Abstract
Objective
Children differ greatly in their ability to self-regulate food intake for reasons that are poorly understood. This laboratory-based twin study tested genetic and environmental contributions to self-regulatory eating and body fat in early childhood.
Methods
Sixty-nine 4 to 7 year-old same-sex twin pairs, including 40 monozygotic (MZ) and 29 dizygotic (DZ) pairs, were studied. Self-regulatory eating was operationalized as the percentage compensation index (COMPX%), assessed by a “preload” challenge in which lunch intake was measured following a low- (3 kcal) or high-calorie (159 kcal) drink. Body fat indexes also were measured. The familial association for COMPX% was estimated by an intraclass correlation, and biometric analyses estimated heritability.
Results
Children ate more at lunch following the low- compared to high-energy preload (p< 0.001), although variability in COMPX% was considerable. Compensation was significantly poorer among African American and Hispanic compared to European American children, and among girls compared to boys. There was a familial association for self-regulatory eating (ρ= 0.23, p= 0.03) but no significant genetic component. Twenty two percent of the variance in COMPX% was due to shared environmental (‘household’) factors, with the remaining variance attributable to child-specific (‘unique’ or ‘random’) environments. Poorer self-regulatory eating was associated with greater percent body fat (r= −0.21, p= 0.04).
Conclusions
Self-regulatory eating was influenced by environmental factors, especially those differing among siblings. The absence of a significant genetic effect may reflect age of the sample or could be artifactual due to measurement issues that need to be considered in future studies.
Keywords: Heritability, Self-regulatory eating, Compensation, Pediatric Obesity, Twins, Behavior Genetics
Introduction
Why do some children spontaneously regulate their food intake in response to environmental perturbations so much better than others? Is this a skill that can be taught or acquired as a result of some optimal rearing practice? Or is it largely the result of differences in genetic predisposition 1? Indeed, children vary greatly in their capacity to self-regulate food intake for reasons that are poorly understood 2, 3. Environmental experiences are believed to disrupt ‘compensation’ 3–5, however parents transmit DNA to their children in addition to providing structure to the home environment. Genes influence many behavioral traits during development 6, including child eating patterns 7–11. Moreover, increased child weight status, which itself is highly heritable 12–15, has been linked to poorer self-regulatory eating 16, 17. The need to control for genetic factors in studies of child development is paramount in order to draw valid inferences about the role of the environment in shaping these behaviors 18.
Few investigations have examined the genetic-environmental architecture of children’s self-regulatory eating. Two recent studies reported that genes accounted for 72% of the variance in reported ‘satiety responsiveness’ in the first three months of life 19 and 63% of the variance at ages 8–11 years 9. These important investigations used parent-report methods to assess the child eating phenotype. It is unclear whether similar results would emerge if using a laboratory-based preloading paradigm in which food intake is directly measured. Thus, the first aim of the present study was to estimate genetic and environmental influences on the self-regulatory eating of 4 to 8 year-old youth, using a laboratory preloading paradigm. We used a classic twin design, which compares the phenotypic similarity of monozygotic (MZ) twin pairs, who effectively share 100% of their genes, against dizygotic (DZ) twin pairs, who share 50% of their genes on average20, 21. We hypothesized that there would be a significant genetic influence based on the two prior studies using parent-report questionnaire9, 19. The second aim of our study was to test whether children with poorer compensatory eating have increased body fat and, if so, whether these associations were due to genetic or environmental factors. We also evaluated the heritability of three body fat indices (body mass index, BMI: kg/m2; waist circumference, and percent body fat) to confirm the sensitivity of our study design to detect these established genetic effects.
Methods
Participants
Sixty-nine same-sex twin pairs, 4 – 7 years of age, and their mothers participated. Families were recruited through twins’ clubs, Twins Magazine, and mailings to parents of twins. All ethnicities and both sexes were eligible. Only same-sex twin pairs were recruited. Participating twins were in good health and had no food allergies or other medical conditions that would prohibit participation. Additional details of the sample are provided below.
Measures
Energy Compensation Ability
We measured energy compensation ability using a preloading paradigm, an established tradition in the literature 2, 17, 22, 23. Specifically, the difference in ad libitum lunch intake following a low- (3 kcal) and high- (159 kcal) energy preload was measured across two laboratory visits, with the order of presentation randomized (see below). The preloads were cherry-flavored carbohydrate drinks matched for mass (173 g) and sensory properties, served in plastic cups at room temperature. The fundamental difference between preloads was the total energy density, which was achieved by serving either standard cherry Kool-Aid® supplemented with maltodextran (high-energy preload) or Kool-Aid® with aspartame artificial sweetener (low-energy preload) 17, 24.
After drinking the preload, twins had a 25 minute play period during which staff post-weighed the plastic cups to compute preload consumption. This also was done to ensure that most or all of the preload was consumed. Mean (SD) intake of the low- and high-energy preloads were 2.97 (0.05) kcals and 147.32 (9.69) kcals, respectively, indicating that children consumed over 95% of the preloads on average in both conditions. Twins were then served a multi-item lunch that included: macaroni and cheese (133 g), canned string beans (57 g), string cheese (30 g), graham crackers (25 g), green grapes (113 g), baby carrots (35 g), and whole milk (513 g). Children could eat as much as they wished and were allowed to request additional servings. Research assistants read stories to the twins during lunch as their mothers sat in a chair that was located within several feet of the children’s table. (The influence of mothers’ presence on children’s lunch intake could not be determined, because this was the same for all children and not experimentally manipulated.) Twins sat together to simulate typical eating conditions at home, but were not allowed to share foods. During the meal, research assistants read non-food related books to the children to serve as a neutral distraction.
Compensation ability was operationalized as the percent compensation index (COMPX%)17, 24:
where Meallow = test meal intake following the low-energy preload, Mealhigh = test meal intake following the high-energy preload, Preloadhigh = high-energy preload intake, Preloadlow = low-energy preload intake. Energy intake was expressed as kcal units. COMPX% is a continuous measure with 100% reflecting “perfect” compensation. Progressively lower scores (“undercompensation”) reflect the tendency to overeat following the high-energy preload relative to the low-energy preload. Progressively higher scores (“overcompensation”) reflect the tendency to undereat following the high energy relative to the low energy preload.
Anthropometry and Body Composition
Children’s weight and height were measured by digital scale and stadiometer, respectively, and converted to body mass index (BMI). BMI values, in turn were converted to BMI z-scores and percentiles according to appropriate age- and sex-specific cut-offs 25. Children’s waist circumferences were measured in the standing position from midway between the last rib and the iliac crest. Because waist circumference is positively correlated with child age, we computed an age-adjusted (“residualized”) waist circumference score using regression analysis 26. Dual energy X-ray absorptiometry (DXA) scans were conducted to measure children’s percent (%) body fat. Height, weight, and BMI measures were collected on 132 children, waist circumference was measured on 109 children, and DXA body fat measures were obtained on 99 children, because certain parents/children declined these measures. Children with and without DXA scans did not significantly differ in self-regulatory eating (p= 0.96), BMI z-score (p= 0.65), or waist circumference (p= 0.97). Mothers were asked to self-report their weights and heights, from which BMI was calculated.
Socio-demographic measures
Mothers reported their education level (≤ high school, college, or graduate/professional school), marital status (never, married, or separated), and current employment status (employed or unemployed). Marital status was recoded as Never or Currently Unmarried (coded 0) or Currently Married (coded 1). Child sex was dummy coded for final analyses. Child ethnicity categories were: European American, African American, Hispanic, Asian, Native American, and Other/Mixed. A dummy-coded variable of European American (0) and non-European American (1) was constructed for analyses.
Zygosity
Zygosity determination was based on analysis of 10 highly polymorphic genetic markers obtained from cheek cells using bucal swabs. Genetic analyses were conducted by an independent laboratory (http://www.affiliatedgenetics.com/). Twin pairs who were identical for all markers were classified as monozygotic (MZ); all others were classified as dizygotic (DZ). Genetic analyses did not yield interpretable results for 8 twin pairs, for whom a parent-report questions determined child zygosity 27. Twin pairs whose zygosity was assessed by swabs vs. parent-report did not differ in COMPX% (p= 0.17), BMI z-score (p=0.91), waist circumference (p= 0.99), or percent body fat (p=0.67).
Procedures
Families came to the New York Obesity Research Center, St. Luke’s-Roosevelt Hospital, for 4 assessments over two weeks 28. The first two visits were primarily dedicated to the energy compensation protocol, with body composition assessed either on the third or fourth visit. Visit 1 lasted from ~11:30AM until ~1:00PM, with parents instructed not to feed their twins for at least two hours prior to the visit. Upon arrival, families were greeted by staff, reviewed the study consent form and provided consent, and acclimated to the laboratory. Next, mothers were provided a questionnaire packet to complete as twins consumed the low- or high-energy preload drink preload. Preloads were presented to twin pairs in a randomized order, with each twin pair assigned to one of three conditions: Low/Low – High/High (i.e., twins A and B both receive low-energy preloads on visit 1 and high-energy preloads on visit 2); High/High – Low/Low (i.e., twins A and B both receive high-energy preloads on visit 1 and low-energy preloads on visit 2); Low/High – High/Low (i.e., twin A receives the low-energy preload and twin B receives the high-energy preload on visit 1, with the order being reversed on visit 2). Twenty-five minutes following preload administration, the protocol lunch meal was served to the twins. Following lunch, children had additional play time and received prizes before departing the laboratory.
Procedures for visit 2 were identical except that questionnaires were not administered to parents and children received the alternate preload. Parents received financial compensation for their participation, to help offset travel and related expenses for their four visits. This study received full approval from the Institutional Review Board of St. Luke’s-Roosevelt Hospital.
Data Analytic Plan
Descriptive statistics are presented as means ± standard deviations (SD) or percentages. A paired t-test compared total energy intake at lunch (kcals) following the low and high calorie preloads, to assess for the compensation effect. The distribution of COMPX% score was examined to characterize variability in self-regulatory eating. We tested whether COMPX% scores differed by child sex and ethnicity (dummy coded). For any significant findings that were detected, mixed effects linear models were conducted to ensure that the finding was preserved when controlling for family membership. We comment on any effect that became non-significant (p> 0.05) when adjusting for family membership.
To test the familiality of self-regulatory eating, an intraclass correlation coefficient (ρ) was estimated for COMPX% across all 69 twin pairs. A significant estimate of ρ would indicate that compensation ability “runs in families,” but would not distinguish genetic from environmental effects. Thus, MZ and DZ twin pair correlations were computed for COMPX% scores and body fat indexes to determine whether these traits were more similar among identical than fraternal twins.
Biometrical genetic analyses 29 formally estimated the magnitude of genetic, shared environmental, and non-shared environmental influences on COMPX% and body fat indexes. The ‘shared environment’ refers to aspects of the environment that are identical for twins, such as foods in home cupboards and meal-time rules for all children (eg, “no dessert until vegetables are eaten”). The ‘non-shared’ environment refers to aspects of the home environment that differ among twins, including differential treatment by or interactions with parents at home as well as peers and unrelated adults out of the home. The non-shared environment also reflects measurement error.
Biometric analyses tested the goodness of fit of five competing models that fit the following parameters in different combinations: Additive Genetic influences (A), referring to multiple alleles that work additively to impact the trait; Dominant Genetic influences (D), referring to multiple alleles at the same genetic locus that work interactively to impact the trait; Shared Environment influences (C), and; Non-Shared Environment influences (E). The five competing biometrical models were: (i) A-C-E; (ii) A-D-E; (iii) A-E; (iv) C-E and (v); and E. The χ2 statistic and Akaike Information Criterion (AIC) were used to evaluate the goodness-of-fit of the competing models. A non-significant χ2 value suggests that the data do not significantly deviate from the posited model and signifies a relatively “good” fitting model. A progressively lower AIC value signifies a progressively better fitting model. The difference in fit between competing models was tested by a likelihood ratio test asymptotically distributed as χ2. We did not conduct separate analyses stratified by child sex or ethnicity, given our sample size.
With respect to statistical power, our study had sufficient power to detect an additive genetic effect of 20% or greater, when assuming an “A-E” model fit to the data 29. As noted previously, two prior behavioral genetics studies of self-regulatory eating in infancy and later childhood, using parent report questionnaire, reported heritability estimates in excess of 60% 9, 19. This lends plausibility to the notion that the heritability of COMPX% should be at least 50%, which is typical of behavioral phenotypes in humans 20, 21. On the other hand, we recognized that genetic influences on this laboratory-assessed phenotype may be smaller in magnitude and potentially below our threshold for statistical detection. We return to this point in the Discussion.
Pearson’s correlations examined the associations between COMPX% and child body fat indexes. Where significant correlations were detected, bivariate biometric analyses tested whether the association was attributable to underlying genetic factors (ie, a “genetic correlation”), shared home environmental factors (ie, a “shared environmental correlation”), or non-shared environmental factors (ie, a “non shared-environmental correlation”). All biometical analyses were conducted using the Mx software 29.
Results
Sample Characteristics
Descriptive data for twins are presented by sex and zygosity (Table 1). The mean ±standard deviation (SD) child age was 59.7±18.2 months and 54% of the sample was female. The ethnicity breakdown was 52% European American, 17% African American, 13% Hispanic, 3% Asian, and 15% other or mixed racial background. Fourteen percent of the children were overweight or obese, ie, BMI-for-age ≥ 85th %. There were no significant differences by ethnicity (ie, European American vs. non-European American children) in BMI (p= 0.62), waist circumference (p=0.85), or percent body fat (p= 0.72). There were no sex differences in BMI z-score (p=0.42) and waist circumference (p= 0.22), although girls had a greater percent body fat than boys (17.1±4.9 vs. 12.9±6.1, p<0.001). Descriptive data for participating mothers are presented in Table 2. Most mothers were college educated or higher (80.9%), married (73.9%), currently working out of the home (55.1%), and not overweight or obese (63.8%).
Table 1.
Characteristics of participating children by sex and zygosity.
| Variable | Boys | Girls | ||
|---|---|---|---|---|
|
| ||||
| MZ: | DZ: | MZ: | DZ: | |
| N (children) | 28 | 36 | 54 | 20 |
| Age (months) | 63.8 (17.4) | 57.6 (18.1) | 57.6 (18.4) | 58.10 (14.7) |
| Ethnicity (%) | ||||
| Non-Hispanic White | 38.5 | 66.7 | 51.9 | 40.0 |
| Other | 61.5 | 33.3 | 48.1 | 60.0 |
| Lunch Intake, w/Low-energy Preload (Kcals) | 458.6 (254.2) | 410.7 (318.5) | 342.4 (206.6) | 344.1 (263.9) |
| Lunch Intake, w/High-energy Preload (Kcals) | 302.4 (272.8) | 247.2 (203.1) | 248.0 (175.5) | 247.5 (186.4) |
| COMPX (%) | 107.0 (139.0) | 112.7 (126.2) | 64.9 (108.8) | 68.4 (119.3) |
| BMI: (kg/m2) | 15.6 (0.8) | 16.6 (3.04) | 15.7 (1.3) | 16.1 (2.0) |
| BMI Percentile | 53.8 (19.6) | 58.2 (27.9) | 52.2 (29.1) | 51.2 (29.6) |
| BMI z-score | 0.0 (0.8) | 0.3 (1.0) | 0.0 (1.0) | 0.2 (1.3) |
| Waist Circumference (cm) | 51.2 (4.8) | 53.9 (8.0) | 50.9 (3.9) | 52.6 (5.3) |
| Percent Body Fat (%) | 10.8 (2.3) | 14.78 (7.6) | 17.0 (4.6) | 17.6 (5.8) |
Note. For MZ boys, the sample sizes for body composition measures were: N=26 (BMI, BMI z-score, and BMI percentile), N= 22 (waist circumference), and N= 20 (total and percent body fat from DXA). For DZ boys, the sample sizes for body composition measures were: N=34 (BMI, BMI z-score, and BMI percentile), N= 32 (waist circumference), and N= 23 (total and percent body fat from DXA). For MZ girls, the sample sizes for body composition measures were: N=54 (BMI, BMI z-score, and BMI percentile), N= 41 (waist circumference), and N= 42 (total and percent body fat from DXA). For DZ girls, the sample sizes for body composition measures were: N=18 (BMI, BMI z-score, and BMI percentile), N= 14 (waist circumference), and N= 14 (total and percent body fat from DXA).
Table 2.
Characteristics of participating mothers.
| Variable | |
|---|---|
| Age (yrs) | |
| Body Mass Index (BMI: kg/m2) | 26.2(7.0) |
| Overweight or Obese (%) | 36.2 |
| Education Level (%) | |
| ≤High School | 19.1 |
| College | 58.9 |
| > Grad/Prof School | 22.0 |
| Marital Status (%) | |
| Single/Never Married | 13.0 |
| Married | 73.9 |
| Separated or Divorced | 11.5 |
| Widow | 1.4 |
| Currently Employed (%) | |
| No | 44.9 |
| Yes | 55.1 |
Note. The number of valid responses for maternal measures was 69 except for age (N= 29), BMI and overweight status (N=65), and educational level (N= 68).
Evidence for energy compensation
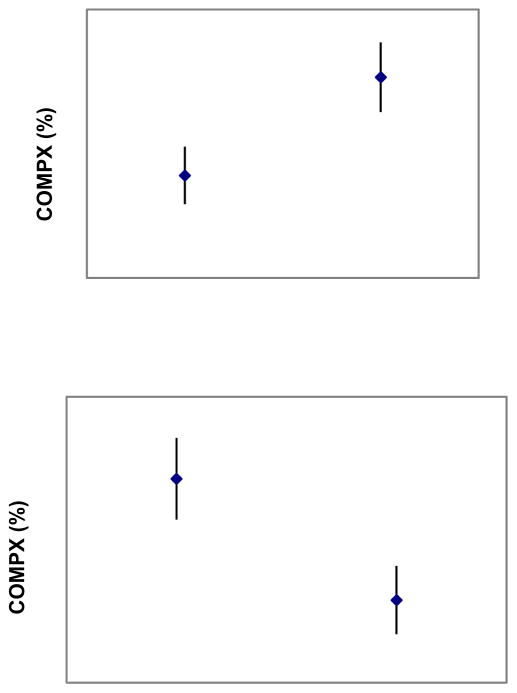
Lunch intake was greater following the low-energy compared to the high-energy preload across all 139 children (380.9 ± 257.5 kcals vs. 255.9 ± 204.1 kcals), with a mean difference of 124.9 kcals (p< 0.001). However, there was considerable variability in self-regulatory eating, with the mean percent compensation being 86.2 ± 119.2%, indicating that the average child undercompensated. Part of this variability was due to child sex and ethnicity. COMPX% scores were lower among girls than boys (65.9% vs. 109.7%, p= 0.03) (Figure 1), and also lower among non-European American than European American children (60.0% vs. 100.2%, p= 0.01) (Figure 1). The ethnicity difference remained significant (p= 0.03) in multiple regression models that controlled for maternal educational status (p= 0.53) and work status (p= 0.58), as well as paternal educational status (p= 0.43) or work status (p= 0.70). The ethnicity difference was comparable when comparing only Hispanic and African American to European American children (54.8% vs. 110.2%, p=0.02). COMPX% did not significantly differ as a function of the preload order to which twins were randomized (p= 0.11).
Figure 1.
Mean (±SD) COMPX% as a function of child sex (upper panel) and ethnicity (lower panel).
Note: For COMPX% scores, 100% represents perfect self-regulatory eating or compensatory eating. Progressively lower scores (<100%) represent undercompensation and progressively higher scores (>100%) represent overcompensation.
Familiality and heritability of child self-regulatory eating
There was a significant familial association for our primary outcome measure, COMPX% scores (ρ= 0.23, p= 0.03). The correlation for COMPX% scores was r= 0.13 and r= 0.35 among MZ and DZ twin pairs, respectively, suggesting the absence of genetic influences on the trait (Table 3). Biometric analyses indicated that the model including shared and non-shared environmental influences provided the best fit to the data (χ2= 1.19, df= 4, p= 0.88, AIC= −6.81). Specifically, 22% of the variance was estimated to be due to shared environmental factors, with the remaining variance estimated to be due to non-shared environmental factors. When using sex/ethnicity-adjusted COMPX% scores, similar results were observed. The shared and non-shared environment accounted for 17% and 83% of the variance, respectively. Heritability was estimated at 0%.
Table 3.
Twin correlations by zygosity, estimates of genetic and environmental influences on phenotypes, and model fit statistics from biometric analyses.
| Measures | r (MZ) | r (DZ) | a2 | c2 | e2 | χ2 | p | AIC |
|---|---|---|---|---|---|---|---|---|
| COMPX (%) | 0.13 | 0.35 | -- | 22% | 78% | 1.19 | 0.88 | −6.81 |
| Adjusted COMPX (%) | 0.05 | 0.31 | -- | 17% | 83% | 1.57 | 0.81 | −6.43 |
| BMI (kg/m2) | 0.72 | 0.45 | 76% | -- | 24% | 4.197 | 0.38 | −3.80 |
| Waist Circumference (cm) | 0.81 | 0.64 | 79% | -- | 21% | 9.24 | 0.06 | 1.24 |
| Adjusted Waist Circumference (cm) | 0.73 | 0. 50 | 73% | -- | 27% | 3.35 | 0.50 | −4.65 |
| Total Body Fat (%) | 0.87 | 0.35 | 91% | -- | 9% | 8.190 | 0.09 | 0.20 |
| Adjusted Body Fat (%) | 0.80 | 0.35 | 90% | -- | 10% | 14.98 | 0.01 | 7.98 |
Notes: COMPX= Compensation Index (%), refers to child self-regulatory eating capacity and measured by laboratory preloading challenge; Adjusted COMPX scores were statistically adjusted for child sex and ethnicity (dummy coded); BMI z-score= Body Mass Index z-score, calculated from the CDC growth charts; Total body fat (%) = Percentage total body fat, measured by dual energy X-ray absorptiometry; Adjusted percent body fat scores were statistically adjusted for child sex.
Heritability of body fat indexes
Correlations for body fat indexes were consistently larger among MZ than DZ twin pairs, suggesting a strong genetic influence (Table 3). The best fitting model for BMI z-score, waist circumference, and percent body fat only fit additive genetic and non-shared environmental influences. Heritability was estimated at 89%, 79%, and 91%, respectively. Results were similar when analyzing age-adjusted waist circumference (h2= 73%) and sex-adjusted percent body fat (h2=90%). Finally, as an alternative to percent body fat, we conducted biometric analyses for the ‘fat mass index’ (FMI), which is defined as total body fat (in kg) divided by the square of height (in meters)30. Heritability of FMI in the best-fitting model was estimated to be 96% (χ2= 35.09, p< 0.001, AIC=29.09).
Associations among COMPX% and body fat indexes
Lower COMPX% (undercompensation) was correlated with greater percent body fat (r=−0.24, p=0.04), an association that remained marginally significant when controlling for child sex (r= −0.19, p= 0.06). The former association was due to a non-shared environmental correlation (re= −0.27), meaning that child-specific environments promoted both poorer self-regulatory eating and increased body fat. COMPX% was not significantly associated with BMI z-scores (r= 0.01, p= 0.95) or waist circumference (r= 0.06, p= 0.52).
Discussion
Young children differ in their capacity to self-regulate food intake for reasons that have been poorly understood. This is an important trait that has been linked to weight status 16, 17 and appears to decline during childhood 23. We found in the present study that all of the variability in this trait was due to environmental factors, providing among the strongest evidence to date for household influences on children’s self-regulatory eating. Twenty-two percent of the variance was estimated to be due to the shared environment, which is known to influence children’s meal patterns 11 and food preferences 31. This accumulating evidence supports household influences in the development of children’s diet and eating patterns, even when controlling for genetic influences.
Child-specific environments explained most of the variability in COMPX% scores. Thus, parents’ greatest influence on children’s self-regulatory eating may reside in how they treat their children differently rather than similarly, which is typical of child development 6, 32. Child-specific environments can include parental feeding practices, which were shown to differ among siblings 33–35, as well as social factors out of the home 36. Peers have a powerful influence on child development 36, including eating behavior 37. Differences among siblings appear critical to the development of self-regulatory eating capacity, and should be identified in future research.
Contrary to our predictions, we did not detect a statistically significant genetic influence on self-regulatory eating. This finding may reflect the young age of our sample. Specifically, there may be age-specific genetic influences on compensatory eating that increase during childhood. The heritability of eating pathology was 0% in 11 year-old youth but increased to 54% at age 17 38. Similarly, another study reported the absence of genetic influences on disordered eating symptoms in middle childhood that emerged in latter adolescence 39. There are age-specific genetic influences on children’s BMI 13 and the same may be true of compensatory eating. Indeed, a recent study using an elegant animal model supports the importance of puberty in the development of disordered eating patterns40. Genes also may interact with children’s food environment in an age-specific manner, resulting in greater genetic expression of self-regulatory eating over time. On the other hand, a recent study reported genetic influences on infants’ satiety responsiveness 19, as measured by parent report. This calls into question whether there is truly no heritable component to self-regulatory eating in early childhood.
There are alternative interpretations of our null genetic finding, which we place into two categories: statistical power/effect size, and measurement procedures. With respect to statistical power/effect size, it is possible that there are relatively small genetic effects on COMPX% that our sample size could not detect. For example, over 500 twin pairs would have been necessary to detect a genetic effect accounting for 2% of the variance in COMPX% scores, if shared and unique environmental factors accounted for 20% and 78% of the variance respectively. Genetic influences on directly measured food intake may be much smaller compared to that for reported food intake. If so, our sample size would have been too small to detect such effects. Interestingly, the two prior studies to examine this trait using parent-report methodology found sizable heritability estimates in excess of 60% 9, 19. To the extent that that method by which this trait is assessed yields discrepant genetic effects, this has implications for the design and powering of future studies. Of note, prior studies have reported a lack of congruence among parents and children with respect to reported aberrant eating patterns by the youth41, 42.
With respect to measurement, our laboratory assessment of compensation, while well established 17, 23, 24, may have induced environmental effects that ‘overwhelmed’ genetic influences. For example, twin pairs were instructed to sit with each other while eating in order to simulate more realistic eating conditions at home and in order to make the children more comfortable on the initial laboratory visits 28. However, we cannot rule out potential “sibling interaction” effects 29 with one twin imitating the food intake of his/her sibling. Also, children were read to by research staff during the meals, which may have influenced children’s food intake and potentially induced similarity among twin pairs irrespective of zygosity. The reading also may have been a distraction that diverted attention away from internal satiety cues, which could have influenced food intake. These considerations collectively speak to the importance of the ‘context of measurement.’ Future research might address this issue by using multiple assessment methods for self-regulatory eating (e.g., observational and parent-report).
We found that 80%–90% of the variance in body fat measures was due to genetic variations. Noteworthy was the high genetic loading for percent body fat, which has been rarely studied in children 12. These findings underscore the critical role of genetic vulnerability in obesity onset. Genes that promote childhood obesity may impact on satiety mechanisms, such as the melanocortin 4 receptor gene (MC4R) 43 and the FTO gene 44, 45. Interestingly, the FTO gene has been implicated in at least one aberrant eating behavior (‘loss of control eating’) in children which has been linked to poor self-regulation46. At the same time, results from genetic studies have not replicated consistently 47. Given the high heritability of body fat, obesity-promoting genes may operate through eating traits other than self-regulatory eating at this age range 48, or may also influence energy expenditure, a variable that was not measured in the present design.
Children who undercompensated had a greater percent body fat. A reduced capacity to recognize satiety cues may lead to longer-term overconsumption of calories 17. For example, responsiveness to internal satiety cues, assessed by parent-report, was poorer among heavier compared to thinner children in a community-based UK study 49. Impaired compensation may be a novel target for obesity prevention, as few satiety training interventions have been tested with children 50. We note that COMPX% was not associated with BMI z-score or waist circumference in our study, underscoring the importance of refined body fat assessment.
Interestingly, COMPX% scores for African American and Hispanic children were ~60% that of European American children. This is noteworthy given current ethnic disparities in childhood obesity rates 51 and nutrition 52. As body fat measures did not significantly differ by ethnic group, poorer self-regulatory eating may be a precursor to the development of obesity in these vulnerable populations 51. Prospective designs need to replicate these findings in a large sample of ethnically diverse subjects. We also found that girls compensated at approximately half the level of boys, replicating a prior finding 17.
Our findings implicate the importance of the family environment for fostering self-regulatory eating practices in early childhood. Parents have a unique opportunity to teach children to recognize internal sensations of hunger 50, as preschool age children can be taught to scale feelings of fullness quantitatively using age-appropriate tools 53, 54. Parenting strategies that foster satiety awareness by children, and a less energy-dense home food environment, need critical evaluation for obesity prevention. Reduced food portion sizes 55–58, and manipulations of cup and bowl dimensions 59, 60, may be environmental strategies to prevent children’s overconsumption in response to external cues 61.
Our findings should be interpreted in light of study limitations. First, the sample size was not large enough to detect potentially small genetic effects on COMPX% scores, as measured in the laboratory. Future studies should consider using larger samples that would be sufficiently powered to detect much smaller genetic effects. Second, measures of 24-hour dietary intake and physical activity were not assessed. Third, as this was a cross-sectional study, inferences cannot be drawn about changes in self-regulatory eating and body fat over time. Fourth, the educational level of our families was relatively high and so generalizability to families with less educated parents is unclear. Fifth, although children were not supposed to eat for two hours prior to coming to the laboratory, we could not confirm adherence beyond parent report. Non-adherence potentially could have impacted on lunch intake and therefore derived COMPX% scores. We did not provide measuring scales or instruct parents in advance to keep a detailed food record for foods eaten earlier that day. Finally, we did not assess children’s habitual self-regulatory eating by parent-report questionnaire 62.
In sum, we found that children’s self-regulatory eating was influenced exclusively by environmental factors, particularly those differing among siblings. Compensatory eating was poorer among African American and Hispanic compared to European American children, who did not differ in body fat, suggesting a potential behavioral precursor to obesity in these at risk youth. Poorer compensation also was associated with greater percent body fat. Identifying child rearing practices that foster self-regulatory eating practices is critically important 50. Finally, the absence of a genetic influence on self-regulatory eating in this study could reflect our laboratory assessment methodology rather than a true null finding. As directly measured food intake has clear advantages over reported intake 63, future research should use multiple methods when possible to assess children’s self-regulatory eating to understand individual differences in this phenotype 49.
Acknowledgments
Funded by grant K08 MH-01530 (MSF)
We thank the families who enrolled in “Project Grow-2-gether” for their generous and enthusiastic participation. This project was supported by NIH grant K08-MH01530 awarded to Dr. Faith.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Johnson SL, Krebs NF. Internal versus external influences on energy intake: are disinhibited eaters born or created? J Pediatr. 2009 Nov;155(5):608–609. doi: 10.1016/j.jpeds.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 2.Birch LL, Deysher M. Conditioned and unconditioned caloric compensation: evidence for self-regulation of food intake by young children. Learning and motivation. 1985;16:341–355. [Google Scholar]
- 3.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998 Mar;101(3 Pt 2):539–549. [PubMed] [Google Scholar]
- 4.Birch LL. The role of experience in children’s food acceptance patterns. J Am Diet Assoc. 1987 Sep;87(9 Suppl):S36–40. [PubMed] [Google Scholar]
- 5.Sullivan SA, Birch LL. Pass the sugar, pass the salt: experience dictates preference. Developmental Psychology. 1990;26(4):546–551. [Google Scholar]
- 6.Rowe DC. The limits of family influence. Genes, experience, and behavior. New York: The Guilford Press; 1994. [Google Scholar]
- 7.Faith MS, Keller KL. Genetic architecture of ingestive behavior in humans. Nutrition. 2004 Jan;20(1):127–133. doi: 10.1016/j.nut.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Keller KL, Pietrobelli A, Must S, Faith MS. Genetics of eating and its relation to obesity. Curr Atheroscler Rep. 2002 May;4(3):176–182. doi: 10.1007/s11883-002-0017-3. [DOI] [PubMed] [Google Scholar]
- 9.Carnell S, Haworth CM, Plomin R, Wardle J. Genetic influence on appetite in children. Int J Obes (Lond) 2008 Oct;32(10):1468–1473. doi: 10.1038/ijo.2008.127. [DOI] [PubMed] [Google Scholar]
- 10.Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: validation of the child eating behaviour questionnaire. Appetite. 2007 Jan;48(1):104–113. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 11.Faith MS, Rhea SA, Corley RP, Hewitt JK. Genetic and shared environmental influences on children’s 24-h food and beverage intake: sex differences at age 7 y. Am J Clin Nutr. 2008 Apr;87(4):903–911. doi: 10.1093/ajcn/87.4.903. [DOI] [PubMed] [Google Scholar]
- 12.Faith MS, Pietrobelli A, Nunez C, Heo M, Heymsfield SB, Allison DB. Evidence for independent genetic influences on fat mass and body mass index in a pediatric twin sample. Pediatrics. 1999 Jul;104(1 Pt 1):61–67. doi: 10.1542/peds.104.1.61. [DOI] [PubMed] [Google Scholar]
- 13.Cardon LR. Genetic influences on body mass index in early childhood. In: Turner JR, Cardon LR, Hewitt JK, editors. Behavior genetic approaches in behavioral medicine. New York London: Plenum Press; 1995. pp. 133–143. [Google Scholar]
- 14.Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008 Feb;87(2):398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- 15.Koeppen-Schomerus G, Wardle J, Plomin R. A genetic analysis of weight and overweight in 4-year-old twin pairs. Int J Obes Relat Metab Disord. 2001 Jun;25(6):838–844. doi: 10.1038/sj.ijo.0801589. [DOI] [PubMed] [Google Scholar]
- 16.Birch LL, Fisher JO. Mothers’ child-feeding practices influence daughters’ eating and weight. Am J Clin Nutr. 2000 May;71(5):1054–1061. doi: 10.1093/ajcn/71.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson SL, Birch LL. Parents’ and children’s adiposity and eating style. Pediatrics. 1994 Nov;94(5):653–661. [PubMed] [Google Scholar]
- 18.Pinker S. The Blank Slate: The Modern Denial of Human Nature. New York: Viking; 2003. [Google Scholar]
- 19.Llewellyn CH, van Jaarsveld CH, Johnson L, Carnell S, Wardle J. Nature and nurture in infant appetite: analysis of the Gemini twin birth cohort. Am J Clin Nutr. May;91(5):1172–1179. doi: 10.3945/ajcn.2009.28868. [DOI] [PubMed] [Google Scholar]
- 20.Plomin R, Crabbe J. DNA. Psychol Bull. 2000 Nov;126(6):806–828. doi: 10.1037/0033-2909.126.6.806. [DOI] [PubMed] [Google Scholar]
- 21.Plomin R, Owen MJ, McGuffin P. The genetic basis of complex human behaviors. Science. 1994 Jun 17;264(5166):1733–1739. doi: 10.1126/science.8209254. [DOI] [PubMed] [Google Scholar]
- 22.Birch LL, Deysher M. Caloric compensation and sensory specific satiety: evidence for self regulation of food intake by young children. Appetite. 1986 Dec;7(4):323–331. doi: 10.1016/s0195-6663(86)80001-0. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SL, Taylor-Holloway LA. Non-Hispanic white and Hispanic elementary school children’s self-regulation of energy intake. Am J Clin Nutr. 2006 Jun;83(6):1276–1282. doi: 10.1093/ajcn/83.6.1276. [DOI] [PubMed] [Google Scholar]
- 24.Faith MS, Keller KL, Johnson SL, et al. Familial aggregation of energy intake in children. Am J Clin Nutr. 2004 May;79(5):844–850. doi: 10.1093/ajcn/79.5.844. [DOI] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002 May;11(246):1–190. [PubMed] [Google Scholar]
- 26.Goran MI, Allison DB, Poehlman ET. Issues relating to normalization of body fat content in men and women. Int J Obes Relat Metab Disord. 1995 Sep;19(9):638–643. [PubMed] [Google Scholar]
- 27.Rietveld MJ, van Der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin Res. 2000 Sep;3(3):134–141. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- 28.Faith MS, Keller KL, Matz P, et al. Project Grow-2-Gether: a study of the genetic and environmental influences on child eating and obesity. Twin Res. 2002 Oct;5(5):472–475. doi: 10.1375/136905202320906309. [DOI] [PubMed] [Google Scholar]
- 29.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht: Kluwer; 1992. [Google Scholar]
- 30.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990 Dec;52(6):953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 31.Breen FM, Plomin R, Wardle J. Heritability of food preferences in young children. Physiol Behav. 2006 Jul 30;88(4–5):443–447. doi: 10.1016/j.physbeh.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Dunn J, Plomin R. Why are siblings so different? The significance of differences in sibling experiences within the family. Fam Process. 1991 Sep;30(3):271–283. doi: 10.1111/j.1545-5300.1991.00271.x. [DOI] [PubMed] [Google Scholar]
- 33.Farrow CV, Galloway AT, Fraser K. Sibling eating behaviours and differential child feeding practices reported by parents. Appetite. 2009 Apr;52(2):307–312. doi: 10.1016/j.appet.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Keller KL, Pietrobelli A, Johnson SL, Faith MS. Maternal restriction of children’s eating and encouragements to eat as the ‘non-shared environment’: a pilot study using the child feeding questionnaire. Int J Obes (Lond) 2006 Nov;30(11):1670–1675. doi: 10.1038/sj.ijo.0803318. [DOI] [PubMed] [Google Scholar]
- 35.Saelens BE, Ernst MM, Epstein LH. Maternal child feeding practices and obesity: a discordant sibling analysis. Int J Eat Disord. 2000 May;27(4):459–463. doi: 10.1002/(sici)1098-108x(200005)27:4<459::aid-eat11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 36.Harris JR. The Nurture Assumption. New York: The Free Press; 1998. [Google Scholar]
- 37.Salvy SJ, Kieffer E, Epstein LH. Effects of social context on overweight and normal-weight children’s food selection. Eat Behav. 2008 Apr;9(2):190–196. doi: 10.1016/j.eatbeh.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal pubertal twins. Int J Eat Disord. 2003;33:287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- 39.Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in preadolescent and adolescent female twins. J Abnorm Psychol. 2000 May;109(2):239–251. [PubMed] [Google Scholar]
- 40.Klump KL, Suisman JL, Culbert KM, Kashy DA, Sisk CL. Binge eating proneness emerges during puberty in female rats: A longitudinal study. J Abnorm Psychol. May 16; doi: 10.1037/a0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson WG, Grieve FG, Adams CD, Sandy J. Measuring binge eating in adolescents: adolescent and parent versions of the questionnaire of eating and weight patterns. Int J Eat Disord. 1999 Nov;26(3):301–314. doi: 10.1002/(sici)1098-108x(199911)26:3<301::aid-eat8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg E, Tanofsky-Kraff M, Cohen ML, et al. Comparison of the child and parent forms of the Questionnaire on Eating and Weight Patterns in the assessment of children’s eating-disordered behaviors. Int J Eat Disord. 2004 Sep;36(2):183–194. doi: 10.1002/eat.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003 Mar 20;348(12):1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 44.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes (Lond) 2009 Jan;33(1):42–45. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- 45.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007 May 11;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanofsky-Kraff M, Han JC, Anandalingam K, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr. 2009 Dec;90(6):1483–1488. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haworth CM, Butcher LM, Docherty SJ, Wardle J, Plomin R. No evidence for association between BMI and 10 candidate genes at ages 4, 7 and 10 in a large UK sample of twins. BMC Med Genet. 2008;9:12. doi: 10.1186/1471-2350-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llewellyn CH, van Jaarsveld CH, Boniface D, Carnell S, Wardle J. Eating rate is a heritable phenotype related to weight in children. Am J Clin Nutr. 2008 Dec;88(6):1560–1566. doi: 10.3945/ajcn.2008.26175. [DOI] [PubMed] [Google Scholar]
- 49.Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr. 2008 Jul;88(1):22–29. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- 50.Johnson SL. Improving Preschoolers’ self-regulation of energy intake. Pediatrics. 2000 Dec;106(6):1429–1435. doi: 10.1542/peds.106.6.1429. [DOI] [PubMed] [Google Scholar]
- 51.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008 May 28;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 52.Kumanyika S. Nutrition and chronic disease prevention: priorities for US minority groups. Nutr Rev. 2006 Feb;64(2 Pt 2):S9–14. doi: 10.1111/j.1753-4887.2006.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 53.Keller KL, Assur SA, Torres M, et al. Potential of an analog scaling device for measuring fullness in children: development and preliminary testing. Appetite. 2006 Sep;47(2):233–243. doi: 10.1016/j.appet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Faith MS, Kermanshah M, Kissileff HR. Development and preliminary validation of a silhouette satiety scale for children. Physiol Behav. 2002 Jun 1;76(2):173–178. doi: 10.1016/s0031-9384(02)00702-3. [DOI] [PubMed] [Google Scholar]
- 55.Fisher JO, Kral TV. Super-size me: Portion size effects on young children’s eating. Physiol Behav. 2008 Apr 22;94(1):39–47. doi: 10.1016/j.physbeh.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 56.Fisher JO, Liu Y, Birch LL, Rolls BJ. Effects of portion size and energy density on young children’s intake at a meal. Am J Clin Nutr. 2007 Jul;86(1):174–179. doi: 10.1093/ajcn/86.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rolls BJ, Engell D, Birch LL. Serving portion size influences 5-year-old but not 3-year-old children’s food intakes. J Am Diet Assoc. 2000 Feb;100(2):232–234. doi: 10.1016/S0002-8223(00)00070-5. [DOI] [PubMed] [Google Scholar]
- 58.Wansink B, van Ittersum K, Painter JE. Ice cream illusions bowls, spoons, and self-served portion sizes. Am J Prev Med. 2006 Sep;31(3):240–243. doi: 10.1016/j.amepre.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Rolls BJ, Roe LS, Halverson KH, Meengs JS. Using a smaller plate did not reduce energy intake at meals. Appetite. 2007 Nov;49(3):652–660. doi: 10.1016/j.appet.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wansink B, van Ittersum K. Bottoms Up! The influence of elongation on pouring and consumption volume. Journal of Consumer Research. 2003;30:455–463. [Google Scholar]
- 61.Wansink B, Painter JE, North J. Bottomless bowls: why visual cues of portion size may influence intake. Obes Res. 2005 Jan;13(1):93–100. doi: 10.1038/oby.2005.12. [DOI] [PubMed] [Google Scholar]
- 62.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001 Oct;42(7):963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 63.Kissileff HR. Where should human eating be studied and what should be measured? Appetite. 1992 Aug;19(1):61–68. doi: 10.1016/0195-6663(92)90237-z. discussion 84-66. [DOI] [PubMed] [Google Scholar]