Abstract
A challenge in Parkinson's disease (PD) is to identify biomarkers of early cognitive change because functioning in some domains may be more prognostic of dementia. Few studies have investigated whether structural magnetic resonance imaging (MRI) correlates in a regionally specific manner with functioning in different cognitive domains. The aim of this study was to identify neuroanatomical correlates of executive functioning, memory, and visual cognition in PD without dementia. 3T MRI was conducted in 51 PD patients and 39 control participants. Brain volumes were measured in structures comprising the frontostriatal cognitive-control system, the medial temporal memory system, the ventral object-based system, and the dorsal spatial-based system. Measures of executive functioning (Stroop Test; Letter Fluency), memory (California Verbal Learning Test), visuospatial cognition (Judgment of Line Orientation), and visuoconstruction (Pentagon Copy) were correlated with volumes comprising each system. Poorer executive functioning largely correlated with decreased frontostriatal volumes. Poorer memory correlated with decreased volumes in all medial temporal regions, but also with frontostriatal volumes. Poorer visuospatial cognition correlated with decreased volumes in the object-based system, whereas poorer visuoconstruction correlated with decreased frontal and object-based system volumes. These relationships were nonsignificant in the control group. This is the first study to demonstrate that subtle changes in multiple cognitive domains in PD without dementia correlate with regional volumes in specific systems implicated in the development of cognitive impairment. The findings suggest that structural MRI holds promise as a marker of early changes in different brain systems, some of which may predict future cognitive deterioration.
Keywords: Parkinson's disease, magnetic resonance imaging, memory, executive function, visual cognition
Parkinson's disease (PD) is associated with cognitive impairment in early stages of the disease,1 can lead to a lower quality of life2 and greater disability,3 and can impact caregiver burden.4,5 An improved understanding of the neural changes associated with cognitive impairment in PD is imperative to improve diagnostic and prognostic accuracy and to identify potential targets for prevention and treatment of cognitive disturbances. To date, the neural correlates of early cognitive decline in PD are not well understood.
Structural magnetic resonance imaging (sMRI) markers of cognitive change are of keen interest because neurodegeneration can be identified in vivo. In mixed samples of PD with and without dementia, scores on global measures of cognition correlated with volumes of the caudate, hippocampus, and ventricles,6-11 and poorer memory correlated with reduced hippocampus and amygdala volumes.8,9,12-15 However, these findings do not address whether neurocognitive relationships can be identified early before the onset of significant cognitive decline, when sMRI markers of cognitive decline would be most useful. Relatively few studies have investigated sMRI correlates of cognition in purely nondemented PD samples. When they have, discrepant findings have been reported. In nondemented PD, hippocampus or amygdala volume12,13,15,16 or fusiform thickness17 correlated with memory in some studies, but not others.18,19 Similarly, poorer performance on some executive functioning measures correlated with decreased frontal and/or temporal-parietal volumes in some studies,20-24 yet not others.17-20,25,26 The reasons for discrepant findings are unclear, but may relate to differences among studies in image processing methods, sample size, and patient characteristics.
Despite these important advances, most studies of nondemented PD patients have examined only 1 or 2 domains of cognition, and sometimes limit the analyses to 1 or 2 brain regions. Hence, whether cortical morphometry correlates in regionally specific ways with functioning in different cognitive domains has not been directly examined. This is important because early changes in some cognitive domains, but not others, may be prognostic of dementia in PD.27,28 Additionally, when neurocognitive associations are found, it is not always clear that they are disease-related because many studies fail to adjust for age and gender, which correlate with brain morphometry.29,30
The present study builds upon past research by using high-resolution 3T MRI to examine sMRI correlates of multiple cognitive domains that are vulnerable to decline in PD. We also adopted an a priori region of interest (ROI) approach based on knowledge about brain systems that mediate different cognitive processes and their role in the development and progression of cognitive impairment in PD. Specifically, we examined regional volumes comprising 4 systems: the frontostriatal cognitive-control system,31,32 which mediates executive functioning; the medial-temporal memory system,33 which governs memory; the ventral object-based system, which mediates object-based visual processing34,35; and the dorsal spatial-based system, which supports spatial cognition.34,35
The frontostriatal system is thought to underlie early changes in executive functioning in PD,36 and some research demonstrates that executive tasks predict future cognitive decline and dementia in PD.37-39 In the present study, measures of executive functioning were phonemic fluency (Letter Fluency) and inhibitory control (Stroop task). Although past studies have not revealed sMRI associations with these tasks in PD,20,24 we predicted that frontostriatal volumes would correlate with performance. As for the medial temporal memory system, regional atrophy correlates with memory functioning in early PD12,16 and predicts future cognitive decline.40 Thus, we used long-delay free recall from the California Verbal Learning Test-II (CVLT-II) to measure memory, and predicted that performance would correlate with volumes comprising the medial temporal system. Last, given the proposal that cognitive measures of posterior brain functioning may better predict future cognitive decline in PD than measures of anterior functioning,27,28 we evaluated the object-based and spatial-based systems using the Judgment of Line Orientation Test (JLOT; version V; number correct out of 15 items) and the Pentagon Copy test from the Mini Mental Status Exam (MMSE). Because both tasks require spatial and object processing, we expected that performance on these tasks would correlate with volumes in both visual systems.
Subjects and Methods
Participants
The University of California, San Diego (UCSD) Human Research Protections Program approved this study. Study participants included 51 patients with idiopathic PD and 39 healthy controls (HC) who were spouses of PD patients or community volunteers. Participants provided written informed consent prior to study procedures. Subjects were excluded from participation if they had metal in their head, neurological diagnoses other than PD, psychiatric diagnoses, history of alcohol or substance abuse, or major cognitive impairment as defined by a score of < 25 on the MMSE.41 PD participants met the PD United Kingdom Brain Bank Criteria and did not exhibit hallucinations. The groups did not differ in gender composition, years of education, or age, although there was a nonsignificant trend for the PD group to be slightly older than the HC group (Table 1). MMSE scores did not differ between the groups. Patients had a PD diagnosis for an average of 6.5 years. Motor symptoms were assessed when patients were on dopamine therapy using Part III of the Unified Parkinson's Disease Rating Scale (UPDRS)42 and the Hoehn and Yahr scale.43
Table 1. Demographic, neuropsychological, and disease characteristics of PD and HC groups.
| Characteristics | PD patients (n = 51) | HCs (n = 39) | F | P |
|---|---|---|---|---|
| Demographics | ||||
| Age | 67.7 (7.2) | 64.8 (7.8) | 3.4 | 0.07 |
| Education (y) | 16.9 (2.9) | 16.8 (2.7) | < 1.0 | 0.87 |
| Female (%) | 41 | 54 | 0.23a | |
| Mini-Mental Status Examination | 28.8 (1.3) | 29.1 (1.0) | < 1.0 | 0.39 |
| Inhibitory control: Stroop Color-Word | 34.8 (9.1) | 39.9 (7.1) | 5.5 | 0.02 |
| Verbal fluency: DKEFS: Letter | 42.5 (11.6) | 48.9 (12.3) | 6.6 | 0.02 |
| Visual cognition | ||||
| Judgment of Line Orientation | 11.8 (2.2) | 12.4 (2.0) | 1.3 | 0.18 |
| Pentagons | 2.3 (0.4) | 2.4 (0.3) | 3.0 | 0.11 |
| Memory: CVLT-II Long-Delay Free-Recall | 6.5 (1.9) | 7.4 (1.7) | 3.5 | 0.11 |
| Disease characteristics | ||||
| Years since diagnosis | 6.5 (4.4) | |||
| UPDRS Motor Subscale (UPDRS III) | 27.6 (9.9) | |||
| Hoehn and Yahr stage | 2.36 (0.5) | |||
| Levodopa dosage equivalenceb | 702.4 (556.3) | |||
Values are raw score means (SDs) for all variables except gender.
Chi-square test.
Calculated using a method of Razmy et al.53
PD, Parkinson's disease; HC, healthy control; DKEFS, Delis-Kaplan Executive Function System; CVLT-II, California Verbal Learning Test II; UPDRS, Unified Parkinson's Disease Rating Scale.
Neuropsychological Measures
Tests of executive functioning included the Stroop Color-Word test (number of items completed in 45 seconds)44 and the Letter Fluency test (number correct) from the Delis-Kaplan Executive Function System (DKEFS).45 Visual cognition was assessed by 2 tests. The JLOT46 measured visuospatial functioning. The total score on a modified scoring system for the overlapping Pentagon Copy task from the MMSE measured visuoconstruction ability. Modified scores ranged from 3 to 0: 3.0 points = 2 distinct 5-sided pentagons intersect forming a 4-sided overlap with all angles meeting appropriately; 2.5 = 2 distinct 5-sided pentagons intersect forming a 4-sided overlap with the majority of angles meeting appropriately; 2.0 = 1 pentagon identifiable, distortion is permitted for 1 pentagon, pentagons intersect forming a 4-sided overlap with the majority of angles meeting appropriately; 1.5 = 1 shape is recognizable as a pentagon and distortion is allowed, the other shape is present, but does not have to be recognized as a pentagon, 2 shapes intersect, but they do not have to form a 4-sided overlap; 1.0 points = 2 shapes are present, but they do not have to be recognizable as pentagons, 2 shapes intersect; 0.5 points = 2 shapes are present, but they do not have to be recognizable as pentagons, no overlap between the 2 shapes is required; 0 points = 1 or 0 shapes are present. Two research assistants rated each participant's pentagon copy. If there was any discrepancy a consensus was reached between the raters. Memory was assessed by the long-delay free-recall test of the CVLT-II Short Form (number of words recalled).47 Larger values for all measures indicated better performance.
MRI Procedures
High-resolution T1-weighted anatomic images were acquired on a GE 3T Excite MRI system equipped with an 8-channel head coil. We used an imaging protocol designed for tissue segmentation that maximizes differentiation of the white and gray matter boundary (3-dimensional [3D] spoiled gradient-recalled at steady state; echo time [TE], 3.0 ms; repetition time [TR], 7.8 ms; inversion time [TI], 600 ms; flip angle, 8 degrees; number of excitations [NEX] 1; slice thickness, 1-mm axial; field of view [FOV] 25.6 cm; matrix size, 256 × 256). Brain volumes were analyzed using FreeSurfer 5.1 automated software (http://surfer.nmr.mgh.harvard.edu/). Each subject's MRI was initially analyzed in original space using the following processing pipeline. Data were motion corrected, normalized for intensity inhomogeneities, and then an affine transform was computed from the original volume to Talairach space, which provides coordinates that are used as seed points in downstream programs. Using the original volume, nonbrain tissue was removed by a hybrid watershed/surface deformation procedure, subcortical structures were segmented,48 and further intensity normalization was conducted.49 This was followed by white-matter segmentation, tessellation of the gray-white matter boundary, and automated topology correction. Then surface deformation following intensity gradients optimally placed the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class. Cortical gray-matter volume was calculated by multiplying cortical thickness by surface area. The cerebral cortex was parcellated into 34 gyral-based regions in each hemisphere using the Desikan atlas,50 which is based on conventional neuroanatomical regions defined by cortical sulci and gyri. Subcortical regions were delineated by an algorithm that examines variations in voxel intensities and spatial relationships.48 Anatomical accuracy of transformations and segmentations were then manually verified and adjusted when necessary.51 This approach provides accurate renderings of regional volumes, without rater bias.48 To adjust for differences in head size, volumes for each region were divided by intracranial volume (ICV).52
Regions of Interest
The analyses focused on a subset of the cortical regions from the Desikan atlas and subcortical regions that are traditionally considered key elements of the 4 systems that were the focus of our hypotheses. The systems and the structures that comprise them included: (1) the frontostriatal cognitive-control system (anterior cingulate, caudal middle frontal gyrus, rostral middle frontal gyrus, superior frontal gyrus, supplementary motor area (SMA), pars triangularis, pars opercularis, pars orbitalis, caudate, and putamen)31,32; (2) the medial temporal memory system (temporal pole, entorhinal cortex, parahippocampus, hippocampus, and amygdala)33; (3) the dorsal spatial-based system (superior parietal and inferior parietal)35; and (4) the ventral object-based system (superior temporal, middle temporal, inferior temporal, fusiform gyrus, lateral occipital cortex, cuneus, and lingualgyrus).35
Statistical Analyses
Cognitive measures were normalized to the control group by subtracting the individual raw scores for each measure from the control group mean and then dividing by the control group standard deviation. The resulting Z scores were then correlated with regional volumes, adjusting for age and gender (partial correlations). Partial correlations were conducted separately for the PD and control groups. Due to our a priori hypotheses that regional volumes in the 4 networks would correlate differently with measures of executive functioning, memory, and visual cognition, a conventional P < 0.05 per comparison threshold was adopted. Nonetheless, the results should be interpreted with caution due to the multiple comparisons.
Results
Neuropsychological Test Performance
An analysis of covariance (ANCOVA) model was used to compare the 2 groups on neuropsychological measures, using age and gender as covariates (Table 1). The PD group performed significantly lower on both tests of executive functioning. Although group differences were not significant for measures of visual cognition or memory, there were trends for poorer performance in the PD group. Partial correlations (age- and gender-adjusted) showed that levodopa dosage equivalence,53 disease duration, and UPDRS scores did not correlate with the cognitive measures.
Group Differences in Regional Volumes
To determine if the PD group showed significant atrophy in the region of interest (ROI) identified for each of the 4 systems, ANCOVAs tested for group differences in regional volumes adjusting for age and gender. Volume loss in the PD group was found in the right putamen (P = 0.029) (frontostriatal system), the right superior parietal cortex (P = 0.05) (spatial-based system), and the bilateral inferior temporal cortex (P = 0.039 and P = 0.006 for the left and right hemispheres, respectively), right lateral occipital cortex (P = 0.009), and right cuneus (P = 0.016) (object-based system). Group differences in medial temporal lobe volumes were not found. When a false discovery rate (FDR) was used to adjust for multiple comparisons, the group effects were nonsignificant. No group differences were found in ventricle volumes.
Correlation of Regional Volumes With Cognitive Functioning
Table 2 lists the significant partial correlations of regional volumes with performance on each of the neuropsychological tests for the PD group. Figure 1 displays the regional maps of these partial correlations. Figure 2 shows scatter plots of the correlations for selected ROIs, in which the plotted values are the residuals from the regression of age and gender onto brain volumes and normalized performance of each neuropsychological measure. The results revealed that worse performance in the PD group on each cognitive measure correlated with decreased volumes, largely in different brain regions. Poorer inhibitory control (Stroop Interference) correlated with decreased volume in 2 frontostriatal regions (left pars triangularis and right putamen) and 1 medial temporal region (right entorhinal cortex). Worse verbal fluency correlated with decreased volume in several frontal regions (left SMA and rostral middle frontal cortex; right caudal middle frontal cortex), the object-based system (right cuneus), and the medial temporal system (right entorhinal cortex). Poorer visuospatial processing (JLOT) correlated with decreased volume in the object-based system (bilateral superior temporal cortex; right lateral occipital cortex). Poorer visuoconstructive ability (Pentagon Copy) correlated with decreased volume largely in frontal regions (right SMA; left rostral middle frontal cortex and pars triangularis), but also in the object-based system (left cuneus). Worse memory performance (CVLT-II, delayed free recall) correlated with reduced volume in the medial temporal (left temporal pole; bilateral entorhinal cortex and parahippocampus; right hippocampus and amygdala) and the frontostriatal (left superior frontal cortex; bilateral pars triangularis and orbitalis; and right putamen) systems. These relationships were nonsignificant in the control group with 1 exception; JLOT correlated with right superior temporal cortex volume (rpartial = 0.30, P < 0.05).
Table 2. Partial correlations of regional volume with neuropsychological test performance in the PD group, adjusting for age and gender.
| System (regions) | H | Stroop | Fluency | JLOT | Pentagons | CVLT |
|---|---|---|---|---|---|---|
| Frontostriatal cognitive-control system | ||||||
| SMA | L | 0.35b | ||||
| R | 0.42d | |||||
| Anterior cingulate | L | |||||
| R | ||||||
| Superior frontal | L | 0.27a | ||||
| R | ||||||
| Caudal middle frontal | L | |||||
| R | 0.27a | |||||
| Rostral middle frontal | L | 0.35b | 0.26a | |||
| R | ||||||
| Pars triangularis | L | 0.36b | 0.30a | 0.25a | ||
| R | 0.30a | |||||
| Pars orbitalis | L | 0.27a | ||||
| R | 0.25a | |||||
| Pars opercularis | L | |||||
| R | ||||||
| Caudate | L | |||||
| R | ||||||
| Putamen | L | |||||
| R | 0.40c | 0.28a | ||||
| Dorsal spatial-based system | ||||||
| Superior parietal | L | |||||
| R | ||||||
| Inferior parietal | L | |||||
| R | ||||||
| Ventral object-based system | ||||||
| Superior temporal | L | 0.34b | ||||
| R | 0.28a | |||||
| Middle temporal | L | |||||
| R | ||||||
| Inferior temporal | L | |||||
| R | ||||||
| Fusiform gyrus | L | |||||
| R | ||||||
| Lateral occipital | L | |||||
| R | 0.24a | |||||
| Cuneus | L | 0.27a | ||||
| R | 0.27a | |||||
| Lingual gyrus | L | |||||
| R | ||||||
| Medial temporal memory system | ||||||
| Temporal pole | L | 0.28a | ||||
| R | ||||||
| Entorhinal | L | 0.26a | ||||
| R | 0.36c | 0.29a | 0.39c | |||
| Parahippocampus | L | 0.31a | ||||
| R | 0.29a | |||||
| Hippocampus | L | |||||
| R | 0.46d | |||||
| Amygdala | L | |||||
| R | 0.28a | |||||
P≤0.05.
P≤0.01.
P≤0.005.
P≤ 0.001.
PD, Parkinson's disease; H, hemisphere; L/R, left/right hemisphere; JLOT, Judgment of Line Orientation Test; CVLT, California Verbal Learning Test; SMA, supplementary motor area.
Fig. 1.
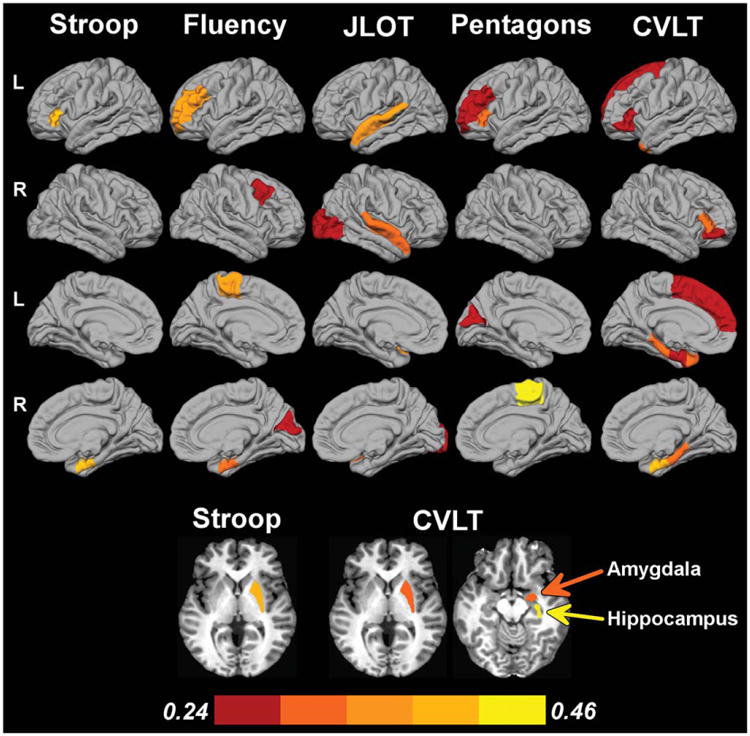
Correlation of regional volumes with cognitive performances in PD. Cortical regions are displayed on lateral and medial surfaces of the left (L)/right (R) hemispheres. Axial views show putamen, amygdala, and hippocampus. Color bar designates the partial correlation coefficient magnitude.
Fig. 2.
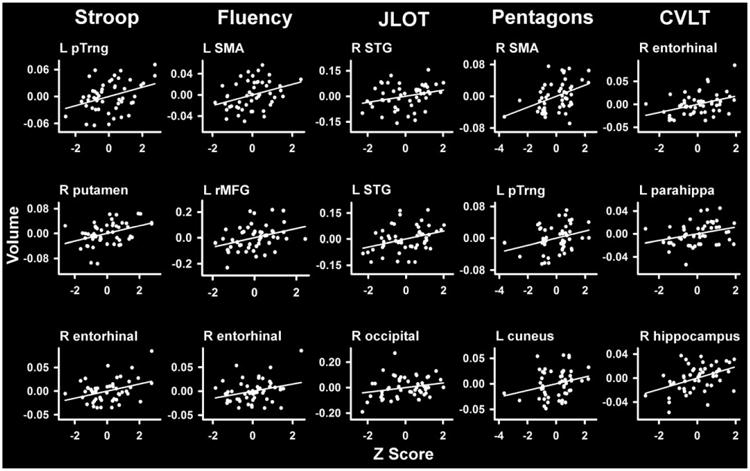
Scatter plots showing neurocognitive correlations in PD. Residuals from the regression of age and gender onto regional volumes and neuropsychological measures are plotted. L/R, left/right hemisphere; parahippa, parahippocampus; pTrng, pars triangularis; rMFG, rostral middle frontal; SMA, supplementary motor area; STG, superior temporal.
Discussion
To our knowledge this is the first study to demonstrate that subtle changes in multiple cognitive domains including executive functioning, memory, visuospatial functioning, and visuoconstruction are associated with distinct patterns of regionally-specific volume changes in nondemented PD patients, but not in controls. These findings suggest that the neurocognitive relationships identified by the present study are related to the disease, rather than to aging or gender.
An important finding was that worse delayed verbal recall (CVLT-II) correlated with decreased volumes in all medial temporal regions including the hippocampus, parahippocampus, entorhinal cortex, temporal pole and amygdala, despite the absence of significant volume loss in these structures. This finding concurs with reports that verbal memory is associated with hippocampus and amygdala volume in early PD,12,13,16 even without atrophy of these structures.16 Our findings extend these earlier results by demonstrating that worse memory in nondemented PD relates to volume loss in all of the medial temporal lobe structures, which govern memory encoding, memory consolidation, and semantic processing. The association between entorhinal cortex volume and memory functioning was notable because entorhinal cortex volume may be especially important in PD, as it distinguishes demented from cognitively normal patients, unlike hippocampal volume.54 No other cognitive measure correlated with volumes in all of the medial temporal structures, and measures of visual cognition were unrelated to volumes in this system. One speculation is that subtle changes in the medial temporal lobes in PD without dementia may be an early marker of progressive memory deterioration.
Stroop and Verbal Fluency performance also correlated with right entorhinal cortex volume, possibly suggesting that executive impairment is partly mediated by some structures of the medial temporal system, and thus changes in memory. However, the entorhinal cortex receives extensive input from the prefrontal cortex, which drives executive functioning. Consistent with our prediction, poorer executive functioning in PD was associated with reduced volumes in the cognitive-control system, especially in the left hemisphere. Worse Stroop interference performance correlated with decreased volumes in Broca's area (left pars triangularis) and the right putamen, whereas worse Verbal Fluency performance was associated with decreased volumes in the left SMA and rostral middle frontal cortex and the right caudal middle frontal cortex. These results are consistent with focal lesion, functional imaging, and repetitive transcranial magnetic stimulation studies of these executive functions.55-60
Performances on most of the other neuropsychological tasks, except JLOT, were also associated with the frontostriatal volumes. This was striking for delayed recall. Past studies of nondemented PD patients have focused on the association between hippocampus and memory. Yet the frontostriatal system also governs aspects of memory including selective attention, memory encoding, and retrieval.61-63 Perhaps more surprising was the finding that poorer Pentagon Copy was predominantly associated with frontal volumes (SMA, left rostral middle frontal, and left pars triangularis). However, visuoconstruction is a multifaceted process that partly depends on executive functions. Our findings question whether the association between Pentagon Copy performance and future dementia in PD is largely due to neurodegeneration of posterior brain regions,27,28 rather than or in addition to frontal lobe dysfunction.
Both measures of visual cognition correlated with volumes of the object-based system. Worse JLOT performance was associated with decreased bilateral superior temporal and right lateral occipital volume. Here it was noteworthy that the PD group showed volume loss in the right superior temporal and lateral occipital cortices (FDR uncorrected), suggesting atrophy of this system earlier in the disease process. In addition, poorer Pentagon Copy performance was associated with decreased left cuneus volume. There was also an association between poorer Verbal Fluency performance and reduced right cuneus volume, which may relate to the multisensory functions of visual-association centers. None of the neuropsychological tests correlated with volumes comprising the spatial-based system. These results emphasize the importance of distinguishing between ventral and dorsal visual systems, and suggest that structural changes in the object system may be an important source of early deterioration in visual cognition in PD patients without dementia. A caveat is that face recognition memory was associated with volume loss in the spatially based (superior parietal cortex) and the object-based systems in PD,64 but in a mixed sample of patients with and without hallucinations.
In summary, our findings suggest that sMRI holds promise as a potential biomarker of changes in different brain systems, some of which may predict future cognitive deterioration in specific domains. More studies using an expanded neuropsychological battery are needed to establish the generalizability of the results. Future studies should include a formal diagnostic criteria for mild cognitive impairment (MCI),65 which was lacking in the present study, to better distinguish patients with normal cognitive functioning. Longitudinal studies are also needed to determine the clinical utility of neurocognitive associations in improving diagnostic accuracy and identifying patients who are at risk for developing PD-MCI or PD-dementia. In this regard, sMRI combined with diffusion weighted imaging may also advance diagnostic and prognostic accuracy, since changes in white-matter tissue are also associated with cognitive functioning in PD patients without dementia.
Acknowledgments
We thank Gabriel Castillo and Christopher Fong for their technical support in acquiring the data for this study. We also thank Dr. David Song for referring patients to the study.
Funding agencies: Department of Veterans Affairs (CX000146 to D.L.H.; CX12004 to J.V.F.).
V.F.: Grant from the Department of Veterans Affairs (CX12004). J.R. and I.L.: None. D.H.: Grants from the Department of Veterans Affairs (CX000146); NIH/NINDS (NS040068); NIH/NINDS U01 (NS082083).
Footnotes
Relevant conflicts of interest/financial disclosures: Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 2.Hurt CS, Landau S, Burn DJ, et al. Cognition, coping, and outcome in Parkinson's disease. Int Psychogeriatr. 2012;24:1656–1663. doi: 10.1017/S1041610212000749. [DOI] [PubMed] [Google Scholar]
- 3.Raggi A, Leonardi M, Ajovalasit D, et al. Disability and profiles of functioning of patients with Parkinson's disease described with ICF classification. Int J Rehabil Res. 2011;34:141–150. doi: 10.1097/MRR.0b013e328344ae09. [DOI] [PubMed] [Google Scholar]
- 4.Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol. 2012;25:208–214. doi: 10.1177/0891988712464823. [DOI] [PubMed] [Google Scholar]
- 5.Morley D, Dummett S, Peters M, et al. Factors influencing quality of life in caregivers of people with Parkinson's disease and implications for clinical guidelines. Parkinsons Dis. 2012;2012:190901. doi: 10.1155/2012/190901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apostolova LG, Beyer M, Green AE, et al. Hippocampal, caudate, and ventricular changes in Parkinson's disease with and without dementia. Mov Disord. 2010;25:687–695. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolova L, Alves G, Hwang KS, et al. Hippocampal and ventricular changes in Parkinson's disease mild cognitive impairment. Neurobiol Aging. 2012;33:2113–2124. doi: 10.1016/j.neurobiolaging.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson's disease is associated with hippocampal atrophy. Mov Disord. 2003;18:784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- 9.Junque C, Ramirez-Ruiz B, Tolosa E, et al. Amygdalar and hippocampal MRI volumetric reductions in Parkinson's disease with dementia. Mov Disord. 2005;20:540–544. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- 10.Melzer TR, Watts R, MacAskill MR, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;83:188–194. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- 11.Oikawa H, Sasaki M, Ehara S, Abe T. Substantia innominata: MR findings in Parkinson's disease. Neuroradiology. 2004;46:817–821. doi: 10.1007/s00234-004-1257-4. [DOI] [PubMed] [Google Scholar]
- 12.Beyer MK, Bronnick KS, Hwang KS, et al. Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson's disease. J Neurol Neurosur Psychiatry. 2013;84:23–28. doi: 10.1136/jnnp-2012-303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouchard TP, Malykhin N, Martin WR, et al. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson's disease. Neurobiol Aging. 2008;29:1027–1039. doi: 10.1016/j.neurobiolaging.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Goldman JG, Weis H, Stebbins G, Bernard B, Goetz CG. Clinical differences among mild cognitive impairment subtypes in Parkinson's disease. Mov Disord. 2012;27:1129–1136. doi: 10.1002/mds.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibarretxe-Bilbao N, Ramirez-Ruiz B, Tolosa E, et al. Hippocampal head atrophy predominance in Parkinson's disease with hallucinations and with dementia. J Neurol. 2008;255:1324–1331. doi: 10.1007/s00415-008-0885-8. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub D, Doshi J, Koka D, et al. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol. 2011;68:1562–1568. doi: 10.1001/archneurol.2011.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellicano C, Assogna F, Piras F, Caltagirone C, Pontieri FE, Spalletta G. Regional cortical thickness and cognitive functions in non-demented Parkinson's disease patients: a pilot study. Eur J Neurol. 2012;19:172–175. doi: 10.1111/j.1468-1331.2011.03465.x. [DOI] [PubMed] [Google Scholar]
- 18.Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, et al. Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson's disease. PLoS One. 2013;8:e54980. doi: 10.1371/journal.pone.0054980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalaker TO, Zivadinov R, Larsen JP, et al. Gray matter correlations of cognition in incident Parkinson's disease. Mov Disord. 2010;25:629–633. doi: 10.1002/mds.22867. [DOI] [PubMed] [Google Scholar]
- 20.Biundo R, Formento-Dojot P, Facchini S, et al. Brain volume changes in Parkinson's disease and their relationship with cognitive and behavioural abnormalities. J Neurol. 2011;310(1-2):64–69. doi: 10.1016/j.jns.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Camicioli R, Gee M, Bouchard TP, et al. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord. 2009;15:187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Ibarretxe-Bilbao N, Tolosa E, Junque C, Marti MJ. MRI and cognitive impairment in Parkinson's disease. Mov Disord. 2009;24(Suppl 2):S748–S753. doi: 10.1002/mds.22670. [DOI] [PubMed] [Google Scholar]
- 23.Nagano-Saito A, Washimi Y, Arahata Y, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64:224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- 24.Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bartres-Faz D, Tolosa E. Structural brain correlates of verbal fluency in Parkinson's disease. Neuroreport. 2009;20:741–744. doi: 10.1097/WNR.0b013e328329370b. [DOI] [PubMed] [Google Scholar]
- 25.Tinaz S, Courtney MG, Stern CE. Focal cortical and subcortical atrophy in early Parkinson's disease. Mov Disord. 2011;26:436–441. doi: 10.1002/mds.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin WR, Wieler M, Gee M, Camicioli R. Temporal lobe changes in early, untreated Parkinson's disease. Mov Disord. 2009;24:1949–1954. doi: 10.1002/mds.22680. [DOI] [PubMed] [Google Scholar]
- 27.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 28.Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson's disease. Neurobiol Dis. 2012;46:590–596. doi: 10.1016/j.nbd.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Mestres-Misse A, Turner R, Friederici AD. An anterior-posterior gradient of cognitive control within the dorsomedial striatum. Neuroimage. 2012;62:41–47. doi: 10.1016/j.neuroimage.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Eichenbaum H, Lipton PA. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18:1314–1324. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- 35.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:35–62. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 36.Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord. 2011;26:289–296. doi: 10.1002/mds.23477. [DOI] [PubMed] [Google Scholar]
- 37.Mahieux F, Fenelon G, Flahault A, Manifacier MJ, Michelet D, Boller F. Neuropsychological prediction of dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1998;64:178–183. doi: 10.1136/jnnp.64.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson's disease: a community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol. 2005;18:149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- 39.Santangelo G, Trojano L, Vitale C, et al. A neuropsychological longitudinal study in Parkinson's patients with and without hallucinations. Mov Disord. 2007;22:2418–2425. doi: 10.1002/mds.21746. [DOI] [PubMed] [Google Scholar]
- 40.Weintraub D, Dietz N, Duda JE, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain. 2012;135(Pt 1):170–180. doi: 10.1093/brain/awr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 42.Fahn S, Elton RLL . UPDRS Development Committee. The United Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham, NJ: Macmillan Health Care Information; 1987. pp. 153–164. [Google Scholar]
- 43.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 44.Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Skoelting; 1978. [Google Scholar]
- 45.Delis DC, Kaplan E, Kramer JH, editors. Delis-Kaplan Executive Function System: Examiner's Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 46.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment: a clinical test. Arch Neurol. 1978;35:364–367. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 47.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 48.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 49.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 50.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 52.Westman E, Aguilar C, Muehlboeck JS, Simmons A. Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer's disease and mild cognitive impairment. Brain Topogr. 2013;26:9–23. doi: 10.1007/s10548-012-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Razmy A, Lang AE, Shapiro CM. Predictors of impaired daytime sleep and wakefulness in patients with Parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch Neurol. 2004;61:97–102. doi: 10.1001/archneur.61.1.97. [DOI] [PubMed] [Google Scholar]
- 54.Goldman JG, Stebbins GT, Bernard B, Stoub TR, Goetz CG, deToledo-Morrell L. Entorhinal cortex atrophy differentiates Parkinson's disease patients with and without dementia. Mov Disord. 2012;27:727–734. doi: 10.1002/mds.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 56.Birn RM, Kenworthy L, Case L, et al. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49:1099–1107. doi: 10.1016/j.neuroimage.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira JB, Junque C, Bartres-Faz D, et al. Modulation of verbal fluency networks by transcranial direct current stimulation (tDCS) in Parkinson's disease. Brain Stimul. 2013;6:16–24. doi: 10.1016/j.brs.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Roberts KL, Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory Stroop tasks. J Cogn Neurosci. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- 60.Robinson G, Shallice T, Bozzali M, Cipolotti L. The differing roles of the frontal cortex in fluency tests. Brain. 2012;135(Pt 7):2202–2214. doi: 10.1093/brain/aws142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 62.Lee AC, Robbins TW, Owen AM. Episodic memory meets working memory in the frontal lobe: functional neuroimaging studies of encoding and retrieval. Crit Rev Neurobiol. 2000;14(3-4):165–197. [PubMed] [Google Scholar]
- 63.Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargallo N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson's disease. Mov Disord. 2009;24:1193–1199. doi: 10.1002/mds.22560. [DOI] [PubMed] [Google Scholar]
- 65.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Theilmann RJ, Reed JD, Song DD, et al. White-matter changes correlate with cognitive functioning in Parkinson's disease. Front Neurol. 2013;4:37. doi: 10.3389/fneur.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]


