Abstract
Background
Pedigree development, family history, and genetic testing are thought to be useful in improving outcomes of chronic illnesses such as hypertension (HTN). However, the clinical utility of pedigree development is still unknown. Further, little is known about African Americans’ (AAs’) perceptions of family history and genetic testing.
Aims
This study examined the feasibility of developing pedigrees for AAs with HTN and explored perceptions of family history and genetic research among AAs with HTN.
Methods
The US Surgeon General’s My Family Health Portrait was administered, and 30–60 minute in-person individual interviews were conducted. Descriptive statistics were used to analyze pedigree data. Interview transcripts were analyzed with content analysis and constant comparison.
Results
Twenty-nine AAs with HTN were recruited from one free clinic (15 women, 14 men; mean age 49 years, SD 9.6). Twenty-six (90%) reported their family history in sufficient detail to develop a pedigree. Perceptions of family history included knowledge of HTN in the family, culturally influenced family teaching about HTN, and response to family history of HTN. Most participants agreed to future genetic testing and DNA collection because they wanted to help others; some said they needed more information and others expressed a concern for privacy.
Conclusion
The majority of AAs in this sample possessed extensive knowledge of HTN within their family and were able to develop a three generation pedigree with assistance. The majority were willing to participate in future genetic research.
Keywords: Family history, pedigree, genetic, African Americans, blacks
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide and hypertension (HTN) is the most prevalent form of CVD.1–3 More than one billion people have HTN and it causes over 16% of all deaths.2,4 African Americans (AAs) bear the greatest burden of CVD and HTN as compared to any other ethnic/racial group in the world.3,5 There are many reasons for this disparity including lower socioeconomic status and reduced access to healthcare shared by many AAs.6 There may also be a genetic basis for this disparity. For instance, genetics contributes to AAs’ tendency to have more salt sensitivity than other groups and some anti-HTN medications are not as efficacious in AAs.3,5 HTN is a complex, multifactorial illness, influenced by numerous genes and the environment.7,8 Therefore, genetics and environmental influences most likely contribute to HTN-related disparities for AAs.
Although genetics and environment influence development of HTN, it is difficult to determine the contribution of each. Each genetic variant (allele) only contributes a small amount to blood pressure level; therefore, numerous alleles have to be examined as well as their interaction with the environment and with each other. Therefore, family history and pedigree analysis may be an easier and less expensive way to examine the effect of these numerous alleles and their interactions on risk of illness than genetic testing. A pedigree is a graphic illustration of at least three generations displaying familial relationships and illnesses and can demonstrate risk of illness through the identification of the number of relatives affected. Since 43% of AA males and 47% of AA females have HTN,3 one would expect several members with the illness in a family pedigree. Identifying people at risk for complex illnesses like HTN through pedigree development is important as it may assist clinicians to focus on prevention and treatment strategies for those most at risk, saving valuable clinic time and resources.
Family history and pedigree development can inform decisions about education, screening, prevention, and treatment of chronic illnesses such as HTN.9 Family history, which has been used for years to determine risk of illness,10 captures important information such as cultural, genetic, behavioral, and environmental factors that tend to be shared by family members.11–14 Having one or more first or second degree relatives with a particular illness is generally considered a positive family history.15 However, as noted by the National Institutes of Health (NIH) State-of-the-Science Conference Statement on Family History and Improving Health (2009), the evidence to support the use of family history and pedigree development for complex illnesses such as HTN is weak, and additional research is needed.16 Also, the history of the AA population may make it difficult for them to accurately report family history. In some areas of the United States (US), segregation was legal until 1965, and AAs were forced to use separate health care facilities. They may not have been diagnosed appropriately or may not have known proper names for illnesses to inform their descendants. This is important because correctly identifying prior illnesses in the family is key to identifying risk.
Family history also influences perceptions of illness through a) shared family experiences; b) shared environmental factors such as cultural foods which may be high in salt and/or fat, levels of exercise, and type of housing; and c) inherited alleles.17,18 Family history obtains risk information concerning these shared environmental factors and genetics.10 One study that explored perceptions of family history found increased perceived risk and worry among those with a family history of diabetes and CVD.19 Family members discuss experiences of illness and perceptions of adherence to treatment for chronic illnesses like HTN. Family history may influence adherence decisions when individuals witness medication side effects and/or HTN complications such as stroke or end-stage renal disease, in family members who adhere to treatment.
Genetic testing may hold promise for early detection of risk for complex illnesses in addition to family history but it is expensive and as stated previously numerous alleles and their interactions would have to be examined.20–22 Also, AAs may not agree to genetic testing because they are reluctant to participate in genetic research, or in research at all23 as a result of injustices inflicted on them in the name of research i.e. the Tuskegee Syphilis Study and the Henrietta Lacks incident. Yet inclusion of this high-risk group in genetic studies of HTN is important to detect risk and improve treatment outcomes. Few studies have examined pedigree development among AAs or their perceptions of family history and genetic research. Therefore, this study examined the feasibility of developing pedigrees for AAs with HTN and explored perceptions of family history and genetic research among AAs with HTN.
Methods
Design
This study was part of a larger study that examined perceptions of adherence to HTN treatment among AAs which has been reported elsewhere.24 Data for this study focused on pedigree development and perceptions of family history and genetic research. We examined the feasibility of pedigree development in AAs with HTN and used ethnographic interviews to gain insight into perceptions of family history and genetic testing.25,26 We could not verify participants’ information regarding family history; therefore, analysis does not include pedigree accuracy. The investigation conforms with the principles of the Declaration of Helsinki.
Participants and setting
Inclusion criteria were a) self-reported as AA; b) treated with HTN medications ≥6 months; c) 21–64 years old; and d) current patient at a free, faith-based urban clinic in central Arkansas. Exclusion criteria were a) profound deafness; b) dementia; and c) severe speech impediment. Also, patients seen at the clinic in the previous year by the first author as a primary care provider were excluded. We administered the Short Blessed Test (SBT) to rule out dementia, which has been found to be reliable and valid with AAs.27–29
Recruitment
After obtaining Institutional Review Board approval, the first author used purposive sampling26 to recruit patients from the free clinic. She placed a culturally sensitive flyer in the waiting area and clinic personnel handed out the flyer to patients at check-in. The author explained the study to those interested, answered questions, obtained initial verbal consent, administered the SBT to rule out dementia, and scheduled an interview. Written consent was obtained prior to data collection.
Data collection
To collect data we used an online family history tool, the MFHP, a demographic form, and individual face-to-face interviews. The first author collected all data. She has over ten years’ experience as a family nurse practitioner including genetics and pedigree development, and has volunteered at the free, faith-based clinic for over three years diagnosing and treating HTN in AAs.
Family history tool
The MFHP is an online, publicly available family history tool developed by the Office of the US Surgeon General in collaboration with the Centers for Disease Control and Prevention and the NIH.30 It is used to collect family history information and displays that information in a standard pedigree format. In 2009, MFHP was revised to be downloadable, customizable, and compatible with electronic health records. The MFHP is written at a college level12 so it may be necessary to help patients complete the tool. Therefore, in the current study, the first author completed the tool for participants and helped them categorize the illnesses of family members. This author completed the MFHP based on the information they provided about adoption and medical history for the participant and family members, including cause and age of death. Next a pedigree was generated by the MFHP software and stored on the computer by participant number. No names or identifying information were put in the online tool and no information was stored online. The pedigrees were not shared with participants or the clinic.
Demographic form
The first author also completed a demographic form compatible with REMARK Classic OMR® software31 based on information provided verbally by each participant, including age, gender, level of education, and length of HTN treatment.
Interview guide
Initial, in-person, semi-structured interviews were conducted by the same author using an interview guide; interviews lasted approximately 30–60 minutes. Questions included, ‘How do you feel about your family history of high blood pressure (BP) or heart problems?’ ‘How would you feel about participating in a study of the genetics of high BP in the future?’ Probe questions were used to clarify information and expand on what participants divulged.26 Probe questions included: a) ‘How has watching family members with high BP affected what you do for your high BP?’ and b) ‘What concerns would you have about being in a genetic study of high BP?’ We contacted a subsample of participants by telephone to schedule a follow-up interview in order to clarify, elaborate, and verify data.26 These interviews lasted approximately 10–15 minutes.
Data analysis
The authors examined each pedigree for completeness and determined the number of participants able to complete a three-generation pedigree. A software program, IBM SPSS (v21.0; IBM Corp., Armonk, New York), assisted with descriptive analysis of the pedigree and demographic data. Interviews were analyzed using Colaizzi’s nine stage framework of qualitative data analysis.32 An experienced transcriptionist transcribed audiotaped interviews verbatim and the first author checked each for accuracy. Initial content analysis was done by a line-by-line review of each transcript.25 Initial code words and definitions were developed and used to code the first two sets of interviews. Codes and definitions were verified by members of the research team, and minor modifications were made. The code book was then used for coding all interviews. Concurrent data collection and analysis continued until data saturation was achieved, defined as repetition of the data and no new relevant data.26
After content analysis and coding, the first author used constant comparison, a technique of comparing and contrasting data to gain theoretical understanding. The data were clustered into increasingly abstract groups to develop themes and identify perceptions of family history and genetic testing among AAs. Findings were validated with participants verbally in second interviews.
Results
Participant characteristics
Twenty-nine AAs with HTN completed data collection (15 women, 14 men; mean age 49 years, SD 9.6; 72% had ≥ high school education; 35% were divorced, 28% were never married, and 28% were married). All met the criteria for poverty, as defined by the free clinic (yearly income < 200% of the US Federal poverty level).
Pedigree development
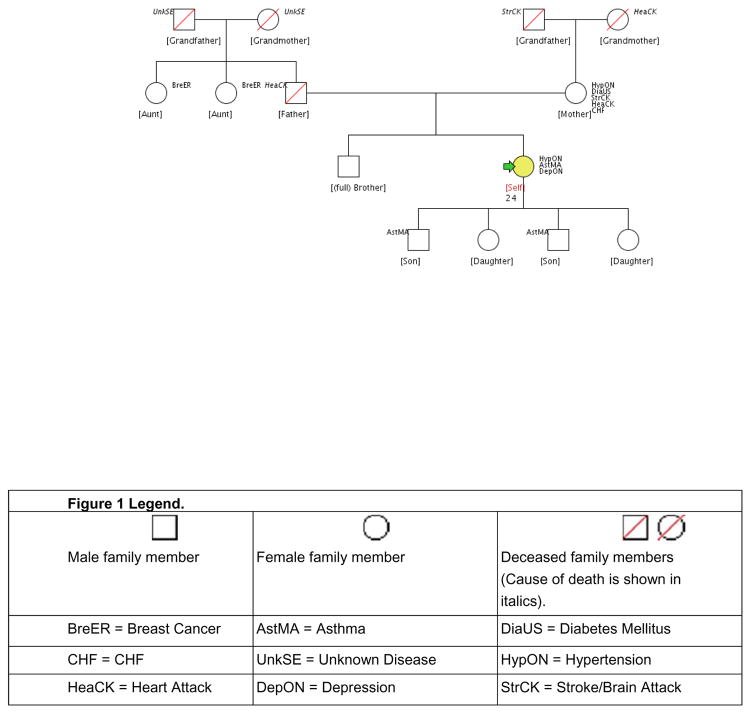
The majority of participants would not have been able to complete the MFHP tool without help. For instance, the tool contains a dropdown box where a disease/condition must be chosen. Possible selections include heart disease, high cholesterol and HTN. Many participants did not know what HTN was. However, with assistance, 26 participants (90%) were able to report their family history in sufficient detail to develop a three-generation pedigree. An example of a pedigree is in Figure 1.
Figure 1.
Example African American pedigree
When asked about their family history, the majority said family history was handed down by word of mouth. However, many did not know medical terms for diseases/conditions. In discussing his family history, one older participant said:
My grandpa, they said he was 96 when he passed but he really didn’t know when he was made and he was born in Macon, Georgia. He was not a slave but he remembers coming out of a slave. I remember going to the hospital with him as a kid and I watched him die. He was hollering for the nurses and they wouldn’t come. At that time they had what they called the ‘colored ward’ and they didn’t have black nurses back then.
This participant said he did not know the cause of death for his grandfather, even though he had been present at his death: no one explained his grandfather’s illness or cause of death to him. This lack of communication on the part of the providers partially explains the participants’ lack of understanding of disease specific terms and classifications. However, participants were effective in describing their family history and the first author was able to complete the MFHP tool for the majority of them.
Perceptions of family history
Perceptions of family history included the themes of a) knowledge of HTN in the family, b) culturally influenced family teaching about HTN, c) response to a family history of HTN, and d) lack of influence of family history of HTN on current actions.
Knowledge of hypertension in family
Factors in knowledge of HTN in the family included family members with HTN, their ‘good’ and ‘bad’ behaviors, and their problems due to HTN. When asked about their knowledge of family history of HTN, many participants immediately identified family members with it. For instance, one woman said, ‘It was on my mom’s side and my dad’s and someone was always in the hospital and if they died, it was always after that we found out it was a stroke.’ Another said, ‘I think my mom, my dad, and my sister, it just runs in the family.’ Some said that they thought it was hereditary. One young woman said, ‘My mother had high BP and my grandmother had high BP, it’s kind of hereditary.’
Good behaviors identified by participants included exercise, eating healthy, taking medicine, and relaxing. One said, ‘They do exercise to keep it in check, keep pressure down, try to keep it at a normal range.’ Many discussed the way older relatives cooked in the past as good for preventing or treating HTN. For instance, one said, ‘When Grandma cooked there wasn’t a lot of processed food like there is now. We didn’t have to watch the high BP.’ Several discussed family members who took medication for HTN such as this man: ‘My dad would always take his medicine.’ Another said, ‘…take meds, exercise, watch what they eat, try not to be so stressed out and just try to take better care of themselves.’
Many also mentioned family members who had died from HTN-related complications. One said, ‘…and then my auntie died and she had hypertension, her kidneys started failing.’ Another mentioned several family members with HTN-related complications, ‘Congestive heart failure, heart attack, and stroke. Those are some of the things that have gone on in my family.’ Still another woman discussed the severity of HTN-related complications her mother had experienced: ‘My mom had six strokes because of her high BP.’
‘Bad’ behaviors identified by participants included non-adherence to prescribed treatment and substance use. For instance, one said, ‘My auntie wouldn’t take her medicine. She would take it and times she would forget to take it and she was in her 50’s when she died.’ Some described barriers family members faced while trying to obtain medications. One said, ‘My mother had high BP and she, I guess she wasn’t taking her medicine and couldn’t afford the medicine.’ Others discussed poor dietary habits, and tobacco and alcohol use of family members as detrimental to their HTN.
Culturally influenced family teaching about hypertension
Culture influenced family teaching about HTN and included home remedies such as vinegar and garlic. One said, ‘I know my mom said something about vinegar, an old home remedy, I guess. Apple cider would get it (BP) down a little faster.’ One man said, ‘My grandfather told me about garlic and vinegar.’
Response to family history of hypertension
No participants said that watching family members with HTN discouraged them from doing what they could to prevent and treat HTN. In fact, 12 participants said their strong family HTN history motivated them to take better care of themselves. One woman said, ‘My mom, my older brother, my sister, just watching them says that I need to take my medicine, you got to take care of yourself, you could die.’ One described watching a family member die from HTN complications: ‘My mom’s sister, and we did go through it and see it and she lost a kidney, but to see it. It’s enough to see it. I take care of myself.’
The lack of influence of family history of hypertension on current actions
Family history of HTN had no influence on current actions; participants said,’ it’s going to happen anyway’ and ‘I don’t know much’. Two spoke about not having control over HTN and related complications. One said, ‘My dad, you know, had a heart attack and a stroke and had congestive heart failure but he would always take his medicine, but it still happened.’ When asked if watching family members with HTN had affected what she did to treat her HTN, another woman said, ‘No, cause if it’s gonna happen, it’s probably gonna happen anyway.’ However, these two participants never said that this interfered with their treating their HTN. In fact, both of them stated that they attempted to adhere to prescribed medications and lifestyle changes. Two participants knew little about their family history and it did not influence their current actions.
Perceptions of genetic testing
Perceptions of genetic testing included willingness to participate in genetic studies. Twenty-four participants (82.7%) said they would participate in a future genetic study of HTN and would be willing to provide DNA with a cheek swab or blood sample. Ten men were agreeable, three were not sure, and one did not answer the question. Fourteen women were agreeable and one was not sure. Participants did express some concerns, however. Men discussed needing to schedule testing around their job, and women were concerned about the test being painful. Participants were agreeable because they wanted to help. However, some said that they would be agreeable but discussed a few things that they would want addressed first, such as needing more information and assurance of privacy.
Wanting to help
Wanting to help included a desire to help others, find a cure for HTN, learn more, and find the right medicine for the right person. Several participants expressed wanting to help others. One woman said, ‘If I could stop the BP thing, I would stop it for everybody.’ One man said, ‘Anything that helps the people that are suffering with this situation, I am all for it.’ Several discussed the possibility of research finding a cure for HTN; one said, ‘If there is anything I can do to find a cure, anything to help anybody in this world, I would do it.’ Another said, ‘We need it. We do, because I don’t want to continue to take high BP medicine.’ Several discussed the fact that they and doctors might be able to learn more through a genetic study; one said, ‘That would be fine because I would like to know more about it.’ Another said, ‘Maybe it would help doctors learn more.’ Others discussed the fact that genetic research might help identify effective medications for people. For instance, one said, ‘To see what medicines would work, I think it would be good.’
Needing more information
Interestingly, only women said that pain would negate their interest in participating. One woman said, ‘Only if there’s no pain involved’ while four (two men and two women) said they would need more information before participating in a genetic study. One woman said, ‘I would have to know what it is used for,’ and a man said, ‘I would have to get more information.’ Two men said testing would have to be scheduled around their job, and two others said they were not sure if a genetic study of HTN would be helpful.
Concern for privacy
One man was especially concerned about privacy. When asked what concerns he would have about a genetic study, he said, ‘That it would only be used for that particular study and not cross-referenced for anything else.’ One woman said she would participate in a genetic study if results would be given to her after the study.
Discussion
In this study of AAs with HTN, most were able to recall their family history in sufficient detail to complete a three-generation pedigree using the MFHP tool. This is important because family history and pedigree development are valuable for clinicians. The MFHP tool is online and is available for anyone to use free of charge. However, most of these participants would not have been able to complete the MFHP tool by themselves because they did not know how to categorize some illnesses or know the correct terminology. Another study reported the MFHP tool is difficult for people to use because it is written at a 12th grade reading level.12 A recent study found that participants were not likely to complete a family history tool at home even when provided a paper form,33 so it would be more effective to have the tool completed during a clinic visit with staff assistance. Clinicians may be amenable to providing staff to help patients complete family history tools if they are educated about their use.34
When queried about perceptions of HTN family history, most participants discussed extensive knowledge of HTN within their family and described health behaviors of family members including home remedies. Clinicians can glean valuable information regarding health behaviors from patients by asking about family health information because they are often shared by families.35 Clinicians can then use family health information for pedigree development; pedigrees are an important addition to the clinic visit because they are a visual representation of illnesses, ages and causes of death of family members. Since HTN is common in the AA population, their family pedigree will most likely contain family members with it. However, those with a family history of end-organ disease such as renal disease or stroke should be targeted for tailored interventions including education regarding medication adherence and lifestyle changes. This is especially important for those most at risk such as AAs.
The visual representation of family history information in pedigree format may also assist people to understand their risk and motivate them to adhere to treatment recommendations. Participants in the current study stated that their family history of HTN motivated them to adhere to treatment recommendations, suggesting that family history and pedigree development could improve adherence to prescribed treatments for chronic illness. This is consistent with other studies of family history and adherence11,17,18,21 and is one reason that clinicians should incorporate the use of family history and pedigree development in their practices.
The example pedigree in this study shows the illustration of familial relationships and illnesses (see Figure 1). Five family members have CVD and four have end-stage organ disease. This participant should receive more frequent monitoring for possible medication changes as well as intense education regarding adherence to medications and lifestyle recommendations. This graphic representation of illness may have more of an impact than verbal information to motivate patients to prevent and treat chronic illnesses such as HTN.
Although family history may motivate patients to prevent and treat chronic illness, the impact of family health information for minorities has not been examined adequately. However, two randomized clinical trials have examined the use of an online family history tool with automatic tailored health messages for several chronic illnesses in whites.36,37 The first randomized clinical trial included 4,248 participants; 91% were white.37 Intervention group participants reported higher consumption of fruit and vegetables and increased physical activity (OR 1.29; 95% CI 1.05–1.58 and OR 1.47; 95% CI 1.08–1.89, respectively). However, screening and other health behaviors were not improved in the intervention group compared with the control group, possibly because a large number of participants in the intervention group were already at goal. The second trial (N=3,786; >90% white) examined perceived illness risk before and six months after completion of the online family history tool.36 Risk perceptions increased for some illnesses such as heart disease and stroke (p<0.05 for all) not for other illnesses such as breast cancer. These studies demonstrate the importance of family history in improving lifestyle changes and risk perception of chronic illness in whites. Further research should be done with minority populations to evaluate the impact of family history for high-risk groups including examining the effect of pedigree development on chronic illness outcomes.
Family history is important but the inclusion of minorities in genetic research is also important. Most participants in the current study said they would be willing to participate in a future genetic study of HTN, consistent with another recent study.38 The most common reason for willingness to participate was a desire to help others. Some had reservations, based primarily on the need for more information or concern for privacy. Researchers must encourage underrepresented AA minorities to participate in research since they may benefit from the findings.38,39
Limitations of this study included a convenience sample from one clinic and results are only generalizable to poor AAs. Also, pedigrees were developed with the information provided by participants and were not verified for accuracy. However, overall the participants had little difficulty relating their family history and said that the information was passed down verbally through the generations.
Conclusion
This study has demonstrated that pedigree development using the MFHP is feasible for AAs, but many individuals need assistance completing it. Future research should examine the effect of pedigree completion on chronic illness outcomes. Participants in this study said family history motivated them to adhere to HTN treatment. Effective interventions must be identified to reduce the impact of HTN in this population and pedigree development can be an important tool to aid clinicians in improving morbidity and mortality for this high-risk group.
Supplementary Material
Acknowledgments
This study was funded by the National Institute for Nursing Research, National Institutes of Health (Pre-doctoral NRSA Fellowship 1-F31-NR012347) and Gamma Xi Chapter of Sigma Theta Tau International.
References
- 1.Deaton C, Froelicher E, Sivarajan, et al. The global burden of cardiovascular disease. Eur J Cardiovasc Nurs. 2011;10:s5–s13. doi: 10.1016/S1474-5151(11)00111-3. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. [accessed 5 June 2014];Cardiovascular diseases. 2013 http://www.who.int/mediacentre/factsheets/fs317/en/
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke Statistics—2014 update: A report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2013;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Leading Health Indicators for Healthy People 2020: Letter Report. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 6.DeNavas-Walt C, Proctor BD, Smith JC. US Census Bureau, Current Population Reports P60-243, Income, Poverty, and Health Insurance Coverage in the United States: 2011. Washington, DC: US Government Printing Office; 2012. [Google Scholar]
- 7.Dungan J, Yucha CB, Artinian NT. Hypertension as a risk factor. In: Moser DK, Riegel B, editors. Cardiac Nursing: A Companion to Braunwald's Heart Disease. St. Louis: Saunsers Elsevier; 2008. pp. 431–445. [Google Scholar]
- 8.Lewis R. Human Genetics: Concepts and Applications. 9. New York: McGraw-Hill; 2010. [Google Scholar]
- 9.Crouch MA, Gramling R. Family history of coronary heart disease: Evidence-based applications. Prim Care. 2005;32:995–1010. doi: 10.1016/j.pop.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Feero WG. Examining the heart of family medicine: Family history. Am Fam Physician. 2010;81:961–962. [PubMed] [Google Scholar]
- 11.Yoon PW, Scheuner MT, Khoury MJ. Research priorities for evaluating family history in the prevention of common chronic diseases. Am J Prev Med. 2003;24:128–135. doi: 10.1016/s0749-3797(02)00585-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang CG, Gallo RE, Fleisher L, et al. Literacy assessment of family health history tools for public health prevention. Public Health Genomics. 2011;14:222–237. doi: 10.1159/000273689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohn WF, Ropka ME, Pelletier SL, et al. Health heritage(c) a web-based tool for the collection and assessment of family health history: Initial user experience and analytic validity. Public Health Genomics. 2010;13:477–491. doi: 10.1159/000294415. [DOI] [PubMed] [Google Scholar]
- 14.Facio FM, Feero WG, Linn A, et al. Validation of My Family Health Portrait for six common heritable conditions. Genetics in Medicine. 2010;12:370–375. doi: 10.1097/GIM.0b013e3181e15bd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdez R, Greenlund KJ, Khoury MJ, et al. Is family history a useful tool for detecting children at risk for diabetes and cardiovascular diseases? A public health perspective. Pediatrics. 2007;120:S78–S86. doi: 10.1542/peds.2007-1010G. [DOI] [PubMed] [Google Scholar]
- 16.Berg AO, Baird MA, Botkin JR, et al. National Institutes of Health state-of-the-science conference statement: Family history and improving health. Ann Intern Med. 2009;151:872–877. doi: 10.7326/0003-4819-151-12-200912150-00165. [DOI] [PubMed] [Google Scholar]
- 17.Omenn GS. Overview of the symposium on public health significance of genomics and eco-genetics. Annu Rev Public Health. 2010;31:1–8. doi: 10.1146/annurev.publhealth.012809.103639. [DOI] [PubMed] [Google Scholar]
- 18.Valdez R, Yoon PW, Qureshi N, et al. Family history in public health practice: A genomic tool for disease prevention and health promotion. Annu Rev Public Health. 2010;31:69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- 19.Dorman JS, Valdez R, Liu T, et al. Health beliefs among individuals at increased familial risk for type 2 diabetes: Implications for prevention. Diabetes Res Clin Pract. 2012;96:156–162. doi: 10.1016/j.diabres.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung MM, Zhang K, Zhang L, et al. Contemporary approaches to genetic influences on hypertension. Curr Opin Nephrol Hypertens. 2011;20:23–30. doi: 10.1097/MNH.0b013e3283406ecf. [DOI] [PubMed] [Google Scholar]
- 21.Paynter NP, Chasman DI, Pare G, et al. Association between a literature-based genetic risk score and cardiovascular events in women. JAMA. 2010;303:631–637. doi: 10.1001/jama.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashani M, Eliasson A, Vernalis M, et al. A systematic approach incorporating family history improves identification of cardiovascular disease risk. J Cardiovasc Nurs. doi: 10.1097/JCN.0000000000000163. Epub ahead of print 30 June 2014. [DOI] [PubMed] [Google Scholar]
- 23.Green BL, Li L, Morris JF, et al. Detailed knowledge of the Tuskegee Syphilis Study: Who knows what? A framework for health promotion strategies. Health Educ Behav. 2011;38:629–636. doi: 10.1177/1090198110391529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettey CM, McSweeney JC, Stewart KE, et al. Factors affecting hypertension treatment adherence among African Americans. J Cardiovasc Nurs. In review. [Google Scholar]
- 25.Rubin HJ, Rubin IS. Qualitative Interviewing: The Art of Hearing Data. 3. Los Angeles: Sage Publications; 2012. [Google Scholar]
- 26.Streubert HJ, Carpenter DR. Qualitative Research in Nursing: Advancing the Humanistic Imperative. 5. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 27.Ball LJ, Bisher GB, Birge SJ. A simple test of central processing speed: An extension of the short blessed test. J Am Geriatr Soc. 1999;47:1359–1363. doi: 10.1111/j.1532-5415.1999.tb07440.x. [DOI] [PubMed] [Google Scholar]
- 28.Katzman R, Brown T, Fuld P, et al. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins CH, Wilkins KL, Meisel M, et al. Dementia undiagnosed in poor older adults with functional impairment. J Am Geriatr Soc. 2007;55:1771–1776. doi: 10.1111/j.1532-5415.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 30.U. S. Surgeon General. [accessed 16 June 2009];My Family Health Portrait. 2009 https://familyhistory.hhs.gov/fhh-web/home.action2009.
- 31.REMARK Classic OMR. Paoli, PA: Gravic, Inc; 1991. [Google Scholar]
- 32.Colaizzi PF. Psychological research as the phenomenologist views it. In: Valle R, King M, editors. Existential Phenomenological Alternative for Psychology. New York: Oxford University Press; 1978. pp. 48–71. [Google Scholar]
- 33.Thompson T, Seo J, Griffith J, et al. ‘You don't have to keep everything on paper’: African American women's use of family health history tools. Journal of Community Genetics. 2013;4:251–261. doi: 10.1007/s12687-013-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owens K, Marvin M, Gelehrter T, et al. Clinical use of the Surgeon General's 'My Family Health Portrait' (MFHP) tool: Opinions of future health care providers. Journal of Genetic Counseling. 2011;20:510–525. doi: 10.1007/s10897-011-9381-x. [DOI] [PubMed] [Google Scholar]
- 35.MacLeod HM, McNally EM. A pilot study of a family history risk assessment tool for cardiovascular disease. Journal of Genetic Counseling. 2008;17:499–507. doi: 10.1007/s10897-008-9174-z. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Sen A, Ruffin MT, et al. Family history assessment: Impact on disease risk perceptions. Am J Prev Med. 2012;43:392–398. doi: 10.1016/j.amepre.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruffin MT, Nease DE, Sen A, et al. Effect of preventive messages tailored to family history on health behaviors: The Family Healthware Impact Trial. The Annals of Family Medicine. 2011;9:3–11. doi: 10.1370/afm.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang R, Kelkar VA, Byrd JR, et al. African American participation in health-related research studies: Indicators for effective recruitment. Journal of Public Health Management and Practice. 2013;19:110–118. doi: 10.1097/PHH.0b013e31825717ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rencher WC, Wolf LE. Redressing past wrongs: Changing the common rule to increase minority voices in research. Am J Public Health. 2013:e1–e5. doi: 10.2105/AJPH.2013.301356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.