Abstract
Introduction
In March, 2006, oral rotavirus vaccine was added to Brazil’s infant immunization schedule with recommended upper age limits for initiating (by age 14 weeks) and completing (by age 24 weeks) the two-dose series to minimize age-specific risk of intussusception following rotavirus vaccination. Several years after introduction, estimated coverage with rotavirus vaccine (83%) was lower compared to coverage for other recommended childhood immunizations (≥94%).
Methods
We analyzed data from Brazil’s national immunization program on uptake of oral rotavirus vaccine by geographic region and compared administrative coverage estimates for first and second doses of oral rotavirus vaccine (Rota1 and Rota2) with first and second doses of diphtheria-tetanus-pertussis-Haemophilus influenzae type b vaccine (DTP-Hib1 and DTP-Hib2). For 27 Brazilian cities, we compared differences between estimated rotavirus and DTP-Hib coverage in 2010 with delayed receipt of DTP-Hib vaccine among a cohort of children surveyed before rotavirus introduction.
Results
In 2010, infant vaccination coverage was 99.0% for DTP-Hib1 versus 95.2% for Rota1 (3.8% difference), and 98.4% for DTP-Hib2 versus 83.0% for Rota2 (15.4% difference), with substantial regional variation. Differences between DTP-Hib and rotavirus vaccination coverage in Brazilian cities correlated with delay in DTP-Hib vaccination among children surveyed. Age restrictions for initiating and completing the rotavirus vaccination series likely contributed to lower coverage with rotavirus vaccine in Brazil.
Conclusion
To maximize benefits of rotavirus vaccination, strategies are needed to improve timeliness of routine immunizations; monitoring rotavirus vaccine uptake and intussusception risk is needed to guide further recommendations for rotavirus vaccination.
Keywords: Rotavirus, Rotavirus vaccine, Immunization coverage, Immunization timing
1. Introduction
Rotavirus is a leading cause of severe diarrhea in children, resulting in >400,000 deaths annually among children <5 years of age worldwide [1]. To prevent these deaths, the World Health Organization (WHO) has recommended inclusion of rotavirus vaccines in all national immunization programs [2]. Use of rotavirus vaccines could have a substantial impact on child mortality, especially in developing countries, which account for >95% of rotavirus-related deaths [1,3]. In 2006, WHO recommended a restricted age range for administration of the first dose of rotavirus vaccine and completion of the vaccine series to minimize risk of intussusception, a rare but severe adverse event [4]. These age restrictions might have resulted in lower coverage with rotavirus vaccines compared with other recommended vaccines, if immunization visits were delayed [5], as occurs in many countries [6]. Monitoring the uptake of oral rotavirus vaccine and timeliness of administration in early adopting countries may be useful as rotavirus vaccines are introduced worldwide.
Countries in Latin American and the Caribbean were among the first to introduce rotavirus vaccines [7,8]. WHO recommended introduction of rotavirus vaccine through national immunization programs in Latin America and the Caribbean in 2007 [9], and by 2010, rotavirus vaccines were introduced through national immunization programs in 14 of 38 countries and territories in the Americas [8]. Brazil is an upper-middle income country in South America with an annual birth cohort of approximately 3 million. The national immunization program is part of Brazil’s universal health system (Sistema Único de Saúde). Vaccines are centrally purchased and delivered through a network of immunization services linked to state and local health departments. Health indicators, including administrative estimates of vaccination coverage (i.e., number of doses administered divided by the estimated target population), are available for all 5,565 municipalities in Brazil, 27 states and 5 geographic regions (North, Northeast, Southeast, South and Central-west). The less developed North and North-east regions have historically had lower vaccination coverage and higher child mortality rates than the more developed South and Southeast regions [10].
Brazil’s national immunization program introduced the single-strain human rotavirus vaccine (Rotarix®, GlaxoSmithKline Biologicals) in the recommended infant immunization schedule in March 2006. The national immunization program set a target of 90% administrative coverage with two doses of rotavirus vaccine in all states and municipalities (the target coverage with 3 doses of combined DTP-Hib vaccine is 95%). Two doses of rotavirus vaccine were recommended at 2 and 4 months of age (approximately 8 and 17 weeks of age) with a minimum interval of 30 days between doses; initiation of the two-dose series was not recommended at age 15 weeks or older, and maximum age for completing the series was 24 weeks of age. Rotavirus vaccination was recommended at the same immunization visit as other childhood immunizations, including oral polio vaccine (OPV) and diphtheria-tetanus-whole cell pertussis-Haemophilus influenzae type b vaccine (DTP-Hib). We assessed uptake of oral rotavirus vaccine in Brazil and evaluated factors associated with lower coverage with rotavirus vaccine compared with other infant immunizations.
2. Methods
2.1. Uptake of oral rotavirus vaccine
To evaluate uptake of rotavirus vaccine by geographic region of Brazil, we compared monthly administrative coverage (i.e., number of doses administered per month divided by one-twelfth of the population <1 year of age) for first and second doses of oral rotavirus vaccine (Rota1 and Rota2) from March 2006 to December 2008. Numbers of doses administered were obtained from the national immunization program [11]. Estimates of the population <1 year of age were obtained from Brazil’s national live birth registration system [12]. We then compared annual administrative coverage (i.e. number of doses administered per calendar year divided by the population <1 year of age) for the first and second doses of DTP-Hib (DTP-Hib1 and DTP-Hib2) with the first and second doses of oral rotavirus vaccine. National and regional differences between DTP-Hib and rotavirus vaccine coverage were similar during 2008 to 2010, so only data for 2010 are presented.
2.2. Timeliness of DTP-Hib administration before introduction of rotavirus vaccine
To investigate delay in administration of routine immunizations before introduction of oral rotavirus vaccine, we analyzed data from a vaccination coverage survey conducted in the capital cities of Brazil’s 26 states and the federal district during 2007 and 2008 [13]. We hypothesized that the percentage of children surveyed who had received DTP-Hib1 after 14 weeks of age or DTP-Hib2 after 24 weeks of age would have predicted lower coverage with rotavirus vaccine compared with DTP-Hib, assuming adherence to recommendations and no improvement in timeliness of DTP-Hib vaccination. The survey was conducted among 19–36 month-old children who were born in 2005, few of whom were age-eligible to receive oral rotavirus vaccine through the national immunization program. Vaccination cards were available for >96% of children surveyed. We calculated the child’s age (in weeks) for each DTP-Hib dose received, according to the date recorded on the vaccination card. For all capital cities in each geographic region, we calculated the percent of children surveyed who received first or second doses of DTP-Hib vaccine after 14 or 24 weeks of age, respectively.
2.3. Correlation between rotavirus and DTP-Hib vaccine uptake
For 27 capital cities, we plotted the percent of children surveyed in 2007–2008 who had received DTP-Hib1 after 14 weeks of age against the difference between DTP-Hib1 and Rota1 administrative coverage among children <1 year old in 2010 (calculated as described in Section 2.1). Similarly, we plotted percent of children surveyed who had received DTP-Hib2 after 24 weeks of age against the difference between DTP-Hib2 and Rota2 coverage in 2010. We calculated Pearson correlation coefficients (r) and corresponding p-values for the comparison between delayed immunization and differences in estimated coverage. For survey data, regional averages for capital cities accounted for sampling weights and proportional population size.
2.4. Human subjects
The vaccination coverage survey was approved by the ethical review board of Santa Casa Medical School, in São Paulo, Brazil. Use of data from this survey for further analysis was approved by the national ethical review committee at the time of protocol approval. The analysis was considered non-research by the U.S. Centers for Disease Control and Prevention.
3. Results
3.1. Uptake of oral rotavirus vaccine
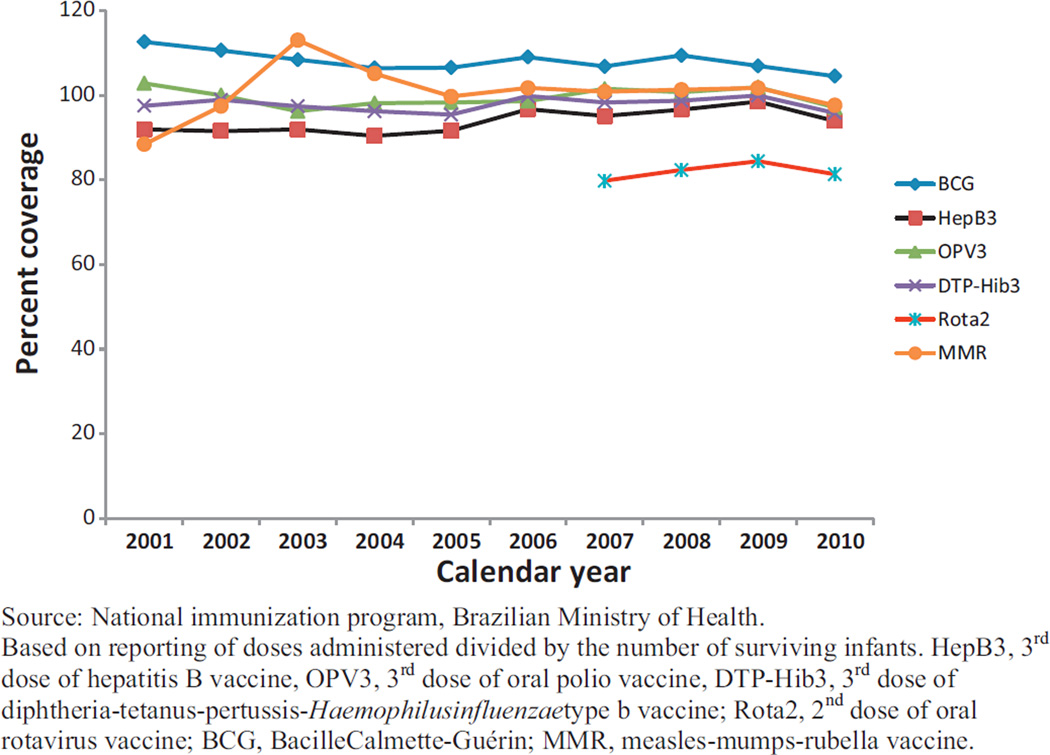
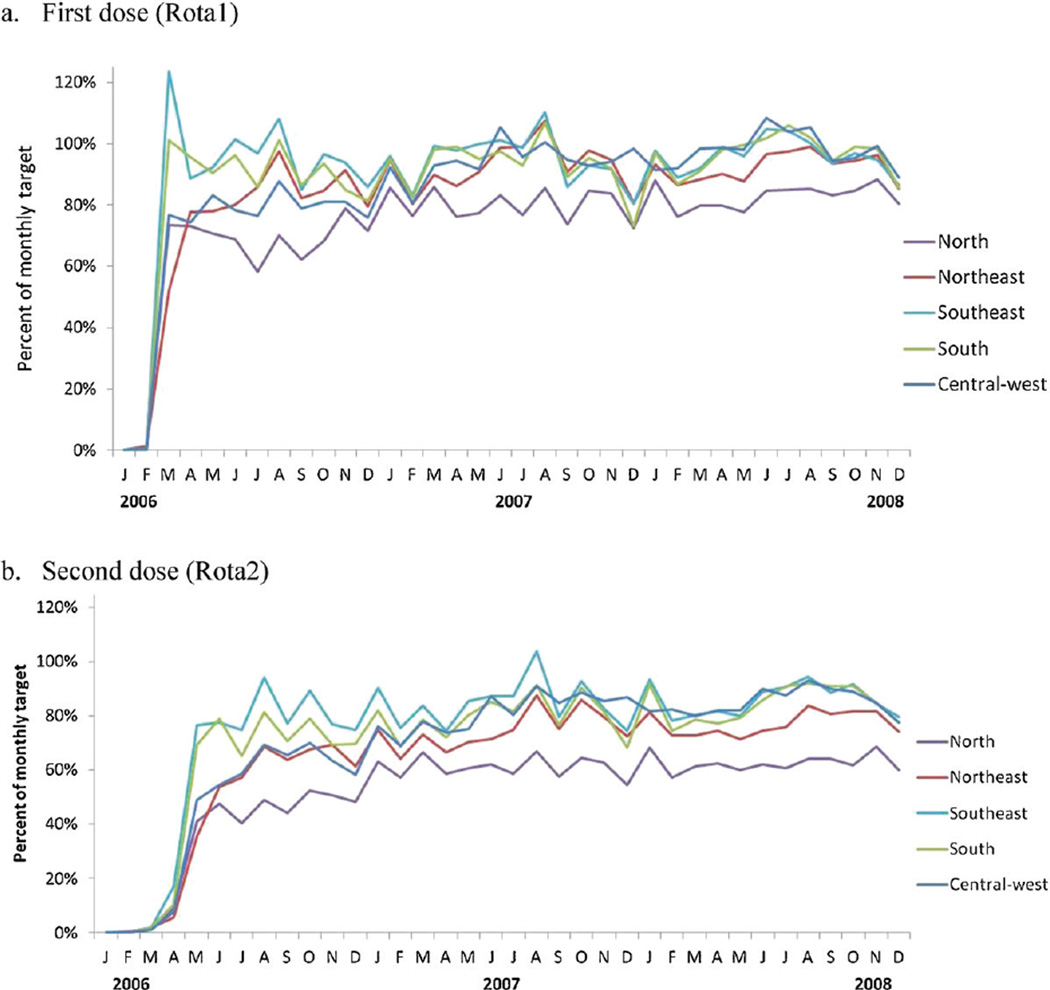
From 2007 to 2010, approximately 20.5 million doses of oral rotavirus vaccine were administered by Brazil’s national immunization program. Administrative coverage with the two-dose series of rotavirus vaccine was the lowest of the six recommended infant immunizations (Fig. 1), ranging from 80 to 84% during this time period. When rotavirus vaccine was introduced in March 2006, monthly administrative coverage with Rota1 exceeded 90% in two regions (South and Southeast) and ranged from 52 to 77% in the remaining three regions (North, Northeast, and Central-west) (Fig. 2a). Monthly administrative coverage increased substantially from March 2006 to December 2008 in the three regions (North, Northeast and Central-west) with lower initial coverage; no increasing trend in monthly coverage for Rota1 was observed in the South and Southeast regions. Through the end of 2008, uptake of the second dose remained 7–20% lower than that of the first dose, with the greatest difference in the North (Fig. 2b).
Fig. 1.
Estimated coverage with recommended immunizations among infants younger than one year of age, Brazil, 2001–2010.
Fig. 2.
Monthly administrative coverage (number of doses administered divided by monthly target population) of oral rotavirus vaccine by geographic region, Brazil, 2006–2008. (a) First dose (Rota1) and (b) Second dose (Rota 2).
In 2010, national administrative coverage with Rota1 was 3.8% lower than DTP-Hib1, while coverage with Rota2 was 15.4% lower than DTP-Hib2 (Table 1). Regionally, Rota1 coverage was lower than DTP-Hib1 coverage in the North, Northeast and Central-west, but slightly higher than DTP-Hib1 coverage in the South and Southeast. Coverage with Rota2 was 31.7% lower than DTP-Hib2 in the North, 21.7% lower in the Northeast and 8.8–10.9% lower in the remaining three regions (Table 1).
Table 1.
Birth cohort and administrative estimatesa of vaccine coverage among infants <12 months of age by geographic region of Brazil, 2010.
| Region | Population <1 year, 2010 | DTP-Hib coverage (%) | Oral rotavirus vaccine coverage (%) |
Difference between DTP-Hib and rotavirus vaccine coverage |
|||
|---|---|---|---|---|---|---|---|
| Dose1 | Dose2 | Dose1 | Dose2 | Δ Dose1b | Δ Dose2c | ||
| North | 309,789 | 103.3 | 99.9 | 85.4 | 68.2 | +17.9 | +31.7 |
| Northeast | 1,005,387 | 100.5 | 100.0 | 93.5 | 78.3 | +7.0 | +21.7 |
| Southeast | 1,119,725 | 96.9 | 97.0 | 97.7 | 88.2 | −0.8 | +8.8 |
| South | 346,555 | 97.7 | 97.5 | 98.3 | 88.6 | −0.6 | +8.9 |
| Central-west | 232,233 | 99.3 | 97.9 | 97.4 | 87.0 | +1.9 | +10.9 |
| All regions | 3,013,689 | 99.0 | 98.4 | 95.2 | 83.0 | +3.8 | +15.4 |
Vaccination coverage estimates based on number of doses administered divided by the population <1 year of age. Source: National Immunization Program, Ministry of Health, Brasilia, Brazil. Data available at http://pni.datasus.gov.br.
Difference between estimated coverage with first dose of DTP-Hib and oral rotavirus vaccine.
Difference between estimated coverage with second dose of DTP-Hib and oral rotavirus vaccine.
3.2. Timeliness of DTP-Hib administration before introduction of rotavirus vaccination
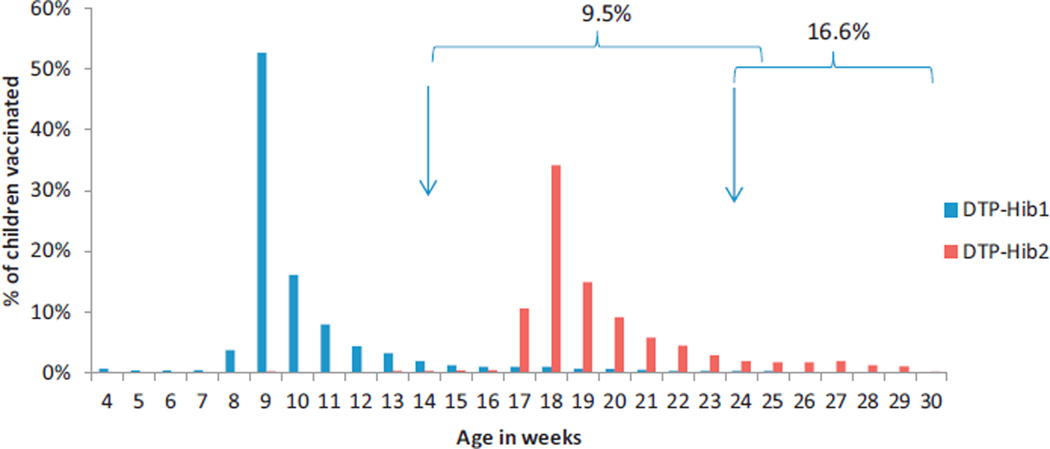
Data on age at receipt of DTP-Hib doses were analyzed for 15,426 (86.9%) of 17,749 children born in 2005 and surveyed in 2007–2008; 9.5% received DTP-Hib1 after 14 weeks of age and 16.6% received DTP-Hib2 after 24 weeks of age (Table 2; Fig. 3).
Table 2.
Percentage of children who received first dose of combined DTP-Hib vaccine (DTP-Hib1) after 14 weeks of age or second dose (DTP-Hib2) after 24 weeks: results of a household survey of vaccination coverage prior to introduction of oral rotavirus vaccine.
| Region | Children who received DTP-Hib1 (N) |
DTP-Hib1 doses received >14 weeks of age (%) |
Children who received DTP-Hib2 (N) |
DTP-Hib2 doses received >24 weeks of age (%) |
|---|---|---|---|---|
| All capital cities | 15,426 | 9.5 | 15,235 | 16.6 |
| North | 3252 | 12.0 | 3193 | 19.7 |
| Northeast | 5521 | 11.8 | 5440 | 19.9 |
| Southeast | 2676 | 6.7 | 2648 | 12.6 |
| South | 2178 | 5.4 | 2171 | 10.7 |
| Central-west | 1799 | 7.8 | 1783 | 13.9 |
Data were obtained from an immunization coverage survey among children born in 2005 in 27 Brazilian cities. Data are presented according to region of Brazil in which cities are located. Age at vaccination was calculated from data recorded on child’s vaccination card.
Fig. 3.
Timeliness of diphtheria-tetanus-pertussis-Haemophilus influenzae type b (DTP-Hib) immunization before introduction of oral rotavirus vaccine: percentage of children vaccinated by week of age according to household survey conducted in 27 capital cities. Arrows indicate 14 and 24 weeks of age. Braces indicate percentage of children who received DTP-Hib1 after 14 weeks of age or DTP-Hib2 after 24 weeks of age.
3.3. Correlation between rotavirus and DTP-Hib vaccine uptake
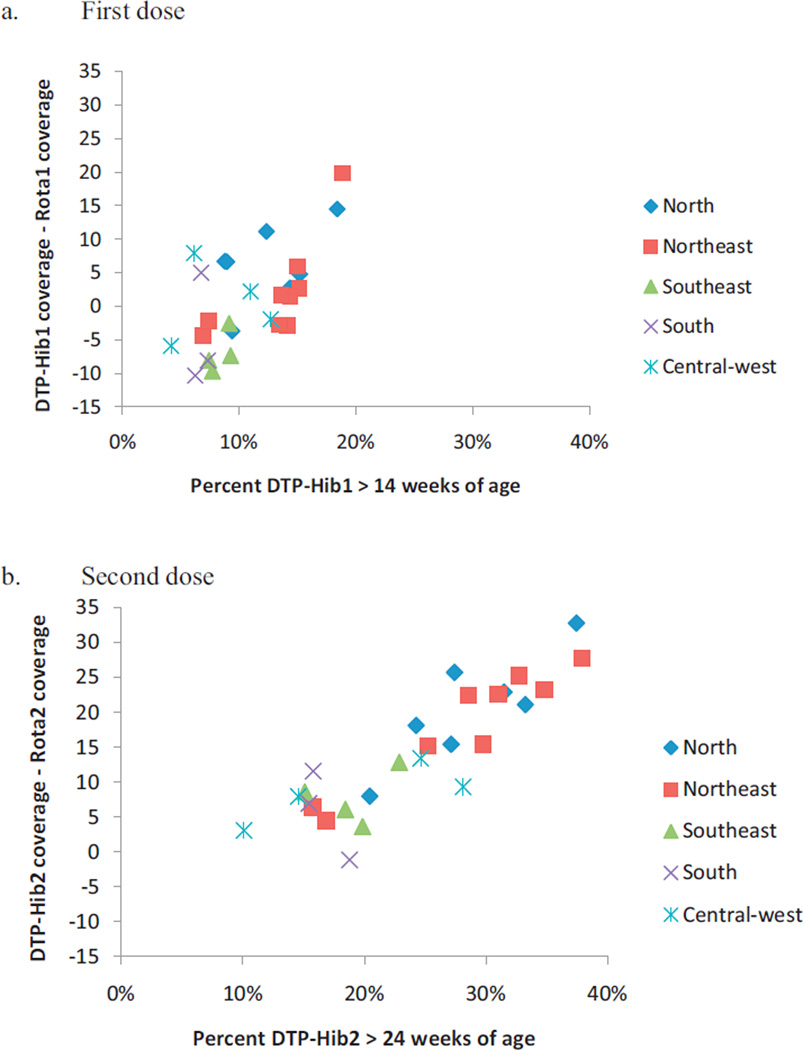
The 2007–2008 survey data were plotted against administrative coverage estimates for DTP-Hib and oral rotavirus vaccine coverage in 2010 for 27 Brazilian cities (Fig. 4). Differences between administrative estimates of DTP-Hib and rotavirus vaccine coverage in 2010 correlated with the percentage of children surveyed in 2007–2008 who had received DTP-Hib1 after 14 weeks of age (r = 0.58, p = 0.002) or DTP-Hib2 after 24 weeks of age (r = 0.88, p < 0.001). When administrative coverage data from cities in each region were aggregated (Table 2), regional differences between DTP-Hib and rotavirus vaccine coverage in 2010 were highly correlated with the percentage of children surveyed in each region who had received DTP-Hib1 after 14 weeks of age (r = 0.82, p = 0.09) or DTP-Hib2 after 24 weeks of age (r = 0.90, p = 0.04).
Fig. 4.
Comparison of administrative vaccine coverage* for 27 Brazilian cities (according to geographic region) in 2010 with timeliness of diphtheria-tetanus-pertussis-Haemophilus influenzae type b (DTP-Hib) vaccination before introduction of oral rotavirus vaccine, from an immunization survey conducted among 19–36 month old children during 2007–2008 (each point represents one city, symbols indicate cities in the same region). a) First dose: percent of children surveyed in 2007–2008 who had received 1st dose of DTP-Hib after 14 weeks of age versus the difference between administrative coverage of 1st dose of DTP-Hib and oral rotavirus vaccine among children <1 year old in 2010; b) Second dose: percent of children surveyed in 2007–2008 who had received 2nd dose of DTP-Hib after 24 weeks of age versus the difference between administrative coverage of 2nd dose of DTP-Hib and oral rotavirus vaccine in 2010. *Administrative coverage refers to the number of doses administered per calendar year divided by the population <1 year of age for each dose of vaccine. DTP-Hib1, diphtheria-tetanus-pertussis-Haemophilus influenzae type b vaccine 1st dose; DTP-Hib2, diphtheria-tetanus-pertussis-Haemophilus influenzae type b vaccine 2nd dose; Rota1, oral rotavirus vaccine 1st dose; Rota2, oral rotavirus vaccine 2nd dose.
4. Comment
Four years after rotavirus vaccine introduction, coverage with two doses of vaccine remained below that of other routine infant immunizations in Brazil. The difference between rotavirus and DTP-Hib coverage varied by geographic region and was greatest in the North and Northeast, regions that have lower routine immunization coverage, difficult-to-reach populations and historically higher diarrhea-related mortality [13,14]. The magnitude of this difference was correlated with delay in DTP-Hib administration among a cohort of children born before rotavirus vaccine introduction and enrolled in a coverage survey [13]. This suggests that delay in routine immunization visits likely contributed to lower rotavirus vaccine coverage, as children who are late for immunizations may have missed opportunities to begin or complete the rotavirus vaccination series. Further, although rotavirus vaccine may be administered at the same immunization visit as DTP-Hib, administrative estimates of rotavirus vaccine coverage were consistently lower than for DTP-Hib for several years following rotavirus vaccine introduction.
Overall strength of the immunization program was associated with uptake of oral rotavirus vaccine; uptake was highest in the South and Southeast regions of Brazil where routine immunization rates were also high [13]. Coverage with newly introduced vaccines may lag behind those of established vaccines for a number of reasons, including insufficient healthcare worker education and information, supply interruptions, inadequate cold chain capacity and missed immunization opportunities. The oral formulation of rotavirus vaccine and the two-dose vaccination schedule should have facilitated simultaneous administration with DTP-Hib vaccine. However, state immunization programs reported that healthcare workers, particularly those in the North and Northeast regions, found recommendations regarding the upper age limits for rotavirus vaccination confusing, which may have led to reluctance to administer the vaccine (National Immunization Program, unpublished data). Limited capacity to store vaccines at health facilities may also have contributed to delayed uptake, but was rapidly resolved by increasing local cold chain capacity to accommodate the large volume required for oral rotavirus vaccines. Further, no major stock-outs of oral rotavirus vaccine were reported to Brazil’s national immunization program from any region. Persistent differences between DTP-Hib and oral rotavirus vaccine coverage after several years suggest that a substantial number of children did not receive rotavirus vaccine due to delayed presentation for vaccination rather than slow scale-up of rotavirus vaccination. The difference between DTP-Hib1 and Rota1 suggests that almost 125,000 infants (4.2% of a birth cohort of approximately 3 million) failed to receive the first dose of rotavirus vaccine despite having access to immunization services. Delayed immunization visits are the most likely explanation for the gap between rotavirus vaccine coverage and coverage of other routinely recommended immunizations.
Although Brazil has not reached coverage targets with oral rotavirus vaccine, dramatic declines have been observed in diarrhea hospitalizations and deaths following rotavirus vaccination, including in the North and Northeast regions with the highest diarrhea-related mortality [14]. Whether improved timeliness of rotavirus vaccination or increased coverage would further decrease rotavirus-associated morbidity and mortality in Brazil is not known. Potential benefits of increased timeliness of routine immunization on prevention of rotavirus diarrhea would depend on the age distribution of rotavirus infections [5], as well as vaccine effectiveness against severe diarrhea due to circulating rotavirus strains after vaccine introduction [15–17].
The age-specific recommendation for administration of rotavirus vaccines was based on a previously licensed rotavirus vaccine (RotaShield®) that was withdrawn from the market after post-licensure studies showed an increased risk of intussusception following vaccination, especially following the first dose [18]. Because of catch-up vaccination with RotaShield® and increased background rates of intussusception in older infants, children older than 12 weeks of age accounted for >80% of intussusception cases following receipt of this vaccine [5]. The maximum age of 14 weeks for the first dose of rotavirus vaccine in Brazil was based on age limits used in clinical trials for Rotarix®, which showed no increased risk of intussusception among vaccinated children [19]. Post-licensure surveillance in several countries has documented an increased risk of intussusception following rotavirus vaccination [4], including a five-fold increased risk within one week of the first dose of rotavirus vaccine in Mexico and a two-fold increased risk within one week of the second dose in Brazil [15]. While the excess risk of intussusception following Rotarix® (1 case per 50,000–70,000 children vaccinated) [20] has been much less than the risk of intussusception following receipt of RotaShield® vaccine (1 case per 4500–9500 children vaccinated) [18], no data are available to assess intussusception risk when the first dose of the newer vaccines is administered after 15 weeks of age.
When WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) first recommended introduction of Rotarix® into national immunization programs in the Americas, administration of the second dose was not indicated beyond 24 weeks of age, the upper age limit used in clinical trials [9]. In 2009, SAGE revised its recommendation and advised completing the rotavirus immunization series by 32 weeks of age, whether using a two-dose or three-dose schedule [4]. In April, 2012, SAGE again revised its recommendations to support removal of upper age limits for administration of each dose of rotavirus vaccine [20]. This decision was based on evidence suggesting that additional deaths could be averted by increasing rotavirus vaccination rates with a small increase in intussusception cases [20,21]. As delays in the timing of vaccination are common in many countries with high childhood diarrhea mortality rates, relaxing age restrictions along with efforts to improve timeliness will help to ensure that vaccine is reaching those children at highest risk of dying from rotavirus diarrhea [5,21].
In the Americas, the Pan American Health Organization’s Technical Advisory Group on Vaccine-preventable Diseases recommended adherence to national immunization schedules to maximize benefits of early vaccination, while recognizing the potential benefit of rotavirus vaccination up to one year of age in settings of difficult access or high diarrhea mortality [22]. In Brazil, the National Immunization Technical Advisory Committee has recommended maintaining upper age limits for rotavirus vaccination, while extending the upper age limit to complete the two-dose rotavirus series to 8 months [23]. Public health officials in Brazil anticipated that maintaining the age limits would encourage immunization program staff to improve timeliness of routine immunizations. Changing the maximum age for administration of the second dose of rotavirus vaccine to 8 months will likely increase two-dose rotavirus vaccination coverage in Brazil. Experience with rotavirus vaccination in countries with different immunization schedules and in countries that relax age restrictions for vaccination may help to evaluate potential risks and benefits of extending upper age limits for receipt of rotavirus vaccine.
This analysis has several limitations. We used administrative data to assess uptake of rotavirus vaccine. Because imprecise population estimates can result in under-or over-estimations of coverage, we compared oral rotavirus vaccine to DTP-Hib coverage in the same population of children under 1 year. However, administrative data are also subject to numerator errors including recording second doses as first doses or unused doses as administered, which may account for greater numbers of first rotavirus doses than DTP-Hib in some regions. In addition, our analysis of timeliness of routine immunizations was limited to the period before rotavirus vaccination, and data on actual timeliness of rotavirus vaccination were not available. The vaccination coverage survey was limited to children residing in 27 capital cities; while these cities represent approximately 20% of the Brazilian population, timeliness of routine immunizations likely differs in rural areas or in areas with limited access to health services, especially in the North and North-east regions. Correlations between regional differences in rotavirus coverage and timeliness of DTP-Hib vaccination were only representative of the cities surveyed.
In conclusion, delayed routine immunizations may have resulted in suboptimal coverage with rotavirus vaccines among children who presented for routine immunizations after reaching the previously recommended upper age limits for beginning or completing the rotavirus vaccine series. Timeliness of immunization needs to be improved to ensure that young infants are protected from rotavirus infection [24], while new recommendations that relax upper age limits should encourage strategies to reach children at highest risk of rotavirus-related morbidity and mortality. Monitoring of rotavirus vaccine safety as well as coverage is needed to inform strategies to improve timeliness of immunization.
Acknowledgments
We would like to thank the immunization program staff in Brazil at municipal, state and national levels for the work that made these analyses possible, as well as those who participated in the immunization coverage survey in Brazilian capital cities during 2007–2008. We would also like to thank Ana Isakov for assistance with the manuscript.
Role of the funding source
The vaccination coverage survey was wholly financed by the Brazilian Ministry of Health.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention (CDC).
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012 Feb;12(2):136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Rotavirus vaccines: an update. Wkly Epi-demiol Rec. 2009 Dec;84(50):533–540. [PubMed] [Google Scholar]
- 3.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010 Jan;362(4):299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global Advisory Committee on Vaccine Safety, report of meeting held 17–18 June 2009. Wkly Epidemiol Rec. 2009 Aug;84(32):325–332. [PubMed] [Google Scholar]
- 5.Patel MM, Clark AD, Glass RI, Greenberg H, Tate J, Santosham M, et al. Broadening the age restriction for initiating rotavirus vaccination in regions with high rotavirus mortality: benefits of mortality reduction versus risk of fatal intussusception. Vaccine. 2009 May;27(22):2916–2922. doi: 10.1016/j.vaccine.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009 May;373(9674):1543–1549. doi: 10.1016/S0140-6736(09)60317-2. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Progress introducing rotavirus vaccine into Latin America and the Caribbean, 2006–2010. Wkly Epidemiol Rec. 2011 Nov;86(48):549–554. [PubMed] [Google Scholar]
- 8.de Oliveira LH, Danovaro-Holliday MC, Sanwogou NJ, Ruiz-Matus C, Tambini G, Andrus JK. Progress in the introduction of the rotavirus vaccine in Latin America and the Caribbean: four years of accumulated experience. Peds Infect Dis J. 2011;30(1 Suppl):S61–S66. doi: 10.1097/INF.0b013e3181fefdd6. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Rotavirus vaccines: WHO position paper. Wkly Epidemiol Rec. 2007 Aug;82(32):285–296. [PubMed] [Google Scholar]
- 10.Barros FC, Matijasevich A, Requejo JH, Giugliani E, Maranhao AG, Monteiro CA, et al. Recent trends in maternal, newborn, and child health in Brazil: progress toward millennium development goals 4 and 5. Am J Public Health. 2010 Oct;100(10):1877–1889. doi: 10.2105/AJPH.2010.196816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Immunization Program. Hepatitis B vaccine, doses administered, vaccination coverage and vaccinated population 1–19 years of age by state of Brazil, 1994–2008. Brasilia: Ministry of Health, Brazil; 2008. [Google Scholar]
- 12.Brazilian Ministry of Health. Nascidos vivos [Live births]. Sistema de Informações sobre Nascidos Vivos (SINASC) Department of Health Statistics (DASIS), Secretariat of Public Health Surveillance. 2009 [Google Scholar]
- 13.Barata RB, Sampaio de Almeida Ribeiro MC, de Moraes JC, Flannery B on behalf of the Vaccine Coverage Survey Group. Socioeconomic inequalities and vaccination coverage: results of an immunisation coverage survey in 27 Brazilian capitals, 2007–2008. J Epidemiol Community Health. 2012 Oct;66(10):934–941. doi: 10.1136/jech-2011-200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.do Carmo GM, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med. 2011 Apr;8(4):e1001024. doi: 10.1371/journal.pmed.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho-Costa FA, Araujo IT, Santos de Assis RM, Fialho AM, de Assis Martins CM, Boia MN, et al. Rotavirus genotype distribution after vaccine introduction, Rio de Janeiro, Brazil. Emerg Infect Dis. 2009 Jan;15(1):95–97. doi: 10.3201/eid1501.071136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009 Jun;301(21):2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 17.Yen C, Figueroa JR, Uribe ES, Carmen-Hernandez LD, Tate JE, Parashar UD, et al. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J Infect Dis. 2011 Sep;204(5):783–786. doi: 10.1093/infdis/jir390. [DOI] [PubMed] [Google Scholar]
- 18.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001 Feb;344(8):564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006 Jan;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Meeting of the Strategic Advisory Group of Experts on immunization. April 2012 – conclusions and recommendations. Wkly Epidemiol Rec. 2012 May;87(21):1–16. [PubMed] [Google Scholar]
- 21.Patel MM, Clark AD, Sanderson CF, Tate J, Parashar UD. Removing the age restrictions for rotavirus vaccination: a benefit risk modeling analysis. PLoS Med. 2012 Oct;9(10):e1001330. doi: 10.1371/journal.pmed.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan American Health Organization. Paving the way for immunization. Report of the Technical Advisory Group on Vaccine-Preventable Diseases 20th annual meeting; 2012. [accessed 2012 Dec 10]. Available at: http://www.new.paho.org. [Google Scholar]
- 23.Brazilian Ministry of Health. [Technical note 193/2012: change in recommended age for administration of measles-mumps-rubella vaccine and oral rotavirus vaccine from January, 2013] Brasilia, Brazil: National Immunization Program, Secretariat of Epidemiologic Surveillance, Ministry of Health; 2012. [Google Scholar]
- 24.Sanderson C, Clark A, Taylor D, Bolanos B, Fine P. Paper for the Initiative for Vaccine Research. Geneva: World Health Organization; 2012. [accessed 2012 Dec 17]. Global review of rotavirus morbidity and mortality data by age and region. Available: http://www.who.int/immunization/sage/meetings/2012/april/Sanderson_et_al_SAGE_April_rotavirus.pdf. [Google Scholar]