Abstract
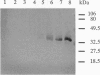
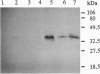
Chlorophyllase (Chlase; EC 3.1.1.14) was extracted from plastid fractions of ethylene-treated orange fruit peel and purified 400-fold to homogeneity by gel filtration, hydrophobic chromatography, and preparative SDS/PAGE of nonheated protein. SDS/PAGE of nonheated purified enzyme indicated that Chlase activity is associated with a single protein band migrating at an apparent molecular mass of 25 kDa whereas the heated purified enzyme had a molecular mass of 35 kDa. The N-terminal sequence of the purified protein was determined. The purified enzyme was used as an immunogen for raising antibodies in rabbits. The antiserum was highly specific and on Western blots recognized both the heated and the nonheated form of Chlase. The antibodies also recognized the solubilized enzyme, as shown by an immunoprecipitation assay and by antigen-antibody capture assays in microtiter plates. Treatment with ethylene, which enhances degreening, increased Chlase activity 12-fold. Immunoblot analyses of crude extracts from ethylene-treated fruit detected a strong signal of the Chlase protein, while only a trace level of the enzyme protein could be detected in air. Gibberellin A3 and N6-benzyladenine partly counteracted the ethylene-induced increase in Chlase activity as well as the immunodetected upsurge of the Chlase protein. Ethylene appears to enhance the degreening of citrus fruit through de novo synthesis of the Chlase protein, which in turn is inhibited by the senescence-delaying regulators, gibberellin A3 and N6-benzyladenine. The Chlase enzyme protein may, therefore, serve as a model system for studying the hormonal molecular regulation of fruit ripening and senescence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir-Shapira D., Goldschmidt E. E., Altman A. Chlorophyll catabolism in senescing plant tissues: In vivo breakdown intermediates suggest different degradative pathways for Citrus fruit and parsley leaves. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1901–1905. doi: 10.1073/pnas.84.7.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapenna D., Alexander D. C., Bennett A. B. Molecular cloning of tomato fruit polygalacturonase: Analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal H. C., Leopold A. C. Gibberellin delays ripening of tomatoes. Science. 1967 Dec 22;158(3808):1579–1580. doi: 10.1126/science.158.3808.1579. [DOI] [PubMed] [Google Scholar]
- Eilati S. K., Goldschmidt E. E., Monselise S. P. Hormonal control of colour changes in orange peel. Experientia. 1969 Feb 15;25(2):209–210. doi: 10.1007/BF01899129. [DOI] [PubMed] [Google Scholar]
- Goldschmidt E. E., Aharoni Y., Eilati S. K., Riov J. W., Monselise S. P. Differential counteraction of ethylene effects by gibberellin a(3) and n(6)-benzyladenine in senescing citrus peel. Plant Physiol. 1977 Feb;59(2):193–195. doi: 10.1104/pp.59.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J., Picton S., Shabbeer J., Schuch W., Grierson D. Molecular biology of fruit ripening and its manipulation with antisense genes. Plant Mol Biol. 1992 May;19(1):69–87. doi: 10.1007/BF00015607. [DOI] [PubMed] [Google Scholar]
- McFeeters R. F., Chichester C. O., Whitaker J. R. Purification and Properties of Chlorophyllase from Ailanthus altissima (Tree-of-Heaven). Plant Physiol. 1971 May;47(5):609–618. doi: 10.1104/pp.47.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R., Porath D. Chlorophyll determination in intact tissues using n,n-dimethylformamide. Plant Physiol. 1980 Mar;65(3):478–479. doi: 10.1104/pp.65.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. C., Barmore C. R. Involvement of ethylene in chlorophyll degradation in peel of citrus fruits. Plant Physiol. 1981 Oct;68(4):854–856. doi: 10.1104/pp.68.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Shioi Y., Sasa T. Purification of solubilized chlorophyllase from Chlorella protothecoides. Methods Enzymol. 1986;123:421–427. doi: 10.1016/s0076-6879(86)23052-9. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Kakuno T., Yamashita J., Horio T. Purification and properties of chlorophyllase from greened rye seedlings. J Biochem. 1982 Dec;92(6):1763–1773. doi: 10.1093/oxfordjournals.jbchem.a134106. [DOI] [PubMed] [Google Scholar]
- Theologis A., Zarembinski T. I., Oeller P. W., Liang X., Abel S. Modification of fruit ripening by suppressing gene expression. Plant Physiol. 1992 Oct;100(2):549–551. doi: 10.1104/pp.100.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M. L., Sexton R., Del Campillo E., Lewis L. N. Bean abscission cellulase : characterization of a cDNA clone and regulation of gene expression by ethylene and auxin. Plant Physiol. 1988 Dec;88(4):1257–1262. doi: 10.1104/pp.88.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. R. Enhancement of immunoblot staining using a mixed chromogenic substrate. J Immunol Methods. 1989 Jul 26;121(2):295–296. doi: 10.1016/0022-1759(89)90174-9. [DOI] [PubMed] [Google Scholar]