Abstract
The neutrophilic dermatoses are a group of disorders characterized by skin lesions for which histological examination reveals intense epidermal and/or dermal inflammatory infiltrates composed primarily of neutrophils without evidence of infection. The myelodysplastic syndromes consist of a heterogeneous group of malignant hematopoietic stem cell disorders characterized by dysplastic and inadequate blood cell production with a variable risk of transformation to acute leukemia. Rarely, histiocytoid Sweet’s syndrome occurring in patients with myelodysplastic syndrome has been described. We present a case of a 66-year-old woman with a history of myelodysplastic syndrome who developed histiocytoid Sweet’s syndrome. We also review the literature and characterize patients with myelodysplastic syndrome who have developed histiocytoid Sweet’s syndrome.
Keywords: histiocytoid Sweet’s, myelodysplasia, myelodysplastic, sweet, sweet’s, syndrome
Introduction
The neutrophilic dermatoses are a group of disorders characterized by skin lesions for which histological examination reveals intense epidermal and/or dermal inflammatory infiltrates composed primarily of neutrophils without evidence of infection [1]. Cutaneous findings of neutrophilic dermatoses vary; lesions can present as nodules, plaques, ulcerations, or vesiculopustules and can be localized or widespread. In some cases, extracutaneous involvement may occur [2,3]. While the exact pathogenesis of neutrophilic dermatoses is unknown, these disorders may represent a state of immunologic reactivity [4]. In patients who have an underlying malignancy, neutrophilic dermatoses may occur as an anti-neoplastic therapy-related disorder, a paraneoplastic syndrome, or, less commonly, as an idiopathic condition [5]. The group of disorders that comprise neutrophilic dermatoses includes Behçet’s disease, bowel (intestinal) bypass syndrome, erythema elevatum diutinum, neutrophilic dermatosis of the dorsal hand, neutrophilic eccrine hidradenitis [6], pyoderma gangrenosum, and Sweet’s syndrome (SS) [1].
The myelodysplastic syndromes (MDS) consist of a heterogeneous group of malignant hematopoietic stem cell disorders characterized by dysplastic and inadequate blood cell production and a variable risk of transformation to acute leukemia. MDS may occur de novo or may arise years after exposure to potentially mutagenic therapy (i.e., chemotherapy, radiation exposure). The patients’ inability to properly produce normal erythrocytes, mature granulocytes, and platelets, often results in an array of systemic consequences such as anemia, bleeding, and an increased risk of infection [7].
Evidence from cancer databases suggests that there are approximately 10,000 new cases of MDS diagnosed annually in the United States [8]. MDS occurs most commonly in older adults with a median age at diagnosis of 65 years and a male predominance. Review of the literature reveals six cases of histiocytoid Sweet’s syndrome with MDS [9–14]. When Sweet’s syndrome is present, it portends a poor prognosis in patients with MDS [12].
Histiocytoid Sweet’s syndrome is a rare variant of SS. Histologically, histiocytoid Sweet’s syndrome can present with an infiltrate containing predominantly mononuclear cells with large, slightly eccentric kidney-shaped or elongated nuclei with single indistinct nucleoli and slightly eosinophilic cytoplasm accompanied by numerous mature neutrophils and some mature lymphocytes; these cells may be misinterpreted as histiocytes. We present the case of a 66-year-old woman with a history of myelodysplasia who developed violaceous papules, some with central ulceration, on her face, bilateral upper extremities, and bilateral lower extremities, which later spread to include her right cheek and center of her chest. Subsequent biopsy of the left posterior forearm confirmed the diagnosis of histiocytoid Sweet’s syndrome. Herein we describe patients with myelodysplastic syndromes who developed histiocytoid Sweet’s syndrome and discuss the therapeutic options for the treatment of SS.
Case report
A 66-year-old woman presented to the dermatology clinic with a one-month history of a rash. The patient reported she was diagnosed with MDS via bone marrow biopsy one year prior; she was started on azacitidine and continued this therapy with minimal improvement. The patient was receiving blood transfusions for anemia and thrombocytopenia. She had not received granulocyte colony-stimulating-factor (G-CSF) at any point during her treatment.
Physical exam revealed violaceous papules and plaques involving the face (Figure 1), upper extremities (Figure 2), and lower extremities (Figure 3). The lesions were slightly tender. The patient reported she had not started any new medications. Complete blood count at time of diagnosis demonstrated: white blood cell count 1.5, hemoglobin 8.2, hematocrit 24.1, and platelets 26.
Figure 1.
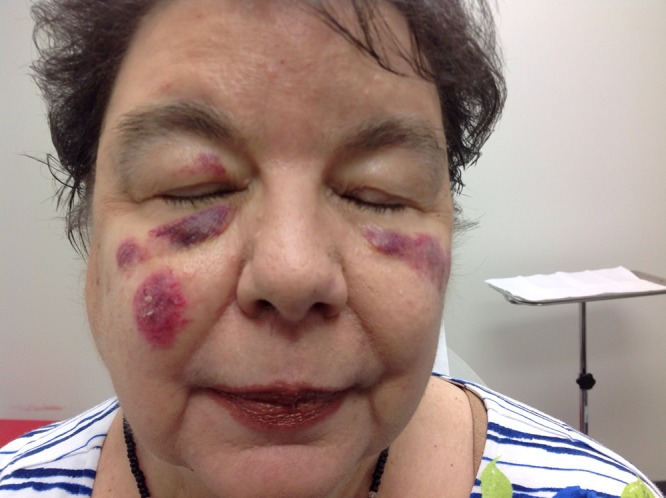
Frontal view of the patient’s face demonstrating deeply erythematous to violaceous, edematous plaques on the bilateral cheeks and right upper eyelid. [Copyright: ©2016 Shalaby et al.]
Figure 2.
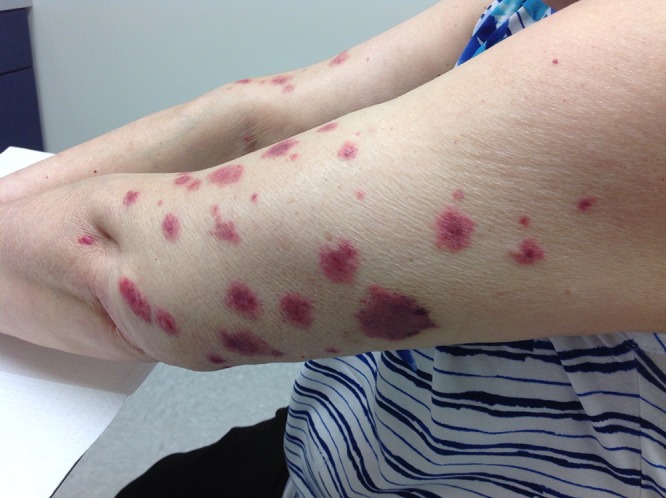
Multiple erythematous, edematous papules and plaques involving the left upper extremity. [Copyright: ©2016 Shalaby et al.]
Figure 3.
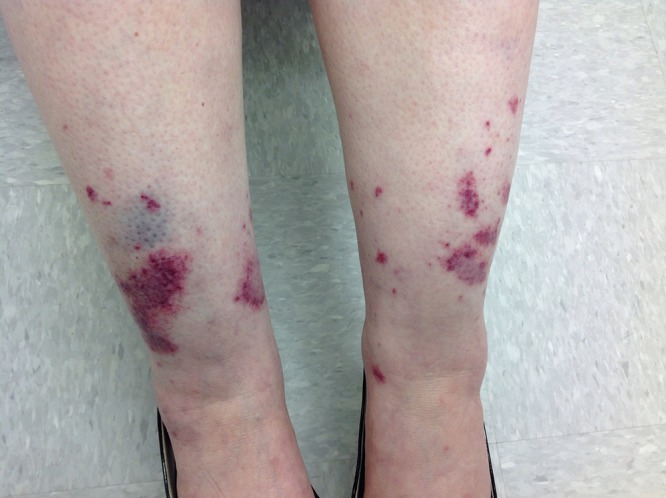
Lower extremities demonstrating ecchymosis and violaceous plaques bilaterally. [Copyright: ©2016 Shalaby et al.]
A 3 mm punch biopsy was performed on the left posterior forearm which showed papillary dermal edema in association with a diffuse dermal infiltrate consisting of lymphocytes, histiocytes, few neutrophils with leukocytoclasis, and occasional eosinophils (Figures 4 and 5). The biopsy was consistent with histiocytoid Sweet’s syndrome. Our patient was initially treated with 90 mg of oral prednisone daily and dapsone 5% gel. The patient experienced resolution of many of her lesions. After four weeks of this regimen, prednisone was tapered and finally stopped.
Figure 4.
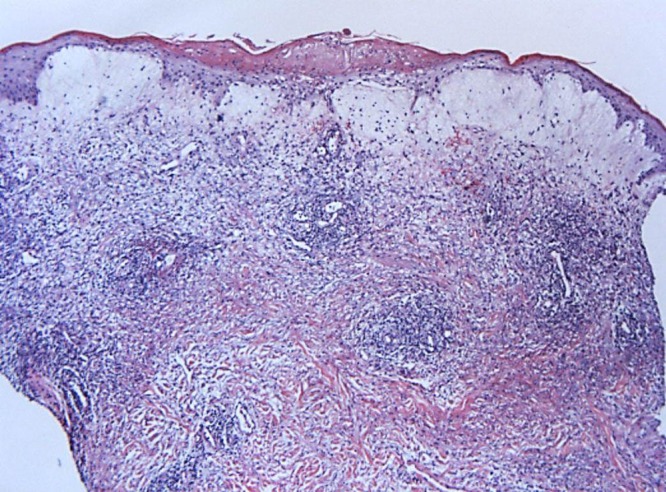
10× view demonstrating focal compact parakeratosis with marked edema of the papillary dermis bordering on vesiculation. A superficial and deep perivascular, interstitial and periadnexal infiltrate consisting of lymphocytes, red blood cells and histiocytoid cells is present. [Copyright: ©2016 Shalaby et al.]
Figure 5.
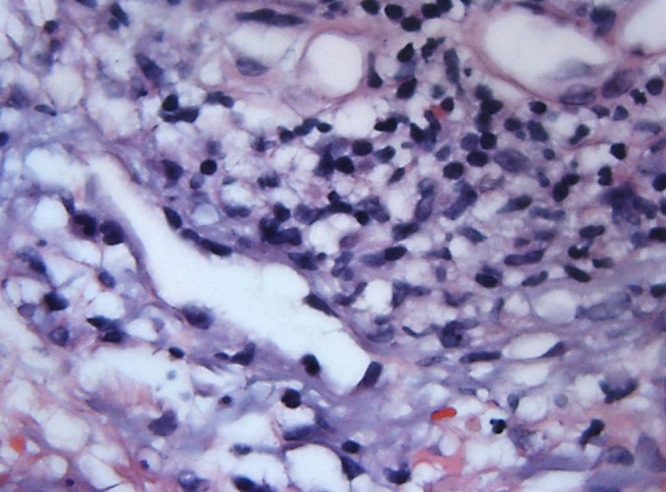
63× view demonstrating showing a perivascular and interstitial infiltrate consisting of lymphocytes, red blood cells and histiocytoid cells. [Copyright: ©2016 Shalaby et al.]
Discussion
SS was first described in 1964 by Robert Sweet as a constellation of clinical and laboratory findings he had observed in eight women as an “acute febrile neutrophilic dermatosis” [4,15]. SS skin lesions are typically tender, red to violaceous papules or nodules. Sites frequently involved include the face, neck, and upper extremities [16]. Salient features of SS include: pyrexia, elevated neutrophil count, painful erythematous cutaneous lesions characterized by an infiltrate of mature neutrophils typically located in the upper dermis, and prompt clinical improvement following the initiation of corticosteroid therapy [4]. Arthralgias, malaise, headache, and myalgia are other symptoms associated with SS.
Subtypes of SS have been described and include: (i) the “classic” presentation, which may be associated with upper respiratory tract or gastrointestinal infection, inflammatory bowel disease, and pregnancy; (ii) the “malignancy-associated” presentation, in which the dermatosis is either the presenting manifestation of a previously undiagnosed cancer or the recurrence of malignancy in an oncology patient; and (iii) the “drug-induced” presentation, when the condition is precipitated by the patient having received a dermatosis-associated medication, most notoriously G-CSF [4]. SS may also result from a hypersensitivity reaction to a bacterial or viral antigen [16]. It has been postulated that photosensitivity may also play a role in the pathogenesis of SS, although the mechanism is unknown [17]. Furthermore, an alteration in the gene encoding protein tyrosine phosphatase nonreceptor 6 appears to be involved in the pathogenesis of SS and other subsets of neutrophilic dermatoses [18].
While the pathogenesis of MDS remains poorly understood, studies suggest that the cell of origin has acquired multiple mutations resulting in dysplasia and ineffective hematopoiesis [19]. MDS genomes are characterized by global DNA hypomethylation with concomitant hypermethylation of gene-promoter regions relative to normal controls [20]. The underlying mechanism of altered DNA methylation in MDS genomes is unclear; however, studies have implicated mutations in genes that encode enzymes, such as TET2 (10,11 translocation), IDH1 (isocitrate dehydrogenase-1), and IDH2 (isocitrate dehydrogenase-2), that influence DNA methylation directly or indirectly [21].
Histiocytoid Sweet’s syndrome is a histopathologic variant of SS characterized by an infiltrate of mononuclear cells that have a histiocytic appearance and represent immature granulocytes [22–25]. Rarely, histiocytoid Sweet’s syndrome has been associated with MDS.
A PubMed search was performed using the keywords: histiocytoid Sweet’s, myelodysplasia, myelodysplastic, sweet, syndrome, which yielded six patients with histiocytoid Sweet’s syndrome and MDS. To the best of our knowledge, our patient is the seventh patient with MDS to develop histiocytoid Sweet’s syndrome [9–14]. These patients have been characterized in [Table 1] [9–14]. Four of the seven patients described were women; the average age of patients with MDS who developed histiocytoid Sweet’s syndrome was 65 years (range 44–75 years of age).
TABLE 1.
| Case | Age/Sex | Clinical Appearance | Time Period to Progression of Leukemia after Diagnosis of Sweet’s Syndrome | MDS Present at Time of Diagnosis of Sweet’s Syndrome | Ref |
|---|---|---|---|---|---|
| 1 | 44W | Multiple papules, plaques, and nodules on the face and extremities | N/A | Yes | [14] |
| 2 | 66W | Violaceous papules and plaques involving the face, upper extremities, and lower extremities | 16 months | Yes | [CR] |
| 3 | 68W | 20 scattered 0.5–2 cm, pink to pink-purple firm, non-tender nodules on the legs and left arm | No progression | Yes | [9] |
| 4 | 73W | Multiple red tender plaques on trunk and upper extremities varying in size from 0.5 to 5 cm | No progression | Yes | [10] |
| 5 | 57M | Multiple tender, raised, annular erythematous lesions on ankles and trunk. | No progression | Yes | [12] |
| 6 | 71 M | Multiple, slightly tender, erythematous nodules and plaques scattered over the neck, thighs, and trunk, and confluent erythematous indurated plaques on the forearms | No progression | Yes | [11] |
| 7 | 75M | Burning, maculopapular, erythematous, sharp-edged lesions, affecting abdomen, arms, back, and face | Leukemia at time of diagnosis | Yes | [13] |
Legend: CR = Current Report; M = male; MDS—myelodysplastic syndrome; N/A—not available; W = woman;
Systemic corticosteroids are the mainstay of therapy for SS. Prednisone, at a dosage of 1 mg/kg/day, may be given as a single morning dose. Intravenous pulse administration of methylprednisone sodium succinate (up to 1000 mg/day) over 1 or more hours, daily for three to five days, may be utilized for patients whose condition is refractory to other therapies [4]. Other therapies include: clofazimine, colchicine, cyclosporine, dapsone, high-potency corticosteroids (such as clobetasol propionate 0.05%), indometacin, intralesional corticosteroids, Lugol’s solution, and potassium iodide [4]. Metronidazole has been effective in patients whose dermatosis is related to inflammatory bowel disease [4]. Tumor necrosis factor (TNF) antagonists such as etanercept, infliximab, and thalidomide have been shown to be effective [4,23].
Conclusion
Histiocytoid Sweet’s syndrome is a rare variant of SS, which was originally described by Robert Sweet and is sometimes associated with MDS. SS belongs to a group of conditions known as neutrophilic dermatoses, which are characterized by skin lesions, which histologically consist of intense epidermal and/or dermal inflammatory infiltrates composed primarily of neutrophils without evidence of infection [1]. The myelodysplastic syndromes consist of a group of malignant hematopoietic stem cell disorders which result in impaired blood cell production and which pose a risk of transformation to acute leukemia. SS has been shown to be associated with gastrointestinal or upper respiratory tract infection, hematopoietic malignancy, and various drugs, notably G-CSF [25]. First-line treatment for SS is systemic corticosteroids. Herein we have presented the case of a 66-year-old woman with a history of myelodysplastic syndrome who developed histiocytoid Sweet’s syndrome.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Callen JP. Neutrophilic dermatoses. Dermatol Clin. 2002;20(3):409–19. doi: 10.1016/s0733-8635(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 2.Jorizzo JL, Solomon AR, Zanolli MD, Leshin B. Neutrophilic vascular reactions. J Am Acad Dermatol. 1988;19(6):983–1005. doi: 10.1016/s0190-9622(88)70264-9. [DOI] [PubMed] [Google Scholar]
- 3.Moschella SL. Review of so-called aseptic neutrophilic dermatoses. Australas J Dermatol. 1983;24(2):55–62. doi: 10.1111/j.1440-0960.1983.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10(5):301–12. doi: 10.2165/11310730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Cohen PR. Neutrophilic dermatoses occurring in oncology patients. Int J Dermatol. 2007;46(1):106–11. doi: 10.1111/j.1365-4632.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 6.Harrist TJ, Fine JD, Berman RS, Murphy GF, Mihm MC., Jr Neutrophilic eccrine hidradenitis. A distinctive type of neutrophilic dermatosis associated with myelogenous leukemia and chemotherapy. Arch Dermatol. 1982;118(4):263–66. doi: 10.1001/archderm.118.4.263. [DOI] [PubMed] [Google Scholar]
- 7.Steensma DP, Bennet JM. The myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc. 2006;81(1):104–30. doi: 10.4065/81.1.104. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109(8):1536–42. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Zhang Q, Chen M. Subcutaneous histiocytoid Sweet’s syndrome in a patient associated with myelodysplastic syndrome-refractory anemia. J Dermatol. 2012;39(1):99–101. doi: 10.1111/j.1346-8138.2011.01290.x. [DOI] [PubMed] [Google Scholar]
- 10.Ten Oever J, Kuijper PH, Kuijpers AL, Dercksen MW, Vreudgenhil G. Complete remission of MDS RAEB following immunosuppressive treatment in a patient with Sweet’s syndrome. Neth J Med. 2009;67(8):347–50. [PubMed] [Google Scholar]
- 11.Pinal-Fernandez I, Ferrer Fabrega B, Ramentol Sintas M, Solans Laque R. Histiocytoid Sweet syndrome and cutaneous polyarteritis nodosa secondary to myelodysplastic syndrome. Int J Rheum Dis. 2013;16(6):777–9. doi: 10.1111/1756-185X.12103. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser R, Connolly K, Linker C, Maldonado J, Fye K. Stem cell transplant for myelodysplastic syndrome-associated histiocytoid Sweet’s syndrome in a patient with arthritis and myalgias. Arthritis Rheum. 2008;59(12):1832–4. doi: 10.1002/art.24061. [DOI] [PubMed] [Google Scholar]
- 13.Srisuttiyakorn C, Reeve J, Reddy S, Imaeda S, Lazova R. Subcutaneous histiocytoid Sweet’s syndrome in a patient with myelodysplastic syndrome and acute myeloblastic leukemia. J Cutan Pathol. 2014;41(5):475–9. doi: 10.1111/cup.12305. [DOI] [PubMed] [Google Scholar]
- 14.Park JY, Park JS, Kim YC. Histiocytoid Sweet’s syndrome potentially related to decitabine in a patient with myelodysplastic syndrome. Eur J Dermatol. 2012;22(6):811–2. doi: 10.1684/ejd.2012.1861. [DOI] [PubMed] [Google Scholar]
- 15.Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349–56. doi: 10.1111/j.1365-2133.1964.tb14541.x. [DOI] [PubMed] [Google Scholar]
- 16.Anzalone CL, Cohen PR. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) Curr Opin Hematol. 2013;20(1):26–35. doi: 10.1097/MOH.0b013e32835ad132. [DOI] [PubMed] [Google Scholar]
- 17.Meyer V, Schneider SW, Bonsmann G, Beissert S. Experimentally confirmed induction of Sweet’s syndrome by phototestin. Acta Derm Venereol. 2011;91(6):720–1. doi: 10.2340/00015555-1139. [DOI] [PubMed] [Google Scholar]
- 18.Nesterovitch AB, Gyorfy Z, Hoffman MD, et al. Alteration in the gene encoding protein tyrosine phosphatase nonreceptor 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. Am J Pathol. 2011;178(4):1434–41. doi: 10.1016/j.ajpath.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang WW, Pluvinage JV, Price EA, et al. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci USA. 2013;110(8):3011–6. doi: 10.1073/pnas.1222861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazzola M. IDH1 and IDH2 mutations in myeloid neoplasms—novel paradigms and clinical implications. Haematologica. 2010;95(10):1623–7. doi: 10.3324/haematol.2010.030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet’s syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathol. 2014;36(3):264–9. doi: 10.1097/DAD.0b013e31828b811b. [DOI] [PubMed] [Google Scholar]
- 23.Maalouf D, Battistella M, Bouaziz JD. Neutrophilic dermatosis: disease mechanism and treatment. Curr Opin Hematol. 2015;22(1):23–9. doi: 10.1097/MOH.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 24.Peroni A, Colato C, Schena D, Rongioletti F, Girolomoni G. Histiocytoid Sweet syndrome is infiltrated predominantly by M2-like macrophages. J Am Acad Dermatol. 2015;72(1):131–9. doi: 10.1016/j.jaad.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 25.So JK, Carlos CA, Frucht CS, Cohen PR. Histiocytoid giant cellulitis-like Sweet’s syndrome: Case report and review of the literature. Dermatology Online Journal. 2015 in press. [PubMed] [Google Scholar]


