Summary
This follow‐up extension of a randomised phase II study assessed differences in long‐term outcomes between bortezomib‐thalidomide‐dexamethasone (VTD) and VTD‐cyclophosphamide (VTDC) induction therapy in multiple myeloma. Newly diagnosed patients (n = 98) were randomised 1:1 to intravenous bortezomib (1·3 mg/m2; days 1, 4, 8, 11), thalidomide (100 mg; days 1–21), and dexamethasone (40 mg; days 1–4, 9–12), with/without cyclophosphamide (400 mg/m2; days 1, 8), for four 21‐day cycles before stem‐cell mobilisation/transplantation. After a median follow‐up of 64·8 months, median time‐to‐next therapy was 51·8 and 47·9 months with VTD and VTDC, respectively. Type of subsequent therapy was similar in both arms. After adjusting for asymmetric censoring, median time to progression was not significantly different between VTD and VTDC [35·7 vs. 34·5 months; Hazard ratio (HR) 1·26, 95% confidence interval: 0·76–2·09; P = 0·370]. Five‐year survival was 69·1% and 65·3% with VTD and VTDC, respectively. When analysed by minimal residual disease (MRD) status, overall survival was longer in MRD‐negative versus MRD‐positive patients with bone marrow‐confirmed complete response (HR 3·66, P = 0·0318). VTD induction followed by transplantation provides long‐term disease control and, consistent with the primary analysis, there is no additional benefit from adding cyclophosphamide. This study was registered at ClinicalTrials.gov (NCT00531453).
Keywords: multiple myeloma, minimal residual disease, transplantation
Bortezomib‐based triplet combinations are among the established standards of care as induction therapy for previously untreated patients with multiple myeloma (MM) who are eligible for high‐dose therapy with autologous stem cell transplantation (HDT‐ASCT) (Anderson et al, 2013; Ludwig et al, 2014). Such combinations include bortezomib plus thalidomide and dexamethasone (VTD) – a regimen which has recently been approved in the European Union, Canada and Australia – and bortezomib plus cyclophosphamide and dexamethasone (VCD), both of which are effective in previously untreated MM (Cavo et al, 2010; Reeder et al, 2010; Moreau et al, 2011; Kumar et al, 2012; Rosiñol et al, 2012a). While the benefits of triplet combinations are proven, it has not been established whether the addition of a fourth agent might further improve the activity of these combinations (Kumar et al, 2012), despite some evidence indicating the potential of quadruplet regimens (Jakubowiak et al, 2011; Palumbo et al, 2014).
We conducted an open‐label, randomised non‐comparative phase II study to evaluate the efficacy and safety of VTD and VTD plus cyclophosphamide (VTDC) as induction therapy prior to HDT‐ASCT in 98 patients with previously untreated MM (Ludwig et al, 2013). Results from the primary analysis conducted after a median follow up of 33·3 months showed that both VTD and VTDC are active induction regimens, resulting in bone marrow‐confirmed complete response (CR) rates of 29% and 31% post‐induction, and 57% and 61% post‐HDT‐ASCT, respectively. We also showed that 35% of VTD and 27% of VTDC patients achieved minimal residual disease (MRD)‐negative status, which is a prognostic indicator of improved outcomes, particularly among patients achieving a ‘conventional’ CR (Korthals et al, 2012; Rawstron et al, 2013; Martinez‐Lopez et al, 2014; Puig et al, 2014). At the time of the primary analysis, no significant differences in survival outcomes were seen, suggesting no benefit from the addition of cyclophosphamide to VTD. However, as outcomes data were not mature at this analysis, with only 21% of patients having progressed and 15% having died, we now report the findings from the protocol‐specified, long‐term extension follow‐up phase of the study, which evaluated final time‐to‐event data after a median follow‐up of more than 5 years. Experience from other trials (Mateos et al, 2014; Palumbo et al, 2014) indicates that subtle differences in outcome may only become detectable after long follow‐up when the treatment impact on good risk patients becomes evident. In addition, data on subsequent MM treatment are reported, together with analyses of outcomes according to MRD status and depth of response.
Methods
Patients and study design
The design of this randomised, non‐comparative multicentre phase II study (clinicaltrials.gov identifier: NCT00531453) has been reported previously (Ludwig et al, 2013). Briefly, transplant‐eligible patients aged 18–70 years with previously untreated, measurable MM and without grade ≥2 peripheral neuropathy or neuropathic pain [National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf)] were randomised [1:1, stratified by International Staging System (ISS) disease stage (Greipp et al, 2005)] to receive initial treatment with four 21‐day cycles of VTD or VTDC. Treatment comprised bortezomib 1·3 mg/m2 intravenously on days 1, 4, 8 and 11, thalidomide 100 mg orally on days 1–21 and dexamethasone 40 mg orally on days 1–4 and 9–12, with or without cyclophosphamide 400 mg/m2 intravenously on days 1 and 8. Patients who remained eligible for transplant then underwent stem cell mobilisation and single or double transplantation, while patients who had become transplant‐ineligible or had achieved a CR post‐induction could receive four additional cycles of VTD or VTDC.
Institutional review boards or independent ethics committees at all participating sites approved the study, which was conducted in accordance with the International Conference on Harmonisation for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent.
Assessments
Responses were determined by independent review per International Myeloma Working Group (IMWG) uniform response criteria (Durie et al, 2006), with the additional response categories of CRflc [defined as CR with a normalised serum free light chain (FLC) ratio; used as a surrogate for stringent CR due to the lack of routine κ/λ bone marrow staining] and near‐CR (defined as absence of M‐protein on electrophoresis and immunofixation‐positive). Post‐transplant, patients were followed every 12 weeks until disease progression, and then every 12 weeks for survival and subsequent therapies. The primary endpoint of the study was the combined rate of CRflc plus CR and near‐CR post‐induction. Secondary endpoints included time to progression (TTP), progression‐free survival (PFS) and overall survival (OS).
Additionally, an exploratory analysis of time‐to‐next therapy (TTNT; defined as the time from randomisation to the start of subsequent MM therapy or death prior to subsequent therapy) was conducted. Outcomes (PFS and OS) among patients achieving bone marrow‐confirmed CR were also investigated according to MRD status. Among patients achieving MRD‐negative status, outcomes were also investigated according to response (CRflc versus other responses).
For MRD assessment, bone marrow aspirates were collected at suspected CR and, where possible, at screening. MRD status was assessed at a central laboratory in Salamanca by immunophenotyping using multiple staining combinations [CD38 (Alexa Fluor®)/‐/CD56‐PE/CD45‐AmCyan/CD19‐PerCP‐Cy5.5/CD138‐APC, and CD38/cλ‐FITC/cκ‐PE/CD45‐AmCyan/CD19‐PerCP‐Cy5.5/CD138‐APC], with the aim of identifying, quantifying and characterising plasma cells. Data were acquired in a FACSCanto™ II flow cytometer (BD Biosciences, San Jose, CA, USA), using FACS Diva™ (BD Biosciences) software to acquire information, and Infinicyt™ software (Cytognos, Salamanca, Spain) for data analysis by a central provider (Hospital Universitario de Salamanca, Spain). Samples were characterised as MRD‐positive if clonal plasma cells were detected [based on increased expression of CD19 and/or CD45 and/or increased expression of CD56, together with immunoglobulin light chain restriction (cytoplasmaticK or cytoplasmaticL)], or MRD‐negative if only normal and polyclonal plasma cells were detected.
Statistical analyses
The Kaplan–Meier method was used to estimate the distribution of time‐to‐event endpoints. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated based on a Cox's model stratified by ISS disease stage, and P‐values for differences between treatment arms and patient groups were calculated using the log‐rank test stratified by ISS disease stage.
For the primary analyses of TTP and PFS, patients who were lost to follow‐up, withdrew consent or received subsequent MM therapy due to early indicators of progression, without fulfilling the standard criteria for disease progression, were censored. To mitigate the effect of asymmetric censoring between arms, sensitivity analyses of TTP and PFS were conducted in which patients who received subsequent therapy without meeting the standard IMWG criteria (Durie et al, 2006) for disease progression were regarded as having had a progression event if reported by the investigator as having ‘relapsed from CR’ or having experienced ‘clinical relapse’.
All analyses were undertaken by the sponsor. All authors had access to the primary clinical trial data.
Results
Patients and follow‐up
As previously reported (Ludwig et al, 2013), a total of 98 patients were enrolled and randomised to receive VTD or VTDC (n = 49 in each arm) (see Fig S1). Median age was 57 years (range 35–65) and 58 years (range 33–68) in the VTD and VTDC arms, respectively, and 24%/45%/31% and 18%/47%/35% of patients, respectively, had ISS stage I/II/III disease. Other baseline characteristics were similarly well balanced between the arms (Ludwig et al, 2013).
All patients had completed VTD or VTDC treatment at the time of the initial report of the study (Ludwig et al, 2013). In both arms, patients received a median of four treatment cycles. Forty‐eight (98%) patients in the VTD arm and 40 (82%) patients in the VTDC arm underwent HDT‐ASCT.
The data cut‐off for this final pre‐specified, long‐term extension analysis was 23 September, 2013; 5 years after the last patient was randomised. The overall median follow‐up, calculated using reverse censoring, was 64·8 months in all 98 patients: 65·3 months in the VTD arm and 64·7 months in the VTDC arm. This represents an additional follow‐up of approximately 32 months in each arm, based on the medians, when compared with the initial report of the study (Ludwig et al, 2013). At the time of data cut‐off, 34 patients had died (15 VTD, 19 VTDC), 1 (2%) VTD patient was lost to follow‐up and 1 (2%) VTD patient had chosen to withdraw from study data collection (see Fig S1). Deaths were primarily due to disease progression: 10 (20%) patients in the VTD arm and 15 (31%) patients in the VTDC arm.
Long‐term outcomes
At data cut‐off for this protocol‐specified final analysis, per investigator assessment in the intent‐to‐treat population, 20/49 (41%) patients in the VTD arm and 32/49 (65%) in the VTDC arm had disease progression events in the TTP analysis, and 24/49 (49%) and 34/49 (69%) patients had PFS events (disease progression or death). Median TTP in this primary analysis was not reached with VTD versus 39·3 months with VTDC [HR 1·55 (95% CI: 0·88–2·72), P = 0·125], and 5‐year progression‐free rates were 54·1% and 30·6%, respectively (Fig 1A). Median PFS was 56·3 vs. 36·3 months with VTD versus VTDC [HR 1·37 (95% CI: 0·81–2·31), P = 0·244], and the respective 5‐year PFS rates were 47·8% and 29·1% (Fig 1C).
Figure 1.
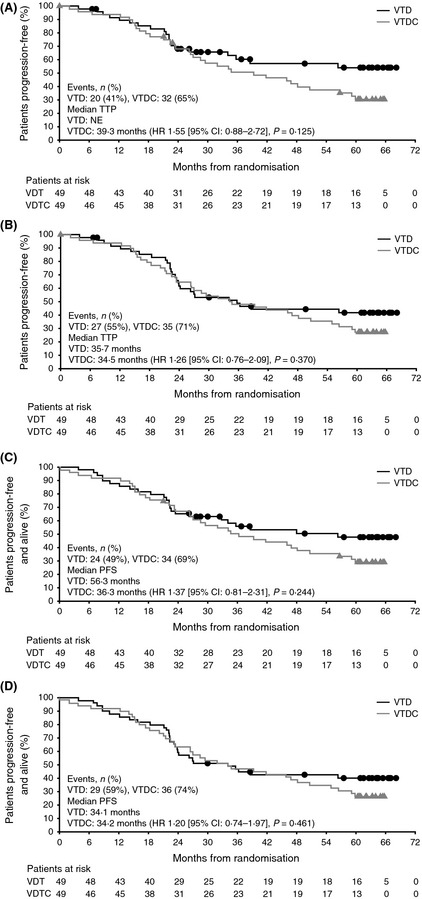
Time to progression (TTP) and progression‐free survival (PFS) with VTD and VTDC. (A) TTP per the primary analysis, with asymmetric censoring between arms. (B) TTP per the sensitivity analysis. (C) PFS per the primary analysis. (D) PFS per the sensitivity analysis. CI: confidence interval; HR: hazard ratio; NE: not estimatable; VTD, bortezomib‐thalidomide‐dexamethasone; VTDC, bortezomib‐thalidomide‐dexamethasone plus cyclophosphamide.
Asymmetric censoring was seen between 24 and 54 months in the primary analysis of TTP/PFS. In the VTD arm, 10 patients were censored in the analysis of TTP (Fig 1A) and 8 in the analysis of PFS (Fig 1C) during this follow‐up period, compared with no patients in the VTDC arm. Of the 10 VTD patients censored in the TTP analysis, 2 were censored due to study data cut‐off and 1 was lost to follow‐up; the other 7 patients were censored due to starting subsequent therapy following a recorded ‘relapse from CR’ or ‘clinical relapse’ in the absence of investigator‐documented disease progression per IMWG criteria (Durie et al, 2006). Therefore, to mitigate the impact of this asymmetric censoring, a sensitivity analysis of TTP and PFS was conducted in which these patients were considered as having an event at the time of recorded ‘relapse from CR’ or ‘clinical relapse’. Results from the sensitivity analysis are summarised in Table 1. Using this approach, median TTP was 35·7 vs. 34·5 months with VTD and VTDC, respectively [HR 1·26 (95% CI: 0·76–2·09), P = 0·370; Fig 1B], and median PFS was 34·1 vs. 34·2 months [HR 1·20 (95% CI: 0·74–1·97), P = 0·461; Fig 1D].
Table 1.
Time to progression (TTP) and progression‐free survival (PFS) per investigator assessment (sensitivity analysis), time‐to‐next therapy (TTNT) and overall survival (OS) in the VTD and VTDC arms, based on Kaplan–Meier product limit estimates
| Outcome | VTD (n = 49) | VTDC (n = 49) | HR (95% CI) | P a |
|---|---|---|---|---|
| TTP (sensitivity analysis) | ||||
| Events, n (%) | 27 (55) | 35 (71) | ||
| Median (95% CI), months |
35·7 (23·9–NE) |
34·5 (23·5 –50·6) |
1·26 (0·76–2·09) |
0·370 |
| 3‐year rate, % (95% CI) |
48·8 (33·9–62·1) |
47·9 (33·3–61·1) |
NA | NA |
| 5‐year rate, % (95% CI) |
41·8 (27·5–55·5) |
27·1 (15·5–40·0) |
NA | NA |
| PFS (sensitivity analysis) | ||||
| Events, n (%) | 29 (59) | 36 (74) | ||
| Median (95% CI), months |
34·1 (23·5–NE) |
34·2 (23·5–48·2) |
1·20 (0·74–1·97) |
0·461 |
| 3‐year rate, % (95% CI) |
46·8 (32·4–59·9) |
46·9 (32·6–60·0) |
NA | NA |
| 5‐year rate, % (95% CI) |
40·1 (26·2–53·5) |
26·5 (15·2 –39·3) |
NA | NA |
| TTNT | ||||
| Events, n (%) | 26 (53) | 30 (61) | ||
| Median (95% CI), months |
51·8 (31·9–NE) |
47·9 (28·7–NE) |
1·21 (0·71–2·05) |
0·484 |
| OS | ||||
| Events, n (%) | 15 (31) | 19 (39) | ||
| Median (95% CI), months |
NE (NE–NE) |
NE (64·3–NE) |
1·15 (0·58–2·27) |
0·692 |
| 3‐year rate, % (95% CI) |
79·6 (65·4–88·5) |
83·7 (70·0–91·5) |
NA | NA |
| 5‐year rate, % (95% CI) |
69·1 (54·1–80·1) |
65·3 (50·3–76·8) |
NA | NA |
HR, hazard ratio; CI, confidence interval; NE: not estimable; NA: not applicable; VTD, bortezomib‐thalidomide‐dexamethasone; VTDC, bortezomib‐thalidomide‐dexamethasone plus cyclophosphamide.
Based on a stratified log‐rank test.
To further evaluate the impact of asymmetric censoring on apparent differences in long‐term outcomes between arms, an exploratory analysis of TTNT was conducted (Table 1; Fig 2A). In this analysis, median TTNT was 51·8 vs. 47·9 months with VTD versus VTDC [HR 1·21 (95% CI: 0·71–2·05), P = 0·484], with 26 (53%) and 30 (61%) patients, respectively, having received subsequent therapy or died due to disease progression prior to receiving subsequent therapy at data cut‐off. Eight (16%) and 6 (12%) patients received at least three more lines of therapy, and 14 (29%) and 12 (25%) received at least two more lines of therapy in the VDT and VDTC treatment groups, respectively. The agents most commonly received as part of subsequent therapy included dexamethasone, lenalidomide, bortezomib, thalidomide and cyclophosphamide (Table 2). The type of subsequent therapy was similar in both treatment groups; 15 (31%) and 16 (33%) patients were retreated with bortezomib, and 9 (18%) and 8 (16%) patients were retreated with thalidomide.
Figure 2.
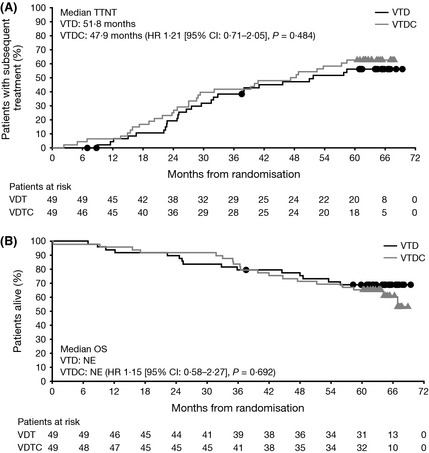
Time‐to‐next therapy (TTNT) and overall survival (OS) with VTD and VTDC. (A) TTNT. (B) OS. CI: confidence interval; HR: hazard ratio; NE: not estimatable; VTD, bortezomib‐thalidomide‐dexamethasone; VTDC, bortezomib‐thalidomide‐dexamethasone plus cyclophosphamide.
Table 2.
Agents commonly received (≥10% of patients overall) as part of subsequent therapy following VTD or VTDC
| Agent | VTD (n = 49) | VTDC (n = 49) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Any subsequent therapy | 24 | 49 | 28 | 57 |
| Dexamethasone | 20 | 41 | 21 | 43 |
| Lenalidomide | 17 | 35 | 16 | 33 |
| Bortezomib | 15 | 31 | 16 | 33 |
| Thalidomide | 9 | 18 | 8 | 16 |
| Cyclophosphamide | 6 | 12 | 10 | 20 |
| Melphalan | 3 | 6 | 8 | 16 |
| Doxorubicin | 4 | 8 | 6 | 12 |
VTD, bortezomib‐thalidomide‐dexamethasone; VTDC, bortezomib‐thalidomide‐dexamethasone plus cyclophosphamide.
Median OS was not reached in either arm (Table 1; Fig 2B). Five‐year survival rates were 69·1% with VTD and 65·3% with VTDC.
Outcomes by depth of response and minimal residual disease status
To assess the relationship between depth of response and long‐term outcomes, PFS and OS were analysed in patients achieving CRflc (CR with normal FLC ratio confirmed by bone marrow plasma cells, but without bone marrow immunohistochemistry; n = 32) at any point in the study versus ≥very good partial response (VGPR) but excluding the 32 patients with CRflc (n = 48) versus <VGPR (n = 18). This analysis was pooled across the VTD and VTDC arms. As expected, there was a trend for better PFS and OS in the true CR (CRflc) versus ≥VGPR versus <VGPR groups. Across both arms combined, median (95% CI) PFS was 56·3 months (35·7–not estimable) in the CRflc group, 38·6 months (26·3–not estimable) in the ≥VGPR group and 26·0 months (10·2–34·7) in the <VGPR group. In the sensitivity analysis, median (95% CI) PFS was 46·9 months (23·9–not estimable) in the CRflc group, 30·6 months (23·2–not estimable) in the ≥VGPR group and 26·0 months (10·2–34·7) in the <VGPR group. Median (95% CI) OS was not reached in the CRflc or ≥VGPR groups, but was 66·9 months (35·0–not estimable) in the <VGPR group (Fig 3A).
Figure 3.
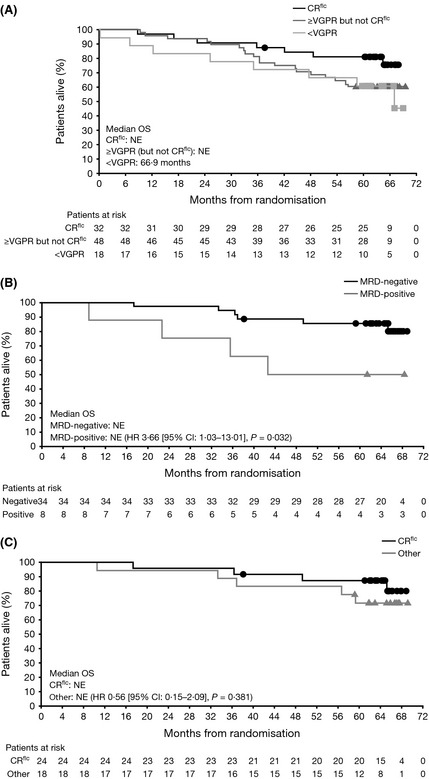
Overall survival (OS) according to minimal residual disease (MRD) status and response (pooled across VTD and VTDC arms). Kaplan–Meier analyses of OS in: (A) patients who achieved CR flc, ≥VGPR but excluding the CR flc patients, or <VGPR; (B) patients with bone marrow‐confirmed CR who were MRD‐negative or MRD‐positive; (C) MRD‐negative patients who achieved CR flc or other responses. CI: confidence interval; CR: complete response; CR flc: CR with normalized serum free light chain ratio; HR: hazard ratio; NE: not estimatable; VGPR: very good partial response; VTD, bortezomib‐thalidomide‐dexamethasone; VTDC, bortezomib‐thalidomide‐dexamethasone plus cyclophosphamide.
A total of 42 patients with bone marrow‐confirmed CR were available for analysis of outcomes (PFS and OS) according to MRD status: 34 were MRD‐negative and 8 were MRD‐positive by multiparameter flow cytometry. In the primary analysis of PFS, median was not reached in MRD‐negative patients versus 38·6 months in MRD‐positive patients [HR 2·29 (95% CI: 0·87–6·04), P = 0·085]. Per the PFS sensitivity analysis, respective medians were 46·9 versus 38·6 months [HR 1·51 (95% CI: 0·61–3·77), P = 0·373]. OS was longer in MRD‐negative versus MRD‐positive patients [median not reached in either group; HR 3·66 (95% CI: 1·03–13·01), P = 0·032; Fig 3B].
Including those patients who achieved <CR, 42 patients achieved MRD‐negative status across the VTD and VTDC groups. Twenty‐four of these patients had a best response of CRflc, 14 had a response of CR (bone marrow‐confirmed) and 4 had a response of <CR [1 near complete response (nCR), 1 VGPR, 2 PR]. PFS and OS were evaluated in the 24 MRD‐negative CRflc patients versus the 18 MRD‐negative patients achieving ≤CR. In the primary analysis of PFS, median was not reached versus 59·9 months in MRD‐negative CRflc versus ≤CR patients, respectively [HR 1·01 (95% CI: 0·40–2·57), P = 0·982]; in the sensitivity analysis, median PFS was 46·9 versus 55·8 months [HR 0·97 (95% CI: 0·44–2·14), P = 0·941]. Median OS was not reached in either group of MRD‐negative patients [HR 0·56 (95% CI: 0·15–2·09), P = 0·381; Fig 3C].
Safety
There were no new adverse events reported in the study database since the initial report of the study. In addition, no second primary malignancies were reported during the long‐term extension phase.
Discussion
At a median follow‐up of 64·5 months, equating to nearly 3 years of additional follow‐up compared with the primary analysis, data from this phase II extension study (Ludwig et al, 2013) demonstrate that VTD or VTDC induction followed by HDT‐ASCT provides long‐term disease control for patients with previously untreated MM. Across both treatment arms, median time until patients required second‐line therapy was approximately 4 years and approximately two‐thirds of patients were alive at 5 years post‐randomisation. Consistent with the primary analysis (Ludwig et al, 2013), there were no statistically significant differences in long‐term outcomes between the two treatment arms, and outcomes (TTP and PFS) were numerically very similar once asymmetric censoring had been accounted for. A total of 42 (43%) patients achieved MRD‐negativity, highlighting the high activity of the VTD and VTDC induction protocols used here. In the prognostic analyses, patients who achieved MRD‐negativity had particularly promising outcomes, with 5‐year OS rates of approximately 80% and significantly better median OS (HR 3·66, P = 0·032) than patients who remained MRD‐positive. Achievement of CRflc versus ‘standard’ CR or less, however, did not confer additional prognostic significance in MRD‐negative patients. The current analysis thus suggests that MRD‐negativity (versus MRD‐positivity) may be a stronger prognostic marker for OS than bone marrow‐confirmed CRflc [versus other responses (CR/nCR/VGPR/PR)].
Despite a lack of statistically significant differences, the numerical differences in TTP and PFS between the two treatment arms prompted us to check for potential confounding factors. It was observed that there was a marked difference between the two arms in the number of patients who were censored between 24 and 54 months’ follow‐up. This ‘asymmetric censoring’ was caused predominantly by VTD patients receiving subsequent therapy due to ‘relapse from CR’ or ‘clinical relapse’ before they had been recorded as having progressive disease per IMWG criteria (Durie et al, 2006). To account for this censoring imbalance, and potential limitation of the study, TTP and PFS analyses were re‐run so that these patients were considered as having an event at the time of recorded ‘relapse from CR’ or ‘clinical relapse’. Using this approach, the sensitivity analyses revealed very similar median TTP and PFS durations in the VTD and VTDC treatment arms [median TTP, 35·7 vs. 34·5 months (HR 1·26, P = 0·370); median PFS 34·1 vs. 34·2 months (HR 1·20, P = 0·461), respectively]. The findings of the exploratory analysis of TTNT [median 51·8 vs. 47·9 months (HR 1·21, P = 0·484)] were also consistent both with those seen in the sensitivity analyses of TTP and PFS, and with the lack of difference in OS between arms, supporting the finding of no meaningful differences in long‐term outcomes between the two treatment arms. Additionally, the number and type of subsequent lines of therapy were similar in both treatment groups.
Our findings of high activity of VTD induction (Ludwig et al, 2013) that persists over the long term are consistent with results reported for the VTD regimen in the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) MMY‐3006 (Cavo et al, 2010, 2012, 2013) and Programa para el Estudio de la Terapéutica en Hemopatías Malignas/Grupo Español de MM (PETHEMA/GEM) phase III (Rosiñol et al, 2012a,b) trials in newly diagnosed MM, and confirm VTD as one of the most clinically active regimens in this setting. This is supported by a recent meta‐analysis showing significant superiority of VTD over VCD, both in terms of activity and of tolerance (Leiba et al, 2014), and by a retrospective comparison of the VTD and VCD arms of large European trials (Cavo et al, 2014). High activity has also been reported with other proteasome inhibitor‐based triplet induction regimens, such as bortezomib‐doxorubicin‐dexamethasone (PAD) (Sonneveld et al, 2012, 2013), bortezomib‐lenalidomide‐dexamethasone (VRD) (Kumar et al, 2012), and carfilzomib‐lenalidomide‐dexamethasone (Jakubowiak et al, 2012). In the present extension study, addition of a fourth agent, cyclophosphamide, to the VTD triplet did not result in any improvement in efficacy, which is in accordance with the primary analysis data (Ludwig et al, 2013). This observation is also supported by evidence from a previous study, in which a quadruplet regimen incorporating lenalidomide instead of thalidomide [VRD‐cyclophosphamide (VRDC)] was found to yield similar outcomes to triplet induction therapy (VCD or VRD) in previously untreated, transplant‐eligible patients with MM (Kumar et al, 2012). In contrast, data from a randomised study conducted in newly diagnosed, transplant‐ineligible patients suggested that the quadruplet regimen of VMP‐thalidomide (VMPT) may be associated with better outcomes (CR rate, PFS, TTNT and OS) than VMP alone (Palumbo et al, 2014). However, not only were these findings in transplant‐ineligible patients, it should also be noted that VMPT induction was followed by maintenance with bortezomib‐thalidomide, whereas no maintenance was used in the VMP arm, and the induction responses were not consolidated with transplantation. Furthermore, when the data were first published after a median follow‐up of 23·2 months, no difference in OS was noted (Palumbo et al, 2010). Survival curves diverged significantly only after prolonged follow‐up (median 54 months) (Palumbo et al, 2014), indicating the importance of long‐term observation for conclusive evaluation of the impact of a treatment strategy. VMPT was also less well tolerated than VMP (Palumbo et al, 2014).
Multiparameter flow cytometry is a highly sensitive technique for evaluation of MRD in MM that seems to offer similar sensitivity to the polymerase chain reaction (Hart et al, 2012; Martinez‐Lopez et al, 2014; Puig et al, 2014). The flow‐MRD assay used here benefited from a 6‐colour approach that confirmed the clonal nature (through light‐chain restriction) of phenotypically aberrant plasma cells. Using this technique, we showed that MRD‐negative patients had significantly longer OS than those who were MRD‐positive, a finding supported by data from other recent studies in the literature (Korthals et al, 2012; Rawstron et al, 2013; Martinez‐Lopez et al, 2014; Puig et al, 2014). Current efforts are now underway to develop an automated flow‐MRD method based on 10‐colour approaches, with similar sensitivity to next‐generation sequencing techniques. The similar PFS or OS among MRD‐negative patients achieving CRflc versus lower responses confirms the superiority of MRD as a marker for long‐term outcomes in newly diagnosed MM over achievement of a true CR, which requires negative immunofixation. As the detection limit of this technique is around 150 mg/l (Tate et al, 2009), the presence of a substantial number of myeloma cells may be missed. Alternatively, M‐protein may be detectable due to the unusually long half‐life of certain M‐proteins, with recycling of IgG by IgG FcRn receptors (Mead et al, 2004; Paiva et al, 2011) being one potential cause of this phenomenon.
In summary, our long‐term follow‐up data support the notion that three‐drug bortezomib‐based induction regimens are the most appropriate therapies for previously untreated, transplant‐eligible MM. They also confirm VTD as a highly active regimen, providing high response rates and notable long‐term outcome data following a limited period of induction therapy of only four cycles. Lastly, these analyses support the prognostic benefit of achieving MRD‐negative status and the importance of this as a goal of first‐line therapy in MM.
Authorship contributions
Contribution: HL and HvDV designed the research; HL, RG, TM, IS, OS, RH, AD, BP, M‐BV, GE, AMS, DR, SC, OA, CE, HB, HvDV and LV performed the research; HL, RG, TM, IS, OS, RH, AD, BP, M‐BV, GE, AMS and LV collected data; SC, OA and HF performed the statistical analysis; HL, BP, DR, SC, OA, CE, HB and HvdV analysed and interpreted the data; and HL, SC, OA, HF and HvDV wrote the manuscript. All authors reviewed the draft manuscript, and approved the final version for submission.
Conflicts of interest
HL: Honoraria and research funding (Janssen‐Cilag, Celgene, Millenium). RG: Consultant/advisory role (Mundipharma); honoraria (Celgene, Mundipharma). TM: Board membership (Janssen‐Cilag). IS: Board membership (Celgene), consultancy (Celgene, Janssen‐Cilag), research funding (Celgene), honorarium (Celgene, Janssen‐Cilag, Novartis, Amgen). OS: Nothing to disclose. RH: Consultancy (Celgene, Merck, Janssen). AD: Honoraria (Janssen‐Cilag). BP: Honoraria (Millennium, Janssen, Celgene, and The Binding Site). M‐BV: Nothing to disclose. GE: Consultancy (Janssen, Celgene); honoraria (Janssen, Celgene). AMS: Nothing to disclose. DR: Employment (Janssen Global Services, LLC). SC: Employment (Janssen Research & Development, LLC), holds stock (Johnson & Johnson). OA: Employment (Janssen‐Cilag Limited). CE: Employment (Janssen Research & Development, LLC), holds stock (Johnson & Johnson). HF: Employment (Janssen Research & Development, LLC). HvdV: Employment (Janssen Pharmaceutica NV), holds stock (Johnson & Johnson). LV: Nothing to disclose.
Supporting information
Fig S1. Updated CONSORT diagram showing patient flow through the study.
Acknowledgments
The authors would like to acknowledge the writing support of Jane Saunders of FireKite, an Ashfield Company, during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc. and Janssen Global Services, LLC. This study was supported by research funding from Janssen Research & Development.
Trial registration: clinicaltrials.gov identifier: NCT00531453.
References
- Anderson, K.C. , Alsina, M. , Bensinger, W. , Biermann, J.S. , Cohen, A.D. , Devine, S. , Djulbegovic, B. , Faber, E.A. Jr , Gasparetto, C. , Hernandez‐Illizaliturri, F. , Huff, C.A. , Kassim, A. , Krishnan, A.Y. , Liedtke, M. , Meredith, R. , Raje, N. , Schriber, J. , Singhal, S. , Somlo, G. , Stockerl‐Goldstein, K. , Treon, S.P. , Weber, D. , Yahalom, J. , Yunus, F. , Shead, D.A. & Kumar, R. (2013) Multiple myeloma, version 1.2013. Journal of the National Comprehensive Cancer Network, 11, 11–17. [DOI] [PubMed] [Google Scholar]
- Cavo, M. , Tacchetti, P. , Patriarca, F. , Petrucci, M.T. , Pantani, L. , Galli, M. , Di, R.F. , Crippa, C. , Zamagni, E. , Palumbo, A. , Offidani, M. , Corradini, P. , Narni, F. , Spadano, A. , Pescosta, N. , Deliliers, G.L. , Ledda, A. , Cellini, C. , Caravita, T. , Tosi, P. & Baccarani, M. (2010) Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. The Lancet, 376, 2075–2085. [DOI] [PubMed] [Google Scholar]
- Cavo, M. , Pantani, L. , Petrucci, M.T. , Patriarca, F. , Zamagni, E. , Donnarumma, D. , Crippa, C. , Boccadoro, M. , Perrone, G. , Falcone, A. , Nozzoli, C. , Zambello, R. , Masini, L. , Furlan, A. , Brioli, A. , Derudas, D. , Ballanti, S. , Dessanti, M.L. , de Stefano, V. , Carella, A.M. , Marcatti, M. , Nozza, A. , Ferrara, F. , Callea, V. , Califano, C. , Pezzi, A. , Baraldi, A. , Grasso, M. , Musto, P. & Palumbo A. (2012) Bortezomib‐thalidomide‐dexamethasone is superior to thalidomide‐dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood, 120, 9–19. [DOI] [PubMed] [Google Scholar]
- Cavo, M. , Galli, M. , Pezzi, A. , di Raimondo, F. , Crippa, C. , Offidani, M. , Tacchetti, P. , Montefusco, V. , Narni, F. , Spadano, A. , Pescosta, N. , Zamagni, E. , Gamberi, B. , Caravita, T. , Falcone, A.P. , Nozzoli, C. , Zambello, R. , Furlan, A. , Brioli, A. & Boccadaro, M. (2013) Persistent improvement in clinical outcomes with bortezomib‐thalidomide‐dexamethasone vs thalidomide‐dexamethasone incorporated into double autologous transplantation for multiple myeloma: an updated analysis of phase 3 GIMEMA‐MMY‐3006 study. Blood, 122, 2090. [Google Scholar]
- Cavo, M. , Pantani, L. , Pezzi, A. , Cavallo, F. , Petrucci, M.T. , Di Raimondo, F. , Patriarca, F. , Waage, A. , Zamagni, E. , Montefusco, V. , Galli, M. , Gamberi, B. , Rossi, G. , Tacchetti, P. , Grasso, M. , Zweegman, S. , Offidani, M. , Ballanti, S. , Zambello, R. , Liberati, A.M. , Bassan, R. , Pregno, P. , Palumbo, A. & Sonneveld, P. (2014) Superior efficacy of VTD over VCD as induction therapy for autotransplantation‐eligible, newly diagnosed, myeloma patients. Blood, 124, 197. [Google Scholar]
- Durie, B.G. , Harousseau, J.L. , Miguel, J.S. , Blade, J. , Barlogie, B. , Anderson, K. , Gertz, M. , Dimopoulos, M. , Westin, J. , Sonneveld, P. , Ludwig, H. , Gahrton, G. , Beksac, M. , Crowley, J. , Belch, A. , Boccadaro, M. , Cavo, M. , Turesson, I. , Joshua, D. , Vesole, D. , Kyle, R. , Alexanian, R. , Tricot, G. , Attal, M. , Merlini, G. , Powles, R. , Richardson, P. , Shimizu, K. , Tosi, P. , Morgan, G. & Rajkumar, S.V. (2006) International uniform response criteria for multiple myeloma. Leukemia, 20, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Greipp, P.R. , San Miguel, J. , Durie, B.G. , Crowley, J.J. , Barlogie, B. , Bladé, J. , Boccadoro, M. , Child, J.A. , Avet‐Loiseau, H. , Kyle, R.A. , Lahuerta, J.J. , Ludwig, H. , Morgan, G. , Powles, R. , Shimizu, K. , Shustik, C. , Sonneveld, P. , Tosi, P. , Turesson, I. & Westin, J. (2005) International staging system for multiple myeloma. Journal of Clinical Oncology, 23, 3412–3420. [DOI] [PubMed] [Google Scholar]
- Hart, A.J. , Jagasia, M.H. , Kim, A.S. , Mosse, C.A. , Savani, B.N. & Kassim, A. (2012) Minimal residual disease in myeloma: are we there yet? Biology of Blood and Marrow Transplantation, 18, 1790–1799. [DOI] [PubMed] [Google Scholar]
- Jakubowiak, A.J. , Griffith, K.A. , Reece, D.E. , Hofmeister, C.C. , Lonial, S. , Zimmerman, T.M. , Campagnaro, E.L. , Schlossman, R.L. , Laubach, J.P. , Raje, N.S. , Anderson, T. , Mietzel, M.A. , Harvey, C.K. , Wear, S.M. , Barrickman, J.C. , Tendler, C.L. , Esseltine, D.L. , Kelley, S.L. , Kaminski, M.S. , Anderson, K.C. & Richardson, P.G. (2011) Lenalidomide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone in newly diagnosed multiple myeloma: a phase 1/2 Multiple Myeloma Research Consortium trial. Blood, 118, 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowiak, A.J. , Dytfeld, D. , Griffith, K.A. , Lebovic, D. , Vesole, D.H. , Jagannath, S. , Al‐Zoubi, A. , Anderson, T. , Nordgren, B. , Detweiler‐Short, K. , Stockerl‐Goldstein, K. , Ahmed, A. , Jobkar, T. , Durecki, D.E. , McDonnell, K. , Mietzel, M. , Couriel, D. , Kaminski, M. & Vij, R. (2012) A phase 1/2 study of carfilzomib in combination with lenalidomide and low‐dose dexamethasone as a frontline treatment for multiple myeloma. Blood, 120, 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthals, M. , Sehnke, N. , Kronenwett, R. , Bruns, I. , Mau, J. , Zohren, F. , Haas, R. , Kobbe, G. & Fenk, R. (2012) The level of minimal residual disease in the bone marrow of patients with multiple myeloma before high‐dose therapy and autologous blood stem cell transplantation is an independent predictive parameter. Biology of Blood and Marrow Transplantation, 18, 423–431. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Flinn, I. , Richardson, P.G. , Hari, P. , Callander, N. , Noga, S.J. , Stewart, A.K. , Turturro, F. , Rifkin, R. , Wolf, J. , Estevam, J. , Mulligan, G. , Shi, H. , Webb, I.J. & Rajkumar, S.V. (2012) Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood, 119, 4375–4382. [DOI] [PubMed] [Google Scholar]
- Leiba, M. , Kedmi, M. , Duek, A. , Freidman, T. , Weiss, M. , Leiba, R. , Nagler, A. & Avigdor, A. (2014) Bortezomib‐Cyclophosphamide‐Dexamethasone (VCD) versus Bortezomib‐Thalidomide‐Dexamethasone (VTD) ‐based regimens as induction therapies in newly diagnosed transplant eligible patients with multiple myeloma: a meta‐analysis. British Journal of Haematology, 166, 702–710. [DOI] [PubMed] [Google Scholar]
- Ludwig, H. , Viterbo, L. , Greil, R. , Masszi, T. , Spicka, I. , Shpilberg, O. , Hajek, R. , Dmoszynska, A. , Paiva, B. , Vidriales, M.B. , Esteves, G. , Stoppa, A.M. , Robinson, D. Jr , Ricci, D. , Cakana, A. , Enny, C. , Feng, H. , van de Velde, H. & Harousseau J.L. (2013) Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. Journal of Clinical Oncology, 31, 247–255. [DOI] [PubMed] [Google Scholar]
- Ludwig, H. , Sonneveld, P. , Davies, F. , Bladé, J. , Boccadoro, M. , Cavo, M. , Morgan, G. , de la Rubia, J. , Delforge, M. , Dimopoulos, M. , Einsele, H. , Facon, T. , Goldschmidt, H. , Moreau, P. , Nahi, H. , Plesner, T. , San‐Miguel, J. , Hajek, R. , Sondergeld, P. & Palumbo, A. (2014) European perspective on multiple myeloma treatment strategies in 2014. Oncologist, 19, 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Lopez, J. , Lahuerta, J.J. , Pepin, F. , Gonzalez, M. , Barrio, S. , Ayala, R. , Puig, N. , Montalban, M.A. , Paiva, B. , Weng, L. , Jimenez, C. , Sopena, M. , Moorhead, M. , Cedena, T. , Rapado, I. , Mateos, M.V. , Rosinol, L. , Oriol, A. , Blanchard, M.J. , Martinez, R. , Blade, J. , San, M.J. , Faham, M. & Garcia‐Sanz, R. (2014) Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood, 123, 3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos, M.V. , Oriol, A. , Martinez‐Lopez, J. , Teruel, A.I. , de la Lopez, G.A. , Lopez, J. , Bengoechea, E. , Perez, M. , Martinez, R. , Palomera, L. , de Arriba, F. , Gonzalez, Y. , Hernandez, J.M. , Granell, M. , Bello, J.L. , Bargay, J. , Penalver, F.J. , Martin‐Mateos, M.L. , Paiva, B. , Montalban, M.A. , Blade, J. , Lahuerta, J.J. , & San‐Miguel J.F. (2014) GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: Do we still need alkylators? Blood, 124, 1887–1893. [DOI] [PubMed] [Google Scholar]
- Mead, G.P. , Carr‐Smith, H.D. , Drayson, M.T. , Morgan, G.J. , Child, J.A. & Bradwell, A.R. (2004) Serum free light chains for monitoring multiple myeloma. British Journal of Haematology, 126, 348–354. [DOI] [PubMed] [Google Scholar]
- Moreau, P. , Avet‐Loiseau, H. , Facon, T. , Attal, M. , Tiab, M. , Hulin, C. , Doyen, C. , Garderet, L. , Randriamalala, E. , Araujo, C. , Lepeu, G. , Marit, G. , Caillot, D. , Escoffre, M. , Lioure, B. , Benboubker, L. , Pegourie, B. , Kolb, B. , Stoppa, A.M. , Fuzibet, J.G. , Decaux, O. , Dib, M. , Berthou, C. , Chaleteix, C. , Sebban, C. , Traulle, C. , Fontan, J. , Wetterwald, M. , Lenain, P. , Mathiot, C. & Harousseau, J.L. (2011) Bortezomib plus dexamethasone versus reduced‐dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood, 118, 5752–5758. [DOI] [PubMed] [Google Scholar]
- Paiva, B. , Martinez‐Lopez, J. , Vidriales, M.B. , Mateos, M.V. , Montalban, M.A. , Fernandez‐Redondo, E. , Alonso, L. , Oriol, A. , Teruel, A.I. , de Paz, R. , Larana, J.G. , Bengoechea, E. , Martin, A. , Mediavilla, J.D. , Palomera, L. , de Arriba, F. , Blade, J. , Orfao, A. , Lahuerta, J.J. & San Miguel J.F. (2011) Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. Journal of Clinical Oncology, 29, 1627–1633. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. , Bringhen, S. , Rossi, D. , Cavalli, M. , Larocca, A. , Ria, R. , Offidani, M. , Patriarca, F. , Nozzoli, C. , Guglielmelli, T. , Benevolo, G. , Callea, V. , Baldini, L. , Morabito, F. , Grasso, M. , Leonardi, G. , Rizzo, M. , Falcone, A.P. , Gottardi, D. , Montefusco, V. , Musto, P. , Petrucci, M.T. , Ciccone, G. & Boccadoro, M. (2010) Bortezomib‐melphalan‐prednisone‐thalidomide followed by maintenance with bortezomib‐thalidomide compared with bortezomib‐melphalan‐prednisone for initial treatment of multiple myeloma: A randomized controlled trial. Journal of Clinical Oncology, 28, 5101–5109. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. , Bringhen, S. , Larocca, A. , Rossi, D. , Di, R.F. , Magarotto, V. , Patriarca, F. , Levi, A. , Benevolo, G. , Vincelli, I.D. , Grasso, M. , Franceschini, L. , Gottardi, D. , Zambello, R. , Montefusco, V. , Falcone, A.P. , Omede, P. , Marasca, R. , Morabito, F. , Mina, R. , Guglielmelli, T. , Nozzoli, C. , Passera, R. , Gaidano, G. , Offidani, M. , Ria, R. , Petrucci, M.T. , Musto, P. , Boccadoro, M. & Cavo, M. (2014) Bortezomib‐melphalan‐prednisone‐thalidomide followed by maintenance with bortezomib‐thalidomide compared with bortezomib‐melphalan‐prednisone for initial treatment of multiple myeloma: Updated follow‐up and improved survival. Journal of Clinical Oncology, 32, 634–640. [DOI] [PubMed] [Google Scholar]
- Puig, N. , Sarasquete, M.E. , Balanzategui, A. , Martinez, J. , Paiva, B. , Garcia, H. , Fumero, S. , Jimenez, C. , Alcoceba, M. , Chillon, M.C. , Sebastian, E. , Marin, L. , Montalban, M.A. , Mateos, M.V. , Oriol, A. , Palomera, L. , de la Rubia, J. , Vidriales, M.B. , Blade, J. , Lahuerta, J.J. , Gonzalez, M. , Miguel, J.F. & Garcia‐Sanz, R. (2014) Critical evaluation of ASO RQ‐PCR for minimal residual disease evaluation in multiple myeloma. A comparative analysis with flow cytometry. Leukemia, 28, 391–397. [DOI] [PubMed] [Google Scholar]
- Rawstron, A.C. , Child, J.A. , de Tute, R.M. , Davies, F.E. , Gregory, W.M. , Bell, S.E. , Szubert, A.J. , Navarro‐Coy, N. , Drayson, M.T. , Feyler, S. , Ross, F.M. , Cook, G. , Jackson, G.H. , Morgan, G.J. & Owen, R.G. (2013) Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: Impact on outcome in the Medical Research Council Myeloma IX Study. Journal of Clinical Oncology, 31, 2540–2547. [DOI] [PubMed] [Google Scholar]
- Reeder, C.B. , Reece, D.E. , Kukreti, V. , Chen, C. , Trudel, S. , Laumann, K. , Hentz, J. , Pirooz, N.A. , Piza, J.G. , Tiedemann, R. , Mikhael, J.R. , Bergsagel, P.L. , Leis, J.F. , Fonseca, R. & Stewart, A.K. (2010) Once‐ versus twice‐weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood, 115, 3416–3417. [DOI] [PubMed] [Google Scholar]
- Rosiñol, L. , Oriol, A. , Teruel, A.I. , Hernandez, D. , Lopez‐Jimenez, J. , de la Rubia, J. , Granell, M. , Besalduch, J. , Palomera, L. , Gonzalez, Y. , Etxebeste, M.A. , Diaz‐Mediavilla, J. , Hernandez, M.T. , de Arriba, F. , Gutiérrez, N.C. , Martín‐Ramos, M.L. , Cibeira, M.T. , Mateos, M.V. , Martínez, J. , Alegre, A. , Lahuerta, J.J. , San Miguel, J. & Blade, J. (2012a) Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: A randomized phase 3 PETHEMA/GEM study. Blood, 120, 1589–1596. [DOI] [PubMed] [Google Scholar]
- Rosiñol, L. , Oriol, A. , Teruel, A.I. , Hernandez, D. , Lopez‐Jimenez, J. , de la Rubia, J. , Granell, M. , Besalduch, J. , Palomera, L. , Gonzalez, Y. , Etxebeste, M.A. , Diaz‐Mediavilla, J. , Hernandez, M.T. , de Arriba, F. , Alegre, A. , Cibeira, M.T. , Mateos, M.V. , Martinez, J. , Lahuerta, J.J. , San Miguel, J.F. & Bladé, J. (2012b) Maintenance therapy after stem‐cell transplantation for multiple myeloma with bortezomib/thalidomide vs. thalidomide vs. alfa2b‐interferon: Final results of a phase III PETHEMA/GEM randomized trial. Blood, 120, 334. [Google Scholar]
- Sonneveld, P. , Schmidt‐Wolf, I.G. , van der Holt, B. , El, J.L. , Bertsch, U. , Salwender, H. , Zweegman, S. , Vellenga, E. , Broyl, A. , Blau, I.W. , Weisel, K.C. , Wittebol, S. , Bos, G.M. , Stevens‐Kroef, M. , Scheid, C. , Pfreundschuh, M. , Hose, D. , Jauch, A. , van der Velde, H. , Raymakers, R. , Schaafsma, M.R. , Kersten, M.J. , van Marwijk‐Kooy, M. , Duehrsen, U. , Lindemann, W. , Wijermans, P.W. , Lokhorst, H.M. & Goldschmidt, H.M. (2012) Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON‐65/GMMG‐HD4 trial. Journal of Clinical Oncology, 30, 2946–2955. [DOI] [PubMed] [Google Scholar]
- Sonneveld, P. , Scheid, C. , van der Holt, B. , el Jarari, L. , Bertsch, U. , Salwender, H. , Zweegman, S. , Vellenga, E. , Broyl, A. , Blau, I.W. , Weisel, K. , Wittebol, S. , Bos, G.M. , Stevens, M. , Schmidt‐Wolf, I.G. , Pfreundschuh, M. , Hose, D. , Jauch, A. , van de Velde, H. , Raymakers, R. , Schaafsma, M.R. , Kersten, M.J. , van Marwijk‐Kooy, M. , Duehrsen, U. , Lindemann, H.W. , Wijermans, P.W. , Lokhorst, H. & Goldschmidt, H. (2013) Bortezomib induction and maintenance treatment improves survival in patients with newly diagnosed multiple myeloma: Extended follow‐up of the HOVON‐65/GMMG‐HD4 trial. Blood, 122, 404. [Google Scholar]
- Tate, J. , Bazeley, S. , Sykes, S. & Mollee, P. (2009) Quantitative serum free light chain assay‐analytical issues. The Clinical Biochemist Reviews, 30, 131–140. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Updated CONSORT diagram showing patient flow through the study.


