Abstract
Case series
Patient: Female, 24 • Male, 35
Final Diagnosis: EBV-induced infectious mononucleosis
Symptoms: Fever • general malaise • lymphadenopathy
Medication: —
Clinical Procedure: Physical examination and serological testing
Specialty: Infectious diseases
Objective:
Rare co-existance of disease or pathology
Background:
Infectious mononucleosis is a clinical syndrome most commonly associated with primary Epstein-Barr virus (EBV) infection. In adults, the symptoms can often be severe and prolonged, sometimes causing serious complications. Analgesic or antipyretic drugs are normally used to relieve the symptoms. However, there is no causal treatment for the disease.
Case Report:
Two cases of adult patients with atopic predispositions developed nocturnal fever, general fatigue, pharyngitis and lymphadenopathy after an exacerbation of atopic symptoms or those of allergic rhinitis. Due to the positive results for EBV viral-capsid antigen (VCA) IgM and negative results for EBV nuclear antigen (EBNA) IgG, diagnoses of infectious mononucleosis induced by EBV were made in both cases. Although oral antibiotics or acetaminophen alone did not improve the deteriorating symptoms, including fever, headache and general fatigue, nonsteroidal anti-inflammatory drugs (NSAIDs), such as tiaramide or loxoprofen, completely improved the symptoms quickly after the initiation.
Conclusions:
In these cases, given the atopic predispositions of the patients, an enhanced immunological response was likely to be mainly responsible for the pathogenesis of the symptoms. In such cases, NSAIDs, that are known to reduce the activity of EBV, may dramatically improve the deteriorating symptoms quickly after the initiation. In the present cases, the immunosuppressive property of these drugs was considered to suppress the activity of lymphocytes and thus provide the rapid and persistent remission of the disease.
MeSH Keywords: Anti-Inflammatory Agents, Non-Steroidal; Dermatitis, Atopic; Epstein-Barr Virus Infections; Immunity, Cellular
Background
Infectious mononucleosis is a clinical syndrome most commonly associated with primary Epstein-Barr virus (EBV) infection [1]. The typical features of infectious mononucleosis include fever, tonsillar pharyngitis, lymphadenopathy, general fatigue and atypical lymphocytosis. In children, the disease is usually subclinical with fewer than 10% developing clinical symptoms despite the higher exposure rates [2]. In adults, however, the symptoms, such as fever, sore throat and general fatigue, are often severe and prolonged, sometimes causing serious complications, including hepatitis, myocarditis, encephalomyelitis and Guillain-Barré syndrome [3–5], or triggering the development of autoimmune or lymphoproliferative disorders [6–9]. During the development of the disease, the transmitted EBV is initially replicated in epithelial cells and B-lymphocytes within the oropharyngeal tissues [10]. Then, the viruses are disseminated systemically through the lymphoreticular system [11], causing local or systemic symptoms. However, since drugs that are effective for the virus itself are not yet developed, there is no causal treatment at present for the EBV-induced infectious mononucleosis. For the relief of the symptoms, analgesic or antipyretic drugs are normally used [1], although they are not effective enough in shortening the duration of the disease. Previously, using EBV genome carrying lymphoblastoid cells, Kapadia et al. provided in vitro evidence that nonsteroidal anti-inflammatory drugs (NSAIDs) reduce the activity of EBV at low doses [12]. This study indicated the therapeutic usefulness of NSAIDs in the treatment of EBV infection. Additionally, besides their anti-inflammatory, antipyretic and analgesic properties, NSAIDs are also known to exert immunosuppressive properties by inhibiting the leukocyte migration or their cytokine production [13–15]. Here, we experienced two case of infectious mononucleosis induced by EBV in adult patients with atopic dermatitis or allergic rhinitis. The patients had previously been diagnosed as allergic rhinitis, due to seasonal and perennial allergic symptoms and the positive results for percutaneous skin testing and radioallergosorbent testing. In these cases, given such atopic predispositions of the patients, an enhanced immunological response was likely to be mainly responsible for the pathogenesis of the symptoms. In such cases, NSAIDs, such as tiaramide and loxoprofen, may dramatically improve the deteriorating symptoms quickly after the initiation. In the present cases, the immunosuppressive property of these drugs was considered to suppress the activity of lymphocytes and thus provide a rapid and persistent remission of the disease.
Case Report
Case 1
A 24-year-old woman with atopic dermatitis noticed the worsening of her atopic eczema despite the use of topical corticosteroids. Several days later, she noticed lymphadenopathy in her neck and developed a nocturnal fever with headache, sore throat, and general fatigue that persisted for 6 days. Because the use of antibiotics (cefditoren pivoxil 300 mg/day), prescribed at a nearby clinic under a diagnosis of “acute bacterial pharyngitis,” did not improve her deteriorating symptoms, she came to our outpatient clinic. On physical examination, the patient appeared very exhausted. Her body temperature was 36.6°C. She had an intensely itchy, dry rash on her face and whole body, indicating generalized atopic eczema. Her oral mucosa was moist and the pharynx was red and swollen. There was white exudate scattered on both of her tonsils. On examination of the neck, bilateral posterior lymphadenopathy was present. As summarized in Table 1, laboratory data showed markedly increased peripheral white blood cell count, in which lymphocytes, most of them atypical lymphocytes, were absolutely increased but neutrophils were almost absent. Liver enzymes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LD), were all significantly elevated, indicating the presence of mild liver injury. Despite the atypical lymphocytosis, the C-reactive protein level was only slightly elevated (0.25 mg/dl). In serological testing, because the IgM antibody directed against EBV viralcapsid antigen (VCA) was strongly positive and the IgG antibody to EBV nuclear antigen (EBNA) was negative, a diagnosis of infectious mononucleosis due to primary EBV infection was made. The possibilities of group A streptococcal pharyngitis or adenoviral tonsillitis were excluded due to the negative results of the commercially available antigen detection kits. After the diagnosis, the antibiotics, which had been administered for 4 days, were immediately discontinued and oral administration of tiaramide (300 mg/day) was alternatively initiated (Figure 1), expecting anti-inflammatory and anti-allergic effects. The patient’s deteriorating symptoms, such as nocturnal fever, headache, sore throat and general fatigue, completely resolved within 2 days after the initiation of the drug, together with a dramatic improvement in the atopic eczema (Figure 1). Tiaramide was continued for 3 days, and there was no recurrence of the symptoms or signs afterwards, indicating complete remission of the disease.
Table 1.
Laboratory data at first visit to our clinic.
| Case 1 | Case 2 | |
|---|---|---|
| White blood cell count | ||
| Total (μl) | 12,800 | 4,200 |
| Neutrophils (μl) | 0 | 1,554 |
| Lymphocytes (μl) | 7,718 | 2,394 |
| Serology | ||
| ALT (IU/l) | 88 | 102 |
| AST (IU/l) | 102 | 80 |
| LD (IU/l) | 556 | 397 |
| EBV VCA-IgM (titer) | 100< | 320 |
| EBNA IgG | (−) | (−) |
Figure 1.
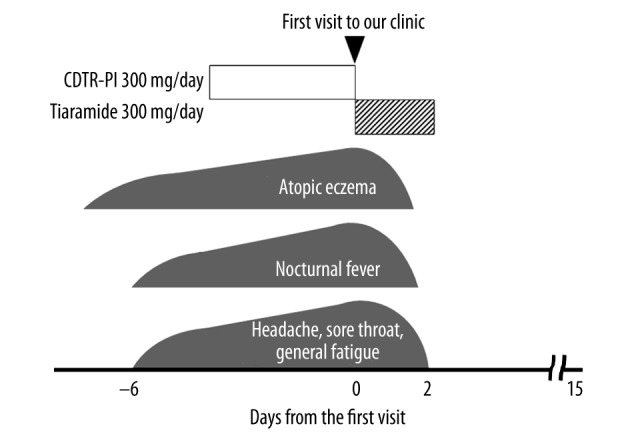
Clinical course of Case 1. Despite the use of cefditoren pivoxil (CDTR-PI), the patient’s symptoms deteriorated. However, tiaramide quickly resolved the symptoms after the administration.
Case 2
A 35-year-old man with a history of allergic rhinitis developed allergic symptoms, such as rhinorrhea, nasal itching and sneezing, followed by a nocturnal fever with headache, general fatigue, and a loss of appetite that persisted for 7 days (Figure 2). Because the symptoms gradually deteriorated despite the repeated administration of 400 mg acetaminophen, he came to our outpatient clinic. On physical examination, the patient appeared tired. His body temperature was 37.4°C. His pharynx was not reddish, but there was a small white exudate spotted on his left tonsil. On examination of the neck, posterior cervical lymphadenopathy was prominent bilaterally. As summarized in Table 1, laboratory data showed slightly decreased peripheral white blood cell count, in which lymphocytes were relatively increased with atypical lymphocytosis (546/μl). The data showed mild thrombocytopenia (platelet count 124 000/μl), indicating the possibility of some viral infection. Liver enzymes were all significantly elevated, suggesting mild liver injury. Despite a weakly positive result for EBV VCA-IgG antibody, a strongly positive result for the VCA IgM antibody and a negative result for the EBNA IgG antibody indicated recent infection with the virus. Because serological tests for other viruses that can also cause infectious mononucleosis, such as cytomegalovirus (CMV) or human immunodeficiency virus (HIV), were both negative, we finally made a diagnosis of infectious mononucleosis due to primary EBV infection. Because the patient’s symptoms deteriorated regardless of the use of acetaminophen, we alternatively started oral administration of loxoprofen (180 mg/day) immediately after the diagnosis (Figure 2). His deteriorating symptoms, such as the allergic symptoms, nocturnal fever, headache, general fatigue and a loss of appetite, quickly disappeared within 2 days after starting the drug. There was no recurrence of the symptoms or signs afterwards. Three weeks later, the EBV VCA-IgM antibody turned negative and the EBNA IgG antibody turned positive, indicating complete remission of the disease.
Figure 2.
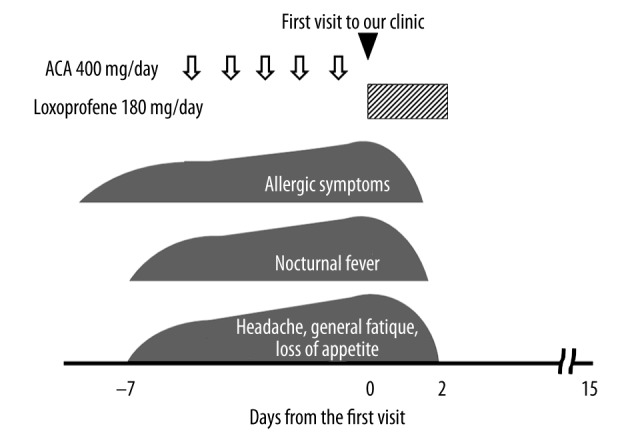
Clinical course of Case 2. Despite the repeated use of acetaminophen (ACA), the patient’s symptoms deteriorated. However, immediately after the initiation of loxoprofen, the symptoms quickly disappeared.
Discussion
Patients with atopic predispositions are vulnerable to the development of autoimmune disorders because of the hypersensitivity of T-helper 2 lymphocytes [16,17] or dysregulated activity of B-lymphocytes [18]. In the present cases, as we previously reported in cases with human parvovirus B19 (HPV-B19) or Mycoplasma pneumoniae (M. pneumoniae) infections [19,20], both patients were predisposed to atopic disorders, such as atopic dermatitis and allergic rhinitis. Therefore, lymphocytes were thought to be easily activated by infectious stimuli, as previously demonstrated in atopic patients [21,22]. Additionally, several studies actually suggested the positive correlation between EBV infection and the pathogenesis of atopic disorders [23,24]. In addition to the induction of circulating antibodies from B-lymphocytes [25], EBV is known to stimulate the activity of T-lymphocytes, facilitating their production of pro-inflammatory cytokines, such as interleukin-2 and interferon-g [26]. Therefore, the symptoms caused by the virus are primarily considered to be immune-mediated rather than induced directly by cellular toxicity [27,28]. In this context, infection with EBV is often associated with the subsequent onset of immune-mediated systemic disorders, such as systemic lupus erythematosus (SLE) and multiple sclerosis [6,7], or lymphoproliferative disorders, such as Hodgkin’s lymphoma [8,9]. In our cases, although they were not complicated with such disorders, the exacerbation of atopic dermatitis or allergic rhinitis, which preceded the onset of infectious mononucleosis, strongly suggested the involvement of an enhanced immunological response in the pathogenesis. As we previously reported in cases with HPV-B19 or M. pneumoniae infections [19,20], EBV infection in the present cases may have also stimulated the activity of lymphocytes in atopic patients.
For adult patients with infectious mononucleosis, acetaminophen is most frequently used to relieve the symptoms, such as fever, sore throat, and malaise [1]. However, the analgesics are not fundamentally effective for the virus itself, nor do they shorten the duration of the disease. Therefore, the majority of patients have to suffer from the symptoms at least for 2 weeks, sometimes for several months, until the disease spontaneously resolves [29–31]. In the present cases, although the antibiotics or acetaminophen alone did not improve the symptoms of the patients, tiaramide or loxoprofen completely improved their deteriorating symptoms immediately after the initiation (Figures 1, 2). Therefore, in the present cases, as we previously reported in patients with HPV-B19 infection [19], these NSAIDs, which are listed in the International Nonproprietary Names (INN) and are available worldwide, were most likely responsible for the resolution of the disease. According to previous case reports, EBV-induced infectious mononucleosis complicated with severe arthritis or pericarditis were also successfully treated by NSAIDs [32,33]. Additionally, in some basic studies, NSAIDs were actually demonstrated to directly reduce the activity of EBV [12] or cause cell lysis of EBV-infected lymphocytes [34]. In common clinical practice, NSAIDs are generally used as anti-inflammatory, antipyretic, and analgesic drugs. Additionally, because of their immunosuppressive properties [35], they have also been used in the treatment of autoimmune disorders, including rheumatoid arthritis [36] and SLE [37]. According to previous studies, NSAIDs exert such immunosuppressive effects by inhibiting the leukocyte migration or their cytokine production, either in cyclooxygenase (COX)-dependent [13] or -independent manner [14,15]. In our patients, since the increased activity of lymphocytes was mainly involved in the pathogenesis, the immunomodulation by the NSAIDs was most likely responsible for the quick resolution of the symptoms and the complete remission of the disease. In our previous basic studies, NSAIDs actually suppressed the activity of lymphocytes by functionally inhibiting delayed rectifier K+-channels (Kv1.3) [38,39]. Since T-lymphocytes predominantly express the channels in their membranes [40,41], such a mechanism may have largely contributed to the immunosuppressive properties of NSAIDs in the present cases. In this context, in addition to the use of immunomodulatory drugs or corticosteroids [42,43], the use of selective blockers for the channel may also be beneficial [39,44,45]. Such drugs would shorten the duration of the disease, if administered early. Additionally, they would prevent serious complications caused by the EBV infection [3–5] or the development of autoimmune or lymphoproliferative disorders triggered by the virus [6–9].
Conclusions
We reported 2 cases of EBV-induced infectious mononucleosis in patients with atopic dermatitis or allergic rhinitis. In these cases, given the atopic predispositions of the patients, an enhanced immunological response was likely the main cause of the pathogenesis of the symptoms. In such cases, NSAIDs, which are known to reduce the activity of EBV, dramatically improved the deteriorating symptoms quickly after the initiation.
Acknowledgments
We thank the staff at Iwakiri Hospital for their assistance.
Footnotes
Conflict of interest
None declared.
This work was supported by a Miyagi Kidney Foundation grant to IK
References:
- 1.Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362(21):1993–2000. doi: 10.1056/NEJMcp1001116. [DOI] [PubMed] [Google Scholar]
- 2.Heath CW, Jr, Brodsky AL, Potolsky AI. Infectious mononucleosis in a general population. Am J Epidemiol. 1972;95(1):46–52. doi: 10.1093/oxfordjournals.aje.a121369. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux CE, Bemiller T, Brann O. Ascites and severe hepatitis complicating Epstein-Barr infection. Am J Gastroenterol. 1999;94(1):236–40. doi: 10.1111/j.1572-0241.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 4.Tselis A, Duman R, Storch GA, Lisak RP. Epstein-Barr virus encephalomyelitis diagnosed by polymerase chain reaction: detection of the genome in the CSF. Neurology. 1997;48(5):1351–55. doi: 10.1212/wnl.48.5.1351. [DOI] [PubMed] [Google Scholar]
- 5.Joki-Erkkila VP, Hietaharju A, Numminen J, et al. Multiple cranial nerve palsies as a complication of infectious mononucleosis due to inflammatory lesion in jugular foramen. Ann Otol Rhinol Laryngol. 2000;109(3):340–42. doi: 10.1177/000348940010900319. [DOI] [PubMed] [Google Scholar]
- 6.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol. 2006;59(3):499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]
- 7.Poole BD, Scofield RH, Harley JB, James JA. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity. 2006;39(1):63–70. doi: 10.1080/08916930500484849. [DOI] [PubMed] [Google Scholar]
- 8.Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med. 2003;349(14):1324–32. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 9.Hjalgrim H, Smedby KE, Rostgaard K, et al. Infectious mononucleosis, childhood social environment, and risk of Hodgkin lymphoma. Cancer Res. 2007;67(5):2382–88. doi: 10.1158/0008-5472.CAN-06-3566. [DOI] [PubMed] [Google Scholar]
- 10.Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85(3):744–50. [PubMed] [Google Scholar]
- 11.Hochberg D, Souza T, Catalina M, et al. Acute infection with Epstein-Barr virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J Virol. 2004;78(10):5194–204. doi: 10.1128/JVI.78.10.5194-5204.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapadia GJ, Azuine MA, Takayasu J, et al. Inhibition of epstein-barr virus early antigen activation promoted by 12-O-tetradecanoylphorbol-13-acetate by the non-steroidal anti-inflammatory drugs. Cancer Lett. 2000;161(2):221–29. doi: 10.1016/s0304-3835(00)00616-9. [DOI] [PubMed] [Google Scholar]
- 13.Iniguez MA, Punzon C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999;163(1):111–19. [PubMed] [Google Scholar]
- 14.Hackstein H, Morelli AE, Larregina AT, et al. Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells. J Immunol. 2001;166(12):7053–62. doi: 10.4049/jimmunol.166.12.7053. [DOI] [PubMed] [Google Scholar]
- 15.Gao JX, Issekutz AC. The effect of ebselen on polymorphonuclear leukocyte migration to joints in rats with adjuvant arthritis. Int J Immunopharmacol. 1993;15(7):793–802. doi: 10.1016/0192-0561(93)90016-r. [DOI] [PubMed] [Google Scholar]
- 16.Feizy V, Ghobadi A. Atopic dermatitis and systemic autoimmune diseases: a descriptive cross sectional study. Dermatol Online J. 2006;12(3):3. [PubMed] [Google Scholar]
- 17.Takeoka K, Hidaka Y, Hanada H, et al. Increase in serum levels of autoantibodies after attack of seasonal allergic rhinitis in patients with Graves’ disease. Int Arch Allergy Immunol. 2003;132(3):268–76. doi: 10.1159/000074309. [DOI] [PubMed] [Google Scholar]
- 18.Astrakhan A, Omori M, Nguyen T, et al. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol. 2007;8(5):522–31. doi: 10.1038/ni1452. [DOI] [PubMed] [Google Scholar]
- 19.Kazama I, Sasagawa N, Nakajima T. Complete remission of human parvovirus b19 associated symptoms by loxoprofen in patients with atopic predispositions. Case Report Med. 2012;2012:703281. doi: 10.1155/2012/703281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazama I, Nakajima T. Dual infection of Mycoplasma pneumoniae and Chlamydophila pneumoniae in patients with atopic predispositions successfully treated by moxifloxacin. Infez Med. 2014;22(1):41–47. [PubMed] [Google Scholar]
- 21.Biedermann T. Dissecting the role of infections in atopic dermatitis. Acta Derm Venereol. 2006;86(2):99–109. doi: 10.2340/00015555-0047. [DOI] [PubMed] [Google Scholar]
- 22.Docke WD, Kiessling C, Worm M, et al. Subclinical activation of latent cytomegalovirus (CMV) infection and anti-CMV immune response in patients with atopic dermatitis. Br J Dermatol. 2003;148(5):954–63. doi: 10.1046/j.1365-2133.2003.05263.x. [DOI] [PubMed] [Google Scholar]
- 23.Okudaira H, Mori A. Concepts of the pathogenesis of allergic disease: possible roles of Epstein-Barr virus infection and interleukin-2 production. Int Arch Allergy Immunol. 1999;120(3):177–84. doi: 10.1159/000024265. [DOI] [PubMed] [Google Scholar]
- 24.Okudaira H, Shuto H, Shuto C, et al. A shadow of Epstein-Barr virus in the pathogenesis of atopic diseases. Clin Exp Allergy. 2001;31(1):18–24. [PubMed] [Google Scholar]
- 25.Thorley-Lawson DA, Mann KP. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corsi MM, Ruscica M, Passoni D, et al. High Th1-type cytokine serum levels in patients with infectious mononucleosis. Acta Virol. 2004;48(4):263–66. [PubMed] [Google Scholar]
- 27.Tomkinson BE, Wagner DK, Nelson DL, Sullivan JL. Activated lymphocytes during acute Epstein-Barr virus infection. J Immunol. 1987;139(11):3802–7. [PubMed] [Google Scholar]
- 28.Giuliano VJ, Jasin HE, Ziff M. The nature of the atypical lymphocyte in infectious mononucleosis. Clin Immunol Immunopathol. 1974;3(1):90–98. doi: 10.1016/0090-1229(74)90026-9. [DOI] [PubMed] [Google Scholar]
- 29.Buchwald DS, Rea TD, Katon WJ, et al. Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med. 2000;109(7):531–37. doi: 10.1016/s0002-9343(00)00560-x. [DOI] [PubMed] [Google Scholar]
- 30.Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auwaerter PG. Infectious mononucleosis in middle age. JAMA. 1999;281(5):454–59. doi: 10.1001/jama.281.5.454. [DOI] [PubMed] [Google Scholar]
- 32.Ng RWC, Lima CED, Cheng NCL. Multiple arthralgia after use of amoxicillin in EBV infection: Could it be adult onset of Still’s disease? J Med Cases. 2014;5(2):105–7. [Google Scholar]
- 33.Akpek M, Yarlioglues M, Durmaz S, Kaya MG. [Pericardial tamponade associated with Epstein-Barr virus in an immunocompetent young patient] Turk Kardiyoloji Dernegi arsivi: Turk Kardiyoloji Derneginin yayin organidir. 2011;39(5):407–9. doi: 10.5543/tkda.2011.01312. [in Turkish] [DOI] [PubMed] [Google Scholar]
- 34.Andreu-Ballester JC, Gil-Borras R, Garcia-Ballesteros C, et al. Epstein-Barr virus is related with 5-aminosalicylic acid, tonsillectomy, and CD19(+) cells in Crohn’s disease. World J Gastroenterol. 2015;21(15):4666–72. doi: 10.3748/wjg.v21.i15.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho JY. Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses. Arch Pharm Res. 2007;30(1):64–74. doi: 10.1007/BF02977780. [DOI] [PubMed] [Google Scholar]
- 36.Gotzsche PC. Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Control Clin Trials. 1989;10(1):31–56. doi: 10.1016/0197-2456(89)90017-2. [DOI] [PubMed] [Google Scholar]
- 37.Wallace DJ. Advances in drug therapy for systemic lupus erythematosus. BMC Med. 2010;8:77. doi: 10.1186/1741-7015-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazama I, Maruyama Y, Murata Y. Suppressive effects of nonsteroidal anti-inflammatory drugs diclofenac sodium, salicylate and indomethacin on delayed rectifier K+-channel currents in murine thymocytes. Immunopharmacol Immunotoxicol. 2012;34(5):874–78. doi: 10.3109/08923973.2012.666249. [DOI] [PubMed] [Google Scholar]
- 39.Kazama I. Physiological significance of delayed rectifier K(+) channels (Kv1.3) expressed in T lymphocytes and their pathological significance in chronic kidney disease. J Physiol Sci. 2015;65(1):25–35. doi: 10.1007/s12576-014-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis RS, Cahalan MD. Potassium and calcium channels in lymphocytes. Annu Rev Immunol. 1995;13:623–53. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- 41.Kazama I, Maruyama Y, Murata Y, Sano M. Voltage-dependent biphasic effects of chloroquine on delayed rectifier K(+)-channel currents in murine thymocytes. J Physiol Sci. 2012;62(3):267–74. doi: 10.1007/s12576-012-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Candy B, Hotopf M. Steroids for symptom control in infectious mononucleosis. The Cochrane database of systematic reviews. 2006;(3):CD004402. doi: 10.1002/14651858.CD004402.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Roy M, Bailey B, Amre DK, et al. Dexamethasone for the treatment of sore throat in children with suspected infectious mononucleosis: a randomized, double-blind, placebo-controlled, clinical trial. Arch Pediatr Adolesc Med. 2004;158(3):250–54. doi: 10.1001/archpedi.158.3.250. [DOI] [PubMed] [Google Scholar]
- 44.Kazama I. Roles of lymphocyte Kv1.3-channels in the pathogenesis of renal diseases and novel therapeutic implications of targeting the channels. Mediators Inflamm. 2015;2015:436572. doi: 10.1155/2015/436572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazama I, Tamada T, Tachi M. Usefulness of targeting lymphocyte Kv1.3-channels in the treatment of respiratory diseases. Inflamm Res. 2015;64(10):753–65. doi: 10.1007/s00011-015-0855-4. [DOI] [PubMed] [Google Scholar]


