Abstract
Objective
To evaluate the short-term response of human pulps to ethanol-wet bonding technique associated with an etch-and-rinse adhesive system.
Methods
Deep class V cavities were prepared on the buccal surface of 17 sound premolars scheduled for extraction for orthodontics. The teeth were assigned into three groups: Ethanol-wet bonding (G1), water-wet bonding (G2) and calcium hydroxide (G3, control). Two teeth were used as intact control. After acid-etching, the cavities from G1 were filled with 100% ethanol for 60s and blot-dried before the application of Single Bond 2. In G2, the cavities were filled with distilled water for 60s previously to adhesive application and in G3, the cavity floor was lined with calcium hydroxide before etching and bonding. All cavities were restored with resin composite. The teeth were extracted 48h after the clinical procedures. From each tooth 6 μm-thick serial sections were obtained and stained with hematoxylin and eosin (H/E) and Masson's trichrome. Bacteria microleakage was assessed using Brown & Brenn. All sections were blindly evaluated and scored for five histological features.
Results
Mean remaining dentin thickness was 463±65μm (G1); 425±184μm (G2); and 348±194μm (G3). Similar pulp reactions followed ethanol- or water-wet bonding techniques. Slight inflammatory responses and disruption of the odontoblast layer related to the cavity floor were seen in all groups. Stained bacteria were not detected in any cavities. Normal pulp tissue was observed in G3 except for one case.
Conclusions
After 48 h, ethanol-wet bonding technique applied on deep cavities prepared in vital teeth does not increase pulpal damage compared to water-wet bonding technique.
Clinical significance
Ethanol-wet bonding has been considered an experimental technique that may increase resin-dentin bond durability. This study reported the in vivo response of human pulp tissue when 100% ethanol was applied previously to an etch-and-rinse simplified adhesive system.
Keywords: Ethanol, dentin bonding agent, dental pulp, biocompatibility
INTRODUCTION
Resin-dentin bonds created by etch-and-rinse adhesives rely on the interlock between collagen network and polymerized monomers.1 After acid-etching, the demineralized dentin zone is composed by 30% organic content and 70% water. Theoretically, adhesive monomers should replace water and encapsulate 100% of collagen fibrils, resulting in a hybrid layer constituted by 30% collagen and 70% resin.2 However, dimethacrylates monomers such as Bis-GMA, TEGDMA and UDMA are not soluble in water, what results in incomplete infiltration and phase separation of adhesive into the hydrated organic matrix of dentin.2-5 The non-infiltrated areas of collagen are susceptible to hydrolytic degradation mediated by water and the proteolytic hydrolases, matrix metalloproteinases (MMPs) and cysteine cathepsins over time, compromising the stability of the adhesive interface.6-11
The incorporation of hydrophilic resins into adhesive systems, such as hydroxyethylmetacrylate (HEMA), favors resin infiltration since this monomer is highly soluble in water.3,12-14 Nevertheless, the increased hydrophilicity of the adhesives may lead to a rapid decrease of their mechanical properties due to the water sorption that lowers the mechanical properties of adhesive resins, high water sorption15-18 and water permeability,19,20 culminating in a unstable hybrid layers.14,21,22
Dehydration of demineralized dentin prior to the application of adhesive monomers may favor dimethacrylates infiltration by removing excess water around collagen and creating a more hydrophobic environment.23 However, when the demineralized dentin is dehydrated by air evaporation, the collagen network collapses and lowers monomer permeation due to a drastic reduction of the size of interfibrillar spaces.2,23 However, if the dehydration is done using ethanol, the collagen becomes stiffer allowing the collagen network to remain expanded, preserving the interfibrillar diffusion pathway for adhesive infiltration.2,24
Ethanol is an organic solvent that can be used as a pretreatment of acid-etched dentin before application of etch-and-rinse adhesive systems (ethanol-wet bonding) that removes free and bound water.10,14,23,25,26 Additionally, it reduces the diameter of collagen fibrils, increasing interfibrillar spaces.23,26 These modifications favor dimethacrylate monomer infiltration, prevent phase separations, and since there are no water molecules available, prevent the cleavage of collagen by hydrolases, such as MMPs and cathepsins.24,27
The ethanol-wet bonding technique produces more stable resin-dentin bonds27,28 capable of sealing the dentin,29 increasing bond strengths23,26 and favoring the infiltration of Bis-GMA.30 Furthermore, this technique can improve bonding to caries-affected dentin.31 Although, ethanol-wet bonding has achieved promising results in vitro, it has still been considered an experimental technique by some authors32 and the effects of the chemical dehydration of dentin with this polar solvent on pulp tissue has not been tested. Thus, the aim of this study was to evaluate the short-term response of human pulps after adhesive restoration of dental cavities in normal dentin using the ethanol-wet bonding technique. The tested null hypothesis was that ethanol-wet bonding produces no adverse effects on human pulp tissue.
MATERIALS AND METHODS
Seventeen caries-free human maxillary first premolars in functional occlusion and scheduled to be extracted for orthodontics reasons were selected from young patients (mean age 16±1.3 years). The parents as well as the volunteers, after reading and receiving all necessary explanations including the experimental rationale, the clinical procedures and possible risks, signed the informed consent document that was approved by the IRB of Georgia Regents University.
The radiographs used in the orthodontics treatment were used to initially evaluate the possible presence of proximal caries or any potential periapical pathology. As a common diagnostic procedure for tooth extraction, periapical radiographs were also taken immediately before the extraction of each tooth. Asepsis of the oral mucosae was performed with 0.12% chlorhexidine solution before the delivery of local infiltration anesthesia. After cleaning the tooth with rubber cup/pumice slurry and rubber dam placement, class V cavities were prepared on the buccal surface using a diamond bur in a high-speed hand-piece under copious water-cooling by one operator. In order to standardize the cavity to a preset depth, cylindrical diamond burs (#1091, KG Sorensen, Cotia, SP, Brazil) had their active tip limited to 2.5 mm with a resin top.33 The bur was replaced after each four cavity preparations to avoid excessive heating due to loss of cutting efficiency. The final dimensions of the buccal cavities were 3.0 mm in length, 2.5 mm in depth, and 1.5 mm in height with no undercuts. The teeth were randomly allocated into four groups (Table 1) using a table of random numbers. Additionally, two sound teeth were used as intact control group. They were demineralized and processed for light microscopy.
Table 1.
Group description and number of teeth per group
| Groups | Treatment | n |
|---|---|---|
| Ethanol-wet bonding (Group 1) | Total etching + ethanol + bonding agent + resin restoration | 6 |
| Water-wet bonding (Group 2) | Total etching + water + bonding agent + resin restoration | 6 |
| Calcium hydroxyde (Group 3) | Dycal as liner + total etching + bonding agent + resin restoration | 3 |
| Intact teeth | No treatment | 2 |
In Groups 1 and 2, enamel and dentin were conditioned with 35% phosphoric acid (Scotchbond Etchant, 3M ESPE, St. Paul, MN, USA) for 30 and 15 seconds, respectively. After etching, the cavities were rinsed thoroughly with water for 30 seconds in order to remove residual acid and its reaction products. All cavosurface margins were in enamel. The cavity walls were gently blot-dried with sterilized absorbent paper to remove excess water. In Group 1, 10 μL of 100% ethanol solution (Sigma-Aldrich, St Louis, MO, USA) were applied in the cavity and left undisturbed for 60 s. During that time, it was constantly checked to insure that the ethanol did not evaporate. The first layer of Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) adhesive system was applied to enamel and dentin and rubbed for 15 s according to the manufacturer's recommendations. Then a second layer was applied, followed by gentle blot-drying with an oil-free stream of air (5 s at 10 cm of distance) and light-activation for 10 seconds (XL1000 Curing Light, 3M ESPE). In Group 2, instead of ethanol, 10 μL of distilled water were applied in the cavity and left undisturbed for 60 seconds. The following steps were the same described previously for the ethanol-wet bonding group (G1). In Group 3 (control), the cavity floor was lined with a hard-setting calcium hydroxide cement (Dycal® - Dentsply Caulk, Milford, DE, USA) before enamel and dentin acid etching and application of the adhesive system, as described above.
All cavities were restored to the enamel cavosurface margin with Filtek Z350 resin composite (3M ESPE, St. Paul, MN, USA) applied in three increments which were individually light-activated for 20 s. A radiometer (Demetron/Kerr – Model 100P/N 10503, Danbury, CT, USA) was used to check the curing light irradiance immediately before each clinical procedure (540±10 mW/cm2). When necessary, any excess material at the cavity margins, specially the cervical margin, was mechanically removed using a fine grit diamond bur at high speed under abundant water-cooling.
Forty-eight hours after the clinical procedures the teeth were extracted under local anesthesia with the use of forceps placed as apically as possible. Immediately after extraction, the roots were sectioned midway between the CEJ and the root apex with a high-speed handpiece under water spray to facilitate the penetration of fixative. The teeth were stored for 48 hours in 10% formalin fixative solution at pH 7.2, decalcified in buffered formic acid/sodium formate (0.2 M formic acid, 0.2 M sodium formate pH 2.3) changed every day, dehydrated, vacuum-infiltrated with paraffin wax and finally embedded in paraffin. The demineralization buffer was tested every day by adding 1 mL of 10% dipotassium oxalate to 1 mL of the demineralized buffer to determine if calcium was still coming out of the tooth. Calcium ions react with soluble potassium oxalate to form insoluble calcium oxalate that produces a white precipitate in the mixture. Generally, after 5-7 days, the potassium oxalate no longer produced a white precipitate that was positive for the presence of calcium. Six μm-thick serial sections, mounted on glass slides, were stained with hematoxylin and eosin (H/E) and Masson's trichrome. Bacteria were evidenced using the Brown & Brenn staining technique.
As previously reported33-35 all sections were evaluated blindly by one experienced examiner for five histological features according to the preset criteria given in Table 2. The pulpal response was evaluated by light microscopy (Carl Zeiss 62774, Oberköchen, West Germany).
Table 2.
Description and scores attributed to the histological features
| Score | Histological features |
|||
|---|---|---|---|---|
| Inflammatory cell response | Tissue disorganization | Reactionary dentin formation | Stained bacteria | |
| 0 | None or a few scattered inflammatory cells present in the pulp area corresponding to the axial wall, characteristic of normal tissue | Normal tissue | Absence | Absence |
| 1 | Slight inflammatory cell infiltrate with polymorphonuclear (PMNs) or mononuclear leukocytes (MNLs) | Odontoblastic layer disorganized but central pulp normal | Modest hard tissue deposition beneath the axial wall | Presence of stained bacteria along the cavity lateral wall |
| 2 | Moderate inflammatory cell infiltrate involving the coronal pulp | Total disorganization of the pulp tissue morphology | Moderate hard tissue deposition beneath the axial wall | Presence of stained bacteria along the cavity lateral walls and axial wall |
| 3 | Severe inflammatory cell infiltrate involving the coronal pulp or characterizing abscess | Pulp necrosis | Intense hard tissue deposition beneath the axial wall | Presence of stained bacteria along the cavity wall and within the cut dentin tubules |
The remaining dentin thickness (RDT) between the cavity floor and the pulp chamber was measured for each tooth using a light microscope (Carl Zeiss, Jena, Germany) connected to a video-camera (Samsung Digital Camera – SSC/131, Samsung Electronics Co. Ltd., Korea) as previously reported.36 The photomicrographs were loaded into a computer and processed using a standard software (ImageLab, Softium Informática, São Paulo, Brazil). RDT data was statistically analyzed by Kruskal-Wallis test considering 5% as a preset level of significance (α=0.05).
RESULTS
The radiographic evaluation of all teeth used in the study demonstrated no periapical pathology before the clinical procedure and extraction. During the experiment, the patients reported no symptoms, including pain. The scores observed for each criterion according to groups are shown in Table 3 and the remaining dentin thickness (RDT) data are presented in Table 4.
Table 3.
Histological score distribution within each group.
| Histopatologic event | Group | Score |
Total | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Inflammatory cell response | Ethanol-wet bonding | 0 | 6 | 0 | 0 | 6 |
| Water-wet bonding | 1 | 5 | 0 | 0 | 6 | |
| Calcium hydroxide | 2 | 1 | 0 | 0 | 3 | |
| Tissue disorganization | Ethanol-wet bonding | 0 | 6 | 0 | 0 | 6 |
| Water-wet bonding | 1 | 5 | 0 | 0 | 6 | |
| Calcium hydroxide | 2 | 1 | 0 | 0 | 3 | |
| Reactionary dentin formation | Ethanol-wet bonding | 6 | 0 | 0 | 0 | 6 |
| Water-wet bonding | 6 | 0 | 0 | 0 | 6 | |
| Calcium hydroxide | 3 | 0 | 0 | 0 | 3 | |
| Stained bacteria | Ethanol-wet bonding | 6 | 0 | 0 | 0 | 6 |
| Water-wet bonding | 6 | 0 | 0 | 0 | 6 | |
| Calcium hydroxide | 3 | 0 | 0 | 0 | 3 | |
Table 4.
Remaining dentin thickness (RDT) in micrometers (μm).
| Group | Teeth |
Mean±sd | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Ethanol-wet bonding | 562 | 378 | 453 | 431 | 443 | 512 | 463±65a |
| Water-wet bonding | 445 | 78 | 578 | 566 | 491 | 393 | 425±184a |
| Calcium hydroxide | 419 | 226 | 401 | --- | --- | --- | 348±194a |
means followed by the same letter do not differ statistically (Kruskal-Wallis, p>0.05).
Group 1: Ethanol-wet bonding
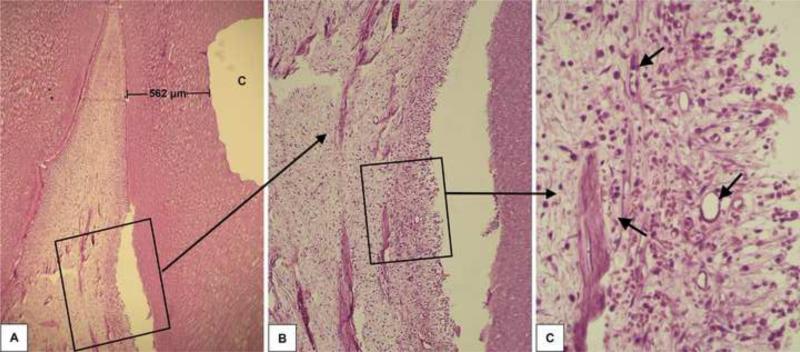
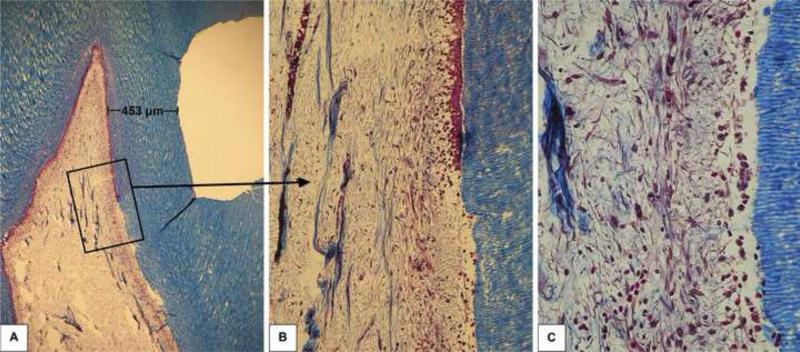
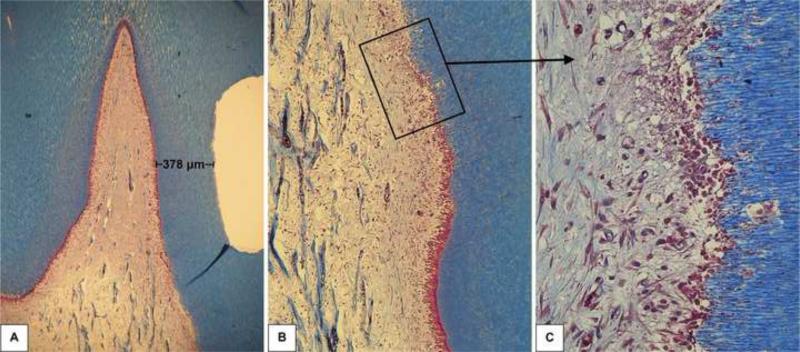
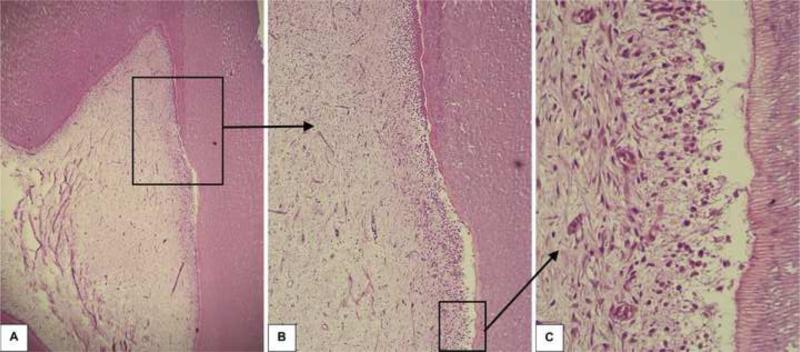
Forty-eight hours after the clinical procedure the odontoblast layer adjacent to the cavity floor was disrupted, in all six specimens, characterizing a superficial discrete pulp tissue disorganization. In these samples, a mild inflammatory response mediated by mononuclear cells and a number of congested small blood vessels were seen (Figures 1, 2 and 3). In all histological sections stained with Brown and Brenn, no bacteria were seen. Additionally, no dentin matrix deposition was observed. The RDT mean value for this group was 463±65 μm.
Fig. 1.
Group 1: Ethanol-wet bonding (RDT = 562 μm). A – Relationship between the class V cavity prepared in the premolar and the subjacent pulp tissue H/E, 32x. B – High magnification of the pulp area selected in the figure 1A. H/E, 64x. C – Detail of the connective pulp tissue selected in Figure 1B. Discrete inflammatory response mediated by mononuclear cells among small congested blood vessels is observed. H/E, 250x.
Fig. 2.
Group 1: Ethanol-wet bonding (RDT = 453 μm). A – Relationship between the class V cavity and the pulp tissue. Masson's trichrome, 32x. B – Higher magnification of the area selected in Figure 2A. Note the transition between pulp areas related and not related to the cavity floor. Only in the upper part of the photomicrograph a continuous odontoblast layer is observed. Masson's trichrome, 64x. C – Detail of the pulp area related to the cavity floor. Note the complete disruption of the odontoblast layer. Discrete local tissue disorganization associated with mild inflammatory response. Masson's trichrome, 250x.
Fig. 3.
Group 1: Ethanol-wet bonding (RDT = 378 μm). A - Relationship between the class V cavity and the pulp tissue. Masson's trichrome, 32x. B – Higher magnification of the area selected in Figure 3A. Note the transition between the pulp areas related or not to the cavity floor. C – Detail of the area where the tubules affected by the procedure terminate into the pulp. Note the disruption of the odontoblast layer characterizing the discrete local tissue disorganization and the mild local inflammatory response. Masson's trichrome, 250x.
Group 2: Water-wet bonding
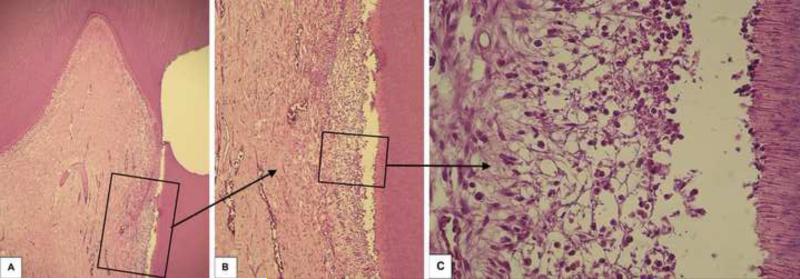
In this group, disruption of the odontoblast layer related to the cavity floor was observed in five of the six samples. In the superficial area of the pulp tissue, a mild inflammatory pulp response mediated by mononuclear cells associated with a number of congested small blood vessels was seen. In only 1 sample, in which the RDT between the cavity floor and the pulp tissue was 78 μm, inner dentin resorption was observed (Figure 4). As recorded for Group 1 (ethanol-wet bonding), no bacteria leakage was evidenced in all histological sections stained with Brown and Brenn in group 2 specimens. In addition, no dentin matrix deposition was observed (Figure 5). The mean RDT for this group was 425±184 μm. Representative histological sections are shown in Figure 7.
Fig. 4.
Group 2: Water-wet bonding (RDT = 78 μm). A - A very thin remaining dentin between the dental cavity and the pulp tissue is observed. H/E, 32x. B – Higher magnification of the pulp area selected in Figure 4A. The empty space between the pulp tissue and the dentin is a histologic artifact. H/E, 64x. C – Detail of the pulp area selected in Figure 4B. Disruption of the odontoblast layer and mild inflammatory pulp response is observed. H/E, 250x.
Fig. 5.
Group 2: Water-wet bonding (RDT = 578 μm). A – Buccal pulp horn related to the cavity floor. H/E, 32x. B – Higher magnification of the pulp area selected in Figure 5A. A shrinkage artifact is present, but the picture allows recognition of the lack of odontoblasts in the affected area. H/E, 64x. C – Detail of the pulp area selected in Figure 5B. Note mild local inflammatory pulp response. H/E, 250x.
Group 3: Calcium hydroxide (Dycal)
In this group, slight disruption of the odontoblast layer related to the cavity floor was observed in only one sample, in which the RDT between the cavity floor and the pulp tissue was 226 μm. In this specific sample, a mild inflammatory pulp response associated with discrete superficial disorganization of the pulp tissue was observed. In the other two samples in which the RDT was 419 μm and 401 μm, no inflammatory reaction or pulp tissue disorganization occurred. The RDT recorded for this group was 348±194 μm. No bacteria were seen in any of the sections.
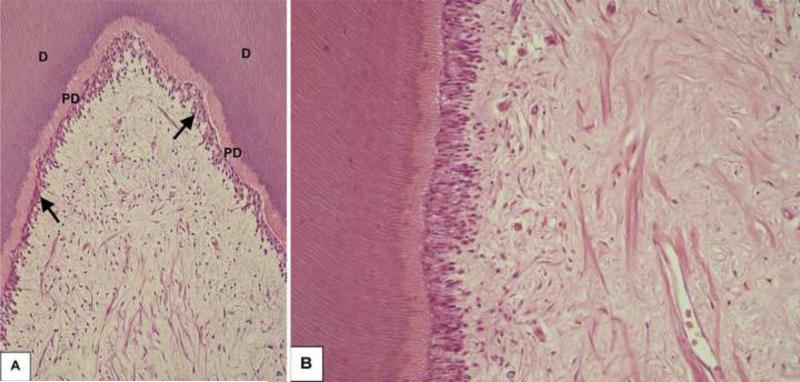
In the intact control group, the pulp tissue exhibited normal histological characteristics. The predentin was lined by a continuous odontoblast layer and the well-defined cell-free and cell-rich zones were clearly observed (Figure 6A/B). These histological findings demonstrate that the adequate laboratorial process of teeth used in this in vivo study which allowed appropriate microscopic assessment of the pulp response caused by the experimental clinical procedures carried out.
Fig. 6.
Intact Control Group. A - Tubular dentin (D) and predentin (PD) lined by a continuous odontoblast layer (arrows) can be observed. H/E, 96x. B - Pulp tissue exhibiting normal histological characteristics. H/E, 250x.
DISCUSSION
The ethanol-wet bonding concept was introduced in 2007 in order to enhance monomer infiltration through demineralized collagen network and improve resin-dentin bond durability.14,24 The saturation of dentin with ethanol allows an increased amount of hydrophobic monomers to infiltrate the demineralized dentin,30 creating a hybrid layer more resistant to water sorption.24 Additionally, it has been shown that the saturation of 0.4 mm etched dentin disks with 100% ethanol did not cause transdentinal cytotoxic effects to cultured odontoblast-like cells.37
In the present study, two intact premolars were used to determine the quality of fixation and laboratory tissue processing techniques on pulpal tissues, as well as a baseline to establish a standard of comparison between the healthy tissue and the any alterations produced by the procedure and materials used (Figure 6A/B). It is known that thermal and non-thermal stimuli generated by burs operated in high-speed handpieces38-40 may produce severe pulpal responses.38,39 In the present study, slight inflammatory cell infiltrate was observed in only one tooth treated with the hard-setting calcium hydroxide cement (G3), in which no reactionary dentin formation or stained bacteria were found. The absence of histological changes in most of the teeth pertaining to this control group shows that when the cavity preparation is properly performed using new burs, intermittent cutting and abundant water cooling changes to pulp tissue are not expected. The only tooth that underwent pulp alterations presented a very thin remaining dentin thickness (RDT= 226 μm).
None of the experimental specimens exhibited moderate to severe inflammatory cell infiltrate or pulp necrosis. All teeth subjected to ethanol-wet bonding technique (G1) exhibited mild inflammatory response, comparable to the inflammatory response seen when the water-wet bonding technique was used (G2). These findings suggest that although ethanol is able to enhance in vitro monomeric infiltration,14,29,30 the ethanol-wet bonding technique used in the present study did not worsen pulpal damage compared to the water-wet bonding treatment performed in vital human teeth. These results require acceptance of the null hypothesis that ethanol-wet bonding produces no adverse immediate effects.
The average of remaining dentin thickness for Groups 1 and 2 were 463±65μm and 425±184μm, respectively. It is known that the mineralized dentin, even at a thickness as thin as 0.5 mm, works as a barrier capable of protecting the pulp against toxic components released from dental materials.41-43 However, it has been reported that the monomer diffusion promoted by ethanol-wet bonding increases necrotic odontoblast-like cell death in vitro.37 This laboratory finding was confirmed in the present study, in that disruption of the odontoblast layer was observed for both water-wet and ethanol-wet bonding groups, that had statistically similar mean RDT. That fact simply confirms that the immunological and lymphatic systems in the pulp-dentin complex, as well as the intrapulpal pressure present in vital pulps are not capable of preventing the mild cytoxicity reactions caused by dental adhesives applied in very deep dentin.36 On the other hand, the subjacent pulp tissue exhibited normal histological characteristics, what determined that the adhesive techniques evaluated in the present study caused only superficial tissue damage at short-term period (48 hours) which was not enhanced by the treatment of dentin with 100% ethanol. Based on this immediate response, we speculate that the pulp tissue would completely recover from the mild injury imposed by the bonding treatments. However, that assumption has yet to be proven by further long-term studies.
In this study, very deep cavities (RDT<500 μm) were prepared in dentin, where application of dental adhesives without prior protection of the dentin-pulp complex is not recommended.33-36,44 The thin RDT offered a major challenge in terms of biocompatibility and yet the saturation of etched dentin with 100% ethanol and the subsequent application of the dental adhesive did not cause intense damage or necrosis of the pulp tissue. Based on the results obtained in the present in vivo study, it appears that the ethanol-wet bonding technique may be safely applied in medium or shallow cavities. However, it is important to bear in mind that this study was performed in sound human teeth. In clinical situations, adhesive restorations are usually performed in cavities following mechanical caries removal. The use of the ethanol-wet bonding in teeth in which an inflammatory reaction is already established in the pulp tissue may result in different outcomes. Therefore, further in vivo studies are needed to assess the response of inflamed human pulps of carious teeth subjected to adhesive restoration using the ethanol-wet bonding technique. This will determine if the initial mild superficial damage is increased or resolved over time. The next step should be the evaluation of the clinical performance of resin dentin bonds produced using the ethanol-wet bonding technique in controlled clinical trials.
CONCLUSION
According to the methodology employed in this study, it may be concluded that after 48 hours the ethanol-wet bonding technique used for adhesive restoration of deep cavities prepared in vital human teeth produced only mild pulp injury that was similar to the pulpal damage produced by contemporary water-wet bonding technique.
ACKNOWLEDGEMENT
This study was supported by CNPq 305204/2010-6 (PI. Josimeri Hebling), CNPq 301291/2010-1 (PI. Carlos Alberto de Souza Costa) and R01 DE015306 from the NIDCR (PI. David H. Pashley).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Débora Lopes Salles Scheffel, Department of Orthodontics and Pediatric Dentistry, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903..
Nancy Tomoko Sacono, Department of Stomatology (Oral Pathology), Dental School, Federal University of Goiás, Goiânia, Goiás, Brazil. Av. Décima Primeira Avenida, 62000 - Setor Leste Universitário, Goiânia, Goiás, Brazil, 74605-020..
Ana Paula Dias Ribeiro, Department of Dentistry, Dental School, Federal University of Brasilia, Brasilia DP, Brazil. Campus Universitário Darcy Ribeiro, Brasília, Distrito Federal, Brazil, 70910-900..
Diana Gabriela Soares, Department of Dental Materials and Prosthodontics, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903..
Fernanda Gonçalves Basso, Department of Physiology and Pathology, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903..
David Henry Pashley, Department of Oral Biology, College of Dental Medicine, Georgia Regents University, Augusta, Georgia, USA. 1120 15th Street, CL- 2112, Augusta, Georgia, USA, 30912-1129..
Carlos Alberto de Souza Costa, Department of Physiology and Pathology, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903..
Josimeri Hebling, Department of Orthodontics and Pediatric Dentistry, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903..
REFERENCES
- 1.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. Journal of Biomedical Materials Research. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 2.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M. Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer P, Wang Y. Adhesive p hase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 4.Cameron IL, Short NJ, Fullerton GD. Verification of simple hydration/dehydration methods to characterize multiple water compartments on tendon type 1 collagen. Cell Biology International. 2007;31:531–9. doi: 10.1016/j.cellbi.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Gu L, Breschi L, Tjäderhane L, Choi KK, Pashley DH, et al. Implication of ethanol wet-bonding in hybrid layer remineralization. Journal of Dental Research. 2010;89:575–80. doi: 10.1177/0022034510363380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 7.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arr ests subclinical degradation of dentin hybrid layers in vivo. Journal of Dental Research. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 8.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Tjäderhane L, Toledano M, et al. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentin by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Mazzoni A, Mannello F, Tay FR, Tonti GAM, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. Journal of Dental Research. 86:436–40. doi: 10.1177/154405910708600509. erratum, Journal of Dental Research 2007; 86:792.
- 10.Nishitani Y, Yoshiyama M, Wadgaonkar B, Elrod D, Breschi L, Mannello F, et al. Activation of gelatinolytic/collagenolytic activity in den-tin by self-etching adhesives . European Journal of Oral Sciences. 2006;114:160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 11.Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio F. Self- etching adhesives increase collagenolytic activity in radicular dentin. Journal of Endodontics. 2006;32:862–8. doi: 10.1016/j.joen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. Interfacial chemistry of the dentin/adhesive bond. Journal of Dental Research. 2000;79:1458–63. doi: 10.1177/00220345000790070501. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Spencer P. Quantifying adhesive penetration adhesive/ dentin interface using confocal Raman microspectroscopy. Journal of Biomedical Materials Research. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 14.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, et al. From dry bonding to water-wet bonding to ethanol wet bonding. A review of the interactions between den tin matrix and solvated resins using a macromodel of the hybrid layer. American Journal of Dentistry. 2007;20:7–20. [PubMed] [Google Scholar]
- 15.Unemori M, Matsuya Y, Matsuya S, Akashi A, Akamine A. Water absorption of poly(methyl methacrylate) containing 4-methacryloxyethyl tri mellitic anhydride. Biomaterials. 2003;24:1381–7. doi: 10.1016/s0142-9612(02)00521-5. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–59. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 17.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22:211–22. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Yiu CK, King NM, Carrilho MR, Sauro S, Rueggeberg FA, Prati C, et al. Effect of resin hydrophilicity and temperature on water sorption of dent al adhesive resins. Biomaterials. 2006;27:1695–703. doi: 10.1016/j.biomaterials.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Sauro S, Pashley DH, Montanari M, Chersoni S, Carvalho RM, Toledano M, et al. Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dental Materials. 2006;23:705–13. doi: 10.1016/j.dental.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Sauro S, Pashley DH, Mannocci F, Tay FR, Pilecki P, Sherriff M, et al. Micropermeability of current self-etching and etch-and-rinse adhesives bonded to deep dentine: a comparison study using a double-staining/confocal microscopy technique. European Journal of Oral Sciences. 2008;116:184–93. doi: 10.1111/j.1600-0722.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 21.Tay FR, Frankenberger R, Krejci I, Bouillaguet S, Pashley DH, Carvalho RM, et al. Single-bottle adhesives behave as permeable membranes after polymerization. I. In vivo evidence. Journal of Dentistry. 2004;32:611–21. doi: 10.1016/j.jdent.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? Journal of the Canadian Dental Association. 2003;69:726–31. [PubMed] [Google Scholar]
- 23.Tay FR, Pashley DH, Kapur RR, Carrilho MR, Hur YB, Garrett LV, Tay KC. Bonding BisGMA to dentin - a proof of concept for hydrophobic dentin bonding. Journal of Dental Research. 2007;86:1034–9. doi: 10.1177/154405910708601103. [DOI] [PubMed] [Google Scholar]
- 24.Becker TD, Agee KA, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Infiltration/evaporation-induced shrinkage of demineralized dentin by solvated model adhesives. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2007;80:156–65. doi: 10.1002/jbm.b.30580. [DOI] [PubMed] [Google Scholar]
- 25.Sadek FT, Pashley DH, Nishitani Y, Carrilho MR, Donnelly A, Ferrari M, et al. Application of hydrophobic resin adhesives to acid-etched d entin with an alternative wet bonding technique. Journal of Biomedical Materials Research Part A. 2008;84:19–29. doi: 10.1002/jbm.a.31290. [DOI] [PubMed] [Google Scholar]
- 26.Hosaka K, Nishitani Y, Tagami J, Yoshiyama M, Brackett WW, Agee KA, Tay FR, Pashley DH. Durability of resin-dentin bonds to water- vs. ethanol-saturated dentin. Journal of Dental Research. 2009;88:146–51. doi: 10.1177/0022034508328910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadek FT, Braga RR, Muench A, Liu Y, Pashley DH, Tay FR. Ethanol wet-bonding challenges current anti-degradation strategy. Journal of Dental Research. 2010;89:1499–504. doi: 10.1177/0022034510385240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadek FT, Castellan CS, Braga RR, Mai S, Tjäderhane L, Pashley DH, Tay FR. One-year stability of resin-dentin bonds created with a hydrophobic ethanol-wet bonding technique. Dental Materials. 2010;26:380–6. doi: 10.1016/j.dental.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Sauro S, Watson TF, Mannocci F, Miyake K, Huffman BP, Tay FR, Pashley DH. Two-photon laser confocal microscopy of micropermeability of resin-dentin bonds made with water or ethanol wet bonding. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;90:327–37. doi: 10.1002/jbm.b.31290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin TP, Yao X, Huenergardt R, Walker MP, Wang Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dental Materials. 2009;25:1050–7. doi: 10.1016/j.dental.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang X, Li L, Huang C, Du X. Effect of ethanol-wet bonding with hydrophobic adhesive on caries-affected dentine. European Journal of Oral Sciences. 2011;119:310–5. doi: 10.1111/j.1600-0722.2011.00830.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. Journal of Dental Research. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebling J, Giro EM, Costa CA. Human pulp response after an adhesive system application in deep cavities. Journal of Dentistry. 1999;27:557–64. doi: 10.1016/s0300-5712(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 34.de Souza Costa CA, Hebling J, Randall RC. Human pulp response to resin cements used to bond inlay restorations. Dental Materials. 2006;22:954–62. doi: 10.1016/j.dental.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 35.de Souza Costa CA, Teixeira HM, Lopes do Nascimento AB, Hebling J. Biocompatibility of resin-based dental materials applied as liners in deep cavities prepared in human teeth. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2007;81:175–84. doi: 10.1002/jbm.b.30651. [DOI] [PubMed] [Google Scholar]
- 36.Costa CA, Ribeiro AP, Giro EM, Randall RC, Hebling J. Pulp response after application of two resin modified glass ionomer cements (RMGICs) in deep cavities of prepared human teeth. Dental Materials. 2011;27:e158–70. doi: 10.1016/j.dental.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Bianchi L, Ribeiro AP, de Oliveira Carrilho MR, Pashley DH, de Souza Costa CA, Hebling J. Transdentinal cytotoxicity of experimental adhesive systems of different hydrophilicity applied to ethanol-saturated dentin. Dental Materials. 2013;29:980–90. doi: 10.1016/j.dental.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trowbridge HO, Kim S. Pulp development, structure and function. In: Cohen S, Burns RC, editors. Pathways of the pulp. 6th ed. CV Mosby; St Louis: 1994. pp. 296–336. [Google Scholar]
- 39.Langeland K, Langeland LK. Cutting procedures with minimized trauma. Journal of the American Dental Association. 1968;76:991–1005. doi: 10.14219/jada.archive.1968.0181. [DOI] [PubMed] [Google Scholar]
- 40.Cavalcanti BN, Lage-Marques JL, Rode SM. Pulpal temperature increases with Er:YAG laser and high-speed handpieces. Journal of Prosthetic Dentistry. 2003;90:447–51. doi: 10.1016/j.prosdent.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Hanks CT, Craig RG, Diehl ML, Pashley DH. Cytotoxicity of dental composites and other materials in a new in vitro device. Journal of Oral Patholology & Medicine. 1988;17:396–403. doi: 10.1111/j.1600-0714.1988.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 42.Bouillaguet S, Wataha JC, Hanks CT, Ciucchi B, Holz J. In vitro cytotoxicity and dentin permeability of HEMA. Journal of Endodontics. 1996;22:244–8. doi: 10.1016/s0099-2399(06)80141-x. [DOI] [PubMed] [Google Scholar]
- 43.Lanza CR, de Souza Costa CA, Furlan M, Alécio A, Hebling J. Transdentinal diffusion and cytotoxicity of self-etching adhesive systems. Cell Biology and Toxicolology. 2009;25:533–43. doi: 10.1007/s10565-008-9110-x. [DOI] [PubMed] [Google Scholar]
- 44.Koliniotou-Koumpia E, Papadimitriou S, Tziafas D. Pulpal responses after application of current adhesive systems to deep cavities. Clinical Oral Investigations. 2007;11:313–20. doi: 10.1007/s00784-007-0121-4. [DOI] [PubMed] [Google Scholar]