Abstract
Background
Evaluation of candidates for living kidney donation relies on screening for individual risk factors for end-stage renal disease (ESRD). To support an empirical approach to donor selection, we developed a tool that simultaneously incorporates multiple health characteristics to estimate a person’s likely long-term risk of ESRD in the absence of donation.
Methods
We used meta-analyzed risk associations from 7 general population cohorts, calibrated to US population-level incidence of ESRD and mortality, to project the estimated long-term incidence of ESRD in the absence of donation according to 10 demographic and health characteristics. We then compared 15-year projections to observed risk among recent US living kidney donors (N=52,998).
Results
There were 4,933,314 participants followed a median of 4 to 16 years. For a 40-year-old person with health characteristics similar to age-matched kidney donors, the 15-year ESRD risk projections in the absence of donation varied by race and sex: 0.24%, 0.15%, 0.06%, and 0.04% in black men, black women, white men, and white women. Risk projections were higher in the presence of lower estimated glomerular filtration rate, higher albuminuria, hypertension, smoking, diabetes, and obesity. In the model-based lifetime projections, ESRD risk was highest at younger age, particularly among African Americans. Risk projections in the absence of donation were 3.5–5.3-fold lower than 15-year observed risk post-donation in US kidney donors.
Conclusions
We suggest multiple health characteristics be considered together to estimate long-term ESRD risk for living kidney donor candidates.
Nearly 30,000 people worldwide become living kidney donors each year.1–3 Traditionally, living donors have been selected, based on the absence of risk factors for poor post-donation outcomes, and without a comprehensive assessment of individualized long-term risk. Although considered safe in healthy, low-risk persons, kidney donation has lifelong implications, and the most direct effect may be a higher long-term risk of end-stage renal disease (ESRD).4–7 A tool to predict a donor candidate’s long-term risk of developing ESRD, based on the combined impact of multiple pre-donation demographic and health characteristics, could help make criteria by which a potential kidney donor is accepted or declined more empiric and transparent.
In the absence of a robust epidemiologic framework for long-term risk assessment, acceptance criteria for living kidney donation have varied widely across transplant centers.8–10 There is controversy over whether donor candidates with certain health characteristics such as older age or hypertension should be accepted for kidney donation. Some transplant centers use more stringent criteria for younger donors compared to middle-aged donors, given the long post-donation life expectancy during which complications may develop.11 Race is also a consideration when evaluating donor candidates, since ESRD risk is higher in black compared to white persons in both the general US and donor populations.2,5,12–14
We developed an online risk tool to help evaluate, counsel, and accept living kidney donor candidates (www.transplantmodels.com/esrdrisk). Using population-based data, we derived equations that quantify the combined effect of 10 routinely available demographic and health characteristics to estimate a kidney donor candidate’s chance of developing ESRD over a 15-year time horizon. These estimates do not incorporate any added risk attributable to kidney donation; kidney donation likely increases ESRD risk, but the increase in risk according to pre-donation characteristics is difficult to quantify reliably with existing data.15–17 We compared risk projections to the observed 15-year incidence of ESRD in recent living kidney donors, hypothesizing that ESRD incidence in the presence of donation would be at least 4-fold higher than projections in the absence of donation, given recent reports.5,6 Because many kidney donors are young, we also projected lifetime risk of ESRD, with the caveat that these lifetime estimates lack precision and were based on relatively short follow-up data.
METHODS
We developed risk equations to estimate the long-term incidence of ESRD in the absence of kidney donation according to a person’s baseline demographic and health characteristics. Data sources included the annual incidence of ESRD for the overall US population, and associations of health characteristics with ESRD in 7 general population studies (Supplementary Appendix, Appendix 1).
Study Design and Oversight
MG, ASL, KM, JC, DLS, BLK, KLL, and AXG conceived of the study concept and design. The CKD Prognosis Consortium (CKD-PCC) Data Coordinating Center (DCC; MG, YS, KM, SB, JC) and the CKD-PC investigators/collaborators listed in the Supplementary Appendix materials acquired the data. The DCC members analyzed the data. MG and JC had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis and all authors had final responsibility for the decision to submit for publication, informed by discussions with collaborators. MG, YS, ASL, KM, SB, JC, KLL, and AXG drafted the manuscript, and ARC, EKC, BLK, CPK, GNN, VS, and DLS provided critical revisions of the manuscript. All collaborators shared data and were given the opportunity to comment on the manuscript. JC obtained funding for CKD-PC and individual cohort and collaborator support is listed in the Supplementary Appendix.
Incidence of ESRD in the US Population
The annual incidence of ESRD, defined as receipt of chronic dialysis or a kidney transplant, was previously estimated for the US population within categories of age, sex, and race.14 These estimates were derived using actual ESRD incidence and mortality collected by the US Renal Data System, and overall mortality rates from the US Census (Supplementary Appendix, Appendix 2);18 annual rates were compounded to determine absolute risk over the desired time horizon. We partitioned the population incidence of ESRD into high-risk (ineligible for kidney donation) and low-risk (potentially eligible for kidney donation) subgroups, with the latter defined to exclude persons with ≥1 absolute contraindication to kidney donation: estimated glomerular filtration rate (eGFR) <45 ml/min/1.73 m2, insulin-dependent diabetes mellitus, the use of ≥4 anti-hypertensive medications, systolic/diastolic blood pressure ≥160/90 mmHg with medication or ≥170/100 mmHg without medication, random urine albumin-to-creatinine ratio ≥300 mg/g, or a history of coronary heart disease, stroke, congestive heart failure, or peripheral arterial disease (Supplementary Appendix, Table S1).
Associations of Individual Health Characteristics with ESRD
We quantified the associations of health characteristics and incident ESRD in the low-risk subgroups of 7 general population cohorts assembled by the CKD-PC19: follow-up of Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994), the Atherosclerosis Risk in Communities Study, Geisinger Health System, Maccabi Health System, Veterans Administration (VA) Health System, Mount Sinai BioMe cohort, and the Institute for Clinical Evaluative Sciences Ontario Kidney, Dialysis and Transplantation Program. To ensure model stability, cohorts were required to have at least 20 ESRD events in the low-risk subgroup. Multiple-imputation was used for missing health characteristic data. Missing data ranged from <1% for all variables in the ARIC cohort to >90% for measures of albuminuria in the cohorts derived from electronic health record data (Supplementary Appendix, Table S2). Coefficients based on data imputed >20% were not used in meta-analysis.
We considered 13 distinct demographic and health characteristics: age, race, sex, eGFR, urine albumin-creatinine ratio, systolic blood pressure, non-insulin dependent diabetes mellitus, anti-hypertensive medication use, smoking status, body mass index (BMI), total cholesterol and low-density lipoprotein (LDL) cholesterol, and a history of kidney stones. Total cholesterol, LDL cholesterol, and history of kidney stones were not statistically significant and thus were excluded from the final model. All models were adjusted for an age-race interaction.
Risk associations were estimated using multivariable Cox proportional hazards models individually in each cohort and then combined using random-effects meta-analysis. The discrimination of meta-analyzed coefficients was evaluated in development cohorts (Supplementary Appendix, Table S3).
Estimating the Long-Term Incidence of ESRD for the Base-Case Scenario
We applied the meta-analyzed coefficients to the low-risk subgroups of NHANES III and continuous NHANES (1999–2010) using sample weights as per analytic guidelines.20 A base-case scenario was defined using average health characteristics of the recent US donor pool: systolic blood pressure 120 mmHg, urine albumin-creatinine ratio 4 mg/g, BMI 26 kg/m2, no smoking, no diabetes or use of anti-hypertensive medications5 (which were fairly uniform in donors, irrespective of age), and an age-specific eGFR (Supplementary Appendix, Appendix 3). The linear function for each participant was centered on that of the base-case scenario within each category of age (10-year increments), sex, and race.21 We calibrated this risk to the estimated ESRD incidence in the low-risk population over a given time period (15-year and lifetime) by dividing the overall estimate by the product-sum of the prevalence of each low-risk participant’s health profile and the exponentiated linear function (Supplementary Appendix, Appendix 4).
Projected Risk Distribution in Recent Donor Populations
We applied the risk equations to 57,508 living kidney donors assembled from the US Organ Procurement and Transplantation Network (OPTN) between January 1, 2005, and July 2, 2014. Donors missing pre-donation serum creatinine or systolic blood pressure were excluded (N=4,510). Albumin-creatinine ratio was imputed as 4 mg/g for those with “negative,” “not done,” or “unknown” urinalysis, and 30 mg/g for those with “positive” urinalysis. Smoking status was imputed as former smoker if “history of cigarette use” or “other tobacco used” was reported. Missing BMI (2.5%), diabetes mellitus (1.7%), and anti-hypertensive medication use (97.5%) were imputed as 26 kg/m2, no diabetes, and no anti-hypertensive medication use, respectively.
Comparison of Projected to Observed Risks and Sensitivity Analyses
We compared recently published 15-year ESRD risk in kidney donors5 with the projected risk in the absence of donation for the average donor and assessed the relative risk. We conducted various sensitivity analyses – first, varying by ±33% the estimated proportion of events occurring in the low-risk subgroup, and second, projecting long-term ESRD risk using coefficients derived from literature review.22,23 Because our meta-analyzed coefficients were similar to those previously published for all variables except BMI, the latter analyses focused on BMI. All analyses were done in Stata/MP 13.1 (College Station, TX).
RESULTS
Baseline Characteristics
Overall, there were 8,325,115 participants in the 7 cohorts, of whom 4,933,314 had no health conditions deemed absolute contraindications to kidney donation. There were 3,900 ESRD events over 31,321,064 person-years of follow-up in this subgroup; median cohort follow-up ranged from 4 to 16 years (Table 1). The average age at cohort entry ranged from 40 to 63 years. The proportion of women ranged from 9.3% in the VA cohort to 52–60% in the remaining cohorts.
Table 1.
Characteristics of the Low-Risk Subgroups of General Population Cohorts*
| Cohort | NHANES | ARIC | VA** | ICES KDT*** | Maccabi | Mount Sinai | Geisinger |
|---|---|---|---|---|---|---|---|
| Country | US | US | US | Canada | Israel | US | US |
| Lower-Risk* Population, N (% of total cohort) | 8775 (81) | 8155 (72) | 1362620 (52) | 2120427 (52) | 1149058 (90) | 8844 (73) | 275435 (80) |
| ESRD events, N (%) | 38 (0.43) | 81 (1.0) | 845 (0.062) | 1146 (0.054) | 1355 (0.12) | 69 (0.78) | 366 (0.13) |
| Follow-up, median years [IQI] | 16 [14–18] | 14 [13–15] | 6 [6–7] | 6 [3–8] | 8 [4–11] | 4 [2–6] | 8 [4–12] |
| Age, mean years (SD) | 41 (16) | 63 (6) | 56 (15) | 40 (15) | 40 (15) | 49 (15) | 46 (17) |
| Age distribution, % | |||||||
| Age 18–24 years | 16 | 0 | 3 | 17 | 16 | 5 | 12 |
| Age 25–34 years | 25 | 0 | 8 | 22 | 24 | 15 | 17 |
| Age 35–44 years | 23 | 0 | 13 | 25 | 25 | 18 | 21 |
| Age 45–54 years | 14 | 6 | 23 | 21 | 18 | 24 | 21 |
| Age 55–64 years | 11 | 57 | 29 | 10 | 10 | 22 | 15 |
| Age 65–74 years | 8 | 36 | 15 | 4 | 5 | 11 | 10 |
| Age 75–84 years | 3 | 0 | 10 | 1 | 2 | 3 | 4 |
| Women, % | 52 | 57 | 9 | 58 | 56 | 60 | 59 |
| Black, % | 12 | 20 | 18 | NA**** | 0 | 46 | 2 |
| eGFR, mean ml/min/1.73 m2 (SD) | 102 (19) | 88 (14) | 87 (16) | 103 (18) | 95 (21) | 92 (22) | 97 (20) |
| Urine ACR, median mg/g [IQI] | 6 [4–9] | 3 [2–7] | 5 [3–11] | NA | 0 [0–12] | 7 [3–22] | 12 [5–29] |
| SBP, mean mmHg (SD) | 119 (14) | 124 (16) | 130 (14) | NA | 119 (14) | 123 (14) | 124 (14) |
| Anti-hypertensive drug use, % | 17 | 33 | 23 | NA | 6 | 15 | 12 |
| Non-insulin dependent DM, % | 8 | 11 | NA | NA | 8 | 11 | 7 |
| BMI, mean kg/m2 (SD) | 26 (5) | 28 (5) | 28 (5) | NA | 26 (5) | 28 (9) | 30 (7) |
| BMI>30 kg/m2, % | 20 | 32 | 32 | NA | 18 | 30 | 41 |
| BMI>35 kg/m2, % | 8 | 11 | 10 | NA | 6 | 14 | 19 |
| Current smoker, % | 25 | 15 | NA | NA | 23 | 14 | 25 |
| Former smoker, % | 35 | 42 | NA | NA | 2 | 18 | 22 |
Abbreviations: ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; ACR, albumin-to-creatinine ratio; SBP, systolic blood pressure; DM, diabetes mellitus; BMI, body mass index; NA, not available; IQI, interquartile interval (25th percentile – 75th percentile); SD, standard deviation; ARIC, Atherosclerosis Risk in Communities Study; Geisinger, Geisinger Health System; Maccabi, Maccabi Health System; Mt Sinai, Mount Sinai BioMe Biobank Platform; NHANES, Third US National Health and Nutrition Examination Survey; ICES KDT, Ontario Institute for Clinical Evaluative Sciences, Provincial Kidney, Dialysis and Transplantation program; VA, Veterans Administration Study
To convert urine ACR from mg/g to mg/mmol divide by 8.84.
The low-risk subgroup excludes persons with eGFR <45 ml/min/1.73 m2, insulin-dependent diabetes mellitus, the use of 4 or more antihypertensive medications, systolic blood pressure ≥160/90 mmHg on medication or ≥170/100 mmHg off medication, random urine albumin-to-creatinine ratio (ACR) ≥300 mg/g, and a history of coronary heart disease, stroke, congestive heart failure, or peripheral arterial disease.
No information on insulin use; thus, lower risk excludes all persons with diabetes
No information on systolic blood pressure or insulin use; thus, lower risk excludes all persons with diabetes and hypertension
Race was not available, but it is estimated that approximately 3% of the Ontario population are black persons.
Associations of Health Characteristics with ESRD
There was a graded association between an eGFR below 90 ml/min/1.73 m2 and a higher risk of ESRD; above eGFR 90 ml/min/1.73 m2, there was no significant association (Table 2). Other characteristics associated with a higher risk of ESRD included non-insulin dependent diabetes (adjusted hazard ratio [HR] 3.01, 95% CI: 1.91–4.74), higher systolic blood pressure (HR 1.42 per 20 mmHg, 95% CI: 1.27–1.58), anti-hypertensive drug use (HR 1.35, 95% CI: 1.01–1.82), former smoking (HR 1.45, 95% CI: 1.23–1.71), current smoking (HR 1.76, 95% CI: 1.29–2.41), and higher urine albumin-creatinine ratio (HR 2.94 per 10-fold change, 95% CI: 0.99–8.75). There was a relatively weak association between BMI and ESRD risk, with a small graded association above 30 kg/m2 (HR 1.16 per 5 kg/m2, 95% CI: 1.04–1.29).
Table 2.
Meta-Analysis of Multivariable-Adjusted Hazard Ratios Estimating the Association of Baseline Characteristics with ESRD*
| Health characteristics |
Meta- analysis HR (95% CI) |
Meta- analysis beta (SE) |
Cohorts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NHANES | ARIC | VA | ICES KDT | Maccabi | Mount Sinai | Geisinger | |||
| eGFR<60, per −15 ml/min/1.73 m2 | 6.61 (4.87, 8.96) | 1.89 (0.16) | 12.82 (0.35, 463.68) | 6.66 (1.85, 23.97) | NA | 10.47 (6.75, 16.24) | 6.00 (4.74, 7.60) | 2.47 (0.64, 9.55) | 5.50 (3.25, 9.30) |
| eGFR 60–89, per −15 ml/min/1.73 m2 | 1.63 (1.53, 1.74) | 0.49 (0.03) | 1.05 (0.33, 3.36) | 1.51 (1.01, 2.25) | 1.50 (1.32, 1.70) | 1.59 (1.39, 1.82) | 1.72 (1.54, 1.93) | 1.65 (1.03, 2.64) | 1.85 (1.51, 2.26) |
| eGFR 90–119, per −15 ml/min/1.73 m2 | 1.02 (0.85, 1.23) | 0.02 (0.09) | 0.83 (0.32, 2.14) | 1.67 (0.87, 3.20) | 0.98 (0.81, 1.17) | 0.77 (0.68, 0.88) | 0.96 (0.81, 1.15) | 1.35 (0.81, 2.27) | 1.27 (0.99, 1.62) |
| eGFR ≥120, per −15 ml/min/1.73 m2 | 0.79 (0.56, 1.10) | −0.24 (0.17) | 1.18 (0.47, 2.94) | NA | 0.50 (0.34, 0.72) | 1.62 (1.04, 2.52) | 0.72 (0.52, 1.00) | 0.82 (0.45, 1.47) | 0.59 (0.47, 0.75) |
| SBP, per 20 mmHg | 1.42 (1.27, 1.58) | 0.35 (0.06) | 2.90 (1.74, 4.82) | 1.40 (1.04, 1.88) | 1.27 (1.15, 1.41) | NA | 1.45 (1.33, 1.57) | 1.29 (0.91, 1.84) | 1.47 (1.25, 1.72) |
| Anti-hypertensive drug use | 1.35 (1.01, 1.82) | 0.30 (0.15) | 0.31 (0.07, 1.31) | 1.18 (0.74, 1.88) | 1.17 (1.01, 1.36) | NA | 1.90 (1.68, 2.16) | 2.04 (1.19, 3.49) | 1.16 (0.90, 1.49) |
| Non-insulin dependent DM | 3.01 (1.91, 4.74) | 1.10 (0.23) | 9.73 (2.97, 31.88) | 2.95 (1.79, 4.85) | NA | NA | 2.21 (1.97, 2.48) | 1.49 (0.79, 2.81) | 4.50 (3.45, 5.88) |
| BMI ≤30 kg/m2, per 5 kg/m2 | 0.98 (0.81, 1.17) | −0.02 (0.09) | 2.40 (1.11, 5.21) | 1.20 (0.74, 1.95) | 0.91 (0.80, 1.02) | NA | NA | 0.94 (0.62, 1.40) | 0.87 (0.71, 1.08) |
| BMI >30 kg/m2, per 5 kg/m2 | 1.16 (1.04, 1.29) | 0.15 (0.054) | 0.95 (0.40, 2.24) | 1.30 (0.95, 1.79) | 1.26 (1.13, 1.40) | NA | NA | 0.99 (0.83, 1.18) | 1.18 (1.06, 1.30) |
| Former smoker | 1.45 (1.23, 1.71) | 0.37 (0.08) | 1.98 (0.73, 5.37) | 1.75 (1.02, 3.00) | NA | NA | 1.35 (1.03, 1.79) | 1.12 (0.62, 2.02) | 1.51 (1.18, 1.94) |
| Current smoker | 1.76 (1.29, 2.41) | 0.57 (0.16) | 4.44 (1.49, 13.27) | 3.51 (1.81, 6.78) | NA | NA | 1.35 (1.17, 1.56) | 1.42 (0.77, 2.63) | 1.60 (1.22, 2.09) |
| ACR, per 10-fold change | 2.94 (0.99, 8.75) | 1.08 (0.56) | 5.48 (2.37, 12.71) | 1.80 (1.26, 2.56) | NA | NA | NA | NA | NA |
Abbreviations: ACR, albumin-to-creatinine ratio; BMI, body mass index; CI, confidence interval; SE, standard error; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HR, hazard ratio; NA, not available; SBP, systolic blood pressure; ARIC, Atherosclerosis Risk in Communities Study; Geisinger, Geisinger Health System; Maccabi, Maccabi Health System; Mt Sinai, Mount Sinai BioMe Biobank Platform; NHANES, Third US National Health and Nutrition Examination Survey; ICES KDT, Ontario Institute for Clinical Evaluative Sciences, Provincial Kidney, Dialysis and Transplantation program; VA, Veterans Administration Study
Additionally adjusted for age, race, and sex. Reference for smoking status is non-smoking.
Variables were imputed with more than 20% missing, thus not included in the meta-analysis
Individualized ESRD Risk Projections
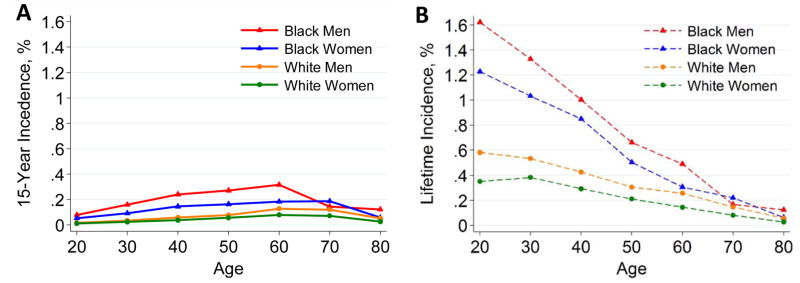
The 15-year pre-donation ESRD risk projection for the average kidney donor candidate varied by age, sex, and race; the highest risks were for middle-aged black men (Fig. 1A). For a base-case candidate, the 15-year projected risk was 0.08%, 0.05%, 0.02%, and 0.01% for a 20-year-old black man, black woman, white man, and white woman, respectively; corresponding estimates for 60-year-old base-case candidates were 0.32%, 0.18%, 0.13%, and 0.08%. As expected, the model-based lifetime projections were higher, especially in younger persons, although these risks were <2% for all base-case scenarios (Fig. 1B).
Figure 1. 15-year (A) and lifetime (B) projections of ESRD incidence in the United States by age, race, and sex for the “base-case” scenario*.
*The base-case scenario is the following: age-specific eGFR (114, 106, 98, 90, 82, 74, and 66 ml/min/1.73 m2 for ages 20, 30, 40, 50, 60, 70, and 80 years, respectively), systolic blood pressure 120 mmHg, urine albumin-creatinine ratio (ACR) 4 mg/g (0.4 mg/mmol), BMI 26 kg/m2, and no diabetes mellitus or anti-hypertensive medication use. These were selected as being representative of recent US living kidney donors. Lifetime projections are based on 15 years of follow-up data and calibrated to the incidence of ESRD in the US low-risk population, and thus lack precision. All estimates reflect the United States population average for latent characteristics; individual risk may be higher or lower. Confidence intervals for each of the estimates are depicted in Appendix 4. Confidence intervals were obtained from simulations sampled from the distribution of meta-analyzed hazard ratios.
The projected risk of ESRD was higher in persons with additional risk factors, particularly high albumin-creatinine ratio (Table 3). Current smoking was also a strong risk factor (Supplementary Appendix, Fig. S1). Risk factors had a larger impact on model-based lifetime projections for young persons (Supplementary Appendix, Fig. S2). Relationships were similar in most sensitivity analyses (Supplementary Appendix, Fig. S3), with the exception of lifetime projected risks in young persons with higher BMI, where coefficients derived from the literature estimated higher risks (Supplementary Appendix, Table S4).
Table 3.
Projected incidence (%) of ESRD in the United States for hypothetical patient profiles in the absence of kidney donation
| Scenario | Age | Race | eGFR (ml/min/ 1.73m2) | ACR (mg/g) | SBP (mmHg) | Smoking | 15-Year Projections (95% CI) | Model-Based Lifetime Projections (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | Black | 115 | 4 | 130 | Never | 0.1% (0.1%–0.1%) | 1.9% (1.2%–2.5%) |
| 2 | 20 | Black | 115 | 4 | 130 | Current | 0.2% (0.1%–0.2%) | 3.4% (2.0%–4.8%) |
| 3 | 20 | Black | 115 | 4 | 140* | Current | 0.3% (0.1%–0.4%) | 5.4% (2.9%–8.5%) |
| 4 | 20 | Black | 115 | 30 | 140* | Current | 0.7% (0.2%–1.5%) | 13.3% (4.8%–27.0%) |
| 5 | 60 | White | 80 | 4 | 140 | Never | 0.2% (0.1%–0.3%) | 0.4% (0.2%–0.6%) |
| 6 | 60 | White | 60 | 4 | 140 | Never | 0.4% (0.2%–0.6%) | 0.7% (0.3%–1.2%) |
| 7 | 60 | White | 60 | 4 | 140* | Never | 0.5% (0.2%–0.8%) | 1.0% (0.5%–1.7%) |
| 8 | 60 | White | 60 | 30 | 140* | Current | 2.2% (1.1%–3.6%) | 4.4% (2.1%–7.0%) |
Taking anti-hypertensive medications. Abbreviations: eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); ACR, random urine albumin-to-creatinine ratio (mg/g), to convert from mg/g to mg/mmol divide by 8.84; CI, confidence interval; SBP, systolic blood pressure (mmHg). Online risk tool available at www.transplantmodels.com/esrdrisk. Lifetime projections are based on 15 years of follow-up data and calibrated to the incidence of ESRD in the US low-risk population, thus are imprecise. All estimates reflect the population average for latent characteristics; individual risk may be higher or lower. Projections are for a man of specified characteristics with body-mass index 25 kg/m2 and no diabetes. Confidence intervals were obtained from simulations sampled from the distribution of meta-analyzed hazard ratios.
Risk Projections in Recent Kidney Donors
When the pre-donation ESRD risk projections were applied to the recent donor population, 99%, 98%, and 94% of recent US donors had <3%, <2%, and <1% projected 15-year pre-donation incidence of ESRD, respectively (Supplementary Appendix, Fig. S4). Pre-donation estimates >3% were most common among middle-aged (53–68 years) donors of black race.
There were similar patterns of ESRD risk according to race and sex in the absence and presence of donation, with 15-year risk projections in non-donors 3.5 to 5.3-lower than those observed for US kidney donors (Supplementary Appendix, Table S5). For example, the projected (in the absence of donation) and observed (after donation) 15-year risks for the average black male donor were 0.21% and 0.96%, respectively. Corresponding projected and observed 15-year risks were 0.12% and 0.59% in black women, 0.07% and 0.34% in white men, and 0.04% and 0.15% in white women.
DISCUSSION
We estimated the long-term risk of ESRD according to ten pre-donation demographic and health characteristics assessed together and then developed an online risk tool to help evaluate and counsel living kidney donor candidates and improve the acceptance process. We demonstrated substantial variation in the ESRD risk projections by age, sex, and race. For the base-case candidate, a scenario reflecting the average US kidney donor, the highest 15-year risks were among middle-aged black men. In model-based lifetime projections, young persons, particularly of African-American race, were at highest risk. Many older persons had low long-term ESRD risk estimates, even in the presence of health characteristics often considered to be contraindications to donation, such as lower eGFR or mild hypertension. These data may provide an empirical foundation for the criteria a transplant center uses to accept or decline a living kidney donor candidate.
A unique aspect of the present study are the estimates of long-term ESRD risk in low-risk persons, which consider together a combination of individual demographic and health characteristics. Our estimates leverage data from over 31 million person-years of follow-up and include persons with health characteristics not well captured in current living kidney donor populations. Use of the online risk tool in kidney donor acceptance protocols may help minimize the number of living kidney donors who develop ESRD after donation, support donation among people whose long-term risk was previously misunderstood, and enhance informed consent and shared decision-making with donor candidates.24 Although the risk tool was developed specifically for the US, the methods used to generate robust estimates may be adapted to other countries using local data sources.
Our risk projections focus on ESRD in the absence of donation over a 15-year time horizon. The protocol and statistical analysis plan are available on www.nejm.org. These estimates may not fully capture relevant risks for younger donors, who may have more than 60 years of remaining life. For this reason we also provided projected lifetime ESRD risk, with the caveat that these estimates lack precision and use relatively short follow-up data. Although we do not specifically model the incidence of risk factors such as diabetes and hypertension, our projections incorporate the natural rate of disease development in a given subset of the population, thereby incorporating all disease pathways to ESRD. However, the projections should be considered the population average. If a person has a higher risk of developing diabetes compared to a peer group with identical demographic and health characteristics (blood pressure, eGFR, albuminuria, BMI, smoking status), the actual risk may be higher than our projection. Similarly, the magnitude of the added risk from donation, and how this risk varies by health characteristics like obesity, remains uncertain. The relative risk of donation compared to non-donation was estimated to be 7.9 (95% CI: 4.6–8.1) and 11.4 (95% CI: 4.4–29.6) in two recent studies.5,6 Our 15-year risk projections in the absence of donation appear consistent with previously published estimates of post-donation risk and the relative risk of donation5,6, with similar patterns of risk variation by sex and race.12,13
The relative associations used in our online tool were derived from 7 cohorts with follow-up ranging from 4 to 16 years. These meta-analyzed estimates were, for the most part, very similar to those previously published in a cohort with 25-year follow-up.22 Risk was higher in black persons than white persons, and slightly higher in men than women, similar to estimates in the general population.14,18 Racial variation in ESRD risk may relate to the incidence of hypertension and diabetes,13,25 access to care and other unmeasured environmental factors, and the distribution of kidney disease risk alleles such as APOL1; our estimates only incorporate the population-average exposure to these factors. However, two studies with long-term follow-up have suggested much stronger risk associations between BMI and ESRD.22,23 Sensitivity analyses suggest that an underestimate of the BMI-ESRD risk association would be significant primarily for younger donor candidates. Thus, we suggest caution be used in evaluating obese donor candidates, particularly when they are young.
Despite excellent outcomes in recipients of kidneys from older living donors,26–28 only 2.8% of US living kidney donors were 65 years or older in 2014.3 Our estimates suggest that healthy older adults may be appropriate donor candidates with respect to their future ESRD risk. Having lived to an older age without the development of high-risk health conditions, a healthy older adult would be relatively unlikely to develop ESRD, even in the presence of suboptimal health characteristics like lower eGFR or higher blood pressure. Other studies have demonstrated the safety of kidney donation among older adults with respect to post-donation outcomes, such as perioperative mortality as well as subsequent cardiovascular events. 26–28
To model ESRD risk in the absence of donation, the present study used established methods, risk estimates derived from actual US incidence, and data from millions of persons. However, certain assumptions must be emphasized, particularly for the projections across a lifetime. First, projections are calibrated to incidence rates of ESRD from US population data. Annual incidence is derived using life-table methods, which assume constant age-, sex-, and race-specific ESRD incidence over decades and a static population sub-structure. Second, information on certain health characteristics of interest was not available. Our estimates reflect the population average for latent characteristics. For example, persons with higher socioeconomic status may have lower risk, and persons with lower socioeconomic status or a family history of kidney disease may have higher risk. Third, our models to estimate 15-year and lifetime risk were based on cohorts of low-risk persons who were followed between 4 and 16 years. Fourth, random effects meta-analysis takes into account potential heterogeneity, but precision is limited. Fifth, our study focuses on a single outcome, ESRD treated with chronic dialysis or transplantation. We did not assess untreated low GFR, a condition particularly common in older persons,29,30 nor did we assess the risk of other diseases linked to kidney donation, such as hypertension or preeclampsia.31,32 Finally, we made no estimate of the age at which a donor candidate would develop ESRD or years with ESRD prior to death, nor did we assess the risk of perioperative or other complications from donation, which may vary by baseline characteristics such as obesity.13,31–33
In summary, our online risk tool incorporates multiple baseline demographic and health characteristics together to project a donor candidate’s 15-year incidence of ESRD in the absence of kidney donation. We speculate that if transplant programs were to incorporate individualized risk assessment using multiple demographic and health characteristics in the evaluation, counseling, and acceptance of all living kidney donor candidates, better estimates of future donor kidney outcomes might be feasible. When data become available, future estimates may be improved by the incorporation of cohorts observed for longer periods of time and from diverse countries, and by adding the risk of ESRD from donation according to multiple pre-donation health characteristics.
Supplementary Material
Acknowledgments
Funding Statement
MG receives support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; K08DK092287). KLL and DLS receive support from the NIDDK (R01DK096008). AXG receives support from the Dr. Adam Linton Chair in Kidney Health Analytics. The CKD-PC Data Coordinating Center is funded in part by a program grant from the US National Kidney Foundation (NKF funding sources include AbbVie and Amgen) and the NIDDK (R01DK100446-01). A variety of sources have supported enrollment and data collection including laboratory measurements, and follow-up in the collaborating cohorts of the CKD-PC. These funding sources include government agencies such as National Institutes of Health and medical research councils as well as foundations and industry sponsors listed in Appendix 6. The funders had no role in the design, analysis, interpretation of this study, and did not contribute to the writing of this report and the decision to submit the article for publication.
The web site (www.transplantmodels.com/esrdrisk) was developed by Eric K. Chow.
Footnotes
A complete list of members of the Chronic Kidney Disease (CKD) Prognosis Consortium is provided in the Supplementary Appendix, available at NEJM.org.
Some of the data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Financial Disclosure: Dr. Garg received an investigator-initiated grant from Astellas and Roche to support a Canadian Institutes of Health Research study in living kidney donors.
References
- 1.Horvat LD, Shariff SZ, Garg AX. Global trends in the rates of living kidney donation. Kidney Int. 2009;75:1088–98. doi: 10.1038/ki.2009.20. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 3.US Department of Health and Human Services, Health Resources and Services Administration. [Accessed March 15, 2015]; http://optn.transplant.hrsa.gov/converge/latestData/rptData.asp.
- 4.Steiner RW, Ix JH, Rifkin DE, Gert B. Estimating risks of de novo kidney diseases after living kidney donation. Am J Transplant. 2014;14:538–44. doi: 10.1111/ajt.12625. [DOI] [PubMed] [Google Scholar]
- 5.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–86. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86:162–7. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 7.Kiberd BA. Estimating the long term impact of kidney donation on life expectancy and end stage renal disease. Transplant Res. 2013;2:2. doi: 10.1186/2047-1440-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandelbrot DA, Pavlakis M, Danovitch GM, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. Am J Transplant. 2007;7:2333–43. doi: 10.1111/j.1600-6143.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- 9.Tong A, Chapman JR, Wong G, de Bruijn J, Craig JC. Screening and follow-up of living kidney donors: a systematic review of clinical practice guidelines. Transplantation. 2011;92:962–72. doi: 10.1097/TP.0b013e3182328276. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadi AR, Lafranca JA, Claessens LA, et al. Shifting paradigms in eligibility criteria for live kidney donation: a systematic review. Kidney Int. 2015;87:31–45. doi: 10.1038/ki.2014.118. [DOI] [PubMed] [Google Scholar]
- 11.Steiner RW. ‘Normal for Now’ or ‘At Future Risk’: A Double Standard for Selecting Young and Older Living Kidney Donors. Am J Transplant. 2010;10:737–41. doi: 10.1111/j.1600-6143.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 12.Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11:1650–5. doi: 10.1111/j.1600-6143.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 13.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363:724–32. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis. 2013;62:245–52. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ommen ES, LaPointe Rudow D, Medapalli RK, Schroppel B, Murphy B. When good intentions are not enough: obtaining follow-up data in living kidney donors. Am J Transplant. 2011;11:2575–81. doi: 10.1111/j.1600-6143.2011.03815.x. [DOI] [PubMed] [Google Scholar]
- 16.Lam NN, Lentine KL, Levey AS, Kasiske BL, Garg AX. Long-term medical risks to the living kidney donor. Nature Rev Nephrol. 2015 doi: 10.1038/nrneph.2015.58. In press. [DOI] [PubMed] [Google Scholar]
- 17.Reese PP, Boudville N, Garg AX. Living Kidney Donation: Outcomes, Ethics, and Uncertainty. Lancet. 2015 doi: 10.1016/S0140-6736(14)62484-3. In press. [DOI] [PubMed] [Google Scholar]
- 18.Kiberd BA, Clase CM. Cumulative risk for developing end-stage renal disease in the US population. Journal of the American Society of Nephrology : JASN. 2002;13:1635–44. doi: 10.1097/01.asn.0000014251.87778.01. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. Analytic and Reporting Guidelines: The Third National Health and Nutritional Examination Survey, NHANES III (1988–1994) Hyattsville, MD: Center for Disease Control and Prevention; 1996. [Google Scholar]
- 21.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 22.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–8. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 23.Vivante A, Golan E, Tzur D, et al. Body mass index in 1. 2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172:1644–50. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiessen C, Gordon EJ, Reese PP, Kulkarni S. Development of a Donor-Centered Approach to Risk Assessment: Rebalancing Nonmaleficence and Autonomy. Am J Transplant. 2015;15:2314–23. doi: 10.1111/ajt.13272. [DOI] [PubMed] [Google Scholar]
- 25.Lentine KL, Schnitzler MA, Xiao H, et al. Consistency of racial variation in medical outcomes among publicly and privately insured living kidney donors. Transplantation. 2014;97:316–24. doi: 10.1097/01.TP.0000436731.23554.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese PP, Bloom RD, Feldman HI, et al. Mortality and cardiovascular disease among older live kidney donors. Am J Transplant. 2014;14:1853–61. doi: 10.1111/ajt.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg AX, Meirambayeva A, Huang A, et al. Cardiovascular disease in kidney donors: matched cohort study. BMJ. 2012;344:e1203. doi: 10.1136/bmj.e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959–66. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 29.Hemmelgarn BR, James MT, Manns BJ, et al. Rates of treated and untreated kidney failure in older vs younger adults. JAMA. 2012;307:2507–15. doi: 10.1001/jama.2012.6455. [DOI] [PubMed] [Google Scholar]
- 30.Rebholz CM, Coresh J, Ballew SH, et al. Kidney Failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) Study: Comparing Ascertainment of Treated and Untreated Kidney Failure in a Cohort Study. Am J Kidney Dis. 2015;66:231–9. doi: 10.1053/j.ajkd.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boudville N, Prasad GV, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185–96. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Garg AX, Nevis IF, McArthur E, et al. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372:124–33. doi: 10.1056/NEJMoa1408932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young A, Storsley L, Garg AX, et al. Health outcomes for living kidney donors with isolated medical abnormalities: a systematic review. Am J Transplant. 2008;8:1878–90. doi: 10.1111/j.1600-6143.2008.02339.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.