INTRODUCTION
Sterile pyuria (SP) is not an uncommon finding in clinical practice. Nine per cent of patients presenting to their GP with lower urinary symptoms, and who are suspected of having urinary tract infections (UTIs), are found to have SP.1 It continues to pose a diagnostic conundrum to physicians, as well as to allied healthcare professionals (HCPs), because there are no guidelines on its management.
Currently no agreed definition for SP exists. It is simply the presence of white blood cells within the urine, in the absence of infection. Typically, the presence of more than 5–8 leucocytes per high-power field on microscopy, in the absence of positive urine cultures, would be classified as an SP. It can also be associated with haematuria, proteinuria, and casts, complicating the diagnosis.
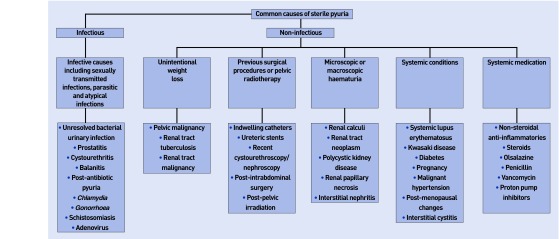
Broadly speaking, SP may be classified as infectious or non-infectious. This article reviews the many causes of SP (Figure 1), and aims to create a clear pathway in the management of these patients.
Figure 1.
Algorithm showing causes of sterile pyuria.
CLASSIFICATION
Infectious causes
Simple bacterial urinary tract infections (UTIs) are extremely common within the general population. UTIs are most often successfully treated in the community with empirical antibiotic therapy, with subsequent positive cultures confirming diagnosis. However, a recently treated UTI, usually within 2 weeks, or even after a single dose of antibiotics, can present as an SP.
In the younger, sexually active population presenting with SP, Chlamydia trachomatis is the most common organism discovered on subsequent cultures.2 Therefore, a sexual history should always be sought in young patients presenting with lower urinary tract symptoms. In the older population, prostatitis, cystouretheritis, and balanitis may present as SP. Furthermore, common viruses such as adenovirus and parasitic infections such as schistosomiasis have been implicated in SP. The clinician should always be vigilant to enquire about recent foreign travel.
In patients with chronic SP, atypical infection should be considered, in particular renal tuberculosis.3 Although a rare manifestation of the disease, its consequences may be disastrous for the patient if not diagnosed and treated early. Suspicion should be borne in mind in patients coming from endemic regions, the immunocompromised, and those presenting with unintentional weight loss.
Non-infectious causes
Pyuria has been noted in the absence of infection. Pelvic inflammation secondary to appendicitis can cause isolated pyuria if the appendix lies in close proximity to the bladder or ureter. In addition, radiotherapy involving the pelvis and urinary tract has also been implicated.4 Pyuria is a common finding after instrumentation of the urinary tract following cystourethroscopy or nephroscopy. Indwelling catheters and stents within the urinary tract are also well-established causes of SP.
However, when there is not a clear cause for the pyuria, the physician must consider other causes such as systemic disease, drug intake, and malignancy.
SP is often implicated in patients with underlying local disease, from benign conditions like renal calculi to neoplasms. When presenting with either microscopic or macroscopic haematuria, the clinician should always try to establish a cause. Malignancy may be associated with weight loss and, depending on the primary site and stage, palpable lymphadenopathy. Other local causes associated with haematuria include polycystic kidney disease and renal papillary necrosis. The latter is typically seen in patients with diabetes mellitus, sickle cell disease, and long-term analgesic use.4
Systemic conditions include systemic lupus erythematosus (SLE), Kawasaki disease, diabetes, sarcoidosis, and malignant hypertension.4 Physiological causes include post-menopausal changes and pregnancy. National Institute for Health and Care Excellence (NICE) guidelines recommend that any female suspected of UTI in pregnancy should be treated empirically according to local policies, with urine cultures sent before and after the antibiotic course.5 Community midwives and clinicians therefore need to know that repeated SP with negative bacteriuria during antenatal checks could imply physiological, benign pathology and help avoid unnecessary antibiotics. Conversely, it could suggest underlying disease for which the HCP should be alert.
Finally, drug intake is one of the forgotten yet common causes of SP. Olsalazine and nitrofurantoin have been reported to cause SP.4 The use of penicillin-based antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs),6 aspirin, proton pump inhibitors (PPIs), and diuretics has also been involved in acute drug reactions, causing tubulointerstitial nephritis with an SP.
MANAGEMENT
History and examination
A thorough clinical history is of the up-most importance in setting the physician on the correct diagnostic pathway. ‘Urinary tract symptoms such as dysuria and haematuria should alarming to the clinician. Furthermore, a detailed clinical examination is mandatory.
General findings like hypertension and pallor are important to consider. Skin rashes, oedema, muffled heart sounds, organomegaly, swollen joints, and lymphadenopathy are signs of more serious underlying pathology. Abdominal and pelvic examination, including a digital rectal examination and vaginal examination in females (except during pregnancy), is recommended.
Investigations and treatment
First, urinalysis is the prime investigation for SP. Importantly, SP is not always sterile, so repeating cultures often yields a positive result on subsequent testing. Contamination, especially with vaginal leukocytes in females, is common, and samples should always be collected as a midstream clean catch. The use of antiseptic solutions prior to collection should be avoided to limit false-negative tests.
Practice nurses may also encounter a previously undiagnosed SP during routine screening tests, or disease monitoring in patients with long-term conditions like diabetes. At this juncture, sending urine for culture and sensitivity is recommended, with onward advice to see their GP for further investigation, pending results.
Second, routine haematological tests including a full blood count, renal, and liver function tests are of paramount importance. Swabs for Chlamydia and Gonorrhoea are recommended for sexually active patients. If urinary tuberculosis is suspected, three consecutive first-void morning samples are required for acid-fast bacilli and polymerase chain reaction (PCR) testing.2
Eosinophilia is an important marker of drug-induced interstitial nephritis, but may also be seen in schistosomiasis. In suspected schistosomiasis, a terminal urine sample should be collected between noon and 3 pm, to coincide with maximal egg excretion.
Third, the choice of imaging modality depends on the history and findings on examination. A renal tract ultrasound scan or computerised tomography is recommended when renal stones, masses, or nephritis are differential diagnoses. Endourological procedures such as rigid or flexible cystourethroscopy and tissue sampling are undertaken if tumours are suspected, but they also have the advantage of diagnosing and treating benign pathologies such as bladder stones.
CONCLUSION
Sterile pyuria can pose a real diagnostic challenge for clinicians and the allied healthcare team. A good clinical history and systematic approach, together with a thorough examination and appropriate investigation, are the mainstay towards reaching a diagnosis and offering the correct treatment. Often, however, no cause can be identified. In this situation, a multidisciplinary approach and referral to a specialist centre should be the way forward for the patient.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Vellinga A, Cormican M, Hanahoe B, et al. Antimicrobial management and appropriateness of treatment of urinary tract infection in general practice in Ireland. BMC Fam Pract. 2011;12(1):108. doi: 10.1186/1471-2296-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nassar FA, Abu-Elamreen FH, Shubair ME, Sharif FA. Detection of Chlamydia trachomatis and Mycoplasma hominis, genitalium and Ureaplasma urealyticum by polymerase chain reaction in patients with sterile pyuria. Adv Med Sci. 2008;53(1):80–86. doi: 10.2478/v10039-008-0020-1. [DOI] [PubMed] [Google Scholar]
- 3.Gibson MS, Puckett ML, Shelly ME. Renal tuberculosis. Radiographics. 2004;24(1):251–256. doi: 10.1148/rg.241035071. [DOI] [PubMed] [Google Scholar]
- 4.Dieter RS. Sterile pyuria: a differential diagnosis. Compr Ther. 2000;26(3):150–152. doi: 10.1007/s12019-000-0001-1. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence Urinary tract infection (lower) — women Clinical Knowledge Summary. 2015. http://cks.nice.org.uk/urinary-tract-infection-lower-women [accessed 21 Jan 2016].
- 6.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60(2):804–817. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]