Abstract
Background
Patient-delivered partner treatment (PDPT) for sexually transmitted infections (STIs) increases rates of partner treatment and decreases reinfection, but has not been evaluated during pregnancy.
Methods
This prospective cohort was nested within a larger study of peripartum HIV acquisition. Participants with microbiologic diagnosis of Chlamydia trachomatis, Neisseria gonorrhoeae, and/or Trichomonas vaginalis were screened for participation. Questionnaires were administered to determine PDPT acceptability and barriers. Women were reassessed at least 30 days to determine partner treatment and reinfection. Women whose partners did or did not receive PDPT were compared.
Results
One hundred twelve (22.2%) women in the parent cohort had a treatable STI; 78 within the PDPT study period, of whom 66 were eligible and 59 (89.3%) accepted PDPT. Fifty-one women had PDPT outcome data, 37 (73%) of whom reported partners treated with PDPT. Fourteen women (27%) refused or did not deliver partner treatment. Median age was 22 years (interquartile range, 20–26 years) and 88% were married. Compared with women who delivered PDPT, those who did not were more likely to have a partner living far away (23% vs. 0%, P = 0.004) and to report current intimate partner violence (14% vs. 0%, P = 0.02). Reported PDPT barriers included fear of partner’s anger/abuse (5%) and accusations of being STI source (5%).
Conclusion
Patient-delivered partner treatment was acceptable and feasible for pregnant/postpartum Kenyan women and may reduce recurrent STIs in pregnancy.
Young women worldwide are disproportionately affected by sexually transmitted infections (STIs), with potential sequelae including pelvic inflammatory disease, ectopic pregnancy, pelvic pain, and infertility.1,2 Sexually transmitted infections are also associated with premature delivery, low-birth-weight infants, and congenital infections.1,3 Screening and treatment of curable STIs in pregnancy can prevent adverse maternal and infant outcomes4; however, efforts are hampered by high rates of reinfection from an untreated male partner.5 A variety of approaches have been used to notify and treat sexual partners of infected persons to manage and prevent future infections. One of those strategies, patient-delivered partner treatment (PDPT), has been shown to increase rates of treated partners and decrease reinfection rates compared with standard partner referrals for testing and treatment in both high- and low-income countries.6,7
Partner treatment approaches in pregnant women have focused on sexual partner notification, where index patients seek care alone and their sexual partners are subsequently referred to care, or concurrent partner treatment, where index patients and partners are treated together in a clinic visit.8,9 In low-income countries, partner notification and referral are hindered by cost and constraints on workforce, infrastructure, and communication services as well as partners often living separately due to work obligations. Patient-delivered partner treatment may facilitate prompt treatment of partner infections and prevent reinfection of the pregnant woman while conserving resources and reaching partners unable or unwilling to attend clinic. Given the additional risk of STIs on pregnancies and neonates, PDPT is a particularly attractive approach in pregnant women given the high rates of partners treated and decreased rates of reinfection. To our knowledge, no studies have demonstrated the feasibility of this approach among pregnant or postpartum women in either high- or low-resource settings. When nonpregnant women in South Africa were given a choice, most chose PDPT to ensure that their partner received treatment.10 It is not clear whether pregnant and postpartum women in low-income countries would find PDPT acceptable and what barriers women would encounter in trying to implement the PDPT approach in a this setting.
We sought to determine the acceptability and feasibility of PDPT for Chlamydia trachomatis (CT), Neisseria gonorrhoeae (GC), and/or Trichomonas vaginalis (TV) and to determine factors that influence PDPTuptake among pregnant and postpartum women.
MATERIALS AND METHODS
Study Setting and Design
“Mama Salama” is a prospective cohort study that aims to determine incidence rates of HIV during pregnancy and postpartum and to identify co-factors for maternal HIV acquisition.11 HIV-uninfected pregnant women 14 years and older were enrolled at 2 study sites in rural Western Kenya from May 2011 to July 2013 if they planned to remain in the area for at least 9 months postpartum. Study participants attended monthly follow-up visits through the duration of their pregnancy and for 9 months postpartum. The nested PDPT study was conducted in a subset of participants enrolled between November 2011 and July 2013 who were diagnosed as having CT, GC, or TV at the Ahero sub-District Hospital, in Nyanza region. Consent was obtained for the larger study including the addition of the PDPT protocol. One woman acquired HIV during follow-up and was excluded from the PDPT study.
Demographic, behavioral, and clinical characteristics were collected using standardized questionnaires administered by a study clinician at enrollment and follow-up visits in the parent study. Women underwent pelvic exams and clinician-collected swabs were obtained for CT, GC, and TV. C trachomatis and GC were assessed using endocervical samples for nucleic acid amplification tests with the APTIMA Combo 2 Assay (HOLOGIC/GEN-PROBE, Inc, San Diego, CA). T vaginalis was detected by identifying motile parasites using standard wet mount microscopy performed by the study laboratory technologist. C trachomatis, GC, and TV were assessed at enrollment in all participants. C trachomatis and GC were reevaluated in women with these infections detected at enrollment to assess treatment effectiveness. T vaginalis was assessed at monthly study visits.
PDPT Delivery
Study clinicians notified participants of a positive CT, GC, or TV test at the study visit subsequent to laboratory detection of the STI. Participants received standardized counseling regarding the STI diagnosis, need for treatment, and prevention of reinfection including condom use and withholding resumption of sexual intercourse until treatment completion. Antimicrobials were administered at no cost as part of the study per the Kenya Ministry of Health’s national treatment guidelines.12 Women received azithromycin 1 g (single dose) for CT, cefixime 400 mg for GC (single dose), and metronidazole 400 mg twice daily for 7 days for TV. All women were counseled to have their partner come to clinic for complete STI testing and treatment. Women with CT, GC, or TV detected at parent study enrollment or at follow-up (TV only) were considered to have a treatable STI and invited to participate in the PDPT program. Women were considered PDPT study participants if they consented to participation in the larger study and were asked and answered questionnaire items about their willingness to notify their sexual partner(s) about the infections and whether they would prefer to have their partner come to clinic for treatment or deliver treatment to their partners via PDPT. Standardized questionnaires ascertaining barriers of delivering treatment to partners were administered. Patient-delivered partner treatment medications were provided to consenting women willing to participate in PDPT at no cost and consisted of doxycycline for CT, cefixime for GC, and metronidazole for TV. Study clinicians inquired about partner allergies and provided information about the medication and instructions to have the partner come in to clinic with any questions or concerns. It was recommended that partners should attend clinic for complete STI testing. If women reported greater than 1 sexual partner, they were offered treatment for all partners.
Participants opting for PDPT were scheduled for follow-up visits for posttreatment interviews and testing at the same time as their parent study follow-up visits. Women diagnosed as having CT, GC, or TV were considered treated if medication administration was documented. At the posttreatment visit, women were asked whether partners were notified about the infection, given treatment, and if women ever witnessed the partner taking treatment. If participants notified their partners and reported delivering the medication, partners were classified as “treated” even if the administration was not witnessed. If the woman was unwilling to inform her partner of the STI, not willing to bring her partner treatment, or expressed inability to execute delivery of treatment to partner at PDPT reassessment, the partner was considered “not treated.” During PDPT study, a subsequent test for CT and GC following PDPT was performed for a subset of 14 women. Reinfection with CT, GC, or TV was defined as a subsequent positive result more than 30 days after treatment.
All study procedures were approved by the institutional review board at the University of Washington and the Ethics Review Committee for the Kenyatta National Hospital and University of Nairobi.
Measures
Acceptability was assessed by participant self-report of desire and willingness to bring STI treatment to their partner at enrollment into the PDPT study. Feasibility was assessed by participant self-report of delivering STI treatment to partners and confirmation that their partner successfully received treatment at PDPT follow-up assessment. As with most PDPT interventions, there was no objective confirmation that a partner received treatment.
Statistical Analysis
Baseline demographic, behavioral, and medical characteristics of PDPT participants and nonparticipants were compared using χ2 tests for proportions and Kruskal-Wallis tests for continuous measures. Fisher exact tests were used for small sample sizes. All women eligible for PDPTwith complete post-PDPT reassessment data were included in an analysis comparing those who delivered treatment to their partners or partner-treated versus those who did not deliver treatment partner not treated, using χ2 tests for proportions and Fisher exact tests for small sample sizes. Kruskal-Wallis tests were used to detect differences in medians for continuous measures. All data were analyzed using Stata Software (StataCorp. 2011, Stata Statistical Software: Release 12; StataCorp LP, College Station, TX).
RESULTS
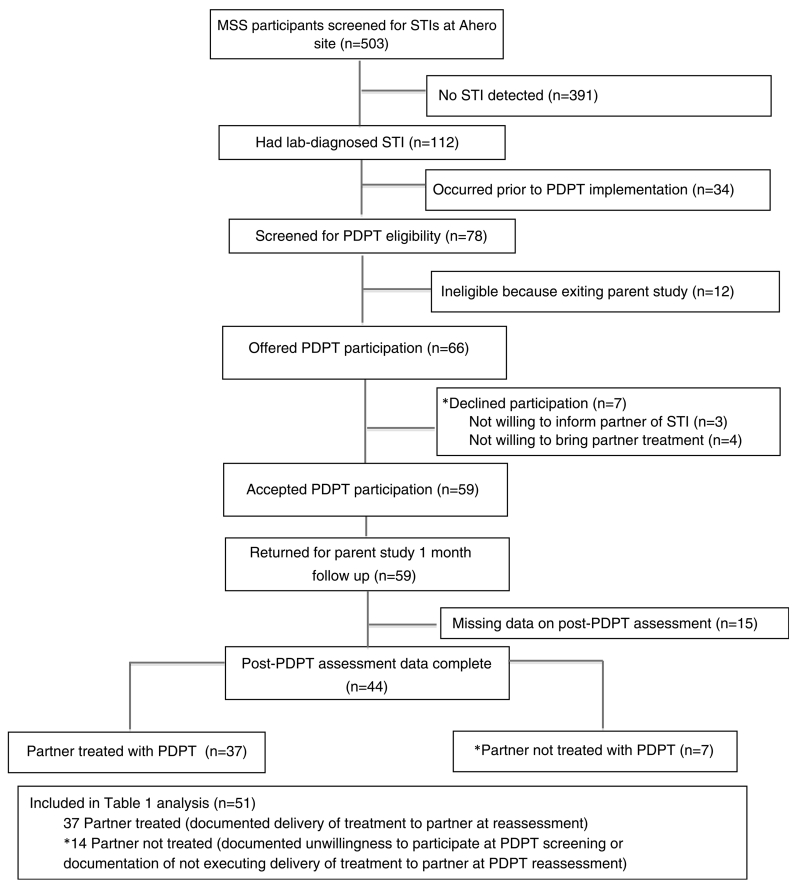
During the entire parent study period, 503 women were assessed for CT, GC, and TV at enrollment of whom 112 (22.2%) had CT, GC, or TV and 78 (69.6% of all women with CT, GC, or TV) occurred during the PDPT study. Of 78 women screened for PDPTeligibility, 12 were excluded because they were screened at final visit in the parent study without a scheduled follow-up visit (Fig. 1). Among the 66 PDPT study–eligible women, 59 reported willingness to participate in PDPT (89% up-take) and 7 (11%) women were unwilling; 3 were unwilling to inform their partner of STI diagnosis and 4 were unwilling to bring their partner treatment. Among the 59 women reporting willingness to participate in PDPT and returning for follow-up study visits, 15 returned for study visits in the parent study but did not receive the post-PDPT questionnaire due to an error in PDPT protocol implementation. Of 44 women who underwent post-PDPT reassessment, 37 (84%) reported delivering treatment to their partners, defined as partner treated, and 7 reported not delivering treatment. We compared the 37 women reporting successful PDPT to 14 women who did not give PDPT—either by refusing PDPT participation (n = 7) or initially expressed acceptability but not delivering partner treatment (n = 7; Fig. 1). Women who agreed to take PDPT but who did not have a post-PDPT evaluation data were excluded from this analysis because partner PDPT uptake data were missing.
Figure 1.
Number of CDC-funded HIV testing events in correctional facilities in the United States, 2009–2013.
Among the 51 participants included in the comparative analysis, GC was detected in 8 women (16%), CT in 6 women (12%), and TV in 37 (72%) women; 11 (22%) infections were symptomatic. No coinfections were detected. Sociodemographic, sexual behavior, and medical characteristics were similar for those with partners treated and partners not treated (Table 1); there were no significant differences in age (median, 22 years vs. 23 years; P = 0.88), years of education (median, 8 vs. 8; P = 0.710), pregnancy status (73% vs. 69% pregnant [vs. postpartum]; P = 0.756), or marriage (89% vs. 79%; P = 0.330). Reported unprotected sex in the past month was high (59%), but we did not detect a difference in unprotected sex between those with treated or untreated partners (66% vs. 50%; P = 0.31). Intimate partner violence in the last month was reported among 2 (13%) of participants with untreated partners and was not reported by any partner-treated participants (0% vs. 13%; P = 0.025). The one woman reporting more than 1 sexual partner in the past month reported that both her partners successfully received treatment.
TABLE 1.
Demographic, Sexual Behavior, and Medical Characteristics of PDPT Completed and Not Completed Among Those Screened for PDPT*
| Partner Treated† (n = 37) | Partner Not Treated† (n = 14) | P ‡ | |
|---|---|---|---|
| Demographic and relationship characteristics | |||
| Age, median (IQR), y | 22 (20–27) | 23 (21–24) | 0.88 |
| Years of school completed, median (IQR) | 8 (7–8) | 8 (7–8) | 0.54 |
| Married, n (%) | 33 (89) | 11 (79) | 0.33 |
| Marriage type, n (%) | |||
| Monogamous | 31 (94) | 10 (91) | 0.73 |
| Polygamous | 2 (6) | 1 (9) | |
| Any reported intimate partner violence, last month, n (%) | 0 (0) | 2 (14) | 0.02 |
| Reports feeling unsafe in relationship | 0 (0) | 1 (7) | 0.10 |
| Sexual behavior | |||
| Age of sexual debut, median (IQR), y | 16 (14–18) | 15 (15–17) | 0.86 |
| No. sexual partners, lifetime, median (IQR) | 3 (2–3) | 3 (1–4) | 0.90 |
| Coital frequency, past month, median (IQR) | 2 (0–3) | 1 (0–2) | 0.35 |
| Reported unprotected sex, past month§, n (%) | 23 (66) | 7 (50) | 0.31 |
| Medical characteristics | |||
| Parity, median (IQR) | 2 (1–4) | 2 (1–3) | 0.88 |
| Gestational age of enrollment pregnancy, median (IQR) | 26 (21–30) | 27 (25–39) | 0.04 |
| Laboratory diagnosis of STI at screening∥, n (%) | |||
| GC | 5 (14) | 3 (21) | 0.49 |
| CT | 5 (14) | 1 (7) | 0.53 |
| TV | 27 (73) | 10 (71) | 0.91 |
| Reported fears or barriers to PDPT | |||
| Feared partner’s anger or abuse, n (%) | 2 (5) | 1 (8) | 0.75 |
| Feared accusations of being STI source, n (%) | 1 (3) | 2 (15) | 0.09 |
| Feared accusations of promiscuity, n (%) | 1 (3) | 0 (0) | 0.56 |
| Feared stigma associated with STIs, n (%) | 0 (0) | 1 (8) | 0.08 |
| Partner lived far away, n (%) | 0 (0) | 3 (23) | 0.002 |
| Clinical outcomes following PDPT acceptance or declination | |||
| Subsequent reinfection¶**, n (%) | |||
| GC | 0/5 (0) | 0/3 (0) | — |
| CT | 0/4 (0) | 1/1 (100) | 0.167 |
| TV | 4/25 (16) | 2/9 (22) | 0.606 |
Missing data not shown; demographic, relationship, medical, and sexual behavior characteristics as reported at
Partner treated defined as those screened for PDPT who were willing to deliver treatment to male partners and reported successful PDPT delivery. Partner not treated defined as those screened for PDPT who were not willing to deliver treatment to male partners or did not report successful PDPT deliver.
χ2 Test and Kruskal-Wallis test with Fisher exact P value.
Among participants who reported sexual intercourse in the past month.
All women were administered treatment at the same visit as STI detection or subsequent follow-up visit.
Subsequent reinfection defined as infection detected at least 30 days after documented treatment administration.
Four women were not reassessed for subsequent infections: 3 partner treated (2 with TV infections before PDPT administration; 1 with CT) and 1 partner not treated (with TV infection before PDPT participation screening).
IQR indicates interquartile range.
Reported fears about delivering treatment to partners were infrequent among PDPT participants (Table 1). Fears reported included fearing partner’s anger or abuse (5%), accusation of being the STI source (5%), and accusation of promiscuity (2%). Those with untreated partners reported fearing partner’s anger or abuse (7%), accusation of being the STI source (14%), and fearing stigma associated with STI (7%) at higher rates than those with treated partners but these were not significant differences. Untreated male partners were more likely to live far away than treated partners (23% vs. 0%; P = 0.002). In addition, 28% of women did not know if their partner had medication allergies but was not associated with delivery of treatment.
At reassessment more than 30 days after participant treatment, no cases of subsequent GC infections were detected in women, regardless of partner treatment (Table 1). Among the 4 partner-treated women with CT who had an STI reassessment, none had CT reinfection, whereas 1 woman with an untreated partner had CT detected at reassessment (P = 0.167). Subsequent TV infection was detected in 4 (15%) of women with a treated partner compared with 2 (22%) reinfections among women with an untreated partner (P = 0.606).
Internal Validity
To address potential selection bias due to incomplete follow-up, we compared women with and without completed PDPT follow-up. No significant differences were detected between women screened for PDPT with and without completed post-PDPT questionnaires in median years of completed education (8 vs. 8; P = 0.671), being married (80% vs. 87%; P = 0.494), experiencing intimate partner violence (4% vs. 3%; P = 0.785), or reporting unprotected sex (58% vs. 58%; P = 0.975).
DISCUSSION
In this study of Kenyan pregnant and postpartum women with laboratory diagnosed STIs, we found that PDPT was acceptable, with 89% of women agreeing to try PDPT, and of those who were willing to deliver PDPT, 73% reported that partners received treatment. To our knowledge, this is the first study that evaluates PDPT for curable STIs during pregnancy and postpartum. Partner treatment rates in our study of PDPT were higher than those reported from concurrent patient-partner treatment programs in pregnancy13 and consistent with studies in other populations using PDPT, including among nonpregnant women in similar settings.6
Few women reported barriers to PDPT, with only 5% reporting concerns about partner’s anger or abuse. This is equal to rates of similar concerns reported in a randomized trial of PDPT among young nonpregnant women in the United States.14 These issues are not unexpected in any type of partner referral or treatment program for STIs, which raise sensitive issues within the partnership. Two participants who did not deliver treatment reported current intimate partner violence, which may have been the reason for declining to disclose the infection and provide treatment. Any partner referral or treatment program must consider and assess for IPV. However, multiple studies have demonstrated that the threat of violence is not a primary concern for the delivery of PDPT.14,15 There are additional challenges to PDPT. For example, women reported living apart from their partner as a barrier to PDPT, which may reflect logistical challenges for PDPT in those couples living apart or in higher-risk partnerships such as those with additional partners. Despite these perceived barriers, most of women in our study were willing to notify partners of their STI diagnosis and did not report significant barriers or concerns about PDPT. The high acceptability reported by women suggests that PDPT could be a feasible part of treatable STI management in pregnant/postpartum women in this setting.
We encouraged participants to have their partners come to the clinic for evaluation and free treatment. Patient-delivered partner treatment was offered as an alternative option. Despite this counseling, no participant reported her partner sought care at the study clinic or went elsewhere for treatment. There is no evidence to date that suggests providing medication prevents or facilitates care seeking by sexual partners. In general, but especially in areas of high HIV prevalence, it is recommended that PDPT is accompanied by counseling advising full STI evaluation for the partner.
Among participants with a treated partner who were reassessed after CT, GC, or TV treatment, there were no reinfections with CT and GC but reinfection for TV (15%) was higher than has been seen in other partner treatment studies (6%–9%).16 Although the lack of CT and GC reinfections among the partner treated group was a positive outcome, these findings were not significant and should be interpreted with caution given the few participants reassessed. Studies from nonpregnant populations have found recurrent infection rates of approximately 10% in PDPT interventions for CT and GC.17 Our PDPT study was designed to demonstrate the acceptability and feasibility of PDPT in this unique population rather than compare effectiveness of PDPT with other methods, although this merits further study.
One important barrier to use of PDPT in pregnancy/postpartum cohorts in low-resourced settings is widespread use of syndromic STI diagnosis and treatment. Use of PDPT after syndromic STI diagnosis is not recommended because partners would potentially be overtreated or undertreated due to low sensitivity and specificity of syndromic diagnosis. It is unclear if acceptability of PDPT among pregnant/postpartum women after syndromic diagnosis would be equivalent to laboratory-confirmed results. However, as STI diagnostics scale up, it will be useful to incorporate PDPT within the STI prevention and care cascade. The World Health Organization notes urgent needs for rapid, point-of-care diagnostic tests for STIs to improve upon current syndromic management, and that these tests are on the immediate horizon for CTand GC.18 Although PDPT has also been shown to be cost-effective and result in fewer quality-adjusted years of life lost in high-resource settings,19 larger-scale PDPT programs in low-income settings with available diagnostics are necessary to demonstrate cost-effectiveness of this approach in different environments.
One report suggests that providers are reluctant to provide PDPT because of concerns around legality and liability.20 Kenya does not have an official policy on PDPT; however, the Centers for Disease Control and Prevention has outlined clear guidelines for PDPT in pregnancy, which are supported by international practice organizations.21
Although we demonstrated the acceptability and feasibility of the PDPT among pregnant and postpartum women in this setting, the study has limitations. Because of challenges with study logistics, 15 women did not have an appropriate post-PDPT assessment, despite continuing in the parent study. The number of women reassessed after PDPT is small and precludes us from making any assessment of PDPT efficacy in this population. This small sample size also limits the ability to detect differences between women with reporting treated partners and those with untreated partners. In addition, a limitation of a PDPT approach is that data regarding partner treatment are dependent on surrogate reporting, which can be susceptible to social desirability bias. However, observations from this study highlight the feasibility of this approach and support further studies of effectiveness.
Sexually transmitted infections in sub-Saharan Africa disproportionately affect young women and STIs during the peripartum period have the double burden of also contributing to infant morbidity including pneumonia and ophthalmia neonatorum.22-24 Patient-delivered partner treatment provides a feasible option for preventing reinfection of curable STIs for couples in which other methods are not appropriate for women or not accessible. Our study was the first evaluation of PDPT during the peripartum period in sub-Saharan African and found low rates of reported barriers and good uptake of PDPT among pregnant and postpartum Kenyan women. If confirmed, PDPT may contribute to reduced peripartum burden of curable STIs in this particularly important population.
Acknowledgments
Support: Dr Unger is supported by the Women’s Reproductive Health Research Career Development Award National Institute of Child Health and Human Development, K12, HD001264. Dr John-Stewart is supported by National Institutes of Health/National Institute of Child Health and Human Development K24 HD054314 (Principal Investigator, Grace John-Stewart) for her mentorship. The project was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases PO1 HSD064915 (Principal Investigator, Julie Overbaugh).
Footnotes
Conflict of interest: None declared.
REFERENCES
- 1.Lewis DA, Latif AS, Ndowa F. WHO global strategy for the prevention and control of sexually transmitted infections: Time for action. Sex Transm Infect. 2007;83:508–509. doi: 10.1136/sti.2007.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haggerty CL, Gottlieb SL, Taylor BD, et al. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201(suppl 2):S134–S155. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 3.Moodley P, Sturm AW. Sexually transmitted infections, adverse pregnancy outcome and neonatal infection. Semin Neonatol. 2000;5:255–269. doi: 10.1053/siny.2000.0026. [DOI] [PubMed] [Google Scholar]
- 4.Meyers DS, Halvorson H, Luckhaupt S. Force USPST. Screening for chlamydial infection: An evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;147:135–142. doi: 10.7326/0003-4819-147-2-200707170-00173. [DOI] [PubMed] [Google Scholar]
- 5.Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: A systematic review of the literature. Sex Transm Dis. 2009;36:478–489. doi: 10.1097/OLQ.0b013e3181a2a933. [DOI] [PubMed] [Google Scholar]
- 6.Trelle S, Shang A, Nartey L, et al. Improved effectiveness of partner notification for patients with sexually transmitted infections: Systematic review. BMJ. 2007;334:354. doi: 10.1136/bmj.39079.460741.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuwaha F, Kambugu F, Nsubuga PS, et al. Efficacy of patient-delivered partner medication in the treatment of sexual partners in Uganda. Sex Transm Dis. 2001;28:105–110. doi: 10.1097/00007435-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Thurman AR, Holden AE, Shain R, et al. Partner notification of sexually transmitted infections among pregnant women. Int J STD AIDS. 2008;19:309–315. doi: 10.1258/ijsa.2007.007295. [DOI] [PubMed] [Google Scholar]
- 9.Gichangi P, Fonck K, Sekande-Kigondu C, et al. Partner notification of pregnant women infected with syphilis in Nairobi, Kenya. Int J STD AIDS. 2000;11:257–261. doi: 10.1258/0956462001915660. [DOI] [PubMed] [Google Scholar]
- 10.Young T, de Kock A, Jones H, et al. A comparison of two methods of partner notification for sexually transmitted infections in South Africa: Patient-delivered partner medication and patient-based partner referral. Int J STD AIDS. 2007;18:338–340. doi: 10.1258/095646207780749781. [DOI] [PubMed] [Google Scholar]
- 11.Kinuthia JDA, Matemo D, Richardson BA, et al. HIV acquisition during pregnancy and postpartm is associated with genital infections and partnership characteristics: A cohort study. AIDS. 2015;29 doi: 10.1097/QAD.0000000000000793. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Mo. Integrating the Management of STIs/RTI Into Reproductive Health Services. NASCOP, NASCP; Nairobi, Kenya: 2010. [Google Scholar]
- 13.Mmeje O, Coleman JS. Concurrent patient-partner treatment in pregnancy: An alternative to expedited partner therapy? Sex Transm Dis. 2012;39:665–670. doi: 10.1097/OLQ.0b013e318259f5a4. [DOI] [PubMed] [Google Scholar]
- 14.Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: A randomized, controlled trial. Sex Transm Dis. 2003;30:49–56. doi: 10.1097/00007435-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Vaidya S, Johnson K, Rogers M, et al. Predictors of index patient acceptance of expedited partner therapy for Chlamydia trachomatis infection and reasons for refusal, sexually transmitted disease clinics, New York City, 2011 to 2012. Sex Transm Dis. 2014;41:690–694. doi: 10.1097/OLQ.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kissinger P, Schmidt N, Mohammed H, et al. Patient-delivered partner treatment for Trichomonas vaginalis infection: A randomized controlled trial. Sex Transm Dis. 2006;33:445–450. doi: 10.1097/01.olq.0000204511.84485.4c. [DOI] [PubMed] [Google Scholar]
- 17.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676–685. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]
- 18.WHO . The importance of a renewed commitment to STI prevention and control in achieving global sexual and reproductive health. Sexual and Reproductive Health. WHO; Geneva: 2013. Sexually transmitted infections (STIs) [Google Scholar]
- 19.Gift TL, Kissinger P, Mohammed H, et al. The cost and cost-effectiveness of expedited partner therapy compared with standard partner referral for the treatment of chlamydia or gonorrhea. Sex Transm Dis. 2011;38:1067–1073. doi: 10.1097/OLQ.0b013e31822e9192. [DOI] [PubMed] [Google Scholar]
- 20.Secura GM, Desir FA, Mullersman JL, et al. Predictors of male partner treatment for sexually transmitted infection. Sex Transm Dis. 2012;39:769–775. doi: 10.1097/OLQ.0b013e31825ec611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57:1–83. quiz CE81-84. [PubMed] [Google Scholar]
- 22.Laga M, Plummer FA, Nzanze H, et al. Epidemiology of ophthalmia neonatorum in Kenya. Lancet. 1986;2:1145–1149. doi: 10.1016/s0140-6736(86)90544-1. [DOI] [PubMed] [Google Scholar]
- 23.Mabey D, Hanlon P, Hanlon L, et al. Chlamydial and gonococcal ophthalmia neonatorum in the Gambia. Ann Trop Paediatr. 1987;7:177–180. doi: 10.1080/02724936.1987.11748502. [DOI] [PubMed] [Google Scholar]
- 24.Beem MO, Saxon EM. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977;296:306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]