Abstract
Severe sepsis is defined by organ failure, often of the kidneys, heart, and brain. It has been proposed that inadequate delivery of oxygen, or insufficient extraction of oxygen in tissue, may explain organ failure. Despite adequate maintenance of systemic oxygen delivery in septic patients, their morbidity and mortality remain high.
The assumption that tissue oxygenation can be preserved by maintaining its blood supply follows from physiological models that only apply to tissue with uniformly perfused capillaries. In sepsis, the microcirculation is profoundly disturbed, and the blood supply of individual organs may therefore no longer reflect their access to oxygen.
We review how capillary flow patterns affect oxygen extraction efficacy in tissue, and how the regulation of tissue blood flow must be adjusted to meet the metabolic needs of the tissue as capillary flows become disturbed as observed in critical illness. Using the brain, heart, and kidney as examples, we discuss whether disturbed capillary flow patterns might explain the apparent mismatch between organ blood flow and organ function in sepsis. Finally, we discuss diagnostic means of detecting capillary flow disturbance in animal models and in critically ill patients, and address therapeutic strategies that might improve tissue oxygenation by modifying capillary flow patterns.
Editorial comment: what this article tells us.
In this narrative review article, the relations of microcirculatory blood flow and oxygen delivery in vital organs during severe sepsis are explored. Disturbances in oxygen extraction efficiency are examined, together with diagnostic and therapeutic aspects in critically ill patients.
Septic patients often develop multi‐organ failure (MOF) despite therapeutic maintenance of normal blood pressure and adequate blood oxygenation.1 Although their systemic circulation is characterized by high cardiac output and low peripheral resistance,2 peripheral oxygen extraction is low, and the elevated oxygen needs of tissue in septic patients can seemingly no longer be met, as they progress into severe sepsis or septic shock.3
Apparently, MOF is not the result of insufficient blood supply to individual organs, and attempts to improve outcome by increasing oxygen delivery have yielded conflicting results.4 In the kidney, acute renal failure (ARF) in sepsis is associated with marked reductions of glomerular filtration rate and urine output. Nevertheless, renal vascular resistance is low and blood flow seemingly normal, or even elevated, in ARF,5 suggesting that an overabundance of oxygen somehow fails to support the energy demands of glomerular filtration. Myocardial depression and elevated serum levels of cardiac troponin are common in sepsis, and associated with poor outcome.6, 7 These findings are difficult to reconcile with the normal or even elevated myocardial blood flow found in sepsis.8, 9 Brain function is often affected very early in sepsis, so‐called septic encephalopathy, and survivors often show ischemic lesions on magnetic resonance imaging (MRI), suffering severe permanent neurological sequelae.10 Despite findings of suppressed oxygen metabolism and cerebral blood flow (CBF) in sepsis patients with neurological symptoms,11, 12 their CBF values remain well above the accepted threshold of cerebral ischemia.13
Mitochondrial dysfunction is a likely contributor to the profound imbalance between oxygen availability and oxygen utilization in sepsis – see Singer for a comprehensive review.14 This review addresses functional shunting 15 as another contributor, noting that tissue oxygen tension (PtO2) is expected to be normal or elevated in mitochondrial dysfunction, but low if oxygenated blood is shunted through the microvasculature. Microvascular shunting can occur either by anatomical arteriolo‐venular shunts, by rapid oxygen diffusion from arterioles to veins,16 or by a pathological redistribution of capillary flows such that some cells are deprived of oxygen while some capillaries are perfused in excess of what can be extracted by the tissue.15, 17 Sepsis is indeed linked to profound, generalized changes in the tissue microcirculation,18 including reductions in the proportion of perfused capillaries, and the appearance of intermittent or sluggish passage of erythrocytes through some capillary paths.19
Using the brain, heart, and kidney as examples, we discuss whether pathological redistribution of capillary flow patterns may suffice to explain the apparent mismatch between tissue blood flow and organ function in sepsis. Finally, we discuss diagnostic means of detecting capillary flow disturbance in animal models and in the critically ill patient, and therapeutic strategies to improve tissue oxygenation by improving capillary flow patterns.
Tissue blood flow, capillary blood flow patterns, and oxygen availability
The relation between tissue blood supply (ml blood per 100 ml tissue per minute) and tissue oxygenation – defined as the maximum metabolic rate of oxygen (ml oxygen per 100 ml tissue per minute) that can be supported by this blood flow – is not linear per se as one might expect. Rather, studies of solute extraction from individual capillaries show that oxygen extraction is less effective at high flow rates because erythrocytes pass through the microvasculature at transit times that are too short to permit complete extraction of their oxygen load – so‐called functional shunting. The text‐book flow‐diffusion equation 20 uses these single‐capillary properties to predict the relation between tissue blood supply and its oxygen availability. While the relation is not linear for the reasons stated above, it has two fundamental properties: (i) there is a one‐to‐one relation between tissue blood supply and tissue oxygenation, and (ii) increase tissue blood supply always improves tissue oxygenation. As illustrated by Fig. 1, these widely held assumptions are only true if all tissue capillaries have identical flow velocities. In the normal tissue, however, capillary flow patterns are highly heterogeneous during rest21, 22, 23 but redistribute to a more homogenous pattern when the flux of erythrocytes increases,24 permitting a more efficient oxygen extraction during hyperemia than one would expect based on the flow‐diffusion equation. Failure of this intrinsic capillary flow homogenization during hyperemia can have dramatic consequences, as shown in Fig. 1D: The resulting increase in functional shunting can cause tissue oxygenation to decrease when blood supply increases. Conversely, a reduction in blood flow can, paradoxically, improve tissue oxygenation if accompanied by a homogenization of capillary flow patterns.
Figure 1.
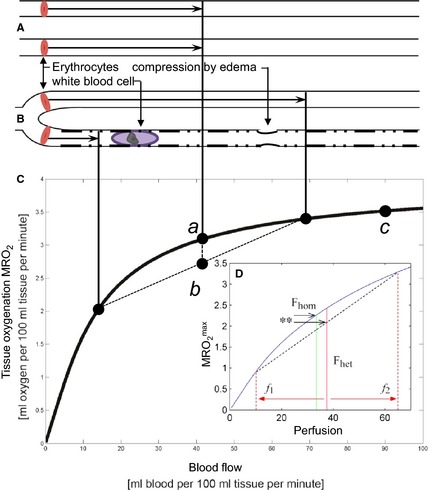
The flow‐diffusion equation for oxygen. The flow‐diffusion equation curve (Panel C) shows the amount of oxygen, which can diffuse into tissue from its capillaries, as a function of tissue blood flow. Until recently, this text‐book relation has been used without attention to its underlying assumption: That all erythrocytes pass through tissue capillaries at identical velocity, as illustrated in Panel A. As shown in Panel B, this ‘hidden assumption’ is critical: Any deviations from this ‘homogeneity requirement’ lead us to overestimate tissue oxygenation if we base our oxygenation assessments on tissue blood flow. This is most easily realized by the following thought experiment: Imagine that blood cell velocities slows down in half of all tissue capillaries – but speed up in the remaining capillaries such that total tissue blood flow remains identical to that in Panel A. The homogeneity requirement applies to both ‘slow’ and ‘fast’ capillaries in Panel B – and the net oxygen availability in Panel B is therefore the average labeled b – and thus always lower than a, the net oxygen availability in Panel B. Note how homogenization + hyperperfusion (b → c) provides a larger increase in tissue oxygenation than hyperperfusion with homogenous capillary flow (a → c). Erythrocyte velocities in fact homogenize during hyperemia, counteracting the tendency of curve C to yield less extra oxygen per unit blood flow increase as flow increases.22 In the next thought experiment, we increase the average flow velocity in Panel A from Fhom to Fhet, and then slow half of the capillaries as in Panel B – giving rise to populations of capillaries with flows f1 and f2, and net tissue blood flow Fhet. Note how tissue oxygen availability in fact decreases although blood flow increased, as indicated by the double asterisk. Conversely, a reduction in blood flow can paradoxically improve tissue oxygenation if capillary flow patterns are homogenized in parallel. Capillary patency may hence be crucial for the validity of fundamental physiological properties that we normally take for granted. The hindered capillary passage indicated in Panel B is the sum of pre‐existing age‐ or disease‐related changes, and sepsis‐related changes such as increased white blood cell numbers, altered endothelial surface properties (loss of glycocalyx, and so forth), and/or external edema pressure.
The oxygenation effects of capillary flow patterns illustrated above underscores the importance of blood rheology and capillary patency in sepsis when considering whether a certain blood supply optimizes organ oxygenation. Such functional shunting effects might indeed add to the mismatch between tissue blood flow and organ function in critical illness.
We extended the classical flow‐diffusion equation to take the biophysical effects of capillary transit time heterogeneity (CTH) into account and refer the reader to Jespersen and Østergaard22 and Østergaard et al.25 for details on the model and its application to brain and heart. To address renal oxygenation in this report, we extended our model to renal cortex and medulla – see Fig. 2 for details and model parameters.
Figure 2.
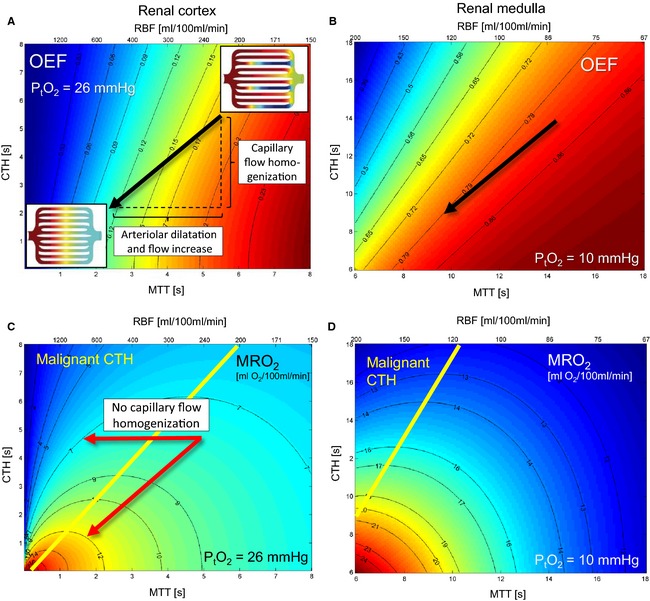
Effects of mean transit time (MTT) and capillary transit time heterogeneity (CTH) on oxygen extraction in the kidney for fixed oxygen tension (PtO2). Rather than the two capillary flow values assumed in Fig. 1, we now use an accepted distribution for capillary transit times (a so‐called gamma‐variate distribution),90 and use the standard deviation of this to quantify what we refer to as CTH. The mean value of this distribution – the MTT – is related to renal blood flow (RBF) and capillary blood volume (CBV) through the central volume theorem, RBF = RBV/MTT.91 Our extended flow‐diffusion equation22 estimates the oxygen extraction fraction (OEF) that corresponds to a given MTT, CTH, and tissue oxygen tension (PtO2) in steady‐state. Then, tissue oxygenation (the metabolic rate of oxygen, MRO 2, supported by a given MTT and CTH at a certain PtO2) is given by OEF times the arterial oxygen concentration (0.19 ml/ml), times RBF. Note that tissue MTT and RBF can be used interchangeably when tissue CBV is known. Figure 2A and B show contour plots of OEF for combinations of MTT and CTH in the renal cortex and medulla, for tissue oxygen tensions of 26 mmHg and 10 mmHg, respectively. In this figure, consider a steady‐state where oxygen availability matches oxygen utilization at a certain PtO2. In Fig. 3, consider this condition over a range of tissue oxygen tensions, and hence values of OEF. Note that the OEF always increases with increasing MTT and with decreasing CTH. The arrow indicates the changes in MTT and CTH that typically occur during hyperemic episodes in brain: It appears to be an inherent property of normal, passive capillary beds that CTH changes in proportion to MTT, which helps maintain efficient oxygen extraction during hyperperfusion, without fluctuations in tissue oxygen tension.22, 24 The corresponding tissue oxygen availability, MRO 2, is shown in units of ml O2/100 ml/min in Fig. 2C and D. Note that the tissue oxygen availability MRO 2 always increases with decreasing CTH – but not necessarily with RBF: Hemodynamic states above the yellow line in 2C and D are unique in that reductions in MTT (increases in RBF) fail to increase tissue oxygen availability: These states are referred to as having malignant CTH. The horizontal arrow in Panel C again demonstrates why the reduction in CTH during hyperemia is so crucial: The oblique arrow shows how oxygenation increases about 50% (from 8 to 12 ml O2/100 ml/min) when MTT and CTH change in parallel. In the absence of capillary flow homogenization (horizontal arrow), however, the same vasodilation and increase in blood flow reduced oxygenation from 8 to 7 ml/100 ml/min. For the renal cortex, we fixed k, the bidirectional rate constant for oxygen transport across the capillary wall – and our extended flow‐diffusion equation's only unknown parameter22 – by literature values obtained in dog and man: At a RBF of 440 ml/100 ml/min, an OEF of 0.12 was used, assuming CBV = 20 ml/100 ml (corresponding to MTT = 2.7 s), and PtO2 = 26 mmHg.92, 93 For the renal medulla, the corresponding values were OEF = 0.8, RBF = 110 ml/100 ml/min, and PtO2 = 10 mmHg, at a similar CBV (corresponding to MTT = 11 s).94 In both tissue types, CTH was set to 0.95∙MTT during this calibration step, based on experimental data used in our previous analysis.22 Figure 2C and D were constructed assuming normal arterial blood oxygen concentrations (0.19 ml/ml) and absence of capillary occlusions (fraction of open capillaries = 1.0). These MRO values can easily be corrected for any deviations from these assumptions in that MRO 2 scales with both the fraction of perfused capillaries (e.g., as observed by side‐stream dark‐field imaging) and arterial oxygen saturation. For example, a reduction in oxygen saturation from 100% to 95% and a reduction in the proportion of perfused capillaries from 100% to 90%, leads to a correction factor of 0.95∙0.9 = 0.855, i.e., a 14.5% reduction in the oxygen availabilities in Fig. 2C and D.
Matching blood flow to the metabolic needs of kidney, heart, and brain during capillary dysfunction
The combined effect of capillary flow disturbances (elevated CTH), tissue blood flow (TBF), and tissue oxygen tension (PtO2), are shown in Fig. 3 for the renal cortex and medulla, the heart, and the brain.26 The green surface in each plot surrounds combinations of MTT, CTH, and PtO2 that, biophysically, can support the metabolic needs of these organs in the resting, awake state. The red plane marks the boundary, left of which vasodilation no longer improves tissue oxygen availability – so‐called malignant CTH.
Figure 3.
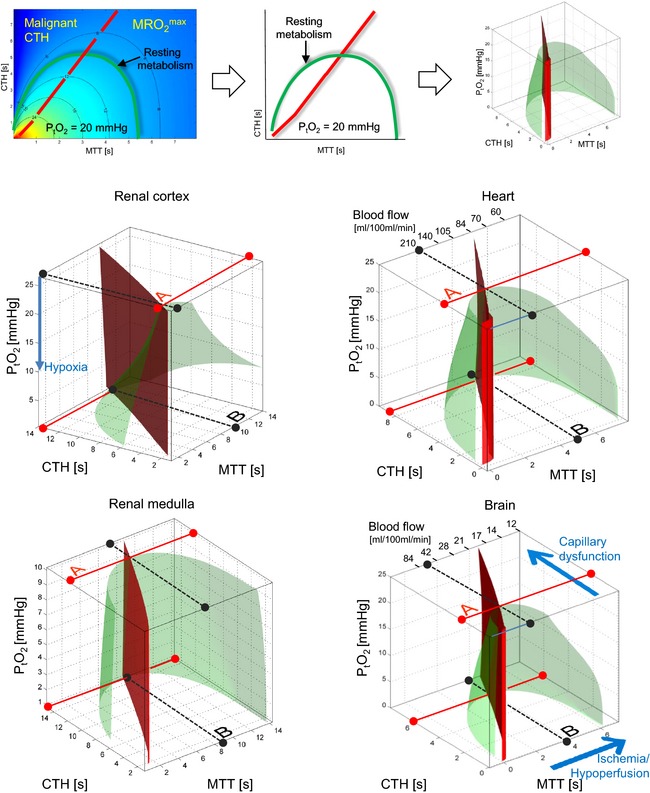
Tissue hypoperfusion, hyperperfusion, and capillary dysfunction (elevated CTH) can all lead to critical reductions in oxygen availability. The top row illustrates how the green surface in each plot is generated by joining the MRO 2 iso‐contours (cf. Fig. 2C and D) that correspond to these organs’ resting metabolic rate of oxygen across all values of PtO2. The green half‐cone therefore contain combinations of MTT, CTH, and PtO2 that, biophysically, can support the metabolic needs of renal cortex and medulla, heart, and brain, in the resting, awake state. The red plane marks the boundary, left of which vasodilation no longer improves tissue oxygen availability (malignant CTH). The full, red lines (labeled A) illustrates how much capillary flow patterns can be disturbed before the tissue's loss of oxygenated blood due functional shunting threatens each organs resting metabolism at its ‘normal’ PtO2. In reality, tissue oxygen utilization increases OEF and result in a parallel decrease in PtO2 as oxygen delivery approaches the rate of utilization in tissue. As capillary flow patterns become more disturbed, PtO2 is therefore expected to fall. Biophysically, tissue can maintain sufficient oxygen supplies to support its resting metabolism until CTH reaches a critical, upper limit (full, red line at zero PtO2). Note how this requires the loss of oxygenated blood to be attenuated – by a reduction in blood flow. Accordingly, the ‘optimal blood flow’ as capillary flows become critically heterogeneous (labeled B), is lower than each organs normal, resting blood flow. The blood flow that optimizes total oxygen extraction at critically elevated CTH is thus roughly 85 ml/100 ml/min in the heart and 21 ml/10 ml/min in the brain, compared to normal resting flow values of roughly 100 ml/100 ml/min in the heart and 45 ml/10 ml/min in the brain. Note that resting oxygen utilization in the renal medulla can only be supported within a narrow range of flow values only (corresponding to MTT between 8 and 12 s) when CTH becomes high (larger than 14 s). Surprisingly, relative hypoperfusion is therefore energetically favorable in conditions of elevated CTH, as we expect in sepsis. Also note that blood flow must stay within increasingly narrow limits as CTH approaches its critical, upper limit and PtO2 approaches zero: Here, any further increase in CTH, any changes in blood flow (both increases and reductions), any reductions in arterial oxygen content, and any loss of perfused capillaries (See legend of Fig. 2 for the effects of reduced capillary volume and oxygen saturation) will reduce oxygen availability below the needs of normal tissue function. The figures for brain and heart were adapted from Jespersen and Østergaard22 and Østergaard et al.25.
All tissue types share common features: At normal tissue oxygen tension, the metabolic needs of resting tissue can no longer be met if CTH grows beyond a tissue‐specific threshold, defined by the full red lines labeled ‘A’ in the figures. For higher CTH values, these metabolic needs can only be met because of parallel reductions in blood flow (to reduce functional shunting) and in PtO2, caused by continued oxygen utilization in the tissue and more efficient oxygen extraction. Biophysically, these mechanisms can only support tissue function until PtO2 becomes negligible. The corresponding CTH thresholds are indicated as full red lines at zero PtO2 in Fig. 3. Note that as CTH approaches this threshold, even small changes – decreases as well as increases – in blood flow can cause critical reductions in tissue oxygenation and thereby affect tissue function.
The dotted black lines (labeled B) in Fig. 3 indicate the ‘optimal blood flow’ in terms of maintaining tissue function during critical CTH increases. Note that the highest degree of capillary flow disturbances can be sustained if blood flow is suppressed considerably compared to resting, normal values. One notable exception is the renal medulla, in which oxygen tension is already low, and oxygen extraction high, in the normal state. The maximum tolerable CTH (red solid line at PtO2) is therefore only slightly higher than ‘physiological’ CTH values at normal tissue oxygen tension (labeled A), and sufficient oxygenation can therefore only be maintained within a narrow range of flow values once CTH increases. These properties imply that that once the function of the medulla (such as urine production) is affected by disturbances in the microcirculation (i.e., CTH becomes critically high), then subsequent maintenance of renal function is contingent on the maintenance of renal medullar perfusion within a very narrow range.
The relation between microcirculatory changes, organ function, and hypoperfusion in critical illness
The extended flow‐diffusion gives rise to three important predictions with regard to hemodynamics and tissue oxygenation when the microcirculation is disturbed, e.g., in sepsis: First, increases in CTH, even in the absence of capillary occlusions, can reduce oxygen availability below the metabolic needs of the tissue and thereby cause organ failure or even tissue damage. Second, increasing CTH can be compensated for by reductions in tissue blood flow and tissue oxygen tension relative to their values in healthy tissue. Our analysis therefore suggests that attenuated blood pressure and organ perfusion, paradoxically, may facilitate the maintenance of tissue oxygenation in conditions where CTH is elevated. The development of endothelial dysfunction in human endotoxemia and endotoxin tolerance occur in parallel with disturbances in their microcirculation,27, 28 suggesting that vascular tone may in fact undergo adaptations during changes in CTH to maintain tissue oxygenation. If vascular tone remains intrinsically regulated to optimize tissue oxygenation in severe sepsis, then severely elevated CTH might contribute both to the loss of vasopressor responses in patients, and to MOF and death by critically reducing tissue oxygenation.
Third, the range of blood flow values that can support organ function and tissue survival gradually narrows as CTH increases. When CTH becomes critically elevated, both increases in tissue blood flow above and reductions in blood flow below the threshold shown in Fig. 3 can therefore cause organ failure and tissue injury.
Taken together, these predictions suggest that disturbances in capillary flow patterns can contribute to the development of ARF, myocardial depression, and septic encephalopathy, despite maintenance of normal blood flow values, and even capillary patency, in these organs.
The origins of capillary flow disturbances or occlusion in sepsis
Systemic inflammation in general, and sepsis in particular, have long been known to cause profound changes in capillary flow patterns due to changes in the size, number, deformability, and endothelial adhesion of blood cells.29 In the normal state, the endothelium serves as mechanical barrier, regulates microvascular blood flow, and inhibits inflammation and coagulation. During sepsis, the endothelium is activated by bacterial components, toxins, and inflammatory mediators. The release of inflammatory mediators induces morphological changes of the endothelium and its protective glycocalyx.30, 31 Endothelial exposure to oxidative stress, oxidized lipoproteins,32, 33, 34 and hyperglycemia35 cause further glycocalyx injury which in turn give rise to profound changes in capillary flows and hematocrit.33, 36 The glycocalyx constitutes a fluid barrier, and degradation of the glycocalyx is hence associated with edema and capillary compression.37, 38
The endothelial activation in relation to sepsis also triggers increased expression of several adhesion molecules that promote leukocyte rolling and adhesion, and further disturb capillary flows. Red blood cell deformability is also reduced early in the course of sepsis.39, 40 Meanwhile, the normal anti‐thrombotic state of the endothelium shifts toward a pro‐coagulant state, as evidenced by reduced expression of anti‐thrombotic factors and increased expression of tissue factors.41 Upon activation, endothelial cells and adhesion molecules are shed. The increased levels of circulating endothelial cells and adhesion molecules in septic patients may therefore reflect factors of importance to parallel changes in the capillary circulation.42, 43, 44, 45, 46
The control of pericyte tone remains much less studied than that of arteriolar tone.47 Oxidative stress causes irreversible capillary constriction in cerebral pericytes,48, 49 while nitric oxide (NO) is obligatory for pericyte relaxation.47 NO production often require oxygen as a substrate,47 and the high levels of oxidative stress and the tissue hypoxia associated with sepsis are therefore likely to contribute to the development of capillary dysfunction.
Evidence of changes in capillary density and CTH from direct studies of the microcirculation in sepsis
The microcirculation in the sublingual mucosa of septic patients has been studied by orthogonal polarization spectral (OPS) imaging50 or side‐stream dark‐field (SDF) imaging51 at some institutions, and reported according to the standardized scores.52 Patients with sepsis show reductions in the proportion of perfused capillaries,53 and in animal models of normotensive sepsis, the density of capillaries with either intermittent or no flow is elevated, while the normal response to vasodilators is reduced.54, 55 Notably, death from MOF after septic shock is antedated by reductions in the proportion of perfused sublingual capillaries compared to survivors, in spite of identical systemic hemodynamic and oxygenation variables.56 With respect to evidence of a separate role of CTH in sepsis, disturbed capillary flow patterns are associated with poor oxygen extraction in models of endotoxemia,15, 17 and early sublingual capillary flow velocities are lower and more heterogeneous in patients who die after severe sepsis or septic shock than in patients who survive.57, 58 Early improvement in the microvascular flow patterns of septic patients seems to be associated with a reduced risk of subsequent MOF.59
Therapeutic implications
The effects of capillary flow patterns on tissue oxygenation re‐emphasizes the microcirculation as a potential target of therapy in critical illness, either through active control of capillary and arteriolar tone, by maintenance of appropriate blood viscosity, or by protection of the endothelium.60, 61, 62, 63 The prevention of mitochondrial injury, however, remains a crucial goal: Pathological redistribution of capillary flows exposes some cells to oxygen in excess of their metabolic needs while others are deprived of oxygen – both of which are associated with mitochondrial reactive oxygen species production and the initiation of apoptotic pathways.64
Our analysis shows that the oxygenation of certain organs may improve if their blood supply is lowered relative to their pre‐sepsis values, whereas relative increases in organ blood supply, which may not have the desired effect. Interventional studies attempting to increase oxygen delivery to supra‐normal levels have indeed not shown beneficial effects on outcomes, and in some cases even the opposite.4 Notably, not all patients and organs are predicted to tolerate relative reductions in blood flow: The renal medulla seems particularly prone to hypoxic injury, especially in patients with pre‐existing capillary flow disturbances. Here, kidney function may already depend on blood flow values within a narrow range – whereas any sepsis‐related increase in CTH or reductions in RBF are likely to elicit renal failure. A randomized controlled trial comparing a mean arterial pressure (MAP) of 80–85 mmHg to one of 65–70 mmHg in septic patients demonstrated no survival benefit for the group randomized to the higher MAP, but a lower need for renal replacement therapy in patients with prior hypertension.65 We speculate that these findings may reflect the specific vulnerability to hypoperfusion of kidney medulla in patients with pre‐existing renal capillary dysfunction. The analysis thus re‐emphasizes the need for early goal‐directed therapy (EGDT), but suggest that subsequent evaluation of the microcirculation in individual organs may guide organ‐specific, optimal macro‐, and microcirculatory endpoints for therapy in individual patients.
Blood rheology affects CTH, and thereby tissue oxygenation. Phosphodiesterase (PDE) inhibitors reduce platelet aggregation,66 decrease blood viscosity,67 and increase the flexibility of erythrocytes,67 and would therefore be expected to improve tissue oxygenation in sepsis. Consistent with this prediction, the PDE inhibitor pentoxifylline normalizes the rheology of human blood exposed to endotoxin in vitro 68 while improving net oxygen extraction in experimental sepsis.69
Studies of retinal pericytes suggest that these cells react to pharmacological stimuli in much the same way as smooth muscle cells (SMCs). They may constrict in response to mechanical stretch, angiotensin II (via AT1 receptors),70 and endothelin‐1 (via ETA receptors),71 by a Ca2+‐dependent mechanism.72 Interestingly, pre‐hospital prescription of angiotensin II receptor blockers is associated with reduced sepsis‐related mortality.73 Studies of the dose‐dependent effects of vasopressors on both SMCs and capillary pericyte tone in various organs may help us better understand how they affect tissue oxygenation in vivo. When translating such results into humans, it should be kept in mind that age and cardiovascular risk factors such as hypertension and diabetes are associated with profound changes in capillary morphology, especially in terms of basement membrane thickening.74, 75, 76 The microcirculation of patients may therefore respond differently to vasoactive substances than that of animal models with fully functional capillaries, just as patients may tolerate less, sepsis‐induced increase in CTH before organ failure or tissue damage ensues.
Diagnostic implications
The extended flow‐diffusion equation may be useful in converting OPS‐ and SDF‐based observations of erythrocyte velocity distributions, and the fraction of perfused capillaries, into estimates of tissue oxygenation by using tissue‐specific nomograms such as those shown in Fig. 2. Knowledge of the tissue oxygen tension is necessary for the absolute quantification of metabolic deficit, in that PtO2 reflects the residual oxygen reserve in terms of the blood–tissue concentration gradient. In the experimental setting, such data are available from NAD fluorescence imaging techniques,15 and may in principle be obtained by needle sensors in both animals and human.77 In brain, CTH and MTT can be measured non‐invasively by monitoring the clearance of intravascular contrast agents as part of perfusion weighted MRI or CT.78 In organs with substantial leakage of MRI and CT contrast agent, contrast‐enhanced ultrasound may be refined for future, bed‐side assessment of tissue microcirculation.79
Other organs and other metabolites
It should be kept in mind that changes in capillary transit time patterns of the lung affects blood saturation in the same way as they affect blood deoxygenation in other tissues.22, 80 Successful strategies for the protection of capillary wall integrity are therefore expected to improve both blood saturation and tissue oxygenation.81 Also, capillary flow disturbances cause a general reduction in the extraction of diffusible solutes,22 including tracers used when quantifying glucose metabolism82 and, we fear, tissue hypoxia.83 We analyzed the effects of capillary flow disturbance on oxygen uptake, glucose uptake, and ATP production in tumor and brain tissue,84, 85 and discovered that the uptake of glucose becomes limited, yet remains favored over that of oxygen, as CTH increases. Further studies should therefore address whether microcirculatory disturbances contribute to the hyperglycemia,86 aerobic glycolysis,87 and lactate production88, 89 observed in sepsis.
Conclusion
Capillary flow disturbances, such as those observed in sepsis, can cause oxygen availability to fall below the requirements of normal renal, heart, and brain function, although their blood supply remain normal or elevated. Paradoxically, one cannot predict whether reductions in blood flow improve oxygenation or not in organs with severely disturbed microcirculation. Non‐invasive methods to quantify capillary transit time characteristics may serve as means to test these predictions, and to examine whether they can guide future strategies to optimize organ oxygenation in individualized sepsis management.
Østergaard L, Granfeldt A, Secher N, Tietze A, Iversen NK, Jensen MS, Andersen KK, Nagenthiraja K, Gutiérrez‐Lizardi P, Mouridsen K, Jespersen SN, Tønnesen EK. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiologica Scandinavica 2015.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by the Danish National Research Foundation (LØ, KM, SNJ) and by the Danish Ministry of Science, Technology and Innovation's University Investment Grant (LØ, AT, NKI, MSJ, KM, SNJ).
References
- 1. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003; 348: 138–50. [DOI] [PubMed] [Google Scholar]
- 2. Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med 1987; 15: 923–9. [DOI] [PubMed] [Google Scholar]
- 3. Kreymann G, Grosser S, Buggisch P, Gottschall C, Matthaei S, Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit Care Med 1993; 21: 1012–9. [DOI] [PubMed] [Google Scholar]
- 4. Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 1994; 330: 1717–22. [DOI] [PubMed] [Google Scholar]
- 5. Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med 2008; 36: S198–203. [DOI] [PubMed] [Google Scholar]
- 6. Spies C, Haude V, Fitzner R, Schroder K, Overbeck M, Runkel N, Schaffartzik W. Serum cardiac troponin T as a prognostic marker in early sepsis. Chest 1998; 113: 1055–63. [DOI] [PubMed] [Google Scholar]
- 7. Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 1984; 100: 483–90. [DOI] [PubMed] [Google Scholar]
- 8. Cunnion RE, Schaer GL, Parker MM, Natanson C, Parrillo JE. The coronary circulation in human septic shock. Circulation 1986; 73: 637–44. [DOI] [PubMed] [Google Scholar]
- 9. Dhainaut JF, Huyghebaert MF, Monsallier JF, Lefevre G, Dall'Ava‐Santucci J, Brunet F, Villemant D, Carli A, Raichvarg D. Coronary hemodynamics and myocardial metabolism of lactate, free fatty acids, glucose, and ketones in patients with septic shock. Circulation 1987;75:533–41. [DOI] [PubMed] [Google Scholar]
- 10. Pytel P, Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol 2009; 22: 283–7. [DOI] [PubMed] [Google Scholar]
- 11. Maekawa T, Fujii Y, Sadamitsu D, Yokota K, Soejima Y, Ishikawa T, Miyauchi Y, Takeshita H. Cerebral circulation and metabolism in patients with septic encephalopathy. Am J Emerg Med 1991; 9: 139–43. [DOI] [PubMed] [Google Scholar]
- 12. Bowton DL, Bertels NH, Prough DS, Stump DA. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit Care Med 1989; 17: 399–403. [DOI] [PubMed] [Google Scholar]
- 13. Moustafa RS, Baron JC. Perfusion thresholds in cerebral ischemia In: Donnan GA, Baron JC, Davis SM, Sharp FR, eds. The Ischemic Penumbra. New York: Informa Healthcare USA, Inc., 2007; 31–6. [Google Scholar]
- 14. Singer M. Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med 2007; 35: S441–8. [DOI] [PubMed] [Google Scholar]
- 15. Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med 1999; 27: 1369–77. [DOI] [PubMed] [Google Scholar]
- 16. Gardiner BS, Thompson SL, Ngo JP, Smith DW, Abdelkader A, Broughton BR, Bertram JF, Evans RG. Diffusive oxygen shunting between vessels in the preglomerular renal vasculature: anatomic observations and computational modeling. Am J Physiol Renal Physiol 2012; 303: F605–18. [DOI] [PubMed] [Google Scholar]
- 17. Humer MF, Phang PT, Friesen BP, Allard MF, Goddard CM, Walley KR. Heterogeneity of gut capillary transit times and impaired gut oxygen extraction in endotoxemic pigs. J Appl Physiol 1996; 81: 895–904. [DOI] [PubMed] [Google Scholar]
- 18. Hinshaw LB. Sepsis/septic shock: participation of the microcirculation: an abbreviated review. Crit Care Med 1996; 24: 1072–8. [DOI] [PubMed] [Google Scholar]
- 19. Lundy DJ, Trzeciak S. Microcirculatory dysfunction in sepsis. Crit Care Clin 2009; 25: 721,31, viii. [DOI] [PubMed] [Google Scholar]
- 20. Renkin EMBW. Zweifach Award lecture. Regulation of the microcirculation. Microvasc Res 1985; 30: 251–63. [DOI] [PubMed] [Google Scholar]
- 21. Cousineau DF, Goresky CA, Rose CP, Simard A, Schwab AJ. Effects of flow, perfusion pressure, and oxygen consumption on cardiac capillary exchange. J Appl Physiol 1995; 78: 1350–9. [DOI] [PubMed] [Google Scholar]
- 22. Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 2012; 32: 264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Presson RG Jr, Graham JA, Hanger CC, Godbey PS, Gebb SA, Sidner RA, Glenny RW, Wagner WW Jr. Distribution of pulmonary capillary red blood cell transit times. J Appl Physiol 1995; 79: 382–8. [DOI] [PubMed] [Google Scholar]
- 24. Rasmussen PM, Jespersen SN, Østergaard L. The effects of transit time heterogeneity on brain oxygenation during rest and functional activation. J Cereb Blood Flow Metab 2015; 35: 432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Østergaard L, Kristiansen SB, Angleys H, Frokiaer J, Michael Hasenkam J, Jespersen SN, Botker HE. The role of capillary transit time heterogeneity in myocardial oxygenation and ischemic heart disease. Basic Res Cardiol 2014; 109: 409,014‐0409‐x. Epub 2014 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Østergaard L, Jespersen SN, Mouridsen K, Mikkelsen IK, Jonsdottir KY, Tietze A, Blicher JU, Aamand R, Hjort N, Iversen NK, Cai C, Dupont K, Simonsen CZ, Weitzel‐Mudersbach PV, Modrau B, Nagenthiraja K, Ribe LR, Hansen MB, Bekke SL, Dahlman MG, Puig J, Pedraza S, Serena J, Cho TH, Siemonsen S, Thomalla G, Fiehler J, Nighoghossian N, Andersen G. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab 2013; 33: 635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Backer D, Donadello K, Favory R. Link between coagulation abnormalities and microcirculatory dysfunction in critically ill patients. Curr Opin Anaesthesiol 2009; 22: 150–4. [DOI] [PubMed] [Google Scholar]
- 28. Draisma A, Bemelmans R, van der Hoeven JG, Spronk P, Pickkers P. Microcirculation and vascular reactivity during endotoxemia and endotoxin tolerance in humans. Shock 2009; 31: 581–5. [DOI] [PubMed] [Google Scholar]
- 29. Mazzoni MC, Schmid‐Schonbein GW. Mechanisms and consequences of cell activation in the microcirculation. Cardiovasc Res 1996; 32: 709–19. [PubMed] [Google Scholar]
- 30. Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 1996; 79: 581–9. [DOI] [PubMed] [Google Scholar]
- 31. Donati A, Damiani E, Domizi R, Romano R, Adrario E, Pelaia P, Ince C, Singer M. Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc Res 2013; 90: 86–9. [DOI] [PubMed] [Google Scholar]
- 32. Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet‐endothelial cell adhesion. Circulation 2000; 101: 1500–2. [DOI] [PubMed] [Google Scholar]
- 33. Constantinescu AA, Vink H, Spaan JA. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol 2001; 280: H1051–7. [DOI] [PubMed] [Google Scholar]
- 34. Czarnowska E, Karwatowska‐Prokopczuk E. Ultrastructural demonstration of endothelial glycocalyx disruption in the reperfused rat heart. Involvement of oxygen free radicals. Basic Res Cardiol 1995; 90: 357–64. [DOI] [PubMed] [Google Scholar]
- 35. Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006; 55: 1127–32. [DOI] [PubMed] [Google Scholar]
- 36. Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol 1990; 258: H647–54. [DOI] [PubMed] [Google Scholar]
- 37. van Haaren PM, VanBavel E, Vink H, Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol 2003; 285: H2848–56. [DOI] [PubMed] [Google Scholar]
- 38. van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res 2003; 92: 592–4. [DOI] [PubMed] [Google Scholar]
- 39. Reggiori G, Occhipinti G, De Gasperi A, Vincent JL, Piagnerelli M. Early alterations of red blood cell rheology in critically ill patients. Crit Care Med 2009; 37: 3041–6. [DOI] [PubMed] [Google Scholar]
- 40. Baskurt OK, Gelmont D, Meiselman HJ. Red blood cell deformability in sepsis. Am J Respir Crit Care Med 1998; 157: 421–7. [DOI] [PubMed] [Google Scholar]
- 41. Ait‐Oufella H, Maury E, Lehoux S, Guidet B, Offenstadt G. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med 2010; 36: 1286–98. [DOI] [PubMed] [Google Scholar]
- 42. Sessler CN, Windsor AC, Schwartz M, Watson L, Fisher BJ, Sugerman HJ, Fowler AA 3rd. Circulating ICAM‐1 is increased in septic shock. Am J Respir Crit Care Med 1995; 151: 1420–7. [DOI] [PubMed] [Google Scholar]
- 43. Hein OV, Misterek K, Tessmann JP, van Dossow V, Krimphove M, Spies C. Time course of endothelial damage in septic shock: prediction of outcome. Crit Care 2005; 9: R323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mutunga M, Fulton B, Bullock R, Batchelor A, Gascoigne A, Gillespie JI, Baudouin SV. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med 2001; 163: 195–200. [DOI] [PubMed] [Google Scholar]
- 45. Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers 2011; 16(Suppl 1): S11–21. [DOI] [PubMed] [Google Scholar]
- 46. Moussa MD, Santonocito C, Fagnoul D, Donadello K, Pradier O, Gaussem P, De Backer D, Vincent JL. Evaluation of endothelial damage in sepsis‐related ARDS using circulating endothelial cells. Intensive Care Med 2015; 41: 231–8. [DOI] [PubMed] [Google Scholar]
- 47. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yemisci M, Gursoy‐Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative‐nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–7. [DOI] [PubMed] [Google Scholar]
- 49. Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med 1999; 5: 1209–12. [DOI] [PubMed] [Google Scholar]
- 51. Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring‐based imaging modality for clinical assessment of the microcirculation. Opt Express 2007; 15: 15101–14. [DOI] [PubMed] [Google Scholar]
- 52. De Backer D, Hollenberg S, Boerma C, Goedhart P, Buchele G, Ospina‐Tascon G, Dobbe I, Ince C. How to evaluate the microcirculation: report of a round table conference. Crit Care 2007; 11: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002; 166: 98–104. [DOI] [PubMed] [Google Scholar]
- 54. Lam C, Tyml K, Martin C, Sibbald W. Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest 1994; 94: 2077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Farquhar I, Martin CM, Lam C, Potter R, Ellis CG, Sibbald WJ. Decreased capillary density in vivo in bowel mucosa of rats with normotensive sepsis. J Surg Res 1996; 61: 190–6. [DOI] [PubMed] [Google Scholar]
- 56. Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004; 32: 1825–31. [DOI] [PubMed] [Google Scholar]
- 57. Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM, Microcirculatory Alterations in Resuscitation and Shock Investigators . Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 2007; 49: 88. [DOI] [PubMed] [Google Scholar]
- 58. Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med 2012; 40: 1443–8. [DOI] [PubMed] [Google Scholar]
- 59. Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, Shapiro NI, Parrillo JE, Hollenberg SM, Microcirculatory Alterations in Resuscitation and Shock (MARS) investigators . Early increases in microcirculatory perfusion during protocol‐directed resuscitation are associated with reduced multi‐organ failure at 24 h in patients with sepsis. Intensive Care Med 2008; 34: 2210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Donati A, Damiani E, Botticelli L, Adrario E, Lombrano MR, Domizi R, Marini B, Van Teeffelen JW, Carletti P, Girardis M, Pelaia P, Ince C. The aPC treatment improves microcirculation in severe sepsis/septic shock syndrome. BMC Anesthesiol 2013; 13: 25, 2253‐13‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 2006; 34: 403–8. [DOI] [PubMed] [Google Scholar]
- 62. Boldt J, Ince C. The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: a review. Intensive Care Med 2010; 36: 1299–308. [DOI] [PubMed] [Google Scholar]
- 63. Boerma EC, Ince C. The role of vasoactive agents in the resuscitation of microvascular perfusion and tissue oxygenation in critically ill patients. Intensive Care Med 2010; 36: 2004–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 2003; 552: 335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard‐Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Herve F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P, SEPSISPAM Investigators . High versus low blood‐pressure target in patients with septic shock. N Engl J Med 2014; 370: 1583–93. [DOI] [PubMed] [Google Scholar]
- 66. Horlington M, Watson PA. Inhibition of 3'5’‐cyclic‐AMP phosphodiesterase by some platelet aggregation inhibitors. Biochem Pharmacol 1970; 19: 955–6. [DOI] [PubMed] [Google Scholar]
- 67. Jacoby D, Mohler ER 3rd. Drug treatment of intermittent claudication. Drugs 2004; 64: 1657–70. [DOI] [PubMed] [Google Scholar]
- 68. Mollitt DL, Poulos ND. The role of pentoxifylline in endotoxin‐induced alterations of red cell deformability and whole blood viscosity in the neonate. J Pediatr Surg 1991; 26: 572–4. [DOI] [PubMed] [Google Scholar]
- 69. Zhang H, Spapen H, Benlabed M, Nguyen DN, Buurman WA, Vincent JL. Pentoxifylline improves the tissue oxygen extraction capabilities during endotoxic shock. Shock 1994; 2: 90–7. [DOI] [PubMed] [Google Scholar]
- 70. Kawamura H, Kobayashi M, Li Q, Yamanishi S, Katsumura K, Minami M, Wu DM, Puro DG. Effects of angiotensin II on the pericyte‐containing microvasculature of the rat retina. J Physiol 2004; 561: 671–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schonfelder U, Hofer A, Paul M, Funk RH. In situ observation of living pericytes in rat retinal capillaries. Microvasc Res 1998; 56: 22–9. [DOI] [PubMed] [Google Scholar]
- 72. Diaz‐Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin‐Vasallo P, Diaz‐Flores L Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 2009; 24: 909–69. [DOI] [PubMed] [Google Scholar]
- 73. Mortensen EM, Restrepo MI, Copeland LA, Pugh JA, Anzueto A, Cornell JE, Pugh MJ. Impact of previous statin and angiotensin II receptor blocker use on mortality in patients hospitalized with sepsis. Pharmacotherapy 2007; 27: 1619–26. [DOI] [PubMed] [Google Scholar]
- 74. Fischer VW, Barner HB, Leskiw ML. Capillary basal laminar thickness in diabetic human myocardium. Diabetes 1979; 28: 713–9. [DOI] [PubMed] [Google Scholar]
- 75. Junker U, Jaggi C, Bestetti G, Rossi GL. Basement membrane of hypothalamus and cortex capillaries from normotensive and spontaneously hypertensive rats with streptozotocin‐induced diabetes. Acta Neuropathol 1985; 65: 202–8. [DOI] [PubMed] [Google Scholar]
- 76. Kalaria RN. Cerebral vessels in ageing and Alzheimer's disease. Pharmacol Ther 1996; 72: 193–214. [DOI] [PubMed] [Google Scholar]
- 77. Pittman RN. Oxygen gradients in the microcirculation. Acta Physiol (Oxf) 2011; 202: 311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mouridsen K, Hansen MB, Østergaard L, Jespersen SN. Reliable estimation of capillary transit time distributions using DSC‐MRI. J Cereb Blood Flow Metab 2014; 34: 1511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schneider AG, Goodwin MD, Schelleman A, Bailey M, Johnson L, Bellomo R. Contrast‐enhanced ultrasonography to evaluate changes in renal cortical microcirculation induced by noradrenaline: a pilot study. Crit Care 2014; 18: 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ramakrishna M, Gan Z, Clough AV, Molthen RC, Roerig DL, Audi SH. Distribution of capillary transit times in isolated lungs of oxygen‐tolerant rats. Ann Biomed Eng 2010; 38: 3449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012; 18: 1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Knudsen GM, Pettigrew KD, Paulson OB, Hertz MM, Patlak CS. Kinetic analysis of blood‐brain barrier transport of D‐glucose in man: quantitative evaluation in the presence of tracer backflux and capillary heterogeneity. Microvasc Res 1990; 39: 28–49. [DOI] [PubMed] [Google Scholar]
- 83. Hotchkiss RS, Rust RS, Dence CS, Wasserman TH, Song SK, Hwang DR, Karl IE, Welch MJ. Evaluation of the role of cellular hypoxia in sepsis by the hypoxic marker [18F]fluoromisonidazole. Am J Physiol 1991; 261: R965–72. [DOI] [PubMed] [Google Scholar]
- 84. Østergaard L, Dreier JP, Hadjikhani N, Jespersen SN, Dirnagl U, Dalkara T. Neurovascular coupling during cortical spreading depolarization and ‐depression. Stroke 2015; 46: 1392–401. [DOI] [PubMed] [Google Scholar]
- 85. Østergaard L, Tietze A, Nielsen T, Drasbek KR, Mouridsen K, Jespersen SN, Horsman MR. The relationship between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic glycolysis. Cancer Res 2013; 73: 5618–24. [DOI] [PubMed] [Google Scholar]
- 86. Andersen SK, Gjedsted J, Christiansen C, Tonnesen E. The roles of insulin and hyperglycemia in sepsis pathogenesis. J Leukoc Biol 2004; 75: 413–21. [DOI] [PubMed] [Google Scholar]
- 87. Gore DC, Jahoor F, Hibbert JM, DeMaria EJ. Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability. Ann Surg 1996; 224: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 2005; 365: 871–5. [DOI] [PubMed] [Google Scholar]
- 89. James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999; 354: 505–8. [DOI] [PubMed] [Google Scholar]
- 90. King RB, Raymond GM, Bassingthwaighte JB. Modeling blood flow heterogeneity. Ann Biomed Eng 1996; 24: 352–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stewart GN. Researches on the circulation time in organs and on the influences which affect it. Parts I.‐III. J Physiol 1893; 15: 1–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hatch FE, Johnson JG. Intrarenal blood flow. Annu Rev Med 1969; 20: 395–408. [DOI] [PubMed] [Google Scholar]
- 93. Cohn JN, Velasquez MT, Notargiacomo A, Khatri IM. Cortical blood flow, cortical fraction, and cortical blood volume in the dog kidney. Am J Physiol 1971; 221: 877–82. [DOI] [PubMed] [Google Scholar]
- 94. Evans RG, Goddard D, Eppel GA, O'Connor PM. Stability of tissue PO2 in the face of altered perfusion: a phenomenon specific to the renal cortex and independent of resting renal oxygen consumption. Clin Exp Pharmacol Physiol 2011; 38: 247–54. [DOI] [PubMed] [Google Scholar]


