Abstract
Following T cell encounter with antigen, multiple signals are integrated to collectively induce distinct differentiation programs within antigen-specific CD8+ T cell populations. Several factors contribute to these cell fate decisions including the amount and duration of antigen, exposure to inflammatory cytokines, and degree of ligation of cosignaling molecules. The inducible costimulator (ICOS) is not expressed on resting T cells but is rapidly upregulated upon encounter with antigen. However, the impact of ICOS signaling on programmed differentiation is not well understood. In this study we therefore sought to determine the role of ICOS signaling on CD8+ T cell programmed differentiation. Through the creation of novel ICOS retrogenic antigen-specific TCR transgenic CD8+ T cells, we interrogated the phenotype, functionality, and recall potential of CD8+ T cells that receive early and sustained ICOS signaling during antigen exposure. Our results reveal that these ICOS signals critically impacted cell fate decisions of antigen-specific CD8+ T cells, resulting in increased frequencies of KLRG-1hiCD127lo cells, altered BLIMP-1, T-bet, and eomesodermin expression, and increased cytolytic capacity as compared to empty vector controls. Interestingly, however, ICOS retrogenic CD8+ T cells also preferentially homed to non-lymphoid organs, and exhibited reduced multi-cytokine functionality and reduced ability to mount secondary recall responses upon challenge in vivo. In sum, our results suggest that an altered differentiation program is induced following early and sustained ICOS expression, resulting in the generation of more cytolyticly potent, terminally differentiated effectors that possess limited capacity for recall response.
Introduction
During the initiation of an antigen-specific CD8+ T cell response, the integration of multiple signals from the extracellular microenvironment collectively serves to induce distinct differentiation programs in antigen-specific T cells, such that the response is optimally adapted to respond appropriately to the insult. The factors that can influence this programmed differentiation include the amount and affinity of antigen, the type and duration of costimulation, and the level of inflammatory cytokines present (1–5). One aspect of programmed differentiation includes the cell fate decision to become either a short lived effector cell (SLEC), which exhibit potent cytolytic effector function during the peak of the response but are destined to undergo apoptosis during the contraction phase of the response, or a long-lived memory precursor cell (MPEC), which may have less potent effector function but will go on to form the memory CD8+ T cell population that persists following clearance of antigen (5). Phenotypically these differentiation programs can be identified on the basis of expression of KLRG-1 and CD127, which are reciprocally expressed on SLECs (KLRG-1hi CD127lo) and MPECs (KLRG-1lo CD127hi) (6, 7). Molecularly, the program is carried out by the expression of key transcription factors; in particular, high expression of T-bet and BLIMP-1 are thought to confer potent effector function but limited recall potential (8–15), while high expression of eomesodermin is thought to confer cytokine-secreting polyfunctionality as well as enhanced memory T cell recall potential (13, 14). Within a given T cell population, both SLEC and MPEC cell fates are induced following activation. The factors that dictate the induction of either a SLEC or MPEC differentiation program in a given T cell are thought to include the amount and duration of antigen exposure and the amount of inflammatory cytokine signaling, in particular IL-12 (also type 1 IFN)(5). However, the role of costimulatory signals in dictating SLEC vs. MPEC cell fate decisions during CD8+ T cell differentiation is less well understood.
It is clear, however, that the type and duration of costimulatory signaling during T cell activation critically impacts the magnitude and quality of antigen-specific CD8+ T cell responses. One of the best-studied families of T cell costimulatory molecules is the CD28 family, and the programmed differentiation that ensues as a result of CD28 signals has been elucidated (16). CD28 signals lead to sustained IL-2 production and CD25 expression, promote cell division and survival, and enhance memory T cell development (17–20). The inducible costimulator (ICOS) is a CD28 family member bearing some sequence homology to CD28 (21). In contrast to the constitutive expression of CD28 on T cells, ICOS is not expressed on resting CD4+ or CD8+ T cells but is induced upon encounter with antigen (22). It is also dynamically regulated such that during activation there is a range of ICOS expression within a given population of antigen-specific CD8+ T cells. However, the impact of ICOS signaling on programmed differentiation is not well understood. It is thought that following upregulation and encounter of its ligand B7-h1 (ICOS-L), ICOS delivers additional co-stimulatory signals to further enhance T-cell activation and differentiation into cytokine-producing effector cells (22, 23).
Models of autoimmunity revealed that ICOS signaling is critical for T cell-mediated pathogenicity in experimental autoimmune encephalomyelitis and the development of type 1 diabetes (24), and that ICOS blockade could be efficacious in treating on-going activated T cell responses and reversing autoimmunity during active disease (25, 26). Similarly, research in experimental transplant models have demonstrated that costimulation through ICOS is required for the development of both acute and chronic rejection (27, 28). In a recent study, ICOS antagonism synergized with CTLA-4-Ig to inhibit the effector function of donor-reactive memory T cells and prolong graft survival (29).
In this study we therefore sought to determine the role of increased and sustained ICOS expression on CD8+ T cell differentiation programs. Through the creation of ICOS retrogenic antigen-specific TCR tg T cells, which constitutively express high levels of ICOS, we interrogated the phenotype, functionality, and recall potential of cells that receive high and sustained ICOS signalling during antigen exposure. Results indicated that ICOS signals critically impacted cell fate decisions of antigen-specific CD8+ T cells, and imparted a differentiation program that rendered cells highly cytolytic but completely unable to sustain secondary recall responses upon heterologous rechallenge in vivo.
Materials And Methods
Mice
C57BL/6 (H-2b) mice were obtained from the National Cancer Institute (Frederick, MD). OT-I (30) and OT-II (31) transgenic mice, purchased from Taconic Farms (Germantown, NY), were bred to Thy1.1+ background at Emory University. mOVA mice (C57BL/6 background, H-2b) (32) were a gift from Dr. Marc Jenkins (University of Minnesota, Minneapolis, MN). All animals were maintained in accordance with Emory University Institutional Animal Care and Use Committee guidelines (Atlanta, GA). All animals were housed in pathogen-free animal facilities at Emory University.
Donor-Reactive T Cell Adoptive Transfers and Listeria infection
For adoptive transfers of donor-reactive T cells, spleen and mesenteric LNs isolated from Thy1.1+ OT-I mice were processed and stained with monoclonal antibodies for CD4 and CD8 (both from Invitrogen), Thy1.1, and Vα2 (BD Pharmingen) for flow cytometry analysis. Cells were resuspended in PBS and 1.0×104 of Thy1.1+ OT-I were injected i.v. 24–48 h prior to inoculation with 104 CFU of Listeria monocytogenes-OVA (33) by intraperitoneal injection. For quantitative cultures of spleen, weighed tissue specimens were ground in 200μl of sterile 0.85% saline using 15 mL sterile disposable tissue grinders (Covidien, Mansfield, MA). Serial 10-fold dilutions of the ground specimen from 10−1 to 10−3 were prepared and 100 μl aliquots were plated on blood agar plates (Remel, Lenexa, KS) and incubated at 35°C in a 5% CO2 atmosphere for 24 hours. Colony counts were obtained from plates containing fewer than 300 colonies. The number of colony forming units per gram of original sample was determined by multiplying the number of colonies by the reciprocal of the dilution counted and adjusted for the volume of sample plated. The organisms recovered were identified as Listeria using MALDI-TOF mass spectrometry (bioMerieux, Durham, NC).
ICOS Plasmid Construction and Transfection
The murine Icos gene was derived from mouse cDNA (Thermo, Cat. MMM1013-7510186) and produced by PCR using the primers: (Forward primer: gcGAATTCgccacc ATG AAG CCG TAC TTC TGC CGT GTC TTT G, Reverse primer: cgCTCGAG TTA TGA GGT CAC ACC TGC AAG). The resulting PCR fragment was cloned into the pMY-IRES-GFP retroviral vector (Cell Biolabs, RV-021) using EcoRI and XhoI cut sites. The Platinum-E retroviral packaging cell line (Cell Biolabs, RV-101) was used to produce the ICOS-containing retrovirus. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 1ug/ml puromycin, 10ug/ml blasticidine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2, humidified atmosphere. The packaging cells were incubated in 10 cm plates at 4.5 × 106/plate overnight at 37 °C. Transfections were performed using Lipofectamine LTX (Invitrogen, 15338-100). Cells were transiently transfected with 10 μg DNA (ICOS plasmid DNA or empty vector control). After 48 hours incubation the culture supernatant was harvested and virus was concentrated per manufacturer’s instructions (Cell Biolabs, RV-201).
Retroviral Transduction and Generation of ICOSrg OT-I T cells
Two days before transduction, bone marrow cells were harvested from 8–12 week old OT-I transgenic mice and cultured at 1.5×107 cells per 10 cm plate in 15 ml DMEM supplemented with 15% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, and 100 μg/mL streptomycin, 10mM hepes, 20 ng/ml murine IL-3 (IL-3), 50 ng/ml human IL-6, and 50 ng/ml murine stem cell factor (SCF) (R&D Systems). The concentrated virus was transduced into the pre-cultured BMCs. After 48 hours incubation bone marrow cells were collected and washed. Sub lethally irradiated (800 rads) WT B6 recipients were injected IV with 4 × 106 bone marrow cells in PBS. Splenocytes from these BM chimeras were harvested 6–8 weeks post-transplant and were enriched by negative selection using a CD8a+ T cell Isolation Kit II (Miltenyi Biotec). Purity of CD8a+ T cells was over 80%. Cells were then stained with anti-CD8 Pac Orange, anti-Thy1.1 PerCP, and anti-ICOS APC and CD8+ Thy1.1+ ICOS+ cells were purified by FACS sorting on a BD FACS Aria. Post-sort ICOS-OT-I T cell populations were over 95% pure.
Flow Cytometry and Intracellular Cytokine Staining
Blood, spleens, graft-draining axillary and brachial LNs, liver, and lung were isolated and stained for CD8 (both from Invitrogen) and Thy1.1 (BD Pharmingen). To isolate lymphocytes from liver, liver was perfused in situ via the portal vein, cells were made into a single cell suspension, and Percoll gradient centrifugation was used as described (34). For isolation of lymphocytes from lung, murine lungs were perfused in situ with 3 ml PBS via the heart right ventricle, placed in digestion buffer (Eagle’s MEM + 10 %FCS, 1.5 mg/ml type IA Collagenase (Sigma Aldrich, St. Louis, MO), and 0.75 mg/ml type I hyaluronidase (Sigma), chopped into fragments of 0.5–2.0 mm3, and incubated at 37°C for 60 min as described (34). For phenotypic analysis cells were also surface-stained with anti-ICOS, anti-KLRG-1, anti-CD127, anti-CD44, anti-CD62L, anti-FasL, anti-CD103, and anti-CD69 (all Pharmingen). BLIMP-1, T-bet, eomesodermin, and Granzyme B expression were measured intracellularly using an intracellular staining kit (BD Pharmingen). For 7-AAD staining, cells were resuspended in 20 ul 7-AAD per 1×106 cells (BD Pharmingen) and analyzed by flow cytometry. Absolute numbers were calculated using TruCount bead analysis according to the manufacturer’s instructions. Samples were analyzed on an LSRII flow cytometer (BD Biosciences). Data was analyzed using FlowJo software (Treestar, San Carlos, CA). For intracellular cytokine staining, splenocytes were stimulated with 10 nM OVA257-264 (SIINFEKL) (Genscript, Inc.) where indicated, in the presence of 10 μg/mL Brefeldin A for 4 hours. An intracellular staining kit was used to detect IL-2, TNF and IFN-γ (all from BD Pharmingen), according to manufacturer’s instructions.
In vivo cytolysis assay
As previously published (35), CD45.1-congenic splenocyte target cells were labeled with high (1 μM) or intermediate (100 nM) concentrations of CFSE. The CFSEint target cells were pulsed at 10 nM OVA257-264 peptide; CFSEhi target cells were incubated without peptide. 106 target cells in a 50:50 mixture of unloaded and peptide-loaded target cells were adoptively transferred i.v. into each LM-OVA infected recipient. Four hours after adoptive transfer, splenocytes were harvested and assessed for CD45.1 expression and CFSE labeling. For CD107a degranulation assay, splenocyte suspensions were incubated in R10 media at 37°C in a 96-well plate (4 × 106 cells/well) for five hours with monensin and anti-CD107a-FITC in the presence or absence of 10 nM OVA257-264 peptide as previously described (36). After incubation, surface staining with anti-Thy1.1 and anti-CD8a- was performed. Degranulation was measured as the frequency of CD8+ Thy1.1+ cells that were CD107a+.
Anti-ICOS mAb Treatment
Where indicated, animals were injected with 250ug/dose of an anti-ICOS monoclonal antibody (clone 17G9, BioXCell, West Lebanon, NJ) diluted in 500 ul of sterile PBS on days 0, 2, 4, and 6 post-infection.
Skin Transplantation
Full thickness tail and ear skins were transplanted onto dorsal thorax of recipient mice and secured with adhesive bandages as previously described (37). Where indicated, animals were treated with 100 ug of anti-CD28 domain antibody on days 0, 2, 4, and 6 (38). Mice were monitored for graft survival, and rejection was defined as <10% viable donor tissue remaining.
Statistical Analysis
Survival data were plotted on Kaplan-Meier curves and log-rank tests were performed. For analysis of T cell responses, non-parametric Mann-Whitney U-tests were performed. Results were considered significant if p<0.05. All analyses were done using GraphPad Prism software (GraphPad Software Inc.). In all legends and figures, *p<0.05, **p<0.01, ***p<0.001.
Results
Differential expansion and contraction in ICOShi vs. ICOSlo antigen-specific CD8+ T cell populations in vivo
We sought to determine the impact of ICOS expression on programmed T cell differentiation. Initial experiments demonstrated that following in vivo antigenic stimulation, Thy1.1+ CD8+ OT-I T cells specific for SIINFEKL divide and upregulate ICOS (Figure 1A). Data from CFSE analysis revealed that highly divided (CFSElo) CD8+ OVA-specific T cells exhibited increased ICOS expression (MFI) relative to less divided (CFSEhi) CD8+ OVA-specific T cells (Figure 1B). Importantly, we observed that while antigen-specific ICOShi cells significantly outnumbered antigen-specific ICOSlo cells at day 10 (the peak of the response) (Figure 1C–D), by day 14 there was no significant difference in the number of ICOShi vs. ICOSlo antigen-specific CD8+ T cells (Figure 1D). The relative enrichment for ICOSlo cells compared to ICOShi cells at day 14 (Figure 1E) suggests one of two possibilities: 1) ICOShi cells underwent a greater fold contraction from day 10 to day 14 relative to ICOSlo cells or 2) ICOS was downregulated such that ICOShi cells converted into ICOSlo cells by day 14. In order to differentiate between these two possibilities, we created TCR tg T cells that expressed ICOS via a constitutively active retroviral promoter, such that they could not modulate the expression of ICOS following activation. We hypothesized that high expression of ICOS may result in a T cell differentiation program that is more short-lived effector (SLEC) like, resulting in increased contraction within the population of antigen-specific CD8+ T cells that express the highest levels of ICOS.
Figure 1. Differential expansion and contraction in ICOShi vs. ICOSlo antigen-specific CD8+ T cell populations in vivo.

Wild-type Thy1.1+ OT-I T cells were CFSE-labeled and adoptively transferred into naïve B6 (Thy1.2) recipients which were then challenged with LM-OVA as described in materials and methods. Animals were sacrificed at the indicated time points and splenocytes were stained for flow cytometry analyzed for CFSE fluorescence and ICOS expression. A, CFSE fluorescence vs. ICOS expression from representative animals analyzed at days 5, 7, 10, and 14 post-infection. B, ICOS mean fluorescence intensity (MFI) of highly divided (CFSElo), intermediate divided (CFSE intermediate), and undivided (CFSEhi) Thy1.1+ CD8+ T cells. CFSE gates used are depicted in A. ICOS expression on non-antigen reactive CD8+ Thy1.1- T cells is shown as a negative control. C, Flow cytometry plots showing gating strategy defining ICOShi vs. ICOSlo cells within the CD8+ Thy1.1+ gate. D, Absolute number of CD8+ Thy1.1+ ICOShi vs. CD8+ Thy1.1+ ICOSlo isolated from splenocytes at the indicated time point. E, Relative contraction of ICOShi vs. ICOSlo Thy1.1+ CD8+ populations is compared by depicting the percentage of day 10 ICOShi cells remaining at day 14 (i.e. # ICOShi Thy1.1+ CD8+ cells at day 14/# ICOShi Thy1.1+ CD8+ cells at day 10 × 100). Data shown are representative of two independent experiments with a total of 5–8 mice per group. *p<0.05
Retrogenic expression of ICOS on antigen-specific CD8+ T cells results in enhanced expansion and accumulation at day 7 post-infection
In order to determine the impact of early and sustained ICOS expression during T cell activation and programmed differentiation, we utilized a retrogenic approach to constitutively express ICOS within the antigen-specific CD8+ T cell population. Briefly, CD45.2+ Thy1.1+ OT-I bone marrow was transduced with a construct that expresses ICOS under a constitutively active promoter. The construct also contained an IRES-GFP to facilitate tracking the cells. Approximately 10% of Thy1.1 OT-I BM cells expressed either GFP alone (pMY control vector-transduced cells) or both GFP and ICOS (for ICOS vector-transduced cells) on day 3 following transduction (not shown). BM cells were then adoptively transferred into irradiated CD45.2+ Thy1.2+ animals. At 8–10 weeks post-in vivo BM transfer, Thy1.1+ pMY and ICOSrg cells OT-I T cells were detectable in the blood at comparable frequencies (Figure 2A, left panels). Mature Thy1.1+ OT-I T cells from spleen and LN of pMY (GFP+ ICOS−) or ICOSrg (GFP+ ICOS+) chimeric animals were FACS sorted (Figure 2A, right panels) and adoptively transferred (104/recipient) into naïve B6 hosts. Animals were then infected with LM-OVA. Prior to adoptive transfer, we confirmed that expression of ICOS was significantly higher on ICOSrg as compared to pMY cells (Figure 2B). Expression of CD62L and CD44 on ICOSrg OT-I vs. pMY empty vector control T cells were not different (Figure 2C), demonstrating that there was no difference in the naïve vs. memory status of ICOSrg OT-I T cells prior to secondary transfer, and suggesting that the expression of ICOS during T cell development did not dramatically alter baseline activation status of these cells. Furthermore, in order to ascertain the degree of surface ICOS expressed by the retrogenic T cells as compared to those cells that upregulate ICOS as a consequence of activation, we compared the MFI of ICOS expressed on ICOSrg OT-I T cells (at day 10 post-infection) to the MFI of ICOS expressed on wild-type OT-I T cells at day 10 post-infection. As shown in Figure 2D, ICOS expression was similar between groups. These data suggest that the level of ICOS expression on ICOSrg OT-I T cells falls within the physiologic range.
Figure 2. Retrogenic expression of ICOS on antigen-specific CD8+ T cells results in enhanced expansion and accumulation.
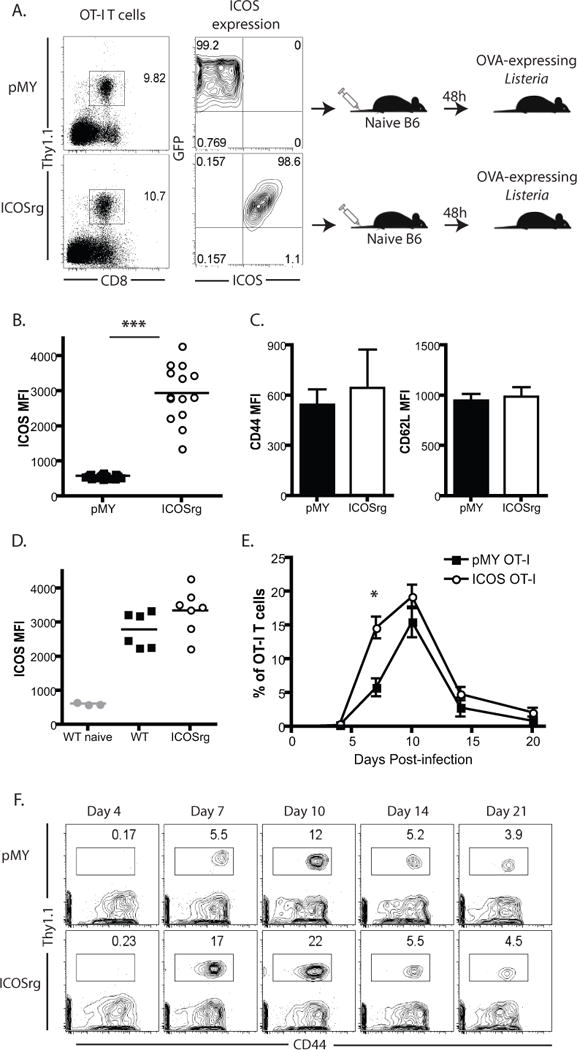
To generate donor-reactive T cells that constitutively over-express ICOS, CD45.2+ Thy1.1+ OT-I bone marrow was transduced with a construct that expresses ICOS under a constitutively active viral promoter. The construct also contained an IRES-GFP to facilitate tracking the cells. At day 2 post transduction, ~5–10% of Thy1.1+ OT-I BM cells expressed either GFP alone (pMY control vector-transduced cells) or both GFP and ICOS (for ICOS vector-transduced cells). BM cells were then adoptively transferred into irradiated CD45.1+ Thy1.2+ animals. At 8–10 weeks post-in vivo transfer, Thy1.1+ pMY and ICOSrg cells OT-I T cells were detectable in the blood at comparable frequencies (A, left panels). Thy1.1+ OT-I T cells from spleen and LN of pMY (GFP+ ICOS−) or ICOSrg (GFP+ ICOS+) chimeric animals were FACS sorted (A, right panels) and adoptively transferred (104/recipient) into naïve B6 hosts. Animals were then infected with LM-OVA. B) Assessment of ICOS expression on pMY vs. ICOSrg cells at the time of transfer. C) Assessment of CD44 and CD62L expression on pMY vs. ICOSrg cells at the time of transfer. D) Comparison of ICOS expression on ICOSrg vs WT OT-I T cells on day 10 post-infection. E-F) Mice were bled at serial time points post infection and PBL were analyzed for Thy1.1+ CD44+ pMY or ICOSrg cells. Representative animals for each time point are depicted. Summary data of frequencies of pMY vs. ICOS rg Thy1.1+ cells within the CD8+ compartment are depicted over time. Representative of 3 independent experiments with a total of 10–25 mice per group. *p<0.05, ***p<0.0001.
Animals containing pMY-OT-I or ICOSrg-OT-I were then infected with LM-OVA and peripheral blood was assessed for antigen-specific T cell expansion over time. Results indicated that ICOS rg Thy1.1+ CD8+ T cells initially expanded to a greater extent than control pMY-OT-I (Figure 2E–F), such that by day 7 there was a significantly increased population of cells in the ICOSrg mice (Figure 2E). However this increase in frequencies of ICOS rg Thy1.1+ CD8+ T cells was not sustained, such that there was no difference in the observed frequencies of ICOS rg Thy1.1+ CD8+ cells as compared to pMY-OT in these animals on days 10, 14, and 20 post-infection. In addition, there was no difference in bacterial loads measured in the spleen at day 3 post-infection (Supplemental Figure 1A).
Retrogenic ICOS overexpression on antigen-specific CD8+ T cells results in increased KLRG-1hi CD127lo phenotype and enhanced cytolytic function
In order to determine whether ICOS overexpression altered the programmed differentiation of the antigen-specific CD8+ T cell response, we assessed phenotypic markers normally associated with short-lived effector cells (SLECs) vs. memory precursor effector cells (MPECs). Results revealed that the frequencies of ICOS rg Thy1.1+ CD8+ cells expressing the SLEC-associated marker KLRG-1 were increased at the peak of the response (day 10 post-infection, Figure 3A), while the frequencies of ICOS rg Thy1.1+ CD8+ cells expressing the MPEC-associated marker CD127 were decreased at day 10 post-infection relative to pMY-transduced OT-I controls (Figure 3B). These data demonstrate the early and sustained ICOS expression contribute to antigen-specific CD8+ T cell differentiation, and increase the proportion of SLEC-like cells.
Figure 3. Retrogenic ICOS overexpression on antigen-specific CD8+ T cells results in increased KLRG-1hi CD127lo phenotype and increased presence in non-lymphoid tissues.
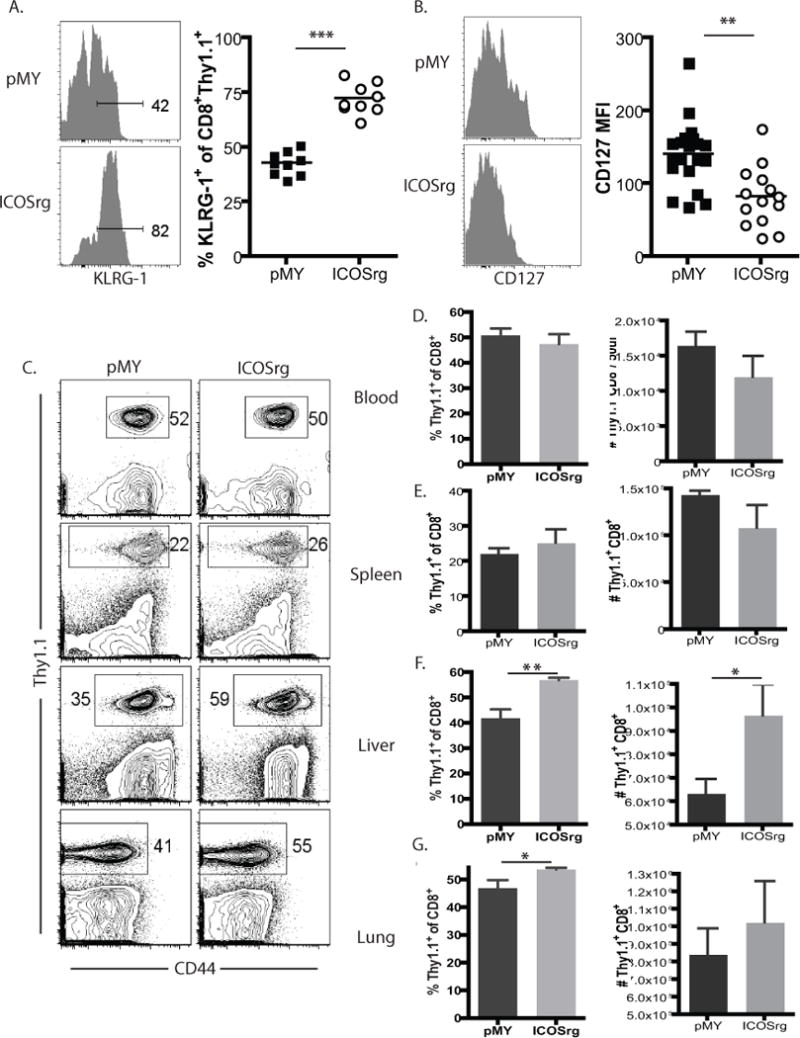
Thy1.1+ OT-I T cells from spleen and LN of pMY (GFP+ ICOS−) or ICOSrg (GFP+ ICOS+) 8–10 week chimeric animals were FACS sorted and adoptively transferred (104/recipient) into naïve B6 hosts. Animals were then infected with LM-OVA. A, B) Thy1.1+ CD8+ pMY or ICOSrg cells in peripheral blood were stained with anti-KLRG-1 (A) and anti-CD127 (B) and analyzed by flow cytometry. Summary data are shown from three independent experiments with a total of 9 mice per group. C, Animals described above were sacrificed on day 10 post-transplant and CD8+ Thy1.1+ pMY or ICOSrg OT-I T cells were assessed in the blood, spleen, liver, and lung. D, Summary data of frequencies and absolute numbers of the CD8+ Thy1.1+ pMY or ICOSrg OT-I T cells described in C (n=5 mice/group). *p<0.05, **p<0.01, ***p<0.0001
Given these observed changes in KLRG-1 and CD127 expression on ICOS rg cells following Listeria infection, we sought to characterize the tissue distribution of pMY and ICOSrg cells in this system. Results demonstrated that while the frequencies and number of Thy1.1+ cells in isolated from these two groups was identical in the spleen and blood (Figure 3C, D, E), there were significantly more ICOSrg cells in the liver (Figure 3C, 3F), and a trend toward more ICOSrg OT-I in the lung (Figure 3C, 3G). These data further corroborate the idea that ICOSrg cells are more effector memory-like.
Retrogenic ICOS expression on antigen-specific CD8+ T cells results in increased cytolytic capacity in vivo but reduced multi-cytokine producing functionality
We next sought to determine whether this increased effector-like phenotype of the ICOSrg CD8+ T cells was associated with any change in functionality. In order to accomplish this, we performed an in vivo CTL assay where in naïve B6 mice were adoptively transferred with either ICOS rg Thy1.1+ OT-I T cells or pMY Thy1.1+ OT-I controls, and were infected with LM-OVA. On day 10 post-infection, 5×106 CD45.1+ CFSEint SIINFEKL-pulsed target cells were transferred into the recipients along with 5×106 CD45.1+ CFSEhi unpulsed control targets (Figure 4A). Four hours later, mice were sacrificed and the relative proportion of peptide pulsed vs. unpulsed CD45.1+ CFSE-labeled targets was determined and the percent specific lysis was calculated as described in materials and methods. Results revealed that mice containing ICOS rg OT-I T cells exhibited significantly higher percent specific lysis than mice containing pMY OT-I controls (Figure 4B, 4C). Importantly, the frequency and absolute number of Thy1.1+ CD8+ OT-I T cells in the spleens of pMY vs. ICOSrg recipients were similar at this time point (Figure 3E), suggesting that the cells possessed increased cytolytic capacity on a per cell basis; interestingly, however in vitro measures of cytolytic function including granzyme B expression and CD107a degranulation assay failed to demonstrate any difference between pMY and ICOSrg OT-I T cells isolated from these animals (Supplemental Figure 1B–D). Taken together, these data indicate that ICOS rg CD8+ antigen-specific T cells exhibit both phenotypic and functional characteristics of more highly activated, potent effectors in vivo during the course of an immune response.
Figure 4. Retrogenic ICOS expression on antigen-specific CD8+ T cells results in increased cytolytic capacity in vivo but reduced multi-cytokine producing functionality.
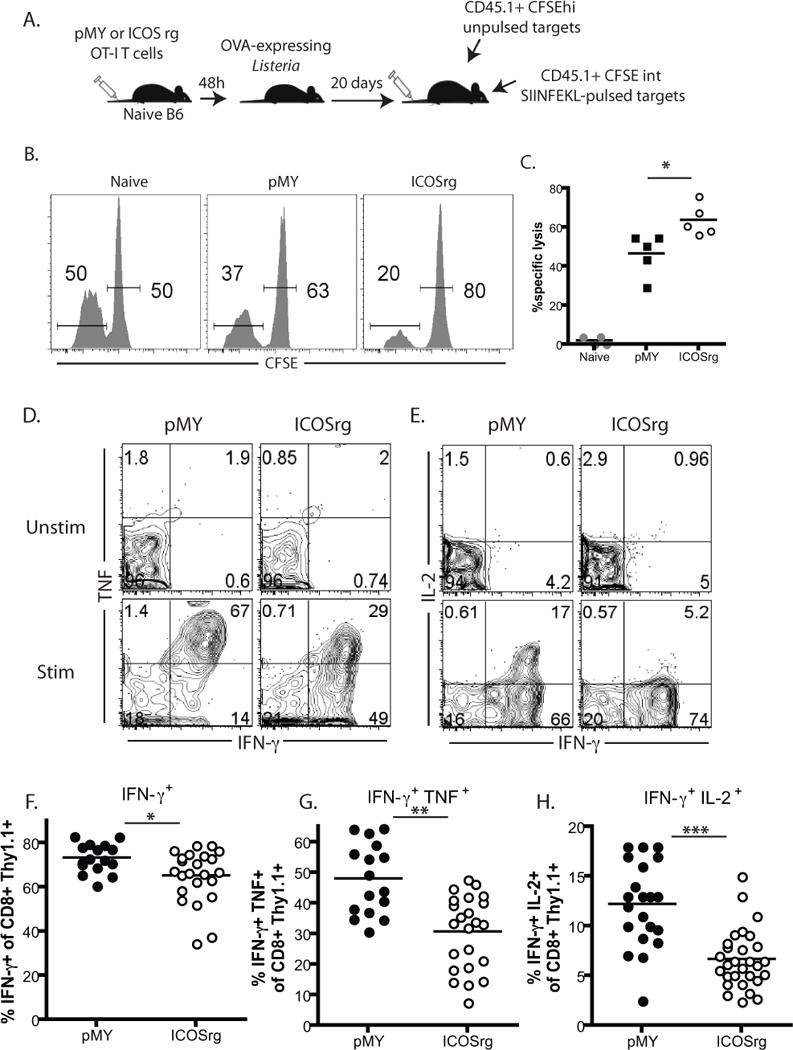
A) Naive B6 mice were adoptively transferred with either ICOS rg Thy1.1+ OT-I T cells or pMY Thy1.1+ OT-I controls, and were infected with LM-OVA. On day 10 post-infection, 5×106 CD45.1+ CFSEint SIINFEKL-pulsed target cells were transferred into the recipients along with 5×106 CD45.1+ CFSEhi unpulsed control targets. Four hours later, mice were sacrificed and the relative proportion of peptide pulsed vs. unpulsed CD45.1+ CFSE-labeled targets was determined (representative animals shown in B) and the percent specific lysis was calculated as described in materials and methods. C) Summary data of cytolysis experiments from two independent experiments with a total of 5 mice per group. D, Day 10 splenocytes of the animals described above were restimulated ex vivo with SIINFEKL antigen for 4 hours in the presence of Brefeldin A and were then stained intracellularly for the presence of cytokines. Unstimulated cells that were not exposed to peptide served as negative controls. D, E) Plots are gated on CD8+ Thy1.1+ cells and depict IFN-γ+ single producers (F), IFN-γ+ TNF+ double producers (G), and IFN-γ+ IL-2+ double producers (H). Representative animals from pMY and ICOSrg animals are shown. F) Summary data depicting frequencies of total IFN-γ+ Thy1.1+ CD8+ cells in both groups. G) Summary data depicting frequencies of IFN-γ+ TNF+ Thy1.1+ CD8+ cells in both groups. H, Summary data depicting frequencies of IFN-γ+ IL-2+ Thy1.1+ CD8+ cells in both groups. D–H, Data shown are cumulative from three independent experiments with a total of 14–29 animals/group. *p<0.05, **p<0.01, ***p<0.0001
Given the above results demonstrating enhanced in vivo cytolytic function of ICOS rg CD8+ T cells, we next interrogated their ability to produce the inflammatory cytokines TNF, IFN-γ, and IL-2. Cells isolated from the spleens of day 10 LM-OVA infected recipients of 104 ICOS rg or pMY control OT-Is were restimulated ex vivo with SIINFEKL antigen for 4 hours in the presence of Brefeldin A and were then stained intracellularly for the presence of cytokines. Interestingly, results revealed that ICOS rg OT-I T cells were compromised in their ability to make cytokines, in that the frequencies of cells making IFN-γ were reduced (Figure 4D–F). Strikingly, however, the frequencies of both IFN-γ+ TNF+ (Figure 4D, 4G) and IFN-γ+ IL-2+ (Figure 4E, 4H) multicytokine producers were even more diminished in the Thy1.1+ CD8+ ICOSrg population as compared to the pMY controls. Live/dead staining performed on these samples did not detect any difference in the frequency of apoptotic Thy1.1+ CD8+ OT-I T cells between the groups (Supplemental Figure 1E). Taken together, these data demonstrate that although ICOSrg CD8+ T cells are more potent cytolytic effectors, their ability to differentiate into high quality multi-cytokine producers is diminished.
Blockade of ICOS signaling during Listeria infection results in fewer KLRG-1hi cells and delayed contraction of the antigen-specific CD8+ T cell response
Given the above results suggesting that constitutive ICOS signaling resulted in the generation of more KLRG-1+ short-term effector-like cells, we next queried whether blockade of ICOS signals during the elicitation of an anti-Listeria T cell response would result in the opposite effect. To address this, naïve B6 animals were adoptively transferred with 104 WT OT-I T cells and infected with LM-OVA 2 days later (Figure 5A). Immediately prior to infection and on days 2, 4, and 6 thereafter, mice were injected with an anti-ICOS blocking antibody as described in materials and methods. Results indicated that on day 7 post-infection, OT-I T cell responses in animals treated with anti-ICOS trended toward decreased frequencies of KLRG-1hi cells in the peripheral blood, and a statistically significant reduction in the absolute number of KLRG-1hi Thy1.1+ CD8+ T cells (Figure 5B, C). Interestingly, however, while frequencies of Thy1.1+ OT-I T cells in anti-ICOS treated animals were not different on day 7 post-infection relative to untreated controls (Figure 5D, E), by day 10 there were significantly more Thy1.1+ OT-I T cells in the anti-ICOS-treated animals relative to untreated controls, both in terms of frequencies (Figure 5D, E) and absolute numbers (not shown). Taken together, these data suggested that blockade of ICOS signals during the primary CD8+ T cell response to Listeria-OVA resulted in diminished contraction of the response between day 7 and 10 post infection (Figure 5F). Similar results were also observed in the spleen (Figure 5G–H).
Figure 5. Anti-ICOS mAb treatment results in reduced KLRG-1hi cells and delayed contraction of antigen-specific CD8+ T cells.
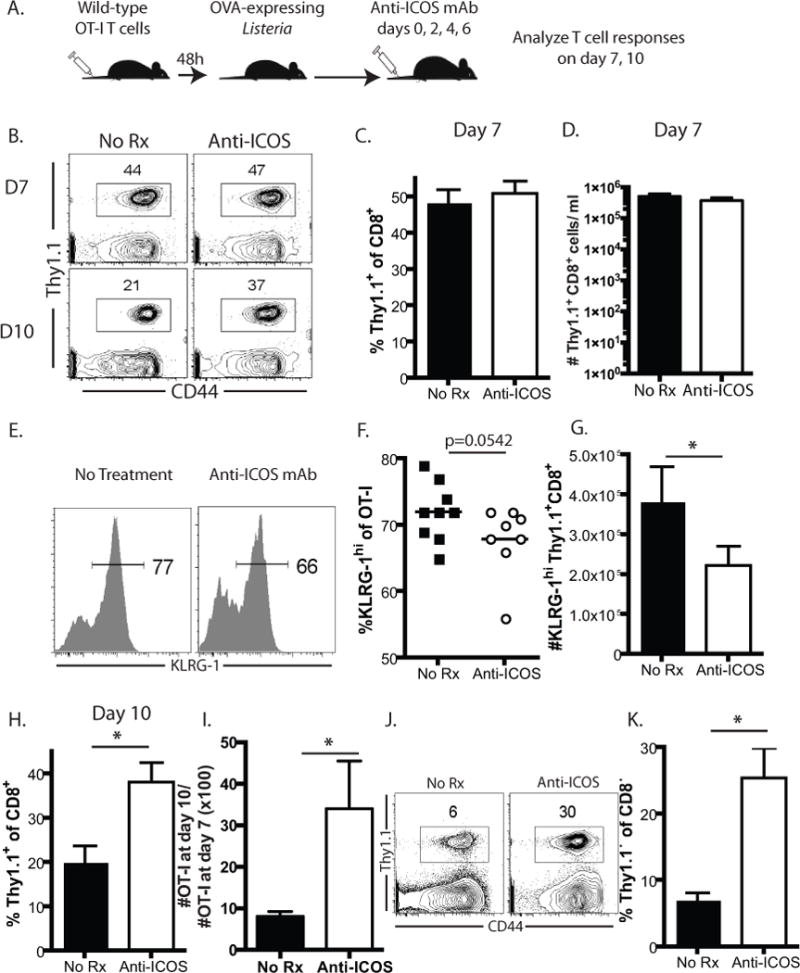
Thy1.1+ OT-I T cells from spleen and LN of WT mice were adoptively transferred (104/recipient) into naïve B6 hosts. Animals were then infected with LM-OVA. A, Where indicated, animals were treated with anti-ICOS antibody (clone 17G9, 250 ug/dose) on days 0, 2, 4, and 6 post-infection. B–C, Frequency of KLRG-1hi Thy1.1+ OT-I T cells in peripheral blood on day 7 post-infection. D-E, Representative flow cytometry plots and summary data depicting frequencies of Thy1.1+ OT-I T cells in peripheral blood of anti-ICOS treated animals and untreated controls on days 7 and 10 post-infection. F, Relative retention of Thy1.1+ CD8+ cells in anti-ICOS treated animals relative to untreated controls is shown by depicting the percentage of day 7 Thy1.1+ OT-I T cells remaining at day 10 in both groups (i.e. # Thy1.1+ CD8+ cells at day 10/# Thy1.1+ CD8+ cells at day 7 × 100). G–H, Frequencies of Thy1.1+ OT-I T cells in the spleens of anti-ICOS treated animals as compared to untreated controls at day 10 post-infection. Data shown are representative of a total of 8–9 mice per group. *p<0.05
Impact of ICOS blockade on antigen-specific CD8+ T cell cytolytic and cytokine effector function
To further corroborate our findings from the retrogenic model, we interrogated OT-I T cell acquisition of cytolytic and cytokine function in the setting of ICOS blockade. LM-OVA infected recipients of OT-I T cells were treated with anti-ICOS mAb as described above, and ten days later CFSE-labeled CD45.1+ OVA-pulsed target cells and unpulsed controls were transferred into animals as described in Materials and Methods. As shown in Figure 6A, anti-ICOS treated animals exhibited reduced cytolytic function on a per cell basis as compared to untreated controls. Interestingly, antigen-specific Thy1.1+ CD8+ T cells isolated at this time point (day 10) exhibited reduced expression of FasL, suggesting that the reduced cytolytic function observed in anti-ICOS-treated animals may be a result of decreased engagement of the Fas/FasL death pathway. Furthermore, consistent with our findings that retrogenic ICOS expression resulted in diminished multi-cytokine effectors (Figure 4D–H), OT-I T cells isolated from anti-ICOS treated animals exhibited increased frequencies of IFN-γ+ IL-2+ multi-cytokine producers on day 10 post-infection (Figure 6D, 6E).
Figure 6. ICOS blockade impacts antigen-specific CD8+ T cell cytolytic and cytokine effector function.

Thy1.1+ OT-I T cells from spleen and LN of WT mice were adoptively transferred (104/recipient) into naïve B6 hosts. Animals were then infected with LM-OVA. Where indicated, animals were treated with anti-ICOS antibody (clone 17G9, 250 ug/dose) on days 0, 2, 4, and 6 post-infection. A) On day 10 post-infection, 5×106 CD45.1+ CFSEint SIINFEKL-pulsed target cells were transferred into the recipients along with 5×106 CD45.1+ CFSEhi unpulsed control targets. Four hours later, mice were sacrificed and the relative proportion of peptide pulsed vs. unpulsed CD45.1+ CFSE-labeled targets was determined and the percent specific lysis was calculated as described in materials and methods. Summary data of cytolysis experiments from two independent experiments with a total of 5 mice per group. B–C, Day 10 splenocytes of the animals described above were stained ex vivo for FasL. B, Representative flow cytometry staining and C, Summary data depicting frequencies of FasL+ cells of Thy1.1+ CD8+ T cells in untreated vs anti-ICOS treated animals. (n=4–5 animals/group). D–E), Day 10 splenocytes of the animals described above were restimulated ex vivo with SIINFEKL antigen for 4 hours in the presence of Brefeldin A and were then stained intracellularly for the presence of cytokines. Unstimulated cells that were not exposed to peptide served as negative controls. D) Representative flow cytometry plots are gated on CD8+ Thy1.1+ cells and depict IFN-γ+ TNF+ double producers, E) Summary data depicting frequencies of IFN-γ+ IL-2+ Thy1.1+ CD8+ cells in both groups (n=5/group). F–G, Analysis of CD103 and CD69 expression on antigen-specific CD8+ Thy1.1+ T cells isolated from lungs and liver of untreated or anti-ICOS treated animals on day 10 post-infection. Data depict representative flow cytometry plots of CD8+ Thy1.1+ T cells isolated from the lung (F) and summary data of n=5 animals per group from the lung (G, left panel) or liver (G, right panel).
Our data suggested that retrogenic ICOS expression resulted in increased effector responses within tissues such as lung and liver (Figure 3C–G). To further understand the role ICOS signaling in antigen-specific CD8+ T cell responses within peripheral tissues, we interrogated the impact of ICOS blockade on the development of CD69+ CD103+ tissue-resident effector-memory T cells following Listeria infection. As depicted in Figure 6F–G (left panel), a significantly reduced frequency of CD69+ CD103+ cells was observed within the antigen-specific CD8+ Thy1.1+ T cell population in the lungs of anti-ICOS treated animals as compared to untreated controls. Similarly, while CD103+ CD8+ T cells were very rare in the liver, data revealed a significantly reduced frequency of CD69+ cells within the antigen-specific CD8+ Thy1.1+ T cell population in the livers of anti-ICOS treated animals as compared to untreated controls (Figure 6G, right panel). Taken together, these data suggest that blockade of ICOS signals may result in diminished generation of CD69+ CD103+ tissue-resident effector-memory T cells following Listeria infection.
ICOSrg CD8+ T cells exhibit impaired recall responses following challenge
Our data thus far has suggested that ICOS expression on antigen-specific CD8+ T cells confers a potent effector phenotype characterized by enhanced early expansion and cytolytic effector function but compromised ability to produce critical T cell cytokines, including IL-2. We therefore sought to determine the impact of increased ICOS expression on antigen-specific CD8+ T cell recall responses. To accomplish this, we utilized a skin allograft model in which LM-OVA-infected recipients of either ICOS rg OT-I or control pMY-OT-I were heterologously rechallenged on day 20 post-infection with skin derived from mice that constitutively express membrane-bound OVA under the β-actin promoter (mOVA mice) (Figure 7A). At day 5 post-challenge, mice were sacrificed and graft-draining lymph node cells were harvested for analysis. As observed in our earlier studies there was no difference in either the frequency or absolute number of ICOS rg CD8+ Thy1.1+ OT-I T cells as compared to pMY OT-I controls in ungrafted (no rechallenge) recipients (Figure 7B). In the recipients that received a skin graft rechallenge, mice containing control pMY-OT-I exhibited significant recall response to the graft challenge with an approximate 7-fold increase in the absolute number of graft-specific Thy1.1+ OT-I T cells. In contrast, however, animals containing ICOSrg CD8+ memory T cells failed to mount a recall response upon heterologous rechallenge, in that there was no observed increase in either the frequency or absolute number of ICOS rg OT-I T cells in the draining LN following skin transplantation (Figure 7B, 7C). To determine whether this effect was restricted to heterologous skin transplant rechallenge, we assessed recall responses to secondary rechallenge with a high dose of Listeria-OVA. Animals that had received pMY or ICOSrg OT-I T cells and been infected with LM-OVA 20 days earlier were rechallenged with a high dose (106 CFU) of LM-OVA and OT-I T cell responses were assessed 5 days later in the blood. As shown in Figure 7D, recipients of pMY OT-I T cells exhibited strong Thy1.1+ CD8+ T cell responses in the blood, whereas Thy1.1+ CD8+ T cells were barely detectable in recipients of ICOSrg OT-I T cell responses. Comparison of absolute numbers of OT-I T cells in the blood of these animals revealed a significant, two-log reduction in the magnitude of the ICOS rg vs. pMY OT-I secondary recall response (Figure 7E).
Figure 7. ICOS rg CD8+ T cells exhibit impaired recall responses following challenge.

Thy1.1+ OT-I T cells from spleen and LN of pMY (GFP+ ICOS−) or ICOSrg (GFP+ ICOS+) 8–10 week chimeric animals were FACS sorted and adoptively transferred (104/recipient) into naïve B6 hosts. Animals were then infected with LM-OVA. On day 20 post-infection, animals were heterologously rechallenged with skin derived from mice that constitutively express membrane-bound OVA under the β-actin promoter (mOVA mice) (A). B) Mice were sacrificed at day 5 post-transplant and graft-draining lymph node cells were harvested for analysis. Representative data shown are gated on CD8+ T cells. C) Summary data of the absolute numbers of CD8+ Thy1.1+ pMY vs. ICOSrg cells from two independent experiments with a total of 7–10 mice per group. D-E, Recipients of either pMY or ICOSrg OT-I T cells were infected with LM-OVA, and then re-infected with a higher dose (106 CFU) on day 20 post-infection. D, Representative flow cytometry plots showing frequencies of Thy1.1+ of CD8+ T cells in the peripheral blood at day 5 post secondary rechallenge with high-dose LM-OVA. E. Summary data of n=5 animals/group. F–H, CD8+ Thy1.1+ T cell effectors isolated on day 7 or 10 post primary Listeria-OVA infection were fixed, permeabilized, and stained intracellularly for the transcription factors BLIMP-1 (F), T-bet (G), and eomesodermin (H). Cumulative data from 2 independent experiments with a total of 10–14 animals per group are depicted. I, Mice containing memory pMY (n=6) or ICOSrg (n=7) T cells were challenged with an OVA-expressing skin graft as described above, and were treated with 100 ug of anti-CD28 domain antibody on days 0, 2, 4, and 6. Mice were monitored for graft rejection. *p<0.05, ** p<0.01.
We hypothesized that altered programmed differentiation in the presence of high ICOS expression was underlying the observed diminished recall responses. We therefore interrogated the expression of BLIMP-1, T-bet and eomesodermin, T cell transcription factors known to be expressed in memory T cells and which help determine potency of recall responses. We observed that ICOS rg CD8+ Thy1.1+ OVA-specific effectors (day 10 post-infection) expressed reduced levels of BLIMP-1 (Figure 7F), T-bet (Figure 7G) and eomesodermin (Figure 7H) as compared to pMY-OT-I controls. Taken together, these data suggest that high expression of ICOS during a primary response integrate to decrease expression of memory T cell-associated transcription factors and thus diminish recall potential. We next queried the impact of this altered differentiation and recall potential on the ability of these cells to carry out graft rejection. Naïve recipients of pMY or ICOSrg OT-I T cells were infected with Listeria-OVA. On day 30 post-infection, animals received an OVA-expressing skin graft and were treated with anti-CD28 domain antibody immunosuppression as described in materials and methods. As shown in Figure 7I, we found that recipients containing ICOSrg memory OT-I T cells experienced accelerated rejection as compared to those possessing WT pMY memory OT-I T cells (p=0.0394). These data indicate that while ICOSrg memory T cells possess poorer recall potential in the draining node, they possess a superior ability to mediate rejection of peripheral tissues.
Discussion
In this study we demonstrated that early and sustained ICOS expression is instructive for enhanced early expansion and differentiation into KLRG-1hi, CD127lo short-lived effector cells, followed by more exaggerated contraction and failure to mount recall responses upon secondary rechallenge. The data are corroborated by blocking studies in which the absence of ICOS signaling during T cell activation resulted in reduced differentiation into KLRG-1hi, short-lived effector-like cells and diminished contraction of the antigen-specific CD8+ T cell response. These studies highlight a key role for ICOS expression in programmed differentiation, and confirm and extend previous studies on the role of ICOS in the development and maintenance of T cell memory. For example, it is known that both ICOS−/− and ICOS-L−/− mice exhibit reduced frequencies of CD4+ TEM as the mice age (39). Intriguingly, this deficit was confined to the TEM compartment and was not observed within the ICOS−/− or ICOS-L−/− TCM population (39). Furthermore, antigenic stimulation of ICOS−/− cells results in less downregulation of CD62L and CCR7 as compared to wild-type cells (40). Together with these findings, our data suggest that ICOS expression is critical for effector/effector memory differentiation following infection. These conclusions are also supported by data from a published study human patients with a rare genetic disorder in ICOS signaling (41). Nine patients have been identified worldwide possessing an identical mutation in the Icos gene, and all exhibit immunologic abnormalities ranging from immune deficiency to autoimmunity.
Our data also demonstrate enhanced cytolysis but reduced multi-functional cytokine secreting cells within ICOS rg CD8+ T cell populations, and conversely reduced cytolytic function and increased multi-functional cytokine secreting cells in OT-I T cells in the setting of ICOS blockade. The observation that ICOSrg CD8+ T cells, which have higher propensity to differentiate into KLRG-1hi CD127lo SLEC-like cells and are found in higher numbers in non-lymphoid tissues such as liver and lung, also have reduced multi-cytokine producing ability is for the most part consistent with many published reports on the general functional capacities of SLECs vs. MPECs (5, 42, 43). For instance, it is known that KLRG-1hi antigen-specific CD8+ T cells have diminished capacity to produce IL-2 relative to antigen-specific KLRG-1lo CD8+ T cells (5, 44, 45). However, most studies show that KLRG-1lo and KLRG-1hi antigen-specific CD8+ T cells are equivalent in their abilities to induce cytolysis and produce IFN-γ (5, 44, 45). Thus, we conclude that while some aspects of the loss of cytokine producing functionality observed in the population of CD8+ ICOS rg cells could be entirely dependent on their increased frequency of KLRG-1hi cells, other aspects of this loss of cytokine functionality are likely independent of the SLEC/MPEC paradigm and represent a distinct aspect of ICOS-mediated T cell differentiation. This impact of ICOS on multicytokine-producing ability has also previously been described in the literature, in that populations of influenza virus-specific CD4+ T cells possessing higher IFN-γ/TNF/IL-2 multicytokine producing functionality also exhibited reduced ICOS expression (46). Taken together with these results, our study demonstrates a role for ICOS signaling in driving T cells towards a less multi-functional, more terminally differentiated effector phenotype.
Overall, our study suggests that ICOS functions on antigen-specific CD8+ T cells to increase early expansion and cytolytic function, but somewhat paradoxically results in reduced long-term memory and recall potential. These results may have clinical implications in that ICOS blocking reagents being developed for the treatment of both autoimmunity and transplantation have met with somewhat limited success (47). Indeed, ICOS blockade studies presented in this manuscript demonstrated an increased proportion of KLRG-1lo MPEC-like cells and increased multi-functional cytokine producers in the setting of ICOS blockade. Thus, light of the findings put forth in this manuscript, is interesting to speculate that perhaps the observed limited efficacy of these reagents was due in part to the generation of more multi-functional CD8+ T cell responses with enhanced recall potential in the setting of reduced ICOS signaling. Conversely, these results may also have implications for protective immunity and vaccine design, in that blockade of ICOS signals during the induction of antigen-specific CD8+ T cell responses may result in more long-lived, multi-functional cellular immunity. One important caveat to note, however, is that these findings pertain to antigen-specific CD8+ T cells, and the critical role of ICOS on CD4+ TFH for the provision of help during the generation of antibody responses is likely indispensible (48).
Our data revealed an increase in the frequency of antigen-specific CD8+ T cells in ICOS rg cells as compared to pMY controls at day 7 post- Listeria infection; however, this initially augmented response failed to persist by day 10. Conversely, blockade of ICOS signals resulted in increased persistence of OT-I T cells at days 10. Together, these data suggest that strong or preserved ICOS signals may promote early T cell contraction while the absence of ICOS signaling favors T cell survival. Previous studies examining the impact of ICOS deficiency on the expansion and accumulation of CD8+ effector T cells have shown dichotomous results, with some studies demonstrating decreased CD8+ T cell proliferation in ICOS−/− animals (49) and other studies concluding that accumulation of activated CD8+ T cells is not altered in ICOS−/− animals (50). Interestingly, we also assessed the expansion of ICOS retrogenic CD8+ T cells in response to a skin transplant as opposed to pathogen infection, and found that under these conditions ICOS rg OT-I T cells did not exhibit enhanced accumulation, and did not exhibit an altered KLRG-1/CD127 profile (data not shown). Taken together, these results suggest that the role of ICOS in CD8+ T cell expansion and differentiation may be dependent on the precise stimulation conditions present. It is interesting to speculate that one factor impacting whether ICOS functions to significantly enhance CD8+ T cell accumulation during an immune response may be expression patterns of ICOS-L. Unlike CD80/CD86, which are primarily restricted to APC, ICOS-L can be expressed on parenchymal cells (51, 52) and is upregulated in the presence of inflammatory cytokines such as TNF (52). Thus, different types of inflammatory conditions such as those present during transplantation vs. pathogen infection might result in differential availability of ICOS-L; this possibility warrants further investigation.
One of the most striking aspects of retrogenic ICOS expression in our study was a reduced ability of ICOSrg memory T cells to mount recall responses when subjected to rechallenge, via either a second LM-OVA infection or heterologous challenge with an OVA-expressing skin graft. This finding is consistent with the decreased proportion of KLRG-1lo CD127hi memory precursor cells in these animals, however it is important to note that we did not observe a difference in the number of antigen-specific pMY vs. ICOSrg CD8+ T cells present at the time of rechallenge. Thus, while ICOS overexpression did not result in a quantitative difference in the generation of memory, it did result in the altered expression of transcription factors eomesodermin and BLIMP-1, both transcription factors known to be important for memory T cell differentiation (8–14). Reduced expression of eomesodermin in these cells is consistent with several published reports that demonstrated the critical role of this molecule in the generation and maintenance of CD8+ memory T cells. Interestingly, previous reports on BLIMP-1 and T-bet in CD8+ T cells have demonstrated the increased expression of these molecules in KLRG-1hi CD127lo short-lived effector cells (10, 12), while our data showed a decrease in BLIMP-1 and T-bet on ICOS rg cells relative to pMY controls. This possible discrepancy may be reconciled by a recent report that demonstrated a critical role for BLIMP-1 during recall responses in influenza virus-infected animals (9), but is also possible that intense ICOS signaling induces the differentiation of cells that are distinct from classical SLECs. We conclude that a distinct profile of transcription factor expression is induced following early and sustained ICOS expression, which results in the generation of more cytolyticly potent, terminally differentiated effectors that possess limited capacity for recall response.
Supplementary Material
Acknowledgments
The authors would like to acknowledge members of the Ford Lab for helpful discussion, and Dr. Aaron Rae and the Emory+Childrens’ Pediatric Flow Cytometry Core for assistance with cell sorting.
Footnotes
This work was supported by NIH grants R01 AI104699 and AI073707 to MLF and GM104323 to MLF and CMC.
References
- 1.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104:15045–50. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Floyd TL, Koehn BH, Kitchens WH, Robertson JM, Cheeseman JA, Stempora L, Larsen CP, Ford ML. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. J Immunol. 2011;186:2033–41. doi: 10.4049/jimmunol.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wherry EJ, McElhaugh MJ, Eisenlohr LC. Generation of CD8(+) T cell memory in response to low, high, and excessive levels of epitope. J Immunol. 2002;168:4455–61. doi: 10.4049/jimmunol.168.9.4455. [DOI] [PubMed] [Google Scholar]
- 5.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 7.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–71. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, Nutt SL. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–74. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 9.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–95. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–20. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin HM, Kapoor VN, Guan T, Kaech SM, Welsh RM, Berg LJ. Epigenetic modifications induced by Blimp-1 Regulate CD8(+) T cell memory progression during acute virus infection. Immunity. 2013;39:661–75. doi: 10.1016/j.immuni.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–92. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 15.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–21. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 17.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 18.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–83. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ML, Stempora LL, Larsen CP. CD28 blockade induces division-dependent downregulation of interleukin-2 receptor alpha. Transpl Immunol. 2011;24:94–9. doi: 10.1016/j.trim.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watts TH, DeBenedette MA. T cell co-stimulatory molecules other than CD28. Curr Opin Immunol. 1999;11:286–93. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 21.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 22.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 23.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T cell- dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 24.Ansari MJ, Fiorina P, Dada S, Guleria I, Ueno T, Yuan X, Trikudanathan S, Smith RN, Freeman G, Sayegh MH. Role of ICOS pathway in autoimmune and alloimmune responses in NOD mice. Clin Immunol. 2008;126:140–7. doi: 10.1016/j.clim.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Sporici RA, Beswick RL, von Allmen C, Rumbley CA, Hayden-Ledbetter M, Ledbetter JA, Perrin PJ. ICOS ligand costimulation is required for T-cell encephalitogenicity. Clin Immunol. 2001;100:277–88. doi: 10.1006/clim.2001.5074. [DOI] [PubMed] [Google Scholar]
- 26.Sporici RA, Perrin PJ. Costimulation of memory T-cells by ICOS: a potential therapeutic target for autoimmunity? Clin Immunol. 2001;100:263–9. doi: 10.1006/clim.2001.5093. [DOI] [PubMed] [Google Scholar]
- 27.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–6. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 28.Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, Anderson CC, Shapiro AM. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55:27–33. [PubMed] [Google Scholar]
- 29.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am J Transplant. 2009;9:64–73. doi: 10.1111/j.1600-6143.2008.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 31.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 32.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–62. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 33.Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–45. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 34.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 35.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 36.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 37.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–22. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, Suchard SJ, Nadler SG, Ford ML. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med. 2014;211:297–311. doi: 10.1084/jem.20130902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore TV, Clay BS, Ferreira CM, Williams JW, Rogozinska M, Cannon JL, Shilling RA, Marzo AL, Sperling AI. Protective effector memory CD4 T cells depend on ICOS for survival. PLoS One. 2011;6:e16529. doi: 10.1371/journal.pone.0016529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore TV, Clay BS, Cannon JL, Histed A, Shilling RA, Sperling AI. Inducible costimulator controls migration of T cells to the lungs via down-regulation of CCR7 and CD62L. Am J Respir Cell Mol Biol. 2011;45:843–50. doi: 10.1165/rcmb.2010-0466OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi N, Matsumoto K, Saito H, Nanki T, Miyasaka N, Kobata T, Azuma M, Lee SK, Mizutani S, Morio T. Impaired CD4 and CD8 effector function and decreased memory T cell populations in ICOS-deficient patients. J Immunol. 2009;182:5515–27. doi: 10.4049/jimmunol.0803256. [DOI] [PubMed] [Google Scholar]
- 42.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–66. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–15. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–40. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuzefpolskiy Y, Baumann FM, Kalia V, Sarkar S. Early CD8 T-cell memory precursors and terminal effectors exhibit equipotent in vivo degranulation. Cell Mol Immunol. 2014 doi: 10.1038/cmi.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strutt TM, McKinstry KK, Kuang Y, Bradley LM, Swain SL. Memory CD4+ T-cell-mediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc Natl Acad Sci U S A. 2012;109:E2551–60. doi: 10.1073/pnas.1205894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merrill JT. Co-stimulatory molecules as targets for treatment of lupus. Clin Immunol. 2013;148:369–75. doi: 10.1016/j.clim.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, Zhang L, Qi H. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–7. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 49.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY, Mak TW, Serody JS, Blazar BR. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–80. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 50.Vidric M, Suh WK, Dianzani U, Mak TW, Watts TH. Cooperation between 4–1BB and ICOS in the immune response to influenza virus revealed by studies of CD28/ICOS-deficient mice. J Immunol. 2005;175:7288–96. doi: 10.4049/jimmunol.175.11.7288. [DOI] [PubMed] [Google Scholar]
- 51.Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–13. [PubMed] [Google Scholar]
- 52.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–32. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


