Abstract
Purpose
To study the association of the single nucleotide polymorphism (SNP) rs4073 in the interleukin‐8 (IL‐8) promoter region with the diagnosis and age of onset of exudative age‐related macular degeneration (AMD) in association with the known genetic risk factors for AMD and tobacco smoking.
Methods
Medical records, smoking history and angiograms or fundus photographs of 301 patients with exudative AMD, 72 patients with dry AMD and 119 control subjects were analysed retrospectively. The associations of IL‐8 rs4073 A→T, CFH rs1061170 T→C, ARMS2 rs10490924 G→T and C3 rs2230199 C→G SNPs with the presence of AMD and with the age of onset of exudative AMD were analysed.
Results
Younger age of exudative AMD onset was associated with the homozygous AA genotype of IL‐8 rs4073 (p = 0.009, Mann–Whitney U‐test), CC genotype of CFH rs1061170 (p = 0.016), TT genotype of ARMS2 rs10490924 (p = 0.001) and with current smoking (p = 0.002). The risk alleles C in CFH rs1061170 (p < 0.0001, Pearson chi‐square) and T in ARMS2 rs10490924 (p < 0.0001), as well as smoking (p < 0.0001), were more prevalent in AMD patients compared with controls. No association was found between the IL‐8 rs4073 genotype and the presence of AMD.
Conclusion
Out of the factors associated with the earlier onset of exudative AMD, only the genotype of IL‐8 rs4073 did not appear as a risk factor for AMD in general. IL‐8 may have a role in accelerating the development of the choroidal neovascularization in exudative AMD.
Keywords: exudative age‐related macular degeneration, genetic associations, interleukin‐8, single nucleotide polymorphism
Introduction
Inflammation plays an important role in the pathogenesis and progression of age‐related macular degeneration (AMD). Higher circulatory levels of inflammatory mediators C‐reactive protein (CRP) and interleukin‐6 (IL‐6) have been shown to be associated with the progression of AMD (Seddon et al. 2005; Hong et al. 2011). Genetic association studies have emphasized the role of individual variations in the alternative complement cascade in increasing the risk of AMD development (Edwards et al. 2005; Haines et al. 2005; Klein et al. 2005; Gold et al. 2006; Maller et al. 2006, 2007; Yates et al. 2007; Seddon et al. 2013). The role of interleukin‐8 (IL‐8) is more controversial. Single nucleotide polymorphisms (SNPs) in the IL‐8 gene have been variably associated with the prevalence of atrophic and exudative AMD (Goverdhan et al. 2008; Tsai et al. 2008; Ricci et al. 2013). The intraocular IL‐8 concentrations, however, have been elevated in patients with exudative AMD (Jonas et al. 2012) and correlated with the size of an active choroidal neovascularization (CNV) and with the macular volume after bevacizumab treatment (Roh et al. 2009; Miao et al. 2012).
Promoter region SNP IL‐8 rs4073 A→T (‐251A/T) has been connected to the regulation of transcriptional activity of the IL‐8 gene and to the levels of IL‐8 production (Hull et al. 2000; Ohyauchi et al. 2005; Taguchi et al. 2005; Hildebrand et al. 2007). In our previous studies in patients with exudative AMD treated with bevacizumab, the IL‐8 rs4073 genotype was associated with both the initial anatomic treatment response and with the persistence of intra‐ and subretinal fluid in macular optical coherence tomographies (OCTs) during 2‐year follow‐up (Hautamäki et al. 2013, 2014). In that data, the risk alleles of IL‐8 rs4073 A→T and CFH rs1061170 T→C were also associated with earlier onset of exudative AMD (data not published). Therefore, we wanted to study further the association of the IL‐8 SNP with the prevalence of AMD and with the age of onset of exudative AMD in a larger patient material, in association with the known AMD risk SNPs CFH rs1061170 T→C (Y402H), ARMS2 rs10490924 G→T (LOC387715, A69S) and C3 rs2230199 C→G (R102G) previously found to be associated with AMD also in the Finnish population (Seitsonen et al. 2006, 2008).
Materials and Methods
The total number of 492 Finnish subjects was included: 301 patients with exudative AMD, 72 with dry AMD and 119 control subjects. The patients with exudative AMD, 90 control subjects and 28 patients with dry AMD were recruited to our three previous studies on AMD genetics and treatment response in exudative AMD from the Medical Retina (cases) and Cataract Surgery (controls) units of the Departments of Ophthalmology of Helsinki (n = 368), Oulu (n = 6), and Tampere (n = 1) University Hospitals, or private offices and outpatient clinics (n = 44), between January 2003 and April 2010 (Seitsonen et al. 2006; Hautamäki et al. 2013, 2014). Additionally, 44 diabetic patients with large drusenoid macular deposits visible in fundus photographs taken for screening of diabetic (DM) retinopathy between January 2006 and February 2007 were included in the analysis of IL‐8 rs4073 as having dry AMD, and 29 men recruited between October 2004 and June 2007 to an ongoing study of risk factors for AMD in a cohort of male executives or academic professionals, originally examined for cardiovascular health, cognition and quality of life (Strandberg et al. 2004), were included as additional control subjects.
The control group consisted of 119 subjects (74 female, 45 male) who were >69 years of age, with no large drusen, and no, or minimal focal pigmentary abnormalities within the radius of one disc diameter from the fovea in fundus photographs graded according to the AREDS classification (Davis et al. 2005). The maximum total area of pigment abnormalities corresponded to a circle with diameter of 250 μm, and a maximum of five hard small (<63 μm) drusen was allowed. Sixty‐five subjects had neither pigment abnormalities nor drusen, 50 had only pigment abnormalities, three had only small drusens, and one subject had both drusens and pigment abnormalities.
Patients with dry AMD were >50 years of age (43 female, 29 male), had at least 10 soft drusen >125 μm, a confluent area of drusen >1 disc area within the radius of one disc diameter from the fovea or a central geographic atrophy related to AMD.
The diagnosis of exudative AMD (204 female, 97 male) was made by an experienced medical retina specialist. The age of onset of exudative AMD was determined as the age at the diagnosis in cases with a recent onset of symptoms based on the medical records, and no substantial subretinal fibrosis detected in clinical examination or angiograms. The age of onset of the exudative AMD in the first affected eye was possible to determine in 259 patients (259/303, 85%). In 42 patients, the diagnosis was considered to be delayed: patients had been symptomatic for longer than a year, or the diagnosis was made only in a late stage of the disease with subretinal fibrosis. In these patients, the age of onset of the exudative AMD in the first eye could not be estimated, and their data were included only in the genetic analyses comparing AMD patients with the control group. The diagnosis of exudative AMD was based on fluorescein angiograms (FA) in 253 patients. The CNV lesions were classified according to the TAP‐study criteria (Barbazetto et al. 2003); 39 were predominantly classic (15.1%), 129 occult (50.0%), 53 minimally classic (20.5%), 1 polypoidal (0.4%) and 23 not defined (>50% of the lesion area composed of haemorrhage or serous pigment epithelial detachment, 8.9%). In eight patients (3.1%), the angiograms were recorded, but were not available for retrospective analysis, and no classification of the lesion was found in the medical records. Five patients had received no treatment (poor visual acuity in four patients and a pigment epithelial tear in one patient), and FAs were, therefore, not recorded.
The patients were classified as never, ex‐ or current smokers. Never smokers were determined as having smoked less than one pack‐year, ex‐smokers as having quit smoking at least 10 years ago. If a participant had ever smoked, the smoking pack‐years were calculated (pack‐year = [cigarettes per day] × [years of smoking]/[20 cigarettes per pack]).
The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the local ethics committee. A written informed consent was obtained from all participants.
SNP genotyping
The blood DNA samples of the patients and control subjects (301 exudative AMD, 28 dry AMD patients and 119 controls) were analysed for the SNPs IL‐8 rs4073 A→T, CFH rs1061170 T→C, ARMS2rs10490924 G→T and C3 rs2230199 C→G (Seitsonen et al. 2008; Hautamäki et al. 2013), except for the additional 44 samples of dry AMD patients recruited from photographic screening of DM retinopathy, which were analysed only for the IL‐8 rs4073.
The SNP analyses were carried out using phenol–chloroform extraction for DNA and PCR‐based genotyping with big dye terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) as described before. (Seitsonen et al. 2008; Hautamäki et al. 2013). The DNA fragments were purified from excess dye terminators with performa® dtr v3 filter plates (Edge Biosystems, Gaithersburg, MD, USA) and resolved on an ABI 3730 capillary sequencer. All the methods were used as recommended by the manufacturer.
Statistical analysis
ibm spss Statistics version 22 (IBM Corp., Armonk, NY, USA) was used for statistical testing. Non‐parametric tests were used in univariate comparisons between the groups. The simultaneous effects of genetic markers and smoking on the diagnosis and the age of onset of exudative AMD were estimated first with multivariate models built with the generalized linear model (GLZ) procedure in spss 22. To take into account the differences in the age distributions in the patients and control subjects, the effects of the studied variables were also estimated with the Cox regression analysis as suggested by van der Net and associates (2008). The use of Cox regression in cross‐sectional genetic association studies is based on the principle that as the genotype status does not change over time, age at event can be considered as follow‐up time (van der Net et al. 2008). A two‐tailed p‐value <0.05 was considered statistically significant, no correction for multiple comparisons was used. We estimated that with this sample size 13% differences in allele frequencies between the groups, and difference of 3 years in the age of onset could be detected with a power of 0.8 and a p‐value of 0.05 (calculator for sample size provided at https://www.statstodo.com/).
Results
We first analysed the associations of the genotypes of IL‐8 rs4073, CFH rs1061170, ARMS2 rs10490924 and C3 rs2230199 and smoking behaviour with the diagnosis of any AMD or exudative AMD in the whole study population.
As reported before for a subgroup of the patients included in the analyses, also in this combined sample the risk alleles C in CFH rs1061170 (p < 0.0001, Pearson chi‐square) and T in ARMS2 rs10490924 (p < 0.0001), as well as smoking (p < 0.0001), were more prevalent both in the exudative AMD group and in all AMD patients compared with the control group (Table 1) (Seitsonen et al. 2006, 2008). No significant differences were found in the frequencies of IL‐8 rs4073 or C3 rs2230199 genotypes between the groups. The effects of the studied SNPs and smoking remained unchanged in multivariate modelling (GLZ, data not shown).
Table 1.
Genotype frequencies and smoking history in exudative age‐related macular degeneration (AMD) patients and all AMD patients (dry or exudative) compared with control subjects
| Polymorphisms | Controls (n = 119) | Exudative AMD (n = 301) | Pearson chi‐square | Any AMD (n = 373) | Pearson chi‐square |
|---|---|---|---|---|---|
| IL‐8 rs4073 A→T | |||||
| AA | 26 (21.8%) | 45 (15.0%) | p = 0.120 | 61 (16.4%) | p = 0.275 |
| AT | 52 (43.7%) | 161 (53.5%) | 190 (50.9%) | ||
| TT | 41 (34.5%) | 95 (31.6%) | 122 (32.7%) | ||
| NA | 0 | 0 | 0 | ||
| CFH rs1061170 T→C | |||||
| CC | 13 (11.5%) | 109 (36.2%) | p < 0.0001 | 129 (39.2%) | p < 0.0001 |
| CT | 59 (52.2%) | 154 (51.2%) | 162 (49.2%) | ||
| TT | 41 (36.3%) | 38 (12.6%) | 38 (11.6%) | ||
| NA | 6 | 0 | 44 | ||
| ARMS2 rs10490924 G→T | |||||
| TT | 2 (1.8%) | 63 (21.1%) | p < 0.0001 | 70 (21.5%) | p < 0.0001 |
| TG | 42 (36.8%) | 157 (52.7%) | 170 (52.1%) | ||
| GG | 70 (61.4%) | 78 (26.2%) | 86 (26.4%) | ||
| NA | 5 | 3 | 47 | ||
| C3 rs2230199 C→G | |||||
| GG | 4 (3.6%) | 9 (3.0%) | p = 0.437 | 11 (3.4%) | p = 0.422 |
| GC | 27 (24.3%) | 92 (30.8%) | 101 (30.9%) | ||
| CC | 80 (72.1%) | 198 (66.2%) | 215 (65.7%) | ||
| NA | 8 | 2 | 46 | ||
| Tobacco | |||||
| Current smoker | 3 (2.6%) | 50 (17.5%) | p < 0.0001 | 64 (18.0%) | p < 0.0001 |
| Ex‐smoker | 25 (21.9%) | 81 (28.3%) | 95 (26.8%) | ||
| Never smoker | 86 (75.4%) | 155 (54.2%) | 196 (55.2%) | ||
| NA | 5 | 15 | 18 | ||
NA = data not available.
We then analysed the associations of the studied factors with the age of onset of exudative AMD including only patients with exudative AMD in the analyses. The effects of the homozygous risk genotypes AA in IL‐8 rs4073, CC in CFH rs1061170 and TT in ARMS2 rs10490924, as well as smoking were significant both in the univariate comparisons between the groups (Table 2) and in the conventional multivariate modelling with the GLZ (Table 3).
Table 2.
Age of onset of exudative age‐related macular degeneration (AMD)
| Polymorphisms | Age of Onset of Exudative AMD Years, median (range) | Mann–Whitney U test |
|---|---|---|
| IL‐8 rs4073 A→T | ||
| AA | 71.7 (55.5–84.3) | AA versus AT and TT |
| AT | 75.5 (56.1–91.2) | p = 0.009 |
| TT | 75.6 (53.1–87.6) | |
| CFH rs1061170 T→C | ||
| CC | 73.8 (53.1–91.2) | CC versus CT and TT |
| TC | 76.3 (59.4–87.6) | p = 0.016 |
| TT | 76.2 (63.9–87.0) | |
| ARMS2 rs10490924 G→T | ||
| TT | 71.3 (55.5–87.6) | TT versus TG and GG |
| GT | 76.2 (53.1–91.2) | p = 0.001 |
| GG | 75.8 (54.5–88.1) | |
| C3 rs2230199 C→G | ||
| GG | 74.7 (63.1–85.1) | CC versus CG and GG |
| CG | 74.8 (55.4–87.6) | p = 0.267 |
| CC | 75.8 (53.1–91.2) | |
| Tobacco | ||
| Current smoker | 70.1 (54.5–82.3) | Current versus never and ex‐smokers |
| Ex‐smoker | 76.5 (62.8–88.1) | |
| Never Smoker | 75.0 (53.1–91.2) | p = 0.002 |
Table 3.
Multivariate model built with the generalized linear model procedure of SPSS 22 to estimate the effects of studied variables on the age of onset of exudative AMD
| Parameter | B | SE | 95% Wald CI | Hypothesis Test | ||
|---|---|---|---|---|---|---|
| Lower | Upper | Wald chi‐square | p‐value | |||
| IL‐8 rs4073 A→T | ||||||
| AA | −2.502 | 1.1448 | −4.746 | −0.258 | 4.776 | 0.029 |
| AT or TT | Reference | |||||
| CFH rs1061170 T→C | ||||||
| CC | −2.171 | 0.8170 | −3.773 | −0.570 | 7.064 | 0.008 |
| CT or TT | Reference | |||||
| ARMS rs10490924 G→T | ||||||
| TT | −2.973 | 0.9811 | −4.896 | −1.050 | 9.181 | 0.002 |
| TG or GG | Reference | |||||
| Smoking | ||||||
| Current smoker | −3.885 | 1.0613 | −5.965 | −1.805 | 13.398 | 0.0003 |
| Non‐ or ex‐smoker | Reference | |||||
| Intercept | 2.240 | 0.5966 | 1.071 | 3.409 | 14.100 | <0.0001 |
Dependent variable: age – 75.0 years (median age of the exudative study patients).
To take into account the difference in the age distribution in the groups of exudative AMD patients and control subjects, the Cox proportional hazards models were used (Fig. 1) as described in the methods section. First, both patients with exudative AMD and control subjects were included to estimate the effects of the studied variables on the prevalence of exudative AMD (Fig. 1, left column). Age was used as time scale: age at the onset of exudative AMD for the cases and age at data collection for the controls. Patients with dry AMD were not included. The estimated effects remained similar compared to the univariate analyses and multivariate models built with the GLZ procedure, significant effects seen with the CFH rs1061170 [TC versus TT, hazard ratio (HR) = 1.7, p = 0.021 and CC versus TT, HR = 2.6, p < 0.0001], ARMS2 rs10490924 (GT versus GG, HR = 1.6, p = 0.024 and TT versus GG, HR = 3.3, p < 0.0001) and smoking (ex‐ versus never smokers, HR = 1.4, CI 1.06 –1.90, p = 0.020 and current versus never smokers, HR = 2.4, p < 0.0001). Then, the effects of the studied factors on the age of onset of exudative AMD were analysed separately in the group of patients with the exudative disease (Fig. 1, middle column). The homozygous risk genotypes AA in IL‐8 rs4073 (HR = 1.7, p = 0.005), CC in CFH rs1061170 (HR = 1.3, p = 0.05) and TT in ARMS2 rs10490924 (HR = 1.8, p = 0.0003) were associated with the younger onset of exudative AMD, whereas the effects of the heterozygous genotypes did not differ from the non‐risk homozygous genotypes. Also, current smoking was significantly associated with the younger age at the onset of the disease (HR = 1.6, p = 0.005), whereas ex‐smokers did not differ from never smokers. No significant associations were detected with the genotypes of C3 rs2230199.
Figure 1.
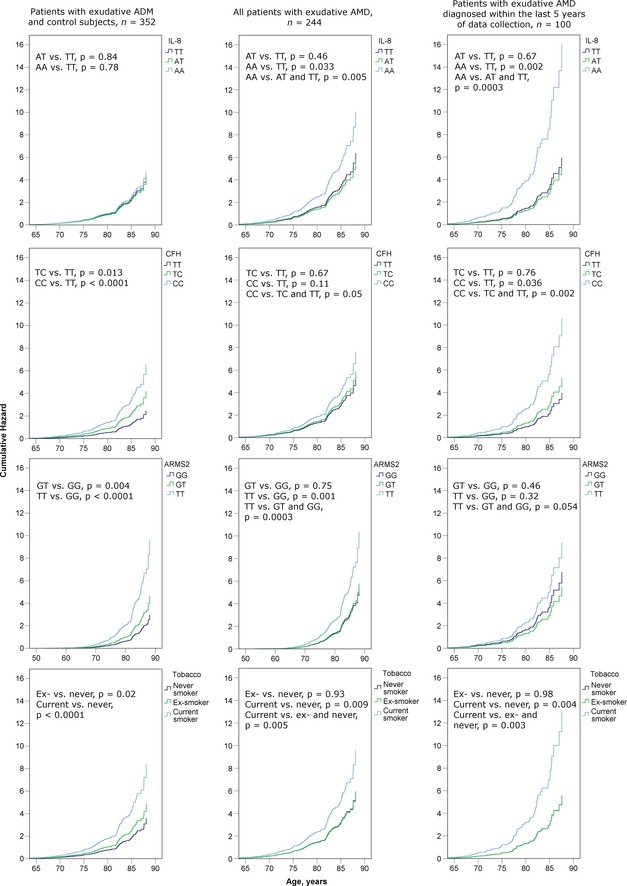
The effects of single nucleotide polymorphisms (SNPs) IL‐8 rs4073, CFH rs1061170 and ARMS2 rs10490924, as well as smoking, were estimated with Cox proportional hazards models in exudative AMD patients together with control subjects (left column), in all patients with exudative AMD (middle column) and in exudative AMD patients diagnosed within the last 5 years of data collection (right column). n, number of cases available in the analysis (no missing values).
We considered the possibility of a more delayed diagnosis of exudative AMD before the era of widely used OCTs and availability of more effective treatments and analysed the data of patients diagnosed during the last 5 years of data collection (n = 101, between April 2005 and April 2010) separately. The effects of the studied SNPs and tobacco smoking on the diagnosis of exudative AMD remained the same when the subgroup was analysed with the control subjects (data not shown). When only the patients with exudative AMD were included, the effect of the IL‐8 rs4073 on the age at diagnosis, however, was even more pronounced (HR = 2.9, p = 0.00031), and became the most significant of the factors associated with the earlier onset of the exudative AMD (Fig. 1, right column).
The homozygous risk genotypes and current smoking had also a cumulative effect on the age of onset of exudative AMD. The best correlation with the age of onset was found when the sum of the homozygous risk genotypes of IL‐8, CFH and ARMS2, and smoking (current smoker = 1 point, ex‐ or non‐smoker = 0 points) were calculated for each patient (Spearman's rho −0.348, p < 0.0001). Significant difference in the age of onset existed between the patients with zero risk factors (n = 84, median age 77.6 years, range 62.8–87.6 years) compared with the patients with one (n = 107, 74.5 years, 53.1–91.2 years, p = 0.0001), two to four (n = 52, 71.8 years, 54.5–83.8 years, p < 0.0001) or three to four (n = 6, 68.3 years, 63.3–69.8 years, p = 0.0002) risk factors. A significant difference existed also between the patients with one and two (n = 46, 72.9 years, 54.5–83.8 years, p = 0.015) risk factors (Fig. 2).
Figure 2.
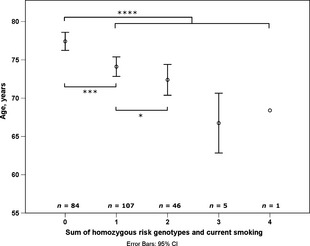
The sum of homozygous risk genotypes in IL‐8,CFH and ARMS2, and current smoking on the age of onset of exudative AMD (*p < 0.05, ***p < 0.001, ****p < 0.0001, Mann–Whitney U‐test).
No clear associations were found between the CNV lesion types and studied SNPs or tobacco smoking. Minimally classic lesions, however, were less frequent in patients with the homozygous TT risk genotype of ARMS2 (p = 0.001). The type of the exudative lesion had no effect on the age of onset of exudative AMD. Gender had no effect in any of the analyses. No interactions between the studied factors were found.
Discussion
In this study, we could show the earlier onset of exudative AMD to be associated not only with current smoking and the homozygous risk genotypes of the CFH rs1061170 and ARMS2 rs10490924 as reported earlier in other populations (Shuler et al. 2007, 2008; Keilhauer et al. 2013; Lechanteur et al. 2015), but also with the risk genotype AA of the IL‐8 rs4073. Out of these factors, only the AA genotype of IL‐8 rs4073 is not a well‐established risk factor for AMD and did not associate with the overall prevalence of AMD in our material. The IL‐8 rs4073 genotype may thus be connected with the mechanisms leading to the growth of CNV in the AMD disease process.
The IL‐8 rs4073 A→T has been associated with variations in IL‐8 production, and significantly higher levels of circulating and mucosal IL‐8 have been detected in patients carrying the A allele or the AA homozygous genotype in IL‐8 rs4073 (Hull et al. 2000; Ohyauchi et al. 2005; Taguchi et al. 2005; Hildebrand et al. 2007). In addition to being a powerful inflammatory cytokine, a chemotactic factor for migratory immune cells and an activating factor for neutrophilic granulocytes, IL‐8 is a potent proangiogenic factor. IL‐8 is expressed not only by the cells of immune system but also by vascular endothelial and retinal pigment epithelial (RPE) cells. It has a critical role in vascular formation both in physiological and pathological conditions (Belperio et al. 2000). Expression of IL‐8 is upregulated in response to various stimuli, including CRP (Wang et al. 2010), oxidative stress (DeForge et al. 1993), saturated fatty acids (Stentz & Kitabchi 2006), preoxidized photoreceptor outer segments (Higgins et al. 2003), amyloid‐β (Kurji et al. 2010) and angiopoietin‐1 (Abdel‐Malak et al. 2008). IL‐8 induces angiogenesis through both VEGF‐dependent and VEGF‐independent pathways and increases endothelial permeability (Mizukami et al. 2005; Martin et al. 2009). IL‐8 receptors CXCR1 and CXCR2 are expressed in endothelial cells. The activation of the CXCR2‐receptor elevates the levels of intracellular VEGF and enhances VEGF secretion (Martin et al. 2009). The activated CXCR2 is also capable of forming a complex with the VEGF receptor, VEGFR2, and causes transactivation of the VEGF receptor without the presence of VEGF (Petreaca et al. 2007). In aged cells, disturbance of calcium homeostasis may lead to induced IL‐8 gene expression and IL‐8 secretion (Yang et al. 2015). Chronic oxidative stress has also been found to trigger the RPE cells to produce higher levels of IL‐8 stimulating both inflammation and angiogenesis, which, in turn, may lead to the development of CNV and exudative AMD (Zhu et al. 2009).
In our data, the associations found between the presence of AMD lesions and the known genetic risk factors for AMD, CFH rs1061170, ARMS2 rs10490924 and current smoking were clear (Christen et al. 1996; Seddon et al. 1996, 2013; Edwards et al. 2005; Haines et al. 2005; Jakobsdottir et al. 2005; Klein et al. 2005; DeWan 2006; Yang et al. 2006; Maller et al. 2007; Yates et al. 2007). We could not, however, show any association between the prevalence of AMD and the risk genotype of IL‐8 rs4073. The A allele in IL‐8 SNP rs4073 has previously been associated with an increased risk of AMD in Caucasian population in Britain (Goverdhan et al. 2008). Also, the IL‐8 rs2227306 T→C (+781 C/T) has been associated with an increased risk of developing exudative AMD in Taiwan Chinese and in Italian populations (Tsai et al. 2008; Ricci et al. 2013). In Europeans, a strong linkage disequilibrium exists between the IL‐8 SNPs rs4073 and rs2227306 (Hull et al. 2001; Ricci et al. 2013), and therefore, we would have expected to find a same kind of association with the IL‐8 rs4073 in our material. Our sample size, however, is limited, and with the power of 0.8 only differences over 13% in allele frequencies between the groups could be detected. The differences reported earlier have been less than that both in the British and in the Italian populations (Goverdhan et al. 2008; Ricci et al. 2013). The IL‐8 rs2227306 may be the SNP that is, after all, the main functional polymorphism responsible for the enhanced interaction with the transcription factor binding complex and the higher levels of secreted IL‐8 and may therefore show somewhat stronger associations with pathologies than the IL‐8 rs4073 (Hacking et al. 2004). Despite our limited sample size, however, the younger age of onset of the exudative AMD was quite clearly associated not only with the known risk genotypes in the CFH rs1061170 and ARMS2 rs10490924 and current smoking but also with the AA genotype of IL‐8 rs4073.
The younger age of onset of the disease in the exudative AMD patients was associated only with the homozygous, not heterozygous risk genotypes in the IL‐8 rs4073, CFH rs1061170 and ARMS2 rs10490924. It was also associated with only current smoking, not ex‐smoking compared with non‐smoking behaviour. The associations with the prevalence of AMD, on the other hand, resemble more of a dose–response pattern with higher hazard ratios associated also with heterozygous genotypes and ex‐smoking compared with non‐risk homozygous genotypes and non‐smoking. The same kind of phenomenon has also been seen in the previous studies showing the homozygous risk genotypes of CFH rs1061170 and ARMS2 rs10490924 and tobacco smoking to be associated with the younger onset of exudative AMD (Shuler et al. 2007, 2008; Keilhauer et al. 2013; Lechanteur et al. 2015). One possible explanation could be that when in the analyses of the age of onset only diseased subjects are included, the protective effects of the non‐risk genotypes and non‐smoking behaviour are not fully seen when healthy controls are not represented.
The limitations of this study are its retrospective nature, the relatively small sample size, the estimate of onset of the disease being based on the medical records and the diagnosis being based on clinical findings and angiograms in the era before OCTs. The study population also represents a combined sample of subjects of our previous studies. The results, especially the distinct difference between the effects of IL‐8 rs4073 on the prevalence of AMD and on the age of onset of exudative disease, need to be confirmed in larger and independent patient materials.
The association of the IL‐8 rs4073 A→T with the earlier onset of exudative AMD increases the evidence of the role of IL‐8 in the exudative AMD disease process. The results of this study suggest that the IL‐8 polymorphism may have a stronger effect on the age of onset of exudative AMD than on the risk of developing AMD. An explanation for that could be that the IL‐8 enhances vascular leakage. An increased tendency to develop retinal oedema with higher IL‐8 levels could lead to more symptomatic lesions and earlier diagnosis of the disease. Also, increased local IL‐8 production may augment the proinflammatory effects of the established AMD risk factors and link inflammation and angiogenesis in the transformation of a dry AMD lesion into the exudative phenotype.
This work was supported by grants from The Eye Foundation, Helsinki, Finland; The Evald and Hilda Nissi Foundation, Helsinki, Finland; The Eye and Tissue Bank Foundation, Helsinki, Finland, and Mary and Georg C. Ehrnrooth Foundation, Helsinki, Finland.
References
- Abdel‐Malak NA, Srikant CB, Kristof AS, Magder SA, Di Battista JA & Hussain SN (2008): Angiopoietin‐1 promotes endothelial cell proliferation and migration through AP‐1‐dependent autocrine production of interleukin‐8. Blood 111: 4145–4154. [DOI] [PubMed] [Google Scholar]
- Barbazetto I, Burdan A, Bressler NM et al. (2003): Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment–TAP and VIP report No. 2. Arch Ophthalmol 121: 1253–1268. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD & Strieter RM (2000): CXC chemokines in angiogenesis. J Leukoc Biol 68: 1–8. [PubMed] [Google Scholar]
- Christen WG, Glynn RJ, Manson JE, Ajani UA & Buring JE (1996): A prospective study of cigarette smoking and risk of age‐related macular degeneration in men. JAMA 276: 1147–1151. [PubMed] [Google Scholar]
- Davis MD, Gangnon RE, Lee LY et al. (2005): The Age‐Related Eye Disease Study severity scale for age‐related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 123: 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA & Remick DG (1993): Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem 268: 25568–25576. [PubMed] [Google Scholar]
- DeWan A (2006): HTRA1 promoter polymorphism in wet age‐related macular degeneration. Science 314: 989. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C & Farrer LA (2005): Complement factor H polymorphism and age‐related macular degeneration. Science 308: 421–424. [DOI] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J et al. (2006): Variation in factor B (BF) and complement component 2 (C2) genes is associated with age‐related macular degeneration. Nat Genet 38: 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverdhan SV, Ennis S, Hannan SR, Madhusudhana KC, Cree AJ, Luff AJ & Lotery AJ (2008): Interleukin‐8 promoter polymorphism ‐251A/T is a risk factor for age‐related macular degeneration. Br J Ophthalmol 92: 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacking D, Knight JC, Rockett K, Brown H, Frampton J, Kwiatkowski DP, Hull J & Udalova IA (2004): Increased in vivo transcription of an IL‐8 haplotype associated with respiratory syncytial virus disease‐susceptibility. Genes Immun 5: 274–282. [DOI] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S et al. (2005): Complement factor H variant increases the risk of age‐related macular degeneration. Science 308: 419–421. [DOI] [PubMed] [Google Scholar]
- Hautamäki A, Kivioja J, Vavuli S et al. (2013): Interleukin 8 promoter polymorphism predicts the initial response to bevacizumab treatment for exudative age‐related macular degeneration. Retina 33: 1815–1827. [DOI] [PubMed] [Google Scholar]
- Hautamäki A, Kivioja J, Seitsonen S, Savolainen ER, Liinamaa MJ, Luoma A, Jarvela I & Immonen I (2014): The IL‐8, VEGF, and CFH polymorphisms and bevacizumab in age‐related macular degeneration. Ophthalmology 121: 973.e2–974.e2. [DOI] [PubMed] [Google Scholar]
- Higgins GT, Wang JH, Dockery P, Cleary PE & Redmond HP (2003): Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest Ophthalmol Vis Sci 44: 1775–1782. [DOI] [PubMed] [Google Scholar]
- Hildebrand F, Stuhrmann M, van Griensven M, Meier S, Hasenkamp S, Krettek C & Pape HC (2007): Association of IL‐8‐251A/T polymorphism with incidence of Acute Respiratory Distress Syndrome (ARDS) and IL‐8 synthesis after multiple trauma. Cytokine 37: 192–199. [DOI] [PubMed] [Google Scholar]
- Hong T, Tan AG, Mitchell P & Wang JJ (2011): A review and meta‐analysis of the association between C‐reactive protein and age‐related macular degeneration. Surv Ophthalmol 56: 184–194. [DOI] [PubMed] [Google Scholar]
- Hull J, Thomson A & Kwiatkowski D (2000): Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax 55: 1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull J, Ackerman H, Isles K, Usen S, Pinder M, Thomson A & Kwiatkowski D (2001): Unusual haplotypic structure of IL8, a susceptibility locus for a common respiratory virus. Am J Hum Genet 69: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE & Gorin MB (2005): Susceptibility genes for age‐related maculopathy on chromosome 10q26. Am J Hum Genet 77: 389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Tao Y, Neumaier M & Findeisen P (2012): Cytokine concentration in aqueous humour of eyes with exudative age‐related macular degeneration. Acta Ophthalmol 90: e381–e388. [DOI] [PubMed] [Google Scholar]
- Keilhauer CN, Fritsche LG, Guthoff R, Haubitz I & Weber BH (2013): Age‐related macular degeneration and coronary heart disease: evaluation of genetic and environmental associations. Eur J Med Genet 56: 72–79. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY et al. (2005): Complement factor H polymorphism in age‐related macular degeneration. Science 308: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurji KH, Cui JZ, Lin T, Harriman D, Prasad SS, Kojic L & Matsubara JA (2010): Microarray analysis identifies changes in inflammatory gene expression in response to amyloid‐beta stimulation of cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 51: 1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechanteur YT, van de Camp PL, Smailhodzic D et al. (2015): Association of smoking and CFH and ARMS2 risk variants with younger age at onset of neovascular age‐related macular degeneration. JAMA Ophthalmol 133: 533–541. [DOI] [PubMed] [Google Scholar]
- Maller J, George S, Purcell S, Fagerness J, Altshuler D, Daly M & Seddon J (2006): Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age‐related macular degeneration. Nat Genet 38: 1055. [DOI] [PubMed] [Google Scholar]
- Maller J, Fagerness J, Reynolds R, Neale B, Daly M & Seddon J (2007): Variation in complement factor 3 is associated with risk of age‐related macular degeneration. Nat Genet 39: 1200. [DOI] [PubMed] [Google Scholar]
- Martin D, Galisteo R & Gutkind JS (2009): CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem 284: 6038–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Tao Y & Li XX (2012): Inflammatory cytokines in aqueous humor of patients with choroidal neovascularization. Mol Vis 18: 574–580. [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Jo WS, Duerr EM et al. (2005): Induction of interleukin‐8 preserves the angiogenic response in HIF‐1alpha‐deficient colon cancer cells. Nat Med 11: 992–997. [DOI] [PubMed] [Google Scholar]
- van der Net JB, Janssens AC, Eijkemans MJ, Kastelein JJ, Sijbrands EJ & Steyerberg EW (2008): Cox proportional hazards models have more statistical power than logistic regression models in cross‐sectional genetic association studies. Eur J Hum Genet 16: 1111–1116. [DOI] [PubMed] [Google Scholar]
- Ohyauchi M, Imatani A, Yonechi M et al. (2005): The polymorphism interleukin 8 ‐251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut 54: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreaca ML, Yao M, Liu Y, Defea K & Martins‐Green M (2007): Transactivation of vascular endothelial growth factor receptor‐2 by interleukin‐8 (IL‐8/CXCL8) is required for IL‐8/CXCL8‐induced endothelial permeability. Mol Biol Cell 18: 5014–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci F, Staurenghi G, Lepre T et al. (2013): Haplotypes in IL‐8 gene are associated to age‐related macular degeneration: a case–control study. PLoS ONE 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh MI, Kim HS, Song JH, Lim JB, Koh HJ & Kwon OW (2009): Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with amd after bevacizumab treatment. Retina 29: 523–529. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Willett WC, Speizer FE & Hankinson SE (1996): A prospective study of cigarette smoking and age‐related macular degeneration in women. JAMA 276: 1141–1146. [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B & Rifai N (2005): Progression of age‐related macular degeneration: prospective assessment of C‐reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol 123: 774–782. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Yu Y, Miller EC et al. (2013): Rare variants in CFI, C3 and C9 are associated with high risk of advanced age‐related macular degeneration. Nat Genet 45: 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitsonen S, Lemmela S, Holopainen J et al. (2006): Analysis of variants in the complement factor H, the elongation of very long chain fatty acids‐like 4 and the hemicentin 1 genes of age‐related macular degeneration in the Finnish population. Mol Vis 12: 796–801. [PubMed] [Google Scholar]
- Seitsonen SP, Onkamo P, Peng G et al. (2008): Multifactor effects and evidence of potential interaction between complement factor H Y402H and LOC387715 A69S in age‐related macular degeneration. PLoS ONE 3: e3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler RK Jr, Hauser MA, Caldwell J et al. (2007): Neovascular age‐related macular degeneration and its association with LOC387715 and complement factor H polymorphism. Arch Ophthalmol 125: 63–67. [DOI] [PubMed] [Google Scholar]
- Shuler RK Jr, Schmidt S, Gallins P et al. (2008): Phenotype analysis of patients with the risk variant LOC387715 (A69S) in age‐related macular degeneration. Am J Ophthalmol 145: 303–307. [DOI] [PubMed] [Google Scholar]
- Stentz FB & Kitabchi AE (2006): Palmitic acid‐induced activation of human T‐lymphocytes and aortic endothelial cells with production of insulin receptors, reactive oxygen species, cytokines, and lipid peroxidation. Biochem Biophys Res Commun 346: 721–726. [DOI] [PubMed] [Google Scholar]
- Strandberg A, Strandberg TE, Salomaa VV, Pitkälä K, Häppölä O & Miettinen TA (2004): A follow‐up study found that cardiovascular risk in middle age predicted mortality and quality of life in old age. J Clin Epidemiol 57: 415–421. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, Niwa Y & Goto H (2005): Interleukin‐8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev 14: 2487–2493. [DOI] [PubMed] [Google Scholar]
- Tsai YY, Lin JM, Wan L et al. (2008): Interleukin gene polymorphisms in age‐related macular degeneration. Invest Ophthalmol Vis Sci 49: 693–698. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bian ZM, Yu WZ, Yan Z, Chen WC & Li XX (2010): Induction of interleukin‐8 gene expression and protein secretion by C‐reactive protein in ARPE‐19 cells. Exp Eye Res 91: 135–142. [DOI] [PubMed] [Google Scholar]
- Yang Z, Camp NJ, Sun H et al. (2006): A variant of the HTRA1 gene increases susceptibility to age‐related macular degeneration. Science 314: 992–993. [DOI] [PubMed] [Google Scholar]
- Yang IH, Wong JH, Chang CM et al. (2015): Involvement of intracellular calcium mobilization in IL‐8 activation in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 56: 761–769. [DOI] [PubMed] [Google Scholar]
- Yates JR, Sepp T, Matharu BK et al. (2007): Complement C3 variant and the risk of age‐related macular degeneration. N Engl J Med 357: 553–561. [DOI] [PubMed] [Google Scholar]
- Zhu D, Deng X, Xu J & Hinton DR (2009): What determines the switch between atrophic and neovascular forms of age related macular degeneration? – the role of BMP4 induced senescence Aging 1: 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]


