Abstract
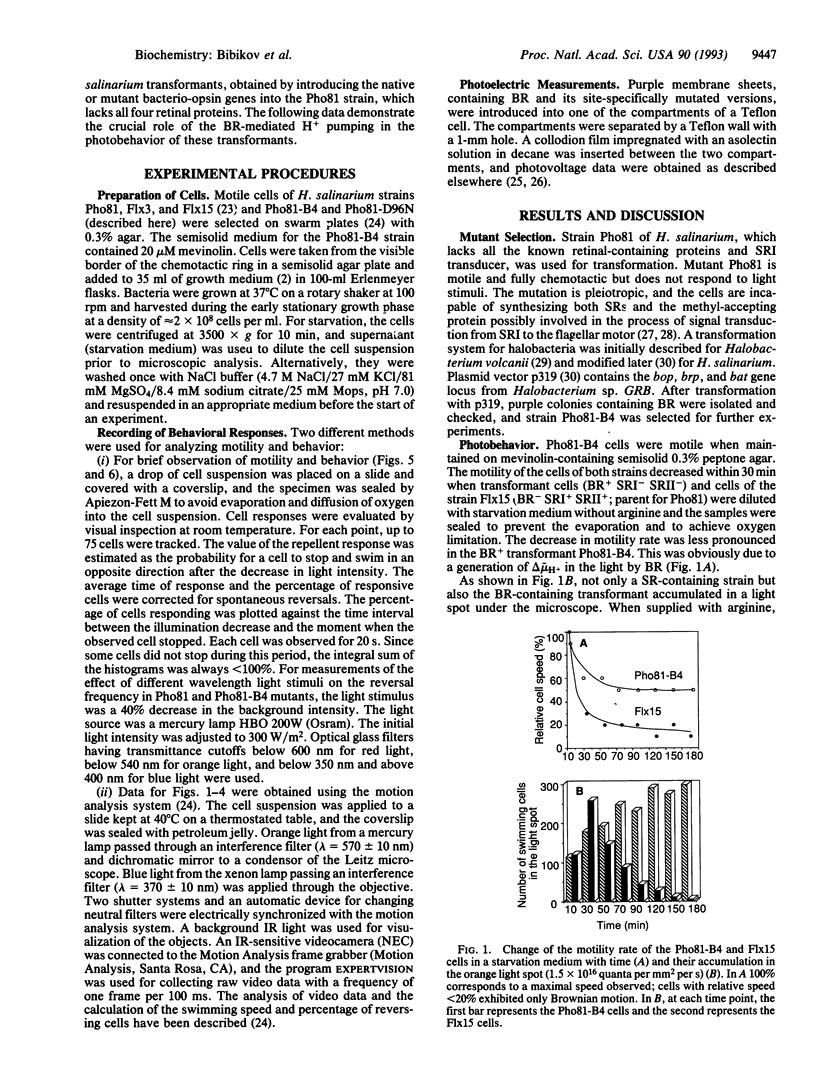
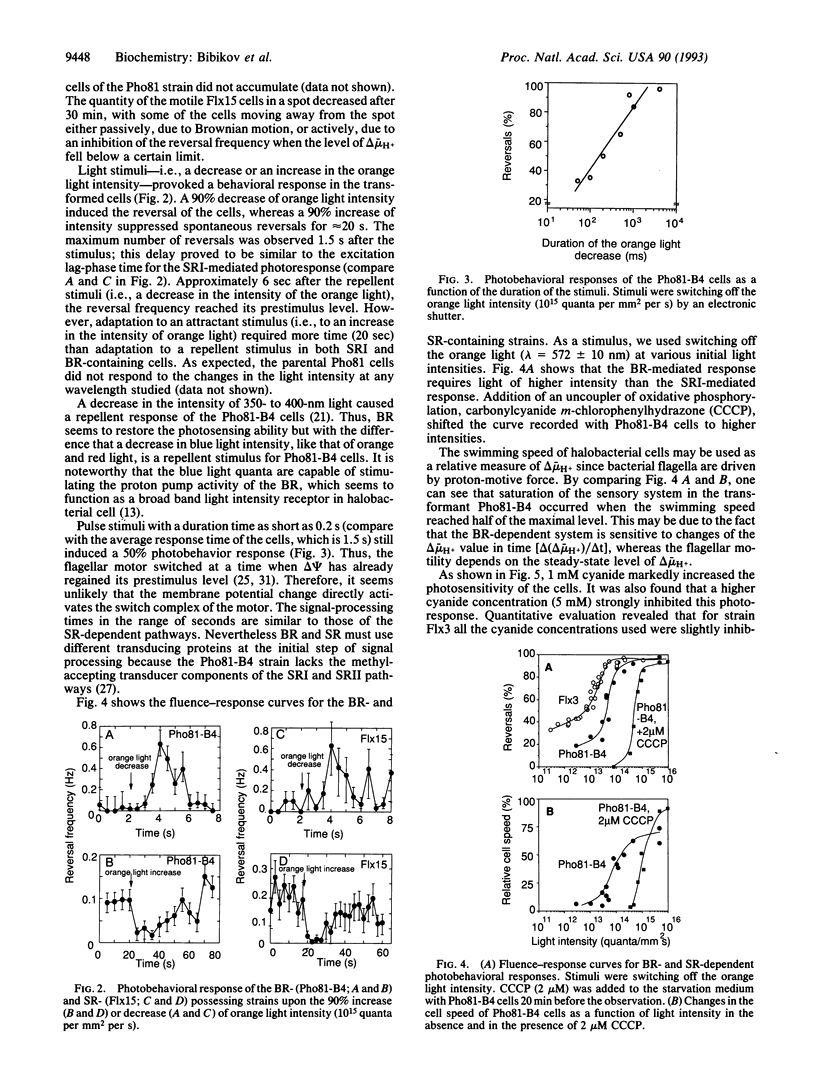
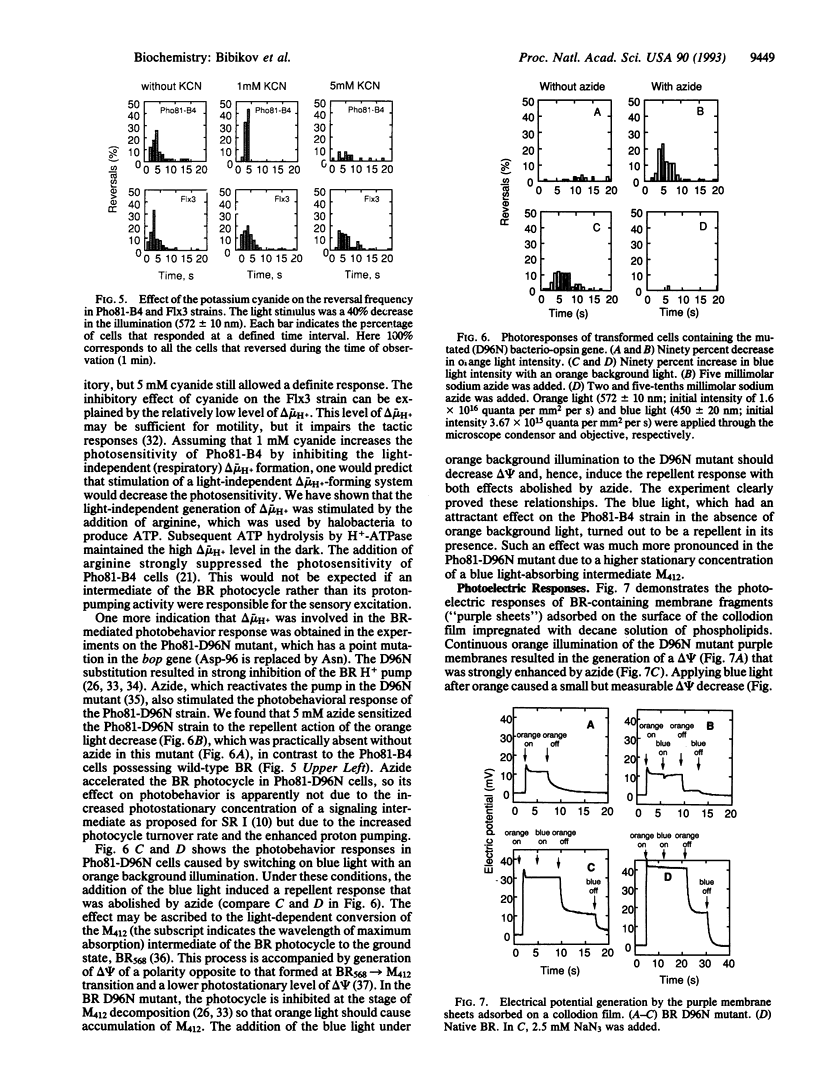
The bacterio-opsin gene was introduced into a "blind" Halobacterium salinarium mutant that (i) lacked all the four retinal proteins [bacteriorhodopsin (BR), halorhodopsin, and sensory rhodopsins (SRs) I and II] and the transducer protein for SRI and (ii) showed neither attractant response to long wavelength light nor repellent response to short wavelength light. The resulting transformed cells acquired the capability to sense light stimuli. The cells accumulated in a light spot, demonstrating the BR-mediated orientation in spatial light gradients. As in wild-type cells, a decrease in the intensity of long wavelength light caused a repellent response by inducing reversals of swimming direction, but, in contrast to wild-type cells, a decrease in the intensity of short wavelength light also repelled the cells. An increase in light intensity evoked an attractant response (i.e., a transient suppression of spontaneous reversals). Signal processing times and adaptation kinetics were similar to the SRI-mediated reactions. However, compared to SR-mediated photoresponses, higher light intensities were necessary to induce the BR-mediated responses. The light sensitivity of the transformant was increased by adding 1 mM cyanide and decreased by the addition of arginine, agents that respectively reduce and increase the light-independent generation of the electrochemical potential difference of H+ ions (delta mu H+). A decrease in irradiance to an intensity that was still high enough to saturate BR-initiated delta mu H+ changes failed to induce the repellent effect, but the addition of a protonophorous uncoupler sensitized the cell to these light stimuli. The BR D96N mutant (Asp-96 is replaced by Asn) with decreased proton pump activity showed strongly reduced BR-mediated responses. Azide, which increases this mutant's H+ pump efficiency, increased the photosensitivity of the mutant cells. Moreover, azide diminished (i) the membrane potential decreasing and (ii) repellent effects of blue light added to the orange background illumination in this mutant. We conclude that the BR-mediated photoreception is due to the light-dependent generation of delta mu H+. Our data are consistent with the assumption that the H. salinarium cell monitors the membrane energization level with a "protometer" system measuring total delta mu H+ changes or its electric potential difference component.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibikov S. I., Grishanin R. N., Marwan W., Oesterhelt D., Skulachev V. P. The proton pump bacteriorhodopsin is a photoreceptor for signal transduction in Halobacterium halobium. FEBS Lett. 1991 Dec 16;295(1-3):223–226. doi: 10.1016/0014-5793(91)81423-6. [DOI] [PubMed] [Google Scholar]
- Birge R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim Biophys Acta. 1990 Apr 26;1016(3):293–327. doi: 10.1016/0005-2728(90)90163-x. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline S. W., Lam W. L., Charlebois R. L., Schalkwyk L. C., Doolittle W. F. Transformation methods for halophilic archaebacteria. Can J Microbiol. 1989 Jan;35(1):148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- Dancsházy Z., Drachev L. A., Ormos P., Nagy K., Skulachev V. P. Kinetics of the blue light-induced inhibition of photoelectric activity of bacteriorhodopsin. FEBS Lett. 1978 Dec 1;96(1):59–63. doi: 10.1016/0014-5793(78)81062-x. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Kaulen A. D., Khitrina L. V., Skulachev V. P. Fast stages of photoelectric processes in biological membranes. I. Bacteriorhodopsin. Eur J Biochem. 1981 Jul;117(3):461–470. doi: 10.1111/j.1432-1033.1981.tb06361.x. [DOI] [PubMed] [Google Scholar]
- Gerwert K., Hess B., Soppa J., Oesterhelt D. Role of aspartate-96 in proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4943–4947. doi: 10.1073/pnas.86.13.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glagolev A. N. Reception of the energy level in bacterial taxis. J Theor Biol. 1980 Jan 21;82(2):171–185. doi: 10.1016/0022-5193(80)90097-1. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Sickinger H. D., Oesterhelt D. Quantitative aspects of energy conversion in halobacteria. FEBS Lett. 1977 Oct 1;82(1):1–6. doi: 10.1016/0014-5793(77)80873-9. [DOI] [PubMed] [Google Scholar]
- Holz M., Drachev L. A., Mogi T., Otto H., Kaulen A. D., Heyn M. P., Skulachev V. P., Khorana H. G. Replacement of aspartic acid-96 by asparagine in bacteriorhodopsin slows both the decay of the M intermediate and the associated proton movement. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2167–2171. doi: 10.1073/pnas.86.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley E. V., MacDonald R. E. A second mechanism for sodium extrusion in Halobacterium halobium: a light-driven sodium pump. Biochem Biophys Res Commun. 1979 May 28;88(2):491–499. doi: 10.1016/0006-291x(79)92075-8. [DOI] [PubMed] [Google Scholar]
- Manor D., Hasselbacher C. A., Spudich J. L. Membrane potential modulates photocycling rates of bacterial rhodopsins. Biochemistry. 1988 Aug 9;27(16):5843–5848. doi: 10.1021/bi00416a004. [DOI] [PubMed] [Google Scholar]
- Marwan W., Hegemann P., Oesterhelt D. Single photon detection by an archaebacterium. J Mol Biol. 1988 Feb 20;199(4):663–664. doi: 10.1016/0022-2836(88)90309-9. [DOI] [PubMed] [Google Scholar]
- Marwan W., Oesterhelt D. Quantitation of photochromism of sensory rhodopsin-I by computerized tracking of Halobacterium halobium cells. J Mol Biol. 1990 Sep 20;215(2):277–285. doi: 10.1016/S0022-2836(05)80346-8. [DOI] [PubMed] [Google Scholar]
- Ni B. F., Chang M., Duschl A., Lanyi J., Needleman R. An efficient system for the synthesis of bacteriorhodopsin in Halobacterium halobium. Gene. 1990 May 31;90(1):169–172. doi: 10.1016/0378-1119(90)90456-2. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Marwan W. Change of membrane potential is not a component of the photophobic transduction chain in Halobacterium halobium. J Bacteriol. 1987 Aug;169(8):3515–3520. doi: 10.1128/jb.169.8.3515-3520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982 Sep 10;257(17):10306–10313. [PubMed] [Google Scholar]
- Skulachev V. P. Membrane-linked energy buffering as the biological function of Na+/K+ gradient. FEBS Lett. 1978 Mar 15;87(2):171–179. doi: 10.1016/0014-5793(78)80326-3. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Hasselbacher C. A., Spudich J. L. Methyl-accepting protein associated with bacterial sensory rhodopsin I. J Bacteriol. 1988 Sep;170(9):4280–4285. doi: 10.1128/jb.170.9.4280-4285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984 Dec 6;312(5994):509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg S. A., Alam M., Lebert M., Spudich J. L., Oesterhelt D., Hazelbauer G. L. Characterization of Halobacterium halobium mutants defective in taxis. J Bacteriol. 1990 May;172(5):2328–2335. doi: 10.1128/jb.172.5.2328-2335.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Watanabe M., Kamo N., Kobatake Y. Negative phototaxis from blue light and the role of third rhodopsinlike pigment in halobacterium cutirubrum. Biophys J. 1985 Aug;48(2):235–240. doi: 10.1016/S0006-3495(85)83776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff E. K., Bogomolni R. A., Scherrer P., Hess B., Stoeckenius W. Color discrimination in halobacteria: spectroscopic characterization of a second sensory receptor covering the blue-green region of the spectrum. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7272–7276. doi: 10.1073/pnas.83.19.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Cline S. W., Doolittle W. F., Spudich J. L. Transformation of a bop-hop-sop-I-sop-II-Halobacterium halobium mutant to bop+: effects of bacteriorhodopsin photoactivation on cellular proton fluxes and swimming behavior. Photochem Photobiol. 1992 Oct;56(4):553–561. doi: 10.1111/j.1751-1097.1992.tb02200.x. [DOI] [PubMed] [Google Scholar]
- Yao V. J., Spudich J. L. Primary structure of an archaebacterial transducer, a methyl-accepting protein associated with sensory rhodopsin I. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11915–11919. doi: 10.1073/pnas.89.24.11915. [DOI] [PMC free article] [PubMed] [Google Scholar]


