Abstract
Background
Some patients with cancer experience multiple pre-diagnostic consultations in primary care, leading to longer time intervals to specialist investigations and diagnosis. Patients with rarer cancers are thought to be at higher risk of such events, but concrete evidence of this is lacking.
Aim
To examine the frequency and predictors of repeat consultations with GPs in patients with rarer cancers.
Design and setting
Patient-reported data on pre-referral consultations from three English national surveys of patients with cancer (2010, 2013, and 2014), pooled to maximise the sample size of rarer cancers.
Method
The authors examined the frequency and crude and adjusted odds ratios for ≥3 (versus 1–2) pre-referral consultations by age, sex, ethnicity, level of deprivation, and cancer diagnosis (38 diagnosis groups, including 12 rarer cancers without prior relevant evidence).
Results
Among 7838 patients with 12 rarer cancers, crude proportions of patients with ≥3 pre-referral consultations ranged from >30.0% to 60.0% for patients with small intestine, bone sarcoma, liver, gallbladder, cancer of unknown primary, soft-tissue sarcoma, and ureteric cancer. The range was 15.0–30.0% for patients with oropharyngeal, anal, parotid, penile, and oral cancer. The overall proportion of responders with any cancer who had ≥3 consultations was 23.4%. Multivariable logistic regression indicated concordant patterns, with strong evidence for variation between rarer cancers (P <0.001).
Conclusion
Patients with rarer cancers experience pre-referral consultations at frequencies suggestive of middle-to-high diagnostic difficulty. The findings can guide the development of new diagnostic interventions and ‘safety-netting’ approaches for symptomatic presentations encountered in patients with rarer cancers.
Keywords: consultation, diagnosis, investigation, neoplasm, primary care, referral
INTRODUCTION
Most patients with cancer first present with symptoms, typically to non-specialists;1,2 consequently, ongoing initiatives in several countries aim to improve diagnostic timeliness for these patients.3–6 Promptly suspecting the diagnosis in patients with rarer cancers who are symptomatic may be particularly challenging, given their overall rarity and heterogeneous nature. In recent years, international initiatives have focused attention on the management of rarer cancers but improvements in diagnosis are also needed.7
A key marker of diagnostic timeliness in patients who are symptomatic and subsequently diagnosed with cancer is the number of consultations they have with a GP before a specialist referral is made, which is highly correlated with the ‘primary care interval’ (time from first presentation to referral).8,9 National audit data indicate primary care intervals of approximately 1, 1.5, and 3 months in patients who experience 3, 4, and ≥5 or more pre-referral consultations.
Describing variation in pre-referral consultations between different patient groups and cancers can provide insights into aetiological mechanisms responsible for diagnostic delay, and help inform future policies and research.10,11 In some cancers, most patients present with symptoms of relatively high predictive value; for example, in the case of breast cancer this is a lump, or in melanoma a visible skin lesion.10 In some other cancers, most patients present with poorly predictive symptoms; as an example, most patients with pancreatic cancer and multiple myeloma present with abdominal or musculoskeletal pain, respectively.12,13 Consequently, variation in the proportion of patients who experience multiple consultations reflects differences in the ‘symptom signature’ of different cancers.10,11 Based on these considerations, it has been suggested that different cancers can be broadly categorised for their diagnostic difficulty, based on the respective frequency of multiple (≥3) consultations: cancers in which ≥30% of patients experience multiple consultations are considered ‘harder to suspect’, whereas those for which <15% of patients have multiple consultations are classed as ‘easier to suspect’.10
As cancer is a heterogeneous disease, evidence about the burden of pre-diagnostic consultations across the range of common and rare cancers is desirable. Nonetheless, currently available evidence (chiefly based on information from responders to a national patient survey in England) excludes patients with several rarer cancers.11 Pooling of data from different waves of surveys of patients with cancer can help to overcome sample size limitations for patients with rarer cancers. As a result, a study was conducted with the principal objective of addressing current evidential gaps about the burden of multiple pre-referral consultations in patients with rarer cancers.
How this fits in
Patients subsequently diagnosed with rarer cancers are often thought to experience multiple pre-referral primary care consultations, but evidence for this assertion is limited. The frequency and predictors of multiple pre-diagnostic consultations in over 7800 patients with 12 rarer cancers were examined. For patients with small intestine, bone sarcoma, liver, gallbladder, cancer of unknown primary, soft-tissue sarcoma, and ureteric cancer, crude proportions of patients with ≥3 pre-diagnostic consultations of >30.0–60.0% were observed. The findings support the development of decision support tools, new diagnostic pathways, and ‘safety-netting’ approaches for patients with possible symptoms of rarer cancers.
METHOD
Data from three waves of the National Cancer Patient Experience Survey (2010, 2013, and 2014), which surveyed patients treated for cancer in English NHS hospitals during sampling periods of 3 months, were analysed.14–16 All three surveys were commissioned by the UK Department of Health and carried out by Quality Health, a specialist survey provider, using identical sampling methods. Survey questions were cognitively validated on samples of volunteer patients.
Patients were sent a survey questionnaire by post a few weeks after discharge and after vital status checks, with up to two reminders sent to non-responders. Response rates were 67%, 64%, and 64% respectively for the 2010, 2013, and 2014 surveys. Anonymous data from the surveys, as used in this study, are made available for research purposes from the UK Data Archive.
There is no universal definition of what constitutes a rarer cancer.7 In this article, the authors specifically focus on rarer cancers without prior published evidence, 10 of which have an annual incidence of <4000/year in England or <1.5% of the annual incidence of all malignant neoplasms, excluding non-melanoma skin cancer. Data from patients with any cancer were included to support direct comparisons between these patient groups and to better contextualise the findings.
Data analysis
Outcome and exposure variables
The information used was provided in response to the first question in the questionnaire: ‘Before you were told you needed to go to hospital about cancer, how many times did you see your GP (family doctor) about the health problem caused by cancer?’ Possible answers were:
none — I did not see my GP before going to hospital;
once;
twice;
three or four times;
≥5 times; and
don’t know/can’t say.
For the analysis, the binary outcome of ≥3 versus 1 or 2 pre-referral consultations were used; patients who responded that they had not seen their GP before going to hospital or responded with ‘Don’t know/can’t say’ were excluded. This binary categorisation is concordant with how data from this survey are reported publicly, and reflects the consideration that some second appointments are generated by the need to review the findings of investigations ordered at the first consultation.10,14–17 Exposure variables considered were:
age;
sex;
deprivation group (an ecological measure of socioeconomic status based on quintile groups of the Index of Multiple Deprivation scores of the lower super output area of patients’ residence18);
International Classification of Diseases, 10th edition diagnosis code based on hospital records (Table 1);
self-assigned Office for National Statistics classification of ethnic group (based on responses to a survey item19); and
survey wave.
Table 1.
ICD-10 definitions of cancer types used in analysis a
| Cancer type | ICD-10 code | ICD-10 code description | Incident count (2012), England |
|---|---|---|---|
| Oropharyngeal | C01, C09, C10 | Malignant neoplasms of base of tongue (C01), tonsil (C09), and oropharynx (C10) | 2037 |
| Oral | C02, C03, C04, C06 | Malignant neoplasm of other and unspecified parts of tongue (C02), gum (C03), floor of mouth (C04), and palate (C06) | 2544 |
| Parotid | C07, C08 | Malignant neoplasm of parotid gland (C07) and other and unspecified major salivary glands (C08) | 556 |
| Oesophageal | C15 | Malignant neoplasm of oesophagus | 7243 |
| Stomach | C16 | Malignant neoplasm of stomach | 5637 |
| Small intestine | C17 | Malignant neoplasm of small intestine | 1065 |
| Colon | C18 | Malignant neoplasm of colon | 22 401 |
| Rectal | C19, C20 | Malignant neoplasm of recto-sigmoid junction (C19) and of rectum (C20) | 11 921 |
| Anal | C21 | Malignant neoplasm of anus and anal canal | 1043 |
| Liver | C22 | Malignant neoplasm of liver and intrahepatic bile ducts | 3867 |
| Gallbladder | C23 | Malignant neoplasm of gallbladder | 686 |
| Pancreatic | C25 | Malignant neoplasm of pancreas | 7371 |
| Laryngeal | C32 | Malignant neoplasm of larynx | 1876 |
| Lung | C33, C34 | Malignant neoplasm of trachea (C33), and bronchus and lung (C34) | 35 903 |
| Bone sarcoma | C40, C41 | Malignant neoplasm of bone and articular cartilage of limbs (C40) and other and unspecified sites (C41) | 412 |
| Melanoma | C43 | Malignant melanoma of skin | 11 281 |
| Mesothelioma | C45 | Mesothelioma | 2347 |
| Soft-tissue sarcoma | C49 | Malignant neoplasm of other connective and soft tissue | 1521 |
| Breast | C50 | Malignant neoplasm of breast | 42 773 |
| Vulval/vaginal | C51, C52 | Malignant neoplasm of vulva (C51) and vagina (C52) | 1262 |
| Cervical | C53 | Malignant neoplasm of cervix uteri | 1262 |
| Endometrial | C54, C55 | Malignant neoplasm of corpus uteri (C54), malignant neoplasm of uterus, unspecified (C55) | 7192 |
| Ovarian | C56 | Malignant neoplasm of ovary | 5582 |
| Penile | C60 | Malignant neoplasm of penis | 505 |
| Prostate | C61 | Malignant neoplasm of prostate | 37 136 |
| Testicular | C62 | Malignant neoplasm of testis | 1874 |
| Renal | C64 | Malignant neoplasm of kidney, except renal pelvis | 7366 |
| Ureteric | C65, C66 | Malignant neoplasm of renal pelvis (C65) and ureter (C66) | 1082 |
| Bladder | C67 | Malignant neoplasm of bladder | 9124 |
| Brain | C71 | Malignant neoplasm of brain | 3959 |
| Thyroid | C73 | Malignant neoplasm of thyroid gland | 2595 |
| Cancer of unknown primary | C77, C78, C79, C80 | Secondary and unspecified malignant neoplasm of lymph nodes (C77), secondary malignant neoplasm of respiratory and digestive organs (C78), secondary malignant neoplasm of other and unspecified sites (C79), and malignant neoplasm, without specification of site (C80) | 7965 |
| Hodgkin lymphoma | C81 | Hodgkin lymphoma | 1555 |
| Non-Hodgkin lymphoma | C82, C83, C85 | Follicular (nodular) non-Hodgkin lymphoma (C82), diffuse non-Hodgkin lymphoma (C83), other and unspecified types of non-Hodgkin lymphoma (C85) | 10 144 |
| Multiple myeloma | C90 | Multiple myeloma and malignant plasma cell neoplasms | 4190 |
| Leukaemia | C91, C92, C93, C94, C95 | Lymphoid leukaemia (C91), myeloid leukaemia (C92), monocytic leukaemia (C93), other leukaemias of specified cell type (C94), other leukaemias of unspecified cell type (C95) | 7354 |
| Ductal carcinoma in situ | D05 | Carcinoma in situ of breast | 5517 |
| Other cancers | All other codes |
The number of incident cases in England is also provided as a measure of cancer frequency; bold italics denote rarer cancers without prior relevant evidence.
ICD =10 = International Classification of Diseases, 10th edition.
Analysis sample derivation
Of 206 591 responders to all three surveys, the analysis sample was restricted a priori to patients who indicated (in response to a survey question) that they were diagnosed with cancer during the year before completing the survey (62.4% of the initial responders’ sample). This was done to minimise the potential of ‘double-counting’ the few responders who may have been treated during the sampling periods of more than one survey.
Among patients who saw the GP at least once, there was complete information on age, sex, and cancer diagnosis, but 2.2% had missing information on ethnicity or deprivation group — these records were excluded from subsequent analyses, resulting in an analysis sample of 95 582 patients. Figure 1 outlines the derivation of the analysis sample.
Figure 1.
Analysis sample derivation flowchart.
Statistical analysis
The crude proportion of patients who had ≥3 consultations are described by exposure variable category; that is, by age group, sex, ethnicity, deprivation group, and cancer diagnosis. Subsequently, using logistic regression, the crude and adjusted (for all above variables) odds ratios (ORs) for ≥3 pre-referral consultations are reported. Standard errors were calculated with a robust estimator. Rectal cancer was used as the reference category for cancer, as a result of it having a large sample size and occurring in both sexes. Additionally, crude proportions of patients for each individual category of number of pre-referral consultations by cancer diagnosis are described. All analyses were performed using Stata (version 13).
RESULTS
After exclusions, 95 582 responders were included in the analysis, of whom 7838 were patients with the following 12 rarer cancers: oropharyngeal, oral, parotid, small intestine, anal, liver, gallbladder, bone sarcoma, soft-tissue sarcoma, penile, ureteric, and cancer of unknown primary. Overall, 22 387 (23.4%) patients had ≥3 consultations.
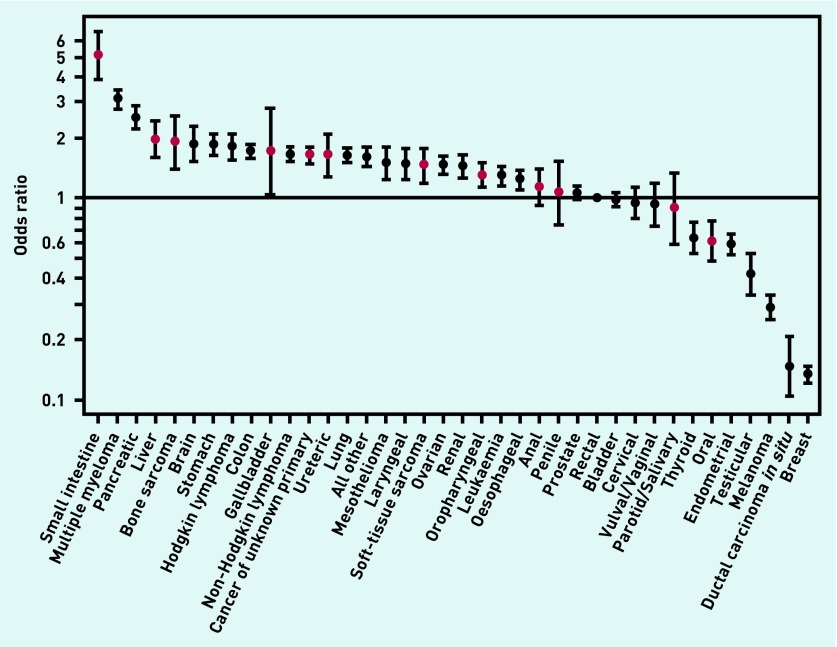
There was strong evidence for very large variation in crude proportions, and crude and adjusted ORs of ≥3 pre-referral consultations between patients with different cancers (P <0.001, Table 2, Figure 2). The proportion of patients with ≥3 consultations was highest for small intestine (60.1%) and multiple myeloma (47.2%), and lowest for melanoma (8.3%), ductal carcinoma in situ (5.5%), and breast cancer (5.0%). There was a notably large — 40-fold — difference in the adjusted odds between small intestine cancer and breast cancer in terms of patients having ≥3 consultations.
Table 2.
Crude proportions, crude ORs, and adjusted ORs for ≥3 pre-diagnostic GP consultations, by cancera
| Patient characteristics | All responders who saw GP at least once, n | Responders with ≥3 consultations, n (%) | Crude OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Cancer diagnosis | ||||||
| Small intestine | 218 | 131 (60.1) | 5.18 (3.92 to 6.84) | 5.09 (3.85 to 6.73) | ||
| Multiple myeloma | 2052 | 969 (47.2) | 3.08 (2.77 to 3.42) | 3.08 (2.77 to 3.43) | ||
| Pancreatic | 1137 | 485 (42.7) | 2.56 (2.24 to 2.92) | 2.52 (2.21 to 2.89) | ||
| Liver | 451 | 167 (37.0) | 2.02 (1.65 to 2.47) | 1.96 (1.60 to 2.40) | ||
| Bone sarcoma | 196 | 81 (41.3) | 2.42 (1.81 to 3.24) | 1.89 (1.39 to 2.56) | ||
| Brain | 474 | 186 (39.2) | 2.22 (1.83 to 2.70) | 1.86 (1.53 to 2.26) | ||
| Stomach | 1828 | 623 (34.1) | 1.78 (1.58 to 2.00) | 1.86 (1.65 to 2.09) | ||
| Hodgkin lymphoma | 969 | 417 (43.0) | 2.60 (2.25 to 2.99) | 1.79 (1.54 to 2.08) | ||
| Colon | 8053 | 2640 (32.8) | 1.68 (1.55 to 1.81) | 1.71 (1.58 to 1.85) | ||
| Gallbladder | 66 | 23 (34.8) | 1.84 (1.10 to 3.06) | 1.70 (1.03 to 2.80) | ||
| Non-Hodgkin lymphoma | 5523 | 1844 (33.4) | 1.72 (1.59 to 1.87) | 1.65 (1.52 to 1.80) | ||
| Cancer of unknown primary | 3537 | 1189 (33.6) | 1.74 (1.59 to 1.91) | 1.63 (1.49 to 1.80) | ||
| Ureteric | 331 | 100 (30.2) | 1.49 (1.17 to 1.90) | 1.63 (1.28 to 2.08) | ||
| Lung | 6405 | 2068 (32.3) | 1.64 (1.51 to 1.78) | 1.63 (1.50 to 1.77) | ||
| All other | 2281 | 753 (33.0) | 1.70 (1.52 to 1.89) | 1.60 (1.43 to 1.78) | ||
| Mesothelioma | 641 | 180 (28.1) | 1.34 (1.12 to 1.61) | 1.49 (1.24 to 1.80) | ||
| Laryngeal | 667 | 198 (29.7) | 1.45 (1.22 to 1.73) | 1.49 (1.24 to 1.78) | ||
| Soft-tissue sarcoma | 508 | 162 (31.9) | 1.61 (1.32 to 1.96) | 1.46 (1.19 to 1.78) | ||
| Ovarian | 2717 | 913 (33.6) | 1.74 (1.57 to 1.93) | <0.0001 | 1.45 (1.31 to 1.61) | <0.0001 |
| Renal | 1436 | 431 (30.0) | 1.48 (1.30 to 1.68) | 1.43 (1.26 to 1.63) | ||
| Oropharyngeal | 1155 | 338 (29.3) | 1.42 (1.24 to 1.64) | 1.30 (1.13 to 1.50) | ||
| Leukaemia | 2043 | 582 (28.5) | 1.37 (1.22 to 1.54) | 1.29 (1.14 to 1.45) | ||
| Oesophageal | 2887 | 737 (25.5) | 1.18 (1.06 to 1.31) | 1.23 (1.11 to 1.37) | ||
| Anal | 495 | 133 (26.9) | 1.26 (1.03 to 1.56) | 1.14 (0.92 to 1.40) | ||
| Penile | 174 | 38 (21.8) | 0.96 (0.67 to 1.38) | 1.07 (0.74 to 1.53) | ||
| Prostate | 8538 | 1863 (21.8) | 0.96 (0.89 to 1.04) | 1.06 (0.97 to 1.15) | ||
| Rectal | 5616 | 1265 (22.5) | Ref | Ref | ||
| Bladder | 6837 | 1397 (20.4) | 0.88 (0.81 to 0.96) | 0.99 (0.90 to 1.07) | ||
| Cervical | 706 | 203 (28.8) | 1.39 (1.17 to 1.65) | 0.95 (0.79 to 1.14) | ||
| Vulval/vaginal | 405 | 95 (23.5) | 1.05 (0.83 to 1.34) | 0.93 (0.73 to 1.18) | ||
| Parotid | 140 | 32 (22.9) | 1.02 (0.68 to 1.52) | 0.89 (0.60 to 1.34) | ||
| Thyroid | 940 | 197 (21.0) | 0.91 (0.77 to 1.08) | 0.63 (0.53 to 0.76) | ||
| Oral | 567 | 94 (16.6) | 0.68 (0.54 to 0.86) | 0.61 (0.49 to 0.77) | ||
| Endometrial | 3255 | 549 (16.9) | 0.70 (0.62 to 0.78) | 0.59 (0.53 to 0.66) | ||
| Testicular | 665 | 96 (14.4) | 0.58 (0.46 to 0.73) | 0.42 (0.33 to 0.53) | ||
| Melanoma | 3470 | 289 (8.3) | 0.31 (0.27 to 0.36) | 0.29 (0.25 to 0.33) | ||
| Ductal carcinoma in situ | 650 | 36 (5.5) | 0.20 (0.14 to 0.28) | 0.15 (0.10 to 0.21) | ||
| Breast | 17 549 | 883 (5.0) | 0.18 (0.17 to 0.20) | 0.13 (0.12 to 0.15) | ||
|
| ||||||
| Sex | ||||||
| Male | 45 151 | 11 613 (25.7) | Ref | <0.0001 | Ref | <0.0001 |
| Female | 50 431 | 10 774 (21.4) | 0.78 (0.76 to 0.81) | 1.24 (1.19 to 1.29) | ||
|
| ||||||
| Age group, years | ||||||
| 16–24 | 536 | 231 (43.1) | 2.40 (2.02 to 2.85) | 2.12 (1.75 to 2.56) | ||
| 25–34 | 1754 | 508 (29.0) | 1.29 (1.16 to 1.44) | 1.82 (1.61 to 2.06) | ||
| 35–44 | 5277 | 1046 (19.8) | 0.78 (0.73 to 0.84) | 1.46 (1.34 to 1.58) | ||
| 45–54 | 13 113 | 2937 (22.4) | 0.91 (0.87 to 0.96) | 1.38 (1.31 to 1.46) | ||
| 55–64 | 22 789 | 5881 (25.8) | 1.10 (1.06 to 1.15) | 1.18 (1.14 to 1.23) | ||
| 65–74 | 30 517 | 7327 (24.0) | Ref | <0.0001 | Ref | <0.0001 |
| 75–84 | 18 442 | 3860 (20.9) | 0.84 (0.80 to 0.88) | 0.84 (0.81 to 0.88) | ||
| ≥85 | 3154 | 597 (18.9) | 0.74 (0.67 to 0.81) | 0.78 (0.71 to 0.86) | ||
|
| ||||||
| Ethnic group | ||||||
| White | 91 824 | 21 024 (22.9) | Ref | Ref | ||
| Mixed | 456 | 161 (35.3) | 1.84 (1.52 to 2.23) | 1.79 (1.44 to 2.23) | ||
| Asianb | 1662 | 611 (36.8) | 1.96 (1.77 to 2.17) | <0.0001 | 2.20 (1.96 to 2.46) | <0.0001 |
| Black | 1280 | 483 (37.7) | 2.04 (1.82 to 2.29) | 2.17 (1.91 to 2.47) | ||
| Chinese | 236 | 71 (30.1) | 1.45 (1.10 to 1.91) | 1.30 (0.96 to 1.76) | ||
| Other | 124 | 37 (29.8) | 1.43 (0.97 to 2.10) | 1.57 (1.02 to 2.43) | ||
|
| ||||||
| Deprivation groupc | ||||||
| Affluent | 22 351 | 4883 (21.8) | Ref | Ref | ||
| Deprivation group 2 | 22 644 | 5127 (22.6) | 1.05 (1.00 to 1.09) | 1.04 (0.99 to 1.09) | ||
| Deprivation group 3 | 20 446 | 4743 (23.2) | 1.08 (1.03 to 1.13) | <0.0001 | 1.05 (1.00 to 1.10) | <0.0001 |
| Deprivation group 4 | 16 868 | 4117 (24.4) | 1.16 (1.10 to 1.21) | 1.08 (1.03 to 1.14) | ||
| Deprived | 13 273 | 3517 (26.5) | 1.29 (1.23 to 1.36) | 1.16 (1.10 to 1.22) | ||
|
| ||||||
| Survey year | ||||||
| 2010 | 30 498 | 7211 (23.6) | Ref | Ref | ||
| 2013 | 32 608 | 7775 (23.8) | 1.01 (0.97 to 1.05) | 0.0035 | 1.02 (0.98 to 1.06) | 0.0409 |
| 2014 | 32 476 | 7401 (22.8) | 0.95 (0.92 to 0.99) | 0.97 (0.93 to 1.01) | ||
|
| ||||||
| Total | 95 582 | 22 387 (23.4) | ||||
The table is sorted in descending order of adjusted odds ratios. Rarer cancers without prior published evidence are denoted in bold italics.
The Asian group excludes Chinese, which was treated as a separate category in all three surveys.
As classified by the Index of Multiple Deprivation. OR = odds ratio.
Figure 2.
Odds ratios for ≥3 GP consultations before hospital referral, by cancer type. Central estimates for 12 rarer cancers without prior relevant published evidence shown in red.
There was also substantial variation between patients with any of the 12 rarer cancers of prime interest to this study (P <0.001 for test of variation between these 12 cancers). Specifically, patients with seven such cancers (small intestine, bone sarcoma, liver, gallbladder, cancer of unknown primary, soft-tissue sarcoma, and ureteric cancer) had proportions of ≥3 consultations that were >30.0%; whereas patients with five other rarer cancers (oropharyngeal, anal, parotid, penile, and oral cancer) had respective proportions of between 15% and 30% (Table 2). Multivariable analysis indicated concordant patterns (Table 2, Figure 2).
Relatedly, although bone and soft-tissue sarcomas tend to present with fairly specific symptoms (for example, bony or a soft-tissue lump), they principally affect teenagers and young adults, and are associated with relatively high crude proportions of multiple consultations.20 In multivariable analysis, however, after adjustment for age group and other patient characteristics, the odds of ≥3 consultations for these two cancers are notably reduced, although remaining comparatively high (Table 1).
Regarding sociodemographic variation, there was evidence of increasing frequency of ≥3 consultations with increasing deprivation (P<0.001): 26.5% versus 21.8% for patients in the groups of most and least deprivation respectively. As previously described,11 there was strong evidence for crude variation in pre-referral consultations by sex, age, and ethnicity (P<0.001 for all): with males, younger patients, and those from a minority ethnic group having a higher proportion of multiple pre-referral consultations (Table 2). Multivariable logistic regression analysis indicated concordant patterns of variation by patient characteristic, with the exception of a reversal of the sex difference (Table 2, Figure 3).
Figure 3.
Odds ratios for ≥3 GP consultations before hospital referral, by patient characteristic. Ref = reference
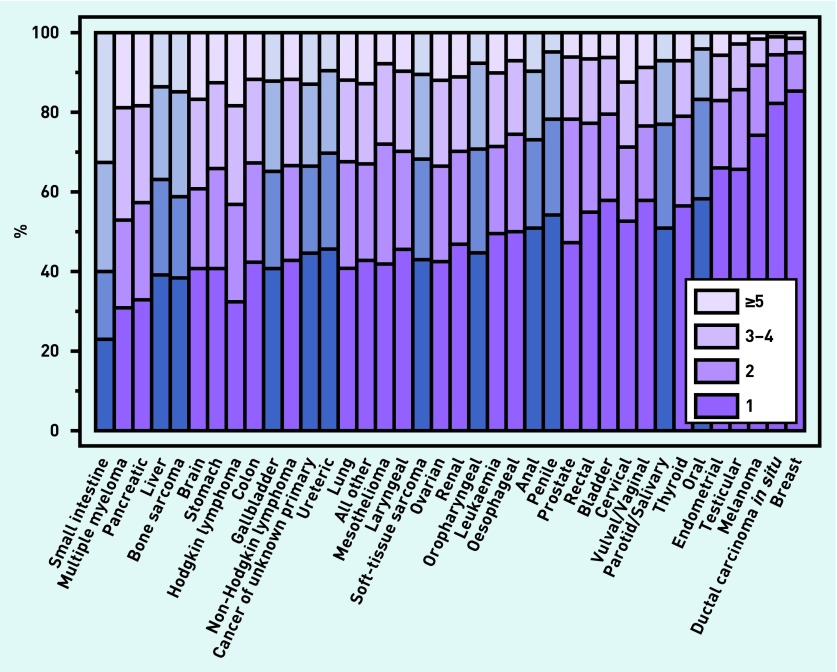
The distribution of the crude proportions of all categories of the number of pre-referral consultation by cancer diagnosis is shown in Figure 4 and Table 3. The overall pattern of variation by cancer diagnosis in respect of the binary measure of ≥3 consultations is, for the most part, similar to the pattern that would have been observed if alternative cut-off points (for example, ≥2 consultations or ≥5 consultations) had been used.
Figure 4.
Distribution of crude proportions of all categories of number of pre-referral consultation, by cancer diagnosis. The blue bars represent rarer cancers without prior relevant evidence.
Table 3.
Patients by cancer diagnosis and number of pre-diagnostic GP consultationsa
| n | Consultations before referral n (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3–4 | ≥5 | ||
| Small intestine | 218 | 50 (22.9) | 37 (17.0) | 60 (27.5) | 71 (32.6) |
| Multiple myeloma | 2052 | 632 (30.8) | 451 (22.0) | 584 (28.5) | 385 (18.8) |
| Pancreatic | 1137 | 374 (32.9) | 278 (24.5) | 277 (24.4) | 208 (18.3) |
| Liver | 451 | 177 (39.2) | 107 (23.7) | 105 (23.3) | 62 (13.7) |
| Bone sarcoma | 196 | 75 (38.3) | 40 (20.4) | 52 (26.5) | 29 (14.8) |
| Brain | 474 | 193 (40.7) | 95 (20.0) | 107 (22.6) | 79 (16.7) |
| Stomach | 1828 | 746 (40.8) | 459 (25.1) | 394 (21.6) | 229 (12.5) |
| Hodgkin lymphoma | 969 | 314 (32.4) | 238 (24.6) | 239 (24.7) | 178 (18.4) |
| Colon | 8053 | 3424 (42.5) | 1989 (24.7) | 1713 (21.3) | 927 (11.5) |
| Gallbladder | 66 | 27 (40.9) | 16 (24.2) | 15 (22.7) | 8 (12.1) |
| Non-Hodgkin lymphoma | 5523 | 2372 (42.9) | 1307 (23.7) | 1187 (21.5) | 657 (11.9) |
| Cancer of unknown primary | 3537 | 1585 (44.8) | 763 (21.6) | 733 (20.7) | 456 (12.9) |
| Ureteric | 331 | 151 (45.6) | 80 (24.2) | 69 (20.8) | 31 (9.4) |
| Lung | 6405 | 2628 (41.0) | 1709 (26.7) | 1301 (20.3) | 767 (12.0) |
| All other | 2281 | 979 (42.9) | 549 (24.1) | 465 (20.4) | 288 (12.6) |
| Mesothelioma | 641 | 268 (41.8) | 193 (30.1) | 130 (20.3) | 50 (7.8) |
| Laryngeal | 667 | 303 (45.4) | 166 (24.9) | 133 (19.9) | 65 (9.7) |
| Soft-tissue sarcoma | 508 | 218 (42.9) | 128 (25.2) | 109 (21.5) | 53 (10.4) |
| Ovarian | 2717 | 1157 (42.6) | 647 (23.8) | 588 (21.6) | 325 (12.0) |
| Renal | 1436 | 673 (46.9) | 332 (23.1) | 273 (19.0) | 158 (11.0) |
| Oropharyngeal | 1155 | 517 (44.8) | 300 (26.0) | 250 (21.6) | 88 (7.6) |
| Leukaemia | 2043 | 1016 (49.7) | 445 (21.8) | 373 (18.3) | 209 (10.2) |
| Oesophageal | 2887 | 1437 (49.8) | 713 (24.7) | 539 (18.7) | 198 (6.9) |
| Anal | 495 | 252 (50.9) | 110 (22.2) | 85 (17.2) | 48 (9.7) |
| Penile | 174 | 94 (54.0) | 42 (24.1) | 29 (16.7) | 9 (5.2) |
| Prostate | 8538 | 4045 (47.4) | 2630 (30.8) | 1329 (15.6) | 534 (6.3) |
| Rectum | 5616 | 3094 (55.1) | 1257 (22.4) | 888 (15.8) | 377 (6.7) |
| Bladder | 6837 | 3961 (57.9) | 1479 (21.6) | 974 (14.2) | 423 (6.2) |
| Cervical | 706 | 372 (52.7) | 131 (18.6) | 115 (16.3) | 88 (12.5) |
| Vulval/vaginal | 405 | 235 (58.0) | 75 (18.5) | 59 (14.6) | 36 (8.9) |
| Parotid | 140 | 71 (50.7) | 37 (26.4) | 22 (15.7) | 10 (7.1) |
| Thyroid | 940 | 532 (56.6) | 211 (22.4) | 133 (14.1) | 64 (6.8) |
| Oral | 567 | 330 (58.2) | 143 (25.2) | 71 (12.5) | 23 (4.1) |
| Endometrial | 3255 | 2145 (65.9) | 561 (17.2) | 365 (11.2) | 184 (5.7) |
| Testicular | 665 | 437 (65.7) | 132 (19.8) | 77 (11.6) | 19 (2.9) |
| Melanoma | 3470 | 2576 (74.2) | 605 (17.4) | 233 (6.7) | 56 (1.6) |
| Ductal carcinoma in situ | 650 | 535 (82.3) | 79 (12.2) | 32 (4.9) | 4 (0.6) |
| Breast | 17 549 | 14 988 (85.4) | 1678 (9.6) | 639 (3.6) | 244 (1.4) |
Cancer diagnoses are ordered as they appear in Table 2; rarer cancers without prior published evidence are denoted in bold italics.
DISCUSSION
Summary
The proportion of pre-referral consultations in patients subsequently diagnosed with several rarer cancers was particularly high (>30.0%) in patients with small intestine, bone sarcoma, liver, gallbladder, cancer of unknown primary, soft-tissue sarcoma, and ureteric cancer. The proportion of patients with any one of five rarer cancers (oropharyngeal, anal, parotid, penile, and oral cancer) who had ≥3 consultations was between 15% and 30%. Using a previously suggested classification, therefore, all of these cancers could be classified as either being harder to suspect or belonging to the intermediate diagnostic difficulty category.10
Strengths and limitations
The main strengths of this study are its large sample size — enabling the examination of data on several rarer cancers with adequate precision — and the availability of data on patient characteristics, which allowed for the estimation of independent predictors of variation.
However, there are also potential limitations. Some patients may have recalled the number of relevant pre-referral consultations inaccurately, although prior evidence indicates that both patients and clinicians provide concordant frequencies of pre-referral consultations in patients with a given cancer.8 Further, the number of consultations that patients themselves judge relevant to cancer has high face validity, which cannot be dismissed. Relatedly, there was no information on actual circumstances surrounding the multiple consultation events, which may have included patient preferences for delayed referral or investigation, primary care-led investigations, and clinically justified expectant (‘safety-netting’) management, particularly for patients with non-specific symptoms.10,17 It is foolhardy to consider that multiple consultations represent suboptimal management: care is likely to have been concordant with good clinical practice in most circumstances, and multiple consultations represent scientific limitations in current medical knowledge, along with a lack of available, easy-to-use, reliable tests.10
Data relate to patients with recent hospital treatment for cancer (typically 3–6 months before survey participation). This sampling method, combined with non-response and early mortality patterns, results in a sample that is different in its cancer diagnosis and sociodemographic case mix compared with all incident patients with cancer in the population.21 As such, the reported proportions of ≥3 pre-referral consultations are not fully representative of incident patients with cancer and it is, therefore, recommended that interpretation focuses on patterns of relative variation between patients with different cancers and characteristics, rather than on the exact frequency of ≥3 pre-referral consultations by variable category.
Comparison with existing literature
The authors know of no previous studies that examine the number of pre-referral consultations among patients with the studied rarer cancers of prior interest. However, in respect of patients with bone or soft-tissue sarcoma, the findings amplify those from a recent study of diagnostic pathways in patients with those cancers, indicating that most such patients who were diagnosed after a GP referral were referred to specialists non-urgently and without cancer being suspected as a diagnosis.22 Similarly, in respect of patients with cancer of unknown primary, the findings complement those of a recent Australian study that indicated delayed recognition of the importance of presenting symptoms in patients with those cancers.23
Previous evidence indicates that repeat consultations occur for between one-fifth and one-quarter of all patients with cancer, although this overall proportion varies greatly between patients with different cancers and characteristics.8,11 The present study extends prior relevant evidence on the frequency of multiple pre-diagnostic consultations to patients with 12 rarer cancers. Adding to previous evidence that indicates a higher risk of multiple pre-referral consultations in younger patients, those of minority ethnic groups, and males, the study presented here also identifies significantly higher risks of multiple consultations in patients of greater deprivation: prior relevant evidence was inconclusive due to power limitations.11
The frequency of multiple pre-diagnostic consultations varies greatly by cancer, being greatest for cancers that often present with symptoms that are common among patients consulting in primary care; consequently, these have low predictive value for cancer.10 For example, ≥3 pre-referral consultations occur in between one-third and one-half of all patients who are subsequently diagnosed with multiple myeloma or pancreatic cancer,8,11 which commonly present with musculoskeletal and abdominal pain, respectively, both of which are very common reasons for primary care consultations.12,13
This prior evidence provides insights into likely reasons for variation in the frequency of ≥3 pre-referral consultations in patients with rarer cancers: although some patients with small intestine, liver, and gallbladder cancers will present with ‘red-flag’ symptoms that have a relatively high predictive value, many will have symptoms such as diarrhoea or abdominal pain, which have relatively low predictive values for these (or other) cancers.24 Similarly, cancer of unknown primary typically presents with non-specific symptoms. Conversely, patients with oral and parotid cancer typically present with visible and/or palpable lesions (oral ulceration or facial lump) and, relatedly, have the lowest proportions of multiple consultations among the 12 rarer cancers described in this article. Therefore, concordant with prior evidence, the findings suggest that the frequency of multiple pre-referral consultations for different cancers reflects their ‘symptom signature’ (that is, the relative frequency and predictive value of the most common presenting symptoms); this observation holds true for both common and rarer cancers. Further, for a given cancer, the frequency of pre-referral consultations can be considered to denote its average diagnostic difficulty at first presentation to primary care.
Two further considerations can help to ascertain why the rarer cancers studied tend to fall within average or high diagnostic difficulty categories:10
as rarer cancers are, by definition, very infrequent, doctors are particularly unfamiliar with their symptomatic presentations; and
specifically for certain rarer cancers, greater incidence in younger age groups may exacerbate diagnostic difficulties as the predictive value of symptoms is particularly low compared with the same symptom in adults.
Implications for research and practice
The findings underpin the need for the development, evaluation, and implementation of effective interventions aimed at decreasing the average number of pre-referral consultations in patients with cancer. In recent years, such interventions encompassed clinical practice guidelines for suspected cancer in primary care, the development and introduction of decision support tools during the consultation, and widening access to specialist investigations, such as imaging and endoscopy.24–27 Until recently, most interventions aimed at decreasing diagnostic intervals have focused on patients with relatively common cancers, such as initiatives aiming to increase endoscopic or imaging investigations for patients with suspected colorectal, lung, or ovarian cancer.28
Indeed, increased emphasis is being paid on system-wide approaches for supporting the diagnostic process. For example, ‘one-stop shop’ multispecialist diagnostic services for patients with serious, but unexplained, symptoms are being implemented in Denmark and the UK. Further, revised clinical guidelines for the referral of patients with suspected cancer have recently been introduced in England, encompassing a wide range of presenting symptoms for 34 different cancers in adulthood, including all cancers included in our study.24 Both the introduction of one-stop shop, diagnostic, clinic models and the implementation of new clinical guidelines encompassing a wider range of symptomatic presentations are likely to help improve diagnostic timeliness for patients with rarer cancers. In the longer term, the development of biomarker-enabled, point-of-care diagnostic tests is likely to be the most effective intervention for reducing multiple pre-referral consultations, particularly for cancers with a high level of diagnostic difficulty; and, therefore, for several rarer cancers.10
In spite of such developments, breakthroughs in diagnostic technologies are unlikely in the short term, and evidence on interventions such as decision support tools and new diagnostic care pathways is still in emergence. Safety netting patients with non-specific symptoms can have a role in improving diagnosis in the interim.29 The 2015 guidelines from the National Institute for Health and Care Excellence for the referral of patients with suspected cancer recommend the review for people with symptoms associated with increased risk of cancer who do not meet the criteria for referral or investigative action. The guidelines also encourage patient-initiated follow-up visits or arranging planned followup consultations in the presence of recurring, persistent, or worsening symptoms.24
These recommendations build on previous guidance from the Royal College of General Practitioners on safety netting.30 It should be noted that safety-netting approaches, by their nature, are likely to increase the number of consultations before referral or investigative actions in some patients; however, they can also shorten between-consultation intervals and improve the patient experience of the diagnostic process. Robust evaluation of safety-netting is nonetheless required, particularly as there is currently a plethora of definitions and variable practice. If effective, safety-netting approaches could be of particular benefit for patients subsequently diagnosed with rarer cancers.
This study has described the burden of pre-diagnostic consultations in primary care for patients with a range of rarer cancers. As implied by the respective proportions of patients having ≥3 consultations, most such cancers appear to have at least average, and often higher-than-average, diagnostic difficulty. The findings presented here could guide research and policy initiatives to increase diagnostic timeliness for patients with rarer cancers, including the introduction of decision support tools that encompass common relevant symptomatic presentations, the development of easily accessible multidisciplinary diagnostic services, and the wider introduction and evaluation of safety-netting approaches.
Acknowledgments
The authors thank the UK Data Archive for access to the anonymous survey data (UKDA study numbers 6742, 7400, and 7562 for the 2010, 2013, and 2014 surveys, respectively), the Department of Health as the depositor and principal investigator of the National Cancer Patient Experience Surveys, Quality Health as data collector, and all NHS acute trusts in England, for the provision of data samples. Thanks also to all patients who responded to the surveys.
Funding
This work was supported by a Cancer Research UK Clinician Scientist Fellowship (A18180) to Georgios Lyratzopoulos.
Ethical approval
Ethical approval was not required as the data used are anonymous and accessible for bona fide research from UK Data Archive.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests. The views expressed in this publication are those of the authors and not necessarily those of any funder or other organisation or institution.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer — determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107(8):1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen H, Tørring ML, Olesen F, et al. Diagnostic intervals before and after implementation of cancer patient pathways — a GP survey and registry based comparison of three cohorts of cancer patients. BMC Cancer. 2015;15(1):308. doi: 10.1186/s12885-015-1317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiom SC. Diagnosing cancer earlier: reviewing the evidence for improving cancer survival. Br J Cancer. 2015;112(Suppl):S1–S5. doi: 10.1038/bjc.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prades J, Espinàs JA, Font R, et al. Implementing a Cancer Fast-track Programme between primary and specialised care in Catalonia (Spain): a mixed methods study. Br J Cancer. 2011;105(6):753–759. doi: 10.1038/bjc.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vedsted P, Olesen F. A differentiated approach to referrals from general practice to support early cancer diagnosis — the Danish three-legged strategy. Br J Cancer. 2015;112(Suppl):S65–S69. doi: 10.1038/bjc.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentín-López B, Ferrándiz-Santos J, Blasco-Amaro J-A, et al. Assessment of a rapid referral pathway for suspected colorectal cancer in Madrid. Fam Pract. 2012;29(2):182–188. doi: 10.1093/fampra/cmr080. [DOI] [PubMed] [Google Scholar]
- 7.Keat N, Law K, Seymour M, et al. International Rare Cancers Initiative. Lancet Oncol. 2013;14(2):109–110. doi: 10.1016/S1470-2045(12)70570-3. [DOI] [PubMed] [Google Scholar]
- 8.Lyratzopoulos G, Abel GA, McPhail S, et al. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer. 2013;108(3):686–690. doi: 10.1038/bjc.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–1267. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyratzopoulos G, Wardle J, Rubin G. Rethinking diagnostic delay in cancer: how difficult is the diagnosis? BMJ. 2014;349:g7400. doi: 10.1136/bmj.g7400. [DOI] [PubMed] [Google Scholar]
- 11.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13(4):353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 12.Stapley S, Peters TJ, Neal RD, et al. The risk of pancreatic cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2012;106(12):1940–1944. doi: 10.1038/bjc.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shephard EA, Neal RD, Rose P, et al. Quantifying the risk of multiple myeloma from symptoms reported in primary care patients: a large case-control study using electronic records. Br J Gen Pract. 2015 doi: 10.3399/bjgp15X683545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Department of Health National Cancer Patient Experience Survey, 2010. [data collection]. UK Data Service SN: 6742, 2011. http://dx.doi.org/10.5255/UKDA-SN-6742-1. [Google Scholar]
- 15.Department of Health National Cancer Patient Experience Survey, 2012–2013. [data collection]. UK Data Service SN: 7400, 2013. http://dx.doi.org/10.5255/UKDA-SN-7400-1. [Google Scholar]
- 16.Department of Health National Cancer Patient Experience Survey, 2013–2014. [data collection]. UK Data Service SN: 7562, 2014. http://dx.doi.org/10.5255/UKDA-SN-7562-1. [Google Scholar]
- 17.Rubin GP, Saunders CL, Abel GA, et al. Impact of investigations in general practice on timeliness of referral for patients subsequently diagnosed with cancer: analysis of national primary care audit data. Br J Cancer. 2015;112(4):676–687. doi: 10.1038/bjc.2014.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Communities and Local Government Indices of Deprivation 2007. http://webarchive.nationalarchives.gov.uk/20100410180038/http://communities.gov.uk/communities/neighbourhoodrenewal/deprivation/deprivation07/ (accessed 18 Jan 2016). [Google Scholar]
- 19.Saunders CL, Abel GA, El Turabi A, et al. Accuracy of routinely recorded ethnic group information compared with self-reported ethnicity: evidence from the English Cancer Patient Experience survey. BMJ Open. 2013;3:e002882. doi: 10.1136/bmjopen-2013-002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyrop HB, Vedsted P, Safwat A, et al. Alarm symptoms of soft-tissue and bone sarcoma in patients referred to a specialist center. Acta Orthop. 2014;85(6):657–662. doi: 10.3109/17453674.2014.957086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel GA, Saunders CL, Lyratzopoulos G. Sample characteristics and non-response patterns in the English Cancer Patient Experience Survey: implications for epidemiological studies based on patient survey data. Cancer Epidemiol. 2016;41:34–41. doi: 10.1016/j.canep.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerrand C, Francis M, Dennis N, et al. Routes to diagnosis for sarcoma — describing the sarcoma patient journey. Eur J Surg Oncol. 2015;14(10):1393–1399. doi: 10.1016/j.ejso.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Vajdic CM, Schaffer AL, Dobbins TA, et al. Health service utilisation and investigations before diagnosis of cancer of unknown primary (CUP): a population-based nested case-control study in Australian Government Department of Veterans’ Affairs clients. Cancer Epidemiol. 2015;39(4):585–592. doi: 10.1016/j.canep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton W, Hajioff S, Graham J, Schmidt-Hansen M. Suspected cancer (part 2 — adults): reference tables from updated NICE guidance. BMJ. 2015;350:h3044. doi: 10.1136/bmj.h3044. [DOI] [PubMed] [Google Scholar]
- 25.Green T, Martins T, Hamilton W, et al. Exploring GPs’ experiences of using diagnostic tools for cancer: a qualitative study in primary care. Fam Pract. 2015;32(1):101–105. doi: 10.1093/fampra/cmu081. [DOI] [PubMed] [Google Scholar]
- 26.Guldbrandt LM, Rasmussen TR, Rasmussen F, Vedsted P. Implementing direct access to low-dose computed tomography in general practice — method, adaption and outcome. PLoS One. 2014;9(11):e112162. doi: 10.1371/journal.pone.0112162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graber ML. Medical diagnosis — the promise. Diagnosis. 2014;1(1):5–9. doi: 10.1515/dx-2013-0005. [DOI] [PubMed] [Google Scholar]
- 28.Department of Health Direct access to diagnostic tests for cancer: best practice referral pathways for general practitioners. 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216503/dh_133511.pdf (accessed 18 Jan 2016).
- 29.Almond S, Mant D, Thompson M. Diagnostic safety-netting. Br J Gen Pract. 2009 doi: 10.3399/bjgp09X472971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell E, Rubin G, Macleod U. Improving diagnosis of cancer: a toolkit for general practice. 2012 http://www.rcgp.org.uk/clinical-and-research/clinical-resources/~/media/Files/CIRC/Cancer/Improving%20Cancer%20Diagnosis%20-%20A%20Toolkit%20for%20General%20Practice%20%282%29.ashx (accessed 18 Jan 2016). [Google Scholar]