Abstract
Over the past decade, various enzyme/prodrug systems such as thymidine kinase/ganciclovir (TK/GCV), yeast cytosine deaminase/5-fluorocytosine (yCD/5-FC) and nitroreductase/CB1954 (NTR/CB1954) have been used for stem cell mediated suicide gene therapy of cancer. Yet, no study has been conducted to compare and demonstrate the advantages and disadvantages of using one system over another. Knowing that each enzyme/prodrug system has its own strengths and weaknesses, we utilized mesenchymal stem cells (MSCs) as a medium to perform for the first time a comparative study that illustrated the impact of subtle differences among these systems on the therapeutic outcome. For therapeutic purposes, we first genetically modified MSCs to stably express a panel of four suicide genes including TK (TK007 and TKSR39 mutants), yeast cytosine deaminase: uracil phosphoribosyltransferase (yCD:UPRT) and nitroreductase (NTR). Then, we evaluated the anticancer efficacies of the genetically engineered MSCs in vitro and in vivo by using SKOV3 cell line which is sensitive to all four enzyme/prodrug systems. In addition, all MSCs were engineered to stably express luciferase gene making them suitable for quantitative imaging and dose-response relationship studies in animals. Considering the limitations imposed by the prodrugs’ bystander effects, our findings show that yCD:UPRT/5-FC is the most effective enzyme/prodrug system among the ones tested. Our findings also demonstrate that theranostic MSCs are a reliable medium for the side-by-side evaluation and screening of the enzyme/prodrug systems at the preclinical level. The results of this study could help scientists who utilize cell-based, non-viral or viral vectors for suicide gene therapy of cancer make more informed decisions when choosing enzyme/prodrug systems.
Keywords: mesenchymal stem cells, cancer gene therapy, enzyme prodrug, suicide genes, cytosine deaminase, thymidine kinase
Introduction
Stem cell mediated gene delivery is emerging as a new strategy to improve the safety and efficacy of current cancer gene therapy methods. Recent evidence indicates that systemically administered mesencymal stem cells (MSCs) can migrate and deliver therapeutic genes to tumors [1-3]. It is envisioned that this inherent tumor tropism of MSCs can be exploited to develop effective and well-tolerated treatments for patients with malignant solid tumors [4, 5]. For this purpose, MSCs are first genetically modified ex-vivo to stably express a prodrug-converting enzyme (e.g., thymidine kinase, cytosine deaminase, nitroreductase, etc.) and then injected back into the body to migrate into tumors. Subsequently, a prodrug is administered which gets converted into its cytotoxic form by the enzyme inside the MSCs. This in turn results in the death of the stem cells as well as neighboring cancer cells through a phenomenon known as “bystander effect” [6-8]. Therefore, in addition to the number of MSCs that reach the tumor target, prodrug’s bystander effect also plays a major role in the success of this approach. Although in the past decade several different enzyme/prodrug systems are utilized for this purpose, literature search shows that thymidine kinase/ganciclovir (TK/GCV) and yeast cytosine deaminase/5-fluorocytosine (yCD/5-FC) are the most widely used systems [9-12]. Unfortunately, in many cases no clear rationale is provided to justify the use of one enzyme/prodrug system over another and it has been unclear which enzyme/prodrug system is the most effective one. As we have discussed elsewhere and knowing that each enzyme/prodrug system has its own strengths and weaknesses[13], it is important to be able to perform a study at the preclinical level that can reliably illustrate the strengths and weaknesses of using different enzyme/prodrug systems and/or compare the efficacies of different enzyme mutants.
The overall objective of this research was to genetically engineer a panel of MSCs that stably express TK (TK007 and TKSR39 mutants), yCD:UPRT and nitroreductase (NTR) suicide genes and evaluate their anticancer efficacies side-by-side by using a sensitive tumor model. To achieve the objective, we genetically modified bone-marrow derived MSCs to stably express the aforementioned suicide genes and evaluated their ability to kill xenografts of SKOV3 ovarian cancer tumors after administration of an appropriate prodrug. This model cancer cell line was chosen because of its sensitivity to the enzyme/prodrugs systems used in this study [14-16]. The use of a cancer cell line that is sensitive to the enzyme/prodrug systems is essential as it helps to eliminate the cell-related bias. As a result, the observed differences in terms of therapeutic outcome will not be due to the cell’s biological traits but the enzyme/prodrug systems’ properties. Therefore, cell lines that are not sensitive (resistant) to one system or another will not be suitable for such comparative studies.
TK007 and TKSR39 are the most efficient mutants of wild-type TK with the ability to rapidly convert GCV into its cytotoxic form inside the TK expressing cells [17, 18]. Bacterial nitroreductase (NTR) is able to convert CB1954 prodrug into its potent cytotoxic form [19, 20]. In comparison to yCD alone, yCD:UPRT, which is a combination of yCD and UPRT, has a higher sensitivity to 5-FC. Therefore, yCD:UPRT can convert this prodrug into its cytotoxic form in a faster rate resulting in higher efficacy [21, 22]. Using an in vitro cell toxicity assay, we first examined the sensitivity of the suicide gene expressing MSCs to prodrugs followed by studying their ability to kill SKOV3 cancer cells through their bystander effects. From the in vitro studies, three of the most efficient suicide gene expressing MSCs were selected and then used to evaluate their ability in killing SKOV3 xenograft tumors in nude mice. To correlate dose with response, all MSCs were engineered to stably express luciferase gene and the in vivo viability of MSCs were tracked and monitored before and after prodrug administration.
Materials and Methods
Genetic engineering of suicide gene expressing MSCs
All the recombinant DNA work presented here has been reviewed and approved by the Rutgers University Environmental Health and Safety office. The genes encoding yCD:UPRT and wild-type herpes simplex virus thymidine kinase (HSVTK) were purchased from Invivogen (San Diego, CA). Using site-directed mutagenesis wild-type HSVTK was mutated into TKSR39 as previously reported [17]. The full length NTR gene based on previously published data was synthesized by IDTDNA technologies (Coralville, IA) [19]. The gene encoding TK007 enzyme was obtained from Professor B. Fehse (University Medical Centre Hamburg-Eppendorf, Germany) through Material Transfer Agreement. Using pBudCE4.1 dual promoter mammalian expression vector (Invitrogen), all suicide genes were cloned separately under EF1α promoter, whereas a firefly luciferase-GFP fusion gene was cloned under CMV promoter to facilitate colony selection and in vivo imaging. The sequences of all genes and fidelity to the original design were verified by DNA sequencing.
In the next step, human bone-marrow derived MSCs were first seeded in 58 cm2 culture dishes at 3000-6000 cells/cm2 density and propagated in DMEM media supplemented with 10% fetal bovine serum, 10,000 units/ml of penicillin and 10,000 μg/ml of streptomycin (Caisson, UT, USA). These cells were originally purchased from the Texas A&M Health Science Center College of Medicine and a generous gift from Professor P. Moghe at Rutgers University to our lab. MSCs were then transfected with the constructed mammalian expression vectors by using GeneIn™ reagent (GlobalStem®, MD, USA) to make MSC-TK007-Luc, MSC-TKSR39-Luc, MSC-yCD:UPRT-Luc, and MSC-NTR-Luc. Transfected MSCs were maintained in full media and treated with 150 μl/ml Zeocin (Invitrogen, NY, USA) for 3-4 weeks to eliminate the nontransfected cells. Several colonies from each plate were picked and then propagated for further analysis.
In vitro and in vivo analysis of luciferase expression in genetically modified MSCs
The levels and stable expression of luciferase in all colonies were first evaluated in vitro by using luciferase assay kit and protocol (Promega). MSC-TK007-Luc, MSC-TKSR39-Luc, MSC-yCD:UPRT-Luc and MSC-NTR-Luc were seeded separately in 96 well plates at the density of 2×104 cells per well. After 24 h the media were removed, cells were washed with Dulbecco’s Phosphate Buffer Saline (DPBS) and 20 μl of lysis buffer was added to each well. Equal amounts of lysate were mixed with 50 μl of D-luciferin (Promega, WI, USA) in glass tubes and luminescence was measured by a luminometer (Berthold, Germany). Colonies with statistically similar luciferase expression were selected and propagated for in vivo evaluation.
The minimum number of genetically modified MSCs that can be detected in mice by IVIS imaging system (PerkinElmer, Waltham, MA, USA) after subcutaneous (SC) injection was examined by using MSC-TKSR39 clone. This was done by first suspending different number of cells ranging from 5×103 to 1×106 in 100 μl of DPBS:Matrigel (Corning, MA, USA) (50:50 v/v) and injecting it subcutaneously (SC) in the dorsal regions of female nude mice. D-luciferin (Goldbio®, St. Louis, MO, USA) was then injected intraperitoneally (IP) into the mice at the dose of 150 mg/kg. Five minutes after D-luciferin injection, mice were anesthetized by isoflurane inhalation and placed in IVIS imaging system to detect the cells. The in vivo bioluminescent images were displayed in “photon” mode; therefore, the signal intensity is represented by radiance (p/sec/cm2/sr), which refers to the number of photons per second that are leaving a square centimeter of tissue and radiating into a solid angle of one steradian (sr).
Evaluation of the sensitivity of suicide gene expressing MSCs to prodrugs
To study the expression and sensitivity of the suicide gene expressing MSCs to prodrugs, they were first seeded in 96-well plates at the density of 1×104 cells per well. The next day, the corresponding prodrug for each suicide gene expressing MSC; i.e., 5-FC for MSC-yCD:UPRT-Luc; CB1954 for MSC-NTR-Luc; and GCV for MSC-TK007-Luc and MSC-TKSR39-Luc were added to each well. 5-FC (PureChemsTM, TX, USA), CB1954 (Medkoo Biosciences, NC, USA) and GCV (PureChemsTM, TX, USA) prodrugs were added and incubated with the cells for 5 days in the following ranges: 0.1-1.5 μM, 10-200 μM and 0.1-5.0 μM, respectively. Cell viability was evaluated by WST-1 assay (Roche, Nutley, NJ) after addition of the reagent and measuring the absorbance at 450 nm. The viability of untransfected MSCs (naïve) treated with prodrug was used as control. The data are reported as mean±SD (n=3).
Evaluation of MSC tropism towards cancer cells by migration assay
The tropism of genetically modified MSCs towards cancer cells was studied by using migration assay. SKOV3 ovarian cancer cells and HEK 293 human embryonic kidney cells (control) were seeded in 24-well plates at the density of 1×105 cells per well. After 24 hours, inserts of 8-μm pore size (Greiner bio-one, NC, USA) were placed on the wells followed by transferring 2×104 genetically modified MSCs to each insert. After 48 hours, the inserts were taken out for staining and visualization. Briefly, cells inside each insert were removed with a cotton tip and the inserts were soaked in cold methanol for 10-20 min to fix the cells on the outer layer. The membrane was carefully cut off after washing with PBS and placed on a slide with the bottom side up. DAPI solution (Southern Biotech, Alabama, USA) was added onto the membranes to stain cells’ nucleus. The cells were then visualized using a fluorescent microscope (Olympus, USA). The number of migrated cells was measured by counting five random fields of view per well under the microscope (ocular lens 10x, objective 10x, field of view 220 μm). The data are reported as mean±SD (n=5).
In vitro evaluation of the cancer cell killing efficiency of suicide gene expressing MSCs
Using a previously published method, we evaluated the ability of the suicide gene expressing MSC clones to kill SKOV3 cancer cells through the bystander effect [4, 5]. In brief, SKOV3 cells (doubling time: ~35 hours) were seeded in 96-well plates either alone or mixed with suicide gene expressing MSCs (doubling time: ~40 hours) at the density of 5×103 cells per well to generate MSC to SKOV3 ratios of 0:100 (SKOV3 alone), 1:50, 1:10, 1:5, and 1:2. The next day, the corresponding prodrugs at the concentrations of 50, 100 or 200 μM for each suicide gene were added to the wells. WST-1 assay was performed after 5 days to measure the cell viability. The absorbance of each MSC to SKOV3 co-culture without any prodrug treatment was considered as 100% viable. The data are reported as mean±SD (n=3).
In vivo evaluation of the tumor killing efficiency of the suicide gene expressing MSCs
Five-week old female nude mice were purchased from Jackson laboratory (Bar Harbor, ME) and used for the in vivo studies. All animals were cared for in accordance with the Rutgers Institutional Animal Care and Use Committee approved protocols. Mice were anesthetized by isoflurane inhalation and 3×106 SKOV3 cells suspended in 100 μl DPBS (50% Matrigel®) were injected SC in dorsal flank regions (two tumors per mouse). The tumor size growth was monitored by pressure sensitive caliper and when reached 100-150 mm3 all mice were randomly distributed into 10 groups each containing 5 mice. First treatment group received intratumoral injection of 1×106 MSC-TKSR39-Luc cells once a week plus daily injection of GCV (25 mg/kg). Second treatment group received intratumoral injection of 1×106 MSC-yCD:UPRT-Luc cells once a week plus daily injection of 5-FC (600 mg/kg). Third treatment group received intratumoral injection of 1×106 MSC-NTR-Luc cells once a week plus daily injection of CB1954 (20 mg/kg). Cell control groups received intratumoral injection of 1×106 MSC-TKSR39-Luc, MSC-yCD:UPRT-Luc and MSC-NTR-Luc once a week, respectively (without prodrug). Prodrug control groups received daily injections of GCV (25 mg/kg), 5-FC (600 mg/kg) and CB1954 (20 mg/kg), respectively. Vehicle control group received intra-tumoral injection of PBS once a week. Genetically modified MSCs were administered intratumorally on days 0, 6, 12, 18, 24 and 30 (6 doses). Before each cell injection, tumor sizes and body weights were measured, D-luciferin injected (150 mg/Kg) and mice were imaged by IVIS imaging system. Five minutes after each cell injection, animals were imaged again to determine the total luciferase expression from the viable MSCs. In each weekly cycle, the prodrugs were administered one day post cell injection, continued for six days and stopped on the day of cell injection before the next cycle. The data are reported as mean±SD (n=10).
Results and Discussion
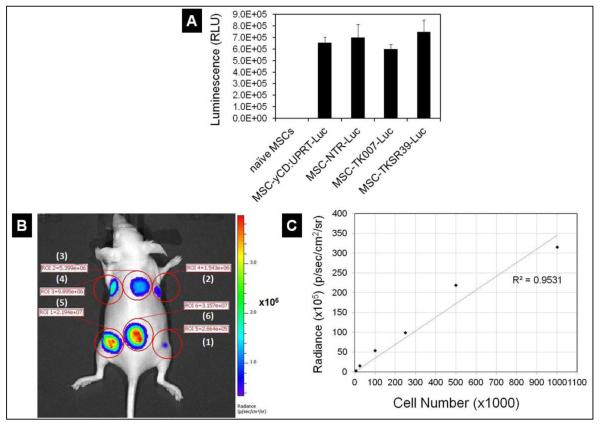
Intrinsic tumor tropism of stem cells has provided this opportunity for scientists to use them as a tool for delivery of various anticancer agents into the tumor environment [23-25]. There are two important factors that play significant roles in determining the success of stem cell mediated suicide gene therapy. One is the number of viable MSCs that come in contact with the tumor cells and the other is the potency of the drug’s bystander effect. Similar to viral and non-viral vectors which get trapped in non-tumor tissues such as liver, a significant portion of the MSCs gets trapped in lungs after IV administration and only a subpopulation of them can reach the tumors [26, 27]. Therefore, the drug’s bystander effect becomes the most prominent factor that could determine the rate of success. To compare the efficiency of the four enzyme/prodrug systems in stem cell mediated suicide gene therapy of cancer, we used a dual promoter mammalian expression vector. This was done by cloning four different suicide genes separately (i.e., TK007, TKSR39, yCD:UPRT and NTR) under an EF1α promoter and a luciferase gene under CMV promoter. The fidelity of the each sequence to the original design was verified by DNA sequencing and translation into the corresponding amino acid sequence (Supplementary Figures S1 and S2). The plasmids were then used to create stably transfected MSC-TK007-Luc, MSC-TKSR39-Luc, MSC-yCD:UPRT-Luc and MSC-NTR-Luc clones suitable for simultaneous therapy and quantitative in vivo imaging. In the next step, we determined the expression of luciferase protein in all four stably transfected clones and selected those with statistically similar luciferase expression levels. As it can be observed in Figure 1A, all four selected clones could express luciferase gene at statistically similar levels (ANOVA, p>0.05). To examine whether this level of luciferase expression is high enough to be detected in nude mice, one clone was selected (i.e., MSC-TKSR39-Luc) and various numbers of MSCs were injected subcutaneously. The results showed that the expression of luciferase was sufficient to allow us detect as low as 5,000 MSCs and there was a good correlation between the cell number and Radiance (Figure 1B and C). The ability to detect MSCs in vivo with high degree of sensitivity is important because it makes it possible to perform more accurate dose-response relationship studies.
Figure 1.
Measurement of bioluminescence intensity of luciferase expressing MSCs in vitro and in vivo. A) In vitro measurement of the bioluminescence of MSC-TK007-Luc, MSC-TKSR39-Luc, MSC-yCD: UPRT-Luc and MSC-NTR-Luc clones. B) Radiance of various numbers of subcutaneously injected MSCs in nude mouse. Numbers 1 to 6 correspond to 5000, 25,000, 100,000, 250,000, 500,000, and 1,000,000 cells, respectively. C) Plot of Cell Number versus Radiance and its correlation.
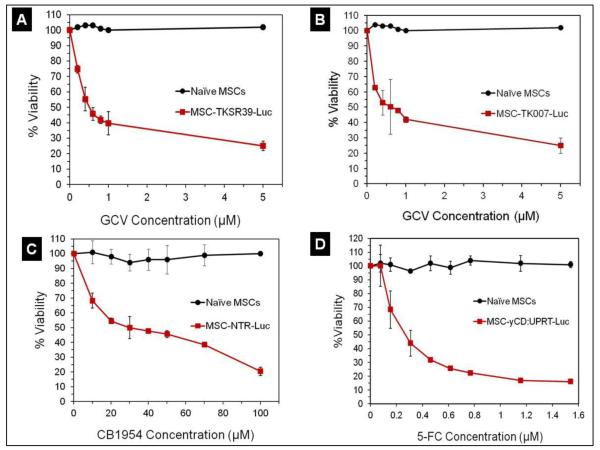
We then examined the sensitivity of the genetically modified MSCs to prodrugs using a cell toxicity assay. This was to verify that the engineered MSCs could express suicide genes at levels sufficiently high that could induce significant cell death in the presence of the corresponding prodrug. The results of this study showed that all four suicide gene expressing MSC clones were sensitive to prodrugs in a dose-dependent manner while naïve MSCs remained insensitive (Figure 2). Among all MSC clones, it was apparent that the MSC-yCD:UPRT-Luc was the most sensitive one (5-FC IC50 0.2 μM). In addition, we did not observe any significant difference between MSC-TK007-Luc and MSC-TKSR39-Luc clones in terms of sensitivity to GCV (IC50 ~0.5 μM). Overall, the results of the cytotoxicity study show that all four genetically modified MSCs were able to convert prodrugs into their cytotoxic forms which resulted in the death of suicide gene expressing MSCs. Although in many cases it may be desirable to see MSCs die as a result of prodrug conversion but it is worth noting that in some cases it may even be advantageous for the MSCs not to die and remain active so that they can produce more cytotoxic drugs in the tumor environment.
Figure 2.
Sensitivity of the MSC-TKSR39-Luc, MSC-TK007-Luc, MSC-NTR-Luc and MSC-yCD: UPRT-Luc clones to prodrugs as measured by a cell toxicity assay. Naïve unmodified MSCs which do not express any suicide gene were used as control.
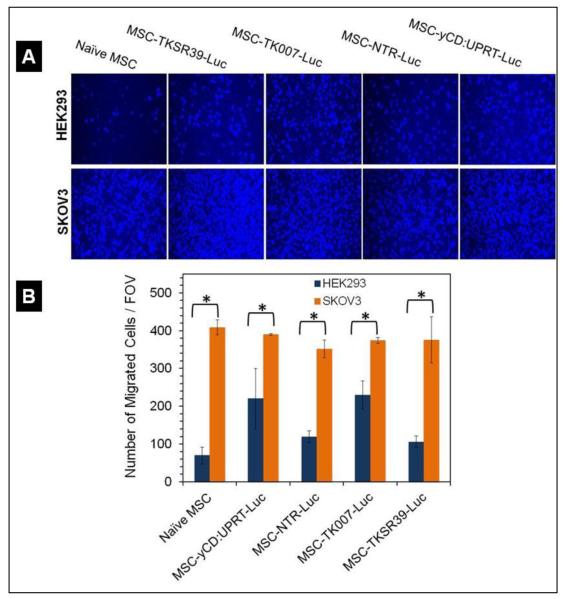
Learning that all the genetically modified MSC clones could efficiently express not only the luciferase gene but also the suicide genes, we utilized a migration assay to examine whether the genetic manipulation of MSCs affected their inherent tropism towards cancer cells. Thus, we studied the tropism of MSCs towards SKOV3 cancer cells and compared it with tropism towards HEK293 normal cells. The results of this assay demonstrated that all genetically modified MSC clones maintained their cancer tropism by migrating at significantly higher numbers towards cancer cells in comparison to HEK293 cells (t-test, p<0.05) (Figure 3). Based on published data by other groups, this observation was expected [28].
Figure 3.
Qualitative and quantitative analysis of MSCs’ cancer tropism by using Migration assay. A) Representative fluorescent images of the MSCs that migrated towards normal HEK293 and SKOV3 cancer cells. B) Quantitative analysis of the number of migrated cells per field of view (FOV) towards SKOV3 cancer cells and HEK293 cells.
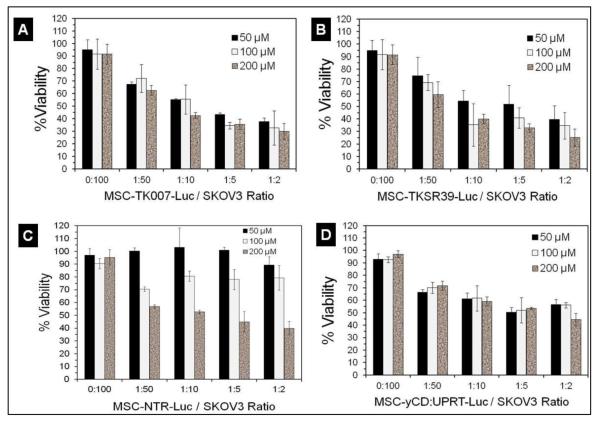
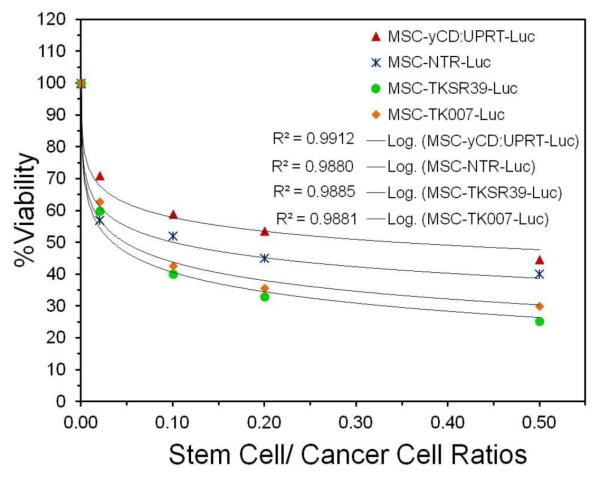
Next, we examined whether the prodrugs after conversion into their cytotoxic form inside the MSCs can diffuse out and kill neighboring cancer cells (bystander effect). For this purpose, we separately co-cultured each suicide gene expressing MSC clone with SKOV3 ovarian cancer cells at different MSC to cancer cell ratios followed by administration of prodrugs. The results of this study illustrated that all genetically modified MSCs could significantly induce cell death in SKOV3 cancer cells (Figure 4A-D).
Figure 4.
Evaluation of the bystander effect. A-D) Cancer cell killing efficiency of MSCs that were co-cultured with SKOV3 cancer cells at different MSC/cancer cell ratios and prodrug concentrations.
More specifically, we did not observe any significant increase in cancer cell killing efficiency by MSC-TKSR39-Luc, MSC-TK007-Luc and MSC-yCD:UPRT-Luc clones when prodrug concentration increased from 50 μM to 200 μM. This could be due to the fact that the IC50 of GCV and 5-FC for all these clones as shown in Figure 2 were less than 1 μM. In contrast, MSCNTR-Luc clone showed dose dependent cancer cell killing efficiency with maximum efficacy at 200 μM of prodrug. This observation correlates very well with the results in Figure 2 which showed more than 100 μM of CB1954 is needed to kill at least 70% of MSC-NTR-Luc cells. To evaluate the potency of the bystander effect with these enzyme/prodrug systems, we used the data in Figure 4 and plotted the best curve fit for all four clones when exposed to 200 μM of prodrug. Comparison of the slopes of the lines and statistical analysis of data revealed the following order of the bystander effect: TKSR39=TK007>NTR>yCD:UPRT (ANOVA followed by posthoc Tukey test, p<0.05) (Figure 5). The similarity of sensitivity to prodrug and bystander effects for MSC-TKSR39-Luc and MSC-TK007-Luc clones signifies that both suicide genes could effectively convert GCV into its charged cytotoxic form GCV-triphosphate (GCV-TP). Moreover, it indirectly suggests that gap junctions were present in between MSCs and SKOV3 cells because the bystander effect induced by GCV-TP is largely dependent on active transport via gap junctional intercellular communication (GJIC) [29]. In a mechanistic study by Matuskova et al. (2010), the formation of gap junctions between MSCs and cancer cells after co-culturing is demonstrated [6].
Figure 5.
Plot of cell viability versus cell ratios for all clones treated with 200 μM prodrug and comparison of the best curve fits.
As we mentioned in the introduction section, in previous published studies with suicide gene expressing MSCs no clear rationale is provided to justify the use of one enzyme/prodrug system over another. The data in Figure 5 which shows the high anticancer activity of TK/GCV system provides the rationale for using this system to effectively kill cancer cells at least at the in vitro level. However, this observation does not explain why the only ongoing two MSC mediated suicide gene therapy protocols in the clinic are based on CD/5-FC system and not TK/GCV or NTR/CB1954. Therefore, we continued the evaluation of these systems at the in vivo level to get a better understanding of the limiting factors.
Based on these results which showed no significant difference between MSC-TKSR39-Luc and MSC-TK007-Luc clones, we selected MSC-TKSR39-Luc as a candidate along with MSCNTR-Luc for the in vivo studies. Although MSC-yCD:UPRT-Luc clone showed lower levels of anticancer efficacy in comparison to others, but we decided to evaluate its in vivo efficacy since its bystander effect is not GJIC dependent. Therefore, MSC-TKSR39-Luc, MSC-NTR-Luc and MSC-yCD:UPRT-Luc were propagated and used to evaluate their efficacy in killing SKOV3 tumors in nude mice.
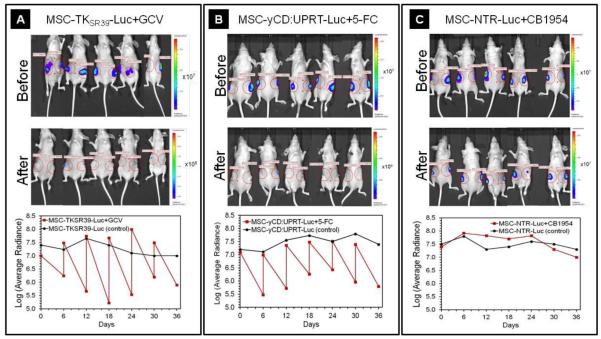
Besides the prodrug’s bystander effect the number of MSCs that come in contact with tumor cells plays a crucial role in determining the therapeutic outcome of the MSC mediated suicide gene therapy [26]. Therefore, we had to choose the most appropriate route of administration to ensure that all tumors receive equal number of MSCs. It has been shown that after each systemic administration a significant number of MSCs get cleared by pulmonary first pass effect [27]. In addition to pulmonary clearance, other factors such as tumor type and stem cell lineage and size could also impact the number of stem cells that reach tumors [30]. Therefore, to eliminate the impact of stem cell lineage/size and pulmonary first pass effect on the number of MSCs that reach tumors we injected all MSC clones intratumorally. This ensures that all tumors receive consistent and known number of MSCs. Since our data in Figure 3 show that all the genetically modified MSCs maintained their cancer tropism, therefore it is highly likely that the MSCs after intratumoral injection remain in the tumor vicinity. To validate the delivery of viable MSCs to the tumors, all mice were imaged and luciferase expression quantified immediately after receiving one million MSCs intratumorally. After treatment with prodrugs for six days, all mice were imaged again and luciferase expression quantified to examine whether the MSCs responded to prodrugs. As soon as the effect of prodrug on MSC viability was measured, the next doses of viable MSCs were injected. For each treatment group (MSC plus prodrug) we used the corresponding cell treated group (no prodrug) as a control. This was to evaluate whether MSCs can remain alive in mice and confirm that the decrease in bioluminescent signal is due to prodrug treatment and not other factors such as clearance by the mice natural killer cells. Qualitative and quantitative analysis of the luciferase signal before and after treatment with prodrugs demonstrated that the GCV and 5-FC at the administered dose could significantly kill the MSCTKSR39-Luc and MSC-yCD:UPRT-Luc clones, respectively (Figure 6A and B). However, the MSC-NTR-Luc was mildly responsive to the maximum tolerable dose of CB1954 and the luciferase signal did not change as significant as others before and after prodrug administration (Figure 6C). It is worth noting that the bioluminescent signal did not increase overtime in the group treated with MSC-NTR-Luc plus CB1954 suggesting that a portion of the MSCs were responding to prodrug treatment although it was not sufficient to cause dramatic change in signal strengths similar to other groups. Unfortunately, we could not exceed the 20 mg/Kg/day for CB1954 because at higher concentrations mice showed significant hepatotoxicity and started to lose weight. Dose-dependent hepatotoxicity is one of the major side effects of CB1954 which has limited its use [31].
Figure 6.
Evaluation of luciferase expression before and after prodrug treatment. A-C) Representative images of mice before (top panels) and after (mid panels) receiving the genetically modified MSCs plus prodrugs. The bottom panels show the quantitative analysis of luciferase expression over the 36 days treatment period. This figure shows that after each intratumoral MSC injection, the luciferase signal significantly increased. It also shows that in each 6 day prodrug treatment (GCV and 5-FC) cycle, the luciferase signal in MSCs significantly dropped.
It is well-documented that CB1954 is a potent DNA chelating agent which can freely diffuse to surrounding cells and trigger extensive DNA damage and P53-independent apoptosis in both replicating and non-replicating cells [32]. However, it has also been reported that the conversion rate of CB1954 by NTR is somewhat low as CB1954 is not the natural substrate of NTR [20]. Therefore, the fact that we didn’t observe significant reduction in luciferase signal before and after treatment with CB1954 in MSC-NTR-Luc treated mice was explicable. As a result of this observation, we reduced the number of injected MSC-NTR-Luc to 500,000 cells after day 12 because the majority of the injected MSC-NTR-Luc cells from previous dose were still alive. Nonetheless, we continued the treatment of all mice for 36 days until the size of tumors in control groups exceeded the 1000mm3 limits. At this point, the tumor sizes were big enough to interfere with the mice natural movements causing distress. Therefore, mice were euthanized at this point and the study was stopped on day 36.
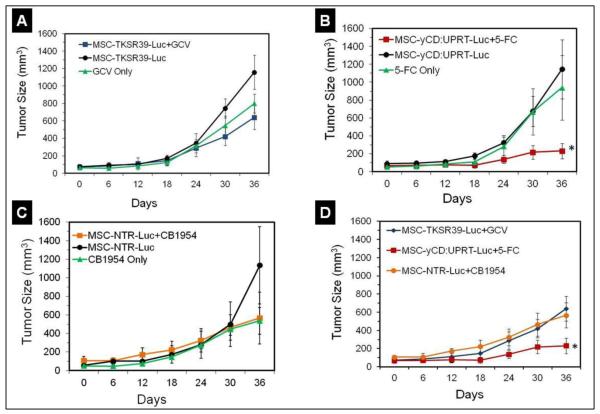
The tumor measurement studies during the period of treatment showed that in comparison to the control groups, mice treated with MSC-TKSR39-Luc plus GCV or MSC-NTR-Luc plus CB1954 did not respond to therapy and the tumor size growth did not get affected significantly (Repeated measure analysis, p>0.05) (Figure 7A and C). Based on the data in Figure 6, the lack of response to therapy with MSC-NTR-Luc plus CB1954 was expected since the conversion rate of CB1954 into its cytotoxic form was not high enough to induce significant MSC death. The fact that we did not observe any significant difference between groups treated with MSC-TKSR39-Luc plus GCV or GCV alone indicates that significantly higher numbers of MSCs are required to come in contact and cause considerable damage to cancer cells in a fast growing tumor model. It appears that to slow down the growth of a tumor that has surpassed the 200mm3 volume, administration of more than 1 million MSCs per week is necessary. This is sensible as the efficacy of GCV-TP is entirely dependent on GJIC and in tumors gap junctions in between cancer cells are either highly compromised or in many cases non-existent [29, 33]. Literature search also shows that TK/GCV enzyme/prodrug therapy has been effective when tumors were small (<100mm3) and treated early [34, 35]. Most importantly, these results could also explain why despite significant success at the preclinical level [6, 7, 36], no MSC mediated suicide gene therapy protocol with TK/GCV system has reached the clinic. This is also consistent with the results of virus-based suicide gene therapy protocols with TK/GCV system which passed Phase I clinical trials (safety) but later failed at efficacy [13, 37].
Figure 7.
Evaluation of tumor size growth in mice treated with suicide gene expressing MSCs and corresponding prodrugs. A) Tumor size growth in mice treated with MSC-TKSR39-Luc cells plus GCV and associated control groups. B) Tumor size growth in mice treated with MSC-yCD:UPRT-Luc cells plus 5-FC and associated control groups. C) Tumor size growth in mice treated with MSC-NTR-Luc cells plus CB1954 and associated control groups. D) Comparison of tumor size growth in mice treated with suicide gene expressing MSCs plus prodrugs and statistical difference among groups.
In contrast to TK/GCV system, mice that were treated with 1 million per week MSC-yCD:UPRT-Luc plus daily administration of 5-FC responded well to therapy and the tumor growth could be inhibited significantly (Repeated measure analysis, *p<0.05) (Figure 7B and D). This response could be attributed to two important factors; one is GJIC-independent diffusion of 5-FU which significantly enhances drug’s bystander effect and the other is the efficiency of yCD:UPRT enzyme in converting 5-FC to 5-FU. Here, we administered one million MSCs to each tumor once a week which could inhibit tumor growth but not eradicate completely. It is worth mentioning that at low concentrations 5-FU has been shown to kill mostly dividing cancer cells; however, at high concentrations it could kill even non-dividing cancer cells through disruption of mRNA processing and protein synthesis [38]. Therefore, a combination of enzyme efficiency and prodrug’s physicochemical properties has been responsible for effective killing of the tumor cells. To achieve complete tumor remission, either injection of higher doses of MSCs or use of other enzyme/prodrug systems with better bystander effect may be needed. The fact that the only MSC mediated suicide gene therapy protocols that have reached clinical trials are based on CD/5-FC system (NCT02015819 and NCT01172964), points at the potentially higher efficacy of this system over others and in line with our findings. Since there are several other enzyme/prodrug systems also available, use of theranostic MSCs for preclinical screening could be expanded to compare the anticancer efficacy of yCD:UPRT/5-FC with other potentially effective systems such as carboxylesterase/irinotecan. For preclinical screening, it is important to choose a cell line that is sensitive to all the enzyme/prodrug systems of interest so that the outcome is not biased. The sensitivity test as shown in Figures 4 and 5 ensures the chosen cell line is suitable for screening process and identification of a system that can cause maximum damage to cancer cells. Non-sensitive cell lines (resistant) can be used in cases where enzyme/prodrug systems are being screened to find one that can generate sufficiently high concentrations of activated prodrug to overcome resistance. For example, non-dividing breast cancer cells are shown to be resistant to therapy with low concentrations of 5-FU but responsive when the 5-FU concentration is high [38]. Therefore, various yCD mutants can be screened through use of theranostic MSCs as shown in this study to find one that can generate high concentrations of 5-FU and overcome resistance.
Conclusions
Success of stem cell mediated suicide gene therapy of cancer is dependent to a large extent on the conversion rate of the prodrug into its cytotoxic form and also the bystander effect. Among the enzyme/prodrug systems tested in this study, our quantitative imaging and tumor size measurement studies show that yCD:UPRT/5-FC is the most effective system. Our findings also show that before choosing an enzyme/prodrug system for cancer therapy, important factors such as prodrug’s conversion rate and bystander effect and limitations imposed by tumor pathophysiology (e.g., absence of gap junctions) need to be carefully considered.
Since this study provides evidence that genetically modified MSCs can be used as a means for side-by-side evaluation of the efficacy of enzyme/prodrug systems, it may open the door to utilization of this approach for screening a large number of enzyme mutants or prodrugs with different levels of bystander effects at the preclinical level. Given that in the clinic the suicide gene expressing MSCs are going to be injected intravenously where a significant portion of the cells may be lost, such studies at the preclinical level would help identify the most effective enzyme/prodrug systems before moving into costly clinical trials. Regardless of vector types used (viral, non-viral or cell based), these findings could help scientists in the field of suicide gene therapy of cancer make more informed decisions when choosing enzyme/prodrug systems and effectively expand this field of study.
Supplementary Material
Acknowledgements
This study was supported in part by a grant from the National Institutes of Health/ National Institute of Biomedical Imaging and Bioengineering (R21EB016792). This work was also made possible by the Animal Imaging and Housing Core Facility in the Center for Cancer Prevention Research at the School of Pharmacy, Rutgers University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yang J, Lam DH, Goh SS, Lee EX, Zhao Y, Tay FC, Chen C, Du S, Balasundaram G, Shahbazi M, Tham CK, Ng WH, Toh HC, Wang S. Tumor tropism of intravenously injected human-induced pluripotent stem cell-derived neural stem cells and their gene therapy application in a metastatic breast cancer model. Stem Cells. 2012;30:1021–1029. doi: 10.1002/stem.1051. [DOI] [PubMed] [Google Scholar]
- [2].Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, Lin RJ, Yang DM, Chang CW, Chen WH, Wei HJ, Gelovani JG. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- [3].Aboody KS, Najbauer J, Metz MZ, D’Apuzzo M, Gutova M, Annala AJ, Synold TW, Couture LA, Blanchard S, Moats RA, Garcia E, Aramburo S, Valenzuela VV, Frank RT, Barish ME, Brown CE, Kim SU, Badie B, Portnow J. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5:184ra159. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z, Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther. 2010;18:223–231. doi: 10.1038/mt.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- [6].Matuskova M, Hlubinova K, Pastorakova A, Hunakova L, Altanerova V, Altaner C, Kucerova L. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010;290:58–67. doi: 10.1016/j.canlet.2009.08.028. [DOI] [PubMed] [Google Scholar]
- [7].Song C, Xiang J, Tang J, Hirst DG, Zhou J, Chan KM, Li G. Thymidine kinase gene modified bone marrow mesenchymal stem cells as vehicles for antitumor therapy. Hum Gene Ther. 2011;22:439–449. doi: 10.1089/hum.2010.116. [DOI] [PubMed] [Google Scholar]
- [8].Kucerova L, Matuskova M, Pastorakova A, Tyciakova S, Jakubikova J, Bohovic R, Altanerova V, Altaner C. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med. 2008;10:1071–1082. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- [9].Altaner C, Altanerova V, Cihova M, Ondicova K, Rychly B, Baciak L, Mravec B. Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int J Cancer. 2014;134:1458–1465. doi: 10.1002/ijc.28455. [DOI] [PubMed] [Google Scholar]
- [10].Gu C, Li S, Tokuyama T, Yokota N, Namba H. Therapeutic effect of genetically engineered mesenchymal stem cells in rat experimental leptomeningeal glioma model. Cancer lett. 2010;291:256–262. doi: 10.1016/j.canlet.2009.10.020. [DOI] [PubMed] [Google Scholar]
- [11].Kosaka H, Ichikawa T, Kurozumi K, Kambara H, Inoue S, Maruo T, Nakamura K, Hamada H, Date I. Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther. 2012;19:572–578. doi: 10.1038/cgt.2012.35. [DOI] [PubMed] [Google Scholar]
- [12].Yi BR, Hwang KA, Aboody KS, Jeung EB, Kim SU, Choi KC. Selective antitumor effect of neural stem cells expressing cytosine deaminase and interferon-beta against ductal breast cancer cells in cellular and xenograft models. Stem Cell Res. 2014;12:36–48. doi: 10.1016/j.scr.2013.09.010. [DOI] [PubMed] [Google Scholar]
- [13].Karjoo Z, Ganapathy V, Hatefi A. Gene Directed Enzyme Prodrug Cancer Therapy. In: Lattime EC, Gerson SL, editors. Gene Therapy of Cancer. Elsevier Inc.; 2013. pp. 77–91. [Google Scholar]
- [14].Wang Y, Canine BF, Hatefi A. HSV-TK/GCV cancer suicide gene therapy by a designed recombinant multifunctional vector. Nanomedicine. 2011;7:193–200. doi: 10.1016/j.nano.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim KY, Kim SU, Leung PC, Jeung EB, Choi KC. Influence of the prodrugs 5-fluorocytosine and CPT-11 on ovarian cancer cells using genetically engineered stem cells: tumor-tropic potential and inhibition of ovarian cancer cell growth. Cancer science. 2010;101:955–962. doi: 10.1111/j.1349-7006.2009.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].White CL, Menghistu T, Twigger KR, Searle PF, Bhide SA, Vile RG, Melcher AA, Pandha HS, Harrington KJ. Escherichia coli nitroreductase plus CB1954 enhances the effect of radiotherapy in vitro and in vivo. Gene therapy. 2008;15:424–433. doi: 10.1038/sj.gt.3303081. [DOI] [PubMed] [Google Scholar]
- [17].Black ME, Kokoris MS, Sabo P. Herpes simplex virus-1 thymidine kinase mutants created by semi-random sequence mutagenesis improve prodrug-mediated tumor cell killing. Cancer Res. 2001;61:3022–3026. [PubMed] [Google Scholar]
- [18].Preuss E, Muik A, Weber K, Otte J, von Laer D, Fehse B. Cancer suicide gene therapy with TK.007: superior killing efficiency and bystander effect. J Mol Med (Berl) 2011;89:1113–1124. doi: 10.1007/s00109-011-0777-8. [DOI] [PubMed] [Google Scholar]
- [19].Grohmann M, Paulmann N, Fleischhauer S, Vowinckel J, Priller J, Walther DJ. A mammalianized synthetic nitroreductase gene for high-level expression. BMC Cancer. 2009;9:301. doi: 10.1186/1471-2407-9-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Prosser GA, Copp JN, Syddall SP, Williams EM, Smaill JB, Wilson WR, Patterson AV, Ackerley DF. Discovery and evaluation of Escherichia coli nitroreductases that activate the anti-cancer prodrug CB1954. Biochem Pharmacol. 2010;79:678–687. doi: 10.1016/j.bcp.2009.10.008. [DOI] [PubMed] [Google Scholar]
- [21].Shi DZ, Hu WX, Li LX, Chen G, Wei D, Gu PY. Pharmacokinetics and the bystander effect in CD::UPRT/5-FC bi-gene therapy of glioma. Chin Med J (Engl) 2009;122:1267–1272. [PubMed] [Google Scholar]
- [22].Richard C, Duivenvoorden W, Bourbeau D, Massie B, Roa W, Yau J, Th’ng J. Sensitivity of 5-fluorouracil-resistant cancer cells to adenovirus suicide gene therapy. Cancer Gene Ther. 2007;14:57–65. doi: 10.1038/sj.cgt.7700980. [DOI] [PubMed] [Google Scholar]
- [23].Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, Ponnazhagan S. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008;15:1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moniri MR, Sun XY, Rayat J, Dai D, Ao Z, He Z, Verchere CB, Dai LJ, Warnock GL. TRAIL-engineered pancreas-derived mesenchymal stem cells: characterization and cytotoxic effects on pancreatic cancer cells. Cancer Gene Ther. 2012;19:652–658. doi: 10.1038/cgt.2012.46. [DOI] [PubMed] [Google Scholar]
- [25].Khan Z, Knecht W, Willer M, Rozpedowska E, Kristoffersen P, Clausen AR, Munch-Petersen B, Almqvist PM, Gojkovic Z, Piskur J, Ekstrom TJ. Plant thymidine kinase 1: a novel efficient suicide gene for malignant glioma therapy. Neuro Oncol. 2010;12:549–558. doi: 10.1093/neuonc/nop067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luetzkendorf J, Mueller LP, Mueller T, Caysa H, Nerger K, Schmoll HJ. Growth inhibition of colorectal carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem cells requires their substantial intratumoral presence. J Cell Mol Med. 2010;14:2292–2304. doi: 10.1111/j.1582-4934.2009.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pendleton C, Li Q, Chesler DA, Yuan K, Guerrero-Cazares H, Quinones-Hinojosa A. Mesenchymal stem cells derived from adipose tissue vs bone marrow: in vitro comparison of their tropism towards gliomas. PLoS ONE. 2013;8:e58198. doi: 10.1371/journal.pone.0058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xiao J, Zhang G, Qiu P, Liu X, Wu Y, Du B, Li J, Zhou J, Tan Y. Tanshinone IIA increases the bystander effect of herpes simplex virus thymidine kinase/ganciclovir gene therapy via enhanced gap junctional intercellular communication. PLoS ONE. 2013;8:e67662. doi: 10.1371/journal.pone.0067662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Belmar-Lopez C, Mendoza G, Oberg D, Burnet J, Simon C, Cervello I, Iglesias M, Ramirez JC, Lopez-Larrubia P, Quintanilla M, Martin-Duque P. Tissue-derived mesenchymal stromal cells used as vehicles for anti-tumor therapy exert different in vivo effects on migration capacity and tumor growth. BMC Med. 2013;11:139. doi: 10.1186/1741-7015-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tang MH, Helsby NA, Goldthorpe MA, Thompson KM, Al-Ali S, Tingle MD. Hepatic nitroreduction, toxicity and toxicokinetics of the anti-tumour prodrug CB 1954 in mouse and rat. Toxicology. 2007;240:70–85. doi: 10.1016/j.tox.2007.07.018. [DOI] [PubMed] [Google Scholar]
- [32].Green LK, Storey MA, Williams EM, Patterson AV, Smaill JB, Copp JN, Ackerley DF. The Flavin Reductase MsuE Is a Novel Nitroreductase that Can Efficiently Activate Two Promising Next-Generation Prodrugs for Gene-Directed Enzyme Prodrug Therapy. Cancers (Basel) 2013;5:985–997. doi: 10.3390/cancers5030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nicholas TW, Read SB, Burrows FJ. Suicide gene therapy with Herpes simplex virus thymidine kinase and ganciclovir is enhanced with connexins to improve gap junctions and bystander effects. Histol Histopathol. 2003;18:495–507. doi: 10.14670/HH-18.495. [DOI] [PubMed] [Google Scholar]
- [34].Wiewrodt R, Amin K, Kiefer M, Jovanovic VP, Kapoor V, Force S, Chang M, Lanuti M, Black ME, Kaiser LR, Albelda SM. Adenovirus-mediated gene transfer of enhanced Herpes simplex virus thymidine kinase mutants improves prodrug-mediated tumor cell killing. Cancer Gene Ther. 2003;10:353–364. doi: 10.1038/sj.cgt.7700589. [DOI] [PubMed] [Google Scholar]
- [35].Ardiani A, Sanchez-Bonilla M, Black ME. Fusion enzymes containing HSV-1 thymidine kinase mutants and guanylate kinase enhance prodrug sensitivity in vitro and in vivo. Cancer gene therapy. 2010;17:86–96. doi: 10.1038/cgt.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rath P, Shi H, Maruniak JA, Litofsky NS, Maria BL, Kirk MD. Stem cells as vectors to deliver HSV/tk gene therapy for malignant gliomas. Current stem cell research & therapy. 2009;4:44–49. doi: 10.2174/157488809787169138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van Putten EH, Dirven CM, van den Bent MJ, Lamfers ML. Sitimagene ceradenovec: a gene-based drug for the treatment of operable high-grade glioma. Future Oncol. 2010;6:1691–1710. doi: 10.2217/fon.10.134. [DOI] [PubMed] [Google Scholar]
- [38].Garcia-Sanchez F, Pizzorno G, Fu SQ, Nanakorn T, Krause DS, Liang J, Adams E, Leffert JJ, Yin LH, Cooperberg MR, Hanania E, Wang WL, Won JH, Peng XY, Cote R, Brown R, Burtness B, Giles R, Crystal R, Deisseroth AB. Cytosine deaminase adenoviral vector and 5-fluorocytosine selectively reduce breast cancer cells 1 million-fold when they contaminate hematopoietic cells: a potential purging method for autologous transplantation. Blood. 1998;92:672–682. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.