Abstract
Chloride intracellular channel 1 (CLIC1) has been demonstrated to be overexpressed in gastric cancer, and elevated CLIC1 expression levels are markedly associated with the processes of tumor cell migration and invasion. However, the regulatory mechanism and signaling pathway underlying these processes have remained to be elucidated. The present study examined the impact of N-acetyl cysteine (NAC), indanyloxyacetic acid (IAA)-94 and SB203580, inhibitors of reactive oxygen species (ROS), as well as CLIC1 and p38 mitogen-activated protein kinase (MAPK) on the migration and invasion of SGC-7901 gastric cancer cells in a hypoxia-reoxygenation (H-R) microenvironment. The results demonstrated that intracellular ROS and CLIC1 levels were increased under H-R conditions, and that functional inhibition of CLIC1 significantly decreased the H-R-elevated ROS generation and p-p38 MAPK levels in SGC-7901 cells, as well as inhibited the migration and invasion of SGC-7901 cells. In addition, the expression levels of MMP-2 and MMP-9 were inhibited by NAC, IAA-94 and SB203580. These results indicated that CLIC1 regulates gastric cancer-cell migration and invasion via the ROS-mediated p38 MAPK signaling pathway.
Keywords: gastric cancer, chloride intracellular channel 1, reactive oxygen species, invasion, p38 mitogen-activated protein kinase signaling pathway
Introduction
Gastric cancer remains the fifth most common type of cancer, and the third leading cause of cancer-associated mortality worldwide (1). In China, gastric cancer has a five-year relative survival rate of ~20% or less, due to the fact that the majority of patients are diagnosed with metastatic gastric cancer at presentation (2). The early diagnosis of individuals at a high risk of metastasis is hindered by a lack of understanding of the underlying molecular mechanisms. Previous studies have recognized novel biochemical functions of chloride intracellular channel (CLIC) in the regulatory mechanism of tumor cells (3–5). CLIC proteins are components or regulators of novel intracellular anion channels in mammalian cells (6). CLIC1, one of the members of CLIC, has been demonstrated to be overexpressed in gastric tumor cells, and elevated CLIC1 are markedly associated with the metastasis of lymph nodes, and lymphatic and perineural invasion (7,8). In addition, downregulation in the expression of CLIC1 following transfection with CLIC1 small interfering (si)RNAs efficiently inhibits the migration and invasion of gastric cancer cells in vitro (9). However, the potential regulatory mechanism and signaling pathway underlying the migration and invasion of gastric cancer cells remains to be elucidated.
CLIC1 is also reported to be a sensor and effector in oxidative stress (10), which is characterized by the overproduction of reactive oxygen species (ROS). Emerging evidence has suggested that elevated ROS levels function as a secondary signaling molecule and are involved in the cellular processes of cancer migration and invasion (11,12). Previous studies have demonstrated that intracellular ROS levels activate the mitogen-activated protein kinase (MAPK) signaling pathway (13–15), and trigger the ROS/eukaryotic protein kinase signaling pathway, which is involved in the CLIC1-mediated regulation of cell migration and invasion in colon cancer (16). Whether similar effects and mechanisms are present in gastric cancer remains to be elucidated.
It is well-known that ROS can be produced in a hypoxia and reoxygenation (H-R) microenvironment (17), and CLIL1 was reported to be involved in colon cancer metastasis under H-R conditions (18). The present study hypothesized that CLIC1 may mediate the migration and invasion of gastric cancer cells via the ROS/p38 MAPK signaling pathway. To test this hypothesis, the present study assessed the migration and invasion of SGC-7901 gastric cancer cells following downregulation of intracellular ROS levels under H-R conditions, and investigated whether this process is regulated by the ROS/p38 MAPK signaling pathway.
Materials and methods
Materials and reagents
The SGC-7901 human gastric cancer cell line was obtained from the Shanghai Institute for Biological Sciences of the Chinese Academy of Sciences (Shanghai, China). The specific inhibitor of ROS, N-acetyl cysteine (NAC), was purchased from Beyotime Institute of Biotechnology (Nantong, China). The inhibitor of CLIC1, indanyloxyacetic (IAA)-94 was purchased from Sigma-Aldrich (St. Louis, MO, USA). The chemical inhibitor of p38-MAPK (SB203580) was purchased from Merck Millipore (Darmstadt, Germany). Antibodies targeting p38 MAPK, phosphorylated (p)-p38 MAPK, matrix metalloproteinase (MMP)-2 and MMP-9 were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
SGC-7901 cell culture and treatment
The SGC-7901 human gastric cancer cell line was incubated in 1% Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 100 U/ml penicillin and 100 µg/ml streptomycin (both from Cosmo Bio Co., Ltd., Tokyo, Japan), and cultured at 37°C in an atmosphere containing 5% CO2 with saturated humidity. H-R conditions were produced, as previously described (19,20). Briefly, for H-R treatment, the SGC-7901 cells (1×104cells/well) cultured in serum-free media were placed in a humidified incubator under hypoxia (95% N2 and 5% CO2) for 6 h. Following the period of hypoxia, the cells were rapidly transferred to a humidified incubator containing 95% air and 5% CO2 for 18 h. In the normoxic control treatment group, the cells were maintained in an incubator with a humidified atmosphere of 95% air and 5% CO2 for the same period of time as the H-R group.
Intracellular ROS measurement
The production of intracellular ROS was measured using a dichloro-dihydro-fluorescein diacetate (DCFH-DA) fluorescent probe (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The high intensity fluorescent compound, dichlorofluorescein (DCF), is formed following the passive entry of DCFH-DA into the cell to react with ROS. Briefly, the cells were trypsinized with a solution of 0.25% trypsin (containing 0.02% EDTA; Sigma-Aldrich) and cultivated in 96-well plates (1×104cells/well), and pre-processed with NAC (30 mmol/l) or IAA-94 (1, 20 or 40 µmol/l) for 1 h to determine the effect of specific inhibitors on the production of ROS. Following treatment with H-R or normoxic conditions, the cells were washed twice with DMEM without FBS, and were then treated with 10 µM DCFH-DA at 37°C in an atmosphere containing 5% CO2 for 20 min. The cells were then washed a further three times with phosphate-buffered saline (PBS) and observed under a fluorescence microscope (CK40-F200; Olympus, Tokyo, Japan). The fluorescence intensity of DCFH-DA was measured using a Victor 3 microplate reader (PerkinElmer, Inc., Waltham, MA, USA). The cells were dissolved using 1% SDS (Bio-Rad Laboratories, Hercules, CA, USA) and centrifuged at 14,000 x g for 5 min at 4°C, prior to the removal of the supernatant and plating into a 96-well plate. Measurements were made at an excitation wavelength of 485 nm and emission wavelength of 530 nm. Values are expressed as the fold change, compared with the normoxic group.
Wound healing assay
A wound healing assay was performed to examine the capacity of cell migration. Following trypsinization with a solution of 0.25% trypsin and EDTA, SGC-7901 cells in the logarithmic growth phase were cultivated in 6-well plates at a density of 5×104 cells/well. When the cells grew to 80% confluence, the cell monolayers were scratched using a sterile pipette tip to form a wound. The cells were subsequently treated with NAC (30 mmol/l), IAA-94 (40 µmol/l), or SB203580 (10 µmol/l) for 1 h, and then exposed to H-R or normoxic conditions for 24 h at 37°C. Images of the wound healing process were captured under a microscope (Nikon Eclipse TS 100; Nikon Corporation, Tokyo, Japan) at 0 and 24 h, respectively. The wound recovery rates (%) were calculated using ImagePro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) and were used to indicate the migration of the SGC-7901 cells. The experiments were performed triplicate.
Cell invasion assay
SGC-7901 cell invasiveness was assayed using Biocoat Matrigel Invasion Chambers (BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer's protocol. Briefly, the cells were trypsinized and plated in the upper chamber at a density of 1×105 cells/ml, and 5% FBS was added to the lower chamber to act as a chemoattractant. To determine the effect of specific inhibitors, the cells were pre-treated with NAC (30 mmol/l), IAA-94 (40 µmol/l) or SB203580 (10 µmol/l) for 1 h prior to their addition to the chamber. Following exposure to H-R or normoxic conditions, the non-invading cells were removed by wiping with a cotton swab, and the invaded cells, located on the lower surface of the membrane, were fixed with paraformaldehyde (Bio-Rad Laboratories, Inc.) and stained with crystal violet (Bio-Rad Laboratories, Inc.). For quantification, the cells were counted under a microscope (X41; Olympus) in five randomly-selected fields. Data are expressed as the relative number of invaded cells, compared with those in normoxic conditions.
Western blot analysis
SGC-7901 cells were cultured until sub-confluence and protein was extracted using a mammalian cell lysis kit (Bio Basic Inc., Markham, OT, Canada). In brief, cells were collected and washed twice by cold PBS, and lysed in 50 µl lysis buffer (2 mmol/l Tris-HCl pH 7.4, 50 mmol/l NaCl, 25 mmol/l EDTA, 50 mmol/l NaF, 1.5 mmol/l Na3VO4, 1% Triton X-100, 0.1% SDS supplemented with protease inhibitors, 1 mmol/l phenylmethylsulfonylfluoride, 10 mg/l pepstatin, 10 mg/l aprotinin and 5 mg/l leupeptin; all from Sigma-Aldrich). Then cells were centrifuged at 13,000 x g for 15 min at 4°C, prior to collection of the supernatant. Protein concentration was measured using a Bicinchoninic Acid Protein Assay kit (Sigma-Aldrich). The proteins were then separated by 10% SDS-PAGE (Bio-Rad Laboratories, Inc.) and electrotransferred onto immunoblot nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked in 3% Tris-buffered saline containing 0.05% Tween-20 and 5% fat-free dry milk, prior to incubation with primary antibodies overnight at 4°C, followed by horseradish peroxidase-conjugated secondary antibody for 1 h. Primary antibodies against CLIC1 (1:400), MAPK (1:400), p-MAPK (1:400), MMP-2 (1:200), MMP-9 (1:200) and GAPDH (1:1,000) were used. Western blots were visualized by enhanced chemiluminescence reagent (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and the density of the bands was quantified using Quantity One software (version 4.2.2; Bio-Rad Laboratories, Inc.). Experiments were performed three times and band intensities were normalized to GAPDH.
Statistical analysis
All data are presented as the mean ± standard deviation of three experiments. Statistical analysis was performed using SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Statistical comparisons were performed using one-way analysis of variance. P<0.05 between the H-R group and control group was considered to indicated a statistically significant difference.
Results
H-R conditions induce intracellular ROS production in SGC-7901 gastric cancer cells
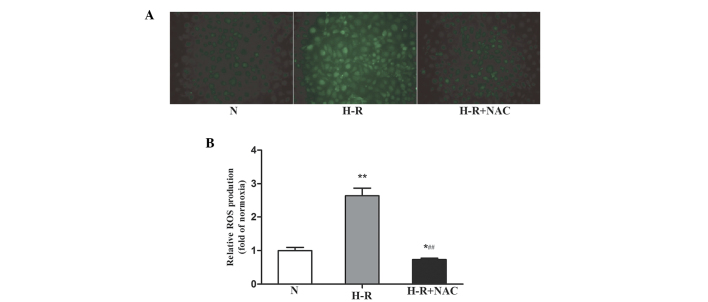
To determine whether H-R conditions induced the production of ROS, an intracellular ROS-sensitive fluorescence indicator, DCFH-DA, was used. The oxidation of non-fluorescent DCFH to highly fluorescent DCF, which emits green fluorescence, provides a quantitative assay of ROS generation (21). Following exposure to H-R conditions, DCF+ fluorescence was markedly increased (Fig. 1A), and was 2.64 times higher than that in the normoxic group (F=11.63; P<0.001; Fig. 1B), indicating that H-R conditions induced the production of ROS in the SGC-7901 gastric cancer cells. Following exposure to the NAC antioxidant, the levels of ROS in the SGC-7901 gastric cancer cells were 0.73-fold of the normoxic group and were significantly decreased, compared with the H-R group (F=14.48; P<0.001). These results suggested that the levels of intracellular ROS in SGC-7901 gastric cancer cells increased under H-R conditions.
Figure 1.
H-R conditions induce the production of ROS. (A) SGC-7901 human gastric cancer cells were pre-treated with the antioxidant NAC (30 mmol/l) for 1 h prior to exposure to H-R conditions for 24 h. ROS production was analyzed using DCFH-DA staining under a fluoresence microscope (magnification, ×40). DCF fluorescence was markedly increased under H-R conditions, and after exposure to the NAC anti-oxidant, the DCF fluorescence was significantly decreased compared with that in the H-R group. (B) Fluorescence intensity of DCFH-DA was measured using a Victor 3 microplate reader. Values are expressed as the fold of normoxia of three independent experiments and are presented as the mean ± standard deviation. *P<0.05 and **P<0.01, vs, normoxic group; ##P<0.01, vs. H-R group. N, normoxia; H-R, hypoxia-reoxygenation; ROS, reactive oxygen species; NAC, N-acetyl cysteine; DCFH-DA, dichloro-dihydro-fluorescein diacetate.
Functional inhibition of CLIC1 downregulates ROS production in SGC-7901 gastric cancer cells
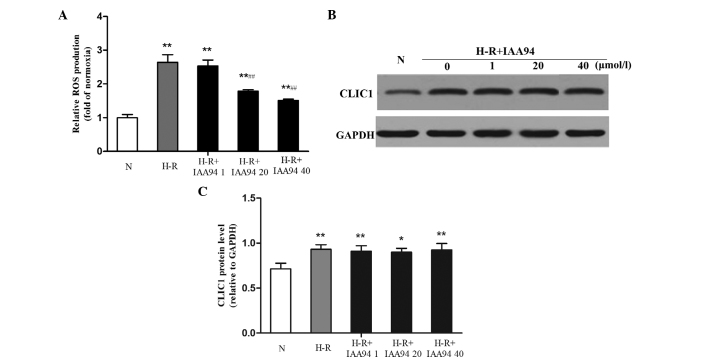
As CLIC1 may function as a 'sensor' and 'effector' of the oxidative stress in cells (10), a specific CLIC1 inhibitor, IAA-94, was introduced into the SGC-7901 gastric cancer cells to further determine the factors involved in the production of ROS. Following incubation with IAA-94 at different concentrations (1, 20 and 40 µmol/l), ROS generation was reduced in a concentration-dependent manner. IAA-94 (20 and 40 µmol/l) induced marked inhibition of ROS, compared with the H-R group (F=13.59; P<0.001; F=15.14; P<0.001), as shown in Fig. 2A. Western blot analysis revealed that the protein expression levels of CLIC1 in the SGC-7901 gastric cancer cells under H-R conditions were significantly increased, compared with those in the normoxic control group, (F=4.658; P=0.009). However, IAA-94 did not downregulate the elevated protein expression levels of CLIC1 (Fig. 2B and C). These results suggested that the functional inhibition of CLIC1 may be involved in downregulating the production of ROS in SGC-7901 gastric cancer cells.
Figure 2.
CLIC1 regulates the production of ROS in SGC-7901 gastric cancer cells under H-R conditions. SGC-7901 cells were pre-treated with the CLIC1 inhibitor, IAA-94 (1, 20 and 40 µmol/l), for 1 h followed by H-R condition exposure for 24 h. ROS production was analyzed using DCFH-DA staining and the fluorescence intensity of DCFH-DA was measured using a Victor 3 microplate reader. (A) Data are expressed as the fold of normoxia. (B and C) Protein expression levels of CLIC1 were measured using western blot analysis, with band intensities were normalized to GAPDH. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01, vs, normoxic group; ##P<0.01, vs. H-R group. N, normoxia; H-R. H-R, hypoxia-reoxygenation; ROS, reactive oxygen species; DCFH-DA, dichloro-dihydro-fluorescein diacetate; IAA-94, indanyloxyacetic acid 94; CLIC1, chloride intracellular channel 1.
CLICl is involved in the regulation of SGC-7901 gastric cancer cell migration
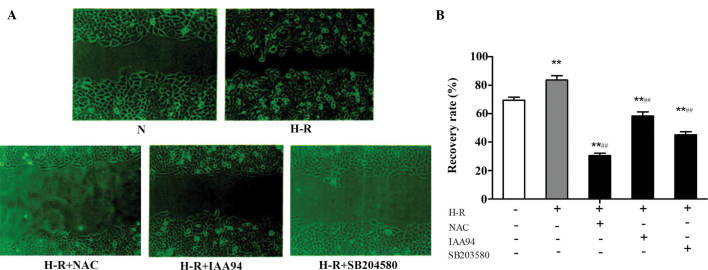
The metastatic process of cancer cells is usually accompanied by morphological changes, to enable the cells to pass through narrow extracellular spaces in order to metastasize (22). In the present study, a wound healing assay was performed to examine the capacity of cell migration. As shown in Fig. 3, compared with the normoxic control group, H-R conditions caused a significant increase in wound recovery (F=6.745; P=0.003). In addition, NAC and IAA-94 (40 µmol/l) inhibited this wound healing effect. These results suggested that CLICl was involved in regulating the migration of SGC-7901 gastric cancer cells, and this effect may be achieved via the ROS-mediated signaling pathway. As p38 MAPK has been demonstrated to be an important signaling molecule for cell invasion and migration in human breast epithelial cells (23), 10 µmol/l SB203580, a chemical inhibitor of p38, was used to determine whether the ROS/p38 MAPK signaling pathway contributed to the migration of the SGC-7901 gastric cancer cells. SB203580 also inhibited the elevated cell motility induced by H-R conditions, indicating that the ROS/p38 MAPK signaling pathway is involved in the migration of SGC-7901 gastric cancer cells.
Figure 3.
CLICl is involved in the regulation SGC-7901 gastric cancer cell migration. A wound healing assay was performed to H-R or normoxic conditions for 24 h. (A) Images of the wound healing process were captured using a microscope (magnification, ×100). (B) Wound recovery rates (%) were quantified using ImageProPlus 6.0 software, and presented as the mean ± standard deviation of three experiments. **P<0.01, vs, normoxic group; ##P<0.01, vs. H-R group. N, normoxia; H-R. H-R, hypoxia-reoxygenation; IAA-94, indanyloxyacetic acid 94; NAC, N-acetyl cysteine.
CLICl is involved in the regulation of SGC-7901 gastric cancer cell invasion
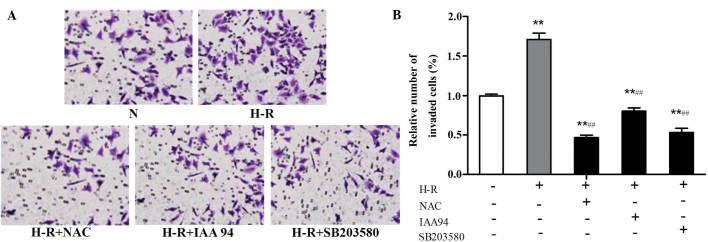
The effect of CLIC1 on SGC-7901 gastric cancer cell invasiveness was examined using Matrigel invasion assays in vitro. As shown in Fig. 4, compared with the normoxic control group, the invasiveness of the SGC-7901 gastric cancer cells was markedly increased under H-R conditions (F=15.063; P<0.001). Following pre-treatment with NAC (30 mmol/l) or IAA-94 (40 µmol/l), the elevated invasiveness caused by H-R exposure was inhibited. Treatment with the SB203580 (10 µmol/l) inhibitor of p38 MAPK also inhibited the elevated cell invasiveness induced by H-R conditions. These results suggested that CLICl was involved in the regulation of SGC-7901 gastric cancer cell invasion, and this process may activate the ROS-p38 MAPK signaling pathway.
Figure 4.
CLICl is involved in the regulation of SGC-7901 gastric cancer cell invasion. Cells were treated with NAC (30 mmol/l), IAA-94 (40 µmol/l) or SB203580 (10 µmol/l) for 1 h and then exposed to H-R or normoxic conditions for 24 h. A Matrigel invasion assay was performed to examine SGC-7901 gastric cancer cell invasiveness. (A) Invaded cells were fixed and stained with crystal violet and observed under a microscope (magnification, ×100). (B) Numbers of invaded ceslls were quantified under a microscope in five randomly-selected fields. Data are expressed as the relative number of invading cells compared with those under normoxic conditions and are presented as the mean ± standard deviation. **P<0.01, vs, normoxic group; ##P<0.01, vs. H-R group. N, normoxia; H-R. H-R, hypoxia-reoxygenation; IAA-94, indanyloxyacetic acid 94; NAC, N-acetyl cysteine.
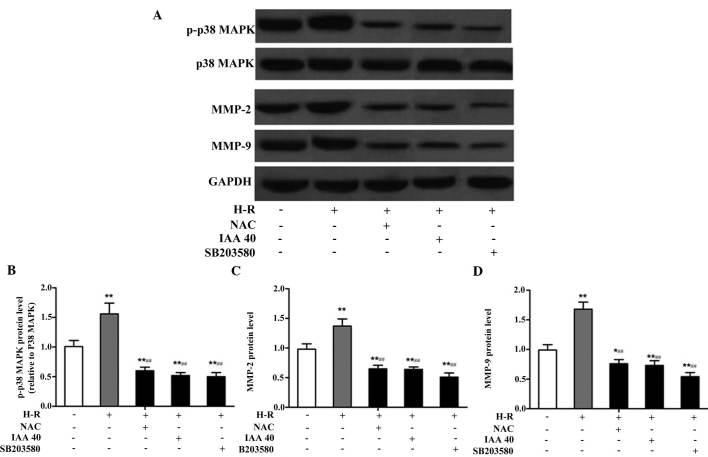
CLICl regulates gastric cancer cell migration and invasion via the ROS-mediated p38 MAPK signaling pathway
It has been demonstrated that the p38 MAPK signaling pathway is associated with cancer migration. In order to further examine whether CLICl regulates gastric cancer cell migration and invasion via the ROS-mediated p38 MAPK signaling pathway, the expression levels of p-p38 MAPK were examined. In addition, the activities of MMP-2 and MMP-9, two important mediators of cancer metastasis were quantified using western blotting. H-R exposure led to a significant increase in the expression levels of p-p38, MMP-2 and MMP-9, and these effects were be inhibited by treatment with NAC, IAA-94 and SB203580 (Fig. 5A). Compared with the normoxic group, the protein expression levels of p-p38 (F=4.626; P=0.009), MMP-2 (F=4.503; P=0.011) and MMP-9 (F=7.967; P=0.001) were significantly increased following H-R exposure (Fig. 5B–D). By contrast, the protein expression levels of p-p38 (F=8.764; P<0.001), MMP-2 (F=5.284; P=0.006) and MMP-9 (F=11.476; P<0.001) were significantly reduced following treatment with NAC, compared with the H-R treatment group, which indicated that ROS activated the p38 MAPK signaling pathway under H-R conditions. Similar effects were observed following treatment with IAA-94 and SB203580. These results suggested that CLIC1 regulated gastric cancer cell metastasis, which was activated via the ROS-mediated p38 MAPK signaling pathway.
Figure 5.
CLICl regulates gastric cancer cell migration and invasion via the ROS-mediated p38 MAPK signaling pathway. The cells were treated with NAC (30 mmol/l), IAA-94 (40 µmol/l) or SB203580 (10 µmol/l) for 1 h, and then exposed to H-R or normoxic conditions for 24 h. (A) Protein expression levels of p-p 38 MAPK (relative to p38 MAPK), MMP-2 and MMP-9 (normalized to GAPDH) were measured using western blot analysis. (B–D) Quantified band intensities are presented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01, vs, normoxic group; ##P<0.01, vs. H-R group. ROS, reactive oxygen species; p, phospho; MAPK, mitogen-activated protein kinase; N, normoxia; H-R, hypoxia-reoxygenation; IAA-94, indanyloxyacetic acid 94; NAC, N-acetyl cysteine; MMP, matrix metalloproteinase.
Discussion
Metastasis of cancer cells is a complex biological process, which is associated with changes in specific cytokines and signaling pathways (24,25). Tumor hypoxia promotes the malignant tumor cell phenotype, and is one of the mechanisms underlying increased tumor aggressiveness (26). Previously, studies have demonstrated that tumor cells exist in the H-R environment due to its irregular microvascular network and blood flow patterns (27,28). Based on these biological hypotheses, the present study exposed gastric cancer cells to H-R treatment, and the results demonstrated that the migration and invasion of gastric cancer cells were significantly increased under H-R conditions. These findings were concordant with those of previous studies, in which H-R was demonstrated to markedly regulate cell migration in breast cancer (29) and cell invasiveness in pancreatic cancer (19).
CLIC1 is markedly associated with the migration and invasion of gastric cancer, which is supported by evidence presented in previous studies. Chen et al (8) reported that the expression levels of CLIC1 in tumor regions increased 1.95-fold, compared with adjacent non-cancerous tissue samples, and elevated CLIC1 was associated with lymph node metastasis, lymphatic and perineural invasion and pathological staging. Ma et al (9) revealed that transfection of the SGC-7901 gastric cancer cell line with CLIC1 siRNA effectively downregulated the protein expression levels of CLIC1, which led to the inhibition of invasion and migration by 54.32 and 29.26%, respectively. However, the molecular mechanisms underlying these processes remain to be elucidated. CLIC1 may act as a 'sensor' and 'effector' of the process of oxidative stress (10), in which CLIC1 reacts to the transformation of the membrane, resulting in its overexpression and the enhancement of channel activity. Based on these findings, the present study further investigated whether the expression of CLICl was associated with oxidative stress in gastric cancer. The results demonstrated that H-R conditions induced a marked increase in the expression levels of CLIC1 and ROS. Although the inhibitor of CLIC1, IAA-94, did not downregulate the elevated protein expression levels of CLIC1, the H-R-induced elevation in intracellular ROS levels were significantly inhibited by IAA-94, suggesting that the functional inhibition of the activity of the CLIC1 signaling pathway may be involved in downregulating ROS production in SGC-7901 gastric cancer cells. Therefore, CLICl was involved in the metastasis and invasion of gastric cancer cells, and these processes were produced through the regulation of intracellular ROS.
ROS are constantly generated and eliminated to maintain equilibrium under biological conditions, and are associated with the regulation of various physiological and pathological processes, including cell differentiation, proliferation and apoptosis (30,31). Previous studies have suggested that ROS and their associated redox-sensitive signaling pathways may be involved in tumor metastasis (32–35). Chronic and sustained generation of ROS can activate certain metastasis-associated proteins, including MMPs, which are regulated by MAPK signal transduction pathways (36–38). The p38 MAPK signaling pathway has been identified as an important member of the MAPK family. It is activated by environmental and genotoxic stresses, and is involved, not only in the regulation of cell growth, differentiation, death and synchronization between cellular function, but also in the proliferation, differentiation, migration and invasion of specific cell types with positive regulation (39,40). MMPs can affect the degradation of the extracellular matrix (ECM), permitting cancer cells to migrate across the basal membrane prior to invading adjacent or distant tissues and organs (41). As MMP-2 and MMP-9 can degrade the majority of ECM components forming the basal membrane, the expression levels of MMP-2 and MMP-9, as well as p-p38 MAPK were examined in the present study using western blotting. The results demonstrated that exposure to H-R conditions led to a significant increase in the expression levels of p-p38, MMP-2 and MMP-9, and this increase was inhibited by NAC, IAA-94 and SB203580, indicating that the ROS-mediated p38 MAPK signaling pathway was involved in CLIC1-regulated gastric cancer cell metastasis.
In conclusion, the involvement of the ROS-mediated p38 MAPK signaling pathway in SGC-7901 cell metastasis was further supported by investigations in the present study using ROS, CLIC1 and p38 MAPK inhibitors, which demonstrated that CLIC1 activated the p38 MAPK signaling pathway during gastric cancer cell migration and invasion, and this process was mediated by ROS. Although the use of SGC-7901 cell lines as a model system to investigate gastric cancer metastasis may not represent the tumor in its entirety, the information provided in the present study contributes to an improved understanding of the molecular mechanisms underlying CLIC1-regulated gastric cancer metastasis.
References
- 1.Fock KM. Review article: The epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250–260. doi: 10.1111/apt.12814. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, Inoue M. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol. 2011;17:4421–4428. doi: 10.3748/wjg.v17.i39.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko JH, Ko EA, Gu W, Lim I, Bang H, Zhou T. Expression profiling of ion channel genes predicts clinical outcome in breast cancer. Mol Cancer. 2013;12(106) doi: 10.1186/1476-4598-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poulsen KA, Andersen EC, Hansen CF, Klausen TK, Hougaard C, Lambert IH, Hoffmann EK. Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: Role of chloride channels. Am J Physiol Cell Physiol. 2010;298:C14–C25. doi: 10.1152/ajpcell.00654.2008. [DOI] [PubMed] [Google Scholar]
- 5.Shiozaki A, Otsuji E, Marunaka Y. Intracellular chloride regulates the G(1)/S cell cycle progression in gastric cancer cells. World J Gastrointest Oncol. 2011;3:119–122. doi: 10.4251/wjgo.v3.i8.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley RH. Challenging accepted ion channel biology: p64 and the CLIC family of putative intracellular anion channel proteins (Review) Mol Membr Biol. 2003;20:1–11. doi: 10.1080/09687680210042746. [DOI] [PubMed] [Google Scholar]
- 7.Zheng DL, Huang QL, Zhou F, Huang QJ, Lin JY, Lin X. PA28β regulates cell invasion of gastric cancer via modulating the expression of chloride intracellular channel 1. J Cell Biochem. 2012;113:1537–1546. doi: 10.1002/jcb.24022. [DOI] [PubMed] [Google Scholar]
- 8.Chen CD, Wang CS, Huang YH, Chien KY, Liang Y, Chen WJ, Lin KH. Overexpression of CLIC1 in human gastric carcinoma and its clinicopathological significance. Proteomics. 2007;7:155–167. doi: 10.1002/pmic.200600663. [DOI] [PubMed] [Google Scholar]
- 9.Ma PF, Chen JQ, Wang Z, Liu JL, Li BP. Function of chloride intracellular channel 1 in gastric cancer cells. World J Gastroenterol. 2012;18:3070–3080. doi: 10.3748/wjg.v18.i24.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Averaimo S, Milton RH, Duchen MR, Mazzanti M. Chloride intracellular channel 1 (CLIC1): Sensor and effector during oxidative stress. FEBS Lett. 2010;584:2076–2084. doi: 10.1016/j.febslet.2010.02.073. [DOI] [PubMed] [Google Scholar]
- 11.Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. doi: 10.1016/j.semcancer.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Okoh V, Deoraj A, Roy D. Estrogen-induced reactive oxygen species-mediated signalings contribute to breast cancer. Biochim Biophys Acta. 2011;1815:115–133. doi: 10.1016/j.bbcan.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 14.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J Signal Transduct. 2011;2011(792639) doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tormos AM, Taléns-Visconti R, Nebreda AR, Sastre J. p38 MAPK: A dual role in hepatocyte proliferation through reactive oxygen species. Free Radic Res. 2013;47:905–916. doi: 10.3109/10715762.2013.821200. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Zeng Y, Liu T, Zhang C, Yu PW, Hao YX, Luo HX, Liu G. Chloride intracellular channel 1 regulates colon cancer cell migration and invasion through ROS/ERK pathway. World J Gastroenterol. 2014;20:2071–2078. doi: 10.3748/wjg.v20.i8.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Zhang C, Yu P, Tang B, Liu T, Cui H, Xu J. Regulation of colon cancer cell migration and invasion by CLIC1-mediated RVD. Mol Cell Biochem. 2012;365:313–321. doi: 10.1007/s11010-012-1271-5. [DOI] [PubMed] [Google Scholar]
- 19.Binker MG, Binker-Cosen AA, Richards D, Gaisano HY, de Cosen RH, Cosen-Binker LI. Hypoxia-reoxygenation increase invasiveness of PANC-1 cells through Rac1/MMP-2. Biochem Biophys Res Commun. 2010;393:371–376. doi: 10.1016/j.bbrc.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 20.Kokura S, Yoshida N, Imamoto E, Ueda M, Ishikawa T, Uchiyama K, Kuchide M, Naito Y, Okanoue T, Yoshikawa T. Anoxia/reoxygenation downregulates the expression of E-cadherin in human colon cancer cell lines. Cancer Lett. 2004;211:79–87. doi: 10.1016/j.canlet.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Su HL, Chou CC, Hung DJ, et al. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. 2009;30:5979–5987. doi: 10.1016/j.biomaterials.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 23.Kim MS, Lee EJ, Kim HR, Moon A. p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res. 2003;63:5454–5461. [PubMed] [Google Scholar]
- 24.Negus RP, Balkwill FR. Cytokines in tumour growth, migration and metastasis. World J Urol. 1996;14:157–165. doi: 10.1007/BF00186895. [DOI] [PubMed] [Google Scholar]
- 25.Versteeg HH, Spek CA, Peppelenbosch MP, Richel DJ. Tissue factor and cancer metastasis: The role of intracellular and extracellular signaling pathways. Mol Med. 2004;10:6–11. doi: 10.2119/2003-00047.Versteeg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avni R, Cohen B, Neeman M. Hypoxic stress and cancer: Imaging the axis of evil in tumor metastasis. NMR Biomed. 2011;24:569–581. doi: 10.1002/nbm.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bendinelli P, Maroni P, Matteucci E, Luzzati A, Perrucchini G, Desiderio MA. Microenvironmental stimuli affect Endothelin-1 signaling responsible for invasiveness and osteomimicry of bone metastasis from breast cancer. Biochim Biophys Acta. 2014;1843:815–826. doi: 10.1016/j.bbamcr.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, Jansen MK, Csiszar K, Hendrix MJ, Kirschmann DA. Hypoxia/reoxygenation: A dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008;103:1369–1378. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- 30.Circu Ml, Aw TY. Reactive oxygen species, cellular redox systems and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 32.Inokuma T, Haraguchi M, Fujita F, Torashima Y, Eguchi S, Kanematsu T. Suppression of reactive oxygen species develops lymph node metastasis in colorectal cancer. Hepatogastroenterology. 2012;59:2480–2483. doi: 10.5754/hge10383. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer lett. 2008;266:53–59. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci USA. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/S0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 36.Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact. 2005;156:141–150. doi: 10.1016/j.cbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Kwon GT, Cho HJ, Chung WY, Park KK, Moon A, Park JH. Isoliquiritigenin inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased JNK/AP-1 signaling. J Nutr Biochem. 2009;20:663–676. doi: 10.1016/j.jnutbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Park SS, Lee US, Kim WJ, Moon SK. Signaling pathway for TNF-alpha-induced MMP-9 expression: Mediation through p38 MAP kinase and inhibition by anti-cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int Immunopharmacol. 2008;8:1821–1826. doi: 10.1016/j.intimp.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Wang WH, Hullinger RL, Andrisani OM. Hepatitis B virus X protein via the p38MAPK pathway induces E2F1 release and ATR kinase activation mediating p53 apoptosis. J Biol Chem. 2008;283:25455–25467. doi: 10.1074/jbc.M801934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MS, Lee EJ, Kim HR, Moon A. p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res. 2003;63:5454–5461. [PubMed] [Google Scholar]
- 41.Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci USA. 1994;91:4293–4297. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]