112:378–381. doi: 10.1080/00015458.2012.11680856. [DOI] [PubMed] [Google Scholar]
- 15.Fox E, Aplenc R, Bagatell R, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28:5174–5181. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmkamp BF, Beecham JB, Wandtke JC, Keys H. Spontaneous pneumothorax in gynecologic malignancies. Am J Obstet Gynecol. 1982;142:706–707. doi: 10.1016/s0002-9378(16)32446-2. [DOI] [PubMed] [Google Scholar]
- 17.Hoag JB, Sherman M, Fasihuddin Q, Lund ME. A comprehensive review of spontaneous pneumothorax complicating sarcoma. Chest. 2010;138:510–518. doi: 10.1378/chest.09-2292. [DOI] [PubMed] [Google Scholar]
- 18.Katta A, Fesler MJ, Tan A, et al. Spontaneous bilateral pneumothorax in metastatic renal cell carcinoma on sunitinib therapy. Cancer Chemother Pharmacol. 2010;66:409–412. doi: 10.1007/s00280-010-1291-3. [DOI] [PubMed] [Google Scholar]
- 19.Laurencet FM, Zulian GB, Dietrich PY. Pneumothorax following induction chemotherapy for a germ cell tumour. Eur J Cancer. 1997;33:169–170. doi: 10.1016/s0959-8049(96)00296-1. [DOI] [PubMed] [Google Scholar]
- 20.Lote K, Dahl O, Vigander T. Pneumothorax during combination chemotherapy. Cancer. 1981;47:1743–1745. doi: 10.1002/1097-0142(19810401)47:7<1743::aid-cncr2820470703>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21.Maniwa T, Nakagawa K, Isaka M, et al. Pneumothorax associated with treatment for pulmonary malignancy. Interact Cardiovasc Thorac Surg. 2011;13:257–261. doi: 10.1510/icvts.2011.273201. [DOI] [PubMed] [Google Scholar]
- 22.Smevik B, Klepp O. The risk of spontaneous pneumothorax in patients with osteogenic sarcoma and testicular cancer. Cancer. 1982;49:1734–1737. doi: 10.1002/1097-0142(19820415)49:8<1734::aid-cncr2820490833>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Steinhauslin CA, Cuttat JF. Spontaneous pneumothorax. A complication of lung cancer? Chest. 1985;88:709–713. doi: 10.1378/chest.88.5.709. [DOI] [PubMed] [Google Scholar]
- 24.Upadya A, Amoateng-Adjepong Y, Haddad RG. Recurrent bilateral spontaneous pneumothorax complicating chemotherapy for metastatic sarcoma. South Med J. 2003;96:821–823. doi: 10.1097/01.SMJ.0000047624.10190.3D. [DOI] [PubMed] [Google Scholar]
- 25.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Yang H, Zhao M, He J. Bilateral pneumothorax after bevacizumab-containing chemotherapy in fibrosarcoma. J Thorac Dis. 2012;4:229–231. doi: 10.3978/j.issn.2072-1439.2012.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moskovic E, MacSweeney E, Law M, Price A. Survival, patterns of spread and prognostic factors in uterine sarcoma: a study of 76 patients. Br J Radiol. 1993;66:1009–1015. doi: 10.1259/0007-1285-66-791-1009. [DOI] [PubMed] [Google Scholar]
- 28.Bini A, Zompatori M, Ansaloni L, et al. Bilateral recurrent pneumothorax complicating chemotherapy for pulmonary metastatic breast ductal carcinoma: report of a case. Surg Today. 2000;30:469–472. doi: 10.1007/s005950050628. [DOI] [PubMed] [Google Scholar]
- 29.Stein ME, Haim N, Drumea K, et al. Spontaneous pneumothorax complicating chemotherapy for metastatic seminoma. A case report and a review of the literature. Cancer. 1995;75:2710–2713. doi: 10.1002/1097-0142(19950601)75:11<2710::aid-cncr2820751112>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 30.Marom EM, Martinez CH, Truong MT, et al. Tumor cavitation during therapy with antiangiogenesis agents in patients with lung cancer. J Thorac Oncol. 2008;3:351–357. doi: 10.1097/JTO.0b013e318168c7e9. [DOI] [PubMed] [Google Scholar]
- 31.Crabb SJ, Patsios D, Sauerbrei E, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009;27:404–410. doi: 10.1200/JCO.2008.16.2545. [DOI] [PubMed] [Google Scholar]
- 32.Kao HL, Lin WC, Hsu HH, Huang GS. Docetaxel (Taxotere(R))-induced cavitary change of pulmonary metastatic lesions complicated by bilateral spontaneous pneumothoraces in a patient with primary adenocarcinoma of the lung. Singapore Med J. 2013;54:e133–e134. doi: 10.11622/smedj.2013114. [DOI] [PubMed] [Google Scholar]
- 33.Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 34.Shah S, Weitman S, Langevin AM, et al. Phase I therapy trials in children with cancer. J Pediatr Hematol Oncol. 1998;20:431–438. doi: 10.1097/00043426-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Cousino MK, Zyzanski SJ, Yamokoski AD, et al. Communicating and understanding the purpose of pediatric phase I cancer trials. J Clin Oncol. 2012;30:4367–4372. doi: 10.1200/JCO.2012.42.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]