Abstract
In August 2012, the Brazilian Ministry of Health introduced inactivated polio vaccine (IPV) as part of sequential polio vaccination schedule for all infants beginning their primary vaccination series. The revised childhood immunization schedule included 2 doses of IPV at 2 and 4 months of age followed by 2 doses of oral polio vaccine (OPV) at 6 and 15 months of age. One annual national polio immunization day was maintained to provide OPV to all children aged 6 to 59 months. The decision to introduce IPV was based on preventing rare cases of vaccine-associated paralytic polio, financially sustaining IPV introduction, ensuring equitable access to IPV, and preparing for future OPV cessation following global eradication. Introducing IPV during a national multivaccination campaign led to rapid uptake, despite challenges with local vaccine supply due to high wastage rates. Continuous monitoring is required to achieve high coverage with the sequential polio vaccine schedule.
Keywords: acute flaccid paralysis, Brazil, inactivated polio vaccine, poliomyelitis, vaccination schedule
The World Health Organization (WHO) recommends that all children worldwide be immunized against polio and that all countries achieve and maintain high levels of coverage with polio vaccine [1]. Until global polio eradication is achieved, WHO and the Pan American Health Organization (PAHO) guidance for national policy on polio immunization is based on evaluation of the potential for wild poliovirus (WPV) importation and transmission [1, 2]. Important factors include risk of WPV importations resulting from international travel, polio vaccination coverage, quality of surveillance for acute flaccid paralysis (AFP), sanitation, and socioeconomic conditions [1]. In the pre-eradication period, WHO recommends inactivated polio vaccine (IPV) as an alternative to oral polio vaccine (OPV) only in countries that have the lowest risk of both WPV importation and transmission [1]. In countries that do not achieve homogeneous vaccination coverage of 95% or greater in every district, PAHO recommends conducting annual polio vaccination campaigns targeting all children <5 years of age regardless of prior vaccination status [2].
Brazil is an upper-middle-income country in South America with a population of 199 million and approximately 3 million annual births. Since its creation in 1973, Brazil’s National Immunization Program has exclusively used OPV for routine infant immunizations and supplemental immunization activities (SIAs) [3]. Use of OPV successfully interrupted transmission of wild poliovirus in Brazil and eliminated polio from the Americas. Brazil has maintained high polio vaccination coverage nationwide since polio elimination, although heterogeneous vaccination coverage may leave pockets of individuals susceptible to WPV infection in the event of a WPV importation.
In August 2012, the Brazilian Ministry of Health introduced inactivated polio vaccine (IPV) as part of sequential IPV-OPV vaccination, including 2 doses of IPV at 2 and 4 months of age followed by 2 doses of trivalent OPV at 6 and 15 months of age for all infants beginning their primary vaccination series [4]. A sequential IPV-OPV schedule was chosen to prevent vaccine-associated paralytic poliomyelitis (VAPP) and provide mucosal immunity to reduce the potential for transmission of wild or vaccine-derived polioviruses (VDPVs). With the introduction of the sequential IPV-OPV schedule, the Ministry of Health revised the strategy for National Polio Immunization Days to conduct a single round of polio vaccination, during the month of June, offering OPV to all children aged 6 to 59 months regardless of prior polio vaccination status [4]. The introduction of a sequential IPV-OPV schedule and transition to a single supplemental polio immunization day were seen as preparatory steps for a posteradication polio vaccination strategy in Brazil. Here we review considerations for IPV introduction in Brazil’s National Immunization Program and describe early uptake of IPV.
BRIEF HISTORY OF POLIO VACCINATION IN BRAZIL, 1961–2012
Polio vaccination with OPV began in the early 1960s in response to polio outbreaks [5]. OPV was one of the recommended routine childhood vaccines when the National Immunization Program was created in 1973 (Table 1), although coverage with 3 doses of OPV among children aged <1 year reached only 51% in 1979 [5]. From 1971 to 1973, nationally coordinated mass vaccination campaigns were conducted in most states and resulted in dramatic declines in polio incidence [5]. Discontinuation of mass campaigns in 1974 led to resurgence in polio cases, which peaked in 1979 and resulted in institution of biannual national polio immunization days (NIDs) in 1980 [5–7]. The objective of polio NIDs was to vaccinate all children aged <5 years with OPV, regardless of prior vaccination history. NIDs played an important role in polio elimination in Brazil and the Americas [7]. The last confirmed case of wild poliovirus in Brazil occurred in 1989, followed by the last case in the Americas in 1991. WHO certified polio elimination from Brazil and the Americas in 1994 [8, 9].
Table 1.
Selected Revisions of Recommended Childhood Immunization Schedule in Brazil’s National Immunization Program, 1973–2012
| Year | Milestone |
|---|---|
| 1973 | Creation of National Immunization Program (recommended immunizations: BCG, OPV, measles, DTwP, smallpox) |
| 1980 | Polio Elimination Plan—2 national polio immunization days (OPV) |
| 1986 | Sustainability and National Self-Sufficiency Initiative (production of DTwP by national vaccine manufacturers) |
| 1989 | HepB vaccine campaigns in high-risk areas |
| 1990 | Established goals for 90% routine vaccination coverage (OPV, DTwP) and 95% coverage for OPV campaigns and measles vaccination |
| Multivaccination in National Immunization Days (all recommended vaccines) | |
| 1992 | Measles elimination plan (measles 2nd dose) |
| Universal infant immunization against HepB | |
| 1992–2000 | Phased introduction of MMR vaccine |
| 1994 | Yellow fever vaccination in high-risk areas incorporated into National Immunization Program |
| 1995–2000 | Rubella control strategy (measles-rubella or MMR campaigns, targeting persons aged 1–11 y in most states) |
| 1999 | Introduction of Hib conjugate vaccine |
| 2002 | Quadrivalent DTwP-Hib replaces DTwP and monovalent Hib conjugate vaccine |
| 2006 | Introduction of oral rotavirus vaccine (Human reassortment vaccine) |
| 2010 | Introduction of pneumococcal conjugate vaccine (10-valent) |
| Introduction of MenC conjugate vaccine | |
| 2012 | Sequential IPV-OPV vaccination schedule for polio |
| Pentavalent DTwP-Hib-HepB replaces DTwP-Hib and HepB vaccines for infant vaccination |
Abbreviations: BCG, Bacillus Calmette–Guérin; HepB, hepatitis B; Hib, Haemophilus influenzae type b; DTwP, diphtheria-tetanus-pertussis (whole cell); IPV, inactivated polio vaccine; MenC, meningococcal serogroup C; MMR, measles-mumps-rubella; OPV, oral polio vaccine.
Investigations of polio outbreaks in Brazil and serologic studies helped identify factors that influenced immunogenicity of OPV and led to changes in the OPV formulation adopted for the Global Polio Eradication Initiative [10–13]. In addition, research in Brazil on IPV in the 1980s identified potential advantages of IPV for routine immunization, including higher seroconversion rates and prevention of VAPP, while OPV was preferable for mass vaccination [14].
CONSIDERATIONS FOR IPV INTRODUCTION
Prior to IPV introduction into Brazil’s childhood immunization schedule, revision of the national polio vaccination policy was discussed at multiple meetings of the national technical advisory committee on immunizations, composed of immunization experts and representatives of professional societies. Considerations included risk of WPV importation, vaccine safety, sustainability, equity, vaccination strategies, and optimal schedule. In 2008, the National Immunization Program began developing a plan for IPV introduction through routine immunization services. The main components of the revised polio vaccination policy included use of a sequential IPV-OPV schedule, continuation of polio vaccination strategies (NIDs and routine vaccination) until global polio eradication, and sustainability of polio vaccination in the recommended childhood immunization schedule.
Potential for WPV Importation and Transmission
As long as wild poliovirus circulates anywhere in the world, all polio-free countries are at risk for WPV importation [1]; countries immediately bordering endemic countries and those with low routine immunization coverage are at highest risk [1]. Transmission potential following an importation is also higher in tropical countries with suboptimal sanitation [1]. Brazil has not had any WPV importations since certification of elimination, and all reported cases of paralytic poliomyelitis in the Americas have been caused by vaccine viruses or VDPVs [15]. However, importations of WPV into polio-free countries have occurred as a result of international air travel [16], and previously polio-free regions have experienced extensive outbreaks resulting from WPV importation [17].
Routine coverage with 3 doses of OPV (OPV3) in Brazilian infants, based on administrative data, has been maintained above 95% nationally since 2000 (Table 2). Due to the limitations of administrative data to monitor immunization coverage at the municipal level, state and municipal immunization programs increasingly use rapid coverage monitoring (used in large scale following mass measles-rubella vaccination in 2008 [18]) to identify undervaccinated populations. A survey of children in state capital cities showed high OPV3 coverage at all socioeconomic levels [19]. In addition, introduction of a national immunization registry in 2012 will eventually provide more accurate estimates of vaccination coverage and reduce the reliance on administrative data [20].
Table 2.
Estimated Coverage With 3 Doses of OPV Among Children Aged <1 Year in Routine Immunization Services and Results of 2 Annual National Polio Immunization Days Targeting Children Aged <5 Years, Brazil, 2002–2012
| Calendar Year | Routine Services
|
National Polio Immunization Days
|
|||
|---|---|---|---|---|---|
| OPV3 Coveragea
|
1st Round
|
2nd Round
|
|||
| Doses Administered (% Pop <1 y) | Doses Administered (% Pop <5 y) | No. Municipalities Reporting ≥95% Coverageb (%) | Doses Administered (% Pop <5 y) | No. Municipalities Reporting ≥95% Coverageb (%) | |
| 2002 | 3 212 618 (105.0) | 16 828 472 (100.7) | 4607 (82.8) | 16 706 362 (99.9) | 4694 (84.4) |
|
| |||||
| 2003 | 3 209 756 (105.6) | 16 792 599 (99.9) | 4655 (83.7) | 16 679 283 (99.2) | 4737 (85.2) |
|
| |||||
| 2004 | 3 152 042 (104.3) | 16 489 247 (97.3) | 4259 (76.5) | 16 489 380 (97.3) | 4361 (78.4) |
|
| |||||
| 2005 | 3 188 216 (105.1) | 16 397 934 (94.5) | 3744 (67.3) | 16 540 230 (95.3) | 3936 (70.7) |
|
| |||||
| 2006 | 3 086 120 (104.8) | 16 126 323 (94.9) | 3562 (64.0) | 16 289 363 (95.9) | 3685 (66.2) |
|
| |||||
| 2007 | 3 032 286 (104.9) | 16 079 786 (100.8) | 4423 (79.5) | 15 941 552 (99.9) | 4376 (78.6) |
|
| |||||
| 2008 | 2 920 562 (99.5) | 15 522 157 (99.2) | 3951 (71.0) | 14 883 257 (95.1) | 3647 (65.6) |
|
| |||||
| 2009 | 2 962 167 (102.8) | 15 028 995 (97.3) | 4022 (72.3) | 15 116 210 (97.9) | 4083 (73.4) |
|
| |||||
| 2010 | 2 838 743 (98.6) | 14 295 965 (92.4) | 3449 (62.0) | 14 743 488 (95.3) | 3836 (68.9) |
|
| |||||
| 2011 | 2 891 340 (100.4) | 14 186 318 (100.2) | 4939 (88.7) | 14 102 506 (99.7) | 5049 (90.7) |
|
| |||||
| 2012 | 2 781 341 (96.6) | 14 004 200 (98.9) | 4665 (84.0) | NA | NA |
Abbreviations: OVP, oral polio vaccine; OPV3, 3 doses of OPV; Pop, population.
Based on the number of doses administered divided by the target population. Data for 2012 include doses of inactivated polio vaccine registered as the third dose of a polio vaccination series.
Based on the number of OPV doses administered in the municipality during National Polio Immunization Days divided by the municipal population aged <5 years.
Between 1970 and 2010, indicators of sanitation infrastructure and socioeconomic conditions improved dramatically in Brazil [21]. However, sanitation, development, and immunization coverage are heterogeneously distributed throughout Brazil, and a large number of municipalities report less than 95% coverage with OPV3, especially in tropical areas (Figure 1). In these areas, continued OPV use in routine immunizations and SIAs provides advantages for boosting mucosal immunity, facilitating administration in remote areas, and providing herd immunity through secondary spread of vaccine viruses.
Figure 1.
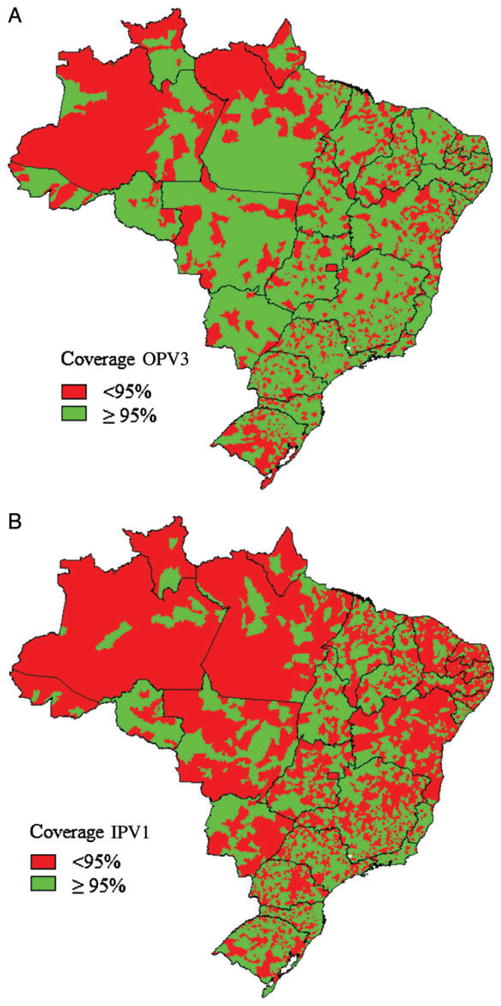
A, Municipalities reporting <95% or ≥95% coverage with 3 doses of oral polio vaccine (OPV3) among children aged <1 year, January–December, 2011. B, Municipalities reporting <95% or ≥95% coverage (based on monthly target population) with 1 dose of inactivated polio vaccine (IPV1) among children aged <1 year, September–December, 2012.
Prevention of VAPP
Prevention of VAPP and risk of VDPVs in immunocompromised children, despite their rare occurrence, was considered important for maintaining public confidence in the national immunization program. Reported incidence of VAPP in Brazil of 1 case per 10.7–13 million OPV doses administered (or 1 case per 2.4–5.1 million first OPV doses) [22, 23] was lower than estimates from the United States (2.5 cases per million OPV doses administered or 0.7 cases per million first OPV doses) [24], raising concerns about completeness of VAPP ascertainment in Brazil.
Limitations of Surveillance
In Brazil, AFP surveillance is conducted by state and municipal health departments and coordinated by the Secretariat for Health Surveillance of the Ministry of Health. While the main objective of AFP surveillance is early detection of WPV importation, it is also essential for detection of vaccine-associated cases and VDPVs. All cases of AFP in individuals younger than 15 years, as well as any suspected poliomyelitis case in individuals of any age with travel history in the previous 30 days to countries with circulation of WPV, must be reported to state and municipal health departments, investigated immediately, and entered into the national surveillance system for notifiable diseases [Sistema de Informação de Agravos de Notificação (Sinan)]. Follow up includes examination of neurological function and laboratory examination of stool specimens (ideally collected within 14 days of onset of paralysis).
Review of AFP surveillance indicators highlighted the need for maintaining surveillance quality and timeliness of diagnosis of AFP cases to rapidly detect and respond to poliovirus importations [25]. During 2003–2012, the national nonpolio AFP reporting rate was slightly above 1.0 case per 100 000 population aged <15 years, PAHO’s target reporting rate for AFP surveillance in the Americas (Table 3). However, fewer than 80% of reported cases had collection of adequate stool specimens, falling below the target indicator. Maintaining surveillance quality is challenging and requires coordination between health professionals, surveillance officers, laboratory staff, and directors of the Unified Health System (SUS) at all levels.
Table 3.
Acute Flaccid Paralysis Surveillance Quality Indicators, Brazil, 2003–2012
| Year | Population <15 y | Expected Number of Cases | Number of Reported AFP Cases | Percent of Municipalities Reporting | Investigation Within 48 h of Notification | Adequate Stool Specimen Collectiona | AFP Reporting Rateb |
|---|---|---|---|---|---|---|---|
| 2003 | 52 411 063 | 522 | 654 | 93 | 96 | 73 | 1.2 |
| 2004 | 53 087 921 | 525 | 642 | 91 | 98 | 70 | 1.2 |
| 2005 | 54 626 743 | 545 | 609 | 94 | 98 | 74 | 1.1 |
| 2006 | 55 411 292 | 554 | 614 | 90 | 97 | 71 | 1.2 |
| 2007 | 56 189 000 | 562 | 636 | 93 | 98 | 79 | 1.1 |
| 2008 | 49 476 645 | 497 | 585 | 95 | 98 | 72 | 1.2 |
| 2009 | 49 138 121 | 491 | 547 | 92 | 98 | 77 | 1.1 |
| 2010 | 45 932 295 | 459 | 504 | 94 | 97 | 78 | 1.1 |
| 2011 | 45 932 295 | 459 | 561 | 96 | 98 | 76 | 1.2 |
| 2012 | 46 740 845 | 467 | 502 | 96 | 98 | 74 | 1.1 |
Abbreviation: AFP, acute flaccid paralysis.
Collection of 2 stool specimens 24 hours apart within 14 days of onset of paralysis.
Reported AFP cases per 100 000 persons <15 years.
Polio Vaccination Strategies
From 1980 to 2011, Brazil held biannual NIDs (usually in June and August) for all children under 5 years of age, regardless of prior immunization status. With the introduction of the sequential IPV-OPV schedule, the National Immunization Program maintained 1 annual NID (in June) with OPV, targeting children aged 6–59 months, regardless of prior immunization status. The previous NID in August was replaced with a multivaccination campaign to provide children up to their fifth birthday with missing vaccinations and to update child health cards.
The decision to replace 1 NID day with a multivaccination campaign was based on potential benefits of social mobilization to improve routine immunization coverage and complete vaccination schedules. In the 1980s, Brazil’s National Immunization Program encouraged the use of NIDs to provide opportunities for “catch-up” vaccination of children missing recommended doses, as long as multivaccination did not have a negative impact on vaccination against poliomyelitis [26]. The decision regarding which antigens to offer during NIDs was left up to state and municipal immunization programs. An immunization survey of children born in 2005 showed that 15% had received recommended vaccines needed to complete immunization schedules during the most recent NID [26].
Revision of Recommended Childhood Immunization Schedule
IPV introduction was part of a revision of the childhood immunization calendar in 2012 (Table 4), including the sequential IPV-OPV schedule and 3 doses of pentavalent DTwP–Haemophilus influenzae type b conjugate-recombinant hepatitisvaccine (pentavalent vaccine, Bio-Manguinhos Institute, Rio de Janeiro, Brazil and Butantan Institute, São Paulo, Brazil). Pentavalent vaccine replaced quadrivalent DTwP-Hib vaccine and eliminated the need for 2 injections of monovalent hepatitisvaccine to complete the primary hepatitisschedule (previously recommended at birth, 1 month, and 6 months of age). The birth dose of monovalent hepatitisvaccine was maintained for the prevention of vertical transmission. Launching the sequential IPV-OPV schedule with pentavalent vaccine introduction (replacing separate injections of hepatitis and DTwP-Hib vaccines) resulted in the same number of injections a child would receive to complete the recommended immunization schedule.
Table 4.
Childhood Immunizations Included in Brazil’s National Immunization Program, August 2012
| Vaccine | Age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth | 2 m | 3 m | 4 m | 5 m | 6 m | 9 m | 12 m | 15 m | 4 y | |
| BCG | 1 dose | |||||||||
| HepB | 1 dose | |||||||||
| Polio (sequential schedule) | 1st dose (IPV) | 2nd dose (IPV) | 3rd dose (OPV) | 1st booster (OPV) | ||||||
| Pentavalenta | 1st dose | 2nd dose | 3rd dose | |||||||
| Pneumococcal 10-valent conjugate | 1st dose | 2nd dose | 3rd dose | Booster | ||||||
| Rotavirus (2-dose schedule) | 1st dose | 2nd dose | ||||||||
| MenC conjugate | 1st dose | 2nd dose | ||||||||
| Yellow fever | 1 dose | |||||||||
| MMR | 1st dose | 2nd dose | ||||||||
| DTwP | 1st booster | 2nd booster | ||||||||
Abbreviations: BCG, Bacillus Calmette–Guérin; DTwP, diphtheria-tetanus-pertussis (whole cell); HepB, hepatitis B; MenC, meningococcal serogroup C; MMR, measles-mumps-rubella; OPV, oral polio vaccine
DTwP (whole cell), Haemophilus influenzae type b, HepB.
An interval of 60 days was recommended between the first and second IPV doses, as well as between the second IPV dose and the first OPV dose in the sequential series. During the first 6 months of life, a minimum interval between doses of 30 days was recommended for infants traveling to endemic countries or at risk of exposure to WPV. Additional guidance was provided for vaccination of children who had received OPV or for whom OPV was not recommended (Table 5).
Table 5.
Polio Immunization Schedule for Children Who Have Already Received 1 OPV Dose and for Children for Whom OPV Is Not Recommended, National Immunization Program, Brazil, 2012
| Child’s Age and Prior Vaccination Status | Polio Vaccination | Note |
|---|---|---|
| At least 2-months of age, received ≥1 dose of OPV | Complete series with OPV | Children who begin schedule with OPV may continue with OPV only |
| At least 2 mo of age, received 1 dose of OPV between birth and 60 d of life | Begin sequential IPV-OPV series | OPV doses administered from birth to 60 d of life are not counted for the primary series |
| Age <12 mo, previously unvaccinated against polio | Begin sequential IPV-OPV series | Previously unvaccinated children ≥12 mo may initiate polio vaccination series with OPV |
| Child with medical indication to receive IPV at CRIEa | IPV only series | Sequential IPV-OPV schedule not recommended |
Source: National Immunization Program, Ministry of Health, Brasília, Brazil.
Abbreviations: IPV, inactivated polio vaccine; OPV, oral polio vaccine; CRIE, Special Immunobiological Reference Center.
Acronym for name in Portuguese: Centro de Referência para Imunobiologicos Especiais.
Equity
The additional cost of IPV was compared with introduction of new vaccines and increases in the National Immunization Program budget [20]. Equity was an important consideration, as IPV became recommended by professional societies [27], while children of higher socioeconomic status were more likely to receive IPV in the private sector [19] and less likely to receive OPV during NIDs [26]. In the public sector, IPV had been recommended for specific groups of children for whom OPV was contraindicated and was provided at specialized vaccine reference centers (CRIE, for the acronym in Portuguese) since 1993 [28]. Increased referral of children to specialized reference centers for IPV resulted in a jump in IPV doses administered from 20 145 in 2008 to 37 305 in 2010.
Sustainability
In 2010, the Science and Technology Secretariat of the Ministry of Health, together with public vaccine manufacturer Bio-Manguinhos Institute, Rio de Janeiro, developed technical guidelines for incorporating standalone IPV or IPV-containing combination vaccines into the National Immunization Program, considering options of international purchase, national production, or acquisition of technology for national production. These technical guidelines estimated costs of IPV introduction, as well as mapping strategies to achieve sustainable IPV use in Brazil.
To comply with national legislation requiring self-sustainability in vaccine production as well as international regulations on IPV manufacture, Brazil’s Ministry of Health and Bio-Manguinhos Institute signed an agreement with Sanofi Pasteur to supply IPV types 1, 2, and 3 for formulation and distribution in Brazil after 2012. Bio-Manguinhos Institute would also begin evaluating combination products, including imported IPV and domestically produced DTwP, hepatitis(HepB), and H. influenzae type b (Hib) antigens for future use in Brazil.
EARLY UPTAKE OF IPV
Beginning with the national multivaccination campaign in August 2012, IPV was administered to children at 2 months of age (60 days), initiating the primary immunization series with pentavalent DTwP-Hib-HepB, pneumococcal conjugate vaccine, and oral rotavirus vaccine. Vaccination histories of children aged <5 years documented in child health cards were evaluated by health workers at fixed vaccination posts in health facilities or mobile posts that functioned during the campaign. Vaccination series were initiated in previously unvaccinated children and those without documented vaccination history. Immunizations offered included all vaccines in the recommended childhood vaccination calendar of the National Immunization Program. Vaccination was selective, based on evaluation of each child’s vaccination history. IPV and pentavalent DTwP-Hib-HepB vaccines were administered according to the revised childhood vaccination schedule.
The 2012 national multivaccination campaign was organized along the same principles as an NID to expand access to vaccines by providing immunizations at a large number of fixed and mobile vaccine posts, as well as through outreach. The multivaccination campaign was conducted over a 7-day period from 18 to 24 August and involved approximately 350 000 health workers at 115 000 vaccination posts (including approximately 30 000 permanent vaccination posts at health facilities) and 40 000 vehicles. Federal funding for the campaign was the same as for the national polio immunization day (18.6 million reais [US $9.3 million]) in addition to contributions from state and municipal health departments.
Administrative Estimates of IPV Coverage
Brazil has an annual birth cohort of approximately 2.8 million surviving infants. In 2012, the monthly vaccination target (based on registered live births in 2011) was 240 006 children aged <1 year for each vaccine dose recommended in the first year of life. During the initial multivaccination campaign from 18 to 24 August 2012, a total of 114 803 IPV doses were administered to children aged <1 year (Table 6); 102 784 were registered as first doses and 12 019 as second doses for children who received IPV in the private sector or had medical indications for receipt of IPV at the Ministry of Health’s CRIE. In routine immunization services, a total of 770 942 first IPV doses were administered from September to December 2012, reaching 80% of the quarterly target of 960 024 doses among children aged <1 year nationally and >95% of the quarterly target in 2658 (48%) of 5564 municipalities (Figure 1B), while 319 579 second doses were administered in November and December, reaching 67% of the target population (Table 6).
Table 6.
Number of First and Second Doses of IPV Administered in Brazil’s National Immunization Program, as a Percentage of Monthly Target Population,a Brazil, August–December, 2012
| Calendar Month | No. of IPV Doses Reported (% of Target) | |
|---|---|---|
| 1st dose | 2nd dose | |
| August | 102 784 (43) | 12 019 (5) |
| September | 173 554 (72) | 25 955 (11) |
| October | 205 153 (85) | 89 761 (37) |
| November | 204 333 (85) | 159 099 (66) |
| December | 187 902 (78) | 160 480 (67) |
Abbreviation: IPV, inactivated polio vaccine.
Monthly target population = 240 006 children aged <1 year (1/12th of registered live births in 2011 from national live birth registration system [SINASC].).
Vaccine Supply
Standalone IPV was included in the children’s immunization calendar in 2012. A total of 11 million doses of standalone IPV in 10-dose vials were purchased in 2012 at a cost of 55 million reais (approximately US $26 million in 2012). Although the 10-dose presentation of IPV includes 2-phenoxyethanol as a preservative, vials were to be discarded 6 hours after opening. IPV vaccination occurred simultaneously in all states and the federal district.
During the first months of IPV use, immunization programs reported substantially increased vaccine wastage due to requirements to discard opened vials after 6 hours. Unexpectedly high wastage resulted in IPV stock-outs in some health centers, requiring constant management and redistribution of available vaccine to avoid running out of IPV over larger areas. In cases of IPV stock-outs at health centers, the National Immunization Program advised rescheduling children to maintain sequential IPV-OPV schedules rather than returning to an OPV-only schedule. In November 2012, Brazil’s national regulatory authority approved a label change, permitting the use of 10-dose vials for 7 days after opening. The label change reduced IPV wastage and resolved problems with vaccine supply.
Surveillance for Adverse Events Following Immunization
IPV is well tolerated and has not been associated with severe adverse events [29]. Based on reported rates of adverse events following immunization (AEFI), mild, local reactions were expected in a small proportion of vaccinees, including erythema at the injection site (<3%), induration (<12%), and tenderness (<30%). Reporting of systemic reactions (such as fever) and other AEFIs associated with any of the vaccines coadministered with IPV, including DTwP-Hib-HepB, oral rotavirus, and pneumococcal conjugate vaccine, was expected in infants who had received IPV. Immunization providers were also alerted to the possibility of hypersensitivity reactions in infants due to the presence in the IPV formulation of trace amounts of the antibiotics streptomycin, neomycin, and polymyxin B. Following IPV introduction, no increase was observed in rates of reported AEFIs, including fever, convulsions, and hypotonic-hyporesponsive episodes (data not shown). A single AEFI (classified as a moderate local reaction) was reported in an infant who received IPV with no concomitant injections.
Communications Strategies
Communication strategies for the multivaccination campaign targeted 2 main audiences: information for healthcare workers, professional societies, and opinion leaders provided rationale for the new vaccination schedule; while public messages encouraged parents to take children younger than 5 years of age to an immunization post during the campaign to review the child’s vaccinations, even if the child was considered “up to date.” Messages emphasized prevention of vaccine-preventable diseases, as well as the introduction of 2 new vaccines (pentavalent vaccine and IPV). Prior to IPV introduction, state and municipal immunization programs conducted trainings for healthcare professionals on the sequential IPV-OPV schedule; high acceptance of IPV, and the revised immunization calendar was reported. As in other vaccination campaigns, the Brazilian Minister of Health held a press conference prior to the campaign launch to explain the objectives of the multivaccination campaign to the media, reaching a broad audience. Social networks and electronic media were also used as in previous campaigns to provide information on vaccination activities [30].
DISCUSSION
Brazil is one of a growing number of countries that have introduced IPV in national immunization programs [15, 31]. As of December 2012, 66 WHO member states included IPV in national immunization programs, including 7 in the Americas (Bahamas, Brazil, Canada, Costa Rica, Mexico, United States, and Uruguay) [32]. In an additional 18 member states (15 of which are in the Americas), IPV was recommended for children at increased risk of VAPP, including immunocompromised children [32]. IPV use is expected to increase as countries implement WHO recommendations for the polio endgame strategy [33].
With the introduction of a sequential IPV-OPV polio vaccination schedule, Brazil’s National Immunization Program initiated plans for IPV use following worldwide eradication of polio, when only IPV use will be recommended [34]. Introduction of IPV in Brazil’s National Immunization Program also meets updated recommendations from WHO’s Strategic Advisory Group of Experts in November 2012 that all countries introduce at least 1 dose of IPV in routine infant immunization schedules prior to withdrawal of type-2 OPV virus from trivalent OPV to mitigate risks of poliomyelitis associated with type-2 VDPVs [35].
Several considerations were important for the choice of a sequential IPV-OPV schedule in Brazil’s National Immunization Program. Despite limited experience with sequential IPV-OPV schedules for routine infant immunizations in Latin America [15], experiences from the United States and countries in other regions have demonstrated success in maintaining elimination of wild poliovirus and preventing VAPP [29]. Several other Latin American countries, including Costa Rica and Mexico, have maintained national polio immunization days with OPV following an IPV-only routine infant immunization schedule [29], as recommended by PAHO [2]. In Brazil, the decision to maintain 1 NID with OPV was based on reducing risk of poliovirus transmission in the event of a WPV or VDPV importation and maintaining annual outreach activities to difficult-to-access populations during campaigns. Financial sustainability of IPV introduction was also considered in the context of increased government commitment to the national immunization program with the introduction of new childhood vaccines in the past decade, including oral rotavirus vaccine, and pneumococcal and meningococcal serogroup C conjugate vaccines [20]. Finally, IPV introduction in Brazil contributes to equitable access to recommended vaccines among all Brazilian children, in accordance with the founding principles of Brazil’s national immunization program [3, 19].
Interpretation of immunization coverage and AFP surveillance data from Brazil is subject to several limitations. Declining reporting rates of VAPP since certification of polio elimination likely reflect underreporting of AFP cases [22, 23, 36]; incidence of VAPP in Brazil, while still rare, was believed to be higher than reported. Administrative coverage estimates are based on numbers of doses administered rather than children vaccinated, and municipal estimates of coverage are unreliable because doses are recorded by health center rather than place of residence. Brazil’s national immunization program has initiated a national immunization registry to provide more reliable immunization coverage data [20]. Data from immunization coverage surveys were only available for selected urban populations [19]. Concerns about heterogeneous polio immunization coverage and pockets of susceptible populations, especially in rural areas with conditions favorable to poliovirus transmission, motivated maintenance of 2 OPV doses in the routine polio immunization schedule and continuation of 1 annual polio immunization day for children aged <5 years.
With the international purchase of IPV, Brazil’s Ministry of Health launched an initiative with 3 national vaccine manufacturers—Bio-Manguinhos Institute of the Oswaldo Cruz Foundation in Rio de Janeiro, Butantan Institute in São Paulo, and Ezequiel Dias Foundation in Belo Horizonte—to produce a heptavalent vaccine for the national immunization program, containing diphtheria, tetanus, pertussis, Hib, HepB, IPV, and meningococcal serogroup C conjugate antigens. Availability of a nationally produced combination product containing IPV would reduce the number of vaccination visits and injections, and potentially reduce medical waste. In addition, production of combination vaccines in single-dose vials can reduce vaccine wastage without substantially increasing requirements for cold storage capacity. Brazil’s national immunization program continually works to expand access to safe and effective vaccines for all Brazilian children.
Acknowledgments
We would like to thank health professionals in immunizations, disease surveillance, and public health laboratories at all levels in the Brazilian health system, and the communications staff for their work over the past 29 years to maintain Brazil polio free; the work of these individuals demonstrates their commitment to public health and exemplifies the principles of the Brazilian Unified Health System. We would especially like to acknowledge the work of vaccination teams to reach children and achieve high vaccine coverage, both in routine immunizations and during campaigns throughout the country. The Brazilian National Technical Advisory Committee for Immunizations has provided essential support for the National Immunization Program, as well as important linkages with professional organizations and the Brazilian population. We thank Concepción Estívariz from the US Centers for Disease Control and Prevention (CDC) for background information and helpful suggestions for revision of the manuscript.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Supplement sponsorship. This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. Carla Domingues, Sirlene Pereira, Ana Carolina Marreiros, and Nair Menezes are employed by the Brazilian Ministry of Health, which purchases inactivated polio vaccine for use in the national immunization program. In Brazil, inactivated polio vaccine is purchased in bulk from Sanofi Pasteur and distributed by Bio-Manguinhos Institute, a public vaccine manufacturer funded by the Brazilian Ministry of Health. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.World Health Organization. Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Wkly Epidemiol Rec. 2010;85:213–28. [PubMed] [Google Scholar]
- 2.Pan American Health Organization. Final report of the XVIII Technical Advisory Group (TAG) meeting on vaccine-preventable diseases of the Pan American Health Organization. Washington, DC: Pan American Health Organization; 2011. Immunization: prioritizing vunerable populations; pp. 15–6. [Google Scholar]
- 3.Ministry of Health. Série C Projetos e Programas e Relatórios. Brasília, Brazil: Ministry of Health; 2003. Programa Nacional de Imunizações—30 anos. [Google Scholar]
- 4.Ministry of Health. Informe Técnico da Introdução da Vacina Inativada Poliomielite. Departamento de Vigilância Epidemiológica; [Accessed 1 October 2013]. http://portal.saude.gov.br/portal/arquivos/pdf/informe_introducao_vacina_inativada_polio_vip_2012.pdf. [Google Scholar]
- 5.Risi JB., Jr The control of poliomyelitis in Brazil. Rev Infect Dis. 1984;6 (suppl 2):S400–3. doi: 10.1093/clinids/6.supplement_2.s400. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento DR. As campanhas de vacinação contra a poliomielite no Brasil (1960–1990) Ciência e Saúde Coletiva. 2011;16:501–11. doi: 10.1590/s1413-81232011000200013. [DOI] [PubMed] [Google Scholar]
- 7.Olive JM, Risi JB, Jr, de Quadros CA. National immunization days: experience in Latin America. J Infect Dis. 1997;175(suppl 1):S189–93. doi: 10.1093/infdis/175.supplement_1.s189. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health. Série A Normas e Manuais Técnicos. Brasília, Brazil: Fundação Nacional de Saúde, Brazilian Ministry of Health; 1998. Programa Nacinal de Imunizações—25 anos. [Google Scholar]
- 9.Silva SJM. A vigilância da Poliomielite—Paralisias Flácidas Agudas. Revista Brasileira de Enfermagem. 2005;58:110–1. doi: 10.1590/s0034-71672005000100022. [DOI] [PubMed] [Google Scholar]
- 10.de Brito Bastos NC, Carvalho Filho ES, Schatzmayr H, Homma A, Chaves J. Antipoliomyelitis program in Brazil; a serologic study of immunity levels. Bull Pan Am Health Organ. 1974;8:54–65. [PubMed] [Google Scholar]
- 11.Patriarca PA, Laender F, Palmeira G, et al. Randomised trial of alternative formulations of oral poliovaccine in Brazil. Lancet. 1988;1:429–33. doi: 10.1016/s0140-6736(88)91229-9. [DOI] [PubMed] [Google Scholar]
- 12.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–39. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Collaborative Study Group on Oral Poliovirus Vaccine. Factors affecting the immunogenicity of oral poliovirus vaccine: a prospective evaluation in Brazil and the Gambia. J Infect Dis. 1995;171:1097–106. doi: 10.1093/infdis/171.5.1097. [DOI] [PubMed] [Google Scholar]
- 14.Schatzmayr HG, Maurice Y, Fujita M, de Fillipis AM. Serological evaluation of poliomyelitis oral and inactivated vaccines in an urban low-income population at Rio de Janeiro, Brazil. Vaccine. 1986;4:111–3. doi: 10.1016/0264-410x(86)90048-4. [DOI] [PubMed] [Google Scholar]
- 15.Salas-Peraza D, Avila-Aguero ML, Morice-Trejos A. Switching from OPV to IPV: are we behind the schedule in Latin America? Expert Rev Vaccines. 2010;9:475–83. doi: 10.1586/erv.10.39. [DOI] [PubMed] [Google Scholar]
- 16.Stewardson AJ, Roberts JA, Beckett CL, et al. Imported case of poliomyelitis, Melbourne, Australia, 2007. Emerg Infect Dis. 2009;15:63–5. doi: 10.3201/eid1501.080791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Outbreaks following wild poliovirus importations—Europe, Africa, and Asia, January 2009–September 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1393–9. [PubMed] [Google Scholar]
- 18.Teixeira AM, Samad SA, Souza MA, Segatto TC, Morice A, Flannery B. Brazilian experience with rapid monitoring of vaccination coverage during a national rubella elimination campaign. Rev Panam Salud Publica. 2011;30:7–14. [PubMed] [Google Scholar]
- 19.Barata RB, Ribeiro MC, de Moraes JC, Flannery B Vaccine Coverage Survey G. Socioeconomic inequalities and vaccination coverage: results of an immunisation coverage survey in 27 Brazilian capitals, 2007–2008. J Epidemiol Community Health. 2012;66:934–41. doi: 10.1136/jech-2011-200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domingues CM, Teixeira AM, Carvalho SM. National immunization program: vaccination, compliance and pharmacovigilance. Rev Inst Med Trop Sao Paulo. 2012;54(suppl 18):S22–7. doi: 10.1590/s0036-46652012000700009. [DOI] [PubMed] [Google Scholar]
- 21.Paim J, Travassos C, Almeida C, Bahia L, Macinko J. The Brazilian health system: history, advances, and challenges. Lancet. 2011;377:1778–97. doi: 10.1016/S0140-6736(11)60054-8. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira LH, Struchiner CJ. Vaccine-associated paralytic poliomyelitis: a retrospective cohort study of acute flaccid paralyses in Brazil. Int J Epidemiol. 2000;29:757–63. doi: 10.1093/ije/29.4.757. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira-Rocha ES, Carmo EH, Tavares-Neto J. The occurrence of vaccine-associated paralytic poliomyelitis in Brazil, 1995 to 2001. Rev Panam Salud Publica. 2005;18:21–4. doi: 10.1590/s1020-49892005000600004. [DOI] [PubMed] [Google Scholar]
- 24.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO-recommended standards for surveillance of selected vaccine-preventable diseases WHO/V&B/0301. Geneva: WHO; 2008. Poliomyelitis; pp. 31–4. [Google Scholar]
- 26.Mello MLR, Moraes JC, Barbosa HA, Flannery B. Participation in national polio immunization days: results of a vaccine coverage survey among children in 27 Brazilian cities. Rev Bras Epidemiol. 2010;13:1–11. [Google Scholar]
- 27.Sociedade Brasileira de Pediatria. Novo Calendário de Vacinas da Sociedade Brasileira de Pediatria SBP Notícias. Vol. 57. Sociedade Brasileira de Pediatria; 2009. [Accessed 1 October 2013]. http://www.sbp.com.br/img/sbp_noticias/sbp57.pdf. [Google Scholar]
- 28.Ministry of Health. Normas e Manuais Técnicos. 3. Brasília, Brazil: Ministry of Health; 2006. Manual dos Centros de Referência para Imunobiológicos Especiais. Série A. [Google Scholar]
- 29.Plotkin SA, Vidor E. Poliovirus vaccine-inactivated. In: Plotkin SA, Orenstein WA, Offit P, editors. Vaccines. 5. Philadelphia, PA: Saunders Elsevier; 2008. pp. 605–29. [Google Scholar]
- 30.Vieira JC, Carvalho MT, Checchia RL, Trombiere M, Flannery B. Survey of rubella knowledge and acceptability of rubella vaccination among Brazilian adults prior to mass vaccination. Rev Panam Salud Publica. 2011;30:335–41. [PubMed] [Google Scholar]
- 31.Schoub BD. Introduction of inactivated polio vaccine (IPV) into the routine immunization schedule of South Africa. Vaccine. 2012;30 (suppl 3):C35–7. doi: 10.1016/j.vaccine.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. [Accessed 1 October 2013];Vaccine introduction [database online] http://www.who.int/immunization_monitoring/data/year_vaccine_introduction.xls.
- 33.Estivariz CF, Pallansch MA, Anand A, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr Opin Virol. 2013;3:309–15. doi: 10.1016/j.coviro.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inactivated poliovirus vaccine following oral poliovirus vaccine cessation. Wkly Epidemiol Rec. 2006;81:137–44. [PubMed] [Google Scholar]
- 35.Meeting of the Strategic Advisory Group of Experts on Immunization, November 2012—conclusions and recommendations. Wkly Epidemiol Rec. 2013;88:1–16. [PubMed] [Google Scholar]
- 36.Andrus JK, Strebel PM, de Quadros CA, Olive JM. Risk of vaccine-associated paralytic poliomyelitis in Latin America, 1989–91. Bull World Health Organ. 1995;73:33–40. [PMC free article] [PubMed] [Google Scholar]


