Abstract
Freezing of gait appears to result from a number of fundamental problems in patients with Parkinson disease. Automaticity is impaired, putting more stress on voluntary mechanisms. Internal drivers of movement are impaired, likely because of deficient basal ganglia function. Deficiency of internal forces to initiate movement is a major factor in freezing. This deficiency gives a greater influence to external or sensory factors. The sensory factors can both help or hinder freezing. Analogous to the problem with set-shifting, there is also some difficulty in regulation of internal versus external factors and in regulation of different external factors.
Keywords: Parkinson disease, freezing of gait, automaticity, self-initiated, externally triggered, attention, dual-task
The pathophysiology of freezing of gait (FOG) has to be understood in the context of the physiology of the initiation and maintenance of movement. Motor blocks occur with hand movements, looking similar to FOG and correlating with the occurrence of FOG. (1) Much of the argument here will deal with what is known about upper extremity movement, but there is no reason to think that basic motor control principles differ for different parts of the body. Gait is a complex movement with multiple contributions from all parts of the brain. While there are always aspects that must remain under thoughtful voluntary control, much is ordinarily automatic. The alpha motoneurons responsible for movement are influenced by segmental reflexes and supraspinal control. There are several supraspinal control pathways, the most important for voluntary movement being the corticospinal tract. Other tracts such as the reticulospinal tract appears to mediate more primitive movements such as reflexes and simple automatic actions. In Parkinson disease (PD), automatic movements are impaired, and this puts pressure on the voluntary system.
The mechanisms of automaticity and the deficits in PD have been better recognized recently. Automaticity is the ability to perform movements without devoting attention to the task, that is, without thinking much about it. This can be tested formally by asking persons to do a second task at the same time as the first. The second task can be another motor task or a different type of task, such as a cognitive task. If the performance of the first task deteriorates under dual task conditions, then it is not automatic. We used functional magnetic resonance imaging (fMRI) and dual tasks to investigate the process of automaticity. (2) Normal subjects made sequential finger movements of different length and practiced until they could perform the tasks automatically. Automaticity was tested by having subjects perform a secondary letter counting task where subjects were asked to identify the number of times a target letter from letter sequences was seen. Before automaticity was reached, subjects made sequence errors in the dual task condition; with automaticity, there were essentially no errors. (No errors were made in the letter counting task at either stage.) The fMRI results before and after automaticity were compared. For both conditions sequential movements activated similar brain regions, and no additional activity was observed in the automatic condition. At the automatic stage, there was less activity in bilateral cerebellum, pre-SMA (supplementary motor area), cingulate cortex, left caudate nucleus, premotor cortex, parietal cortex, and prefrontal cortex. These findings suggest that the brain becomes more efficient as movements become more automatic.
Subsequently, the studies that we did in normal subjects were also done with PD patients. (3) All patients performed sequences correctly. However, only 12 of 15 patients could perform the shorter sequence of 4 elements automatically, and only 3 of the patients could perform the longer sequence of 12 elements automatically. Neuroimaging comparison, therefore, was done only with the shorter sequence. fMRI results showed that for both normal subjects and patients, sequential movements activated similar brain regions before and after automaticity was achieved, but in patients, only the bilateral superior parietal lobes and left insular cortex were less activated. In patients, all other regions remained at about the same level of activity; no region increased activity. At the automatic stage, despite the fact that some areas decreased their activity, patients still had greater activity in most brain regions compared with normal subjects. PD patients can achieve some automaticity with more difficulty, but only with relatively simple tasks. In general, they require more brain activity of the “voluntary type” than normal subjects when trying to perform automatic movements.
That patients must depend more on voluntary mechanisms for movements such as gait is problematic because there are clearly difficulties with voluntary movement. These difficulties include bradykinesia, fatigue and excessive dependence on external triggering of movement compared with intrinsic driving. (4) The latter point is critical for the argument here. Self-initiation of movement is particularly difficult for patients. Physiologically this appears to be due to slowness in ability to increase the excitability of the motor cortex in order to generate a motor command. This has been nicely demonstrated with transcranial magnetic stimulation (TMS) studies, where the growth of the produced motor evoked potential (MEP) is slower than expected. On the other hand, sensory stimulation is more effective in driving movement. Clinically, this is most dramatically shown with the phenomenon of paradoxical kinesia, the frozen PD patient who can run out of the building when someone yells, “fire!”
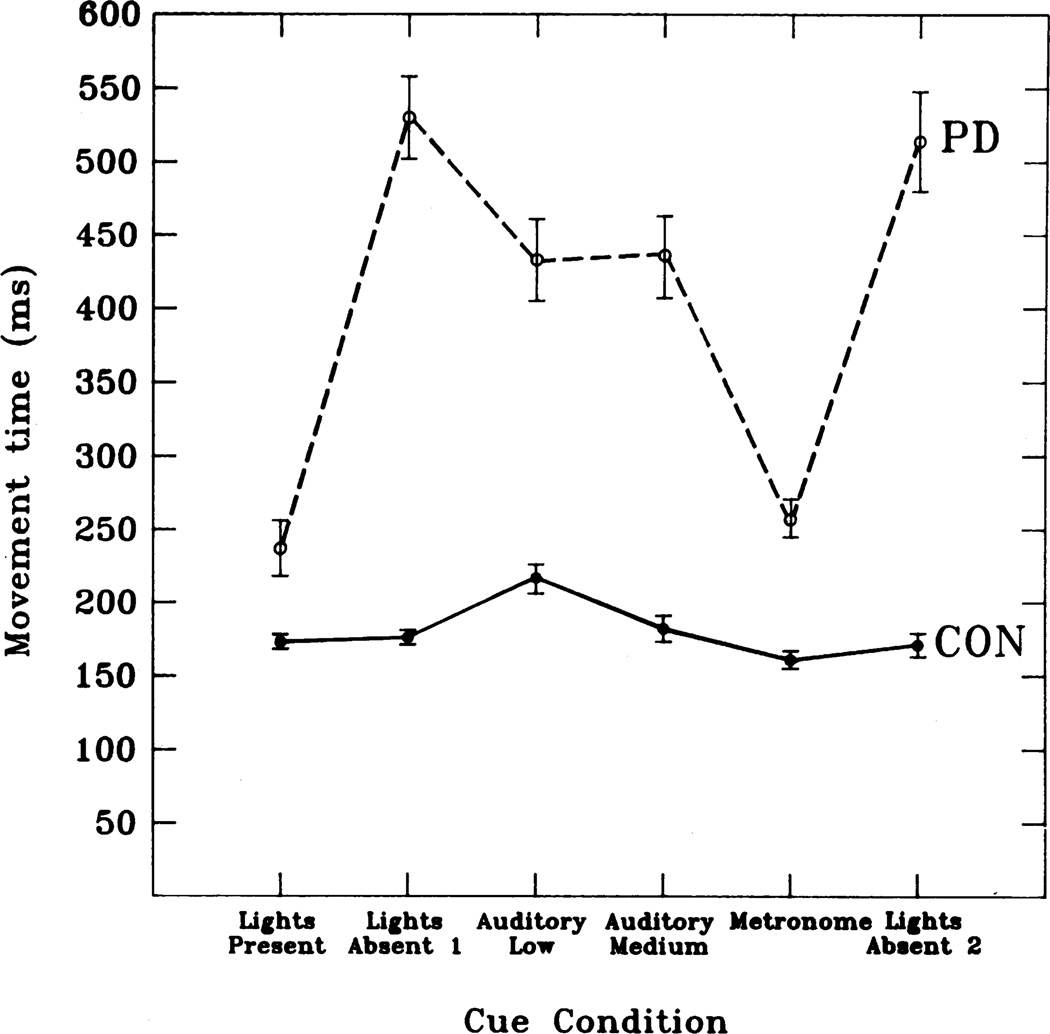
A nice formal demonstration of the deficiency of internal triggering and stronger external triggering is a study of sequential button pressing with variation in the cueing. (5) Patients with PD and matched controls pressed buttons in a series of two-way choice points sequentially down a pathway, both when the latter remained illuminated throughout its length, and when it had to be followed from memory alone. In other experimental conditions, auditory cues were given that added different levels of sensory information. Patients did badly in the absence of external cues and were aided the more sensory information that was provided (Fig. 1).
Fig. 1.
Speed in a serial task when internally driven compared with various amounts of sensory information. Subjects had to press 13 buttons, the first two and last were specified, but the middle 10 were two-choice. The choices were a learned sequence. The buttons could be lit (lights present), reminding the subjects which buttons to press, or not (light absent). Auditory tones were given when the button was released (auditory low), when the button was pressed (auditory medium) or at a fixed rate similar to the speed of button pressing (metronome). The total movement time for initiating each movement in the sequence (called down time in the original article) is plotted for each condition for both PD patients and control subjects (CON). Note faster speed with lights and metronome, where external information was maximally helpful. From Georgiou et al. (5) with permission.
There is some knowledge about the physiology of internal compared with external triggering of movement. Sensory information, providing the external triggering, comes from the posterior part of the brain, somatosensory, auditory and visual cortices. Intrinsic information, providing internal triggering comes from frontal lobe, hypothalamus and limbic systems. Basal ganglia dysfunction underlies PD, and the basal ganglia have a heavy input to frontal lobe. (6) Hence, frontal lobe functions will be more in jeopardy. There are many studies that show a deficiency in frontal function in PD related to attempts at internally generated movement.
Using blood flow positron emission tomography (PET), Deiber and colleagues investigated movement selection, which movement to make. In one study, normal subjects performed five different motor tasks consisting of moving a joystick on hearing a tone. (7) In the control task they always pushed it forwards (fixed condition), and in four other experimental tasks the subjects had to select between four possible directions of movement depending on instructions, including one task where the choice of movement direction was to be freely chosen and random. The greatest activation was seen in this latter task with significant increases in regional cerebral blood flow most prominent in the SMA. In a subsequent study, normal subjects were asked to make one of four types of finger movements depending on instructions. (8) Of the numerous comparisons, the one relevant for the issue of internal triggering is between the fully specified condition and the freely chosen, random movement. The anterior part of the SMA was the main area preferentially involved with the freely chosen movement.
Another aspect of movement selection is the choice of when to move. This was studied by Jahanshahi and colleagues using PET. (9) Normal subjects were asked to make self-initiated right index finger extensions randomly, but on average once every 3 s. The pattern of movement was recorded. A second task was externally triggered finger extension using the recorded pattern from the self-initiated task to generate the stimuli. Greater activation of the right dorsolateral prefrontal cortex (DLPFC) was the only area that significantly differentiated the self-initiated movements from the externally triggered movements. In a subsequent experiment, measurements of regional cerebral blood flow were made under three conditions: rest, self-initiated right index finger extension at a variable rate of once every 2–7 s, and finger extension triggered by pacing tones with the pattern of the self-initiated movements. Compared with rest, the sensory triggered movements activated the contralateral primary sensorimotor cortex, caudal SMA and contralateral putamen. Self-initiated movements additionally activated rostral SMA, adjacent anterior cingulate cortex and bilateral DLPFC.
A similar experiment was conducted by Deiber and colleagues using fMRI focusing on the frontal mesial cortex. (10) There were two types of movements, repetitive or sequential, performed at two different rates, slow or fast. Four regions of interest (pre-SMA, SMA, rostral cingulate motor area, CMAr, and caudal cingulate motor area, CMAc) were identified anatomically on a high-resolution MRI of each subject's brain. Descriptive analysis showed a bilateral activation in the four mesial structures for all movement conditions, but self-initiated movements were more activating than visually-triggered movements. Quantitatively, activation was more for self-initiated than for visually triggered movements in pre-SMA, CMAr and CMAc.
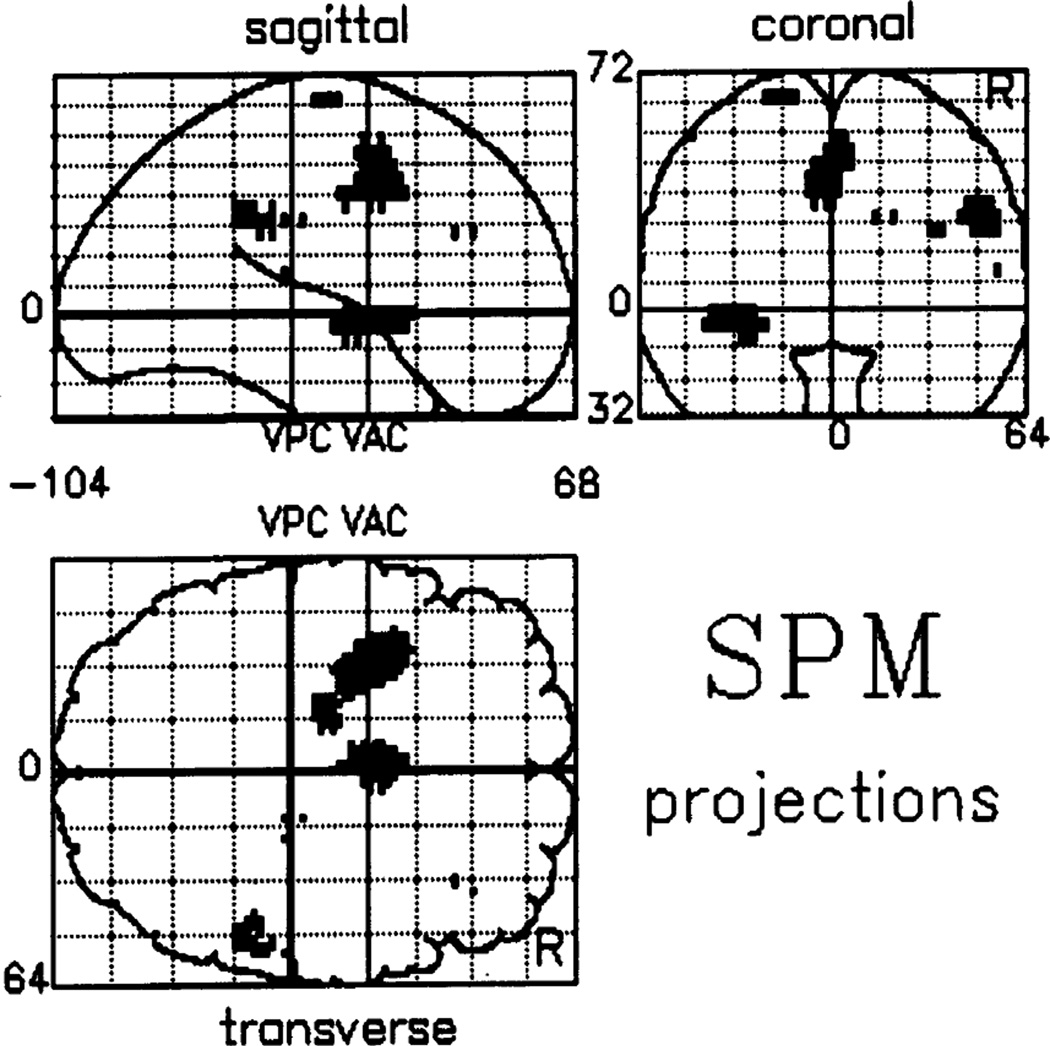
Jahanshahi and colleagues also studied PD patients in their study of self-initiated versus externally triggered movement. (9) When PD patients and normals were compared for the self-initiated movements relative to rest, normals showed greater activation of the SMA and anterior cingulate, left putamen, left insular cortex, right DLPFC and right parietal area 40 (Fig. 2). When the groups were compared for the externally triggered movements relative to rest, the pattern in the two groups did not differ. Such studies show that internally triggered movements in PD patients are abnormal due to inadequate activation of frontal structures such as SMA.
Fig. 2.
Difference in brain activation between normal subjects and PD patients for the difference between self-paced and triggered movements. PET study comparing PD patients and normal subjects making self-paced index finger extensions compared with sensory triggered index finger extensions with the same pattern of timing as with the self-paced movements. Note that normal subjects have greater activation of the SMA, anterior cingulate, left putamen, left insular cortex, right DLPFC and right parietal area 40. From Jahanshahi et al. (9) with permission.
That the control of voluntary movement is shifted more toward sensory influences is clear clinically in PD patients. Sensory stimuli can facilitate movement. Visual stimuli, such as a line on the floor, can aid movement initiation and maintenance. Patients can be instructed to step over the line. Sometimes inverting a cane can be helpful, stepping can be over the handle. Auditory stimuli are likewise effective. Patients are improved with music, particularly march music with a strong beat. Even a metronome can help.
On the other hand, sensory stimuli can interfere with movement. A door frame can cause freezing. A particularly troublesome situation is when a patient is waiting at a traffic light and freezes when the light turns green. Why sensory stimuli can both facilitate and block movement is not at all clear.
Another element that is likely important in the genesis of freezing is the difficulty in shifting control from one driver of movement to another. This difficulty in shifting is seen in studies of attentional systems. When attention is devoted to one task, it is difficult to shift to another task, and the time needed for such shifts is generally prolonged. Hence, there could be difficulty shifting between intrinsic and extrinsic drivers and even between different extrinsic drivers.
One example of difficulty in set shifting behavior is the Wisconsin Card Sorting task, where cards need to be sorted depending on different attributes. Patients have difficulty when trying to shift to a different attribute. One study showed that this deficit was associated with decreased activation in the prefrontal cortex. (11) Other results in the study suggested that both nigrostriatal dopamine depletion and intracortical dopamine depletion might play roles in this dysfunction.
Another aspect of the same problem is the difficulty in dual tasking. Patients clearly have problems doing a second task while walking. (12, 13) Both tasks deteriorate, and it becomes easier to stop walking to do the second task. This is the basis for the “stop walking when talking” test. (14) How people do two tasks simultaneously is not completely known and one possibility is that they constantly shift between the two. In any event, attentional mechanisms are taxed by dual tasking. Dual tasking has been studied with fMRI. In general there is less activation of brain than might be predicted by the sum of the activation of the two individual tasks. However, both in elderly subjects and patients with PD, dual tasking does produce more activation than the sum in the region of the precuneus (Wu and Hallett, submitted). It is of note in this regard that the precuneus is a component of the “default” network, ordinarily active at rest and deactivated during almost any task. (15)
All the information reviewed here then leads to at least a general picture of how freezing of gait might emerge. In PD, there is an increased dependence on voluntary mechanisms of movement. In the voluntary system, there is an imbalance of intrinsic and extrinsic input due to a deficit of intrinsic drivers. Freezing is primarily due to the deficit of these intrinsic factors. The extrinsic sensory information has abnormally strong influence and can both help and hinder freezing in different circumstances. Moreover, dealing with multiple drivers of movement is also a problem because of failures in attentional control. Freezing is a complex consequence of multiple deficiencies.
There must be a role for dopaminergic mechanisms in FOG. While most freezing is seen in the off-state, and can be relieved to some extent with dopaminergic therapy, sometimes freezing is seen in the on-state, called on-freezing. On-freezing is difficult to treat, but may improve with reduction in dopaminergic medication. It is not clear physiologically that on-freezing and off-freezing are similar, but with the assumption that there are, this might be understood by considering the organization of the basal ganglia. The indirect pathway is inhibited by dopamine, and the direct pathway is facilitated. Normal motor function requires a balance of activity in the direct and indirect pathways, and either too little or too much dopamine might upset the balance. Which of the etiologic factors discussed here would be most influenced by this balance is not clear.
References
- 1.Ziv I, Avraham M, Dabby R, Zoldan J, Djaldetti R, Melamed E. Early-occurrence of manual motor blocks in Parkinson's disease: a quantitative assessment. Acta Neurol Scand. 1999;99(2):106–111. doi: 10.1111/j.1600-0404.1999.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 2.Wu T, Kansaku K, Hallett M. How Self-initiated Memorized Movements Become Automatic: A fMRI Study. J Neurophysiol. 2003 doi: 10.1152/jn.01052.2003. [DOI] [PubMed] [Google Scholar]
- 3.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain. 2005;128(Pt 10):2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- 4.Hallett M. Parkinson revisited: pathophysiology of motor signs. Adv Neurol. 2003;91:19–28. [PubMed] [Google Scholar]
- 5.Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA. An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain. 1993;116:1575–1587. doi: 10.1093/brain/116.6.1575. [DOI] [PubMed] [Google Scholar]
- 6.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- 7.Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RSJ. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- 8.Deiber MP, Ibañez V, Sadato N, Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol. 1996;75:233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- 9.Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118(Pt 4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 10.Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 1999;81(6):3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- 11.Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. J Neurosci. 2004;24(3):702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22(5):1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 13.Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The "posture second" strategy: a review of wrong priorities in Parkinson's disease. J Neurol Sci. 2006;248(1–2):196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Lundin-Olsson L, Nyberg L, Gustafson Y. "Stops walking when talking" as a predictor of falls in elderly people. Lancet. 1997;349(9052):617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- 15.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]